Published online Jul 14, 2017. doi: 10.3748/wjg.v23.i26.4788
Peer-review started: March 28, 2017
First decision: May 10, 2017
Revised: May 16, 2017
Accepted: June 9, 2017
Article in press: June 9, 2017
Published online: July 14, 2017
To determine the prevalence of gastrointestinal neoplasia among dermatomyositis patients who underwent an esophagogastroduodenoscopy and/or colonoscopy.
A cross-sectional study examining the results of upper endoscopy and colonoscopy in adults with dermatomyositis at an urban, university hospital over a ten year period was performed. Chart review was performed to confirm the diagnosis of dermatomyositis. Findings on endoscopy were collected and statistical analyses stratified by age and presence of symptoms were performed.
Among 373 adult patients identified through a code based search strategy, only 163 patients had dermatomyositis confirmed by chart review. Of the 47 patients who underwent upper endoscopy, two cases of Barrett’s esophagus without dysplasia were identified and there were no cases of malignancy. Of the 67 patients who underwent colonoscopy, no cases of malignancy were identified and an adenoma was identified in 15% of cases. No significant differences were identified in the yield of endoscopy when stratified by age or presence of symptoms.
The yield of endoscopy is low in patients with dermatomyositis and is likely similar to the general population; we identified no cases of malignancy. A code based search strategy is inaccurate for the diagnosis of dermatomyositis, calling into question the results of prior population-based studies. Larger studies with rigorously validated search strategies are necessary to understand the risk of gastrointestinal malignancy in patients with dermatomyositis.
Core tip: Dermatomyositis is associated with an increased risk of gastrointestinal (GI) malignancies based on large-population based studies. These prior studies utilized code-based search strategies and did not perform individual chart review. The yield of endoscopy in this patient population is not known. In this study, endoscopy identified no cases of malignancy and was of low yield, likely similar to the general population, in the identification of pre-malignant findings. Code-based searched strategies were inaccurate in the identification of dermatomyositis, calling into question the results of prior population-based studies. The association between increased GI malignancy and dermatomyositis may be lower than previously reported.
- Citation: Kidambi TD, Schmajuk G, Gross AJ, Ostroff JW, Terdiman JP, Lee JK. Endoscopy is of low yield in the identification of gastrointestinal neoplasia in patients with dermatomyositis: A cross-sectional study. World J Gastroenterol 2017; 23(26): 4788-4795
- URL: https://www.wjgnet.com/1007-9327/full/v23/i26/4788.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i26.4788
Patients with dermatomyositis, a common idiopathic inflammatory myopathy characterized by muscle weakness and cutaneous findings affecting approximately 1 per 100000 people are generally considered to have an increased prevalence of gastrointestinal (GI) malignancy[1,2]. However, the reported prevalence of GI malignancy varies depending on the level of chart review or diagnostic codes used to verify the diagnoses[3-9]. Large population-based studies[3-5] utilizing diagnostic codes for identifying dermatomyositis patients have reported a prevalence of GI malignancy as high as five percent. Additionally, a study using extensive testing for malignancy in dermatomyositis patients reported a 15% prevalence of GI malignancy[6]. In contrast, small retrospective studies[7-9] utilizing individual level chart review have reported the prevalence of gastric or colorectal cancer (CRC) to be closer to one percent among dermatomyositis patients.
Currently, there are no guidelines that recommend an initial endoscopic workup for evaluation of an underlying GI malignancy in dermatomyositis patients. However, given the reported increased prevalence of GI neoplasia, many providers recommend an upper esophagogastroduodenoscopy (EGD) and colonoscopy as part of routine clinical care among dermatomyositis patients despite the lack of signs, symptoms, or laboratory abnormalities to suggest a GI source. To date, there are no data regarding the utility of this practice among dermatomyositis patients. Therefore, the aim of this study was to determine the prevalence of GI neoplasia among dermatomyositis patients who underwent an EGD and/or colonoscopy.
This retrospective, cross-sectional study was conducted at the University of California, San Francisco (UCSF), an academic, tertiary care medical center serving over 1.2 million people in the San Francisco Bay Area. Patients with dermatomyositis were referred to either UCSF’s rheumatology, dermatology, or gastroenterology clinics.
The study was approved by the UCSF institutional review board, which waived the requirement for informed consent. The listed authors had sole responsibility for the study design, data collection, decision to submit the manuscript for publication, and drafting of the manuscript.
We included all dermatomyositis patients 18 years of age and above seen at UCSF between January 2005-February 2016, who had an endoscopy (EGD and/or colonoscopy) at or after their dermatomyositis diagnosis. We selected this time period because of the availability of electronic health records. To identify patients with dermatomyositis seen at UCSF, we performed a comprehensive search of our electronic medical record using International Classification of Diseases (ICD)-9 and ICD-10 diagnoses codes (Supplementary Table 1). We also reviewed a separate query of the electronic medical record initiated by UCSF’s rheumatology division, which utilized a combination of ICD-9 and ICD-10 codes as well as clinic modifiers used for research purposes by the rheumatology clinic, to identify any additional dermatomyositis patients that may have been missed by our diagnostic code search. After the initial search, two reviewers (TDK, JKL) manually reviewed each chart to confirm the diagnosis of dermatomyositis. We defined a dermatomyositis diagnosis as any patient seen in either rheumatology and/or dermatology clinic with a diagnosis of dermatomyositis made by histological and/or clinical criteria; patients with an overlap rheumatologic condition that included dermatomyositis were included. For any charts where the diagnosis of dermatomyositis was unclear or with discordant results by the two reviewers, a rheumatologist was used for adjudication.
| Baseline characteristics | Had endoscopy (n = 79) |
| Age (mean ± SD) | 56.7 (14.4) |
| Male gender | 21 (27) |
| Age at dermatomyositis diagnosis (mean ± SD) | 50.1 (15.9) |
| Disease duration (mean ± SD) at time of endoscopy | 6.8 (6.6) |
| Personal history of cancer | 8 (10.1) |
| Melanoma | 1 |
| Prostate | 1 |
| Endometrial | 1 |
| NCC | 1 |
| HCC | 1 |
| RCC | 1 |
| AdenoCA of unknown origin | 2 |
| Indication for EGD | Had EGD (n = 47) |
| Screening | 19 (40.4) |
| Dysphagia | 13 (27.7) |
| Dyspepsia/pain | 9 (19.1) |
| IDA | 2 (4.3) |
| Weight loss | 3 (6.4) |
| Abnormal CT scan | 1 (2.1) |
| Indication for colonoscopy | Had colonoscopy (n = 67) |
| Screening | 49 (73.1) |
| Surveillance | 2 (3.0) |
| Diarrhea, abdominal pain | 11 (16.4) |
| IDA | 2 (3.0) |
| Weight loss | 1 (1.5) |
| Blood in stool | 1 (1.5) |
| Abnormal CT | 1 (1.5) |
We obtained patient demographic and clinical information for each dermatomyositis patient included in this study from our electronic medical records, which included clinic notes, consultation notes, endoscopy reports, pathology reports, radiology reports, and cancer diagnoses. Age and disease duration were defined as the age at the end of the study period or the difference in years between the patients’ date of birth and the end of the study period (February 2016), respectively. For patients who underwent an endoscopy, data on age and disease duration at the time of endoscopy was collected. Findings on EGD were categorized as normal, pre-malignant, malignant, or non-malignant. Gastric and duodenal erosions with non-specific inflammation on histology were included as normal. Pre-malignant findings on EGD included Barrett’s esophagus (with or without dysplasia), gastric intestinal metaplasia (with or without dysplasia) and duodenal adenoma. Malignant findings on EGD included carcinoma of the esophagus, stomach or duodenum. Non-malignant findings included erosive esophagitis, infectious esophagitis (such as candidiasis or cytomegalovirus infection) and helicobacter pylori infection, all of which were confirmed on histology, as well as esophago-gastric varices. If subsequent EGDs were performed after the initial endoscopy, the findings were collected and the highest risk finding was recorded.
Findings on colonoscopy were categorized as normal, pre-malignant, malignant, or non-malignant. Diverticulosis, distal hyperplastic polyps and non-specific findings on endoscopy with normal histology (such as “thickened folds” or “nodules”) were included as normal. Pre-malignant findings included any colonic adenoma, proximal serrated lesions, and were further categorized as advanced adenomas or low-risk adenomas. We defined an advanced adenoma as any adenoma of any size with high grade dysplasia or a villous component or an adenoma greater than ten millimeters in size, low risk tubular adenomas, defined as less than three adenomas not meeting criteria for an advanced adenoma, or higher risk tubular adenomas, defined as three of more tubular adenomas found on a single colonoscopy. Malignancy was defined as carcinoma of the colon, including adenocarcinoma and neuroendocrine neoplasms. Non-malignant findings included inflammatory bowel disease and microscopic colitis. If subsequent colonoscopies were performed after the initial colonoscopy, the findings were collected and the highest risk finding was recorded.
The primary outcome of the study was the yield of endoscopy to identify GI neoplasia among dermatomyositis patients. We further stratified the findings on endoscopy by age and whether signs or symptoms were present at the time of endoscopy. Statistical analyses comparing differences amongst the patients who underwent endoscopy stratified by age or presence of symptoms was performed using χ2 test and Fisher’s exact test was used for categorical variables with less than five outcomes. Results were displayed as either mean ± SD) or the number of outcomes (percentage who underwent endoscopy). All analyses were performed using SPSS (IBM version 23) and a two-tailed P value of < 0.05 was considered statistically significant.
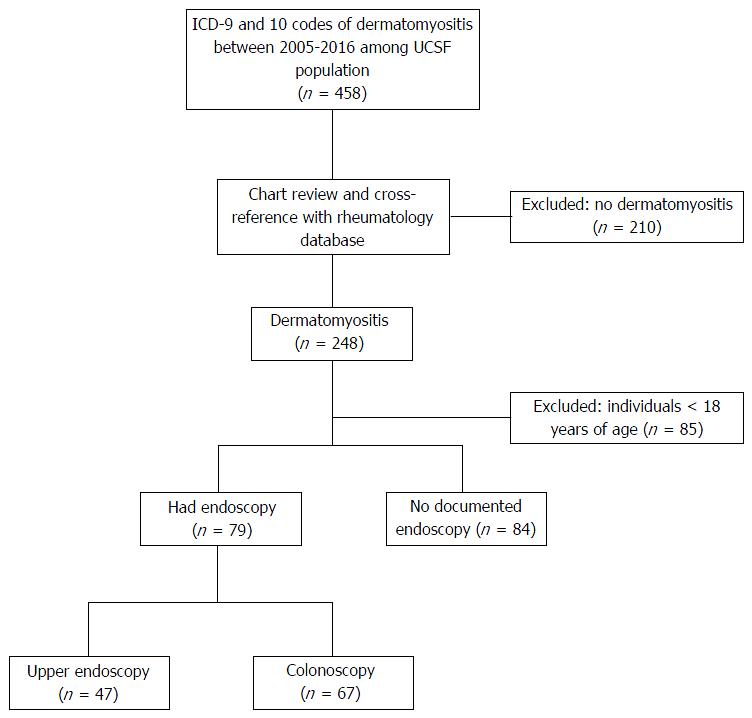
A total of 458 patients were identified by querying the electronic medical records for ICD-9 and ICD-10 codes of dermatomyositis between January 2005 and February 2016. We did not identify any additional dermatomyositis patients after reviewing the rheumatology initiated query of the electronic medical record. After chart review of the 458 patients, 295 of these patients (64%) were excluded because they either had dermatomyositis incorrectly coded (n = 210, 46%) or they were younger than 18 years of age (n = 85, 18%). Thus, a total of 163 patients with dermatomyositis were included and, of these, 79 patients had an endoscopy documented within the electronic medical record (EGD and/or colonoscopy) as shown in Figure 1.
Baseline demographic data for the patients who underwent endoscopy is shown in Table 1. The mean age of patients at the time of endoscopy was 56.7 years with a range from 21 to 88 years and a SD of 14.4 years; 73% were women. On average, patients had dermatomyositis for 6.8 years at the time of endoscopy with a SD of 6.6 years; 39 patients (49%) had endoscopy within 5 years of their diagnosis. Of the two patients with an adenocarcinoma of unknown origin, one of these patients was diagnosed based on biopsy of a large lymph node found on cross sectional imaging while the other patient’s diagnosis was made based on biopsy of a liver lesion, with genome sequencing of the tumor suggestive of a lung primary. The most common indication for EGD and colonoscopy was screening in an otherwise asymptomatic patient (40.4% and 73.1%, respectively); dysphagia was the most common symptom present at time of EGD (27.7%) and diarrhea and abdominal pain were the most common symptoms prompting colonoscopy (16.5%).
Chart review was performed on the patients with a diagnosis of dermatomyositis who did not undergo endoscopy and the results comparing demographic data for these patients compared to those who did undergo endoscopy is shown in Supplementary Table 2. Patients who underwent endoscopic evaluation were significantly older at the time of their dermatomyositis diagnosis than those who did not (50.1± 15.9 vs 40.6 ± 15.9, respectively, P < 0.01). No significant differences were identified in any of the other variables, including personal history of cancer. The single patient with CRC who did not undergo endoscopy was diagnosed with CRC 10 years prior to her dermatomyositis diagnosis and no records of the initial or subsequent surveillance colonoscopies were available in the electronic medical record.
| Findings on EGD, n = 47 | |
| Malignancy | 0 (0) |
| Pre-malignancy | 3 (6.4) |
| Barrett's esophagus without dysplasia | 2 |
| Gastric intestinal metaplasia | 1 |
| Non-malignant findings | 8 (17) |
| Esophageal varices | 2 |
| H. pylori gastritis | 4 |
| CMV esophagitis | 1 |
| Candida esophagitis | 1 |
| Normal | 36 (76.6) |
| Findings on colonoscopy, n = 67 | |
| Colorectal cancer | 0 (0) |
| Any adenoma | 10 (14.9) |
| Inflammatory bowel disease | 4 (6.0) |
| Normal | 53 (79.1) |
Of the 47 patients who underwent EGD, no cases of malignancy were identified on either the initial exam, or if performed, on subsequent exams as shown in Table 2. EGD identified two cases of Barrett’s esophagus without dysplasia and subsequent EGD at one year identified no progression of the Barrett’s in either patients. In another patient, gastric intestinal metaplasia without dysplasia on random gastric biopsies was identified on EGD and the patient underwent a total of seven subsequent surveillance EGDs over ten years without progression of the gastric intestinal metaplasia.
There were no cases of malignancy detected in the 67 patients who underwent colonoscopy as shown in Table 2. Of the 12 patients who underwent surveillance exams, the majority underwent a single surveillance exam in the study period while one patient underwent three exams over a ten-year period for surveillance purposes because of a previous finding of an advanced adenoma (> 10 mm sessile serrated adenoma) and a family history of CRC. Seventy-nine percent of the colonoscopies were normal and at least one adenoma was identified on 14.9% of the initial colonoscopies, with the majority being low risk tubular adenomas (9.0%).
The yield of endoscopy, stratified by age less than or greater than 50 years is shown in Table 3. We selected age 50 as the cutoff because many United States guidelines commonly recommend average-risk CRC screening at this age[10]. In addition, multiple gastroenterology societies recommend esophageal cancer or Barrett’s esophagus screening for high-risk individuals (i.e., long-standing gastro-esophageal reflux disease, Caucasian race, obesity, etc.) at age 50[11]. There were no statistical differences in the yield of EGD for pre-malignant conditions stratified by age.
| Age < 50 (n = 21) | Age ≥ 50 (n = 26) | Age ≥ 50 (n = 46) | P value | |
| Findings on EGD | ||||
| Normal | 16 (76.2) | 20 (76.9) | - | 0.95 |
| Pre-malignancy | 1 (4.8) | 2 (7.7) | - | 1.00 |
| Malignancy | 0 (0) | 0 (0) | - | 1.00 |
| Non-malignant | 4 (19.0) | 4 (15.4) | - | 1.00 |
| Findings on Colonoscopy | ||||
| Normal | 17 (81.0) | - | 36 (78.3) | 0.80 |
| Any adenoma | 1 (4.8) | - | 9 (20.0) | 0.15 |
| Colorectal cancer | 0 (0) | - | 0 (0) | 1.00 |
| Inflammatory bowel disease | 2 (9.5) | - | 2 (4.3) | 0.58 |
The yield of endoscopy, stratified by the presence of symptoms is shown in Table 4. There were no identified differences in the yield of EGD when symptoms were present. Asymptomatic patients undergoing colonoscopy were more likely to have a normal exam than those with symptoms (86.3% vs 56.3%, respectively, P = 0.01), which was attributable to the finding of inflammatory bowel disease or microscopic colitis in symptomatic patients (25% vs 0% in the asymptomatic patients, P < 0.01).
| Asymptomatic | Symptomatic | P value | |||
| n = 19 | n = 51 | n = 28 | n = 16 | ||
| Findings on EGD | |||||
| Normal | 15 (78.9) | - | 21 (75.0) | - | 0.75 |
| Pre-malignancy | 0 (0) | - | 3 (10.7) | - | 0.26 |
| Barrett's without dysplasia | - | - | 2 (7.1) | - | - |
| Gastric intestinal metaplasia without dysplasia | - | - | 1 (3.6) | - | - |
| Malignancy | 0 (0) | - | 0 (0) | - | - |
| Non-malignant | 4 (21.1) | - | 4 (14.3) | - | 0.70 |
| Varices | 2 (10.5) | - | - | - | - |
| Helicobacter pylori gastritis | 2 (10.5) | - | 2 (7.1) | - | - |
| Cytomegalovirus esophagitis | - | - | 1 (3.6) | - | - |
| Candida esophagitis | - | - | 1 (3.6) | - | - |
| Findings on Colonoscopy | |||||
| Normal | - | 44 (86.3) | - | 9 (56.3) | 0.01 |
| Pre-malignancy | - | 7 (13.7) | - | 3 (18.8) | 0.69 |
| Advanced adenoma | - | 1 (2.0) | - | 1 (6.3) | - |
| Low risk tubular adenoma | - | 5 (9.8) | - | 1 (6.3) | - |
| ≥ 3 tubular adenomas | - | 1 (2.0) | - | 1 (6.3) | - |
| Malignancy | - | 0 (0) | - | 0 (0) | - |
| Non-malignant findings | - | 0 (0) | - | 4 (25) | < 0.01 |
| IBD | - | - | - | 3 (18.8) | - |
| Microscopic colitis | - | - | - | 1 (6.3) | - |
Although screening for GI neoplasia is commonly recommended in patients with dermatomyositis, the yield of endoscopy remains unclear. To that end, we report results of the largest study of dermatomyositis patients utilizing individual chart-review and the first to examine the yield of endoscopy on identification of pre-malignant and malignant lesions of the GI tract in the United States. In our study of 79 physician-confirmed dermatomyositis patients, we found the yield of endoscopy to be low for the identification of pre-malignant and malignant GI lesions, even when stratified by age, in both asymptomatic and symptomatic patients, calling into question an aggressive diagnostic approach to identify occult malignancy. No cases of GI malignancy were identified; EGD was of very low yield in the identification of significant pathology and colonoscopy was only useful in identifying pre-malignant lesions (colonic adenomas) in the screening age population (patients 50 years and older).
To date, few studies have evaluated the prevalence of GI malignancy in dermatomyositis patients. Previous large, population-based studies have found an association between dermatomyositis and GI malignancies[3-5]. In addition, a single descriptive study[6] of 40 patients with dermatomyositis and polymositis at a French hospital over a 20 year period suggested that an extensive search for malignancy with CT scans of the chest, abdomen and pelvis increased the identification of malignancy, when compared to routine malignancy screening. In contrast, smaller studies[7-9] have found a low prevalence of GI neoplasia. These studies found gastric cancer in less than 3% of patients[8,9] and CRC in less than 2% of patients[7,8] with dermatomyositis. In our study, we found no GI malignancies after an extensive endoscopic evaluation. One potential explanation for these inconsistent findings could be the identification of dermatomyositis patients. Studies utilizing ICD code based searches[3,5] and key-word search criteria[4] for the diagnosis of dermatomyositis and GI malignancy showed higher prevalence for GI malignancy compared to smaller studies[7-9], including ours, that utilized individual chart review to confirm the dermatomyositis diagnosis. In our study, we found ICD coding based diagnosis of dermatomyositis was inaccurate in 46% of the patients; this calls into question the results of these prior studies and suggests that rigorous validation is necessary for any future population-based dermatomyositis studies.
Common factors for deciding to pursue endoscopic or cross-sectional imaging workup in the general population include advancing age and symptoms. In our study, there were no significant differences in the yield of endoscopy for pre-malignant conditions stratified by age and symptoms. In the French cohort of 40 dermatomyositis and polymositis patients, six patients (15%) were diagnosed with a GI malignancy by endoscopy following an abnormal CT scan. The results of our study are inconsistent with this study, though only two patients in our study had an abnormal CT prior to endoscopy. The presence of concerning clinical symptoms (i.e., constitutional symptoms, anemia, etc.) prompting further evaluation with CT scans in all 40 patients, likely accounts for the difference in results when compared to our study, which largely included asymptomatic patients undergoing screening endoscopy. Thus, we conclude red-flag symptoms in dermatomyositis patients, as in any patient, should prompt investigation for an underlying cause, but endoscopy is probably of similar yield in dermatomyositis patients as in age-matched patients without dermatomyositis. Unfortunately, given the retrospective nature of the study we were unable to control for all potential confounders including the presence of Epstein-Barr virus which in a prior study was shown to be associated with gastric cancer risk[12].
Our study had many strengths. Because the data were generated from routine clinical care, the findings are likely to be accurate and generalizable to other clinical centers caring for patients with dermatomyositis. A particular strength of our data is that individual chart review was conducted to verify the diagnosis of dermatomyositis as well as the findings on endoscopy and pathologic diagnoses. To our knowledge this is the largest sample size of dermatomyositis patients in a study utilizing chart review and therefore is a valuable addition to the literature. As this is the only study examining the yield of endoscopy in both symptomatic and asymptomatic dermatomyositis patients, the results are likely to help inform decisions in the routine clinical care of patients.
There were several limitations to our study. Because the study was conducted in a single tertiary care center, there was loss to follow-up and it is possible that, despite reviewing the electronic medical record including uploaded records from care received outside of UCSF, endoscopies performed elsewhere were not captured and as a result cases of cancer were missed. Furthermore, the age of patients in our study was relatively young which may have reduced the likelihood of identifying malignancy, as older age has been shown to be a risk factor for neoplasia in this patient population[13]. However, this supports our finding that the risk of neoplasia is likely similar to the general, age-matched population and screening outside of the guideline recommendations may not be needed where an adenoma detection rate of 20%-30% is expected in the United States. Additionally, a large number of patients with dermatomyositis did not undergo endoscopy so cases of cancer could have been missed. However, this was mitigated by the fact that the entire medical record was reviewed, since a cancer diagnosis likely would have been mentioned in clinic notes or within the problem lists. Furthermore, because there is wide variation in practice it is possible that patients who underwent endoscopy may have been perceived by their physicians to be at higher risk for malignancy but this would have biased our study towards an overestimate of the prevalence of malignancy, which is less of a concern given the negative findings of our study. While this was the largest study of its kind, the power to identify differences in endoscopy yield stratified by age was low. For example, to detect a 5% difference in the prevalence of colonic adenomas assuming a prevalence 25% would require over 1000 patients 50 years and older and another 1000 patients younger than 50. Given dermatomyositis is a rare disease, an adequately powered study was not feasible. Nevertheless, the absolute values and findings in our study are still meaningful, interpretable and may be more applicable to the clinician caring for dermatomyositis than the previous larger population based studies that did not utilize individual level chart review.
In conclusion, this study suggests that the yield of endoscopy in dermatomyositis for the identification of pre-malignant and malignant GI lesions is low in the United States. In fact, our study identified no cases of upper GI or colon cancers. Moreover, even when stratified by age or the presence of symptoms, endoscopy was of low yield, raising the question of whether routine use of aggressive screening for GI malignancy is necessary and justified. Further studies utilizing rigorously validated search strategies are necessary to determine the absolute risk of GI malignancy in patients with dermatomyositis to inform whether aggressive surveillance is needed beyond what is recommended in the average-risk screening population.
Dermatomyositis is associated with an increased risk of gastrointestinal (GI) malignancies based on large-population based studies. These prior studies utilized code-based search strategies and did not perform individual chart review.
Endoscopy is now used to screen for GI neoplasias, but the yield of endoscopy in this patient population with dermatomyositis is not known. Understanding the yield of endoscopy could help inform clinical care of these patients.
By utilizing individual chart review, we provided an accurate estimate of prevalence of GI neoplasia in this population. They found that endoscopy identified no cases of malignancy and was of low yield, likely similar to the general population, in the identification of pre-malignant findings. Code-based searched strategies were inaccurate in the identification of dermatomyositis, calling into question the results of prior population-based studies.
The association between increased GI malignancy and dermatomyositis may be lower than previously reported.
This is an observational study attempting to quantify the actual risk of GI malignancy in patients with dermatomyositis and the yield of routine screening endoscopy in this population. The study is performed by retrospective chart review after identifying patients with dermatomyositis and reviewing endoscopy results. The paper is well written with good numbers and a thorough search method and provides evidence for management of a rare condition.
Manuscript source: Invited Manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chow CFK, Treeprasertsuk S S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
| 1. | Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1061] [Cited by in F6Publishing: 955] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 2. | Dalakas MC. Polymyositis, dermatomyositis and inclusion-body myositis. N Engl J Med. 1991;325:1487-1498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 753] [Cited by in F6Publishing: 643] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 3. | Hill CL, Zhang Y, Sigurgeirsson B, Pukkala E, Mellemkjaer L, Airio A, Evans SR, Felson DT. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 757] [Cited by in F6Publishing: 635] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 4. | Buchbinder R, Forbes A, Hall S, Dennett X, Giles G. Incidence of malignant disease in biopsy-proven inflammatory myopathy. A population-based cohort study. Ann Intern Med. 2001;134:1087-1095. [PubMed] [Cited in This Article: ] |
| 5. | Chen YJ, Wu CY, Huang YL, Wang CB, Shen JL, Chang YT. Cancer risks of dermatomyositis and polymyositis: a nationwide cohort study in Taiwan. Arthritis Res Ther. 2010;12:R70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 6. | Sparsa A, Liozon E, Herrmann F, Ly K, Lebrun V, Soria P, Loustaud-Ratti V, Bouyssou-Gauthier ML, Boulinguez S, Bédane C. Routine vs extensive malignancy search for adult dermatomyositis and polymyositis: a study of 40 patients. Arch Dermatol. 2002;138:885-890. [PubMed] [Cited in This Article: ] |
| 7. | András C, Ponyi A, Constantin T, Csiki Z, Szekanecz E, Szodoray P, Dankó K. Dermatomyositis and polymyositis associated with malignancy: a 21-year retrospective study. J Rheumatol. 2008;35:438-444. [PubMed] [Cited in This Article: ] |
| 8. | Fang YF, Wu YJ, Kuo CF, Luo SF, Yu KH. Malignancy in dermatomyositis and polymyositis: analysis of 192 patients. Clin Rheumatol. 2016;35:1977-1984. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Limaye V, Luke C, Tucker G, Hill C, Lester S, Blumbergs P, Roberts-Thomson P. The incidence and associations of malignancy in a large cohort of patients with biopsy-determined idiopathic inflammatory myositis. Rheumatol Int. 2013;33:965-971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | US Preventive Services Task Force. Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW Jr, García FAR, Gillman MW, Harper DM, Kemper AR, Krist AH, Kurth AE, Landefeld CS, Mangione CM, Owens DK, Phillips WR, Phipps MG, Pignone MP, Siu AL. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315:2564-2575. [PubMed] [Cited in This Article: ] |
| 11. | Spechler SJ, Souza RF. Barrett’s esophagus. N Engl J Med. 2014;371:836-845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 360] [Cited by in F6Publishing: 343] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 12. | Yamashita K, Hosokawa M, Hirohashi S, Arimura Y, Endo T, Denno R, Ikeda T, Imai K. Epstein-Barr virus-associated gastric cancer in a patient with dermatomyositis. Intern Med. 2001;40:96-99. [PubMed] [Cited in This Article: ] |
| 13. | Marie I, Hatron PY, Levesque H, Hachulla E, Hellot MF, Michon-Pasturel U, Courtois H, Devulder B. Influence of age on characteristics of polymyositis and dermatomyositis in adults. Medicine (Baltimore). 1999;78:139-147. [PubMed] [Cited in This Article: ] |