Published online Mar 28, 2017. doi: 10.3748/wjg.v23.i12.2124
Peer-review started: December 29, 2016
First decision: January 19, 2017
Revised: February 3, 2017
Accepted: March 2, 2017
Article in press: March 2, 2017
Published online: March 28, 2017
Bacterial colonization of the gut shapes both the local and the systemic immune response and is implicated in the modulation of immunity in both healthy and disease states. Recently, quantitative and qualitative changes in the composition of the gut microbiota have been detected in Crohn’s disease and ulcerative colitis, reinforcing the hypothesis of dysbiosis as a relevant mechanism underlying inflammatory bowel disease (IBD) pathogenesis. Humans and microbes have co-existed and co-evolved for a long time in a mutually beneficial symbiotic association essential for maintaining homeostasis. However, the microbiome is dynamic, changing with age and in response to environmental modifications. Among such environmental factors, food and alimentary habits, progressively altered in modern societies, appear to be critical modulators of the microbiota, contributing to or co-participating in dysbiosis. In addition, food constituents such as micronutrients are important regulators of mucosal immunity, with direct or indirect effects on the gut microbiota. Moreover, food constituents have recently been shown to modulate epigenetic mechanisms, which can result in increased risk for the development and progression of IBD. Therefore, it is likely that a better understanding of the role of different food components in intestinal homeostasis and the resident microbiota will be essential for unravelling the complex molecular basis of the epigenetic, genetic and environment interactions underlying IBD pathogenesis as well as for offering dietary interventions with minimal side effects.
Core tip: The gut microbiota has a recognized role in immunity, and changes in its composition, or dysbiosis, may be the basis for the worldwide increased incidence of inflammatory bowel disease (IBD). Dietary constituents have been shown to affect the immune response and the inflammatory status, in great part mediated through the modulation of the microbiota. Environmental compounds, including nutrients, can induce alterations in the epigenome interface, resulting in long lasting phenotypic or even tissue structure and function modifications. Unravelling the complex molecular basis of the epigenetic, genetic and environmental interactions underlying IBD pathogenesis will have implications for the development of novel therapies.
- Citation: Rapozo DCM, Bernardazzi C, de Souza HSP. Diet and microbiota in inflammatory bowel disease: The gut in disharmony. World J Gastroenterol 2017; 23(12): 2124-2140
- URL: https://www.wjgnet.com/1007-9327/full/v23/i12/2124.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i12.2124
The gastrointestinal tract is relentlessly challenged by the luminal contents harbouring innumerable microorganisms and food antigens. To maintain the normal homeostatic equilibrium, it is critical for the system to be capable of identifying whether a stimulus is pathogenic or not and of mounting an appropriate response, resulting in either inflammation or tolerance[1]. Particularly in the context of the gut, defence mechanisms and tolerance should act in concert, allowing the organism to control the inflammatory response and tissue injury that may occur following exposure to a given pathogen[2]. While immune deficiency inevitably culminates in recurrent infections, defective tolerance may result in uncontrolled inflammation and immunopathology[3]. In fact, an abnormal relationship between host and microbiota is believed to result in intestinal immune imbalance[4], leading to the development of conditions such as inflammatory bowel disease (IBD), which consists of two major forms, Crohn’s disease (CD) and ulcerative colitis (UC)[5,6]. In this paper, we discuss basic mechanisms and potential connections between microbiota, diet, and the development of IBD.
A mutually beneficial association between humans and microbes is essential for maintaining homeostasis. Such co-existence highlights the predominantly symbiotic nature of the interaction between humans and microorganisms despite the remarkable variation that occurs over time at diverse body sites[7]. As a consequence, abnormalities of the intestinal microbiota have been implicated in the pathogenesis of several health conditions, including gastrointestinal diseases.
The development and adaptation of the intestinal microbiota represents a continuous process that occurs throughout the lifetime. In this regard, several environmental factors contribute to the microbial colonization of the gastrointestinal tract. The composition of the intestinal microbiota is affected very early in life, beginning with the route of delivery[8]. Shortly after birth, breast-feeding, exposure to food and other environmental factors play a pivotal role in the development of the intestinal microbiota. The microbial composition of the gut, in turn, also shapes the development of both the innate and the adaptive immune system[9]. The commensal microbiota is universally distributed throughout the gastrointestinal tract, with a characteristic progressive increase in both diversity and density from the upper to the lower segments. Studies of the human microbiome have identified more than three million unique genes within the gut, widely outnumbering the human genome and containing more than a thousand bacterial species, most of them of the Bacteroidetes and Firmicutes phyla[10]. In fact, several different groups around the world are currently investigating the composition of the human microbiome. Recently, the phylogenetic composition of faecal samples from different nationalities was investigated in a metagenomic analysis, which demonstrated the presence of robust bacterial clusters, defined as enterotypes. These enterotypes, mostly defined by species composition, were not nation- or continent-specific, supporting the idea of a relatively limited number of established host-microbe symbiotic conditions, which may behave distinctly upon exposure to food or drugs[11].
The complexity of the human gut microbiome is further evidenced by the spatial distribution and alternation of microorganisms throughout the length of the gastrointestinal tract and across the radial axis. It has been demonstrated, for example, that different bacteria inhabit distinct segments of the intestine and are found in different layers of the gut, such as the central lumen, associated with mucosal folds, or embedded in the mucus layer[12]. Together, these findings support the hypothesis that the resident or autochthonous microbiota has been modified to adapt to new functional specializations, therefore playing a distinct role compared to the transient microbiota present in the faecal stream. In this sense, each intestinal niche is thought to shelter the microbes that would be the most convenient to preserve local tissue homeostasis and exhibiting clear beneficial mutualism with the host[12].
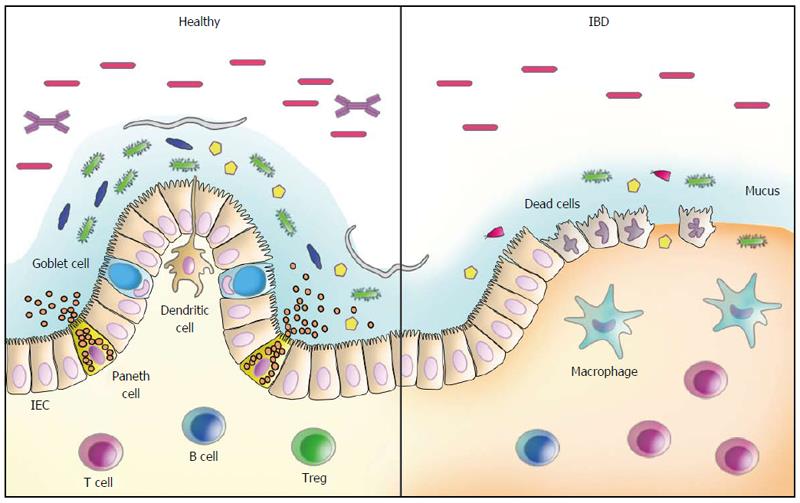
Currently, it is well accepted that one of the key functions of the gut microbiota, in addition to nutrition, metabolism and energy production, consists of the development and maturation of the immune system[13]. In fact, bacterial colonization of the gut is believed to shape not only the local but also the systemic immune response, being implicated in the modulation of immunity in both healthy and disease states[14]. Under normal conditions, gastrointestinal microorganisms are recognized by NOD-like and Toll-like receptors, specialized molecules of the innate immune system predominantly localized in epithelial and immune cells, and this recognition process results in activation of the immune response, which is indispensable to intestinal homeostasis[15].
To maintain homeostasis, the microbiota is regulated by several mechanisms involving epithelial and immune cell molecules, including IgA, RegIIIγ, and defensins, whereas the immune response is reciprocally regulated by the microbiota, with particular microorganisms promoting the growth of distinct T cell subsets[16]. For example, commensal segmented filamentous bacteria were shown to induce Th17 cells[17,18] capable of identifying extraintestinal autoimmune inflammation in experimental models[19,20]. On the other hand, Clostridia and Bacteroides fragilis were shown to favour the induction of Treg cells and type 1 T helper (Th1) cells, respectively[16]. Of note, Clostridia were demonstrated to induce Tregs within the gut with a concomitant down-regulation of Th1 and Th17 responses[21]. Although the exact mechanism by which Tregs are induced by the intestinal microbiota are yet to be determined, there is evidence suggesting a role for microbe-derived short-chain fatty acids[22]. Alimentary fibres are not digested by the human gastrointestinal tract but, instead, they are fermented in the gut by bacteria, which in turn modifies the gut microbiota. The microbial processing of fibres results in the formation of short-chain fatty acids (SCFAs), such as acetate, propionate and butyrate, which are used by colonocytes as crucial energy sources, with important anti-inflammatory activities in vitro and in vivo[23,24]. In particular, butyrate, produced by commensal bacteria, was also shown to participate in Treg differentiation and suppression of pro-inflammatory cytokines from macrophages and dendritic cells[25,26], while its in vivo administration was shown to ameliorate experimental colitis[27], suggesting the importance of specific luminal nutrients in the homeostasis of the colon.
In addition to the effects of the gut microbiota on immunity, dietary factors have also been implicated in gut microbial regulation of intestinal immunity. Therefore, diet has emerged as another critical element that interacts with the microbiota and immunity to actively affect homeostatic control[28].
The influence of food in shaping the intestinal microbiota has been hypothesized for a long time. However, only in recent years have consistent data on this subject been obtained, due in particular to the advent of technologies such as next-generation DNA sequencing and metabolic profiling[29]. As a result, interesting new data have been reported, consequently shaping conceptual changes in the field. For example, the role of early nutrition in moulding the gut microbiota appears to impact the risk of diseases development even late in life[30,31]. Furthermore, it is now clear that the microbiota composition is dynamic, changing with age and oscillating according to environmental modifications, including food intake patterns, among other factors[32].
Network-based studies of microbial communities performed with faecal samples of several mammalian species have confirmed that diet does determine bacterial diversity, which increases from carnivore to omnivore to herbivore, whereas microbial communities diversify concomitantly with their hosts, supporting the hypothesis of the co-evolution of gut microbiotas and their hosts[33]. Although there is a general assumption that the typical modern human intestinal microbiota tends to be one of omnivorous habits, considerable heterogeneity still exists in the world, with some remarkable discrepancies. An interesting study, for example, demonstrated substantial differences in the intestinal microbiota of children living in African rural communities compared with children living in Europe. The guts of African children were rich in Bacteroidetes and poor in Firmicutes and Enterobacteriaceae, while the results obtained from European children were quite the opposite[34]. The investigators suggested that the findings were mostly attributable to radically different dietary patterns (Table 1).
| Phylum | Class | Order | Family | Genus | Species | Characteristics | Action in the GI tract | Ref. |
| Bacteroidetes | Bacteroidetes | Bacteroidales | Prevotellaceae | Prevotella | P. sp | Gram-negative | Diets rich in carbohydrates and fat | [10, 16, 22-24, 34, 35, 37, 38, 43, 44, 80, 82, 83] |
| Bacteroides | B. fragilis | Anaerobic | Involved in colitis | |||||
| B. uniformis | Commensal bacteria | |||||||
| Firmicutes | Clostridia | Clostridiales | Clostridiaceae | Clostridium | C. lavalense | Gram-positive | Play a role in the clinical course of IBD | [10, 16, 21, 34, 37, 38, 43, 44, 80-84, 86, 87, 129, 159, 160, 181-185] |
| C. perfringens | Anaerobic | |||||||
| Ruminococcaceae | Ruminococcus | R. torques | Gram-positive | Fermentation of dietary fibre | ||||
| Anaerobic | ||||||||
| Faecalibacterium/ Fusobacterium | F. prausnitzii | Anaerobic Commensal bacteria | ||||||
| Lachnospiraceae | Roseburia | R. faecis | Gram-positive | Fermentation of dietary fibre | ||||
| R. hominis | Anaerobic | |||||||
| R. cecicola | ||||||||
| R. intestinalis | ||||||||
| R. inulinivorans | ||||||||
| Fusicatenibacter | F. saccharivorans | Present in the intestine | ||||||
| Blautia | B. faecis | |||||||
| Bacilli | Lactobacillales | Streptococcaceae | Streptococcus | S. spp | Gram-positive | Part of the normal animal microbiota | ||
| Lactobacillaceae | Lactobacillus | L. acidophilus | Induces remission in UC patients | |||||
| Negativicutes | Veillonellales | Veillonellaceae | Veillonella | V. spp | Gram-negative | Present in the intestine and oral mucosa | ||
| Anaerobic | ||||||||
| Erysipelotrichia | Erysipelotrichales | Erysipelotrichaceae | Turicibacter | T. sp | Gram-positive | Present in mammal intestines | ||
| Proteobacteria | Gammaproteobacteria | Enterobacteriales | Enterobacteriaceae | Escherichia | E. coli | Gram-negative | Involved in colitis | [34, 36, 80, 83, 186] |
| Desulfovibrionales | Desulfovibrionaceae | Bilophila | B. wadsworthia | Anaerobic | ||||
| Pasteurellales | Pasteurellaceae | Pasteurella | P. sp | Gram-negative | Present in the nose and mouth | |||
| Commensal bacteria | ||||||||
| Facultative anaerobes | ||||||||
| Actinobacteria | Actinobacteria | Bifidobacteriales | Bifidobacteriaceae | Bifidobacterium | B. breve | Gram-positive | Induces remission in UC patients | [159, 187] |
| B. bifidum | Anaerobic | |||||||
| Fusobacteria | Fusobacteria | Fusobacteriales | Fusobacteriaceae | Fusobacterium | F. spp | Gram-negative | Involved in colitis and colon cancer | [83] |
| Anaerobic |
Following the same line of evidence, several other studies have raised the issue of diet potentially affecting the gut microbiota. Of note, animal fat-based diets and carbohydrate-based diets lead to a specific enrichment of Bacteroides and Prevotella in adult individuals. Moreover, it is important to highlight that the gut microbiome composition undergoes relatively rapid changes upon exposure to a low-fat/high-fibre or high-fat/low-fibre diet, for example[35]. In another short-term dietary intervention in humans, in contrast to the effects of a plant-based diet, consumption of strictly animal-based products increased the abundance of bile-tolerant microorganisms and decreased the levels of Firmicutes that metabolize dietary plant polysaccharides. These results reflect the differences between herbivorous and carnivorous habits, depicting specific adjustments between carbohydrate and protein fermentation. In particular, the identification of increases in the abundance and activity of Bilophila wadsworthia as a result of an animal-based diet was interpreted as a probable link between dietary fat, bile acids, and the prominence of microorganisms potentially involved in the development of IBD[36].
Several studies have provided additional information on dietary fibre supplementation and the effect of SCFAs on the intestinal microbiota. In regards to the type of SCFA generated in the intestine, both the type of fibre ingested, usually composed of non-digestible complex carbohydrates, and the metabolizing microbiota are determining factors. While resistant starch promotes the production of relatively more butyrate, pectin leads to more acetate and propionate production. Regarding the gut microbiota, bacteria of the Bacteroidetes phylum produce more acetate and propionate, whereas bacteria of the Firmicutes phylum predominantly produce butyrate[37,38]. In animal models of colitis, for example, dietary fibres, including fermentable fibres and starches, are metabolized by colonic bacteria into SCFAs, with relevant anti-inflammatory effects[39-41]. In addition to the high fat content of Western diets in general, it is also important to call attention to the high levels of dietary omega-6 fatty acids, due to the use of vegetable oils, resulting in a high omega-6 to omega-3 ratio. Omega-6 fatty acids, especially arachidonic acid, are potentially pro-inflammatory, whereas omega-3 fatty acids, such as a-linolenic acid from plants and eicosapentaenoic acid and docosahexaenoic acid from fish, are anti-inflammatory[42].
High caloric intake with a large consumption of carbohydrates, typical of Western diets, has been associated with less microbiome diversity, in contrast to the Mediterranean diet based on fruits, vegetables, and red wine[43]. Nevertheless, recently, exclusion diets such as the specific carbohydrate diet (SCD, which restricts all carbohydrates except monosaccharides) and a diet low in fermentable oligo-, di-, and monosaccharides and polyols (FODMAPs) have produced promising results in IBD[44]. In uncontrolled trials of restriction diets for IBD, SCD-like diets were shown to reduce symptoms and intestinal inflammation[45,46]. These observations support the notion that dietary manipulations might modify the intestinal microbiota despite the presence of resident enterotypes settled by long-term dietary patterns.
The effects of specific nutritional changes on the mammalian system have been increasingly investigated, including the impact of micronutrients on the gut microbiota. For example, in weaned-mouse models of zinc or protein deficiency, considerable changes in the gut microbiota were observed, in addition to reductions in microbial proteolysis and increases in microbial dietary choline processing[47]. Processed foods are usually low in micronutrients and have been associated with a greater risk of developing several diseases. In this sense, zinc and other nutrients such as n-3 fatty acids and vitamins D and E are thought to protect from preclinical and/or clinical type 1 diabetes, for example[48].
In the last decade, the intestinal microbiota-host interaction has gained progressively increasing attention, as it has been associated, directly or indirectly, with a variety of immune, inflammatory, and metabolic disorders[49]. Furthermore, in recent years, the increase in the incidence of autoimmune and chronic inflammatory disorders has been attributed to alterations in the microbial composition and the role of the intestinal microbiota in immune regulation[50]. Modifications in human habits have been implicated in the rise of IBD worldwide[51]. This thought is supported by the evidence showing a consistent increase in the incidence and prevalence of IBD in Western countries and, more recently, in the Asia Pacific area[52].
The idea of “Western lifestyle factors” triggering intestinal inflammation appears to be reinforced by the dramatic increase in the incidence of IBD in last half century, which is likely not paralleled by changes in the human genome[53,54]. In this regard, several factors such as the improvement of general sanitary conditions and antibiotic usage, resulting in a decreased incidence of infectious diseases, coincide with the increase in autoimmune diseases and chronic inflammatory conditions, constituting the basis for the hygiene hypothesis[55,56]. In fact, some events likely related to changes in the gut microbiota appear to be associated with the development of IBD. For example, the risk of IBD has been shown to increase after an episode of acute gastroenteritis[57] and in children repeatedly treated with antibiotics[58]. IBD-associated genetic findings have also provided important evidence for the role of microorganisms in disease pathogenesis. Several sources of information, including genome-wide association studies, have identified more than 200 genetic risk loci as predisposing factors for IBD. Of note, several of the genetic risk alleles for IBD are directly associated with pathways that regulate the adaptive immune system, while many others are involved in innate immune responses or epithelial barrier regulation, crucial mechanisms in the defence against microbial invasion[59,60] (Figure 1).
Interestingly, abnormalities of the gut microbiota are present in common intestinal conditions, including irritable bowel syndrome, chronic idiopathic diarrhoea, and IBD[61-63]. In addition, recent evidence has suggested that the impact of the intestinal microbiota in disease pathogenesis can extend to other immune-mediated conditions beyond the gut including, for example, type 1 diabetes, cardiovascular disease, and autoimmune demyelination[64-66].
In IBD, distinct abnormalities of the intestinal microbiota have been reported, including changes in the microbial composition, an inappropriate immune response towards commensal microorganisms, or even both[67]. In CD, for example, immune reactivity against microbial-derived antigens has long been reported, characterized by several different circulating serum antibodies[68-71]. Another clinically relevant observation to support a role for the gut microbiota in the inflammatory process of CD comes from postsurgical relapses triggered by agents present in the faecal stream[72]. Recently, longitudinal studies have provided evidence implicating dietary patterns as risk factors for IBD. In general, a lower risk of IBD has been associated with habits of consuming more vegetables and fruits, in contrast to a higher risk among people whose diet is based more on animal fats and sugar[73-76]. In particular, the association between the ingestion of fats and the development of UC has been most prominently related to the long-term high intake of trans-unsaturated fats[76], likely due to dietary linoleic acid, an n-6 polyunsaturated fatty acid[75]. Of note, dietary-fat-induced taurocholic acid, secondary to the intake of saturated fats from milk, was shown to boost pathobiont expansion, triggering colitis in IL-10-deficient mice, with the induction of a pro-inflammatory Th1 immune response[77].
Quantitative and qualitative changes in the composition of the gut microbiota have been detected in CD and in UC, reinforcing the hypothesis of dysbiosis as a relevant mechanism underlying IBD pathogenesis[78]. Changes in the composition of the intestinal microbiota have been reported in CD, for example, including an overall decreased diversity[79] but also an increase in Bacteroidetes and Proteobacteria paralleled by a decrease in Firmicutes abundance[80]. Additional evidence corroborating the role of bacteria in intestinal inflammation was the finding of a lower proportion of Faecalibacterium prausnitzii, a member of the phylum Firmicutes with anti-inflammatory properties, in patients with CD with increased risk of postoperative recurrence after resection for ileal disease[81]. At the species level, in addition to Faecalibacterium prausnitzii, several other butyrate-producing bacterial species, such as Blautia faecis, Roseburia inulinivorans, Ruminococcus torques, Clostridium lavalense, and Bacteroides uniformis, were also shown to be significantly reduced in CD patients[82]. Also interesting is the fact that exposure to antibiotics may amplify the microbial dysbiosis associated with CD. In particular, in a large paediatric cohort of new-onset CD, an increased abundance of bacteria including Enterobacteriaceae, Pasteurellaceae, Veillonellaceae, and Fusobacteriaceae and a decreased abundance of Erysipelotrichales, Bacteroidales, and Clostridiales were consistently correlated with disease severity[83]. The changes in microbial composition in CD have been further corroborated by a recent systematic review confirming a relative increase in Bacteroidetes and decrease in Firmicutes abundance. In particular, Enterobacteriaceae were increased, while Faecalibacterium prausnitzii was found at a lower abundance, including in patients with postoperative recurrence[84].
Abnormalities in the intestinal microbiota have also been detected in UC, although to a lesser degree compared to CD patients[85]. Nevertheless, a less diverse microbiota was also demonstrated in samples from patients with UC and, in particular, the finding of increased C. perfringens in faeces suggested its role in disease exacerbation[86]. In another study, investigators identified a decrease in Fusicatenibacter saccharivorans in patients with active UC, in contrast to the increase observed in patients with quiescent disease[87].
Whether dysbiosis consists of a primary or secondary phenomenon in IBD is a question that remains unanswered. There is evidence showing that the intestinal microbiota can be shaped by the host’s genotype[88,89] but also by diet, habits, history of infections, use of antibiotics or other medications, and the inflammatory process[14,90-92]. On the other hand, it is important to call attention to the fact that dysbiosis alone may not be sufficient to induce IBD.
Several defects in the inflammatory response against microbial agents that have been reported in IBD[93,94] lend support to the idea of an inadequate clearance of microbial-associated molecular patterns as an important underlying mechanism of disease[95]. This may be particularly relevant in CD, due to the well-established association of the disease with genetic polymorphisms of NOD2 and ATG16L1, for example, which result in defective autophagy and impaired microbial clearance[96-98]. Another important mechanistic association in intestinal inflammation is believed to occur in response to the accumulation of unfolded proteins in the lumen of the endoplasmic reticulum (ER stress), resulting in the activation of intracellular signal transduction pathways, known as the unfolded protein response (UPR). In addition to the relationship with autophagy, ER stress has been associated with intestinal inflammation and IBD based on studies revealing primary genetic alterations involving XBP1, ARG2, ORMDL3, and other components of the UPR[99,100]. Another example of an inadequate microbial recognition and control comes from the reduced expression of defensins, antimicrobial peptides produced by Paneth cells, in patients with NOD2 mutations, with expected implications for CD[101]. Individual or combined defects involving various genes such as NOD2, ATG16L1 and IRGM might result in inadequate recognition of microorganisms present in the intestinal lumen[102] and subsequently defective induction of autophagy, activation of alternate pathways, and modulation of adaptive immunity[103]. In addition to ATG16L1, polymorphisms of the immunity-related GTPase family M (IRGM) gene, shown to be involved in the process of microbial control, have also been associated with CD[104,105]. Furthermore, the interaction between single nucleotide polymorphisms of ATG16L1 and IRGM has also been demonstrated in CD[106], indicating the probable integration of defective autophagy with mitochondrial dysfunction and apoptosis. Together, the knowledge accumulated in the last few years in the field of IBD, in addition to shedding light on new mechanisms, has revealed the multiple redundant and overlapping pathways underlying the disease pathogenesis. In addition, the information accumulated matches, in great part, the recent epidemiological changes in IBD distribution and reinforce the participation of dysbiosis in disease pathogenesis[56].
Environmental factors have been recognized as fundamental elements in the perinatal maturation of the immune system. In this sense, the microbial colonization of mucosal surfaces becomes of critical importance in the development and maturation of the mucosal immune system[107,108]. At birth, the transition from the sterile foetal environment is marked by exposure to a large number of exogenous stimuli. Interestingly, after natural birth, a newborn’s microbiota composition tends to resemble that of the maternal vaginal or gut microbiota, while after Caesarean section, the microbiota contains a considerable number of environmental agents[109]. The subsequent microbiota that establishes thereafter has an increasingly diverse composition, but individualities are preserved and are relatively stable over time[110]. Among the environmental factors, food components contribute to the development of the immune response both directly and indirectly. Early in life, breast-feeding provides several important elements in the defence against pathogens, such as IgA, cytokines, growth factors, and high concentrations of oligosaccharides that foster the accumulation of lactic acid-producing bacteria in the gut[111]. Moreover, in terms of IBD, the effect of breast-feeding may prove to be more important than previously thought, as the results of a meta-analysis have suggested that it might play a protective role against the development of paediatric disease[112].
Several other data exist to support the participation of dietary elements in the definition of the microbiota itself and the interaction with the immune system. For example, Western-like diets with their ubiquitous food additives were shown to affect the composition and function of the microbiota[113]. Retinoic acid, a derivative of vitamin A, is important in the development of the neonatal immune system, for cellular and subcellular membrane stability and in epithelial surfaces[114], and in adults, where it is required for the expression of gut-homing molecules on immune cells, the induction of Tregs and IgA class switching[115]. Iron, an essential element in haematopoiesis, may also trigger inflammatory processes associated with CD progression, as luminal iron may directly modify epithelial cell function or generate a pathological milieu due to alterations of the intestinal microbiota[116]. Vitamin D induces tolerogenic dendritic cells and is now regarded as an important regulator of mucosal immunity[117]. The availability and functionality of vitamin D depends on both ingestion and exposure to sunlight with natural ultraviolet (UV) radiation. In the case of IBD, it has been suggested that low sunlight exposure constitutes a risk factor, particularly for CD[118,119]. This is in agreement with the notion that the incidence of IBD is higher in the northern hemisphere, where UV exposure is significantly lower[120]. Analysing these data together, it is rational to suggest that not only do early postnatal events influence the priming of the mucosal immune system and the immune response in adult life, but also that there are clearly innumerable other dietary-environmental intervening factors that might impact normal homeostasis and the risk of developing IBD.
In the last few years, epigenetic mechanisms have been implicated in the regulation of gene expression and cellular functions. The epigenome has been regarded as an interface between the environment and the genome, which plays a pivotal role in the definition of phenotypes and their maintenance. In this context, methylation of cytosine in CpG motifs has constituted the most extensively studied epigenetic event[121]. In the nucleus, DNA CpG methylation regulates gene expression through its effects on chromatin states and accessibility of factor binding sites in regulatory regions in gene promoters. While hypermethylation close to promoter regions is associated with gene silencing, in contrast, hypomethylation results in an opposite effect[122]. Recent data have reinforced the thought that epigenetic interactions connecting host DNA with environmental factors might have a key influence in the phenotypical expression of complex diseases such as IBD. This hypothesis is further supported by epidemiologic observations revealing the increased risk of developing IBD among people migrating from low to high incidence areas of the world[123]. Another example highlighting the importance of non-genetic processes in IBD development comes from studies showing a relatively high discordance rate among monozygotic twins[124].
Currently, there are indications that epigenetic mechanisms other than DNA methylation are implicated in the development of IBD, including the differential expression of microRNAs[125] and histone modifications[126]. However, most epigenetic modifications that have been correlated with the pathogenesis of IBD rely on DNA methylation studies[127]. One of these studies, for example, investigated the methylation status in the colonic mucosa from foetuses, control children and children with IBD. The analysis comparing IBD with control samples identified 233 differentially methylated regions (DMR), with a substantial overlap between paediatric IBD and control samples. This study supports probable novel physiological roles for DNA methylation in the human intestinal epithelium and presents data connecting developmentally acquired alterations in the DNA methylation profile to changes seen in paediatric IBD[128].
Regarding the question of whether epigenetic changes during development could be associated with a later onset of IBD, another group studied the colonic mucosa epigenome in association with the microbiome in children and adolescents. The investigators observed a strong connection between age-dependent and IBD-specific DNA methylation variations, remarkably more consistent with UC than CD, and DMRs with decreased methylation during late-onset paediatric disease. Of note, the authors called attention to the finding that the genera with epigenetically plastic DMRs during childhood and adolescence were Roseburia and Streptococcus. In particular, Roseburia, butyrate-producing bacteria, possess the potential to drive epigenetic changes in epithelial stem cells, since butyrate has been shown to be a histone deacetylase inhibitor[129].
Complex interactions between genotype, epigenome and environmental factors, leading to continuous remodelling of the epigenome, determine the phenotype of an individual. Among the environmental factors, food constituents emerge as important stimuli, which have been associated with specific epigenetic signatures and patterns of gene expression[130]. The one-carbon metabolism is dependent on dietary food components (e.g., choline, betaine, folate) that participate in biochemical pathways of DNA methylation and/or supply of methyl groups[131]. Processed food, typical of Western diets, in most cases are deficient in micronutrients, including selenium and folate, which are both implicated in the progression of many diseases, including increased risk of developing colorectal cancer[132-135].
DNA hypomethylation represents an important phenomenon in human health, as it acts as the initial epigenetic alteration associated with carcinogenesis[136]. Since DNA methylation depends on the one-carbon metabolism pathway, requiring the activity of enzymes that depend on micronutrients provided by the diet, it is conceivable that hypomethylation might occur due to the lack of methyl donors. In fact, folate present in the diet, not synthesized endogenously, acts as a donor of one-carbon moieties, critical elements for the synthesis and repair of DNA and methylation that control gene expression[137]. Folate deficiency, in turn, has been demonstrated to induce DNA hypomethylation, while its supplementation has been able to correct some mutations and DNA strand breaks[138]. However, contradictory effects of folate deficiency on DNA methylation also have been reported[139,140]. Nevertheless, the ablation of two receptor/carrier-mediated pathways for folate transport in transgenic mice was shown to increase the risk of developing colitis-associated colorectal cancer in a chemically induced IBD model[141]. On the other hand, controversial results based on human or animal studies add some uncertainty about the actual role of folate in preventing cancer[142-144].
The micronutrient selenium has also been implicated in colorectal cancer susceptibility and DNA methylation. Selenium-deficient diets were shown to result in significantly hypomethylated liver and colon DNA in an experimental model[145]. Moreover, selenium-deficient diets contributed to the formation of more carcinogen-induced aberrant colon crypts in rats[138,146]. In experimental IBD, using a model of chemically induced colitis, selenium supplementation prevented tissue damage through the protection of the mitochondria and interfering in the expression of key genes responsible for inflammation[147]. In another model of experimental IBD, selenium deficiency was shown to worsen inflammation and promote tumour development and progression in inflammatory carcinogenesis[148]. In human IBD, consistent studies regarding selenium and its potential impact in disease development are still limited. Recently, however, decreased serum selenium levels have been detected in patients with IBD[149].
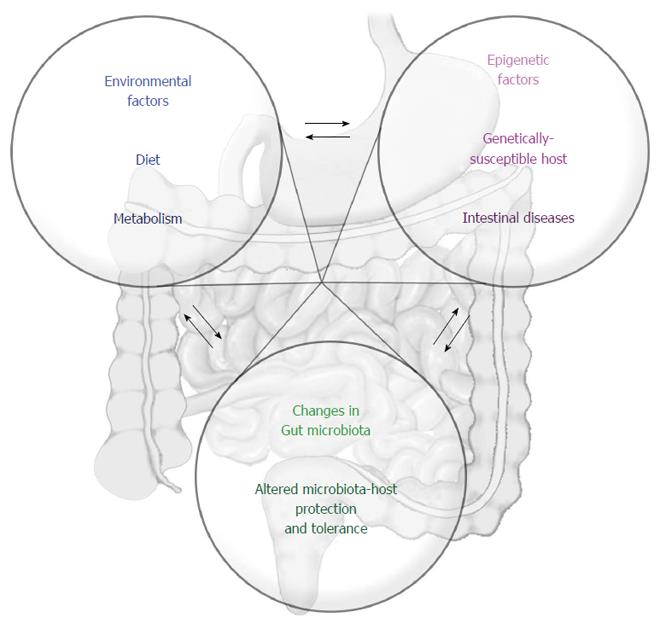
Taken together, the current information available on dietary constituents and the potential effects on the epigenome is not sufficient to establish a clear relationship of cause and effect concerning IBD. Many questions remain unresolved, and it is urgent to address the interactions between the microbiome and epigenome, microbiome and diet, diet and epigenome, and the entire network of simultaneous, overlapping but also dynamic interactions that constitute the basis for intestinal homeostasis (Figure 2).
Currently, consistent evidence to support specific dietary recommendations for patients with IBD is lacking. Nevertheless, it is fundamental to recognize particularities based on the heterogeneity of the patients and their complaints, with the frequent and spontaneous associations of symptoms with dietary habits and specific foods. Although interventional and well-controlled studies of dietary manipulation are still required, it is agreed that the dietary intake should not be excessively restrictive in IBD[150]. However, considering the current knowledge on the direct effects of nutritional elements and the ability of food components to interact with microbial communities, it seems logical to continue pursuing dietary interventions in IBD, especially considering the modulatory potential of diet on the microbiota. On the other hand, a better comprehension of the complex mechanisms that underlie the interaction between the gut and its microbiota may clarify the defective relationships contributing to the development of diseases, such as IBD. Importantly, investigations of the gut-microbiota axis and the intervening modulating factors may unveil new mechanisms and, consequently, novel targets for therapeutic intervention[49]. The knowledge accumulated so far should allow exploration of the therapeutic potential of the intestinal microbiota in the treatment of several immune, metabolic and inflammatory disorders[151].
During the last decade, attempts to modulate the intestinal microbiota through the use of antibiotics, prebiotics, probiotics and synbiotics have represented a rational approach for the treatment of ubiquitous clinical disorders affecting the gastrointestinal tract[152,153]. The use of probiotics, including lactic acid bacteria, such as Lactobacilli and Bifidobacteria, for example, has been extensively studied in recent years. Lactic acid bacteria are commonly present in yogurt and other fermented food products, but they are also commercialized in dietary supplements[154]. Data from the results of clinical trials suggest that probiotics consisting of lactic acid bacteria may be effective in treatment of pouchitis[155] and UC[156] and to a lesser extent in CD[157,158]. In UC, particularly, probiotics containing lactic acid bacteria have generated more promising results, although inconsistencies between studies may render the data difficult to interpret[159]. On the other hand, in CD, only relatively weak evidence exists to support a role for probiotics as effective therapeutic tools[160]. However, a lower rate of recurrence after surgery among CD patients who received early VSL#3 suggests its potential usefulness but also the need for additional studies on this probiotic in CD[161]. Another line of investigation in the field of IBD therapy analyses the potential use of prebiotics, oligosaccharides that are metabolized into SCFAs by commensal bacteria of the intestinal microbiota[162]. Interestingly, a synergistic effect between prebiotics and probiotics for the treatment of CD was proposed in an open-label study, where more effective results were observed when a mix of different lactic acid bacteria was used in combination with the prebiotic psyllium[163]. However, a consequent challenge that arises is how to maintain those lactic acid bacteria probiotics in the gut of patients with IBD, as clinical relapses tend to occur once the probiotic has been discontinued[164].
Recently, in a more audacious approach, another probiotic therapy based on faecal transplantation has been under investigation. Faecal microbiota transplantation (FMT) therapy is a process in which an abnormal, pathological microbiota is replaced by a supposedly normal one[165]. Although this type of intervention may sound like a rather extreme form of therapy, favourable outcomes have already been achieved in patients with recurrent Clostridium difficile infection, for example[166]. In IBD, the results of studies investigating FMT as a potential new alternative therapy are still difficult to interpret, because of distinct study designs and the relatively small number of controlled trials. However, some preliminary information suggests that FMT may be useful in the treatment of IBD, as most patients have exhibited symptomatic relief or even remission in several studies[167]. In a systematic literature search and meta-analysis investigating clinical outcomes, FMT was evaluated as safe, although with variable efficacy in IBD[168]. In a pilot study, high rates of clinical improvement and remission were observed after a single FMT was administered to patients with refractory CD[169]. Using a similar approach, the same group also investigated the efficacy and safety of a designed step-up FMT strategy for steroid-dependent UC. Almost sixty percent of the patients achieved clinical improvement, and the microbiota analysis showed that FMT altered its composition, which became highly similar to that of the donor, particularly in the patients with successful treatment[170]. In a recent randomized controlled trial, FMT was shown to induce remission in a significantly greater percentage of patients with active UC compared to a placebo, with no difference regarding adverse events[171]. Together, these data support the idea that FMT might develop into a promising new alternative for the treatment of IBD.
It is increasingly accepted that dietary constituents can affect the immune response and inflammatory status, in great part mediated through the modulation of the microbiota, as previously discussed in this article. Here, it is worth highlighting the fact that environmental compounds, including nutrients, can modify the genome activity in a manner that, although not changing the DNA sequence, can produce relevant, stable and, possibly, transgenerational alterations in the phenotype[172]. In this sense, alterations to the epigenome interface, which can determine long lasting phenotypic or even tissue structure and function modifications, are believed to be secondary to the nature and potency of the environmental stimuli, including dietary factors, in a dynamic process[173]. Support for the hypothesis of epigenetic programming constituting a permanent and even a transgenerational phenomenon is derived primarily from animal models, including studies involving dietary methyl donors and cofactors such as folic acid, choline and vitamin B12, for example[174,175]. The mechanisms by which environmental stimuli can induce long-term effects and be transmitted across generations are still unclear, and a better understanding of these processes has been regarded as essential for possible future interventions in dramatically increasing diseases such as obesity and diabetes[176], in an approach that hopefully can also be translated to IBD therapy.
In the interim, patients should be advised to pursue a healthier life, including a healthy diet, and avoiding sedentary behaviour, exposure to tobacco, pollutants and drugs in general. In terms of food, specifically, current knowledge suggests that the best approach relies on consuming a well-balanced diet containing predominantly fruits and vegetables and avoiding, as much as possible, processed foods and foods identified by the patient as prejudicial, capable of worsening symptoms or even triggering flares[43]. In this regard, for example, a high intake of red meat and processed meat, protein, alcoholic beverages, sulfur, and sulfate has been associated with an increased risk of flares in UC[177,178]. On the other hand, a high intake of saturated fat, monounsaturated fatty acids, and a higher ratio of omega-6:omega-3 polyunsaturated fatty acids have been associated with CD relapses[179,180].
The increase in and worldwide distribution of autoimmune and complex chronic inflammatory diseases such as IBD, especially in the last half-century, strongly suggest the crucial participation of environmental changes. Among the environmental factors, food and alimentary habits, progressively altered in modern societies, appear to be critical modulators of the microbiota, contributing to or co-participating in dysbiosis, an important component of IBD pathogenesis. In addition, food components have also been shown to modulate epigenetic mechanisms, which can result in increased risk for the development and progression of IBD. Therefore, it seems reasonable to suppose that a better understanding of the role of the different food components in intestinal homeostasis and the resident microbiota will be essential for unravelling the complex molecular basis of the epigenetic, genetic and environment interactions underlying IBD pathogenesis as well as for offering dietary interventions with minimal expected side effects.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Li HD, Sjoberg K, Zhang FM S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 931] [Cited by in F6Publishing: 936] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 2. | Råberg L, Sim D, Read AF. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science. 2007;318:812-814. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 541] [Cited by in F6Publishing: 552] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 3. | Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science. 2012;335:936-941. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1245] [Cited by in F6Publishing: 1112] [Article Influence: 92.7] [Reference Citation Analysis (0)] |
| 4. | Hill DA, Artis D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu Rev Immunol. 2010;28:623-667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 403] [Cited by in F6Publishing: 401] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 5. | Hart AL, Hendy P. The microbiome in inflammatory bowel disease and its modulation as a therapeutic manoeuvre. Proc Nutr Soc. 2014;73:452-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Knights D, Lassen KG, Xavier RJ. Advances in inflammatory bowel disease pathogenesis: linking host genetics and the microbiome. Gut. 2013;62:1505-1510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 323] [Cited by in F6Publishing: 314] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 7. | Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science. 2009;326:1694-1697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2198] [Cited by in F6Publishing: 2081] [Article Influence: 138.7] [Reference Citation Analysis (0)] |
| 8. | Dominguez-Bello MG, Blaser MJ, Ley RE, Knight R. Development of the human gastrointestinal microbiota and insights from high-throughput sequencing. Gastroenterology. 2011;140:1713-1719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 305] [Cited by in F6Publishing: 256] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 9. | Martin R, Nauta AJ, Ben Amor K, Knippels LM, Knol J, Garssen J. Early life: gut microbiota and immune development in infancy. Benef Microbes. 2010;1:367-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 195] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 10. | Zhu B, Wang X, Li L. Human gut microbiome: the second genome of human body. Protein Cell. 2010;1:718-725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 241] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 11. | Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM. Enterotypes of the human gut microbiome. Nature. 2011;473:174-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4310] [Cited by in F6Publishing: 4470] [Article Influence: 343.8] [Reference Citation Analysis (0)] |
| 12. | Nava GM, Stappenbeck TS. Diversity of the autochthonous colonic microbiota. Gut Microbes. 2011;2:99-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 13. | Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, Peterson DA, Stappenbeck TS, Hsieh CS. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 761] [Cited by in F6Publishing: 778] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 14. | Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3154] [Cited by in F6Publishing: 3177] [Article Influence: 211.8] [Reference Citation Analysis (0)] |
| 15. | Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819-826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1864] [Cited by in F6Publishing: 1837] [Article Influence: 108.1] [Reference Citation Analysis (0)] |
| 16. | Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268-1273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3003] [Cited by in F6Publishing: 2739] [Article Influence: 228.3] [Reference Citation Analysis (0)] |
| 17. | Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3529] [Cited by in F6Publishing: 3256] [Article Influence: 217.1] [Reference Citation Analysis (0)] |
| 18. | Gaboriau-Routhiau V, Rakotobe S, Lécuyer E, Mulder I, Lan A, Bridonneau C, Rochet V, Pisi A, De Paepe M, Brandi G. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31:677-689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1045] [Cited by in F6Publishing: 939] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 19. | Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815-827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1157] [Cited by in F6Publishing: 1159] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 20. | Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2011;108 Suppl 1:4615-4622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 881] [Cited by in F6Publishing: 946] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 21. | Geuking MB, Cahenzli J, Lawson MA, Ng DC, Slack E, Hapfelmeier S, McCoy KD, Macpherson AJ. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity. 2011;34:794-806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 599] [Cited by in F6Publishing: 609] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 22. | Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569-573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2937] [Cited by in F6Publishing: 3380] [Article Influence: 307.3] [Reference Citation Analysis (0)] |
| 23. | Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1657] [Cited by in F6Publishing: 1670] [Article Influence: 104.4] [Reference Citation Analysis (0)] |
| 24. | Pacheco RG, Esposito CC, Müller LC, Castelo-Branco MT, Quintella LP, Chagas VL, de Souza HS, Schanaider A. Use of butyrate or glutamine in enema solution reduces inflammation and fibrosis in experimental diversion colitis. World J Gastroenterol. 2012;18:4278-4287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 43] [Cited by in F6Publishing: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2516] [Cited by in F6Publishing: 2923] [Article Influence: 265.7] [Reference Citation Analysis (0)] |
| 26. | Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci USA. 2014;111:2247-2252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1079] [Cited by in F6Publishing: 1258] [Article Influence: 125.8] [Reference Citation Analysis (0)] |
| 27. | Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446-450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2951] [Cited by in F6Publishing: 3272] [Article Influence: 297.5] [Reference Citation Analysis (0)] |
| 28. | Maslowski KM, Mackay CR. Diet, gut microbiota and immune responses. Nat Immunol. 2011;12:5-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 844] [Cited by in F6Publishing: 813] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 29. | Duffy LC, Raiten DJ, Hubbard VS, Starke-Reed P. Progress and challenges in developing metabolic footprints from diet in human gut microbial cometabolism. J Nutr. 2015;145:1123S-1130S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Laitinen K, Collado MC, Isolauri E. Early nutritional environment: focus on health effects of microbiota and probiotics. Benef Microbes. 2010;1:383-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1709] [Cited by in F6Publishing: 1670] [Article Influence: 128.5] [Reference Citation Analysis (0)] |
| 32. | Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258-1270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2236] [Cited by in F6Publishing: 2316] [Article Influence: 193.0] [Reference Citation Analysis (0)] |
| 33. | Ley RE, Hamady M, Lozupone C, Turnbaugh PJ, Ramey RR, Bircher JS, Schlegel ML, Tucker TA, Schrenzel MD, Knight R. Evolution of mammals and their gut microbes. Science. 2008;320:1647-1651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2433] [Cited by in F6Publishing: 2355] [Article Influence: 147.2] [Reference Citation Analysis (0)] |
| 34. | De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. 2010;107:14691-14696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3584] [Cited by in F6Publishing: 3703] [Article Influence: 264.5] [Reference Citation Analysis (0)] |
| 35. | Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4098] [Cited by in F6Publishing: 4113] [Article Influence: 316.4] [Reference Citation Analysis (0)] |
| 36. | David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5625] [Cited by in F6Publishing: 5980] [Article Influence: 543.6] [Reference Citation Analysis (0)] |
| 37. | Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003;62:67-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1150] [Cited by in F6Publishing: 1148] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 38. | Barcenilla A, Pryde SE, Martin JC, Duncan SH, Stewart CS, Henderson C, Flint HJ. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl Environ Microbiol. 2000;66:1654-1661. [PubMed] [Cited in This Article: ] |
| 39. | Hu Y, Le Leu RK, Christophersen CT, Somashekar R, Conlon MA, Meng XQ, Winter JM, Woodman RJ, McKinnon R, Young GP. Manipulation of the gut microbiota using resistant starch is associated with protection against colitis-associated colorectal cancer in rats. Carcinogenesis. 2016;37:366-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 40. | Wang H, Shi P, Zuo L, Dong J, Zhao J, Liu Q, Zhu W. Dietary Non-digestible Polysaccharides Ameliorate Intestinal Epithelial Barrier Dysfunction in IL-10 Knockout Mice. J Crohns Colitis. 2016;10:1076-1086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 41. | Hung TV, Suzuki T. Dietary Fermentable Fiber Reduces Intestinal Barrier Defects and Inflammation in Colitic Mice. J Nutr. 2016;146:1970-1979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 42. | Calder PC. Polyunsaturated fatty acids and inflammation. Biochem Soc Trans. 2005;33:423-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 231] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 43. | Lewis JD, Abreu MT. Diet as a Trigger or Therapy for Inflammatory Bowel Diseases. Gastroenterology. 2017;152:398-414.e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 215] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 44. | Charlebois A, Rosenfeld G, Bressler B. The Impact of Dietary Interventions on the Symptoms of Inflammatory Bowel Disease: A Systematic Review. Crit Rev Food Sci Nutr. 2016;56:1370-1378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 45. | Sigall-Boneh R, Pfeffer-Gik T, Segal I, Zangen T, Boaz M, Levine A. Partial enteral nutrition with a Crohn’s disease exclusion diet is effective for induction of remission in children and young adults with Crohn’s disease. Inflamm Bowel Dis. 2014;20:1353-1360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 164] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 46. | Olendzki BC, Silverstein TD, Persuitte GM, Ma Y, Baldwin KR, Cave D. An anti-inflammatory diet as treatment for inflammatory bowel disease: a case series report. Nutr J. 2014;13:5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 136] [Article Influence: 13.6] [Reference Citation Analysis (1)] |
| 47. | Mayneris-Perxachs J, Bolick DT, Leng J, Medlock GL, Kolling GL, Papin JA, Swann JR, Guerrant RL. Protein- and zinc-deficient diets modulate the murine microbiome and metabolic phenotype. Am J Clin Nutr. 2016;104:1253-1262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 48. | Virtanen SM. Dietary factors in the development of type 1 diabetes. Pediatr Diabetes. 2016;17 Suppl 22:49-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 49. | Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1003] [Cited by in F6Publishing: 862] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 50. | Kranich J, Maslowski KM, Mackay CR. Commensal flora and the regulation of inflammatory and autoimmune responses. Semin Immunol. 2011;23:139-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 51. | Bernstein CN, Shanahan F. Disorders of a modern lifestyle: reconciling the epidemiology of inflammatory bowel diseases. Gut. 2008;57:1185-1191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 199] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 52. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3134] [Cited by in F6Publishing: 3261] [Article Influence: 271.8] [Reference Citation Analysis (1)] |
| 53. | Albenberg LG, Lewis JD, Wu GD. Food and the gut microbiota in inflammatory bowel diseases: a critical connection. Curr Opin Gastroenterol. 2012;28:314-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 54. | Rogler G, Zeitz J, Biedermann L. The Search for Causative Environmental Factors in Inflammatory Bowel Disease. Dig Dis. 2016;34 Suppl 1:48-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 55. | Rook GA. Hygiene hypothesis and autoimmune diseases. Clin Rev Allergy Immunol. 2012;42:5-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 259] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 56. | Saidel-Odes L, Odes S. Hygiene hypothesis in inflammatory bowel disease. Ann Gastroenterol. 2014;27:189-190. [PubMed] [Cited in This Article: ] |
| 57. | García Rodríguez LA, Ruigómez A, Panés J. Acute gastroenteritis is followed by an increased risk of inflammatory bowel disease. Gastroenterology. 2006;130:1588-1594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 237] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 58. | Hviid A, Svanström H, Frisch M. Antibiotic use and inflammatory bowel diseases in childhood. Gut. 2011;60:49-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 348] [Cited by in F6Publishing: 332] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 59. | Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1693] [Cited by in F6Publishing: 1713] [Article Influence: 131.8] [Reference Citation Analysis (1)] |
| 60. | Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3465] [Cited by in F6Publishing: 3322] [Article Influence: 276.8] [Reference Citation Analysis (0)] |
| 61. | Shanahan F. Irritable bowel syndrome: shifting the focus toward the gut microbiota. Gastroenterology. 2007;133:340-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 62. | Swidsinski A, Loening-Baucke V, Verstraelen H, Osowska S, Doerffel Y. Biostructure of fecal microbiota in healthy subjects and patients with chronic idiopathic diarrhea. Gastroenterology. 2008;135:568-579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 63. | Quigley EM. Commensal bacteria: the link between IBS and IBD? Curr Opin Clin Nutr Metab Care. 2011;14:497-503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 64. | Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109-1113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1456] [Cited by in F6Publishing: 1433] [Article Influence: 89.6] [Reference Citation Analysis (0)] |
| 65. | Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3327] [Cited by in F6Publishing: 3627] [Article Influence: 279.0] [Reference Citation Analysis (0)] |
| 66. | Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, Wekerle H, Krishnamoorthy G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 867] [Cited by in F6Publishing: 868] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 67. | Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577-594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1339] [Cited by in F6Publishing: 1319] [Article Influence: 82.4] [Reference Citation Analysis (0)] |
| 68. | Quinton JF, Sendid B, Reumaux D, Duthilleul P, Cortot A, Grandbastien B, Charrier G, Targan SR, Colombel JF, Poulain D. Anti-Saccharomyces cerevisiae mannan antibodies combined with antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease: prevalence and diagnostic role. Gut. 1998;42:788-791. [PubMed] [Cited in This Article: ] |
| 69. | Lodes MJ, Cong Y, Elson CO, Mohamath R, Landers CJ, Targan SR, Fort M, Hershberg RM. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113:1296-1306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 291] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 70. | Dotan I, Fishman S, Dgani Y, Schwartz M, Karban A, Lerner A, Weishauss O, Spector L, Shtevi A, Altstock RT. Antibodies against laminaribioside and chitobioside are novel serologic markers in Crohn’s disease. Gastroenterology. 2006;131:366-378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 190] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 71. | Xiong Y, Wang GZ, Zhou JQ, Xia BQ, Wang XY, Jiang B. Serum antibodies to microbial antigens for Crohn’s disease progression: a meta-analysis. Eur J Gastroenterol Hepatol. 2014;26:733-742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 72. | D’Haens GR, Geboes K, Peeters M, Baert F, Penninckx F, Rutgeerts P. Early lesions of recurrent Crohn’s disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology. 1998;114:262-267. [PubMed] [Cited in This Article: ] |
| 73. | Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, de Silva P, Korzenik JR, Fuchs CS, Willett WC, Richter JM, Chan AT. A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. Gastroenterology. 2013;145:970-977. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 381] [Cited by in F6Publishing: 392] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 74. | Racine A, Carbonnel F, Chan SS, Hart AR, Bueno-de-Mesquita HB, Oldenburg B, van Schaik FD, Tjønneland A, Olsen A, Dahm CC. Dietary Patterns and Risk of Inflammatory Bowel Disease in Europe: Results from the EPIC Study. Inflamm Bowel Dis. 2016;22:345-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 165] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 75. | Tjonneland A, Overvad K, Bergmann MM, Nagel G, Linseisen J, Hallmans G, Palmqvist R, Sjodin H, Hagglund G, Berglund G. Linoleic acid, a dietary n-6 polyunsaturated fatty acid, and the aetiology of ulcerative colitis: a nested case-control study within a European prospective cohort study. Gut. 2009;58:1606-1611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 245] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 76. | Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, de Silva P, Fuchs CS, Willett WC, Richter JM, Chan AT. Long-term intake of dietary fat and risk of ulcerative colitis and Crohn’s disease. Gut. 2014;63:776-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 312] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 77. | Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature. 2012;487:104-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1405] [Cited by in F6Publishing: 1265] [Article Influence: 105.4] [Reference Citation Analysis (0)] |
| 78. | Chassaing B, Darfeuille-Michaud A. The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1720-1728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 326] [Cited by in F6Publishing: 338] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 79. | Hansen R, Russell RK, Reiff C, Louis P, McIntosh F, Berry SH, Mukhopadhya I, Bisset WM, Barclay AR, Bishop J. Microbiota of de-novo pediatric IBD: increased Faecalibacterium prausnitzii and reduced bacterial diversity in Crohn’s but not in ulcerative colitis. Am J Gastroenterol. 2012;107:1913-1922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 206] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 80. | Man SM, Kaakoush NO, Mitchell HM. The role of bacteria and pattern-recognition receptors in Crohn’s disease. Nat Rev Gastroenterol Hepatol. 2011;8:152-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 81. | Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. 2008;105:16731-16736. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2747] [Cited by in F6Publishing: 2881] [Article Influence: 180.1] [Reference Citation Analysis (0)] |
| 82. | Takahashi K, Nishida A, Fujimoto T, Fujii M, Shioya M, Imaeda H, Inatomi O, Bamba S, Sugimoto M, Andoh A. Reduced Abundance of Butyrate-Producing Bacteria Species in the Fecal Microbial Community in Crohn’s Disease. Digestion. 2016;93:59-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 329] [Cited by in F6Publishing: 446] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 83. | Gevers D, Kugathasan S, Denson LA, Vázquez-Baeza Y, Van Treuren W, Ren B, Schwager E, Knights D, Song SJ, Yassour M. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014;15:382-392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1945] [Cited by in F6Publishing: 2054] [Article Influence: 205.4] [Reference Citation Analysis (0)] |
| 84. | Wright EK, Kamm MA, Teo SM, Inouye M, Wagner J, Kirkwood CD. Recent advances in characterizing the gastrointestinal microbiome in Crohn’s disease: a systematic review. Inflamm Bowel Dis. 2015;21:1219-1228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 85. | Andoh A, Imaeda H, Aomatsu T, Inatomi O, Bamba S, Sasaki M, Saito Y, Tsujikawa T, Fujiyama Y. Comparison of the fecal microbiota profiles between ulcerative colitis and Crohn’s disease using terminal restriction fragment length polymorphism analysis. J Gastroenterol. 2011;46:479-486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 86. | Li KY, Wang JL, Wei JP, Gao SY, Zhang YY, Wang LT, Liu G. Fecal microbiota in pouchitis and ulcerative colitis. World J Gastroenterol. 2016;22:8929-8939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 42] [Cited by in F6Publishing: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 87. | Takeshita K, Mizuno S, Mikami Y, Sujino T, Saigusa K, Matsuoka K, Naganuma M, Sato T, Takada T, Tsuji H. A Single Species of Clostridium Subcluster XIVa Decreased in Ulcerative Colitis Patients. Inflamm Bowel Dis. 2016;22:2802-2810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 88. | Rausch P, Rehman A, Künzel S, Häsler R, Ott SJ, Schreiber S, Rosenstiel P, Franke A, Baines JF. Colonic mucosa-associated microbiota is influenced by an interaction of Crohn disease and FUT2 (Secretor) genotype. Proc Natl Acad Sci USA. 2011;108:19030-19035. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 255] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 89. | Rehman A, Sina C, Gavrilova O, Häsler R, Ott S, Baines JF, Schreiber S, Rosenstiel P. Nod2 is essential for temporal development of intestinal microbial communities. Gut. 2011;60:1354-1362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 231] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 90. | Wang J, Linnenbrink M, Künzel S, Fernandes R, Nadeau MJ, Rosenstiel P, Baines JF. Dietary history contributes to enterotype-like clustering and functional metagenomic content in the intestinal microbiome of wild mice. Proc Natl Acad Sci USA. 2014;111:E2703-E2710. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 91. | Rehman A, Rausch P, Wang J, Skieceviciene J, Kiudelis G, Bhagalia K, Amarapurkar D, Kupcinskas L, Schreiber S, Rosenstiel P. Geographical patterns of the standing and active human gut microbiome in health and IBD. Gut. 2016;65:238-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 92. | Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780-13785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3075] [Cited by in F6Publishing: 3195] [Article Influence: 187.9] [Reference Citation Analysis (1)] |
| 93. | Marks DJ, Harbord MW, MacAllister R, Rahman FZ, Young J, Al-Lazikani B, Lees W, Novelli M, Bloom S, Segal AW. Defective acute inflammation in Crohn’s disease: a clinical investigation. Lancet. 2006;367:668-678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 280] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 94. | Smith AM, Rahman FZ, Hayee B, Graham SJ, Marks DJ, Sewell GW, Palmer CD, Wilde J, Foxwell BM, Gloger IS. Disordered macrophage cytokine secretion underlies impaired acute inflammation and bacterial clearance in Crohn’s disease. J Exp Med. 2009;206:1883-1897. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 297] [Cited by in F6Publishing: 325] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 95. | Sewell GW, Marks DJ, Segal AW. The immunopathogenesis of Crohn’s disease: a three-stage model. Curr Opin Immunol. 2009;21:506-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 96. | Cooney R, Baker J, Brain O, Danis B, Pichulik T, Allan P, Ferguson DJ, Campbell BJ, Jewell D, Simmons A. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat Med. 2010;16:90-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 760] [Cited by in F6Publishing: 766] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 97. | Fritz T, Niederreiter L, Adolph T, Blumberg RS, Kaser A. Crohn’s disease: NOD2, autophagy and ER stress converge. Gut. 2011;60:1580-1588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 158] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 98. | Salem M, Ammitzboell M, Nys K, Seidelin JB, Nielsen OH. ATG16L1: A multifunctional susceptibility factor in Crohn disease. Autophagy. 2015;11:585-594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 99. | Kaser A, Martínez-Naves E, Blumberg RS. Endoplasmic reticulum stress: implications for inflammatory bowel disease pathogenesis. Curr Opin Gastroenterol. 2010;26:318-326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 100. | Hosomi S, Kaser A, Blumberg RS. Role of endoplasmic reticulum stress and autophagy as interlinking pathways in the pathogenesis of inflammatory bowel disease. Curr Opin Gastroenterol. 2015;31:81-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 101. | Wehkamp J, Salzman NH, Porter E, Nuding S, Weichenthal M, Petras RE, Shen B, Schaeffeler E, Schwab M, Linzmeier R. Reduced Paneth cell alpha-defensins in ileal Crohn’s disease. Proc Natl Acad Sci USA. 2005;102:18129-18134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 749] [Cited by in F6Publishing: 706] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 102. | Billmann-Born S, Lipinski S, Böck J, Till A, Rosenstiel P, Schreiber S. The complex interplay of NOD-like receptors and the autophagy machinery in the pathophysiology of Crohn disease. Eur J Cell Biol. 2011;90:593-602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 103. | Shaw MH, Kamada N, Warner N, Kim YG, Nuñez G. The ever-expanding function of NOD2: autophagy, viral recognition, and T cell activation. Trends Immunol. 2011;32:73-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 104. | Hampe J, Franke A, Rosenstiel P, Till A, Teuber M, Huse K, Albrecht M, Mayr G, De La Vega FM, Briggs J. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet. 2007;39:207-211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1387] [Cited by in F6Publishing: 1402] [Article Influence: 77.9] [Reference Citation Analysis (0)] |
| 105. | Parkes M, Barrett JC, Prescott NJ, Tremelling M, Anderson CA, Fisher SA, Roberts RG, Nimmo ER, Cummings FR, Soars D. Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn’s disease susceptibility. Nat Genet. 2007;39:830-832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 870] [Cited by in F6Publishing: 867] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 106. | Hoefkens E, Nys K, John JM, Van Steen K, Arijs I, Van der Goten J, Van Assche G, Agostinis P, Rutgeerts P, Vermeire S. Genetic association and functional role of Crohn disease risk alleles involved in microbial sensing, autophagy, and endoplasmic reticulum (ER) stress. Autophagy. 2013;9:2046-2055. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 107. | Elinav E, Strowig T, Henao-Mejia J, Flavell RA. Regulation of the antimicrobial response by NLR proteins. Immunity. 2011;34:665-679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 270] [Cited by in F6Publishing: 242] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 108. | Garrett WS, Gallini CA, Yatsunenko T, Michaud M, DuBois A, Delaney ML, Punit S, Karlsson M, Bry L, Glickman JN. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe. 2010;8:292-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 582] [Cited by in F6Publishing: 592] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 109. | Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. 2010;107:11971-11975. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2922] [Cited by in F6Publishing: 2890] [Article Influence: 206.4] [Reference Citation Analysis (0)] |
| 110. | Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, Angenent LT, Ley RE. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci USA. 2011;108 Suppl 1:4578-4585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1666] [Cited by in F6Publishing: 1619] [Article Influence: 115.6] [Reference Citation Analysis (0)] |
| 111. | Hanson LA. Session 1: Feeding and infant development breast-feeding and immune function. Proc Nutr Soc. 2007;66:384-396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 112. | Barclay AR, Russell RK, Wilson ML, Gilmour WH, Satsangi J, Wilson DC. Systematic review: the role of breastfeeding in the development of pediatric inflammatory bowel disease. J Pediatr. 2009;155:421-426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 113. | Nickerson KP, McDonald C. Crohn’s disease-associated adherent-invasive Escherichia coli adhesion is enhanced by exposure to the ubiquitous dietary polysaccharide maltodextrin. PLoS One. 2012;7:e52132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 114. | Chabra S, Arnold JD, Leslie GI, Bowen JR, Earl J, Wood F. Vitamin A status in preterm neonates with and without chronic lung disease. J Paediatr Child Health. 1994;30:432-435. [PubMed] [Cited in This Article: ] |
| 115. | Sawa S, Cherrier M, Lochner M, Satoh-Takayama N, Fehling HJ, Langa F, Di Santo JP, Eberl G. Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science. 2010;330:665-669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 391] [Cited by in F6Publishing: 412] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 116. | Werner T, Wagner SJ, Martínez I, Walter J, Chang JS, Clavel T, Kisling S, Schuemann K, Haller D. Depletion of luminal iron alters the gut microbiota and prevents Crohn’s disease-like ileitis. Gut. 2011;60:325-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 215] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 117. | Adorini L, Penna G. Dendritic cell tolerogenicity: a key mechanism in immunomodulation by vitamin D receptor agonists. Hum Immunol. 2009;70:345-352. [PubMed] [Cited in This Article: ] |
| 118. | Nerich V, Jantchou P, Boutron-Ruault MC, Monnet E, Weill A, Vanbockstael V, Auleley GR, Balaire C, Dubost P, Rican S. Low exposure to sunlight is a risk factor for Crohn’s disease. Aliment Pharmacol Ther. 2011;33:940-945. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 119. | Jørgensen SP, Hvas CL, Agnholt J, Christensen LA, Heickendorff L, Dahlerup JF. Active Crohn’s disease is associated with low vitamin D levels. J Crohns Colitis. 2013;7:e407-e413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 108] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 120. | Limketkai BN, Bayless TM, Brant SR, Hutfless SM. Lower regional and temporal ultraviolet exposure is associated with increased rates and severity of inflammatory bowel disease hospitalisation. Aliment Pharmacol Ther. 2014;40:508-517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 121. | Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13:484-492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3811] [Cited by in F6Publishing: 3836] [Article Influence: 319.7] [Reference Citation Analysis (0)] |
| 122. | Deaton AM, Bird A. CpG islands and the regulation of transcription. Genes Dev. 2011;25:1010-1022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2107] [Cited by in F6Publishing: 2050] [Article Influence: 157.7] [Reference Citation Analysis (0)] |
| 123. | Benchimol EI, Mack DR, Guttmann A, Nguyen GC, To T, Mojaverian N, Quach P, Manuel DG. Inflammatory bowel disease in immigrants to Canada and their children: a population-based cohort study. Am J Gastroenterol. 2015;110:553-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 151] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 124. | Kellermayer R. Epigenetics and the developmental origins of inflammatory bowel diseases. Can J Gastroenterol. 2012;26:909-915. [PubMed] [Cited in This Article: ] |
| 125. | Koukos G, Polytarchou C, Kaplan JL, Oikonomopoulos A, Ziring D, Hommes DW, Wahed R, Kokkotou E, Pothoulakis C, Winter HS. A microRNA signature in pediatric ulcerative colitis: deregulation of the miR-4284/CXCL5 pathway in the intestinal epithelium. Inflamm Bowel Dis. 2015;21:996-1005. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 126. | Scarpa M, Stylianou E. Epigenetics: Concepts and relevance to IBD pathogenesis. Inflamm Bowel Dis. 2012;18:1982-1996. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 127. | Barnett M, Bermingham E, McNabb W, Bassett S, Armstrong K, Rounce J, Roy N. Investigating micronutrients and epigenetic mechanisms in relation to inflammatory bowel disease. Mutat Res. 2010;690:71-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 128. | Kraiczy J, Nayak K, Ross A, Raine T, Mak TN, Gasparetto M, Cario E, Rakyan V, Heuschkel R, Zilbauer M. Assessing DNA methylation in the developing human intestinal epithelium: potential link to inflammatory bowel disease. Mucosal Immunol. 2016;9:647-658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 129. | Harris RA, Shah R, Hollister EB, Tronstad RR, Hovdenak N, Szigeti R, Versalovic J, Kellermayer R. Colonic Mucosal Epigenome and Microbiome Development in Children and Adolescents. J Immunol Res. 2016;2016:9170162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 130. | Gallou-Kabani C, Vigé A, Gross MS, Junien C. Nutri-epigenomics: lifelong remodelling of our epigenomes by nutritional and metabolic factors and beyond. Clin Chem Lab Med. 2007;45:321-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 131. | Davis CD, Uthus EO. DNA methylation, cancer susceptibility, and nutrient interactions. Exp Biol Med (Maywood). 2004;229:988-995. [PubMed] [Cited in This Article: ] |
| 132. | Garfinkel MD, Ruden DM. Chromatin effects in nutrition, cancer, and obesity. Nutrition. 2004;20:56-62. [PubMed] [Cited in This Article: ] |
| 133. | Al-Awadi FM, Khan I, Dashti HM, Srikumar TS. Colitis-induced changes in the level of trace elements in rat colon and other tissues. Ann Nutr Metab. 1998;42:304-310. [PubMed] [Cited in This Article: ] |
| 134. | Wani NA, Hamid A, Kaur J. Folate status in various pathophysiological conditions. IUBMB Life. 2008;60:834-842. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 135. | McKay JA, Williams EA, Mathers JC. Gender-specific modulation of tumorigenesis by folic acid supply in the Apc mouse during early neonatal life. Br J Nutr. 2008;99:550-558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 136. | Ehrlich M. DNA hypomethylation in cancer cells. Epigenomics. 2009;1:239-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 643] [Cited by in F6Publishing: 687] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 137. | Lu Q, Qiu X, Hu N, Wen H, Su Y, Richardson BC. Epigenetics, disease, and therapeutic interventions. Ageing Res Rev. 2006;5:449-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 138. | Davis CD, Uthus EO. Dietary folate and selenium affect dimethylhydrazine-induced aberrant crypt formation, global DNA methylation and one-carbon metabolism in rats. J Nutr. 2003;133:2907-2914. [PubMed] [Cited in This Article: ] |
| 139. | Choi SW, Friso S, Keyes MK, Mason JB. Folate supplementation increases genomic DNA methylation in the liver of elder rats. Br J Nutr. 2005;93:31-35. [PubMed] [Cited in This Article: ] |
| 140. | Arasaradnam RP, Commane DM, Bradburn D, Mathers JC. A review of dietary factors and its influence on DNA methylation in colorectal carcinogenesis. Epigenetics. 2008;3:193-198. [PubMed] [Cited in This Article: ] |
| 141. | Chapkin RS, Kamen BA, Callaway ES, Davidson LA, George NI, Wang N, Lupton JR, Finnell RH. Use of a novel genetic mouse model to investigate the role of folate in colitis-associated colon cancer. J Nutr Biochem. 2009;20:649-655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 142. | Qin T, Du M, Du H, Shu Y, Wang M, Zhu L. Folic acid supplements and colorectal cancer risk: meta-analysis of randomized controlled trials. Sci Rep. 2015;5:12044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 143. | Tio M, Andrici J, Cox MR, Eslick GD. Folate intake and the risk of upper gastrointestinal cancers: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2014;29:250-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 144. | MacFarlane AJ, Behan NA, Matias FM, Green J, Caldwell D, Brooks SP. Dietary folate does not significantly affect the intestinal microbiome, inflammation or tumorigenesis in azoxymethane-dextran sodium sulphate-treated mice. Br J Nutr. 2013;109:630-638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 145. | Davis CD, Uthus EO, Finley JW. Dietary selenium and arsenic affect DNA methylation in vitro in Caco-2 cells and in vivo in rat liver and colon. J Nutr. 2000;130:2903-2909. [PubMed] [Cited in This Article: ] |
| 146. | Davis CD, Uthus EO. Dietary selenite and azadeoxycytidine treatments affect dimethylhydrazine-induced aberrant crypt formation in rat colon and DNA methylation in HT-29 cells. J Nutr. 2002;132:292-297. [PubMed] [Cited in This Article: ] |
| 147. | Tirosh O, Levy E, Reifen R. High selenium diet protects against TNBS-induced acute inflammation, mitochondrial dysfunction, and secondary necrosis in rat colon. Nutrition. 2007;23:878-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 148. | Barrett CW, Singh K, Motley AK, Lintel MK, Matafonova E, Bradley AM, Ning W, Poindexter SV, Parang B, Reddy VK. Dietary selenium deficiency exacerbates DSS-induced epithelial injury and AOM/DSS-induced tumorigenesis. PLoS One. 2013;8:e67845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 149. | Castro Aguilar-Tablada T, Navarro-Alarcón M, Quesada Granados J, Samaniego Sánchez C, Rufián-Henares JÁ, Nogueras-Lopez F. Ulcerative Colitis and Crohn’s Disease Are Associated with Decreased Serum Selenium Concentrations and Increased Cardiovascular Risk. Nutrients. 2016;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 150. | Richman E, Rhodes JM. Review article: evidence-based dietary advice for patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38:1156-1171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 151. | O’Hara AM, Shanahan F. Gut microbiota: mining for therapeutic potential. Clin Gastroenterol Hepatol. 2007;5:274-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 152. | Sartor RB, Muehlbauer M. Microbial host interactions in IBD: implications for pathogenesis and therapy. Curr Gastroenterol Rep. 2007;9:497-507. [PubMed] [Cited in This Article: ] |
| 153. | Macfarlane GT, Blackett KL, Nakayama T, Steed H, Macfarlane S. The gut microbiota in inflammatory bowel disease. Curr Pharm Des. 2009;15:1528-1536. [PubMed] [Cited in This Article: ] |
| 154. | Mach T. Clinical usefulness of probiotics in inflammatory bowel diseases. J Physiol Pharmacol. 2006;57 Suppl 9:23-33. [PubMed] [Cited in This Article: ] |
| 155. | Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC, Nicholls RJ, Gionchetti P, Campieri M, Kamm MA. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004;53:108-114. [PubMed] [Cited in This Article: ] |
| 156. | Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620-1633. [PubMed] [Cited in This Article: ] |
| 157. | Ewaschuk JB, Dieleman LA. Probiotics and prebiotics in chronic inflammatory bowel diseases. World J Gastroenterol. 2006;12:5941-5950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 103] [Cited by in F6Publishing: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 158. | Mack DR. Probiotics in inflammatory bowel diseases and associated conditions. Nutrients. 2011;3:245-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 159. | Sokol H. Probiotics and antibiotics in IBD. Dig Dis. 2014;32 Suppl 1:10-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 160. | Guslandi M. Role of Probiotics in Crohn’s Disease and in Pouchitis. J Clin Gastroenterol. 2015;49 Suppl 1:S46-S49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 161. | Fedorak RN, Feagan BG, Hotte N, Leddin D, Dieleman LA, Petrunia DM, Enns R, Bitton A, Chiba N, Paré P. The probiotic VSL#3 has anti-inflammatory effects and could reduce endoscopic recurrence after surgery for Crohn’s disease. Clin Gastroenterol Hepatol. 2015;13:928-935.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 135] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 162. | Scaldaferri F, Gerardi V, Lopetuso LR, Del Zompo F, Mangiola F, Boškoski I, Bruno G, Petito V, Laterza L, Cammarota G. Gut microbial flora, prebiotics, and probiotics in IBD: their current usage and utility. Biomed Res Int. 2013;2013:435268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 163. | Fujimori S, Tatsuguchi A, Gudis K, Kishida T, Mitsui K, Ehara A, Kobayashi T, Sekita Y, Seo T, Sakamoto C. High dose probiotic and prebiotic cotherapy for remission induction of active Crohn’s disease. J Gastroenterol Hepatol. 2007;22:1199-1204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 164. | Chandler M, Wollins E, Toles A, Borum M, Doman DB. The emerging therapeutic role of probiotics in inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2008;4:634-640. [PubMed] [Cited in This Article: ] |
| 165. | Khoruts A, Sadowsky MJ. Therapeutic transplantation of the distal gut microbiota. Mucosal Immunol. 2011;4:4-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 166. | Bakken JS, Borody T, Brandt LJ, Brill JV, Demarco DC, Franzos MA, Kelly C, Khoruts A, Louie T, Martinelli LP. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011;9:1044-1049. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 650] [Cited by in F6Publishing: 641] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 167. | Anderson JL, Edney RJ, Whelan K. Systematic review: faecal microbiota transplantation in the management of inflammatory bowel disease. Aliment Pharmacol Ther. 2012;36:503-516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 226] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 168. | Colman RJ, Rubin DT. Fecal microbiota transplantation as therapy for inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. 2014;8:1569-1581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 346] [Cited by in F6Publishing: 319] [Article Influence: 31.9] [Reference Citation Analysis (1)] |
| 169. | Cui B, Feng Q, Wang H, Wang M, Peng Z, Li P, Huang G, Liu Z, Wu P, Fan Z. Fecal microbiota transplantation through mid-gut for refractory Crohn’s disease: safety, feasibility, and efficacy trial results. J Gastroenterol Hepatol. 2015;30:51-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 206] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 170. | Cui B, Li P, Xu L, Zhao Y, Wang H, Peng Z, Xu H, Xiang J, He Z, Zhang T. Step-up fecal microbiota transplantation strategy: a pilot study for steroid-dependent ulcerative colitis. J Transl Med. 2015;13:298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 171. | Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, Armstrong D, Marshall JK, Kassam Z, Reinisch W. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology. 2015;149:102-109.e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 930] [Cited by in F6Publishing: 942] [Article Influence: 104.7] [Reference Citation Analysis (0)] |
| 172. | Lee HS. Impact of Maternal Diet on the Epigenome during In Utero Life and the Developmental Programming of Diseases in Childhood and Adulthood. Nutrients. 2015;7:9492-9507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 137] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 173. | Attig L, Gabory A, Junien C. Nutritional developmental epigenomics: immediate and long-lasting effects. Proc Nutr Soc. 2010;69:221-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 174. | MacLennan NK, James SJ, Melnyk S, Piroozi A, Jernigan S, Hsu JL, Janke SM, Pham TD, Lane RH. Uteroplacental insufficiency alters DNA methylation, one-carbon metabolism, and histone acetylation in IUGR rats. Physiol Genomics. 2004;18:43-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 151] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 175. | Vanhees K, Vonhögen IG, van Schooten FJ, Godschalk RW. You are what you eat, and so are your children: the impact of micronutrients on the epigenetic programming of offspring. Cell Mol Life Sci. 2014;71:271-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 176. | Vickers MH. Early life nutrition, epigenetics and programming of later life disease. Nutrients. 2014;6:2165-2178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in F6Publishing: 218] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 177. | Jowett SL, Seal CJ, Pearce MS, Phillips E, Gregory W, Barton JR, Welfare MR. Influence of dietary factors on the clinical course of ulcerative colitis: a prospective cohort study. Gut. 2004;53:1479-1484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 307] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 178. | Magee EA, Edmond LM, Tasker SM, Kong SC, Curno R, Cummings JH. Associations between diet and disease activity in ulcerative colitis patients using a novel method of data analysis. Nutr J. 2005;4:7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 179. | Guerreiro CS, Ferreira P, Tavares L, Santos PM, Neves M, Brito M, Cravo M. Fatty acids, IL6, and TNFalpha polymorphisms: an example of nutrigenetics in Crohn’s disease. Am J Gastroenterol. 2009;104:2241-2249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 180. | Ferreira P, Cravo M, Guerreiro CS, Tavares L, Santos PM, Brito M. Fat intake interacts with polymorphisms of Caspase9, FasLigand and PPARgamma apoptotic genes in modulating Crohn’s disease activity. Clin Nutr. 2010;29:819-823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 181. | Takada T, Kurakawa T, Tsuji H, Nomoto K. Fusicatenibacter saccharivorans gen. nov., sp. nov., isolated from human faeces. Int J Syst Evol Microbiol. 2013;63:3691-3696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 182. | Miquel S, Martín R, Rossi O, Bermúdez-Humarán LG, Chatel JM, Sokol H, Thomas M, Wells JM, Langella P. Faecalibacterium prausnitzii and human intestinal health. Curr Opin Microbiol. 2013;16:255-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 608] [Cited by in F6Publishing: 633] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 183. | Park SK, Kim MS, Bae JW. Blautia faecis sp. nov., isolated from human faeces. Int J Syst Evol Microbiol. 2013;63:599-603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 184. | Swidsinski A, Loening-Baucke V, Herber A. Mucosal flora in Crohn’s disease and ulcerative colitis - an overview. J Physiol Pharmacol. 2009;60 Suppl 6:61-71. [PubMed] [Cited in This Article: ] |
| 185. | Auchtung TA, Holder ME, Gesell JR, Ajami NJ, Duarte RT, Itoh K, Caspi RR, Petrosino JF, Horai R, Zárate-Bladés CR. Complete Genome Sequence of Turicibacter sp. Strain H121, Isolated from the Feces of a Contaminated Germ-Free Mouse. Genome Announc. 2016;4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 186. | Huys G, Cnockaert M, Janda JM, Swings J. Escherichia albertii sp. nov., a diarrhoeagenic species isolated from stool specimens of Bangladeshi children. Int J Syst Evol Microbiol. 2003;53:807-810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 187. | Ishikawa H, Akedo I, Umesaki Y, Tanaka R, Imaoka A, Otani T. Randomized controlled trial of the effect of bifidobacteria-fermented milk on ulcerative colitis. J Am Coll Nutr. 2003;22:56-63. [PubMed] [Cited in This Article: ] |