Published online Oct 28, 2016. doi: 10.3748/wjg.v22.i40.8956
Peer-review started: June 17, 2016
First decision: July 12, 2016
Revised: July 29, 2016
Accepted: August 23, 2016
Article in press: August 23, 2016
Published online: October 28, 2016
To study the clinicopathological characteristics of neuroendocrine neoplasms (NEN) on liver samples and apply World Health Organization (WHO) 2010 grading of gastroenteropancreatic (GEP) NEN.
Clinicopathological features of 79 cases of NEN of the liver diagnosed between January 2011 to December 2015 were analyzed. WHO 2010 classification of GEP NEN was applied and the tumors were graded as G1, G2 or G3. Two more categories, D1/2 (discordant 1/2) and D2/3 (discordant 2/3) were also applied. The D1/2 grade tumors had a mitotic count of G1 and Ki-67 index of G2. The D2/3 tumors had a mitotic count of G2 and Ki-67 index of G3. The follow up details which were available till the end of the study period (December 2015) were collected.
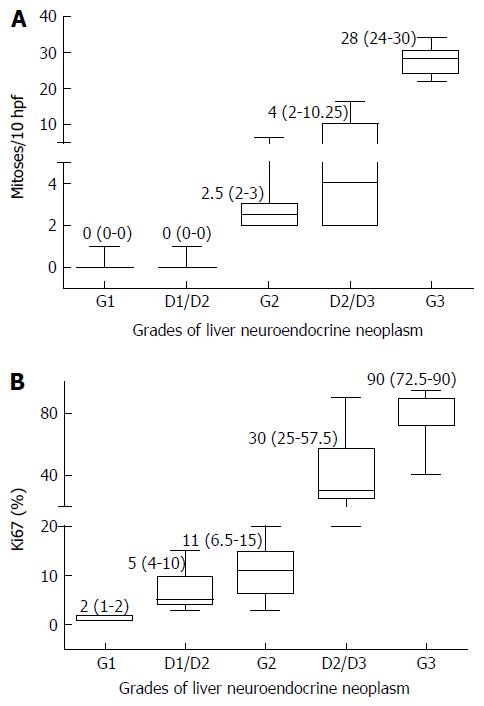
Of the 79 tumors, 16 each were G1 and G2, and 18 were G3 tumors. Of the remaining 29 tumors, 13 were assigned to D1/2 and 16 were D2/3 grade. Male preponderance was noted in all tumors except for G2 neoplasms, which showed a slight female predilection. The median age at presentation was 47 years (range 10-82 years). The most common presentation was abdominal pain (81%). Pancreas (49%) was the most common site of primary followed by gastrointestinal tract (24.4%) and lungs (18%). Radiologically, 87% of the patients had multiple liver lesions. Histopathologically, necrosis was seen in only D2/3 and G3 tumors. Microvascular invasion was seen in all grades. Metastasis occurred in all grades of primary NEN and the grades of the metastatic tumors and their corresponding primary tumors were similar in 67% of the cases. Of the 79 patients, 36 had at least one follow up visit with a median duration of follow up of 8.5 mo (range: 1-50 mo). This study did not show any impact of the grade of tumor on the short term clinical outcome of these patients.
Liver biopsy is an important tool for clinicopathological characterization and grading of NEN, especially when the primary is not identified. Eighty-seven percent of the patients had multifocal liver lesions irrespective of the WHO grade, indicating a higher stage of disease at presentation. Follow up duration was inadequate to derive any meaningful conclusion on long term outcome in our study patients.
Core tip: Neuroendocrine neoplasms (NEN) in liver are commonly metastatic. The clinicopathological features of NEN diagnosed on liver samples were analyzed and graded applying World Health Organization (WHO) 2010 classification of gastroenteropancreatic NEN. A marked male preponderance was noted in all WHO grades except for G2 tumors, wherein a slight female predilection was seen. Necrosis was noted only in higher grade tumors. Most patients had multifocal liver lesions favoring metastasis and higher stage of disease at presentation. Follow up duration was inadequate to derive any meaningful conclusion on long term outcome in our study patients.
- Citation: Burad DK, Kodiatte TA, Rajeeb SM, Goel A, Eapen CE, Ramakrishna B. Neuroendocrine neoplasms of liver - A 5-year retrospective clinico-pathological study applying World Health Organization 2010 classification. World J Gastroenterol 2016; 22(40): 8956-8966
- URL: https://www.wjgnet.com/1007-9327/full/v22/i40/8956.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i40.8956
Neuroendocrine neoplasms (NEN) are tumors arising from the neuroendocrine cells which are distributed throughout the body[1,2]. They commonly originate from gastrointestinal tract, lungs and pancreas and rarely from other sites such as gall bladder, thymus, testes and ovaries[3-6]. NEN are the second most common tumors to metastasize to liver after adenocarcinomas[7,8]. NEN in the liver are usually metastatic and primary tumors are rare[9-13]. Liver is the second most common site of metastatic NEN following lymph nodes[5]. Amongst the metastatic NEN in liver, those originating from gastroenteropancreatic (GEP) region are more common due to spread via the portal vein[14].
The GEP NEN have been classified by the World Health Organization (WHO) 2010[15] into 3 (G1-G3) grades based on mitotic activity and Ki-67/MIB-1 proliferation index. These are G1: mitotic count < 2/10 high power fields (hpf) and/or Ki-67 index ≤ 2%, G2: mitotic count 2-20/10 hpf and/or Ki-67 index 3%-20% and G3: mitotic count > 20/10 hpf and/or Ki-67 index > 20%. If the mitotic count or Ki-67 proliferation index points to different grades, a higher grade has to be given[15,16]. Some studies have shown discordance between mitotic count and Ki-67 index[17,18] in some cases. They have shown that the grade discordant tumors with mitotic count of G1 and Ki-67 index of G2 behave worse than grade concordant tumors[17,18].
Targeted biopsy of the liver lesion is often required to confirm the diagnosis of neuroendocrine neoplasm, as often the primary may not be identified.
Utility of WHO 2010 grading of NEN in liver biopsies has not been carried out and hence, we undertook this study to pathologically characterize these cases and to correlate with clinical findings.
This study included all cases of NEN involving the liver diagnosed in the Department of Pathology, from January 2011 to December 2015. The data was collected from Pathology online data base. A total of 82 cases were available. Of the 82 cases, 3 had only cytology smears without cell block material and hence were excluded from the study. The remaining 79 cases included liver biopsies (59), resection specimens (5), cytology smears with cell block (5) and referred slides and blocks (10). Of the 59 liver biopsies, 58 were ultrasound (US) guided and, 1 was a per-operative wedge biopsy sample. Of the 5 resection specimens, 3 were localized segmentectomy specimens and the remaining 2 were left lateral segmentectomies. All the 5 cytology cases were US guided. Of the 10 cases of referred slides and blocks, 6 were US guided biopsies, 2 were per-operative wedge biopsies and the remaining 2 were resections.
One resection (left lateral segmentectomy) case included in this study was from a 40 year old lady diagnosed with neuroendocrine neoplasm by biopsy in 2009, who received 6 cycles of chemotherapy and metaiodobenzylguanidine (MIBG) ablation prior to resection.
The histopathological features and immunohistochemistry details in 79 cases were analyzed. Based on WHO 2010 classification of GEP NEN, all cases were graded as G1, G2 or G3. In this study, we assigned 2 more categories which included D1/2 (discordant 1/2) and D2/3 (discordant 2/3). The D1/2 grade tumors had a mitotic count of G1 and Ki-67 index of G2. The D2/3 tumor had a mitotic count of G2 and Ki-67 index of G3. We did not have any cases wherein the mitotic count was of a higher grade and Ki-67 index of a lower grade.
The relevant clinical and radiological findings and the follow up details which were available till the end of the study period (December 2015) were collected.
Continuous data was described as number, mean, median, minimum and maximum; and categorical data was described as number with percentage. Categorical data was compared with chi-square test. A two sided P value of < 0.05 was considered statistically significant. All statistical analyses were done using SPSS software version 17.0.
This study was approved by Institutional Review Board.
Of the 79 patients, 71 were of Indian origin, 7 from Bangladesh and 1 from Srilanka. Overall, there was a male preponderance (male:female = 53:26) in this study. G2 tumors showed a slight female predilection (M:F = 7:9) as compared to the other grades (M:F = 46:17).
The median age at presentation was 47 years (range 10-82 years). Fifty-six percent of the cases were seen between 5th to 6th decade.
The study patients had a delay of 3 mo (0-24 mo; median, range) from their first symptoms to their final diagnosis at hospital. The most common presentation was abdominal pain (81%) followed by loss of weight and appetite (41%), altered bowel habits (14%) and mass per abdomen (9%). In 9% of the patients, the tumor was incidentally detected.
A clinical diagnosis of malignancy was given in 77% of the patients.
In 18 patients, the primary site was identified by biopsy and in another 27 patients, a probable primary lesion was identified on radiological examination alone. In both the groups, the most common primary site was pancreas (Table 1). In the remaining 34 patients, a definite primary site could not be identified.
| Site of primary | Biopsy proven (n = 18) | Lesion-on radiology (n = 27) | Total |
| Pancreas | 6 | 16 | 22 (49.0) |
| Lung | 4 | 4 | 8 (18.0) |
| Duodenum | 1 | 3 | 4 (8.8) |
| Rectum | 2 | 1 | 3 (6.6) |
| Ileum | 2 | - | 2 (4.4) |
| Esophagus | 1 | - | 1 (2.2) |
| Cecum | - | 1 | 1 (2.2) |
| Gall bladder | 1 | 1 | 2 (4.4) |
| Anterior mediastinum | - | 1 | 1 (2.2) |
| Kidney | 1 | - | 1 (2.2) |
In this study we encountered three interesting cases. The first case was a 46 year old male presented with 2 years duration of pain in right hip and lower limbs, acromegaly and erectile dysfunction in 2009. Based on radiological, serological and pathological examination, he was diagnosed with multiple endocrine neoplasia type 1 with pituitary macroadenoma, insulinoma, gastrinoma, bilateral inferior parathyroid adenomas, primary hyperparathyroidism, thymic carcinoid and adrenal adenoma. In 2012, computed tomography (CT) of the abdomen revealed a 2.5-cm lesion in segment 3 of liver along with multiple pancreatic lesions. The patient underwent a distal pancreatectomy with splenectomy and resection of segment 3 of liver in 2014, which on pathological examination confirmed neuroendocrine neoplasm of WHO grade 2, in both pancreas and in liver. The tumor was multifocal in pancreas.
The second case was a 22 year old male presented with left flank pain of 2 mo duration in 2013. CT scan showed a 10-cm lobulated mass in the left kidney and a diagnosis of renal cell carcinoma was suspected. Patient underwent left nephrectomy and histologically, diagnosed as large cell neuroendocrine carcinoma. On follow-up, 2 years later, patient was detected to have multiple hypodense lesions in the liver, in segments 6, 4a, and 2, and the largest measured 1.7 cm. An US guided biopsy confirmed metastatic neuroendocrine neoplasm, WHO grade 2.
The third case was a 40 year old female who presented with jaundice, loss of weight and appetite, lower limb weakness and abdominal distension of 2 years duration. She was recently detected to have hypertension. Blood investigations revealed hypokalemia, hypomagnesaemia and elevated serum adrenocorticotropic hormone (ACTH) and cortisol levels. In view of the above findings, she was diagnosed with ACTH dependent Cushing syndrome. CT scan revealed multiple liver lesions in both lobes, largest measuring 2.8 cm. An US guided biopsy of liver lesion was diagnosed as neuroendocrine neoplasm, WHO grade 2.
Radiological findings were available in all 79 cases. In 69 cases (87%), the patients had multiple liver lesions (1.8 cm to 15.9 cm in maximum dimension) involving both the left and the right lobes. Remaining 10 patients (13%) had a single lesion (2.7 cm to 12.5 cm in maximum dimension), 4 of which were in the left lobe and 6 in the right lobe. A radiological diagnosis of metastases was made in 66 (83.5%) cases, hepatocellular carcinoma in 5 (6.3%), hemangioma in 1 (1.3%) and non-neoplastic etiology in 7 (8.9%), which includes 4 cases diagnosed as abscess.
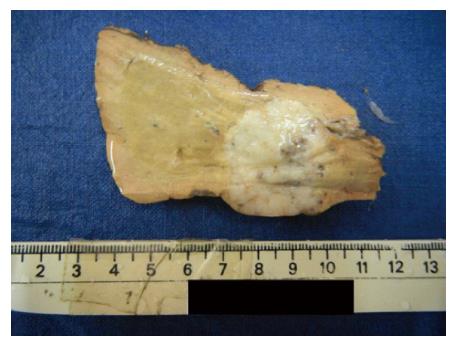
Of the seven liver resections in our study, five were available for gross examination and the remaining two were slides and blocks. Of the five, 3 were segmental resection specimens of segment 3, 6 and 8 respectively and the remaining 2 were left lateral segmentectomies. All resection specimens except one left lateral segmentectomy specimen had a single tumor nodule, ranging in size from 2.5 cm to 7.5 cm with a firm grey white to yellow cut surface (Figure 1). Focal area of hemorrhage was seen in one case. There was no evidence of necrosis or gross vascular invasion. The surrounding liver parenchyma was normal. One left lateral segmentectomy specimen following chemotherapy showed 2 tumor nodules, measuring 6.5 cm and 0.7 cm respectively. The cut surface of the tumor was firm grey white with focal areas of necrosis amounting to approximately 20% of the total tumor volume. There was no gross vascular invasion. The surrounding liver parenchyma was normal.
Based on WHO 2010 grading of the 79 tumors, 16 each were G1 and G2 and 18 were G3 tumors. Of the remaining 29 tumors, 13 were assigned to D1/2 grade and 16 were assigned D2/3 grade.
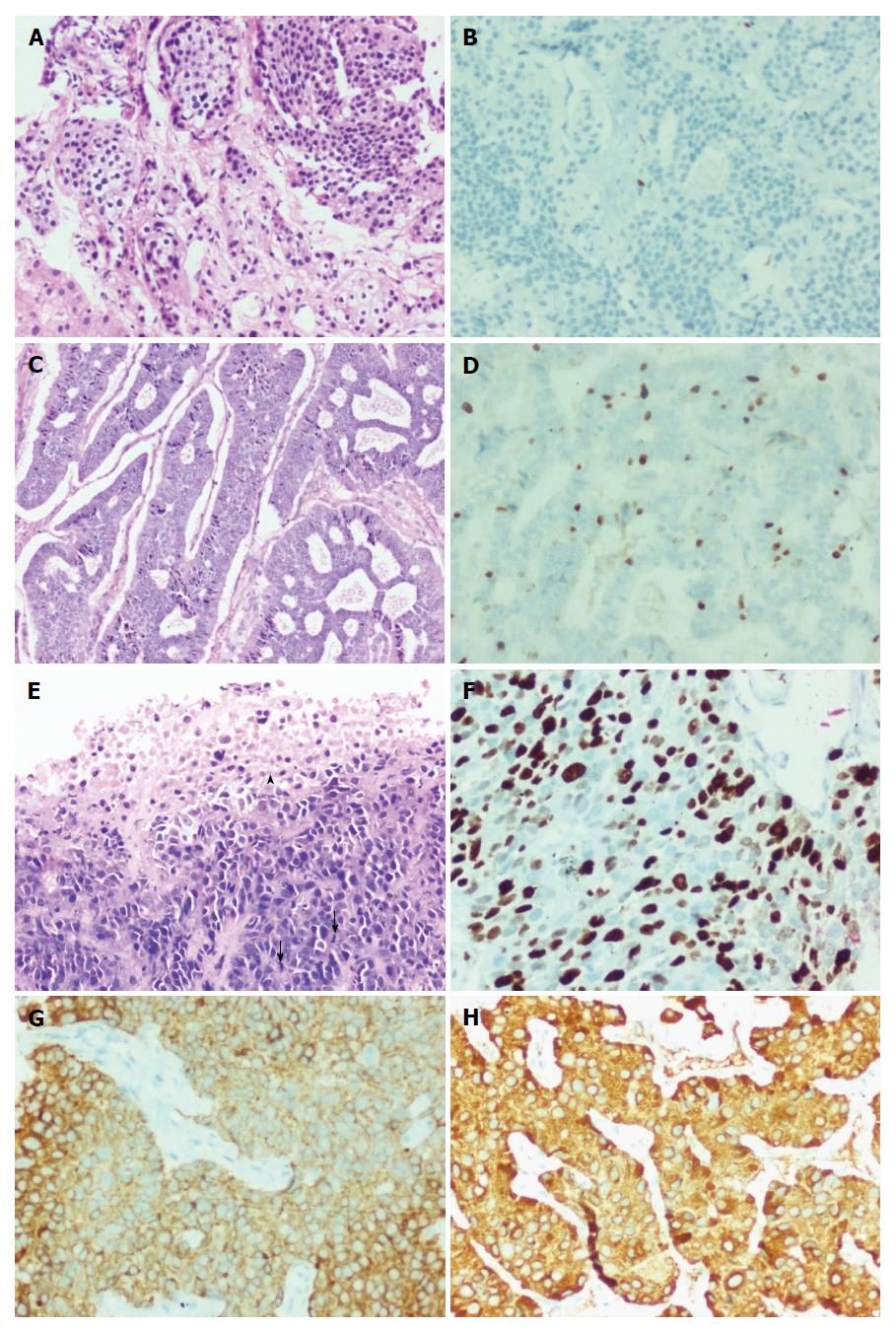
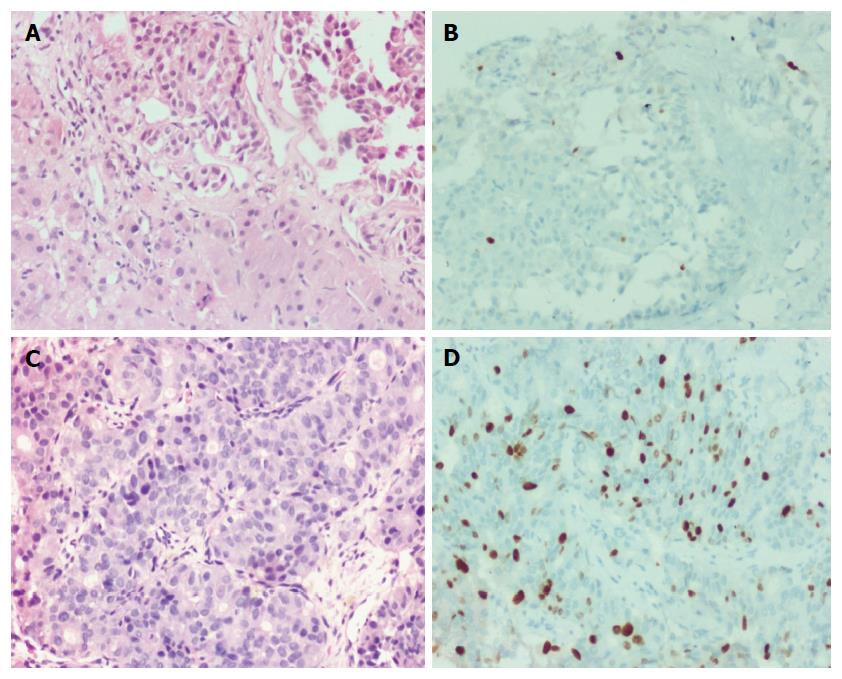
Histologically, the low grade tumors (G1, G2, D1/2) had a classical pattern of arrangement including nests, trabeculae, cords, ribbons, festoons, sheets, gyriform, pseudopapillary and acinar patterns (Figure 2A and C). The cells were round to polygonal with moderate to abundant amounts of eosinophilic granular cytoplasm and uniform to mildly pleomorphic nuclei with uniformly dispersed coarse chromatin and inconspicuous mitotic activity (Figure 3A).
The high grade tumors (G3, D2/3) showed nests and sheets of medium sized polygonal cells with mild to moderately pleomorphic nuclei with finely dispersed chromatin and scant to moderate amounts of eosinophilic cytoplasm. There was increased mitotic and apoptotic activity (Figures 2E and 3C).
Six cases had morphology consistent with small cell carcinoma with sheets and nests of polygonal cells displaying moderate nuclear pleomorphism, moulding, overlapping and increased mitotic and apoptotic activity.
The post chemotherapy and MIBG ablation resection specimen showed presence of occasional peritumoral non necrotizing epithelioid cell granulomas, probably reactive. Special stains for acid fast bacilli and fungal organisms were negative. None of the other 78 cases showed granulomas.
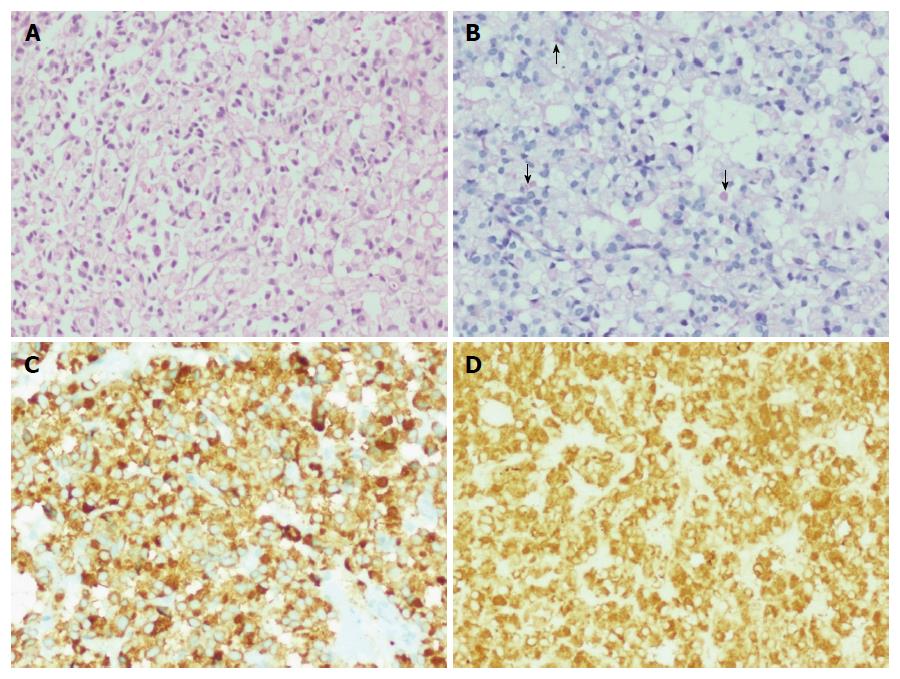
There was one interesting case of a 63 year old female with multiple liver nodules who underwent an US guided liver biopsy. Histologically, the tumor was composed of sheets and closely packed clusters of polygonal cells with eccentrically placed mild to moderately pleomorphic nuclei and abundant amounts of pale eosinophilic cytoplasm, resembling signet ring cells (Figure 4A). Occasional cells contained intracytoplasmic mucin droplets (Figure 4B). This case was diagnosed as signet ring cell neuroendocrine neoplasm.
Necrosis was identified in 12 cases and all were biopsies. Six of these were D2/3 grade and the remaining 6 were G3 grade. In 9 cases, it was present in small foci, while the remaining 3 showed extensive areas of necrosis (1 was G3 and 2 were D2/3). There was no necrosis in any of the resection cases except in the post chemotherapy case which showed necrosis and hyalinization, amounting to 20% of the entire tumor volume. However, there was no necrosis in the initial pre chemotherapy biopsy.
Microvascular invasion (MVI) was seen in 17 cases (4 resection cases and 13 biopsies). Of these 17 cases, 3 were G1, 4 G2, 6 G3 and 4 D2/3 grade tumors. In all the cases, tumor emboli were present in thin walled vascular channels and/or within sinusoids.
MVI was seen more frequently in high grade (G3 and D2/3) tumors (59%) when compared to low grade (G1 and G2) tumors (41%). However, it was not statistically significant. As most of these cases were biopsies, this increased frequency is most probably a chance finding.
We also looked at the presence of MVI in the 18 proven primary cases (12 resection, 4 biopsies and 2 cytology with cell block material). MVI was seen in 10 of 12 resection cases and none of the biopsied cases.
Status of nodal disease was also noted. Of the 79 patients, 7 had biopsy proven nodal metastasis and 35 had significant nodes on radiological examination. The remaining 37 patients did not have any significant lymph node enlargement.
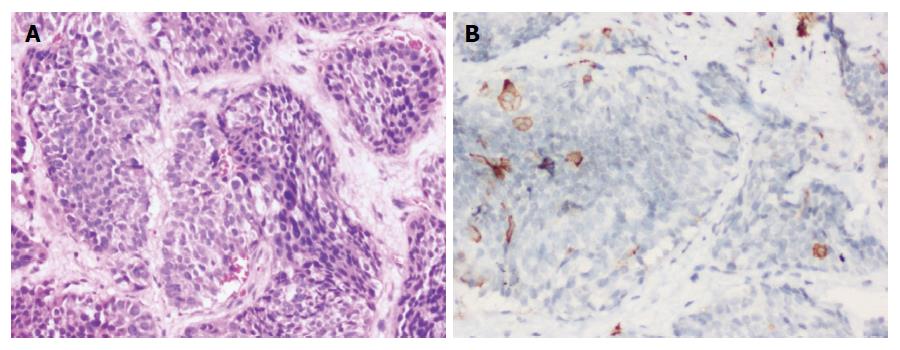
Immunostaining for two markers - synaptophysin and chromogranin were carried out in all 79 cases. Synaptophysin (Figure 2G) was positive in 77 and chromogranin (Figure 2H) in 75 cases, respectively.
In all 6 cases which were negative for either synaptophysin or chromogranin, CD 56 was found to be positive.
Pancytokeratin immunostaining done in 34 cases with high grade (G3:18, D2/3:16) neoplasms, showed positive staining in all except two cases with G3 tumor.
Immunostaining for thyroid transcription factor (TTF-1) was carried out in 9 cases. Six of these had morphology of small cell carcinoma and the remaining three had a lung lesion on radiology. Five out of 6 cases of small cell carcinoma type were TTF1 positive and 2 of these cases had a lung mass indicating a lung primary. One of the 3 cases with radiologically detected lesion in the lung was TTF1 positive.
The patient with signet ring cell NEN showed the tumor cells to be positive for CK7, synaptophysin (Figure 4C) and chromogranin (Figure 4D) and negative for CK20 and CDX2. Ki-67 proliferation index was 15%.
The patient with Cushing’s syndrome showed neuroendocrine neoplasm with patchy cytoplasmic positivity within tumor cells for ACTH on immuno-histochemistry (Figure 5A and B).
Ki-67 proliferation index in various grades of tumors are shown in Figures 2B, 2D, 2F, 3B and 3D. The medians of mitotic and Ki-67 proliferation indices are shown as box plots (Figure 6A and B). We did not have any cases, wherein mitotic count was of higher grade and Ki-67 index was of lower grade.
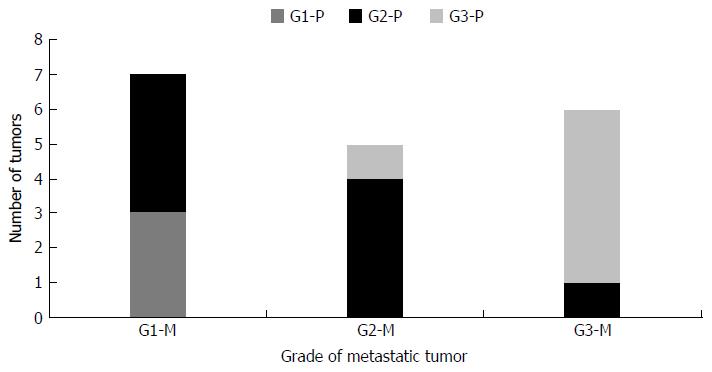
We also compared the WHO grading of tumors metastatic to liver with their biopsy proven 18 primary tumors (Figure 7). The grades of the metastatic tumors and the corresponding primary tumors were similar in 12 /18 cases (67%), lower in 5/18 (28%) and higher in 1/18 (5%) cases.
We noticed that metastasis can occur in NEN irrespective of whether the tumor is a low grade or high grade.
Comparison of G1 tumors (16 cases) with discordant D1/2 tumors (13 cases) revealed slightly lower median age at presentation in (47 years vs 52 years) and presence of MVI (19% vs 0%) in the former. However, there was no difference in sex distribution between the two groups.
Comparison of G2 tumors (16 cases) with discordant D1/2 tumors (13 cases) also revealed slightly lower median age at presentation (47 years vs 52 years), presence of MVI (25% vs 0%) and female predilection (56% vs 23%) in the former. Necrosis was not seen in any case in both grades.
Comparison of discordant D2/3 tumors (16 cases) with G2 tumors (16 cases) showed a slightly higher median age of presentation (51 years vs 47 years), male predilection (75% vs 44%) and presence of necrosis (25% vs 0%) in the former. MVI was seen in 25% of cases in both grades.
There was no statistically significant difference in any of these features between various groups.
Comparison of concordant G3 tumors (18 cases) with discordant D2/3 tumors showed no difference in age of presentation, sex distribution or presence of necrosis or MVI.
Forty (51%) of the 79 study patients were offered specific mode(s) of therapy - 9 (11.4%) underwent surgical resection which were either only liver resection (3 cases), both liver and primary tumor resection (2 cases; one with right hemicolectomy and the other with distal pancreatectomy) or only primary tumor resection (4 cases; 2 distal pancreatectomies, 1 lung resection and 1 subtotal gastrectomy). Thirty-three (43%) had chemotherapy and 7 (9%) had therapeutic 131l-MIBG therapy. 3 patients received a combination of chemotherapy and MIBG therapy, 4 patients underwent surgery in combination with chemotherapy and 1 patient received all three. Only 12 patients (Grade 1:4, Grade 2:4, Grade D2/D3:2, Grade 3:2) were deemed as cured after the initial therapy. There was no association of grade of disease and curative intent/achievement.
Of the 79 study patients, 36 patients had at least one follow up visit after diagnosis of neuroendocrine neoplasm. The median duration of follow up was 8.5 mo (range: 1-50 mo). During the follow up, five (Grade 3:2, Grade 2:2, Grade D1/D2:1) of the initial 12 cured patients suffered recurrence (median duration: 32 mo, range: 13-33 mo). Two patients (Grade 1:1, Grade D1/D2:1) showed continuous downhill course. Rest 29 followed patients (Grade 1:6, Grade D1/D2:5, Grade 2:5, Grade D2/D3:9, Grade 3:4) remained stable till the last follow up. One patient died (Grade D2/D3) within 10 d after the diagnosis.
To the best of our knowledge, this is the largest study describing detailed histological findings and relevant clinical data of 79 patients with hepatic NEN, metastatic in most cases.
Metastases from NEN are much more common than primary NEN in liver[9-13]. In a study of 393 digestive neuroendocrine tumors, only 5% had a primary neuroendocrine neoplasm in liver[19]. In a study of 13715 neuroendocrine tumors, liver was the second most common site of metastasis after lymph nodes[5].
In our study, pancreas was the most common site of primary tumor metastasizing to liver accounting for 49% of the cases followed by GIT (24.4%) and lungs (18%). Other studies have also reported that pancreas to be the most common primary site followed by rectum, stomach and ileum amongst the GEP NEN metastasizing to liver[20-22]. Begum et al[23] have shown that in their study of 2009 cases of GEP NEN, pancreas was the most common site accounting for 34.2% of cases followed by midgut (5.8%), gastric (6.5%), colon (6.9%) and duodenum (4.8%). Dromain et al[24] have shown that small bowel as the common primary site in their study (43%) followed by pancreas (25%), lung (15%), colon (5%), thymus (2%) and unknown primary (10%).
In our study 69 (87%) patients had multiple liver lesions involving both lobes of liver, which is similar to the study done by Niederle et al[25] where multiple liver lesions were seen in 92% of their cases.
It has been shown that the primary hepatic NEN to be predominantly a single lesion when compared to metastatic NEN[26]. In our study, a single lesion was identified in 10/79 cases (13%), of which a biopsy proven primary was confirmed in 7 cases and probable primary was identified by radiology in 2 and in one case, no primary was detected.
In our study, males were twice more commonly affected than females. Male predilection has been shown in both metastatic and primary neuroendocrine tumors by Shen et al[26] and Shin et al[22] in their studies. However, some studies have reported almost similar incidence in males and females[2,5,17,20,27-29]. The median age at diagnosis was 47 years in this study which was slightly lower compared to other studies, which have reported from 55-62 years[2,5,20,23,26].
The most common presentation was abdominal pain (81%). A similar finding was reported by Chan et al[20] in their study on 126 GEP NEN.
Three interesting cases were encountered in this study. One case was a primary renal neuroendocrine carcinoma which is very rare[30,31] and the patient developed liver metastasis 2 years after nephrectomy.
Another case was signet ring cell neuroendocrine neoplasm which is also very rare and is characterized by the presence of cytoplasmic vacuoles that are negative for mucin stain and positive for cytokeratin[32-34].
The third case was a 40 year old female who was diagnosed with ACTH dependent Cushing syndrome with a concomitant neuroendocrine neoplasm, diagnosed on liver biopsy. Ectopic secretion of ACTH by NEN of liver is extremely rare and has been described[6,35].
In our study, necrosis was identified only in high grade tumors, either G3 or D2/3. However, McCall et al[18] have shown in their study of 297 G1 and G2 pancreatic NEN, necrosis in both grades, but the frequency was higher in G2 as compared to G1.
In our study, among the 18 pathology proven primary cases, liver metastases occurred irrespective of the grade of the tumor. In contrast, liver metastases were seen more often in grade 2 or 3 tumors in a study of gastrointestinal neuroendocrine tumors[36].
In our study, the grades of the primary and secondary tumors were similar in 67% of the cases and there was no difference in grade and metastatic potential. This was in contrast to the study by Shi et al[37], in which 65% of the patients with grade 1 primary tumor developed a higher grade (G2 or 3) liver metastasis. This could be explained partly by the fact that the Ki-67 index was determined on multiple resected tumors in a single patient in their study and the highest WHO grade was taken, indicating the importance of intertumoral heterogeneity. Our study involves mainly samples from a single lesion (91% cases) and hence intertumoral heterogeneity could not be assessed. Intratumoral and intertumoral heterogeneity in Ki-67 index has also been described in a study of metastatic NEN of liver by Yang et al[38].
Of the 79 study patients, 36 patients had at least one follow up visit after diagnosis of neuroendocrine neoplasm with a median duration of follow up of 8.5 mo (range: 1-50 mo). However, this duration was not adequate to derive any meaningful conclusion on long term outcomes in our study patients. Shen et al[26] have shown in their study on liver NEN, a better survival in low grade tumors as compared to high grade tumors. This could be due to the fact that this study included only liver resection cases but our study was done predominantly on biopsy samples and also due to lesser number of patients available for follow-up. Also, 87% of our patients had multifocal liver lesions irrespective of the WHO grade, indicating a higher stage of disease at presentation itself.
The limitations of the study were the following; lack of follow-up data on all patients included in this study and though most of the patients had multiple liver lesions, biopsy or cytology sample was obtained from only one lesion. Intratumoral and intertumoral heterogeneity in Ki-67 index also could not be determined, which has been described in few studies[37,38].
In conclusion, liver biopsy is an important tool for clinicopathological characterization and grading of NEN, especially when the primary is not identified. Most of the cases had multifocal disease indicating metastasis. However, a possibility of a primary hepatic neuroendocrine tumor should be kept in mind, especially when it is a single lesion and a definite primary is not identified after extensive workup.
Neuroendocrine neoplasms (NEN) in liver are commonly metastatic. In many cases, a primary cannot be identified and in some cases where primary is identified, the site may not be accessible for biopsy. In such scenarios, a liver biopsy is of great help to characterize these tumors.
The authors have studied the clinicopathological features of NEN in liver and graded them according to the World Health Organization (WHO) 2010 grading of gastroenteropancreatic NEN.
The grades of the metastatic tumors and the corresponding primary tumors were similar in 67% of the cases. Few interesting and rare cases were also identified.
Liver biopsy is an important tool for clinicopathological characterization and grading of NEN, especially when the primary is not identified.
NEN are tumors arising from the neuroendocrine cells which are distributed throughout the body. The WHO 2010 grades these tumors in to grade 1, 2 and 3 based on mitotic activity and Ki-67 proliferation index.
The authors provide a comprehensive study of NEN of liver that includes 79 patients, some interesting cases among them. Neuroendocrine neoplasm in the liver is not a common disease. According to the authors, this is the largest study so far describing detailed histological findings and relevant clinical data of patients with hepatic NEN. The authors had drawn some valuable conclusions for this disease.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Corrales FJ, Peck-Radosavljevic M, Wu SL S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF
| 1. | Langley K. The neuroendocrine concept today. Ann N Y Acad Sci. 1994;733:1-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 62] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-3072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3022] [Cited by in F6Publishing: 3036] [Article Influence: 189.8] [Reference Citation Analysis (0)] |
| 3. | Soga J. Primary endocrinomas (carcinoids and variant neoplasms) of the gallbladder. A statistical evaluation of 138 reported cases. J Exp Clin Cancer Res. 2003;22:5-15. [PubMed] [Cited in This Article: ] |
| 4. | Soga J. Carcinoids and their variant endocrinomas. An analysis of 11842 reported cases. J Exp Clin Cancer Res. 2003;22:517-530. [PubMed] [Cited in This Article: ] |
| 5. | Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934-959. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1848] [Cited by in F6Publishing: 1766] [Article Influence: 84.1] [Reference Citation Analysis (0)] |
| 6. | Watson RG, Johnston CF, O’Hare MM, Anderson JR, Wilson BG, Collins JS, Sloan JM, Buchanan KD. The frequency of gastrointestinal endocrine tumours in a well-defined population--Northern Ireland 1970-1985. Q J Med. 1989;72:647-657. [PubMed] [Cited in This Article: ] |
| 7. | Kasper HU, Drebber U, Dries V, Dienes HP. [Liver metastases: incidence and histogenesis]. Z Gastroenterol. 2005;43:1149-1157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Khadim MT, Jamal S, Ali Z, Akhtar F, Atique M, Sarfraz T, Ayaz B. Diagnostic challenges and role of immunohistochemistry in metastatic liver disease. Asian Pac J Cancer Prev. 2011;12:373-376. [PubMed] [Cited in This Article: ] |
| 9. | Iwao M, Nakamuta M, Enjoji M, Kubo H, Fukutomi T, Tanabe Y, Nishi H, Taguchi KI, Kotoh K, Nawata H. Primary hepatic carcinoid tumor: case report and review of 53 cases. Med Sci Monit. 2001;7:746-750. [PubMed] [Cited in This Article: ] |
| 10. | Nikfarjam M, Muralidharan V, Christophi C. Primary hepatic carcinoid tumours. HPB (Oxford). 2004;6:13-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Fenoglio LM, Severini S, Ferrigno D, Gollè G, Serraino C, Bracco C, Castagna E, Brignone C, Pomero F, Migliore E. Primary hepatic carcinoid: a case report and literature review. World J Gastroenterol. 2009;15:2418-2422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 48] [Cited by in F6Publishing: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Shetty PK, Baliga SV, Balaiah K, Gnana PS. Primary hepatic neuroendocrine tumor: an unusual cystic presentation. Indian J Pathol Microbiol. 2010;53:760-762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Gravante G, De Liguori Carino N, Overton J, Manzia TM, Orlando G. Primary carcinoids of the liver: a review of symptoms, diagnosis and treatments. Dig Surg. 2008;25:364-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Goodman ZD, Terracciano LM, Wee A. Tumours and tumour-like lesions of the liver. 6th ed. In: Burt A, Portmann B, Ferrell L, editors. MacSween's pathology of the liver. Edinburg: Churchill Livingstone, Elsevier, 2012: 761-851. . [Cited in This Article: ] |
| 15. | Klimstra DS, Arnold R, Capella C, Hruban RH, Klöppel G, Komminoth P, Solcia E, Rindi G. Neuroendocrine neoplasms of the pancreas. 4th ed. In: Bosman F, Carneiro, F, Hruban RH, Theise N, editors. WHO Classification of tumours of the digestive system. Lyon: IARC Press, 2010: 322-326. . [Cited in This Article: ] |
| 16. | Yang Z, Tang LH, Klimstra DS. Gastroenteropancreatic neuroendocrine neoplasms: historical context and current issues. Semin Diagn Pathol. 2013;30:186-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Khan MS, Luong TV, Watkins J, Toumpanakis C, Caplin ME, Meyer T. A comparison of Ki-67 and mitotic count as prognostic markers for metastatic pancreatic and midgut neuroendocrine neoplasms. Br J Cancer. 2013;108:1838-1845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 18. | McCall CM, Shi C, Cornish TC, Klimstra DS, Tang LH, Basturk O, Mun LJ, Ellison TA, Wolfgang CL, Choti MA. Grading of well-differentiated pancreatic neuroendocrine tumors is improved by the inclusion of both Ki67 proliferative index and mitotic rate. Am J Surg Pathol. 2013;37:1671-1677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 19. | Maire F, Couvelard A, Vullierme MP, Kianmanesh R, O’Toole D, Hammel P, Belghiti J, Ruszniewski P. Primary endocrine tumours of the liver. Br J Surg. 2005;92:1255-1260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Chan DT, Luk AO, So WY, Kong AP, Chow FC, Ma RC, Lo AW. Natural history and outcome in Chinese patients with gastroenteropancreatic neuroendocrine tumours: - a 17-year retrospective analysis. BMC Endocr Disord. 2016;16:12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Hentic O, Couvelard A, Rebours V, Zappa M, Dokmak S, Hammel P, Maire F, O’Toole D, Lévy P, Sauvanet A. Ki-67 index, tumor differentiation, and extent of liver involvement are independent prognostic factors in patients with liver metastases of digestive endocrine carcinomas. Endocr Relat Cancer. 2011;18:51-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Shin Y, Ha SY, Hyeon J, Lee B, Lee J, Jang KT, Kim KM, Park YS, Park CK. Gastroenteropancreatic Neuroendocrine Tumors with Liver Metastases in Korea: A Clinicopathological Analysis of 72 Cases in a Single Institute. Cancer Res Treat. 2015;47:738-746. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Begum N, Maasberg S, Plöckinger U, Anlauf M, Rinke A, Pöpperl G, Lehnert H, Izbicki JR, Krausch M, Vashist YK. [Neuroendocrine tumours of the GI tract--data from the German NET Registry]. Zentralbl Chir. 2014;139:276-283. [PubMed] [Cited in This Article: ] |
| 24. | Dromain C, de Baere T, Lumbroso J, Caillet H, Laplanche A, Boige V, Ducreux M, Duvillard P, Elias D, Schlumberger M. Detection of liver metastases from endocrine tumors: a prospective comparison of somatostatin receptor scintigraphy, computed tomography, and magnetic resonance imaging. J Clin Oncol. 2005;23:70-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 276] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 25. | Niederle MB, Hackl M, Kaserer K, Niederle B. Gastroenteropancreatic neuroendocrine tumours: the current incidence and staging based on the WHO and European Neuroendocrine Tumour Society classification: an analysis based on prospectively collected parameters. Endocr Relat Cancer. 2010;17:909-918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 298] [Cited by in F6Publishing: 296] [Article Influence: 21.1] [Reference Citation Analysis (1)] |
| 26. | Shen YH, Chen S, Zhang WT, Ji Y, Yu L, Sun HC, Qiu SJ, Ren N, Zhou J. Clinical analysis of gastroenteropancreatic neuroendocrine tumor with liver metastasis, compared with primary hepatic neuroendocrine tumor. J Cancer Res Ther. 2014;10 Suppl:276-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Vagefi PA, Razo O, Deshpande V, McGrath DJ, Lauwers GY, Thayer SP, Warshaw AL, Fernández-Del Castillo C. Evolving patterns in the detection and outcomes of pancreatic neuroendocrine neoplasms: the Massachusetts General Hospital experience from 1977 to 2005. Arch Surg. 2007;142:347-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 165] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 28. | Ahmed A, Turner G, King B, Jones L, Culliford D, McCance D, Ardill J, Johnston BT, Poston G, Rees M. Midgut neuroendocrine tumours with liver metastases: results of the UKINETS study. Endocr Relat Cancer. 2009;16:885-894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 208] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 29. | Panzuto F, Nasoni S, Falconi M, Corleto VD, Capurso G, Cassetta S, Di Fonzo M, Tornatore V, Milione M, Angeletti S. Prognostic factors and survival in endocrine tumor patients: comparison between gastrointestinal and pancreatic localization. Endocr Relat Cancer. 2005;12:1083-1092. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 323] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 30. | Hansel DE, Epstein JI, Berbescu E, Fine SW, Young RH, Cheville JC. Renal carcinoid tumor: a clinicopathologic study of 21 cases. Am J Surg Pathol. 2007;31:1539-1544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Dvorackova J, Macak J, Brzula P, Tomanova R, Dokulil J. Primary neuroendocrine carcinoma of the kidney. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2013;157:257-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Sioutos N, Virta S, Kessimian N. Primary hepatic carcinoid tumor. An electron microscopic and immunohistochemical study. Am J Clin Pathol. 1991;95:172-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 44] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Oh YH, Kang GH, Kim OJ. Primary hepatic carcinoid tumor with a paranuclear clear zone: a case report. J Korean Med Sci. 1998;13:317-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Zhu H, Sun K, Ward SC, Schwartz M, Thung SN, Qin L. Primary hepatic signet ring cell neuroendocrine tumor: a case report with literature review. Semin Liver Dis. 2010;30:422-428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | El Zein M, Vali R, Charron M, Manson D, Perlman K, Shammas A. Neuroendocrine tumor in liver with positive ACTH receptor: a case report. J Pediatr Hematol Oncol. 2014;36:e1-e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Geramizadeh B, Kashkooe A, Malekhosseini SA. Liver Metastasis of Gastrointestinal Neuroendocrine Tumors: A Single Center Experience. Hepat Mon. 2016;16:e37293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Shi C, Gonzalez RS, Zhao Z, Koyama T, Cornish TC, Hande KR, Walker R, Sandler M, Berlin J, Liu EH. Liver metastases of small intestine neuroendocrine tumors: Ki-67 heterogeneity and World Health Organization grade discordance with primary tumors. Am J Clin Pathol. 2015;143:398-404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 38. | Yang Z, Tang LH, Klimstra DS. Effect of tumor heterogeneity on the assessment of Ki67 labeling index in well-differentiated neuroendocrine tumors metastatic to the liver: implications for prognostic stratification. Am J Surg Pathol. 2011;35:853-860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 250] [Article Influence: 19.2] [Reference Citation Analysis (0)] |