Published online Jun 14, 2017. doi: 10.3748/wjg.v23.i22.4112
Peer-review started: February 6, 2017
First decision: March 16, 2017
Revised: March 24, 2017
Accepted: June 1, 2017
Article in press: June 1, 2017
Published online: June 14, 2017
To verify the utility of treatment with fecal microbiota transplantation (FMT) in patients with irritable bowel syndrome (IBS).
We searched EMBASE, Cochrane Library and PubMed in March, 2017. The reviewed literature was based on two systematic searches in each of the databases. The MeSH terms used were IBS and fecal microbiota transplantation and the abbreviations IBS and FMT. Reference lists from the articles were reviewed to identify additional pertinent articles.
A total of six conference abstracts, one case report, one letter to the editor, and one clinical review were included. In the final analysis, treatment of 48 patients was evaluated. Treatment revealed an improvement in 58% of cases. The varying structure of the nine included studies must be taken into consideration.
Data on FMT and IBS are too limited to draw sufficient conclusions. Standardized double blinded randomized clinical trials need to be carried out to evaluate the effect of FMT on IBS.
Core tip: In humans, the gastrointestinal tract represents a large microbial ecosystem, housing several trillion microbial cells named the gut microbiota. Recent advances in sequencing methods have increased our understanding of the role of the gut microbiota in health and disease. Worldwide, interest is growing rapidly for fecal microbiota transplantation (FMT) as an “ecological” therapy for several diseases. Evidence suggests that a disturbance in the gut microbiota may be responsible for the initiation and persistence of symptoms in patients with irritable bowel syndrome. FMT could, therefore, be an ideal treatment option.
- Citation: Halkjær SI, Boolsen AW, Günther S, Christensen AH, Petersen AM. Can fecal microbiota transplantation cure irritable bowel syndrome? World J Gastroenterol 2017; 23(22): 4112-4120
- URL: https://www.wjgnet.com/1007-9327/full/v23/i22/4112.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i22.4112
There is a growing interest in fecal microbiota transplantation (FMT) therapy for several gastrointestinal (GI) disorders. Treating GI disorders with FMT has been attempted as early as the 4th century, where a Chinese physician named Ge Hong advised patients suffering from severe diarrhea to consume fresh stool from a healthy neighbor as a form of treatment[1]. It is thought that the microbiome in our GI system plays an important role in health and disease. Literature in this area has increased markedly over recent years. More than 90% of the nearly 4000 articles indexed by PubMed on the subject have been published in the past five years (data from 2014)[2], indicating the rapidly growing interest within this field. Our knowledge of the microbiome is expanding due to, amongst other things, the development of new genetic technologies that allow us to identify and quantify the organisms of the microbiome at a faster rate[2]. Treatment with FMT has been a huge success when treating Clostridium difficile infection (CDI), with 33 case studies and a single randomized clinical trial (RCT) showing efficacy rates ranging from 81%-94%[1]. An increasing number of studies demonstrate an aberrant gut microbiota composition in irritable bowel syndrome (IBS)[3-6] and raise the question of whether FMT has a place in the treatment of this condition. The microbial pathophysiology of IBS is, however, not clearly understood, as microbiota alterations in IBS might either be a cause of IBS or a consequence of intestinal secretion and motility altered by IBS. FMT may play a significant role in future treatment of several other diseases which are thought to be linked to an abnormal gut microbiota, such as metabolic syndrome[1], inflammatory bowel disease (IBD)[1], obesity, type 2 diabetes mellitus, colonization of the gastrointestinal tract by pathogenic and multi-resistant microorganisms[7], depression, autism spectrum disorders[8], and chronic stress[9].
IBS is the most prevalent functional GI disorder in developed countries. It is estimated that IBS affects 10%-15% of the adult population[10] and strongly impairs quality of life, work productivity, and social function as well as inflicting substantial costs to health care systems[11,12].
The pathogenesis of IBS is complex and not yet fully understood. Accumulating evidence indicates that the gut microbiota plays a significant role[13], and alterations in gut microbiota among IBS patients have been described frequently[14]. It is also postulated that gut motility, enhanced visceral hypersensitivity, post infectious states[15], food sensitivity[2], genetics[16,17], and psychosocial disturbances[14] play a role in the pathophysiology of IBS.
IBS symptoms are characterized by chronic abdominal pain and altered bowel habits, including diarrhea and/or constipation, in the absence of organic or structural causes[2]. To receive the diagnosis IBS, symptoms must concur with the Rome III criteria[18]. Furthermore, IBS can be subcategorized into diarrhea predominant (IBS-D), constipation predominant (IBS-C), and alternating (IBS-A) or mixed (IBS-M), where the last two are sometimes considered synonymous[19]. In most patients, IBS is a chronic relapsing disease in which symptoms and IBS subtype may vary over time. IBS affects women more often than men[10].
The treatment of IBS remains challenging due to the heterogeneity of the disorder, a lack of reliable outcome measures, and high placebo response rates. At present, there is no cure for IBS and, while there are a number of pharmacological therapies available to treat IBS symptoms, they are not effective in many patients[20].
An improved understanding of microbiota in IBS is important not only with regard to its pathogenesis but also in enabling therapeutic modulation of the microbiota. Many medical and alternative therapies have been tested without convincing effects.
Some studies indicate that moderate effects can be achieved by probiotics and prebiotics[21,22]. These products must, however, be taken continuously to obtain a lasting effect[23]. Also, treatment with tricyclic antidepressants, antibiotics, anti-cholinergic drugs, motility regulatory drugs, selective serotonin reuptake inhibitors, melatonin, non-steroid anti-inflammatory drugs, opioids, and even Chinese herbs are suggested in severe IBS cases and underlines the fact that we do not yet know the etiology of the disease.
Many patients report that their symptoms are related to various food items and two-thirds of IBS patients report dietary restrictions on this basis[24]. Many different dietary approaches for the management of IBS symptoms have been tested over the years. Although dietary interventions for IBS are frequently recommended, there is, however, a lack of data to support their use[25]. This impact highlights the need for more effective IBS treatments as current therapies are not successful in many patients. This review aims to investigate current evidence about FMT and its use in IBS.
The reviewed literature was based on two systematic searches performed on March 13th, 2017 in the databases PubMed, Cochrane Library and EMBASE. The MeSH terms used were irritable bowel syndrome and fecal microbiota transplantation and the abbreviations IBS and FMT.
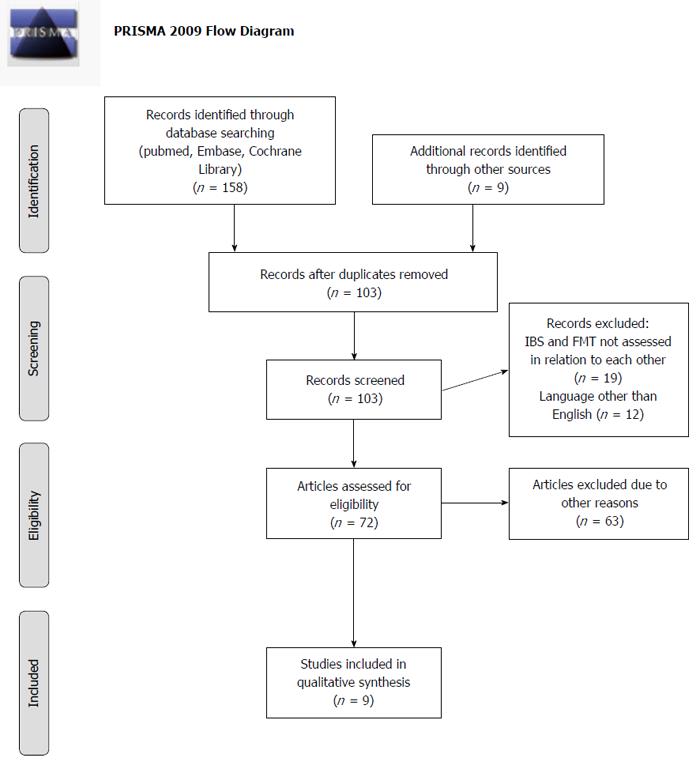
One hundred and fifty-eight papers were discovered in the 6 searches and 9 additional records were found by going through reference lists and other sources. Seventy-two papers were assessed; where one unobtainable and 63 others were excluded since they were reviews and referred to the same 3 original studies. Of these 3 studies, 2 were recovered but the 3rd could not be found[26]. The authors of this 3rd study have made a clinical review, in which they mention their study. This review has been included instead[27]. A total of 9 papers were found from scrutinizing references, 3 articles were retrieved from the 6 searches made, and 3 were found from other sources. Nine papers were, therefore, included in the final review. Of the 9 articles, 6 are conference abstracts[28-33], 1 is a case report[34], 1 is a letter to the editor[35], and 1 is a review[27]. A PRISMA (http://www.prisma-statement.org/statement.htm) flow diagram illustrates the outcome of the search (Figure 1).
All studies evaluating the effects of FMT in IBS patients are included in this paper. To estimate the overall effect of FMT in IBS patients, we decided to include studies with a follow-up longer or equal to 3 mo. Due to the restricted number of FMT studies in IBS patients, we have also decided to include conference abstracts.
A limited number of studies (summarized in Table 1) have examined the therapeutic role of FMT in IBS. The 9 publications reviewed in this paper consisted of 6 conference abstracts, 1 case report, 1 letter to the editor, and 1 clinical review. The studies are all case reports or case series and include a total of 127 patients. Borody et al[35] describe the treatment of 55 patients with FMT. The administration route of the FMT is not specified. What is stated is that the original bowel flora was removed by gastrointestinal lavage and replaced with bowel bacteria from a healthy donor. The 55 patients were suffering from constipation, diarrhoea, abdominal pain, ulcerative colitis, or Crohn’s disease. Patients were included if other forms of therapy had failed to ease their symptoms. Unfortunately, a distinction between patient groups was not made. Of the 55 patients, 20 (36.4%) were described as “cured”, 9 (16.4%) experienced a decrease of symptoms, and 26 (47.3%) experienced no improvement in symptoms.
| Ref. | Year | Type | n | N in regard to IBS | Subcategory | ||
| IBS-D | IBS-C | IBS-M | |||||
| Borody et al[35] | 1989 | Letter to the editor | 55 | Not specified | - | - | - |
| Andrews et al[34] | 1992 | Case report | 1 | 1 | - | 1 | - |
| Borody et al[27] | 2004 | Review | 6 | 3 | - | 3 | - |
| Pinn et al[28] | 2013 | Conference abstract | 13 | 13 | 9 | 3 | 1 |
| Holvoet et al[29] | 2015 | Conference abstract | 12 | 12 | - | - | - |
| Cruz Aguilar et al[30] | 2015 | Conference abstract | 9 | 9 | 5 | 4 | 0 |
| Hong et al[31] | 2016 | Conference abstract | 10 | 10 | - | - | - |
| Syzenko et al[32] | 2016 | Conference abstract | 12 | 12 | 6 | 5 | 1 |
| Mazzawi et al[33] | 2016 | Conference abstract | 9 | 9 | - | - | - |
Andrews et al[34] published a case report of a patient with chronic constipation who they, in a separate publication, diagnosed with constipation-predominant IBS[36]. The patient presented with a history of constipation spanning 3 years after an uncomplicated hysterectomy. Defaecation was once per week and required the use of laxatives. Associated symptoms included abdominal bloating, daily nausea, mild oesophageal reflux symptoms, and frequent headaches. The patient was given a treatment of vancomycin 250 mg thrice daily for 4 wk. Her constipation and associated symptoms disappeared promptly, but returned within 3 d of seponating treatment. Andrews et al[34] then decided to continue treatment with FMT. A fresh suspension of faeces was collected from her spouse and infused by enema for 2 d. Within 3 d of treatment, her stool frequency had shifted to 1-2 times per day without the use of laxatives. The headaches, abdominal bloating, and reflux symptoms had also disappeared. Symptom improvement was sustained at 18 mo follow-up.
The work of Borody et al[26] has not been acquired, despite extensive searching. Instead, the clinical review by Borody et al[27] is included as a substitute and describes the same case series from 2001. In 2001, Borody et al[27] published a case series study of ulcerative colitis and chronic constipation with 3 patients in each series. Chronic constipation was described as constipation-predominant IBS. Patients received single daily retention enemas for 5 d with donor stool suspended in 200 mL water with NaCl and a tablespoon of Psyllium. The patients with chronic constipation experienced a restoration of normal bowel function with a frequency of defaecation of 1-2 times per day. The follow-up period ranged from 8 to 28 mo in both case series.
In 2013, Pinn et al[28] carried out a follow-up study of IBS treatment with FMT. Diagnosis of participants was based on the Rome III criteria and patients, who were otherwise unresponsive to traditional treatment, were included in the study. Traditional treatment included probiotics, antibiotics, dietary changes, and other therapeutic modalities[28,37]. Thirteen of 15 eligible patients completed the study and were grouped in diarrhoea or constipation predominant IBS or mixed IBS. A questionnaire with 41 items addressing demographic data, pre- and post-FMT data, severity of abdominal pain, bloating, flatus, dyspepsia, diarrhoea, constipation, and overall well-being were filled out by the participants. Nine patients had IBS-D, 3 had IBS-C and 1 had IBS-M. The abstract does not include a description of how the FMT was administered[28]. Average time from FMT to data collection was 11 mo (range: 6-18 mo). FMT resolved or improved symptoms in 70% of the included patients. Factors which improved or resolved included abdominal pain (72%), changes in bowel habit (69%), dyspepsia (67%), bloating (50%), flatus (42%), and improved quality of life (42%). Transient increase in flatus was the only adverse effect reported[28,37].
Holvoet et al[29] carried out a prospective pilot study where 12 patients with refractory IBS symptoms underwent FMT. Patients with symptoms of severe bloating were included in the study. Fresh stool, under 6 hours after donation, were administered to the right colon via colonoscopy. The treatment was considered effective if the patient experienced an adequate relief of symptoms at week 12 post-transplantation. As secondary end points, the authors monitored IBS symptom scores and quality of life via questionnaires. At week 4 and week 12, 67% and 75% of patients reported an adequate relief of general IBS symptoms and, in particular, improvements in bloating, respectively. Furthermore, 16s rRNA amplicon sequencing was carried out on stool samples taken at different time points before and following transplantation. No 16s RNA results were presented in this paper[29]. In a subsequent journal letter written by Holvoet et al[38] further results were, however, presented. They found that the positive effects on IBS-related symptoms were linked to changes in the microbiota as a result of FMT treatment. Their stool sample analysis showed no microbiota community differences between patients and donors. Furthermore, no difference in microbial dissimilarity between patient-donor responders and non-responder pairs at baseline was found. A trend of higher Streptococcus counts was seen in donors compared to patients, and successful donors tended to have higher counts of Streptococcus compared with donors without success. In responders, a trend of higher enrichment potential compared with non-responders was also observed. Furthermore, the median number of successfully transferred phylotypes was higher in responders in relation to non-responders. The responders to FMT were assessed at a 1 year follow-up, where 7/9 (78%) still reported a significant relief of symptoms. The use of the Rome III criteria was utilized in this work[38].
In 2015, Cruz Aguilar et al[30] published an abstract in which they summarise the treatment and results of 9 patients suffering from IBS. Five patients had IBS-D and 4 had IBS-C. Patients received a pre-treatment of rifaximin and, 3 wk later, a single FMT was performed during a colonoscopy. Evaluation of the treatment was performed 3 mo after FMT using a standardized questionnaire [Rome III, Patient Health Questionnaires, Short Form Health Survey (36 items)] and clinical evaluation. Furthermore, deep sequencing analysis was performed on the microbiome before and 12 wk after FMT. The IBS-D patients immediately experienced a reduction of 2.5 points in the Bristol Stool Scale (BSS) score. No change in BSS score was reported in the IBS-C patients. A 50% reduction of abdominal pain was reported by 66% of the patients. A 50% reduction in bloating was reported by 16% of the participants. Reduction of symptoms lasted only 8 wk after FMT before a gradual reinstatement of symptoms occurred. Changes of the microbiome were seen in both IBS-D and IBS-C patients. In The IBS-D patients, a more diverse flora were discovered after treatment[30].
In 2016, Hong et al[31] published an abstract on FMT treatment in 10 patients with moderate IBS that did not respond to traditional treatment. Diagnosis was based on the Rome III criteria and healthy donors were selected from family members and screened for infectious diseases before donation. It is not specified through which route the FMT was administrated or how the FMT was performed. Patients answered the IBS severity score before as well as 1 and 3 mo after FMT. Study outcomes included the length of symptom-free intervals, bloating, flatus, abdominal pain, frequency of bowel movements, dyspepsia, and overall well-being before and after FMT. Eighty percent of the study participants experienced resolution or improvement of symptoms after FMT. According to their IBS severity score (231 ± 110), patients’ symptoms did, however, tend to return to their pre-treatment state within 3 mo after FMT. Clinically significant improvements in IBS severity score were observed at only 1 mo follow-up after FMT (132 ± 100) compared to baseline (252 ± 122) (P = 0.027). No long-term side-effects was reported by patients[31].
Mazzawi et al[33] conducted a study with FMT in IBS-D patients in order to investigate the effect of FMT on symptoms and the density of duodenal enteroendocrine cells. Nine patients were included according to the Rome III criteria. The FMT consisted of freshly donated stool from relatives. Details concerning how the FMT was administrated and performed were not included. Apart from the IBS severity score, the IBS symptom questionnaire and Bristol stool form scale were completed before and 3 wk after FMT. IBS symptom scores were significantly reduced 3 wk after FMT treatment; abdominal pain (P = 0.005), diarrhea (P = 0.0002), constipation (P = 0.02), nausea (P = 0.004), and anorexia (P = 0.096). Furthermore, total IBS severity scores and Bristol stool scale scores were significantly reduced 3 wk after FMT (P = 0.0002 and P = 0.02)[33].
Syzenko et al[32] published an abstract in 2016 on a study evaluating the effect of FMT in “treatment resistant” IBS patients. Twelve patients were enrolled according to the Rome III criteria, including 6 with IBS-D, 5 with IBS-C, and 1 with IBS-M. Treatment resistance was defined as continuous GI symptoms after adequate lifestyle modification, as well as antibiotic, pre- and probiotic, and antipsychotic treatment. FMT was accomplished via colonoscopy with or without consecutive enemas. To quantify the severity of GI symptoms, all patients registered eventual abdominal pain, bloating, and flatus according to the VAS scale. Bowel habits were evaluated using the Bristol stool scale and through frequency assessment. The results showed an abdominal pain resolution or significant improvement in 9 (75%) patients (P ≤ 0.01). Only 1 patient reported no change in pain level. Normalization of stool frequency and consistency was reported in all IBS-M and IBS-D patients. In IBS-C patients, a significant reduction in frequency of laxative using was reported (P ≤ 0.01). They also observed a significant improvement or complete resolution of symptoms in 7 (58.3%) and 4 (33.3%) patients, respectively. No date for time of assessment of the study’s data has been provided[32].
In total, this review includes 9 published abstracts that describe 118 patients treated with FMT. Since the criteria for the diagnosis of the 55 included patients were not specified in the study by Borody et al[35], the results have been excluded from this paper. The results from Syzenko et al[32] and Mazzawi et al[33] have also been excluded. Syzenko et al[32] did not describe when the outcome data was measured. Mazzawi et al[33] had their follow-up after 3 wk - a period rated too short according to our criteria for evaluation of treatment effect. Therefore, the total number of treated patients with IBS included in this review is 48. Andrews et al[34], Borody et al[27], Pinn et al[28], and Holvoet et al[29] reported improvements in symptoms in 1, 3, 9, and 9 patients, respectively. Hong et al[31] reported no effect in 10 patients. Because of the manner in which Cruz Aguilar et al[30] present their results, it is difficult to give an exact number of patients who experienced symptom improvement. Since 6 (66%) patients experienced a 50% reduction in abdominal pain, abdominal pain has been chosen as the most significant parameter in evaluating FMT effect. A total of 28 of 48 patients (58%) experienced an improvement of symptoms upon review of the existing literature. No serious adverse effects were reported in any of the 9 included studies.
Current evidence suggests that the microbiota of the GI tract is a significant factor in the aetiology of IBS. Several aspects support this: the onset of IBS after infectious gastroenteritis[15], transient relief of symptoms after antibiotic treatment[15], previous reports of the successful treatment of Clostridium difficile infection with FMT[1], improvements of symptoms in combination with probiotic treatment[22], and findings of an altered gut microbiome combined with improvement in IBS-D patients after FMT[30]. Holvoet et al[38] ascribed the positive effects of FMT on IBS-related symptoms to changes in the microbiota. Furthermore, orally administered antibiotic drugs that are poorly absorbed through the GI tract result in a temporarily reduction of symptoms[39]. The aetiology of IBS is complex and, though it is not certain that it is of bacterial origin, the treatment with FMT appears to be beneficial with an improvement in 58% of patients treated. Several factors make a comparison of existing studies difficult, however. Holvoet et al[29] included patients with refractory IBS symptoms and severe bloating, Hong et al[31] included IBS patients who were moderately or fully unresponsive to traditional treatment, Cruz Aguilar et al[30] and Mazzawi et al[33] included patients with diarrhoea predominant IBS, Pinn et al[28] and Syzenko et al[32] divided their patients into 3 groups of IBS-D, IBS-C and IBS-M, and Borody et al[27] included 3 patients with chronic constipation. None of the included studies specify how patient subgroups were categorised or diagnosed. Additionally, the method used to evaluate symptom relief, if specified at all, varied. Borody et al[27] failed to clarify how improvement was assessed. In the abstract from 1989[35], the group describe their patients as “cured” but the definition of cure was not specified. In the study by Pinn et al[28], a 41-point-questionnaire was used with an average time from FMT to data collection of 11 mo, while Cruz Aguilar et al[30] used clinical evaluation and a standardized questionnaire with data collection 3 mo after FMT. Holvoet et al[29] also used questionnaires for quality of life but did not specify how patients reported relief of IBS symptoms 12 wk post FMT[29]. Cruz Aguilar et al[30], Pinn et al[37], Holvoet et al[29,38] Hong et al[31], Syzenko et al[32], and Mazzawi el al[33] used the ROME III criteria. These criteria are, however, not mentioned in Andrews et al[34] or the articles by Borody[27] and his group.
Differences between the 9 studies make it difficult to verify findings and reproduce results. The absence of the Rome III criteria from some of the included studies[27,34,35] could be explained by the fact that the Rome III criteria were only first published in 2006[19]. The included studies in our review do, however, lay the ground work for larger scale clinical trials by attempting FMT in several subgroups of IBS.
Consensus over the use of internationally accepted guidelines is needed to ensure that patients are sorted into predefined groups according to symptoms and diagnosis in order to accurately elucidate subgroup differences and FMT effect. Additional research should also include the microbiome of donors so that donors with advantageous microbiomes can be matched with a specific subtype of IBS or any other FMT-treatable GI disorder. The RCTs of the future should implement standard criteria, such as the ROME III criteria, when diagnosing patients. Six out of the 8 RCTs currently listed on clinicaltrials.gov (15/3-2017) use or refer to the Rome III criteria.
Holvoet et al[29] and Cruz Aguilar et al[30] made efforts to map the GI-microbiome associated with IBS before, during, and after their trials. This has not been accomplished earlier most likely because of lack of or access to necessary technology. Access to such technology is now becoming more widespread and rapid identification and quantification of the highly diverse organisms that comprise the human microbiome is becoming a reality[2]. In the case of Cruz Aguilar et al[30], their 5 IBS-D patients displayed a higher degree of microbiome diversity after FMT, but it is not specified what kind of bacteria this diversity included. The IBS-C patients also showed a change in gut microbiota, but this change was not further elaborated on[30]. Holvoet et al[29] failed to mention any results regarding the sequencing of 16s rRNA in their trial. In a later letter, they did, however, link the positive effects on IBS-related symptoms to changes in the microbiota as a result of FMT treatment[38]. Further sequencing of the microbiome is needed from larger groups to ascertain a broader picture of what a healthy microbiome consists of. This could be done through screening of healthy stool donors.
In only 1[38] of the 9 core studies, a placebo effect was mentioned. Placebo has a large effect in clinical trials concerning IBS and ranges from 16%-71%[40]. The currently ongoing clinical trials listed above are all placebo controlled and mainly include patients with diarrhoea predominant IBS. Furthermore, there is a risk of positive outcome bias when dealing with small trials and case reports in which researchers only publish cases where improvement was reported.
Several other factors could influence the effect of FMT, such as the route used for FMT, duration of treatment and quantity of fecal microbiota transplanted to the patient. There are many different ways in which FMT can be administered, including capsules, enemas, and colonoscopy. No clinical trials have compared FMT delivery routes in IBS and further trials are needed to determine the ideal route of FMT treatment in these patients[14]. Furthermore, it is unknown whether 1 FMT treatment is enough or if FMT should be repeated for best effect. In the study by Hong et al[31], results suggest that FMT may only be beneficial for 1 mo. The positive effects seemed to decrease over time and symptoms tended to return to their pre-FMT state within 3 mo after FMT treatment.
A systematic review by Wang et al[41] found that FMT could result in serious adverse effects, even death. Looking into the actual cases, however, the adverse effects were mostly related to the mode of delivery rather than the actual FMT, e.g., one death was due to sedation issues before colonoscopy. Using an endoscope to administer the FMT is widely used and will always include the risk of perforation of the intestine. The use of encapsulated FMT as mode of delivery can circumvent this problem and is, therefore, an attractive alternative. There is a general opinion that thorough donor screening is necessary in order to avoid possible transfer of disease or pathogens[42]. No standardized donor screening protocol has yet emerged, however.
Currently, 8 FMT and IBS studies (15/3-2017) are registered on clinicaltrials.gov. These ongoing trials primarily focus on the beneficial effects of FMT on IBS. Secondary goals of these trials include research into the possible bacterial aetiology behind IBS. Several of these studies have included sequencing of bacteria before and after FMT which hopefully will reveal a pattern or give us clues as to where to look next.
Few studies examining FMT in the treatment of IBS have been published. Despite the small number of patients reviewed in this paper and differences in study design between the included studies, it seems that there is an - at least temporary- improvement in a large proportion of FMT treated patients. An improvement was seen in 58% of participating IBS patients. Randomized, double-blinded placebo controlled trials are, however, still lacking. Currently, 8 studies (15/3-2017) fulfilling these aforementioned criteria (clinicaltrials.gov) are underway, and considerable leaps in knowledge on the effect of FMT in IBS is expected within the near future.
The authors would like to thank Victoria Elizabeth de Knegt, MD, from the Department of Paediatrics, Copenhagen University Hospital Hvidovre, Copenhagen, Denmark, for her assistance with proofreading the manuscript.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Denmark
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bashashati M, Li HD S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Rossen NG, MacDonald JK, de Vries EM, D’Haens GR, de Vos WM, Zoetendal EG, Ponsioen CY. Fecal microbiota transplantation as novel therapy in gastroenterology: A systematic review. World J Gastroenterol. 2015;21:5359-5371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 160] [Cited by in F6Publishing: 145] [Article Influence: 16.1] [Reference Citation Analysis (1)] |
| 2. | Khanna S, Tosh PK. A clinician’s primer on the role of the microbiome in human health and disease. Mayo Clin Proc. 2014;89:107-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 3. | Kassinen A, Krogius-Kurikka L, Mäkivuokko H, Rinttilä T, Paulin L, Corander J, Malinen E, Apajalahti J, Palva A. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 711] [Cited by in F6Publishing: 694] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 4. | King TS, Elia M, Hunter JO. Abnormal colonic fermentation in irritable bowel syndrome. Lancet. 1998;352:1187-1189. [PubMed] [Cited in This Article: ] |
| 5. | Malinen E, Rinttilä T, Kajander K, Mättö J, Kassinen A, Krogius L, Saarela M, Korpela R, Palva A. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am J Gastroenterol. 2005;100:373-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 502] [Cited by in F6Publishing: 479] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 6. | Mättö J, Maunuksela L, Kajander K, Palva A, Korpela R, Kassinen A, Saarela M. Composition and temporal stability of gastrointestinal microbiota in irritable bowel syndrome--a longitudinal study in IBS and control subjects. FEMS Immunol Med Microbiol. 2005;43:213-222. [PubMed] [Cited in This Article: ] |
| 7. | Singh R, Nieuwdorp M, ten Berge IJ, Bemelman FJ, Geerlings SE. The potential beneficial role of faecal microbiota transplantation in diseases other than Clostridium difficile infection. Clin Microbiol Infect. 2014;20:1119-1125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, Khoruts A, Geis E, Maldonado J, McDonough-Means S. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017;5:10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 799] [Cited by in F6Publishing: 730] [Article Influence: 104.3] [Reference Citation Analysis (0)] |
| 9. | Dinan TG, Cryan JF. Melancholic microbes: a link between gut microbiota and depression? Neurogastroenterol Motil. 2013;25:713-719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 279] [Cited by in F6Publishing: 270] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 10. | Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712-721.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1251] [Cited by in F6Publishing: 1292] [Article Influence: 107.7] [Reference Citation Analysis (1)] |
| 11. | Spiegel BM. The burden of IBS: looking at metrics. Curr Gastroenterol Rep. 2009;11:265-269. [PubMed] [Cited in This Article: ] |
| 12. | Canavan C, West J, Card T. Review article: the economic impact of the irritable bowel syndrome. Aliment Pharmacol Ther. 2014;40:1023-1034. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 280] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 13. | Tap J, Derrien M, Törnblom H, Brazeilles R, Cools-Portier S, Doré J, Störsrud S, Le Nevé B, Öhman L, Simrén M. Identification of an Intestinal Microbiota Signature Associated With Severity of Irritable Bowel Syndrome. Gastroenterology. 2017;152:111-123.e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 348] [Cited by in F6Publishing: 371] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 14. | Pinn DM, Aroniadis OC, Brandt LJ. Is fecal microbiota transplantation (FMT) an effective treatment for patients with functional gastrointestinal disorders (FGID)? Neurogastroenterol Motil. 2015;27:19-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136:1979-1988. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 489] [Cited by in F6Publishing: 524] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 16. | Kelly CR, Kahn S, Kashyap P, Laine L, Rubin D, Atreja A, Moore T, Wu G. Update on Fecal Microbiota Transplantation 2015: Indications, Methodologies, Mechanisms, and Outlook. Gastroenterology. 2015;149:223-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 420] [Cited by in F6Publishing: 388] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 17. | Choi HH, Cho YS. Fecal Microbiota Transplantation: Current Applications, Effectiveness, and Future Perspectives. Clin Endosc. 2016;49:257-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 162] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 18. | Saha L. Irritable bowel syndrome: pathogenesis, diagnosis, treatment, and evidence-based medicine. World J Gastroenterol. 2014;20:6759-6773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 210] [Cited by in F6Publishing: 225] [Article Influence: 22.5] [Reference Citation Analysis (11)] |
| 19. | Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480-1491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3413] [Cited by in F6Publishing: 3270] [Article Influence: 181.7] [Reference Citation Analysis (1)] |
| 20. | Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. 2015;313:949-958. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 629] [Cited by in F6Publishing: 637] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 21. | Hungin AP, Mulligan C, Pot B, Whorwell P, Agréus L, Fracasso P, Lionis C, Mendive J, Philippart de Foy JM, Rubin G. Systematic review: probiotics in the management of lower gastrointestinal symptoms in clinical practice -- an evidence-based international guide. Aliment Pharmacol Ther. 2013;38:864-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 22. | Didari T, Mozaffari S, Nikfar S, Abdollahi M. Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World J Gastroenterol. 2015;21:3072-3084. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 232] [Cited by in F6Publishing: 205] [Article Influence: 22.8] [Reference Citation Analysis (3)] |
| 23. | Bezkorovainy A. Probiotics: determinants of survival and growth in the gut. Am J Clin Nutr. 2001;73:399S-405S. [PubMed] [Cited in This Article: ] |
| 24. | Hayes PA, Fraher MH, Quigley EM. Irritable bowel syndrome: the role of food in pathogenesis and management. Gastroenterol Hepatol (N Y). 2014;10:164-174. [PubMed] [Cited in This Article: ] |
| 25. | Shah SL, Lacy BE. Dietary Interventions and Irritable Bowel Syndrome: A Review of the Evidence. Curr Gastroenterol Rep. 2016;18:41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Borody T, McGrath K. Treatment of chronic constipation and colitis using human probiotic infusions. 2001;. [Cited in This Article: ] |
| 27. | Borody TJ, Warren EF, Leis SM, Surace R, Ashman O, Siarakas S. Bacteriotherapy using fecal flora: toying with human motions. J Clin Gastroenterol. 2004;38:475-483. [PubMed] [Cited in This Article: ] |
| 28. | Pinn D, Aroniadis O, Brandt L. Follow-up study of fecal microbiota transplantation (FMT) for the treatment of refractory irritable bowel syndrome (IBS). Am J Gastroenterol. 2013;108:S563. [Cited in This Article: ] |
| 29. | Holvoet T, Boelens J, Joossens M, Raes J, Vos MD, Looze DD. Tu2025 Fecal Microbiota Transplantation in Irritable Bowel Syndrome With Bloating: Results From a Prospective Pilot Study. Gastroenterology. 2015;148:S-963-S-964. [DOI] [Cited in This Article: ] |
| 30. | Cruz Aguilar R, Buch T, Bajbouj . Fecal microbiota transplantation as a novel therapy for irritable bowel syndrome with predominant diarrhea. Neurogastroenterol Motil. 2015;27:110. [Cited in This Article: ] |
| 31. | Hong J, Bang B, Shin Y, Kim H, Kwon K. OP257 Treatment of Irritable Bowel Syndrome with Fecal Microbiota Transplantation: A case series of 10 patients. Unit Eur Gastroenterol J. 2016;2 (Suppl 1). [Cited in This Article: ] |
| 32. | Syzenko G, Budovska L, Puchkov K. P0397 Efficiency of FMT in cases of ‘Treatment-resistant’ IBS. Unit Eur Gastroenterol J. 2016;2 (Suppl 1). [Cited in This Article: ] |
| 33. | Mazzawi T, Lied G, El-Salhy M, Gilja O, Hatlebakk J, Hausken T. P1527 Effect of faecal microbiota transplantation on symptoms and duodenal enteroendocrine cells in patients with irritable bowel syndrome. Unit Eur Gastroenterol J. 2016;2 (Suppl 1). [Cited in This Article: ] |
| 34. | Andrews P, Barnes P, Borody T. Chronic constipation reversed by restoration of bowel flora - a case and a hypothesis. Eur J Gastroenterol Hepatol. 1992;4:245-7. [Cited in This Article: ] |
| 35. | Borody TJ, George L, Andrews P, Brandl S, Noonan S, Cole P, Hyland L, Morgan A, Maysey J, Moore-Jones D. Bowel-flora alteration: a potential cure for inflammatory bowel disease and irritable bowel syndrome? Med J Aust. 1989;150:604. [PubMed] [Cited in This Article: ] |
| 36. | Borody TJ, Paramsothy S, Agrawal G. Fecal microbiota transplantation: indications, methods, evidence, and future directions. Curr Gastroenterol Rep. 2013;15:337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 171] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 37. | Pinn DM, Aroniadis OC, Brandt LJ. Is fecal microbiota transplantation the answer for irritable bowel syndrome? A single-center experience. Am J Gastroenterol. 2014;109:1831-1832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 38. | Holvoet T, Joossens M, Wang J, Boelens J, Verhasselt B, Laukens D, van Vlierberghe H, Hindryckx P, De Vos M, De Looze D. Assessment of faecal microbial transfer in irritable bowel syndrome with severe bloating. Gut. 2017;66:980-982. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 39. | Menees SB, Maneerattannaporn M, Kim HM, Chey WD. The efficacy and safety of rifaximin for the irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2012;107:28-35; quiz 36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 205] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 40. | Patel SM, Stason WB, Legedza A, Ock SM, Kaptchuk TJ, Conboy L, Canenguez K, Park JK, Kelly E, Jacobson E. The placebo effect in irritable bowel syndrome trials: a meta-analysis. Neurogastroenterol Motil. 2005;17:332-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 213] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 41. | Wang S, Xu M, Wang W, Cao X, Piao M, Khan S, Yan F, Cao H, Wang B. Systematic Review: Adverse Events of Fecal Microbiota Transplantation. PLoS One. 2016;11:e0161174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 234] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 42. | Cammarota G, Ianiro G, Tilg H, Rajilić-Stojanović M, Kump P, Satokari R, Sokol H, Arkkila P, Pintus C, Hart A. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 601] [Cited by in F6Publishing: 662] [Article Influence: 94.6] [Reference Citation Analysis (0)] |