Published online Jul 28, 2017. doi: 10.3748/wjg.v23.i28.5237
Peer-review started: March 18, 2017
First decision: May 12, 2017
Revised: May 30, 2017
Accepted: July 12, 2017
Article in press: July 12, 2017
Published online: July 28, 2017
To validate prognostic scores for acute decompensation of cirrhosis and acute-on-chronic liver failure in Brazilian patients.
This is a prospective cohort study designed to assess the prognostic performance of the chronic liver failure-consortium (CLIF-C) acute decompensation score (CLIF-C AD) and CLIF-C acute-on-chronic liver failure score (CLIF-C ACLF), regarding 28-d and 90-d mortality, as well as to compare them to other prognostic models, such as Model for End-Stage Liver Disease (MELD), MELD Sodium (MELD-Na), Child-Pugh (CP) score, and the CLIF-C Organ Failure score (CLIF-C OF). All participants were adults with acute decompensation of cirrhosis admitted to the Emergency Department of a tertiary hospital in southern Brazil. Prognostic performances were evaluated by means of the receiver operating characteristic (ROC) curves, area under the curves (AUC) and 95%CI.
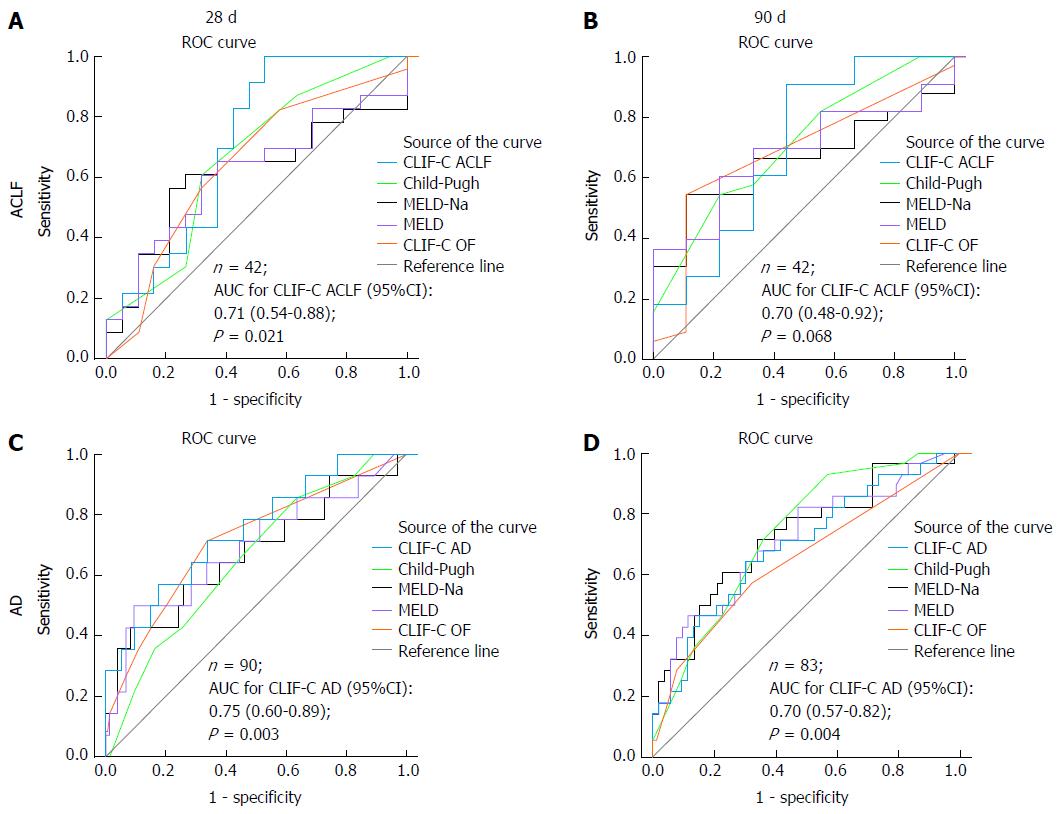
One hundred and thirteen cirrhotic patients were included. At admission, 18 patients had acute-on-chronic liver failure (ACLF) and 95 individuals had acute decompensation (AD) without ACLF, of which 24 eventually developed ACLF during the course of hospitalization (AD evolving to ACLF group). The AD group had significantly lower 28-d (9.0%) and 90-d (18.3%) mortality as compared to the AD evolving to ACLF group and to the ACLF group (both P < 0.001). On the other hand, 28-d and 90-d mortalities were not significantly different between AD evolving to ACLF group and ACLF group (P = 0.542 and P = 0.708, respectively). Among patients with ACLF, at 28 d from the diagnosis, CLIF-C ACLF was the only score able to predict mortality significantly better than the reference line, with an AUC (95%CI) of 0.71 (95%CI: 0.54-0.88, P = 0.021). Among patients with AD, all prognostic scores performed significantly better than the reference line regarding 28-d mortality, presenting with similar AUCs: CLIF-C AD score 0.75 (95%CI: 0.63-0.88), CP score 0.72 (95%CI: 0.59-0.85), MELD score 0.75 (95%CI: 0.61-0.90), MELD-Na score 0.76 (95%CI: 0.61-0.90), and CLIF-C OF score 0.74 (95%CI: 0.60-0.88). The same occurred concerning AUCs for 90-d mortality: CLIF-C AD score 0.70 (95%CI: 0.57-0.82), CP score 0.73 (95%CI: 0.62-0.84), MELD score 0.71 (95%CI: 0.59-0.83), MELD-Na score 0.73 (95%CI: 0.62-0.84), and CLIF-C OF score 0.65 (95%CI: 0.52-0.78).
This study demonstrated that CLIF-C ACLF is the best available score for the prediction of 28-d mortality among patients with ACLF. CLIF-C AD score is also useful for the prediction of mortality among cirrhotic patients with AD not fulfilling diagnostic criteria for ACLF, but it was not superior to other well-established prognostic scores.
Core tip: The present study demonstrated that chronic liver failure-consortium (CLIF-C) acute-on-chronic liver failure score (ACLF) is the best available score for the prediction of 28-d mortality among patients with ACLF, but it was unable to determine the same regarding 90-d mortality. On the other hand, while this study also demonstrated that CLIF-C acute decompensation (AD) was useful for the prediction of 28-d and 90-d mortalities among patients with AD not fulfilling diagnostic criteria for ACLF, it failed to identify superiority when compared to other scores already routinely used worldwide.
- Citation: Picon RV, Bertol FS, Tovo CV, de Mattos AZ. Chronic liver failure-consortium acute-on-chronic liver failure and acute decompensation scores predict mortality in Brazilian cirrhotic patients. World J Gastroenterol 2017; 23(28): 5237-5245
- URL: https://www.wjgnet.com/1007-9327/full/v23/i28/5237.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i28.5237
Cirrhosis is a relevant disease worldwide, both because of its high prevalence and because of its high mortality. In addition, this disease is responsible for substantial use of health care resources[1]. In Brazil, for instance, liver diseases are the eighth leading cause of death, and cirrhosis is the major cause of hospital admissions and lethality among them. Moreover, the burden of cirrhosis in terms of hospital admissions and mortality rate is still increasing, despite considerable medical advances[2]. Most of cirrhosis-related admissions and deaths are related to its acute decompensations.
Recently, a redefinition of acute-on-chronic liver failure (ACLF), an especially severe form of cirrhosis decompensation based on the occurrence of organic dysfunctions, was proposed by the European Association for the Study of the Liver - Chronic liver failure (CLIF) consortium. ACLF represents a syndrome occurring in patients with chronic liver disease, which is characterized by acute deterioration of liver function and one or more extrahepatic organ failures, leading to increased short-term mortality[3]. In the United States, from 2001 to 2011, the proportion of patients discharged from hospitals with a diagnosis of cirrhosis increased from 0.4% to 4.0%, while the proportion of those discharged with a diagnosis of ACLF increased from 0.3% to 6.0%[1]. Similarly, a recent French study demonstrated that, between 2008 and 2013, the annual proportion of cirrhotic patients with ACLF undergoing orthotopic liver transplantation (OLT) increased from 32% to 51%[4].
Considering the need to better predict the prognosis of patients with acute decompensations of cirrhosis, the CLIF Consortium proposed the use of two scores: one for patients with ACLF and the other for patients with acute decompensation (AD), but not ACLF[5,6]. Considering the lack of evidence about the performance of these scores outside Europe and taking into account possible differences regarding the epidemiological aspects of liver diseases and the characteristics of health care systems, this study aimed at validating their use in a Brazilian population.
This was a prospective cohort study conducted with a convenience consecutive sample of patients admitted to a tertiary hospital of Porto Alegre, southern Brazil. All participants were adults admitted to the Emergency Department of Nossa Senhora da Conceição Hospital with either acute decompensation of cirrhosis - AD group - or acute-on-chronic liver failure - ACLF group[3]. The AD group was further subdivided in two: (1) patients with AD that did not develop ACLF during hospital stay - hereafter AD group; and (2) patients admitted with AD that did develop ACLF at some point during the same hospitalization - henceforth AD evolving to ACLF group.
The evaluated outcome was transplant-free survival according to the diagnosis of AD or ACLF.
In order to evaluate the capacity of Chronic Liver Failure-Consortium Acute Decompensation (CLIF-C AD)[6] and Chronic Liver Failure-Consortium Acute-on-Chronic Liver Failure (CLIF-C ACLF) scores[5] in predicting death in 28 d and 90 d after the diagnosis, their sensitivity, specificity and positive and negative predictive values were calculated, and receiver operating characteristic (ROC) curves were developed. Several well-established severity scores employed for cirrhosis assessment were used as comparators: Model for End-Stage Liver Disease (MELD), MELD Sodium (MELD-Na), Child-Pugh (CP) score, and the Chronic Liver Failure-Consortium Organ Failure (CLIF-C OF) score[5-9].
Between January and September 2016, patients over 18 years-old admitted to the Emergency Department were screened by the International Classification of Diseases - 10th revision (ICD-10) codes and deemed eligible if codes K70 to K77 were stated at their hospital admission forms. Participants were cirrhotic patients undergoing a non-elective Emergency Department admission for acute decompensation - i.e., ascites, encephalopathy, gastrointestinal hemorrhage, or bacterial infection according to criteria stated by Moreau et al[3]. Cirrhosis was diagnosed based on histology or on clinical grounds, laboratory tests, imaging and endoscopic findings. Exclusion criteria were (1) elective hospitalization; and (2) non-elective Emergency Department admission for reasons different from acute decompensation of cirrhosis. For patients undergoing more than one hospitalization during the studied period, only data regarding the first admission was considered for analysis. Participants were followed until the end of December 2016.
Data were drawn from the electronic medical records of the patients. Data collection did not affect management of participants during hospital stay. Data extraction was carried-out on a pilot-tested Microsoft Excel™ spreadsheet by two authors (FSB and RVP).
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the institutional Ethical Committee. Informed consent was waived by the Ethical Committee.
Qualitative data were described as proportions, and Pearson’s χ2 or Fisher’s exact test was used for inferential analysis. Quantitative data were described as means ± SD, and analysis of variance (ANOVA) was used for comparison across three groups. Alpha was set at 0.05 and all comparisons were two-tailed.
OLT was regarded as a competing end-point, thus Kaplan-Meier survival analysis was undertaken assessing time to event as days from AD or ACLF diagnosis to death or OLT. Individuals who did not die nor were submitted to OLT were censored at the end of follow-up. Mantel-Cox log rank statistic was employed for inferential analysis across groups. Median survival times and their respective 95%CI according to group were obtained.
ROC curves, their corresponding areas under the curve (AUC) and respective AUC 95%CI were used to assess the performance of the CLIF-C AD score, CLIF-C ACLF score, CLIF-C OF score, MELD, MELD-Na, and CP score at 28 and 90 d from diagnosis. Patients lost to follow-up or with incomplete data were excluded from such analyses.
A univariate analysis was performed using all the scores and selected baseline clinical characteristics not embedded in the prediction scores as covariates and death at any point during the study as the dependent variable. Data analysis was performed using the Statistical Package for Social Sciences™ version 18.0.
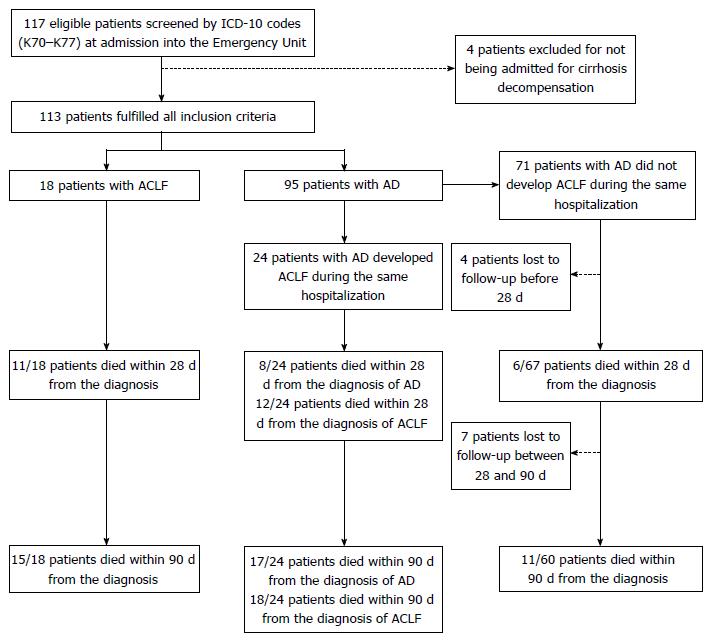
One hundred seventeen patients were considered eligible for the study, and 113 individuals fulfilled all the inclusion criteria. At admission, 18 (15.9%) patients had ACLF and 95 (84.1%) individuals had AD without ACLF, of which 24 eventually develop ACLF during the course of hospitalization (AD evolving to ACLF group). Figure 1 shows the flowchart of patients through the study.
Tables 1 and 2 describe, respectively, baseline clinical characteristics and laboratory findings of patients according to group. Of note, none of the patients from the AD group was admitted for spontaneous bacterial peritonitis (SBP), while 3 (12.5%) patients from the AD evolving to ACLF group and 2 (11.1%) of those from the ACLF group were admitted for SBP (P = 0.035). Over 70.0% of patients who were diagnosed with ACLF had ACLF grade 1. Kidney failure was the single most frequent organic failure, being present in 66.7% of patients with ACLF at admission and in 75.0% of those with AD evolving to ACLF. Moreover, mean serum C-reactive protein was approximately three times higher in the ACLF group compared to the AD group (77.5 mg/dL vs 25.5 mg/dL, P = 0.003).
| AD (n = 71) | AD evolving to ACLF (n = 24) | ACLF (n = 18) | ANOVA/pearson χ2 | ||||
| No. | % | No. | % | No. | % | P value | |
| mean | SD | mean | SD | mean | SD | ||
| Sex | 0.259 | ||||||
| Male | 48 | 67.6% | 13 | 54.2% | 14 | 77.8% | |
| Age (yr) | 57.8 | 10.2 | 62.6 | 8.0 | 61.3 | 12.9 | 0.097 |
| Primary etiology of cirrhosis | 0.592 | ||||||
| Alcohol | 23 | 32.4% | 11 | 45.8% | 9 | 50.0% | |
| HBV | 4 | 5.6% | 0 | 0.0% | 0 | 0.0% | |
| HCV | 34 | 47.9% | 9 | 37.5% | 6 | 33.3% | |
| NASH | 4 | 5.6% | 1 | 4.2% | 2 | 11.1% | |
| Other | 6 | 8.5% | 3 | 12.5% | 1 | 5.6% | |
| Main cause of current hospital admission | 0.035 | ||||||
| SBP | 0 | 0.0% | 3 | 12.5% | 2 | 11.1% | |
| Ascites - not SBP | 20 | 28.2% | 7 | 29.2% | 1 | 5.6% | |
| Encephalopathy | 15 | 21.1% | 6 | 25.0% | 5 | 27.8% | |
| Alcoholic hepatitis | 2 | 2.8% | 2 | 8.3% | 1 | 5.6% | |
| Digestive bleeding | 21 | 29.6% | 2 | 8.3% | 4 | 22.2% | |
| Sepsis | 0 | 0.0% | 0 | 0.0% | 1 | 5.6% | |
| Other | 13 | 18.3% | 4 | 16.7% | 4 | 22.2% | |
| No. of previous hospitalizations | 2.4 | 4.7 | 2.5 | 3.4 | 2.2 | 3 | 0.972 |
| Alcohol consumption in the past three months | 0.683 | ||||||
| Yes | 10 | 14.1% | 6 | 25.0% | 3 | 16.7% | |
| West Haven HE grade at admission | 0.005 | ||||||
| No HE | 54 | 76.1% | 15 | 62.5% | 7 | 38.9% | |
| Grades I and II | 13 | 18.3% | 9 | 37.5% | 7 | 38.9% | |
| Grades III and IV | 4 | 5.6% | 0 | 0.0% | 4 | 22.2% | |
| Vasopressor at admission | 0.070 | ||||||
| Yes | 0 | 0.0% | 0 | 0.0% | 1 | 5.6% | |
| Mechanical ventilation at admission | < 0.001 | ||||||
| Yes | 0 | 0.0% | 0 | 0.0% | 3 | 16.7% | |
| Mean arterial pressure (mmHg), n = 111 | 95.5 | 18.3 | 95.7 | 18.0 | 79.9 | 22.7 | 0.008 |
| PaO2/FiO2 (mmHg), n = 17 | 476.0 | 173.5 | 380.0 | No SD (n = 1) | 352.4 | 152.5 | 0.341 |
| SpO2/FiO2, n = 113 | 461.4 | 21.9 | 457.3 | 16.6 | 399.7 | 130.7 | < 0.001 |
| Child-Pugh score, n = 110 | 8.0 | 2.0 | 9.9 | 1.9 | 10.3 | 1.8 | < 0.001 |
| MELD score, n = 111 | 13.0 | 3.6 | 21.7 | 6.5 | 23.4 | 7.2 | < 0.001 |
| MELD-Na score, n = 111 | 14.0 | 4.6 | 23.8 | 5.8 | 23.9 | 8.9 | < 0.001 |
| CLIF-C OF score, n = 111 | 6.5 | 0.8 | 6.9 | 1.0 | 9.1 | 1.7 | < 0.001 |
| ACLF grade1 | |||||||
| ACLF grade 1 | NA | NA | 17 | 70.8% | 13 | 72.2% | 1.000 |
| ACLF grade 2 | NA | NA | 2 | 8.3% | 4 | 22.2% | 0.3752 |
| ACLF grade 3 | NA | NA | 5 | 20.8% | 1 | 5.6% | 0.2142 |
| AD (n = 71) | AD evolving to ACLF (n = 24) | ACLF (n = 18) | ANOVA | ||||
| Mean | SD | Mean | SD | Mean | SD | P value | |
| Total bilirubin (mg/dL), n = 112 | 2.1 | 2.2 | 5.1 | 5.3 | 5.7 | 7.2 | < 0.001 |
| Serum creatinine (mg/dL), n = 113 | 1.0 | 0.3 | 1.2 | 0.4 | 2.1 | 0.8 | < 0.001 |
| INR, n = 112 | 1.4 | 0.3 | 1.5 | 0.5 | 1.8 | 0.4 | < 0.001 |
| White-cell count (109 cells/L), n = 113 | 6.9 | 3.7 | 8.8 | 3.6 | 12.6 | 6.1 | < 0.001 |
| Serum sodium (mmol/L), n = 113 | 138.4 | 3.9 | 136.1 | 4.3 | 137.6 | 5.6 | 0.070 |
| Serum albumin (g/dL), n = 112 | 3.0 | 0.6 | 2.7 | 0.5 | 2.7 | 0.5 | 0.006 |
| Serum C-reactive protein (mg/L), n = 47 | 25.5 | 33.0 | 41.9 | 32.0 | 77.5 | 61.7 | 0.003 |
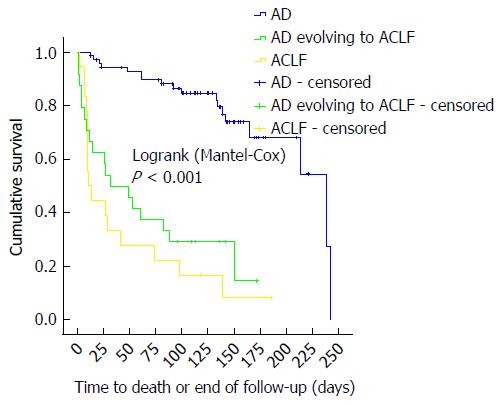
Median survival times for ACLF group (11.0 d) and AD evolving to ACLF group (32.0 d) did not differ significantly (P = 0.247). Merging the ACLF and the AD evolving to ACLF groups into a single cohort yielded a median survival time of 27.0 d (95%CI: 7.97-46.03; data not shown), which differed significantly from the median survival of 239 d of the AD group (P < 0.001). The AD group had significantly lower 28-d (9.0%) and 90-d (18.3%) mortality as compared to the AD evolving to ACLF group and to the ACLF group (both P < 0.001). The latter groups had comparable 28-d and 90-d mortalities (P = 0.542 and P = 0.708, respectively). At 28 d from the diagnosis of ACLF (either at admission or during hospitalization), 54.8% (23/42) of patients had died, whereas, at 90 d, 78.6% (33/42) of them had died. One patient from the ACLF group and seven others from the AD group died after 90 d of follow-up. Kaplan-Meier analysis revealed that patients with ACLF grades 1, 2, and 3 had significantly different survivals (Mantel-Cox P = 0.031), with median survival times of 29.0 d (95%CI: 4.3-53.7), 15.0 d (95%CI: 0.0-52.2), and 3.7 d (95%CI: 0.0-14.2) respectively (data not shown). No patient was submitted to OLT. Figure 2 depicts the Kaplan-Meier survival analysis, and Table 3 shows median survival times and mortalities according to group.
| Group (group symbol) | Survival time1 | Mortality1 | ||||
| Median (d) | Mantel-Cox | 28-d mortality | 90-d mortality | Death at any point during the study | ||
| Estimate | (95%CI) | P value | n (%) | n (%) | n (%) | |
| AD (A), n = 71 | 239 | (166.4-311.6) | Reference group | 6/67 (9.0)2 | 11/60 (18.3)2 | 18/60 (30.1)2 |
| AD evolving to ACLF (B), n = 24 | 32 | (0.0-64.4) | B vs A < 0.001; | 12/24 (50.0)23 | 18/24 (75.0)23 | 18/24 (75.0)23 |
| B vs C = 0.247 | ||||||
| ACLF (C), n = 18 | 11 | (2.7-19.3) | C vs A < 0.001; | 11/18 (61.1)23 | 15/18 (83.3)23 | 16/18 (88.9)23 |
| B + C vs A < 0.001 | ||||||
| Overall, n = 113 | 165 | (117.7-212.3) | Not applicable | 29/109 (26.6) | 44/102 (43.1) | 52/102 (51.0) |
Figure 3 illustrates the ROC curves for mortality of several prognostic scores at 28 and 90 d from the diagnosis of either AD or ACLF. Since 24 patients developed ACLF during hospitalization, they contributed with data to both the CLIF-C AD and the CLIF-C ACLF ROC curves. One patient from the AD group did not have sufficient data to calculate CLIF-C AD score and was excluded from ROC curve analyses. Four patients from the AD group had follow-ups shorter than 28 d, hence they were also excluded from the 28-d and 90-d ROC curves. Furthermore, another seven individuals from the AD group were lost to follow-up between 28 and 90 d and were excluded from the 90-d ROC curve. Among patients with ACLF, at 28 d from the diagnosis, CLIF-C ACLF was the only score able to predict mortality significantly better than the reference line, with an AUC of 0.71 (95%CI: 0.54-0.88, P = 0.021). Considering a pretest probability of death at 28 d of 54.8% (23/42 patients with the diagnosis of ACLF at some point of the study), a CLIF-C ACLF score cut-off of 40 points yielded 100% sensitivity (Se), 37% specificity (Sp), 100% negative predictive value (NPV), and 65.8% positive predictive value (PPV), whereas a cut-off of 60 points yielded 4% Se, 100% Sp, 46.2% NPV, and 100% PPV. The optimum cut-off value for the present study was 43.9 points, which returned 82.6% Se and 57.9% Sp. None of the several prognostic scores was superior to the reference line regarding 90-d mortality.
Regarding 28-d mortality among patients with AD, all the prognostic scores performed significantly better than the reference line and had similar AUCs when compared to each other: CLIF-C AD score 0.75 (95%CI: 0.63-0.88), CP score 0.72 (95%CI: 0.59-0.85), MELD score 0.75 (95%CI: 0.61-0.90), MELD-Na score 0.76 (95%CI: 0.61-0.90), and CLIF-C OF score 0.74 (95%CI: 0.60-0.88). At 90 d from the diagnosis, all prognostic scores also performed significantly better than the reference line, with comparable AUCs: CLIF-C AD score 0.70 (95%CI: 0.57-0.82), CP score 0.73 (95%CI: 0.62-0.84), MELD score 0.71 (95%CI: 0.59-0.83), MELD-Na score 0.73 (95%CI: 0.62-0.84), and CLIF-C OF score 0.65 (95%CI: 0.52-0.78). Considering a pretest probability of death at 90 d of 33.3% (28/84 patients; 95 patients with AD at admission minus 11 individuals lost to follow-up), a CLIF-C AD score cut-off of 45 points yielded 93% Se, 20% Sp, 85.1% NPV, and 36.7% PPV, while a cut-off of 60 points yielded 25% Se, 90.9% Sp, 70.8% NPV, and 57.8% PPV. The optimum cut-off value for this study was 53.4 points, which returned 65.5% Se and 72.2% Sp.
Univariate analysis for death at any given time during the study was performed. The covariates evaluated were sex, alcohol ingestion three months prior to admission, alcohol-induced cirrhosis, digestive bleeding, SBP, history of any previous hospitalization, CLIF-C OF score, CLIF-C AD score, CLIF-C ACLF score, CP score, MELD, and MELD-Na. According to the univariate analysis, the only variable associated to death was CLIF-C ACLF score (P = 0.009). Considering that the univariate analysis identified only one variable associated to this outcome, a multivariate analysis was not performed.
Considering the high prevalence, mortality and impact on the healthcare systems associated to cirrhosis decompensations, it is of great importance to develop tools which could better predict outcomes of cirrhotic patients[1]. The present study demonstrated that CLIF-C ACLF is the best available score for the prediction of 28-d mortality among patients with ACLF, as previously suggested[5], but it was unable to demonstrate the same regarding 90-d mortality, probably because most of the deaths in this group of patients occurred early in their follow-up. On the other hand, while this study also demonstrated that CLIF-C AD was useful for the prediction of 28-d and 90-d mortalities among patients with AD not fulfilling diagnostic criteria for ACLF, it failed to identify superiority when compared to other scores, some of them already routinely used worldwide. This finding differs from that of the study that originally proposed CLIF-C AD score, in which it performed better than other scores, at least for the prediction of 90-d mortality[6]. This could be explained by our sample of patients with AD not being large enough in order to detect a difference between the performance of CLIF-C AD score and that of other prognostic scores. Nevertheless, it is important to remind that our sample was sufficiently large to demonstrate that CLIF-C AD score had an AUC significantly greater than that of the reference line.
In the present study, ACLF was diagnosed in 37% of patients with decompensated cirrhosis, which is similar to what was previously reported[10]. The group of patients that fulfilled the diagnostic criteria for ACLF had a median survival time of 27 d, which differed significantly from the median survival of 239 d of the AD group (P < 0.001), corroborating the idea that ACLF is a distinct syndrome with an elevated short-term mortality[3]. Moreover, as also previously described[3], the prognosis of patients diagnosed with ACLF at hospital admission was not significantly different from that of patients who developed ACLF during hospitalization. Therefore, patients should be managed similarly irrespective of the moment of the diagnosis of ACLF.
The poor prognosis of ACLF was also demonstrated in a North American population of infected cirrhotic patients. In that study, 30-d survival was 51.3% for patients with ACLF and 91.4% for those without ACLF[11], which is similar to what we found (28-d survival of 38.9% for patients with ACLF and 91% for those without ACLF). Nevertheless, it is important to highlight that the definition of ACLF used in that study is not the same as that suggested by the CLIF Consortium and used in the present study.
Using the CLIF Consortium definition of ACLF, Soares e Silva et al[12] also showed that ACLF was a strong predictor of short-term mortality in a Brazilian population. Similar findings were described as well in a recent Argentina study[13]. However, to the best of our knowledge, the present study is the first to validate CLIF-C AD and CLIF-C ACLF scores outside Europe.
Other interesting finding of our study is that patients with ACLF more frequently had alcohol-related cirrhosis and less frequently had hepatitis C virus. These results are similar to those reported in the CANONIC study[3] and also corroborate what Jeong et al[14] have shown in their paper.
ACLF differs from traditional decompensated cirrhosis, not only because of the presence of organ failure(s) and higher mortality rate, but also because it happens more frequently in younger patients and in those with an alcoholic etiology of cirrhosis, as well as because it is associated to higher prevalences of some precipitating events (bacterial infections, active alcoholism, among others), and to a higher level of systemic inflammation[10]. As expected, in our study, we found that serum C-reactive protein and leukocyte count were significantly higher in the ACLF group compared to the AD group. This corroborates the systemic inflammation hypothesis[15], which suggests that ACLF is caused by an aggravation of systemic inflammation and by the associated systemic circulatory dysfunction already present in decompensated cirrhosis. According to this hypothesis, these processes would lead to organ failures as a consequence of hypoperfusion and the direct deleterious effects of inflammatory mediators on microcirculation and on cell physiology homeostasis[15]. The systemic inflammation hypothesis was based on the CANONIC study, in which it was observed a close relationship between blood leukocytes and C-reactive protein levels and the presence and severity of ACLF[3]. In this context, the association between the course of systemic inflammation and the course of ACLF was also recently demonstrated[16]. The relevance of the inflammatory state to the prognosis of cirrhotic patients is such that prognostic models incorporating C-reactive protein are also being studied[17,18].
Among the strengths of the present study, one must highlight its prospective character and the large sample of patients enrolled in a single centre. On the other hand, this study also presents limitations. One of the limitations, contradictorily, regards the fact that this was a single-center study, and its results might not reflect the reality elsewhere. For instance, despite being one of the largest hospitals in southern Brazil, our institution does not have a liver transplantation program, which does not preclude patients from being referred to others hospitals, but might explain why none of the studied patients was submitted to OLT. Nevertheless, we understand that the characteristics of our institution are similar to those of the majority of tertiary hospitals in Brazil and probably in Latin America and, therefore, we believe in the external validity of our findings.
Another limitation is the fact that we did not perform a sequential assessment of ACLF status and of the scores of the patients during hospitalization, which has recently been demonstrated useful[19,20]. This assessment was not planned prior to data collection, and we did not have sufficient data to perform it.
Yet another limitation of this study concerns missing data for some of the evaluated parameters, which is explained by the fact that authors did not interfere with the management of the patients. However, it should be noticed that the main analyses of this study were not affected by missing data.
In conclusion, in order to improve the quality of care of cirrhotic patients, it is of the utmost importance to be able to accurately predict the prognosis of decompensated cirrhosis. This study has shown that CLIF-C ACLF is the most accurate score to predict mortality of patients with ACLF in a Brazilian setting. Moreover, it has demonstrated that CLIF-C AD score is also useful for the prediction of mortality among cirrhotic patients with AD not fulfilling diagnostic criteria for ACLF, but it was not superior to other well-established prognostic scores.
Most of cirrhosis-related admissions and deaths are related to acute decompensations.
Acute-on-chronic liver failure (ACLF) has a significantly higher mortality compared to other forms of acute decompensation (AD) of cirrhosis. Two recently developed scores - CLIF-C ACLF and CLIF-C AD - were proposed as tools for assessing prognosis of ACLF and AD. They were validated in European patients.
To the best of our knowledge, this is the first study to validate CLIF-C AD and CLIF-C ACLF scores outside Europe.
In order to aid clinical decision-making and to improve the quality of care of cirrhotic patients, it is of the utmost importance to accurately predict the prognosis of AD and ACLF. This study has shown that CLIF-C ACLF is the most accurate score to predict mortality of patients with ACLF in a Brazilian setting. Moreover, it has also shown that CLIF-C AD score is also useful to predict mortality among cirrhotic patients with AD not fulfilling diagnostic criteria for ACLF, but it was not superior to other well-established prognostic scores.
ACLF: acute-on-chronic liver failure - i.e., acute decompensation of cirrhosis with at leats two organ failures, or one organ failure plus renal dysfunction, or one organ failure plus mild encephalopathy, or isolated renal failure; AD: acute decompensation of cirrhosis - i.e., digestive bleeding, large-volume ascites, hepatic encephalopathy, or bacterial infection.
The manuscript is novel one describing one of the most important issues in the diagnosis and mangement of decompensated and liver failure cases but some comments are to be considered: As the definition of ACLF it is a specific syndrome characterized by AD, organ failure and high short-term mortality.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): A, A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Arroyo V, El-Bendary MM, Jarcuska P, Lee HC S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF
| 1. | Allen AM, Kim WR, Moriarty JP, Shah ND, Larson JJ, Kamath PS. Time trends in the health care burden and mortality of acute on chronic liver failure in the United States. Hepatology. 2016;64:2165-2172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 2. | Nader LA, de Mattos AA, Bastos GA. Burden of liver disease in Brazil. Liver Int. 2014;34:844-849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1-1437.e9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1720] [Cited by in F6Publishing: 1860] [Article Influence: 169.1] [Reference Citation Analysis (3)] |
| 4. | Levesque E, Winter A, Noorah Z, Daurès JP, Landais P, Feray C, Azoulay D. Impact of acute-on-chronic liver failure on 90-day mortality following a first liver transplantation. Liver Int. 2017;37:684-693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 5. | Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Ginès P, Levesque E, Durand F, Angeli P, Caraceni P. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61:1038-1047. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 557] [Cited by in F6Publishing: 624] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 6. | Jalan R, Pavesi M, Saliba F, Amorós A, Fernandez J, Holland-Fischer P, Sawhney R, Mookerjee R, Caraceni P, Moreau R. The CLIF Consortium Acute Decompensation score (CLIF-C ADs) for prognosis of hospitalised cirrhotic patients without acute-on-chronic liver failure. J Hepatol. 2015;62:831-840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 230] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 7. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3462] [Cited by in F6Publishing: 3432] [Article Influence: 149.2] [Reference Citation Analysis (0)] |
| 8. | Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, Edwards E, Therneau TM. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018-1026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 935] [Cited by in F6Publishing: 948] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 9. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [PubMed] [Cited in This Article: ] |
| 10. | Moreau R, Arroyo V. Acute-on-chronic liver failure: a new clinical entity. Clin Gastroenterol Hepatol. 2015;13:836-841. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Bajaj JS, O’Leary JG, Reddy KR, Wong F, Biggins SW, Patton H, Fallon MB, Garcia-Tsao G, Maliakkal B, Malik R, Subramanian RM, Thacker LR, Kamath PS; North American Consortium For The Study Of End-Stage Liver Disease (NACSELD). Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology. 2014;60:250-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 358] [Cited by in F6Publishing: 379] [Article Influence: 37.9] [Reference Citation Analysis (1)] |
| 12. | Silva PE, Fayad L, Lazzarotto C, Ronsoni MF, Bazzo ML, Colombo BS, Dantas-Correa EB, Narciso-Schiavon JL, Schiavon LL. Single-centre validation of the EASL-CLIF consortium definition of acute-on-chronic liver failure and CLIF-SOFA for prediction of mortality in cirrhosis. Liver Int. 2015;35:1516-1523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 13. | Dominguez C, Romero E, Graciano J, Fernandez JL, Viola L. Prevalence and risk factors of acute-on-chronic liver failure in a single center from Argentina. World J Hepatol. 2016;8:1529-1534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Jeong JH, Park IS, Kim DH, Kim SC, Kang C, Lee SH, Kim TY, Lee SB. CLIF-SOFA score and SIRS are independent prognostic factors in patients with hepatic encephalopathy due to alcoholic liver cirrhosis. Medicine (Baltimore). 2016;95:e3935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Bernardi M, Moreau R, Angeli P, Schnabl B, Arroyo V. Mechanisms of decompensation and organ failure in cirrhosis: From peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63:1272-1284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 347] [Cited by in F6Publishing: 367] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 16. | Clària J, Stauber RE, Coenraad MJ, Moreau R, Jalan R, Pavesi M, Amorós À, Titos E, Alcaraz-Quiles J, Oettl K, Morales-Ruiz M, Angeli P, Domenicali M, Alessandria C, Gerbes A, Wendon J, Nevens F, Trebicka J, Laleman W, Saliba F, Welzel TM, Albillos A, Gustot T, Benten D, Durand F, Ginès P, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL-CLIF Consortium and the European Foundation for the Study of Chronic Liver Failure (EF-CLIF). Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure. Hepatology. 2016;64:1249-1264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 408] [Cited by in F6Publishing: 472] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 17. | Di Martino V, Coutris C, Cervoni JP, Dritsas S, Weil D, Richou C, Vanlemmens C, Thevenot T. Prognostic value of C-reactive protein levels in patients with cirrhosis. Liver Transpl. 2015;21:753-760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Ruf AE, Villamil FG. C-reactive protein and model for end-stage liver disease score: Have we found the fifth element? Liver Transpl. 2015;21:713-715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Gustot T, Fernandez J, Garcia E, Morando F, Caraceni P, Alessandria C, Laleman W, Trebicka J, Elkrief L, Hopf C. Clinical Course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology. 2015;62:243-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 372] [Cited by in F6Publishing: 407] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 20. | Arroyo V, Moreau R. Diagnosis and prognosis of acute on chronic liver failure (ACLF) in cirrhosis. J Hepatol. 2017;66:451-453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |