Published online Jul 21, 2017. doi: 10.3748/wjg.v23.i27.4920
Peer-review started: March 2, 2017
First decision: March 16, 2017
Revised: March 31, 2017
Accepted: June 18, 2017
Article in press: June 19, 2017
Published online: July 21, 2017
To investigate the effects of hydrogen-rich water (HRW) treatment on prevention of ethanol (EtOH)-induced early fatty liver in mice.
In vitro reduction of hydrogen peroxide by HRW was determined with a chemiluminescence system. Female mice were randomly divided into five groups: control, EtOH, EtOH + silymarin, EtOH + HRW and EtOH + silymarin + HRW. Each group was fed a Lieber-DeCarli liquid diet containing EtOH or isocaloric maltose dextrin (control diet). Silymarin was used as a positive control to compare HRW efficacy against chronic EtOH-induced hepatotoxicity. HRW was freshly prepared and given at a dosage of 1.2 mL/mouse trice daily. Blood and liver tissue were collected after chronic-binge liquid-diet feeding for 12 wk.
The in vitro study showed that HRW directly scavenged hydrogen peroxide. The in vivo study showed that HRW increased expression of acyl ghrelin, which was correlated with food intake. HRW treatment significantly reduced EtOH-induced increases in serum alanine aminotransferase, aspartate aminotransferase, triglycerol and total cholesterol levels, hepatic lipid accumulation and inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-6. HRW attenuated malondialdehyde level, restored glutathione depletion and increased superoxide dismutase, glutathione peroxidase and catalase activities in the liver. Moreover, HRW reduced TNF-α and IL-6 levels but increased IL-10 and IL-22 levels.
HRW protects against chronic EtOH-induced liver injury, possibly by inducing acyl ghrelin to suppress the pro-inflammatory cytokines TNF-α and IL-6 and induce IL-10 and IL-22, thus activating antioxidant enzymes against oxidative stress.
Core tip: Hydrogen-rich water (HRW), a safe and effective antioxidant with minimal side effects, is used in preventive and clinical applications. Few studies have investigated the effects of hydrogen on early alcoholic liver disease. The present study evaluated the potential protective effects of HRW against chronic ethanol (EtOH)-induced early liver injury and the underlying mechanisms in female mice after chronic-plus-binge EtOH feeding. HRW pretreatment protected against mild EtOH-induced liver injury, possibly by inducing acyl ghrelin to suppress tumor necrosis factor-alpha and interleukin (IL)-6 and induce IL-10 and IL-22, thereby activating antioxidant enzymes against oxidative stress. These results suggest that HRW helps prevent and treat EtOH-induced early liver injury.
- Citation: Lin CP, Chuang WC, Lu FJ, Chen CY. Anti-oxidant and anti-inflammatory effects of hydrogen-rich water alleviate ethanol-induced fatty liver in mice. World J Gastroenterol 2017; 23(27): 4920-4934
- URL: https://www.wjgnet.com/1007-9327/full/v23/i27/4920.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i27.4920
Sustained excessive alcohol consumption results in a spectrum of liver injury, from hepatic steatosis to hepatitis, fibrosis and cirrhosis, which can ultimately lead to hepatocellular carcinoma[1-4]. Among heavy drinkers, the incidence of hepatic steatosis is about 95%. Risk factors potentially associated with alcoholic liver disease (ALD) include sex, obesity, dietary factors, smoking and non-sex-linked genetic factors. Among humans and rodents, females are more susceptible to ALD, even if they consume less alcohol than males. This may be attributable to lower gastric alcohol dehydrogenase activity, lower distributed volume of alcohol and estrogen, which has a substantial effect on alcohol-induced hepatotoxicity[5-7].
ALD pathogenesis is mediated by increased steatosis, inflammatory factors, oxidative stress and immune responses. Ethanol (EtOH) impairs antioxidant defenses and mitochondrial functions and may trigger a burst of reactive oxygen species (ROS), thus resulting in hepatotoxicity, steatosis, inflammation and fibrosis[1,2]. ROS can also induce hepatocellular responses strongly associated with Kupffer cell activation, which increases inflammatory response and leads to liver injury. Moreover, activated Kupffer cells release ROS and cytokines that are crucial in activating hepatic stellate cells (HSCs) and inducing the pro-fibrogenic pathway[1,2].
Previous studies reported that oxidative stress and sensitization to endotoxins contributing to EtOH-induced liver injury are associated with release of pro-inflammatory mediators, promotion of lipid peroxidation and impaired hepatic antioxidant defense[1,2,8]. Activation of pro-inflammatory cytokines, particularly nitric oxide synthase, cyclooxygenase 2, transcription factor nuclear factor-κB, tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-6 is crucial in ALD progression, which leads to hepatocellular injury and death[1-3,6,9].
IL-22, a novel hepatoprotective factor, is a member of the IL-10 family of cytokines and appears to be an important effector molecule of activated T cells and natural killer cells. The main biological roles of IL-22 are to promote innate immunity, improve regeneration and protect against damage[10]. Evidence from several studies suggests that IL-22 - through antioxidant and anti-apoptotic pathways - exerts protective effects against hepatic injury induced by concanavalin A[11], carbon tetrachloride (CCl4)[12] and EtOH[3]. Ghrelin, a 28-amino acid peptide produced in gastric mucosa, acts in the hypothalamus to promote appetite and inhibit sympathetic activity, thus increasing food intake while lowering metabolic rate[13,14]. Recent studies suggest that ghrelin has various biological functions, including anti-oxidation, anti-inflammation, anti-autoimmunity and promotion of vascular health[13-16].
Because of its effective scavenging of ROS, molecular hydrogen (H2) has potent systemic antioxidant activity[17,18]. Approaches to administering H2 include inhalation, injection, oral administration and immersion. Oral administration of H2-rich water (HRW) was easier, safer and more economical as a means to protect against oxidative stress-induced injury in multiple animal models of human diseases. H2 was successfully used in a number of in vivo studies of hepatic injury, which examined conditions such as ischemia reperfusion injury, obstructive jaundice, acute hepatic failure and nonalcoholic steatohepatitis[19-25].
Our previous research indicated that electrolyzed reduced water and silica hydride, which contains H2, ameliorated CCl4-induced hepatotoxicity in mice by enhancing antioxidant enzyme activity and reducing lipid oxidation[26,27]. Additionally, consumption of more than 20 mL/kg per day of HRW had no observable adverse effects, which suggests a 60-kg human could safely drink at least 1.2 L/d of HRW[28]. Thus, HRW could be used in preventive and clinical applications as a safe and effective antioxidant with minimal side effects[17-21,23,28-31]. Recent clinical studies found that HRW reduced oxidative stress in persons with chronic hepatitis B[32] and metabolic syndrome[31].
The effects of H2 on chronic EtOH-induced liver injury are not well understood. The Lieber-DeCarli liquid diet has been extensively used as the typical approach to establish a chronic-plus-binge EtOH feeding model that mimics some of the molecular and histological features of mild, early-stage human ALD[3,33,34]. This model induces mild steatosis and elevations in serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in C57BL/6 mice. The elevations are much more severe than those seen in mice on chronic EtOH or single EtOH gavage alone diets. This study investigated the potential protective effects of HRW against chronic EtOH-induced early-stage liver injury and the underlying mechanisms of such effects in female mice after chronic-plus-binge EtOH feeding.
HRW was prepared by inducing a chemical reaction between metallic magnesium and water [Mg + 2H2O/Mg(OH)2 + H2][35]. The Daily Inner T505 Hydrogenerator apparatus (Unitiva Applied Materials Corp., Taipei, Taiwan) was used to generate HRW. Briefly, to produce HRW, a metallic magnesium stick (T505, 175 g Mg Chips) containing 99.99% pure metallic magnesium in a polypropylene and ceramic container was placed in distilled water with a flow rate set at 400 mL/min. The resulting H2 content was 500-600 parts per billion (ppb). HRW was freshly prepared and immediately diluted to pre-specified concentrations for use in vitro and in vivo.
HRW was analyzed by multiple devices. To analyze H2 content and oxidation-reduction potential (ORP), HRW was freshly prepared in a capped vial and immediately measured with a dissolved H2 portable meter (ENH-1000; Trustlex Co., Ltd, Tokyo, Japan) and ORP portable meter (MP220; Mettler Toledo, Zurich, Switzerland). Analysis of free radical scavenging activity was performed by modifying previously described methods[35,36]. In brief, a mixture of 0.1 mL of H2O2 solution (97 mmol/L in distilled water) and 0.4 mL of sample was loaded in the stainless steel container of a chemiluminescence analysis system (CLA-2100; Tohoku Electronic Co., Ltd, Sendai, Japan) for 60 s. Next, 0.1 mL of luminol solution (3 mmol/L in phosphate-buffered saline, pH 7.4) was immediately injected into the dark chamber of the chemiluminescence analyzer. Then, chemiluminescence intensity was continuously recorded for 120 s. Scavenging activity (%) was defined as [(Sum1 - Sum2)/Sum1] × 100%.
All procedures involving animals were reviewed by the Institutional Animal Care and Use Committee of Chung-Shan Medical University Experimental Animal Center (IACUC approval no. 1745). Female C57BL/6 mice (age 5 wk) were purchased from BioLasco Taiwan (Ilan, Taiwan) and acclimatized to the environment for 1 wk. All mice were handled under standard laboratory conditions (temperature 22 ± 2 °C, humidity 55% ± 5% and 12-h light-dark cycle). Then, mice were allowed ad libitum access to a controlled Lieber-DeCarli diet for 1 wk, to acclimatize to the liquid diet before the experiment. The liquid diets provided 1 kcal/mL (prepared by Dyets, Inc., Bethlehem, PA, United States), in accordance with the Lieber-DeCarli formulation. This nutritional diet (containing 41.4 g/L casein, 0.5 g/L L-cystine, 0.3 g/L DL-methionine, 8.5 g/L corn oil, 28.4 g/L olive oil, 2.7 g/L safflower oil, 115.2 g/L maltose dextrin, 10 g/L cellulose, 8.75 g/L mineral mix, 2.5 g/L vitamin mix, 0.53 g/L choline bitartrate and 3 g/L xanthan gum) allowed for the prolonged exposure of EtOH in a rodent model and allowed for modification to calories provided by EtOH[2,3,5,33,34].
Mice in the present study were assigned to 5 groups (n = 8-10 each), as follows: (1) control group - mice receiving a controlled liquid diet and gavaged with distilled water; (2) EtOH group-mice receiving 5% EtOH (v/v) containing a liquid diet and gavaged with distilled water; (3) EtOH + silymarin group-mice receiving an EtOH diet and gavaged with silymarin (200 mg/kg); (4) EtOH + HRW group-mice receiving an EtOH diet and gavaged with HRW; and (5) EtOH + silymarin (200 mg/kg) + HRW group-mice receiving an EtOH diet and gavaged with silymarin and HRW.
Chronic and binge EtOH feeding was carried out by modifying a previously described protocol[33,34]. Both liquid diets were freshly prepared daily. HRW was orally administered (500 ppb, 1.2 mL/mouse) thrice daily for 13 continuous weeks. After 1-wk pretreatment with HRW, all mice (except for the control group) underwent a 2-wk acclimation to their modified liquid diets; specifically, the EtOH content in the diet was graded from 7.2% to 36% of energy composition. All mice, including the control diet group, were regularly fed their assigned diets ad libitum for 10 wk. Subsequently, mice in the EtOH groups were gavaged with a single dose of EtOH (5 g/kg) and mice in the control group were gavaged with an isocaloric amount of dextrin maltose in the early morning. Nine hour after gavage, the mice were euthanized by CO2 administration and blood was collected by caudal vena cava sampling. The whole liver was excised and washed immediately with ice-cold saline, to remove residual blood before weighing. The largest right lobe of each liver was fixed in 10% buffered formalin for histopathological assessment and the remaining tissues were immediately frozen at -80 °C for subsequent analysis.
After collection of whole blood, 4-(2-aminoethyl)benzene sulfonyl fluoride (AEBSF) was immediately added to achieve a final concentration of 1 mg/mL for 30 min at room temperature, after which the sample was centrifuged (4 °C, 2000 × g, 15 min). Next, serum was acidified with HCl to a final concentration of 0.1 N and samples were frozen at -80 °C for further analysis. The acyl form of ghrelin was measured in serum by the Active Ghrelin Enzyme-Linked Immunosorbent Assay (ELISA) Kit (Millipore, MA, United States), according to the manufacturer’s protocols.
To evaluate hepatic injury, levels of ALT, AST, triacylglycerol (TG) and total cholesterol (TC) were measured with commercial kits (Randox Laboratories Ltd, Antrim, United Kingdom), according to the assay protocol.
Liver specimens were fixed in 10% neutral buffered formalin and embedded in paraffin, using standard microtechniques. Then, 4-μm-thick liver sections were stained with hematoxylin and eosin and observed with a microscope (IX71S8F-2; Olympus, Tokyo, Japan), to estimate fatty liver progression in hepatocytes. The semi-quantitative histological assessment of hepatic damage was graded 0-4, as follows: none (0), slight (1), mild (2), moderate (3) and severe (4).
Extraction of hepatic lipids was performed by using the method of Folch et al[37], with some modifications. Then, 100 mg of liver tissue was homogenized in chloroform/methanol (v/v: 1/2). Next, chloroform and distilled water (v/v: 1/1) were loaded and mixed thoroughly. After centrifugation (1500 × g, 10 min), the organic layer was removed, placed in another glass tube and dried under nitrogen gas. The dried powder was dissolved in phosphate-buffered saline containing 1% Triton X-100. TG and TC content were measured with a commercial kit (Randox Laboratories Ltd).
For measurement of cytokine profiles, 100 mg of liver tissue was homogenized on ice with RIPA buffer (50 mmol/L Tris,150 mmol/L NaCl,1% Triton, 5 mmol/L EDTA, 0.5% sodium deoxycholate, 0.1% SDS) containing protease inhibitor. After centrifugation (12000 × g, 10 min, 4 °C), TNF-α and IL-6 content in serum supernatant was determined with ELISA kits (Enzo Life Science Inc., Farmingdale, NY, United States), according to the manufacturer’s protocols. Tissue values were normalized to tissue wet weight. Serum IL-10 and IL-22 levels were determined with ELISA kits (Elabscience, Hubei, China), according to the manufacturer’s protocols.
Livers were homogenized on ice with Tris-HCl (5 mmol/L, pH 7.4) containing 2 mmol/L EDTA. After centrifugation (10000 × g, 10 min, 4 °C), the supernatant was immediately stored at -80 °C for additional antioxidant assays. Superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activities were determined by enzymatic assay kits (RANSOD and RANSEL, respectively; Randox Laboratories Ltd), according to the manufacturer’s protocols. Catalase (CAT) activity was determined with the method proposed by Aebi[38]. The above antioxidant activities were normalized to hepatic total protein. GSH content was determined with a colorimetric assay kit (Bioxytech GSH-400; OXIS International Inc., Portland, OR, United States), according to the manufacturer’s protocol.
Malondialdehyde (MDA) is a marker of lipid peroxidation. MDA reaction with thiobarbituric acid was determined by the method of Buege et al[39], with some modifications. In brief, deproteinized homogenates from liver were mixed thoroughly with 0.67% thiobarbituric acid in a 50% acetic acid solution and then placed in a boiling-water bath for 60 min. The supernatant was collected and measured at excitation/emission wave lengths of 515 nm and 555 nm in a microplate reader (Molecular Devices Flexstation 3; Molecular Devices, LLC, Sunnyvale, CA, United States).
Measurement data are expressed as mean ± SD and differences between groups were analyzed with the unpaired t-test. Associations between variables were assessed by the Spearman correlation test. All statistical analyses were performed using SPSS 12.0 software (SPSS Inc. IBM, Chicago, IL, United States). When appropriate, P < 0.05 was considered statistically significant.
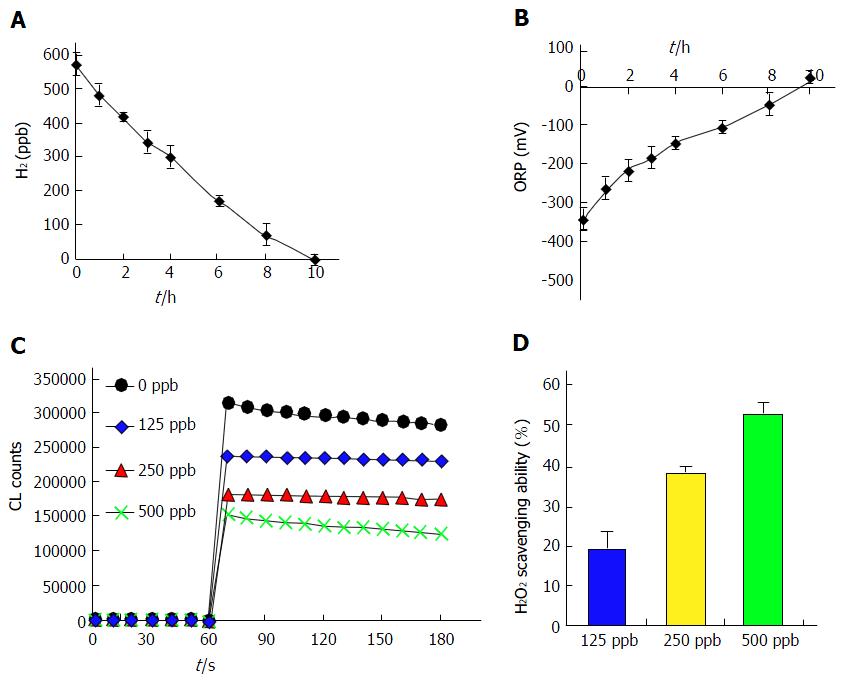
To characterize H2 solubility in water, a specific electrode was used to detect H2 concentrations in freshly prepared HRW. Dissolved H2 concentration decreased from the baseline (freshly prepared) concentration to undetectable in 10 h (Figure 1A). ORP is generally used as a measure of the antioxidant activity of a water sample. The present results show that HRW had a negative ORP and that ORP became positive at the same time that H2 content became undetectable in water (Figure 1B).
H2 markedly reduces ROS. Chemiluminescence emission in vitro was used to verify that HRW scavenged ROS in the present study. H2O2-generated free radicals were significantly and dose-dependently decreased by HRW treatment (125-500 ppb; approximately 7.5% to 30% saturation; Figure 1C). ROS scavenging ability was converted to the integral of the area under the curve. At concentrations of 125, 250 and 500 ppb, HRW treatment enhanced scavenging ability by 19.8%, 38.7% and 52.7%, respectively, as compared with the control group (Figure 1D). The presence of ROS scavenging ability in vitro suggests that HRW has hepatoprotective potential for in vivo EtOH-induced oxidative stress.
Effects of H2-rich water on food intake, acyl ghrelin, body weight and liver weight in chronic-binge ethanol-fed C57BL/6J mice
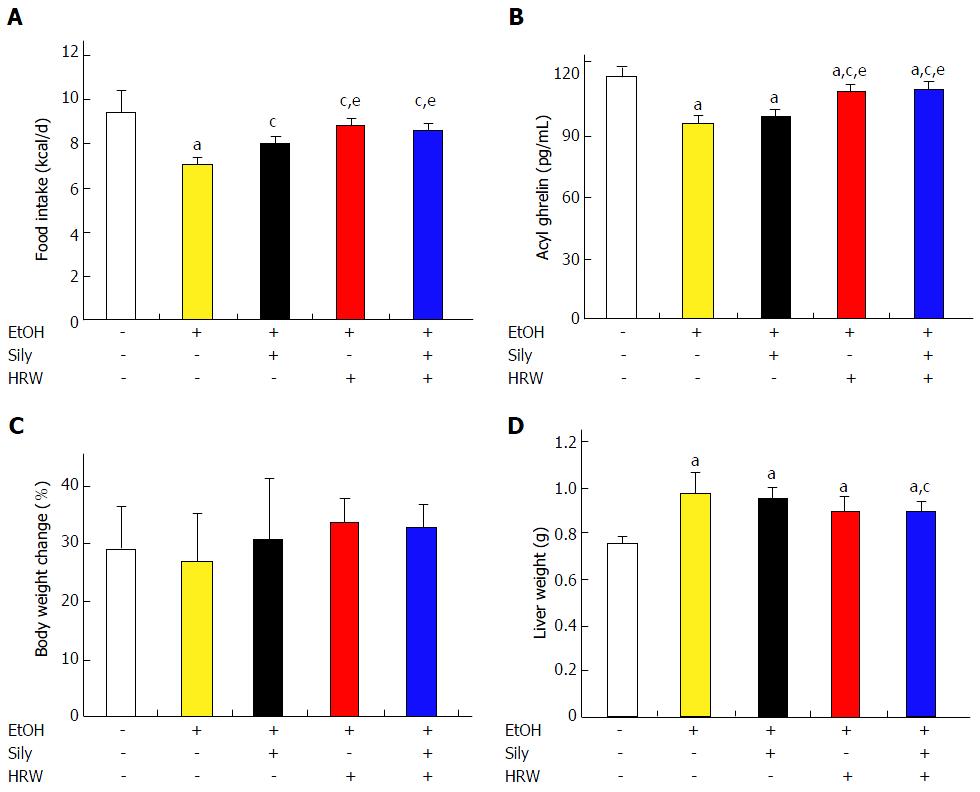
To investigate the hepatoprotective potential of HRW in vivo, C57BL/6 mice were subjected to a chronic-plus-binge EtOH feeding model. Silymarin (200 mg/kg) was used as a positive control. The control and EtOH groups significantly differed in daily food intake (P < 0.05; Figure 2A). Silymarin, HRW and combination treatment significantly reversed the hypophagic effect induced by EtOH (P < 0.01), which indicates that HRW reversed EtOH-induced anorexia. After 12 wk of EtOH exposure, serum was collected for analysis of acyl ghrelin. The control and EtOH groups significantly differed in acyl ghrelin expression (P < 0.001; Figure 2B). Acyl ghrelin expression was significantly higher in the HRW and combination treatment groups than in the EtOH group (P < 0.001).
During the course of the experiment, body weight was lower in the EtOH group than in the control diet group (Supplementary Figure 1). Silymarin, HRW and combination treatment slightly restored body weight, especially from week 4 until week 10. The values for relative body weight gain among groups after 13 wk of feeding were 29%, 26.6% 28.5%, 25.4% and 26.8%, compared to the baseline, respectively (Figure 2C). Body weight gain did not significantly differ among the groups. After the mice were sacrificed, liver tissues were excised and weighed. Liver weight significantly differed between the control and EtOH groups (P < 0.001), which suggests that EtOH administration resulted in liver enlargement (Figure 2D). Silymarin, HRW and combination treatment (P < 0.05) attenuated this EtOH-induced liver enlargement.
ALT and AST activities are biomarkers of liver damage. As shown in Table 1, both significantly differed between controls and the EtOH group (P < 0.001), which indicates that EtOH administration caused hepatocellular injury. As compared with the EtOH group, ALT and AST levels were 15.8% and 14.8% lower, respectively, in the silymarin-treated group (P < 0.01 and P < 0.05) and 11.5% and 10.9% lower, respectively, in the HRW-treated group (P < 0.05 and P > 0.05). Combination treatment reduced ALT and AST by 24.2% and 26.7%, respectively (P < 0.001 for both). In addition, serum TG and TC levels were higher in the EtOH group than in the control diet group (P > 0.05 and P < 0.001, respectively). As compared with the EtOH group, TG and TC levels were 17% and 10.7% lower, respectively, after silymarin treatment (P < 0.05 and P < 0.01) and 13.9% and 10.3% lower, respectively, after HRW treatment (P < 0.05 and P < 0.01). Combination treatment yielded the greatest decreases; as compared with the EtOH group, TG and TC levels were 25.9% and 18.1% lower, respectively (P < 0.001 for both).
| Group | Control | EtOH | EtOH + Sily | EtOH + HRW | EtOH + Sily + HRW |
| ALT, U/L | 43.4 ± 17.2 | 159 ± 16.9a | 133.9 ± 15.3a,c | 140.7 ± 15.5a,c | 120.5 ± 19.7a,c |
| AST, U/L | 98.4 ± 10.9 | 291 ± 43.7a | 247.7 ± 20a,c | 259.3 ± 10.9a | 181.6 ± 22.3a,c,e |
| TG, mg/dL | 153.1 ± 19.8 | 165 ± 26.7 | 136.9 ± 16.8c | 142.1 ± 14.7c | 122.2 ± 16.4a,c |
| TC, mg/dL | 81 ± 15.8 | 106.3 ± 7.4a | 94.9 ± 4.5a,c | 95.4 ± 6.5a,c | 87.1 ± 8.3c,e |
Effects of H2-rich water on hepatic lipid and inflammatory cytokines in chronic-binge ethanol-fed C57BL/6J mice
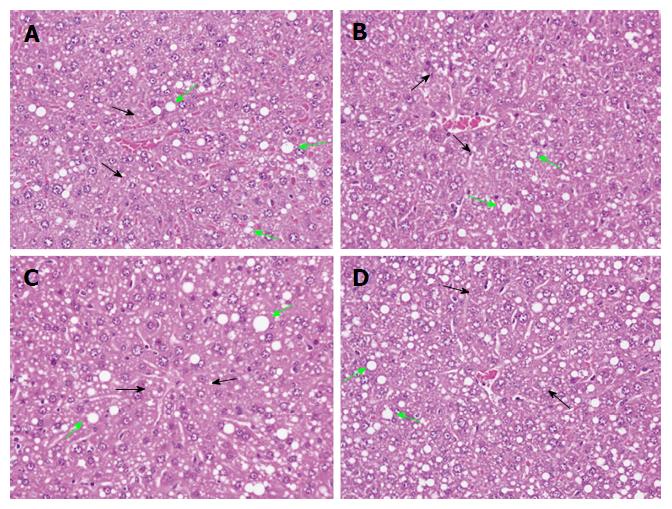
In normal liver, hepatic cells have well-preserved cytoplasm, a prominent nucleolus and portal vein. Hepatic steatosis is the most common EtOH-induced disorder and is characterized by accumulation of abnormal lipid droplets in hepatic cells. After 12 wk of EtOH exposure, hepatic TG and TC levels both significantly differed between the present control and EtOH groups (P < 0.001 for both; Table 2). These findings were consistent with the results of a histopathological examination of liver sections from the EtOH group, which revealed mild, diffuse and multifocal fatty change with microvesicular, macrovesicular and mixed steatosis (Figure 3A). As compared with the EtOH group, hepatic TG and TC levels were 24.5% and 16.9% lower, respectively, in the silymarin-treated group (P < 0.001 for both; Table 2).
| Group | Control | EtOH | EtOH+ Sily | EtOH+ HRW | EtOH + Sily + HRW |
| Hepatic TG, mg/g tissue | 82.4 ± 9.9 | 120.7 ± 6.8a | 91.1 ± 8.8c | 107.5 ± 6.6a,c,e | 113.5 ± 7.3a,c,e |
| Hepatic TC, mg/g tissue | 18.1 ± 2.4 | 41.4 ± 3.1a | 34.4 ± 2.8a,c | 38.0 ± 2.6a,c,e | 36.5 ± 2.1a,c |
| Hepatic TNF-α, pg/mg tissue | 29.0 ± 3.0 | 45.1 ± 3.4a | 39.7 ± 2.5a,c | 42.0 ± 2.4a,c | 37.3 ± 3.8a,c |
| Hepatic IL-6, pg/mg tissue | 10.7 ± 1.2 | 19.0 ± 1.2a | 12.5 ± 2.3a,c | 17.1 ± 2.5a,e | 11.2 ± 2.7c |
Histopathological assessment yielded similar results. Silymarin treatment significantly attenuated EtOH-induced fatty change and macrovesicular steatosis (Figure 3B). HRW treatment resulted in significant reductions in hepatic TG and TC (10.9% and 8.9%; P < 0.01 and P < 0.05, respectively; Table 2), although there was no significant improvement in histopathological characteristics as compared with the EtOH group. Combination treatment resulted in 6% and 11.8% reductions (P < 0.05 and P < 0.01, respectively; Table 2).
We investigated the inflammatory profile of EtOH-induced liver injury. TNF-α and IL-6 levels were significantly higher for the EtOH group than for the control diet group (P < 0.001 for both; Table 2). As compared with the EtOH group, TNF-α and IL-6 levels were significantly lower in the silymarin-treated group (12% and 34.2% lower, respectively; P < 0.01 and P < 0.001). After HRW treatment, TNF-α and IL-6 levels were 6.4% and 10% lower than those of the EtOH group (P < 0.05 and P > 0.05, respectively). Combination treatment yielded the best results, with reductions of 17.3% and 41.1%, respectively (P < 0.001 for both). These results suggest that HRW inhibits EtOH-induced lipid accumulation and hepatic inflammation in the liver.
Anti-inflammatory effect of H2-rich water on cytokine in chronic-binge ethanol-fed C57BL/6J mice
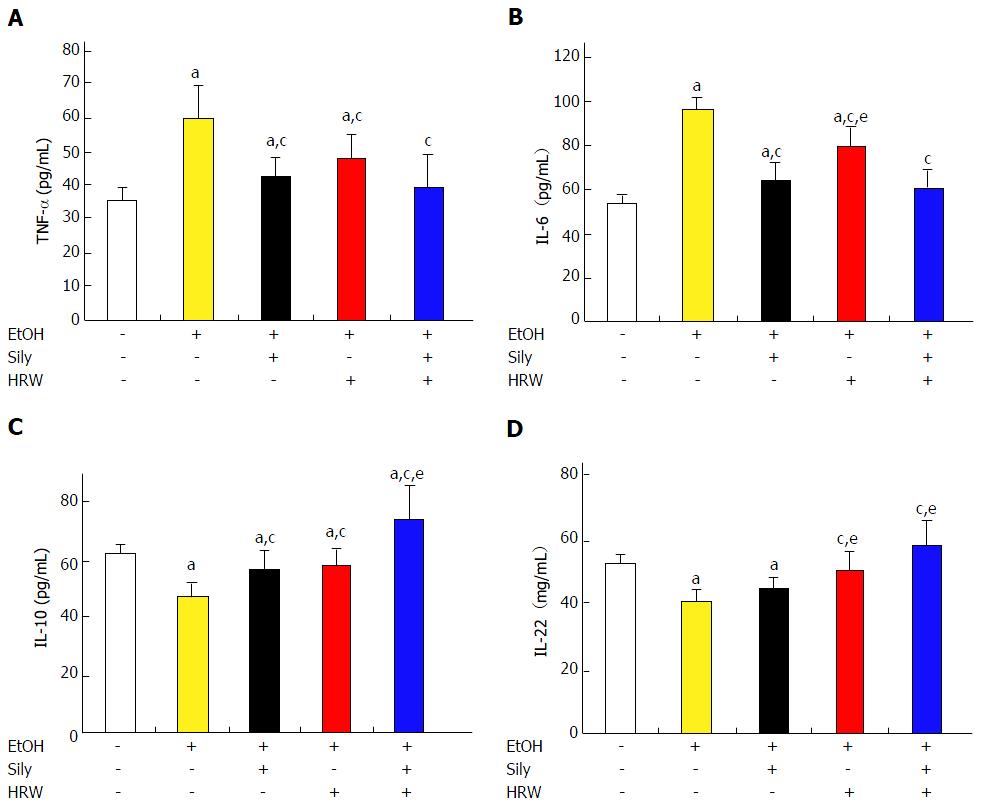
EtOH feeding significantly increased production of serum pro-inflammatory cytokines, including TNF-α and IL-6, as compared with the control diet group (Figure 3). TNF-α level was significantly reduced by silymarin, HRW and combination treatment (P < 0.001, P < 0.05 and P < 0.001, respectively; Figure 4A). In addition, IL-6 level was significantly reduced by silymarin, HRW and combination treatment (P < 0.001; Figure 4B). Moreover, levels of anti-inflammatory cytokines, including IL-10 and IL-22, were significantly lower in the EtOH group than in the control diet group. IL-10 level was significantly increased by silymarin, HRW and combination treatment (P < 0.001; Figure 4C). IL-22 level was also increased by silymarin, HRW and combination treatment (P > 0.05, P < 0.01 and P < 0.001, respectively; Figure 4D). In sum, these results suggest that HRW inhibits pro-inflammatory mediators and induces anti-inflammatory mediators in EtOH-induced liver injury.
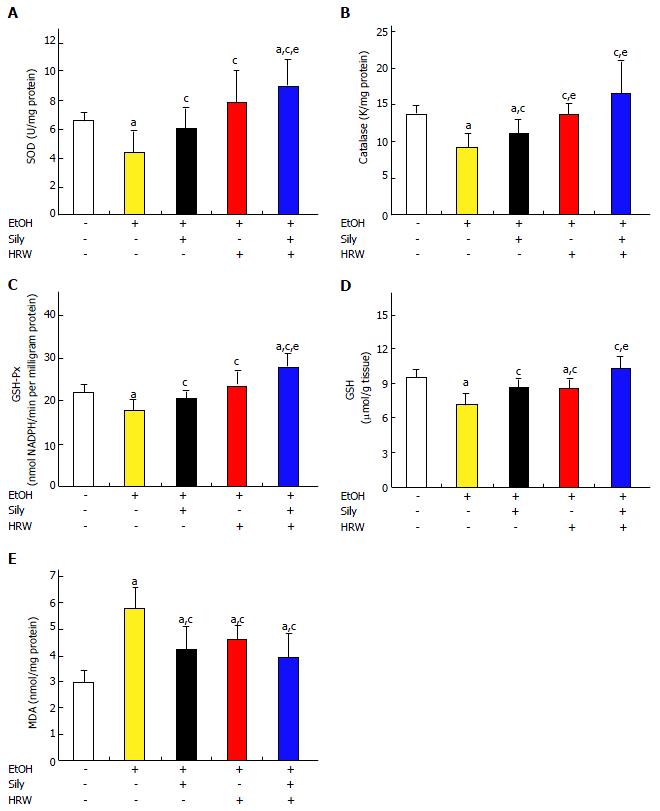
Hepatic antioxidant effects of H2-rich water on activities of related antioxidant enzymes and lipid oxidation in chronic-binge ethanol-fed C57BL/6J mice
EtOH-induced free radical oxidative stress is a hallmark of liver disease. Thus, we tested the hypothesis that HRW decreases oxidative neuronal stress by means of anti-ROS activity. The activities of hepatic antioxidant enzymes, including SOD, CAT, GSH-Px and GSH, in the EtOH group were significantly lower than in the control diet group (Figure 5). In addition, these activities were significantly promoted by silymarin and HRW. Moreover, significantly higher levels of hepatic antioxidant enzymes were observed in mice that received combined treatment with silymarin and HRW.
MDA concentration was used as a marker of oxidative stress. MDA level in hepatic tissue was significantly higher in the EtOH group than in the control diet group (P < 0.001). In addition, MDA was significantly lower in the silymarin group (P < 0.01) and HRW group (P < 0.05) than in the EtOH group. The strongest beneficial effect was seen in the combined treatment group. These findings suggest that HRW promotes antioxidant capacity and reduces lipid peroxidation, thus improving antioxidant defense.
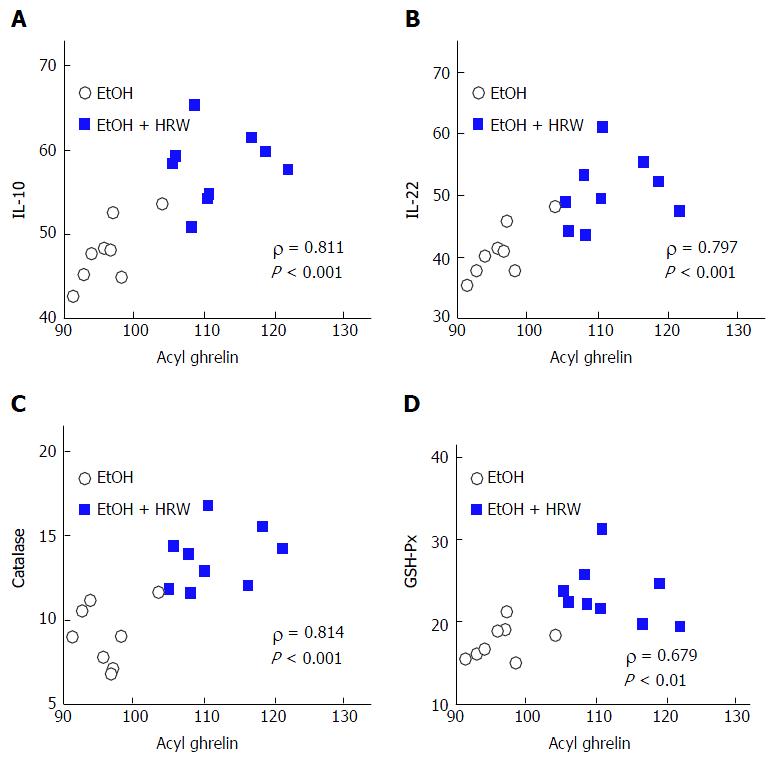
Spearman correlation analysis was used to evaluate associations of HRW-induced alterations in acyl ghrelin with anti-inflammatory and antioxidant markers. Acyl ghrelin concentration was inversely correlated with the following pro-inflammatory and oxidative markers: serum TNF-α (ρ = -0.455, P > 0.05), serum IL-6 (ρ = -0.636, P < 0.01) and hepatic MDA (ρ = -0.542, P < 0.05). In contrast, acyl ghrelin was positively correlated with serum IL-10 (ρ = 0.811, P < 0.001; Figure 6A), serum IL-22 (ρ = 0.797, P < 0.001; Figure 6B), hepatic SOD (ρ = 0.539, P < 0.05), hepatic CAT (ρ = 0.814, P < 0.001; Figure 6C), hepatic GSH-Px (ρ = 0.679, P < 0.01; Figure 6D), hepatic MDA (ρ = 0.542, P < 0.05) and hepatic GSH (ρ = 0.478, P > 0.05). HRW-induced changes in IL-10 and IL-22 resulted in inverse correlations with TNF-α (ρ = -0.304, P > 0.05 and ρ = -0.508, P < 0.05, respectively) and serum IL-6 (ρ = -0.623, P < 0.01 and ρ = -0.703, P < 0.01) and positive correlations with hepatic SOD (ρ = 0.385, P > 0.05 and ρ = 0.630, P < 0.01, respectively), hepatic CAT (ρ = 0.659, P < 0.01 and ρ = 0.723, P < 0.01, respectively) and hepatic GSH-Px (ρ = 0.711, P < 0.01 and ρ = 0.809, P < 0.001, respectively). These findings indicate that HRW-induced alterations in acyl ghrelin and the hepatoprotective cytokines IL-10 and IL-22 were associated with inflammatory and oxidative responses.
ALD not only causes lipopolysaccharide, which activates HSCs, but also affects synthesis and absorption of protein and vitamins, which leads to malnutrition, a secondary factor in hepatocyte damage. The mutual effects of these events eventually result in hepatic fat infiltration, inflammation, necrosis and cirrhosis[1,2,8]. If these conditions are not treated, the inevitable fibrosis and cirrhosis of the liver can result in numerous complications and death. Hence, there is a need for safe and effective agents that prevent or treat ALD. Future research should therefore investigate suppression and blockage of any of the steps that culminate in hepatic injury.
This study investigated whether HRW, alone or combined with silymarin, was beneficial for early-stage EtOH-induced liver injury in female mice. We found that HRW directly scavenged H2O2in vitro. Our in vivo study showed that HRW pretreatment significantly attenuated increases in serum ALT, AST, TG and TC and hepatic lipid accumulation, which were induced by EtOH feeding. Ghrelin expression was higher after HRW treatment and was correlated with restoration of food intake and inflammatory cytokines, including TNF-αand IL-6, which were induced by EtOH feeding. HRW also attenuated MDA level, restored GSH depletion and increased SOD, GSH-Px and CAT activities in liver. Moreover, HRW reduced TNF-α and IL-6 levels and increased IL-10 and IL-22 levels. These results support the hypothesis that HRW has important antioxidant and anti-inflammatory effects in alcohol-related disease in mice. Previous studies reported that HRW treatment for 6 wk or 10 wk significantly attenuated oxidative stress and had the potential to improve liver function in patients with chronic hepatitis B[32] and metabolic syndrome[31], respectively. Therefore, HRW might be effective for prevention and clinical treatment of ALD such as steatosis, steatohepatitis and cirrhosis.
In the present study, a Lieber-DeCarli EtOH liquid diet was used to induce early ALD in female mice. This model closely reproduces the drinking behaviors of humans and the pathogenetic features of ALD[3,5,33,34]. The present mice fed an EtOH diet for 12 wk exhibited mild hepatic damage, as indicated by significant elevations in serum ALT and AST, hepatic TG and TC, which agreed with the findings of previous studies[3,33]. These effects were abolished by HRW pretreatment, particularly in combination with silymarin treatment. HRW prevented progression of nonalcoholic steatohepatitis[19,40] and metabolic syndrome in previous studies which suggests that anti-fatty liver benefits are regulated by fatty acid and steroid metabolism through the peroxisome proliferator-activated receptor α (PPARα) signaling pathway[30]. Our results also indicate that prevention of ALD by HRW is partially mediated by lipid metabolism.
Acyl ghrelin acts within the hypothalamus to promote appetite and inhibit sympathetic activity, which increases food intake while lowering metabolic rate[13,14]. Our findings indicate that, as compared with a control liquid diet, an EtOH-containing liquid diet significantly decreases dietary intake and acyl ghrelin level, which suggests that EtOH affects appetite. This finding is consistent with the loss of appetite seen in long-term heavy drinkers. Silymarin, HRW and combination treatment significantly reversed the hypophagic effect of EtOH, indicating that HRW reversal of EtOH-induced anorexia may be mediated by restoration of acyl ghrelin levels. EtOH administration also altered liver weight but not body weight. This suggests that EtOH impairs body composition by means of liver enlargement and sarcopenia[41], which can be improved by HRW pretreatment. HRW might therefore reverse EtOH-induced effects on acyl ghrelin, which stimulate energy expenditure, thus resulting in loss of muscle mass and hypophagia. A previous study reported that ghrelin has a hepatoprotective role in nonalcoholic fatty liver[9]. In addition, after HRW treatment, acyl ghrelin had a neuroprotective effect in Parkinson’s disease[42].
Acyl ghrelin and des-acyl ghrelin are both active signaling molecules; however, a limitation of the present study is that we did not measure des-acyl ghrelin. Measurement of the total ghrelin is not a surrogate for analysis of acyl ghrelin[13,43]. A recent study found that des-acyl ghrelin specifically binds to and acts on a subset of arcuate nucleus cells in a ghrelin receptor-independent manner and antagonizes the orexigenic effects of peripherally administered acyl ghrelin[44]. Furthermore, in a lethal rat model of burn trauma, survival was significantly better after resuscitation with saline containing des-acyl ghrelin than after resuscitation with saline alone[45].
ALD pathogenetic mechanisms are involved in increased steatosis, oxidative stress, inflammatory factors and immune response. EtOH-induced liver steatosis due to ROS accumulation and bacterial endotoxin leakage from damaged intestine triggers an inflammatory response[1-3,5,8,33]. TNF-α and IL-6 are widely considered to be the most important pro-inflammatory cytokines in ALD. In addition, pro-inflammatory cytokines and adipokines inhibit muscle-mass formation and promote fat-mass accumulation, a state that is associated with sarcopenia and obesity[41]. In the present study, HRW pretreatment reduced hepatic and systemic production of inflammatory mediators induced by EtOH feeding. Thus, HRW had a protective effect against early ALD.
The anti-inflammatory cytokine IL-10 protects against hepatic damage caused by viruses, alcohol and dietary autoimmunity[46]. IL-10 inhibited activation of HSCs and had antifibrogenic effects in rodents[47]. IL-22 is a survival factor for hepatocytes and prevents and repairs liver injury by enhancing pro-growth pathways via STAT3 activation. A previous study revealed that treatment with IL-22 protein contributed to liver regeneration in mice with concanavalin A-induced hepatitis after hepatectomy, which suggests that IL-22 acts as a protective cytokine that attenuates liver injury[11]. In the present study, levels of both anti-inflammatory cytokines were higher after HRW pretreatment than in the EtOH feeding group, which suggests that HRW pretreatment protects against chronic EtOH-induced liver injury and sarcopenia by suppressing the pro-inflammatory cytokines TNF-α and IL-6 and inducing the anti-inflammatory cytokines IL-10 and IL-22.
Oxidative stress is induced by overproduction of ROS, including superoxide anion, hydroxyl radical and H2O2 and has a key role in ALD pathogenesis. EtOH consumption leads to excessive ROS, which results in lipid peroxidation and membrane damage, as well as depletion of mitochondrial reduced GSH and its final precursor in liver[1,2]. Antioxidant enzymes such as SOD, CAT and GSH-Px protect against oxidative damage: SOD converts superoxide anion into H2O2 and GSH-Px and CAT metabolize H2O2 to H2O. The balance between ROS and antioxidant enzymes is an important mechanism in preventing EtOH-induced oxidative damage. Therefore, antioxidant therapy is a potential strategy to improve outcomes in ALD.
In the present study, we found that EtOH decreased activities of hepatic SOD, CAT, GSH-Px and GSH and increased hepatic lipid oxidation, which is consistent with the findings of previous studies that used the chronic-plus-binge model[3]. These changes may be attributable to oxidative inactivation of enzymes by ROS accumulation. Our results suggest that HRW pretreatment abolished ROS induced by EtOH, resulting in enhanced antioxidant effects. This hypothesis is supported by the present in vitro studies (Figure 1C and D) and evidence from previous studies, which indicates that H2 directly reduces ROS[17,35]. Similar antioxidant phenomena were observed in many oxidative stress-related diseases, especially CCl4-, endotoxin-, acetaminophen- and ischemia/reperfusion-induced hepatic injuries in rodents[21-27,32,40]. A number of studies reported that H2 reduces oxidative stress not only directly but also indirectly, by regulating anti-oxidative signal transduction, including nuclear factor erythroid 2-related factor 2 (Nrf-2) and sirtuin 1 (Sirt1)[14,18,20,35]. Antioxidants regulated by Nrf-2 via an antioxidant response element-driven mechanism include SOD, GSH-Px, CAT, heme oxygenase-1 (HO-1), peroxiredoxins, etc[48]. Interestingly, our results indicate that the antioxidant effects of HRW are similar and/or stronger than those of silymarin, indicating that HRW pretreatment protects against EtOH-induced oxidative stress in EtOH-fed mice.
Acyl ghrelin has anti-inflammatory effects and may help mediate autoimmunity. Notably, acyl ghrelin acts on monocytes and T lymphocytes to suppress their production of TNF-α, IL-1β and IL-6 pro-inflammatory cytokines, which can induce anorexia during both infection and cancer progression[13,14,43]. Ghrelin administration had protective effects against high-fat diet-induced liver injury, oxidative stress, inflammation and apoptosis in rats, in part through the action of the LKB1/AMPK and PI3K/Akt pathways[9]. In the present study, HRW-induced alterations in acyl ghrelin were strongly associated with inflammatory and oxidative responses. This suggests that the hepatoprotective effect of HRW in ALD is mediated by acyl ghrelin production, which results in anti-inflammatory and antioxidant effects. However, future studies should investigate if acyl ghrelin directly regulates expression of IL-10 and IL-22.
HRW-induced alterations in IL-10 and IL-22 were strongly associated with inflammatory mediators and anti-oxidative enzymes. A previous study found that IL-10 was an anti-inflammatory cytokine that inhibits both secretion of pro-inflammatory cytokines by monocytes and/or macrophages and release of free oxygen radicals. Recombinant IL-10 blocks release of ROS[49]. IL-22 prevents oxidative and endoplasmic reticulum stress in mouse and human cells, where stress is induced by lipids, inflammatory cytokines or environmental ROS via STAT1- and STAT3-mediated up-regulation of antioxidant genes and suppression of oxidative stress-inducing genes[50].
H2 inhibits secretion of pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6, reduces the severity of intestinal inflammation and improves repair of intestinal cells[51,52]. EtOH-induced gastrointestinal dysfunction is caused by abnormalities in Kupffer cells - which result in reduced ability to detoxify endotoxins, disruption of intestinal barrier function and increased permeability to endotoxins and bacteria - and bacterial overgrowth in the gut, which leads to excessive generation of endotoxins[8]. Unfortunately, the limited sensitivity of the ELISA kit prevented us from analyzing endotoxins in the present study. However, previous studies reported that H2 alleviated endotoxin-induced liver injury in rodents by reducing inflammation and cell apoptosis[20,22,23]. Taken together, the evidence indicates that HRW administration helps maintain permeability, mucosal structure and barrier function in intestine and improves the gastrointestinal microenvironment for bacteria that protect against EtOH-induced liver injury.
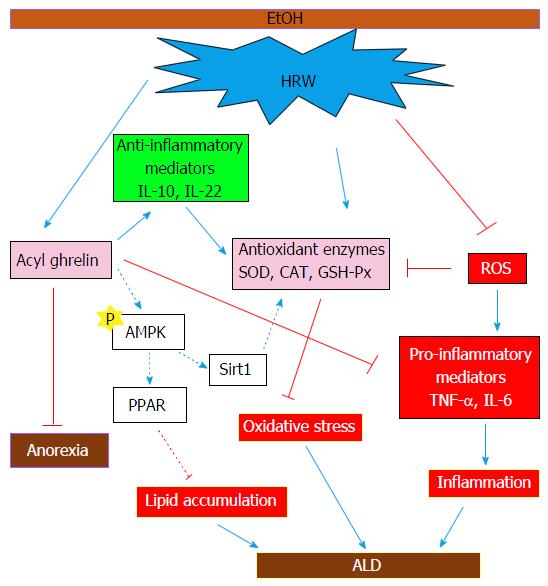
The response to EtOH ingestion in the present study appeared to depend on underlying hypertriglyceridemia and hypercholesterolemia and increased accumulation of hepatic TG and TC. AMPK has been implicated as a major regulator of energy metabolism at the cellular and systemic level, which suggests that it has a role in physiological regulation of lipid and glucose metabolism. Existing evidence indicates that EtOH consumption impairs AMPK-mediated regulation of fatty acid metabolism and results in facilitation of TG and TC accumulation in rodents[4]. By inhibiting AMPK and activating the p38-MAPK pathway, acyl ghrelin inhibits ROS-induced autophagy and cell death[15]. Our previous study revealed that the neuroprotective mechanisms of HRW were mediated via up-regulation of FoxO3a, which stimulated AMPK in a Sirt1-dependent manner[35]. Past and present evidence suggests that HRW inhibits hepatic lipid accumulation through induction of acyl ghrelin, which activates AMPK signaling after suppressing lipogenesis, inhibiting pro-inflammatory mediators and inducing hepatoprotective cytokines to activate anti-oxidative enzymes. In addition, HRW improved EtOH-induced anorexia via acyl ghrelin secretion (Figure 7).
Most importantly, use of HRW in combination with silymarin in the present study resulted in stronger hepatoprotective effects in EtOH-fed mice. These findings are consistent with those of our previous studies in which electronically produced H2 co-administered with GSH increased the apoptosis-inducing effect in leukemia cells[36]. This finding suggests that HRW combination therapy has a beneficial effect. Alcoholic patients often develop protein calorie malnutrition, which can promote bacterial infection. Nutritional support is recommended for patients with ALD and was found to improve liver function in histological analyses and to increase survival in short-term follow-up studies[2]. HRW administration might help improve appetite and treat malnutrition and can thus be regarded as an alternative nutritional strategy for treatment of patients with ALD.
In conclusion, this study is the first to show that, in female mice, HRW protects against early-stage chronic EtOH-induced liver injury, possibly by inducing acyl ghrelin to suppress the pro-inflammatory cytokines TNF-α and IL-6 and activate IL-10 and IL-22, which enhance antioxidant enzymes against oxidative stress. These findings indicate that long-term consumption of HRW is a potential strategy for prevention and clinical complementary treatment of ALD.
We thank Dr. Jiunn-Wang Liao (Institute of Veterinary Pathobiology, National Chung Hsing University, Taichung City, Taiwan) for assistance in histological analysis.
Sustained excessive alcohol consumption results in a spectrum of liver injury, from hepatic steatosis to hepatitis, fibrosis and cirrhosis, which can ultimately lead to hepatocellular carcinoma. Females have an increased susceptibility to alcoholic liver diseases compared with males. This study investigated the potential protective effects of hydrogen-rich water (HRW) against chronic ethanol (EtOH)-induced early-stage liver injury and the underlying mechanisms of such effects in female mice after chronic-plus-binge EtOH feeding.
Molecular hydrogen scavenges free radicals, thereby exerting a hepatoprotective effect. Approaches to administering hydrogen include inhalation, injection, oral administration and immersion. Oral administration of HRW was easier, safer and more economical as a means to protect against EtOH-induced early liver injury.
The authors investigated the effects of HRW on EtOH-induced early liver injury in female mice. The present study concluded that HRW protects against early-stage chronic EtOH-induced liver injury, possibly by inducing acyl ghrelin to suppress the pro-inflammatory cytokines tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-6 and activate IL-10 and IL-22, which enhance antioxidant enzymes against oxidative stress.
Long-term consumption of HRW is a potential strategy for prevention and clinical complementary treatment of alcoholic liver disease.
Acyl ghrelin has anti-inflammatory effects and may help mediate autoimmunity. Notably, acyl ghrelin acts on monocytes and T lymphocytes to suppress their production of TNF-α, IL-1β and IL-6 pro-inflammatory cytokines, which can induce anorexia during both infection and cancer progression. Acyl ghrelin administration had protective effects against high-fat diet-induced liver injury, oxidative stress, inflammation and apoptosis in rodents.
The manuscript is well documented and interesting. The performance of methodology and the statistical analysis of their results are very well established. They analyzed many biomarkers in the serum as well as in the liver tissue, so as to support their basic hypothesis that HRW can prevent progression of steatosis, liver damage and fibrosis. Their research will provide useful guidance for the treatment of alcoholic fatty liver disease in humans.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Taiwan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Pavlidis C, Yamada S S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Wang S
| 1. | Sid B, Verrax J, Calderon PB. Role of oxidative stress in the pathogenesis of alcohol-induced liver disease. Free Radic Res. 2013;47:894-904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572-1585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1244] [Cited by in F6Publishing: 1342] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 3. | Ki SH, Park O, Zheng M, Morales-Ibanez O, Kolls JK, Bataller R, Gao B. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291-1300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 335] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 4. | García-Villafranca J, Guillén A, Castro J. Ethanol consumption impairs regulation of fatty acid metabolism by decreasing the activity of AMP-activated protein kinase in rat liver. Biochimie. 2008;90:460-466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Fulham MA, Mandrekar P. Sexual Dimorphism in Alcohol Induced Adipose Inflammation Relates to Liver Injury. PLoS One. 2016;11:e0164225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Wagnerberger S, Fiederlein L, Kanuri G, Stahl C, Millonig G, Mueller S, Bischoff SC, Bergheim I. Sex-specific differences in the development of acute alcohol-induced liver steatosis in mice. Alcohol Alcohol. 2013;48:648-656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Baraona E, Abittan CS, Dohmen K, Moretti M, Pozzato G, Chayes ZW, Schaefer C, Lieber CS. Gender differences in pharmacokinetics of alcohol. Alcohol Clin Exp Res. 2001;25:502-507. [PubMed] [Cited in This Article: ] |
| 8. | Rao RK, Seth A, Sheth P. Recent Advances in Alcoholic Liver Disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:G881-G884. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 222] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 9. | Li Y, Hai J, Li L, Chen X, Peng H, Cao M, Zhang Q. Administration of ghrelin improves inflammation, oxidative stress, and apoptosis during and after non-alcoholic fatty liver disease development. Endocrine. 2013;43:376-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 10. | Wolk K, Witte E, Witte K, Warszawska K, Sabat R. Biology of interleukin-22. Semin Immunopathol. 2010;32:17-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 311] [Cited by in F6Publishing: 311] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 11. | Zhang YM, Liu ZR, Cui ZL, Yang C, Yang L, Li Y, Shen ZY. Interleukin-22 contributes to liver regeneration in mice with concanavalin A-induced hepatitis after hepatectomy. World J Gastroenterol. 2016;22:2081-2091. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Lu DH, Guo XY, Qin SY, Luo W, Huang XL, Chen M, Wang JX, Ma SJ, Yang XW, Jiang HX. Interleukin-22 ameliorates liver fibrogenesis by attenuating hepatic stellate cell activation and downregulating the levels of inflammatory cytokines. World J Gastroenterol. 2015;21:1531-1545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 48] [Cited by in F6Publishing: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Chen CY, Asakawa A, Fujimiya M, Lee SD, Inui A. Ghrelin gene products and the regulation of food intake and gut motility. Pharmacol Rev. 2009;61:430-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 161] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 14. | McCarty MF. Potential ghrelin-mediated benefits and risks of hydrogen water. Med Hypotheses. 2015;84:350-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Wang X, Wang XL, Chen HL, Wu D, Chen JX, Wang XX, Li RL, He JH, Mo L, Cen X. Ghrelin inhibits doxorubicin cardiotoxicity by inhibiting excessive autophagy through AMPK and p38-MAPK. Biochem Pharmacol. 2014;88:334-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 16. | Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, Lillard JW, Taub DD. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114:57-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 253] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 17. | Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688-694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1346] [Cited by in F6Publishing: 1487] [Article Influence: 87.5] [Reference Citation Analysis (0)] |
| 18. | Ichihara M, Sobue S, Ito M, Ito M, Hirayama M, Ohno K. Beneficial biological effects and the underlying mechanisms of molecular hydrogen - comprehensive review of 321 original articles. Med Gas Res. 2015;5:12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 165] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 19. | Kawai D, Takaki A, Nakatsuka A, Wada J, Tamaki N, Yasunaka T, Koike K, Tsuzaki R, Matsumoto K, Miyake Y. Hydrogen-rich water prevents progression of nonalcoholic steatohepatitis and accompanying hepatocarcinogenesis in mice. Hepatology. 2012;56:912-921. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Iketani M, Ohshiro J, Urushibara T, Takahashi M, Arai T, Kawaguchi H, Ohsawa I. Preadministration of Hydrogen-Rich Water Protects Against Lipopolysaccharide-Induced Sepsis and Attenuates Liver injury. Shock. 2017;48:85-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Koyama Y, Taura K, Hatano E, Tanabe K, Yamamoto G, Nakamura K, Yamanaka K, Kitamura K, Narita M, Nagata H. Effects of oral intake of hydrogen water on liver fibrogenesis in mice. Hepatol Res. 2014;44:663-677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Xu XF, Zhang J. Saturated hydrogen saline attenuates endotoxin-induced acute liver dysfunction in rats. Physiol Res. 2013;62:395-403. [PubMed] [Cited in This Article: ] |
| 23. | Sun H, Chen L, Zhou W, Hu L, Li L, Tu Q, Chang Y, Liu Q, Sun X, Wu M. The protective role of hydrogen-rich saline in experimental liver injury in mice. J Hepatol. 2011;54:471-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 24. | Fukuda K, Asoh S, Ishikawa M, Yamamoto Y, Ohsawa I, Ohta S. Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem Biophys Res Commun. 2007;361:670-674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 292] [Cited by in F6Publishing: 289] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 25. | Zhang JY, Song SD, Pang Q, Zhang RY, Wan Y, Yuan DW, Wu QF, Liu C. Hydrogen-rich water protects against acetaminophen-induced hepatotoxicity in mice. World J Gastroenterol. 2015;21:4195-4209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 23] [Cited by in F6Publishing: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Hsu YW, Tsai CF, Chuang WC, Chen WK, Ho YC, Lu FJ. Protective effects of silica hydride against carbon tetrachloride-induced hepatotoxicity in mice. Food Chem Toxicol. 2010;48:1644-1653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Tsai CF, Hsu YW, Chen WK, Chang WH, Yen CC, Ho YC, Lu FJ. Hepatoprotective effect of electrolyzed reduced water against carbon tetrachloride-induced liver damage in mice. Food Chem Toxicol. 2009;47:2031-2036. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Saitoh Y, Harata Y, Mizuhashi F, Nakajima M, Miwa N. Biological safety of neutral-pH hydrogen-enriched electrolyzed water upon mutagenicity, genotoxicity and subchronic oral toxicity. Toxicol Ind Health. 2010;26:203-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Ishibashi T, Sato B, Shibata S, Sakai T, Hara Y, Naritomi Y, Koyanagi S, Hara H, Nagao T. Therapeutic efficacy of infused molecular hydrogen in saline on rheumatoid arthritis: a randomized, double-blind, placebo-controlled pilot study. Int Immunopharmacol. 2014;21:468-473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Kamimura N, Ichimiya H, Iuchi K, Ohta S. Molecular hydrogen stimulates the gene expression of transcriptional coactivator PGC-1α to enhance fatty acid metabolism. Npj Aging Mech Dis. 2016;2:16008. [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Song G, Li M, Sang H, Zhang L, Li X, Yao S, Yu Y, Zong C, Xue Y, Qin S. Hydrogen-rich water decreases serum LDL-cholesterol levels and improves HDL function in patients with potential metabolic syndrome. J Lipid Res. 2013;54:1884-1893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 32. | Xia C, Liu W, Zeng D, Zhu L, Sun X, Sun X. Effect of hydrogen-rich water on oxidative stress, liver function, and viral load in patients with chronic hepatitis B. Clin Transl Sci. 2013;6:372-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Xu MJ, Cai Y, Wang H, Altamirano J, Chang B, Bertola A, Odena G, Lu J, Tanaka N, Matsusue K. Fat-Specific Protein 27/CIDEC Promotes Development of Alcoholic Steatohepatitis in Mice and Humans. Gastroenterology. 2015;149:1030-1041.e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 34. | Bertola A, Mathews S, Ki SH, Wang H, Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat Protoc. 2013;8:627-637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 558] [Cited by in F6Publishing: 698] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 35. | Lin CL, Huang WN, Li HH, Huang CN, Hsieh S, Lai C, Lu FJ. Hydrogen-rich water attenuates amyloid β-induced cytotoxicity through upregulation of Sirt1-FoxO3a by stimulation of AMP-activated protein kinase in SK-N-MC cells. Chem Biol Interact. 2015;240:12-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 36. | Tsai CF, Hsu YW, Chen WK, Ho YC, Lu FJ. Enhanced induction of mitochondrial damage and apoptosis in human leukemia HL-60 cells due to electrolyzed-reduced water and glutathione. Biosci Biotechnol Biochem. 2009;73:280-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497-509. [PubMed] [Cited in This Article: ] |
| 38. | Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121-126. [PubMed] [Cited in This Article: ] |
| 39. | Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302-310. [PubMed] [Cited in This Article: ] |
| 40. | Hou C, Wang Y, Zhu E, Yan C, Zhao L, Wang X, Qiu Y, Shen H, Sun X, Feng Z. Coral calcium hydride prevents hepatic steatosis in high fat diet-induced obese rats: A potent mitochondrial nutrient and phase II enzyme inducer. Biochem Pharmacol. 2016;103:85-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 41. | Arthur ST, Cooley ID. The effect of physiological stimuli on sarcopenia; impact of Notch and Wnt signaling on impaired aged skeletal muscle repair. Int J Biol Sci. 2012;8:731-760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 42. | Matsumoto A, Yamafuji M, Tachibana T, Nakabeppu Y, Noda M, Nakaya H. Oral ‘hydrogen water’ induces neuroprotective ghrelin secretion in mice. Sci Rep. 2013;3:3273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 43. | Chen CY, Fujimiya M, Laviano A, Chang FY, Lin HC, Lee SD. Modulation of ingestive behavior and gastrointestinal motility by ghrelin in diabetic animals and humans. J Chin Med Assoc. 2010;73:225-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 44. | Fernandez G, Cabral A, Cornejo MP, De Francesco PN, Garcia-Romero G, Reynaldo M, Perello M. Des-Acyl Ghrelin Directly Targets the Arcuate Nucleus in a Ghrelin-Receptor Independent Manner and Impairs the Orexigenic Effect of Ghrelin. J Neuroendocrinol. 2016;28:12349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 45. | Sheriff S, Kadeer N, Friend LA, James JH, Alexander JW, Balasubramaniam A. Des-acyl-ghrelin (DAG) normalizes hyperlactacidemia and improves survival in a lethal rat model of burn trauma. Peptides. 2014;60:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 46. | Cintra DE, Pauli JR, Araújo EP, Moraes JC, de Souza CT, Milanski M, Morari J, Gambero A, Saad MJ, Velloso LA. Interleukin-10 is a protective factor against diet-induced insulin resistance in liver. J Hepatol. 2008;48:628-637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 122] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 47. | Zhang LJ, Zheng WD, Chen YX, Huang YH, Chen ZX, Zhang SJ, Shi MN, Wang XZ. Antifibrotic effects of interleukin-10 on experimental hepatic fibrosis. Hepatogastroenterology. 2007;54:2092-2098. [PubMed] [Cited in This Article: ] |
| 48. | Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401-426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2334] [Cited by in F6Publishing: 2920] [Article Influence: 265.5] [Reference Citation Analysis (0)] |
| 49. | Haddad JJ, Fahlman CS. Redox- and oxidant-mediated regulation of interleukin-10: an anti-inflammatory, antioxidant cytokine? Biochem Biophys Res Commun. 2002;297:163-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 50. | Hasnain SZ, Borg DJ, Harcourt BE, Tong H, Sheng YH, Ng CP, Das I, Wang R, Chen AC, Loudovaris T. Glycemic control in diabetes is restored by therapeutic manipulation of cytokines that regulate beta cell stress. Nat Med. 2014;20:1417-1426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 185] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 51. | Chen H, Sun YP, Hu PF, Liu WW, Xiang HG, Li Y, Yan RL, Su N, Ruan CP, Sun XJ. The effects of hydrogen-rich saline on the contractile and structural changes of intestine induced by ischemia-reperfusion in rats. J Surg Res. 2011;167:316-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 52. | Kajiya M, Silva MJ, Sato K, Ouhara K, Kawai T. Hydrogen mediates suppression of colon inflammation induced by dextran sodium sulfate. Biochem Biophys Res Commun. 2009;386:11-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |