Published online Jan 14, 2017. doi: 10.3748/wjg.v23.i2.345
Peer-review started: August 2, 2016
First decision: September 20, 2016
Revised: September 29, 2016
Accepted: October 31, 2016
Article in press: October 31, 2016
Published online: January 14, 2017
To investigate the diagnostic accuracy of FibroScan (FS) in detecting esophageal varices (EV) in cirrhotic patients.
Through a systemic literature search of multiple databases, we reviewed 15 studies using endoscopy as a reference standard, with the data necessary to calculate pooled sensitivity (SEN) and specificity (SPE), positive and negative LR, diagnostic odds ratio (DOR) and area under receiver operating characteristics (AUROC). The quality of the studies was rated by the Quality Assessment of Diagnostic Accuracy studies-2 tool. Clinical utility of FS for EV was evaluated by a Fagan plot. Heterogeneity was explored using meta-regression and subgroup analysis. All statistical analyses were conducted via Stata12.0, MetaDisc1.4 and RevMan5.
In 15 studies (n = 2697), FS detected the presence of EV with the summary sensitivities of 84% (95%CI: 81.0%-86.0%), specificities of 62% (95%CI: 58.0%- 66.0%), a positive LR of 2.3 (95%CI: 1.81-2.94), a negative LR of 0.26 (95%CI: 0.19-0.35), a DOR of 9.33 (95%CI: 5.84-14.92) and an AUROC of 0.8262. FS diagnosed the presence of large EV with the pooled SEN of 0.78 (95%CI: 75.0%-81.0%), SPE of 0.76 (95%CI: 73.0%-78.0%), a positive and negative LR of 3.03 (95%CI: 2.38-3.86) and 0.30 (95%CI: 0.23-0.39) respectively, a summary diagnostic OR of 10.69 (95%CI: 6.81-16.78), and an AUROC of 0.8321. A meta-regression and subgroup analysis indicated different etiology could serve as a potential source of heterogeneity in the diagnosis of the presence of EV group. A Deek’s funnel plot suggested a low probability for publication bias.
Using FS to measure liver stiffness cannot provide high accuracy for the size of EV due to the various cutoff and different etiologies. These limitations preclude widespread use in clinical practice at this time; therefore, the results should be interpreted cautiously given its SEN and SPE.
Core tip: Esophageal varices (EV) is the main relevant portosystemic collaterals in cirrhotic patients. Hemorrhage from EV remains the leading cause of death in cirrhosis. Although more non-invasive techniques for evaluating the severity of EV have been carried out, the cutoff value and validity are not clear. Hence, this study examining the basis for clinical application of transient elastography [FibroScan (FS)] assessed whether there is sufficient evidence to recommend FS to predict EV. The result demonstrates that the cutoff of FS cannot provide high accuracy due to the various etiologies, and the value of FS should be interpreted cautiously.
- Citation: Pu K, Shi JH, Wang X, Tang Q, Wang XJ, Tang KL, Long ZQ, Hu XS. Diagnostic accuracy of transient elastography (FibroScan) in detection of esophageal varices in patients with cirrhosis: A meta-analysis. World J Gastroenterol 2017; 23(2): 345-356
- URL: https://www.wjgnet.com/1007-9327/full/v23/i2/345.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i2.345
Esophageal varices (EV) is the main relevant portosystemic collaterals and are present in approximately 50% of cirrhotic patients[1]. Hemorrhage from EV remains the leading cause of death in patients with cirrhosis, with an in-hospital mortality of 14.2%-14.5%[2,3]. Endoscopic screening for EV is recommended for the diagnosis, prevention, and management in patients with cirrhosis via surveillance with frequency related to the degree and treatment of varices[1]. Nevertheless, a generalized program of periodical and repeated esophagogastroduodenoscopy (EGD) examination can result in unnecessary economic burden, and subject the patient to an uncomfortable feeling without general anesthesia or profound sedation. All of these reasons lead to decline in patient compliance with treatment and follow-ups. Meanwhile, the endoscopy-related complications reported by a related article is close to 0.1% of incidence[4].
Moreover, approximately 50% of cirrhotic patients may not develop EV in the 10-year period after the initial cirrhosis diagnosis[5], and prophylactic medication with beta-blockers or invasive preventive treatments such as endoscopic sclerosis or band ligation[1] should have been initiated after diagnosis. Actually, according to the point prevalence of medium and significant varices the highest risk of hemorrhage is only 15% to 25%, and the majority of patients with cirrhosis who undergo screening EGD either do not have varices or have small EV that do not require prophylactic therapy[6]. To avoid unnecessary endoscopy in low-risk patients, more noninvasive tests have been carried out as substitution to replace endoscopy for EV screening.
Transient elastography (TE) with FibroScan (FS; Echosens, Paris, France), which measures liver stiffness (LS) depending on the calculation of liver frequency elastic wave inside the liver[7], has been recognized as a rapid, non-invasive technique for evaluating the severity of liver disease, and has been found to be useful in the diagnosis of the underlying stage of fibrosis in recent studies[8-11]. Therefore, FS has the potential to be used for the non-invasive evaluation of EV[12].
Although there are few studies that have focused on the correlation between LS and the presence of EV or the severity of EV, the cutoffs and validities vary in the different factors, including different studies, techniques of measuring LS, fibrosis stages and etiologies of hepatic cirrhosis[13]. Hence, the aim of this meta-analysis of the basis for clinical application and research was to assess whether there is sufficient evidence to recommend FS as a noninvasive screening method as compared with EGD as the reference standard for predicting the presence of EV and high-risk EV in patients with cirrhosis.
Electronic databases, including PubMed, EMBASE, Web of Science and Cochrane Library, were used to perform systematic search for all relevant clinical articles on evaluation of LS for diagnosis of EV in cirrhotic patients from the time of database inception to January 1, 2016 by applying heading terms and key words of “TE”, “EV” and “liver cirrhosis”. The process of trials selection were assessed by two review authors (Wang XJ, Tang KL) independently and blindly. The references were screened by titles and abstracts firstly and then further selected by reading the full-text to exclude irrelevant reports according to the inclusion criteria.
Study inclusion criteria were as follows: (1) performed in patients with liver cirrhosis diagnosed by liver biopsy, due to any etiology with or without evidence of portal hypertension or cirrhosis; (2) offered adequate description of LS using either TE (FS) or real-time tissue elastography; (3) assessment of EV based on upper endoscopy (GIE) as the reference standard; (4) provided sufficient data necessary to calculate the test performance, including sensitivity (SEN), specificity (SPE), false positive and false negative diagnostic results (either in the primary article or after contact with corresponding authors) based on available cutoff point of FS in the presence and large EV. Inclusion was not restricted by study size, language, or publication type.
The primary data from included studies was abstracted as follows: first author’s name and year of publication, number of patients, region, etiology of liver cirrhosis, cutoff point, and the values for true-positive (TP), true-negative (TN), false-positive (FP), false-negative (FN), SEN and SPE results of FS. All discrepancies were resolved by consensus.
The quality assessment of the studies included in this study was performed by two authors independently using the Quality Assessment of Diagnostic Accuracy studies (QUADAS-2)[14] in Systematic Review. This tool consisted of 4 domains, including patient selection, index test, reference standard and flow and timing domain. Each signaling question was judged as “yes”, “no” or “unclear”. Each study’s risk of bias and concern for applicability were estimated as “high”, “low” or “unclear”, except for the flow and timing domain, for which applicability concern does not apply.
According to the TP, FP, FN and TN values from the original papers, the meta-analyses were performed by the Meta-Disc software version 1.4 to evaluate the pooled statistics (95%CI) of SEN, SPE, positive and negative LR [i.e., PLR = SEN/(1 - SPE), NLR = (1 - SEN)/SPE], diagnostic odds ratio (DOR) and area under the summary receiver operating characteristic curves (AUSROC) with standard errors (SE) and Q indexes with SE for the test performance of LS for the presence of EV and large EV diagnosis. If there were not sufficient information, we recalculated these values on the basis of the sensitivities and specificities offered. However, summary statistics observed the diagnostic threshold effect analyzed by Spearman’s correlation coefficient and P value. If there was no significant threshold effect, the diagnostic accuracy was estimated by pooled statistics; on the contrary, the diagnostic accuracy was evaluated by only AUSROC and Q indexes, rather than sensitivities, specificities, PLR, NLR and DOR.
A PLR was the probability of a cirrhotic patient with EV testing positive by the gold standard (i.e., GIE) divided by the probability of a cirrhotic patient without EV testing positive; meanwhile, a NLR was the probability of testing negative for cirrhosis patients with EV divided by the probability of testing negative for cirrhotic patients without EV. The PLR > 5.0 and NLR < 0.2 implied higher diagnostic evidence. The DOR represented the odds of positive LS in cirrhotic patients with EV compared with the odds of cirrhotic patients without EV. AUSROC values of 0.5-0.7, 0.7-0.9 and 0.9-1.0 were used to suggest low, moderate and high diagnostic accuracy, respectively. A smaller Q index indicated a lower diagnostic accuracy.
Heterogeneity was valued by Cochran’s Q statistic based on χ2 test and I2 statistic. I2 values of 0%-40%, 40%-70% and 70%-100% were indicative of low, moderate and high variance, respectively[15]. If moderate heterogeneity existed or different clinical characteristics were noted, the DerSimonian Laird method in random-effects model was applied. Otherwise, the fixed-effects model was used. Considerable heterogeneity was considered if I2 > 50% and/or P < 0.05. Sources of heterogeneity were explored by meta-regression analysis according to the possible characteristics; a subsequent subgroup analysis was conducted in attempt to identify potential covariates.
Post-test probability was calculated with a presumed pre-test probability of 25%, 50% and 75% for EV and high-risk EV via Fagan’s plot. Potential publication bias was evaluated by the asymmetry test of Deek’s funnel plots, which used a regression of the diagnostic logarithm of OR against 1/sqrt [effective sample size (ESS)] and weighting by ESS, with a P value < 0.10 for the slope coefficient indicating asymmetry and suggestive of a significant publication bias[15].
Meta-Disc version 1.4 (Ramon y Cajal Hospital, Madrid, Spain) software was use to generate forest plot, and Stata12.0 (StataCorp, College Station, TX, United States) was applied to perform the SEN analysis and publication bias.
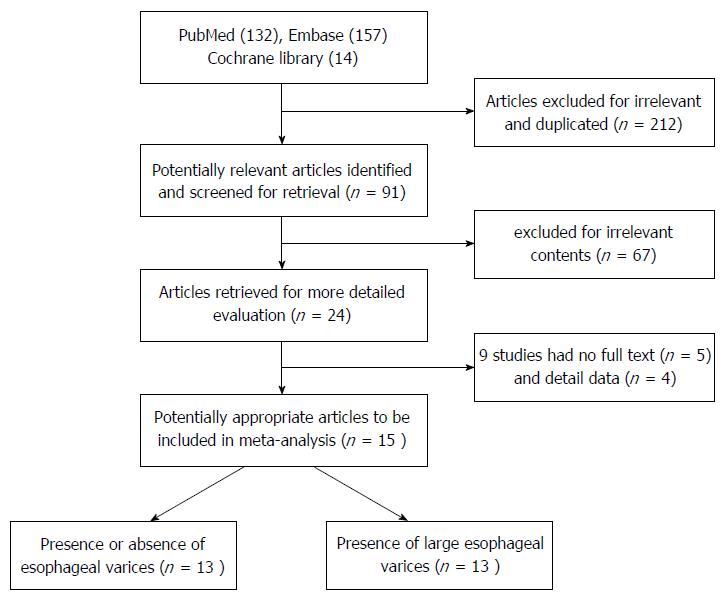
The 303 articles yielded by the study selection process are presented in a flow chart in Figure 1, of which 212 were excluded for irrelevance and duplication following title and abstract screening. The remaining 91 potentially eligible reports were screened for further evaluation. Of those, after exclusion for irrelevant contents, no full-text and insufficient data, ultimately 15 papers[16-30] were included for the meta-analysis and included 12 English papers, 1 Korean[23] paper and 2 Chinese papers[20,21].
The 15 studies, which were performed in Europe (8 papers), Asia (6 papers) and Africa (1 paper), included a total of 2697 cirrhotic patients informing diagnostic performance of LS measure by FS (TE) for the detection of EV and significant EV (Table 1).
| Ref. | Location | Etiology (viral, %) | Simple | Mean age | Childs | Presence of EV | Large EV | Reference | Design | Presence of EV (Grade 0, 1) | Large EV (Grade 2, 3) | ||||||||||
| (male,%) | score (%) | cutoff (kPa) | cutoff (kPa) | standard | method | TP | FP | FN | TN | SEN (%) | SPE (%) | TP | FP | FN | TN | SEN (%) | SPE (%) | ||||
| Sporea et al[16], 2013 | Romania | 73.6 | 697 | 57 (57.20) | NR | NR | 29.5 | GIE | A | 212 | 56 | 61 | 368 | 77.50 | 86.90 | ||||||
| Sharma et al[17], 2013 | India | 29.9 | 174 | 49.3 (88.50) | A/B/C | 27.3 | NR | EGD | A | 113 | 14 | 11 | 36 | 91.00 | 72.00 | ||||||
| 32/57/11 | |||||||||||||||||||||
| Saad et al[18], 2013 | Egypt | 100 | 32 | NR | A/B | 29.7 | 38.2 | GIE | B | 19 | 4 | 1 | 8 | 95.00 | 67.00 | 10 | 5 | 0 | 17 | 100 | 77.30 |
| 72/28 | |||||||||||||||||||||
| Nguyen-Khac et al[19], 2010 | France | 31.7 | 183 | 55.2 (64.50) | A/B/C | NR | 48 | EGD | A | 30 | 38 | 11 | 104 | 73.20 | 73.20 | ||||||
| 63/26/15 | |||||||||||||||||||||
| Li et al[20], 2012 | China | 100 | 158 | 47.4 (82.40) | NR | 23.3 | 31.5 | GIE | A | 72 | 27 | 18 | 41 | 80.00 | 60.30 | 32 | 33 | 9 | 84 | 78.10 | 71.80 |
| Li et al[21], 2014 | China | 84.2 | 260 | 49.4 (67.70) | NR | 22.8 | 30.6 | GIE | A | 129 | 39 | 25 | 67 | 83.80 | 63.20 | 57 | 57 | 12 | 134 | 82.60 | 70.10 |
| Kazemi et al[22], 2006 | France | 69.7 | 165 | 56 (67.30) | NR | 13.9 | 19 | UTE | A | 70 | 52 | 4 | 39 | 95.00 | 91.00 | 43 | 47 | 4 | 71 | 91.00 | 60.00 |
| Jung et al[23], 2008 | Korea | 46.4 | 112 | 53.3 (78.60) | NR | 19.7 | 29.5 | GIE | A | 71 | 9 | 11 | 21 | 87.00 | 70.00 | 27 | 33 | 8 | 44 | 77.00 | 57.00 |
| Hu et al[24], 2015 | China | 100 | 200 | 45.1 (71.00) | B/A + C | 20.25 | 25.55 | GIE | B | 95 | 25 | 15 | 65 | 86.40 | 72.20 | 58 | 36 | 11 | 95 | 84.10 | 72.50 |
| 84/16 | |||||||||||||||||||||
| Castéra et al[25], 2009 | France | 100 | 66 | 54.1 (60.00) | A/B + C | 21.5 | 30.5 | GIE | A | 19 | 9 | 6 | 32 | 76.00 | 78.00 | 10 | 8 | 3 | 45 | 77.00 | 85.00 |
| 70/30 | |||||||||||||||||||||
| Calvaruso et al[26], 2013 | Italy | 100 | 96 | 63.2 (69.80) | A | 17 | 19 | GIE | A | 38 | 18 | 16 | 24 | 71.00 | 57.00 | 19 | 31 | 7 | 39 | 72.00 | 55.00 |
| 100 | |||||||||||||||||||||
| Bintintan et al[27], 2015 | Romania | 45.0 | 60 | 57 (65.00) | A/B/C | 15 | 28.8 | EGD | A | 45 | 0 | 2 | 13 | 95.00 | 100 | 28 | 5 | 4 | 23 | 87.20 | 82.76 |
| 65/22/13 | |||||||||||||||||||||
| Stefanescu et al[28], 2011 | Romania | NR | 137 | 56 (56.20) | A/B/C, | 28 | NR | UTE | A | 86 | 7 | 30 | 14 | 74.36 | 64.29 | ||||||
| 65/28/7 | |||||||||||||||||||||
| Stefanescu et al[29], 2011 | Romania | 61.0 | 231 | 55.9 (58.40) | A/B/C, | 19 | 38 | UTE | A | 132 | 50 | 25 | 24 | 84.00 | 32.39 | 38 | 40 | 30 | 123 | 55.56 | 75.32 |
| 76/18/6 | |||||||||||||||||||||
| Wang et al[30], 2012 | Taiwan | 100 | 126 | 54.5 (73.80) | A, | 12 | 21 | EGD | A | 32 | 18 | 16 | 60 | 67.00 | 77.00 | 10 | 15 | 3 | 98 | 77.00 | 87.00 |
| 100 | |||||||||||||||||||||
All studies included cirrhotic participants who were recently diagnosed or referred to the endoscopic units for screening endoscopy. Almost all of the patients included were stable and did not have any active upper gastrointestinal bleeding. All patients underwent clinical and biochemical evaluation, and underwent ultrasonography to assess the liver diameter and determine the presence of ascites complication. The severity of cirrhosis was classified into class A, B, and C on the basis of Child Turcotte Pugh’s score.
The etiologies of liver cirrhosis included viral hepatitis (hepatitis B virus, hepatitis C virus, and mixture), alcoholic cirrhosis, and miscellaneous etiologies. Viral etiology was the leading cause of liver cirrhosis in the included studies. There were 5 studies performed only in patients with hepatitis B or C, 3 studies performed in cirrhotic patients with 2 etiologies and 7 studies conducted in patients with more than 3 etiologies.
The gold standard for the identification and grading of EV for all studies was GIE or EGD. Except for the 3 studies of respective design, the Chinese Medical Association 2003 classification[31] was used to classify the varices into small, moderate and large, and 2 papers classified F0-3 and Grade 0-4 with Beppu[32] and Thakeb classification while the others used the grading system to classify the varices into 4 Grades[33].
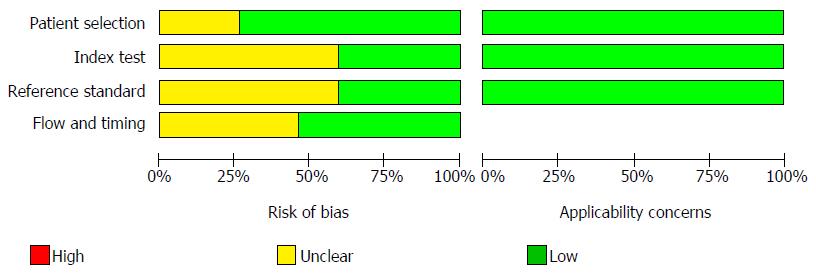
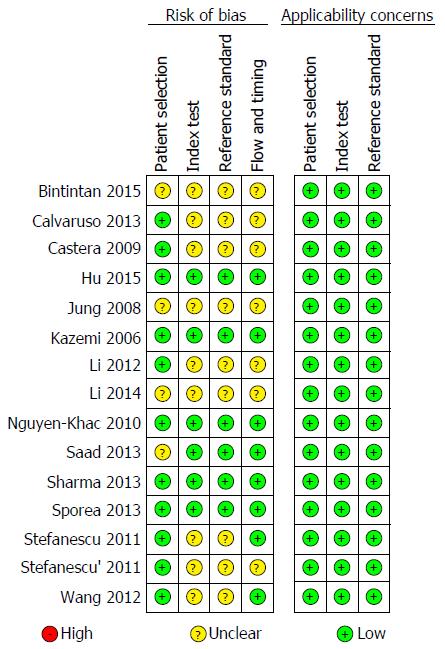
The quality of the eligible studies, as assessed according to the QUADAS-2 criteria, was independently appraised by reviewers, as reported in Figures 2 and 3. Five studies were identified as low-risk for risk of bias and applicability concerns. The remaining studies were estimated as suboptimal for unclear risk in the following domains: index test, reference standard, flow and timing; most of the studies were identified as having a potential bias risk for patient selection and reference standard.
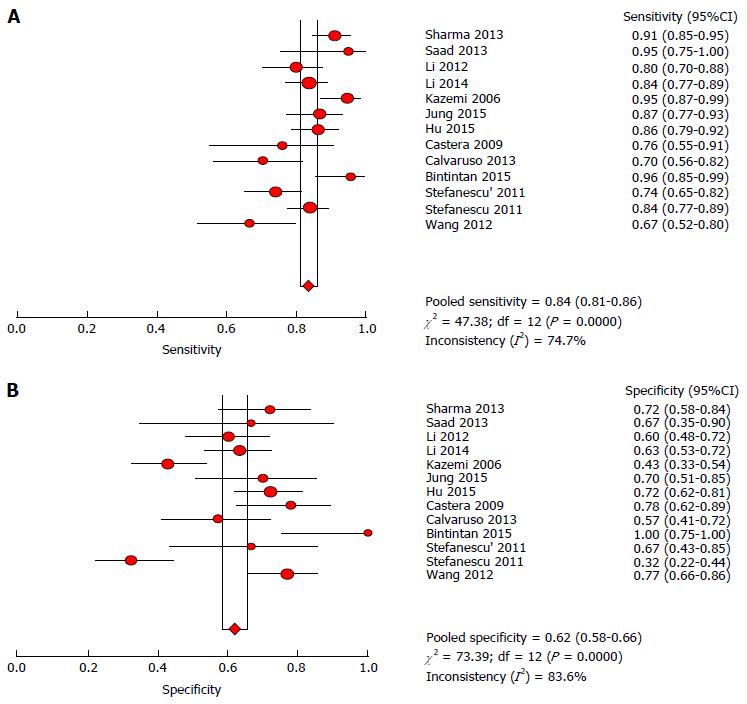
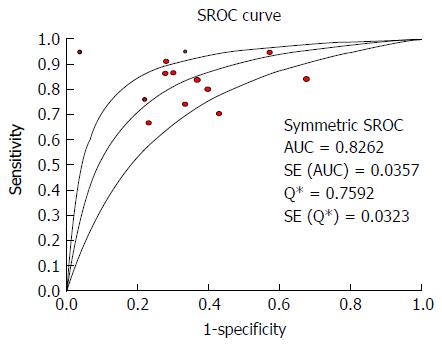
The heterogeneity test indicated that Cochran-Q and I2 of DOR were 40.34 and 70.3% (P = 0.0001) (Supplementary Figure 1); there was significant heterogeneity in the included articles. Therefore, the random-effects model was selected to combine effect quantity. As a result, the pooled SEN of 13 studies was 0.84 (95%CI: 81.0%-86.0%, I2 statistic 74.7%), whereas the pooled SPE was 0.62 (95%CI: 58.0%-66.0%, I2 statistic 83.6%) (Figure 4). The positive and negative LR was 2.3 (95%CI: 1.81-2.94, I2 statistic 82.0%) and 0.26 (95% CI: 0.19-0.35, I2 statistic 71.6%) respectively. The summary diagnostic OR was 9.33 (95%CI: 5.84-14.92) (Supplementary Figure 1). The area under receiver operating characteristics (AUROC) was 0.8262 (SE 0.0357) (Figure 5). Significant heterogeneity was found in the meta-analysis for 13 studies assessing the LS for the prediction of the presence of EV.
In cirrhotic patients with 25% pre-test probability, based on the clinical suspicion of pretest, FS diagnosed the presence of EV. There was 45% probability of being diagnosed correctly by a positive LS measurement (LSM); nevertheless, there was 7% probability of EV for patients with liver cirrhosis following a negative measurement (Supplementary Figure 3A). When 71% probability of diagnosing EV correctly was followed by a positive measurement under the suspicion of 50% pre-test probability, a negative LSM descended to 19%; it also indicated that there was 19% probability of EV in cirrhotic patients with a negative test (Supplementary Figure 3B). When there was a high pre-test index of suspicion (pre-test probability = 75%), the probability of a correct diagnosis following a positive measurement was 88% for EV; however, the misdiagnosis rate would raise 41% for patients with a negative measurement (Supplementary Figure 3C).
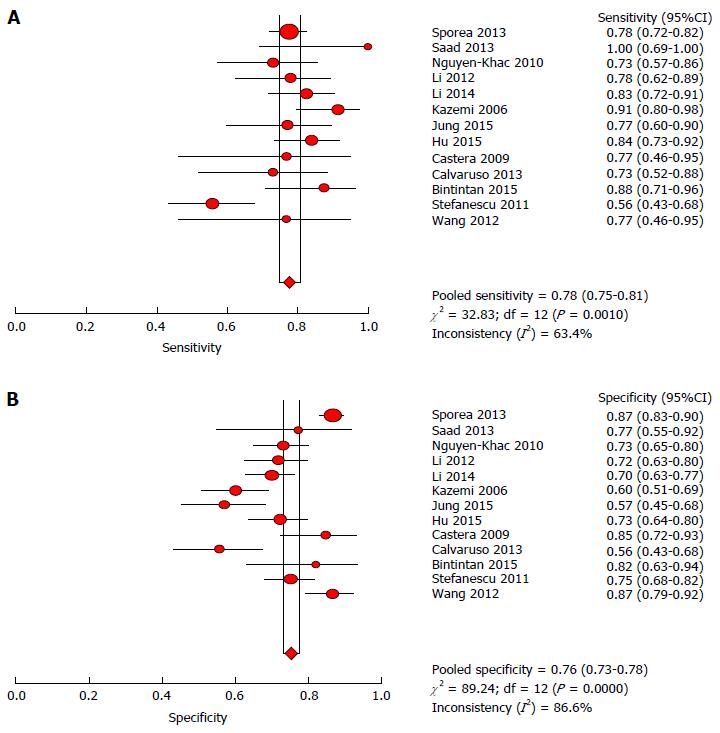
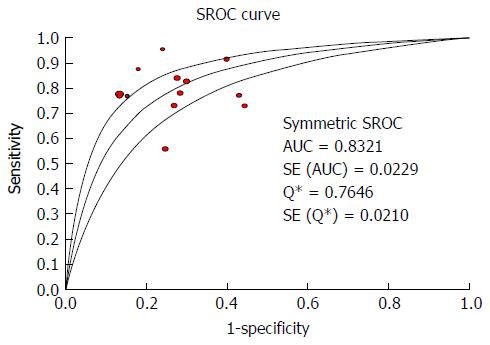
According to the heterogeneity test indicating that Cochran-Q and I2 of DOR were 10.69 and 70.4% (P = 0.0001) (Supplementary Figure 2), there was significant heterogeneity in the included articles. Hence, the random-effects model was selected to combine effect quantity. As a result, the pooled SEN of 13 studies was 0.78 (95%CI: 75.0%-81.0%, I2 statistic 63.4%), whereas the pooled SPE was 0.76 (95%CI: 73.0%-78.0%, I2 statistic 86.6%) (Figure 6). The positive and negative LR was 3.03 (95%CI: 2.38 to 3.86, I2 statistic 83.3%) and 0.30 (95%CI: 0.23-0.39, I2 statistic 65.8%) respectively. The summary diagnostic OR was 10.69 (95%CI: 6.81-16.78) (Supplementary Figure 2). The AUROC was 0.8321 (SE 0.0229) (Figure 7). Significant heterogeneity was found in the meta-analysis for 13 studies assessing the LS for the prediction of the presence of large EV.
In cirrhotic patients with 25% pre-test probability, depending on the clinical hypothesis of pretest, FS diagnosis of significant EV had 51% probability for correct diagnosis by a positive LSM; nevertheless, there still was 7% probability of large EV in patients with liver cirrhosis to be diagnosed with a result of negative measurement (Supplementary Figure 4A). When 75% of correct diagnostic probability of large EV was followed by a positive measurement under the suspicion of 50% pre-test probability, a negative LSM lowered from 50% to 22%; thus, it also implied that there was 22% probability of EV in cirrhotic patients with a negative test (Supplementary Figure 4B). When there was a high pre-test index of hypothesis (pre-test probability = 75%), the probability of a correct diagnosis following a positive measurement was 90% for significant EV; however, the misdiagnosis rate would raise to 45% of patients under a negative measurement (Supplementary Figure 4C).
According to the characteristics of included studies, covariates including etiology (one factor vs two factors vs multiple factors), publication year (2006-2011 year vs 2012-2016 year), location (European vs Asia vs Africa) and LS threshold (< 20 kPa vs > 20 kPa in the presence of EV; < 30 kPa vs >30 kPa in large EV) were applied to investigate heterogeneity by using meta-regression modeling.
In meta-regression analysis, sources of significant heterogeneity suggested statistically that the accuracy for detecting the presence of EV was affected mainly by etiology (P = 0.04) (Supplementary Table 1), and were not significantly affected by the rest of the covariates. The heterogeneity of FS accuracy for detecting large EV was not influenced significantly by other covariates (Supplementary Table 2).
In accordance with the above results, the etiology of studies could be explained as a source of the heterogeneity for the presence of EV classification in meta-regression, and none of the covariates could be statistically elucidated for heterogeneity of the large EV group. Hence, four subgroup analyses (etiology, publication year, location and LS threshold) were attempted to further investigate the heterogeneity (Tables 2 and 3).
| Subgroup | n | SEN (CI) | I2 (%) | SPE (CI) | I2 (%) | PLR (CI) | I2 (%) | NLR (CI) | I2 (%) | DOR (CI) | I2 (%) |
| LC etiology | |||||||||||
| One factor | 5 | 0.76 (0.70-0.81) | 56.5 | 0.68 (0.62-0.74) | 55.6 | 2.26 (1.86-2.74) | 37.3 | 0.37 (0.29-0.48) | 25.3 | 6.17 (4.20-9.06) | 30.4 |
| Two factors | 3 | 0.82 (0.77-0.85) | 68.7 | 0.56 (0.48-0.63) | 92.8 | 2.02 (0.96-4.27) | 93.1 | 0.33 (0.19-0.58) | 76.9 | 6.18 (1.86-20.55) | 86.2 |
| Multiple factors | 5 | 0.89 (0.86-0.92) | 62.0 | 0.61 (0.55-0.66) | 86.2 | 2.48 (1.65-3.73) | 81.0 | 0.16 (0.10-0.25) | 56.8 | 16.74 (8.23-33.84) | 57.9 |
| Location | |||||||||||
| Europe | 6 | 0.82 (0.79-0.86) | 82.3 | 0.52 (0.46-0.58) | 89.1 | 1.84 (1.33-2.55) | 78.6 | 0.29 (0.18-0.49) | 74.1 | 7.14 (3.06-16.66) | 74.0 |
| Asia | 6 | 0.84 (0.81-0.87) | 68.8 | 0.69 (0.64-0.73) | 28.5 | 2.61 (2.25-3.03) | 16.8 | 0.25 (0.21-0.30) | 68.1 | 10.56 (7.93-14.07) | 50.7 |
| Africa | 1 | 0.95 | NA | 0.67 | NA | NA | NA | NA | |||
| Year | |||||||||||
| 2006-2011 | 5 | 0.83 (0.80-0.87) | 75.9 | 0.51 (0.44-0.57) | 87.6 | 1.97 (1.39-2.78) | 82.0 | 0.30 (0.19-0.46) | 62.6 | 7.46 (3.43-16.24) | 69.6 |
| 2012-2016 | 8 | 0.84 (0.81-0.87) | 77.2 | 0.68 (0.64-0.73) | 63.6 | 2.48 (2.0-3.07) | 49.7 | 0.24 (0.16-0.36) | 77.0 | 10.84 (5.94-19.77) | 70.8 |
| LS value (cutoff) | |||||||||||
| < 20 kPa | 6 | 0.84 (0.80-0.87) | 83.2 | 0.55 (0.50-0.61) | 91.0 | 1.94 (1.37-2.74) | 83.2 | 0.27 (0.16-0.47) | 78.6 | 7.82 (3.36-18.24) | 77.4 |
| > 20 kPa | 7 | 0.83 (0.80-0.86) | 65.7 | 0.68 (0.63-0.72) | 1.1 | 2.58 (2.22-3.00) | 9.9 | 0.24 (0.20-0.29) | 58.8 | 10.69 (7.97-14.34) | 45.4 |
| Subgroup | n | SEN (CI) | I2 (%) | SPE (CI) | I2 (%) | PLR (CI) | I2 (%) | NLR (CI) | I2 (%) | DOR (CI) | I2 (%) |
| LC etiology | |||||||||||
| One factor | 5 | 0.79 (0.69-0.86) | 24.7 | 0.75 (0.71-0.80) | 84.3 | 2.82 (2.31-3.45) | 78.9 | 0.30 (0.21-0.44) | 0.0 | 9.05 (5.50-14.90) | 49.3 |
| Two factors | 2 | 0.7 (0.62-0.78) | 92.5 | 0.74 (0.69-0.79) | 0.0 | 2.67 (2.00-3.57) | 38.8 | 0.37 (0.13-1.02) | 90.9 | 7.21 (2.07-25.16) | 85.3 |
| Multiple factors | 6 | 0.80 (0.76-0.83) | 40.9 | 0.76 (0.73-0.79) | 92.1 | 3.02 (2.01-4.55) | 90.7 | 0.27 (0.21-0.34) | 21.2 | 12.46 (6.99-22.18) | 68.5 |
| Location | |||||||||||
| Europe | 7 | 0.76 (0.72-0.80) | 75.2 | 0.77 (0.75-0.80) | 90.3 | 3.09 (2.03-4.70) | 89.6 | 0.31 (0.21-0.48) | 79.5 | 10.55 (5.04-22.07) | 82.2 |
| Asia | 5 | 0.81 (0.75-0.86) | 0.0 | 0.72 (0.69-0.76) | 81.9 | 2.73 (2.37-3.15) | 72.1 | 0.27 (0.21-0.36) | 0.0 | 10.03 (7.01-14.35) | 20.7 |
| Africa | 1 | 0.95 | NA | 0.67 | NA | NA | NA | NA | |||
| Year | |||||||||||
| 2006-2011 | 4 | 0.72 (0.64-0.78) | 84.4 | 0.72 (0.68-0.76) | 78.1 | 2.58 (2.06-3.24) | 39.9 | 0.34 (0.18-0.62) | 76.2 | 8.22 (3.94-17.15) | 61.0 |
| 2012-2016 | 9 | 0.80 (0.76-0.83) | 8.4 | 0.77 (0.74-0.79) | 88.8 | 3.19 (2.28-4.46) | 87.2 | 0.27 (0.23-0.32) | 0.8 | 11.9 (7.10-20.01) | 66.4 |
| LS value (cutoff) | |||||||||||
| < 30 kPa | 7 | 0.80 (0.76-0.84) | 30.1 | 0.77 (0.74-0.79) | 92.6 | 3.11 (2.01-4.81) | 91.4 | 0.27 (0.20-0.35) | 28.3 | 12.39 (6.60-23.27) | 71.6 |
| > 30 kPa | 6 | 0.73 (0.67-0.79) | 74.5 | 0.74 (0.70-0.77) | 12.3 | 2.78 (2.40-3.21) | 1.6 | 0.34 (0.22-0.53) | 67.3 | 8.33 (4.94-14.05) | 48.0 |
Studies conducted in multiple etiologies appeared to be preeminently superior to solitary and double factors [16.74 (8.23-33.84) vs 6.35 (3.77-10.68), and 16.74 (8.23-33.84) vs 6.18 (1.86-20.55)], as shown in Table 2, whereas the heterogeneity of etiology revealed that the one factor (I2 = 30.4%) etiology altered in a decreasing trend. Studies in Asian countries manifested a better diagnostic performance and a lower heterogeneity, as compared to European countries [Asian vs European, 11.06 (7.10-17.23) vs 7.14 (3.06-16.66), and (50.7 vs 74.0)]. Also, articles published from 2012 to 2016 year suggested the preferable performance of FS for the prediction of EV, contrasting with the year from 2012 to 2016 [10.84 (5.94-19.77) vs 7.46 (3.43-16.24)]. The accuracy and heterogeneity of FS applied at cutoff of more than 20 kPa revealed FS for diagnosis of the presence of EV was superior and inferior in contrast to less than 20 kPa [11.11 (7.05-17.49) vs 7.82 (3.36-18.24), and (45.4 vs 77.4)].
According to subgroup analysis, the heterogeneity for the presence of large EV classification is shown in Table 3. In etiology subgroup studies, multiple factors appeared to be superior to one and double factors [12.46 (6.99-22.18) vs 9.05 (5.50-14.90), and 12.46 (6.99-22.18) vs 7.21 (2.07-25.16)], and the heterogeneity was influenced slightly compared to solitary factor. Articles from European and Asian countries showed no different diagnostic performance, [European vs Asian, 10.55 (5.04-22.07) vs 10.03 (7.01-14.35)], but lower heterogeneity was found in Asian countries. Studies published from 2012 to 2016 year suggested the prior performance of FS for the prediction of large EV, contrasting with the year from 2012 to 2016 [11.92 (7.10-20.01) vs 8.22 (3.94-17.15)]. Also, the accuracy of FS for the detection of large EV in the less than 30 kPa classification, which had moderate heterogeneity, was demonstrated superior to the more than 30 kPa classification [12.39 (6.60-23.27) vs 8.33 (4.94-14.05)].
Therefore, although there were differences in diagnostic accuracy of FS for the presence of EV and significant EV based on the etiology, location, diagnostic threshold (cutoff value) and publication year, by combining the results of meta-regression analysis we found that the heterogeneity was not statistically different, excluding the solitary factor in the presence and absence of EV group.
SEN analyses were performed using the leave-one-out approach to investigate the influence of every included study to the pooled result of the DOR of FS for the diagnosis of the presence of EV and significant EV respectively. As is shown in both Supplementary Figure 5A and B, the pooled DOR of the eligible studies after removing every article sequentially, which did not alter the results significantly, fluctuated between the range of CI of the pooled DOR. Meanwhile, the consequence of the figure reflected that the meta-analysis result was robust, and no study dominated the results or contributed to the heterogeneity primarily.
Deek’s funnel plot asymmetry test was used to explore the publication bias of meta-analysis of diagnostic accuracy[15]. According to Deeks’ funnel plot (Supplementary Figure 6), there was no evidence of significant publication bias in FS for the detection of the presence of EV (P = 0.153) and large EV (P = 0.481).
Patients with cirrhosis have high incidence of EV with high morbidity and mortality due to bleeding; active surveillance via upper gastrointestinal examination can represent an unnecessary burden for patients, therefore, the increasing number of noninvasive tests for EV has gained widely attention. Nevertheless, few meta-analyses have involved predicting the presence and absence of EV and large EV measured by the LS value obtained with FS. Therefore, this meta-analysis aimed to assess the diagnostic performance of LS value measured with FS as a TE test to detect the presence of EV and large EV in patients with liver cirrhosis.
In meta-analysis of 15 studies on the diagnostic accuracy of FS-based LSM, the DOR for detecting the presence of EV and large EV was 9.33 and 10.69 respectively, which indicated higher diagnostic accuracy comparing patients without. The results of pooled estimates for SEN and SPE in the presence of EV and large EV groups were separately 84%, 78% and 62%, 76%, with missed diagnosis rate of 16% and 22%, and misdiagnosis rate of 38% and 24%. The pooled LR positive was 2.30 and 3.03, LR negative was 0.26 and 0.30 in two groups respectively, which indicated the likelihood of an accurate positive LSM diagnosis for EV and large EV with FS is 2-fold and 3-fold higher in cirrhotic patients in comparison to cirrhotic patients without EV. Combining the pre-test and post-test probability, we arrived at the following: if pre-test probability was equal to 50%, FS for predicting the absence and presence EV and significant EV could have 71% and 75% probability of correctly diagnosing, and 19% and 22% of patients might have EV and large EV if LSM was negative by FS. A meta-analysis about the FS for diagnosing the presence of EV and large EV, the area under the SROC curve (AUROC) of EV and significant EV were 0.8262 and 0.8321, suggesting the better diagnostic performance of LSM with FS in estimating the cirrhotic patients with EV.
Significant heterogeneity (70.3% and 70.4%) was found in the meta-analysis for 13 studies assessing the FS accuracy for the prediction of the presence of EV and large EV. Meta-regression and subgroup analysis methods were applied and screened conveniently and reliably the relevant factors that are responsible for heterogeneity. Consequently, according to meta-regression, we detected 4 covariates including the etiology, publication year, LS cutoff values, and region. Comparing the FS for the diagnosis of the presence of EV and significant EV, etiology of cirrhosis in covariates was significantly associated with the heterogeneity in the former, and none of covariates accounted for statistical heterogeneity in the latter. To take the unexplained heterogeneity into account, through subgroup analysis we further observed the systematic differences in the performance characteristics of the test across different covariates; however, the difference was not the source of the heterogeneity, excluding the solitary factor in the presence of EV group.
The strength of our study was that we evaluated the diagnostic accuracy of LSM with FS for the detection of EV and large EV with different cirrhotic patients and etiological characteristics, to achieve more real assessment of the test performance. What’s more, we sought to identify systematic differences in the performance characteristics of the test across Asian and Western populations through subgroup analysis. Our results show that FS also had a high accuracy in diagnosing EV and significant EV in patients with cirrhosis.
There were several limitations of our analysis that should be taken into consideration. Firstly, we screened 2697 patients in 15 reports limited to English or Chinese language mostly, but the higher quality articles written in non-English and non-Chinese were not included in our study. In addition, it remains possible that diagnostic performance showing poor accuracy has not been published as results of negative outcome. Secondly, owing to different etiologies, there was not the ability to define a diagnostic threshold value, which could provide the greatest accuracy in predicting the size of EV; meanwhile, the difference in diagnostic threshold value, identified through natural observation or derived on the basis of disease prevalence, may have resulted in the heterogeneity observed with the results. Consequently, it is difficult to value the diagnostic threshold of LSM with FS on the basis of these limited studies.
Finally, although we regarded EGD or GIE as the standard reference for valuing EV, the significant variability that exists unavoidably in different inter-observers confined the validity of gold standard in comparison with FS[34]. Moreover, according to the methodological quality validated assessment, there were inadequate information in most of the included studies to determine whether the results of the FS were blinded to EGD results, or vice versa, and the time period between performance of EGD and FS was not explicit. Similarly, there were insufficient and non-uniform descriptions on the spectrum of cirrhotic patients who received FS test, possibly impacting the overall results for compensated and decompensated cirrhosis with all etiologies in our study. Hence, the unclear information might attribute to the studies at risk for bias and heterogeneity.
In summary, this meta-analysis demonstrates that FS could be considered as a better noninvasive test for EV and significant EV in different histological stages and etiologies of hepatic cirrhosis; meanwhile, it has potential as part of a prediction rule incorporating other clinical characteristics or varying LSM cutoffs and, if used in conjunction with EGD, may help us prevent unnecessary screening by EGD. Nevertheless, the results should be interpreted cautiously given its SEN, SPE and limited utility. The major role of FS, which was suboptimal to substitute EGD as the screening modality for detecting the presence of EV and large EV, should be further validated.
In the future, prospective, well-designed studies for use of noninvasive methods such as EV, which may be a benchmark for diagnostic performance due to its elegant technique, inexpensive cost and wide availability, are needed to improve accuracy.
Recently, many non-invasive techniques for evaluating the severity of esophageal varices (EV) in liver cirrhosis have been used widely as alternatives to avoid the unnecessary endoscopy for EV screening. Transient elastography [FibroScan (FS)], as a non-invasive method to assess the fibrosis stages of hepatic cirrhosis, is applied to evaluate the severity of EV seldomly; moreover, there is no available consensus regarding diagnostic performance of different liver stiffness (LS) values (cutoff value) in the detection of EV in cirrhotic patients.
Despite few studies having investigated the diagnostic accuracy of FS for the detection of EV, no definite result of uniform standard is available to estimate the severity of EV according to the different cutoff values of LS. Thus, the importance of discussion about whether there is sufficient evidence to recommend FS as a noninvasive screening method has been emphasized.
In this study, the authors explored the value of FS for the diagnosis of EV in cirrhotic patients; meanwhile, it is also believed to be the first meta-analysis evaluating the diagnostic accuracy of FS for the detection of EV.
FS has relatively better performance for the detection of EV. Nevertheless, the results should be interpreted cautiously given its sensitivity, specificity and limited utility. In clinical practice, it has potential as part of a prediction rule incorporating other clinical characteristics or varying LS measurement cutoffs and, if used in conjunction with esophagogastroduodenoscopy (EGD), may help to prevent unnecessary screening EGD.
The study aimed to perform a meta-analysis regarding diagnostic accuracy of FS in detection of EV. This study is well performed and well written.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lee HC, Lo GH S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Zhang FF
| 1. | Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922-938. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1229] [Cited by in F6Publishing: 1147] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 2. | Chalasani N, Kahi C, Francois F, Pinto A, Marathe A, Bini EJ, Pandya P, Sitaraman S, Shen J. Improved patient survival after acute variceal bleeding: a multicenter, cohort study. Am J Gastroenterol. 2003;98:653-659. [PubMed] [Cited in This Article: ] |
| 3. | Carbonell N, Pauwels A, Serfaty L, Fourdan O, Lévy VG, Poupon R. Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology. 2004;40:652-659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 568] [Cited by in F6Publishing: 494] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 4. | Eisen GM, Baron TH, Dominitz JA, Faigel DO, Goldstein JL, Johanson JF, Mallery JS, Raddawi HM, Vargo JJ, Waring JP. Complications of upper GI endoscopy. Gastrointest Endosc. 2002;55:784-793. [PubMed] [Cited in This Article: ] |
| 5. | Addley J, Tham TC, Cash WJ. Use of portal pressure studies in the management of variceal haemorrhage. World J Gastrointest Endosc. 2012;4:281-289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 20] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Colli A, Gana JC, Turner D, Yap J, Adams-Webber T, Ling SC, Casazza G. Capsule endoscopy for the diagnosis of oesophageal varices in people with chronic liver disease or portal vein thrombosis. Cochrane Database Syst Rev. 2014;CD008760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. [PubMed] [Cited in This Article: ] |
| 8. | Shaheen AA, Wan AF, Myers RP. FibroTest and FibroScan for the prediction of hepatitis C-related fibrosis: a systematic review of diagnostic test accuracy. Am J Gastroenterol. 2007;102:2589-2600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 259] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 9. | Talwalkar JA, Kurtz DM, Schoenleber SJ, West CP, Montori VM. Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2007;5:1214-1220. [PubMed] [Cited in This Article: ] |
| 10. | Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960-974. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1046] [Cited by in F6Publishing: 1011] [Article Influence: 63.2] [Reference Citation Analysis (1)] |
| 11. | Stebbing J, Farouk L, Panos G, Anderson M, Jiao LR, Mandalia S, Bower M, Gazzard B, Nelson M. A meta-analysis of transient elastography for the detection of hepatic fibrosis. J Clin Gastroenterol. 2010;44:214-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 149] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 12. | Castera L, Pinzani M, Bosch J. Non invasive evaluation of portal hypertension using transient elastography. J Hepatol. 2012;56:696-703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 220] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 13. | Castéra L, García-Tsao G. When the spleen gets tough, the varices get going. Gastroenterology. 2013;144:19-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6953] [Cited by in F6Publishing: 8128] [Article Influence: 625.2] [Reference Citation Analysis (0)] |
| 15. | Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58:882-893. [PubMed] [Cited in This Article: ] |
| 16. | Sporea I, Raţiu I, Bota S, Şirli R, Jurchiş A. Are different cut-off values of liver stiffness assessed by transient elastography according to the etiology of liver cirrhosis for predicting significant esophageal varices? Med Ultrason. 2013;15:111-115. [PubMed] [Cited in This Article: ] |
| 17. | Sharma P, Kirnake V, Tyagi P, Bansal N, Singla V, Kumar A, Arora A. Spleen stiffness in patients with cirrhosis in predicting esophageal varices. Am J Gastroenterol. 2013;108:1101-1107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 18. | Saad Y, Said M, Idris MO, Rabee A, Zakaria S. Liver stiffness measurement by fibroscan predicts the presence and size of esophageal varices in egyptian patients with HCV related liver cirrhosis. J Clin Diagn Res. 2013;7:2253-2257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Nguyen-Khac E, Saint-Leger P, Tramier B, Coevoet H, Capron D, Dupas JL. Noninvasive diagnosis of large esophageal varices by Fibroscan: strong influence of the cirrhosis etiology. Alcohol Clin Exp Res. 2010;34:1146-1153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Li F, Yan T, Zhang J, Shao Q, Li B, Li ZB, Chen GF. [FibroScan can be used to diagnose the size of oesophageal varices in patients with HBV-related cirrhosis]. Zhonghua Shiyan He Linchuang Bingduxue Zazhi. 2012;26:470-473. [PubMed] [Cited in This Article: ] |
| 21. | Li F, Yan T, Shao Q, Ji D, Li B, Li Z, Chen G. [Clinical study of FibroScan efficiency for diagnosing size of oesophageal varices in liver cirrhosis patients]. Zhonghua Ganzangbing Zazhi. 2014;22:600-603. [PubMed] [Cited in This Article: ] |
| 22. | Kazemi F, Kettaneh A, N’kontchou G, Pinto E, Ganne-Carrie N, Trinchet JC, Beaugrand M. Liver stiffness measurement selects patients with cirrhosis at risk of bearing large oesophageal varices. J Hepatol. 2006;45:230-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 299] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 23. | Jung HS, Kim YS, Kwon OS, Ku YS, Kim YK, Choi DJ, Kim JH. [Usefulness of liver stiffness measurement for predicting the presence of esophageal varices in patients with liver cirrhosis]. Korean J Hepatol. 2008;14:342-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Hu Z, Li Y, Li C, Huang C, Ou Z, Guo J, Luo H, Tang X. Using Ultrasonic Transient Elastometry (FibroScan) to Predict Esophageal Varices in Patients with Viral Liver Cirrhosis. Ultrasound Med Biol. 2015;41:1530-1537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Castéra L, Le Bail B, Roudot-Thoraval F, Bernard PH, Foucher J, Merrouche W, Couzigou P, de Lédinghen V. Early detection in routine clinical practice of cirrhosis and oesophageal varices in chronic hepatitis C: comparison of transient elastography (FibroScan) with standard laboratory tests and non-invasive scores. J Hepatol. 2009;50:59-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 272] [Cited by in F6Publishing: 287] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 26. | Calvaruso V, Bronte F, Conte E, Simone F, Craxì A, Di Marco V. Modified spleen stiffness measurement by transient elastography is associated with presence of large oesophageal varices in patients with compensated hepatitis C virus cirrhosis. J Viral Hepat. 2013;20:867-874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | Bintintan A, Chira RI, Bintintan VV, Nagy GA, Manzat-Saplacan MR, Lupsor-Platon M, Stefanescu H, Duma MM, Valean SD, Mircea PA. Value of hepatic elastography and Doppler indexes for predictions of esophageal varices in liver cirrhosis. Med Ultrason. 2015;17:5-11. [PubMed] [Cited in This Article: ] |
| 28. | Stefanescu H, Grigorescu M, Lupsor M, Procopet B, Maniu A, Badea R. Spleen stiffness measurement using Fibroscan for the noninvasive assessment of esophageal varices in liver cirrhosis patients. J Gastroenterol Hepatol. 2011;26:164-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 29. | Stefanescu H, Grigorescu M, Lupsor M, Maniu A, Crisan D, Procopet B, Feier D, Badea R. A new and simple algorithm for the noninvasive assessment of esophageal varices in cirrhotic patients using serum fibrosis markers and transient elastography. J Gastrointestin Liver Dis. 2011;20:57-64. [PubMed] [Cited in This Article: ] |
| 30. | Wang JH, Chuah SK, Lu SN, Hung CH, Chen CH, Kee KM, Chang KC, Tai WC, Hu TH. Transient elastography and simple blood markers in the diagnosis of esophageal varices for compensated patients with hepatitis B virus-related cirrhosis. J Gastroenterol Hepatol. 2012;27:1213-1218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Society of Digestive Endoscopy of Chinese Medical Association. Trial scheme of diagnosing and treating gastroesophageal varices under endoscopy(2003). Zhonghua Xiaohuan Neijing Zazhi. 2004;21:149-151. [Cited in This Article: ] |
| 32. | Beppu K, Inokuchi K, Koyanagi N, Nakayama S, Sakata H, Kitano S, Kobayashi M. Prediction of variceal hemorrhage by esophageal endoscopy. Gastrointest Endosc. 1981;27:213-218. [PubMed] [Cited in This Article: ] |
| 33. | Sarangapani A, Shanmugam C, Kalyanasundaram M, Rangachari B, Thangavelu P, Subbarayan JK. Noninvasive prediction of large esophageal varices in chronic liver disease patients. Saudi J Gastroenterol. 2010;16:38-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | Bendtsen F, Skovgaard LT, Sørensen TI, Matzen P. Agreement among multiple observers on endoscopic diagnosis of esophageal varices before bleeding. Hepatology. 1990;11:341-347. [PubMed] [Cited in This Article: ] |