Published online Sep 14, 2016. doi: 10.3748/wjg.v22.i34.7660
Peer-review started: June 4, 2016
First decision: July 12, 2016
Revised: July 25, 2016
Accepted: August 10, 2016
Article in press: August 10, 2016
Published online: September 14, 2016
After the first description of fatty pancreas in 1933, the effects of pancreatic steatosis have been poorly investigated, compared with that of the liver. However, the interest of research is increasing. Fat accumulation, associated with obesity and the metabolic syndrome (MetS), has been defined as “fatty infiltration” or “nonalcoholic fatty pancreas disease” (NAFPD). The term “fatty replacement” describes a distinct phenomenon characterized by death of acinar cells and replacement by adipose tissue. Risk factors for developing NAFPD include obesity, increasing age, male sex, hypertension, dyslipidemia, alcohol and hyperferritinemia. Increasing evidence support the role of pancreatic fat in the development of type 2 diabetes mellitus, MetS, atherosclerosis, severe acute pancreatitis and even pancreatic cancer. Evidence exists that fatty pancreas could be used as the initial indicator of “ectopic fat deposition”, which is a key element of nonalcoholic fatty liver disease and/or MetS. Moreover, in patients with fatty pancreas, pancreaticoduodenectomy is associated with an increased risk of intraoperative blood loss and post-operative pancreatic fistula.
Core tip: Nonalcoholic fatty pancreas disease is a very common yet neglected pathological condition. It can be considered an early marker of the metabolic syndrome and, as so, its clinical significance spaces between internal and surgical diseases, such as type 2 diabetes mellitus, atherosclerosis, acute pancreatitis and even pancreatic cancer. This review collects current knowledge of worldwide opinion leaders and researchers of this matter.
- Citation: Catanzaro R, Cuffari B, Italia A, Marotta F. Exploring the metabolic syndrome: Nonalcoholic fatty pancreas disease. World J Gastroenterol 2016; 22(34): 7660-7675
- URL: https://www.wjgnet.com/1007-9327/full/v22/i34/7660.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i34.7660
The spreading of obesity is one of the most concerning problems of modern medicine. According to the World Health Organization (WHO), worldwide obesity has nearly doubled since 1980, and in 2008 more than 10% of the world’s adult population was obese[1]. About 3.4 million adults die every year because of overweight or obesity, which are more deaths than underweight.
According to the International Diabetes Federation, the association of abdominal (central) obesity with hypertension, elevated fasting plasma glucose, high serum triglycerides, and low high density lipoproteins (HDL) define the metabolic syndrome (MetS), also known as syndrome X, cardiometabolic syndrome or insulin resistance syndrome. This condition is associated with a pro-inflammatory, pro-thrombotic state, and leads to an increased risk of developing cardiovascular disease and type 2 diabetes mellitus (T2DM)[2,3].
In the last years, more research is focusing on understanding obesity, MetS and the diseases associated.
Obesity, especially when associated with a higher waist circumference, causes ectopic fat deposition in certain organs, such as the liver (nonalcoholic fatty liver disease - NAFLD), heart, muscles, kidney and pancreas[4]. This is called steatosis[5].
As the liver is a key organ in the metabolism, its fatty infiltration has been the most investigated. Large evidence supports the hypothesis of NAFLD as both cause and consequence of the MetS[6].
Although the pancreas is also an important organ in the metabolism, the effects of fatty infiltration of this organ has been less investigated than that of the liver.
The following research was performed on MEDLINE/PubMed: “pancreatic steatosis” or “pancreatic lipomatosis” or “NAFPD” or “fatty pancreas” or “pancreatic fat” or “pancreatic fatty replacement” or “pancreatic fatty infiltration”. A total of 210 results were found and abstracts were examined. Thirty-four papers were excluded because the main topic was not pancreatic fat. While reviewing, further references were added.
The first description of pancreatic fat was made by Ogilvie in 1933[7]. He compared 19 pancreas derived from obese patients with 19 controls. Obese cadavers showed a greater mean pancreatic adiposity (17.1%, range 0%-48.5%) than the controls (9.3% range 2.5%-23.6%).
After more than 40 years, in 1978, Olsen[8] performed a larger study over 394 autopsies. The cadavers were divided into three groups: below normal weight, normal weight and above normal weight. He found a relationship between the content of fat and age, and confirmed the relation with obesity.
Across the years, many synonymous have been used to refer to “pancreatic fat accumulation”, with many different meanings. Those terms have been very well reviewed in 2011, and are summarized in Table 1. According to the authors, the limit of this nomenclature is the lack of distinction between the accumulation of triglycerides in acinar cells, β-cells or intrapancreatic adipocyte tissue[9,10].
| Name | Definition |
| Pancreatic steatosis | General term for pancreatic fat accumulation |
| Pancreatic lipomatosis | |
| Fatty pancreas | |
| Lipomatous pseudohypertrophy | Extreme variant of pancreatic fat accumulation when the pancreas is enlarged uniformly or focally, the exocrine system is replaced by fat, and when no association can be found with obesity[10] |
| Fatty replacement | Death of acinar cells with subsequent replacement with adipocytes |
| Fatty infiltration | Infiltration of adipocytes owing to obesity |
| NAFPD | Pancreatic fat accumulation in association with obesity and metabolic syndrome |
| NASP | Pancreatitis owing to pancreatic fat accumulation |
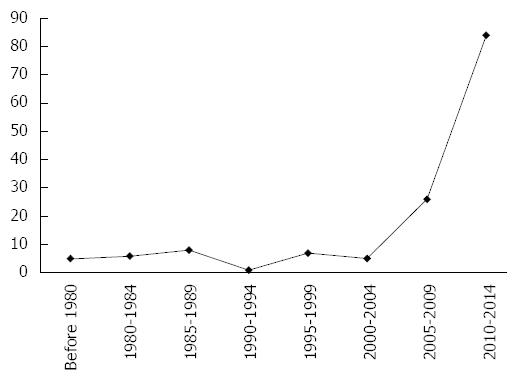
Nowadays, with the development of more sophisticated imaging techniques and data suggesting the clinical importance of obesity and the MetS, the interest of researchers is increasing, as shown in Figure 1.
Mild or massive pancreatic steatosis can be assessed by simple inspection of the organ. That can be useful in the surgical setting, as further explained[11].
Histological examination is the most common way to assess pancreatic fatty infiltration in animal models[12-28]. Human specimen can be obtained from autopsies, operatory remnants or, rarely, fine needle aspiration cytological (FNAC) examination[7,8,16,29-40].
Fat accumulation may be even or uneven[41-43]. Four different types of uneven pancreatic lipomatosis have been described: (1) Type 1a (35% of cases): replacement of the head with sparing of the uncinate process and peribiliary region; (2) Type 1b (35%): replacement of the head, neck, and body, with sparing of the uncinate process and peribiliary region; (3) Type 2a (12%): replacement of the head, including the uncinate process, and sparing of the peribiliary region; and (4) Type 2b (18%): total replacement of the pancreas with sparing of the peribiliary region[41,44].
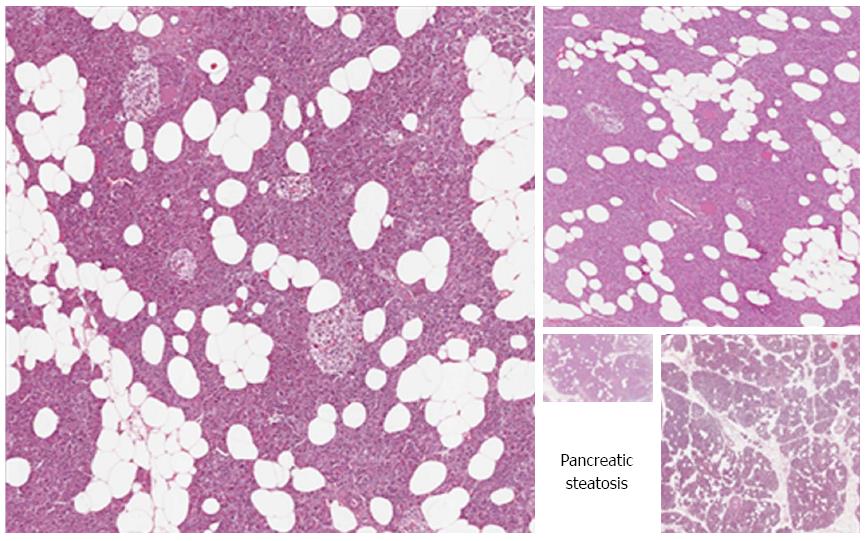
Unlike the liver, where the triglycerides accumulation is mainly intracellular, pancreatic steatosis is histologically characterized by an increased number of adipocytes (Figures 2 and 3)[5,16]. However, intracellular fat accumulation can be visualized by electronic microscopy or immunohistochemistry in both acinar and islet cells and may precede adipocytes infiltration[12,13,16,18,21,23,45-48]. It is unknown if intracellular or extracellular triglycerides have a different clinical significance, but it is possible that adipocytes influence the function of acinar and/or islet cells by a paracrine effect, while intracellular lipids may lead to lipotoxicity and therefore islet or acinar cells injury, as further discussed[9].
Thus now, there is not a shared score to grade the severity of fatty infiltration on histological examination, so each group have used arbitrary subjective parameters, or computer-based morphometric analysis, which gives an objective quantification of pancreatic fat[8,30,31,33,34,38,48-50].
To our knowledge, the only scoring system that has been validated on patients by a rastering method is the pancreatic lipomatosis score (PLS), developed by van Geenen et al[9,33].
PLS modifies the classification made by Olsen in 1978[8], based on the percentage of adipocytes per microscopic field: (1) Group 1: ≥ 51%; (2) Group 2: ≥ 26%; (3) Group 3: ≥ 15%; and (4) Group 4: ≥ 8%.
The group numbers were shifted and a group for patients with less than 8% fat was added. Furthermore, intralobular fat, interlobular fat and total fat were scored separately. A last group was added for pancreases with > 75% of total fat.
The majority of radiological techniques available have been used to study pancreatic steatosis. So far, there is not cut-off points validated on patients, nor valid comparative trials that are able to assess which technique is the most accurate.
Ultrasonography: Both transabdominal ultrasound and endoscopic ultrasound (EUS) can be used to observe the pancreas[42,51-66]. A fatty pancreas looks hyperechogenic (hyperechogenic pancreas - HP) compared to the liver or, if the liver is also hyperechogenic, with the spleen or the kidney. Since the kidney and the pancreas cannot often be seen in the same window, one could use an indirect comparison between the kidney with the liver, and then the liver with the pancreas[57,58].
By EUS, the echogenicity of the pancreas can also be compared with the one of retroperitoneal fat[65]. In addition, EUS may also be associated with FNAC for cytological analysis[36].
Some authors have even graded the severity of pancreatic fat infiltration basing on echogenicity only, or on a grading system based on the aspect of the pancreatic duct and the presence of parenchymal “salt and pepper” dots[56,59].
Transabdominal ultrasound is cheap, fast and non-invasive, but the pancreas can’t always be visualized, especially in obese patients. Another limitation of both transabdominal and endoscopic ultrasonography is that they are operator dependent.
More important, HP may not be a certain indicator of pancreatic fat infiltration, as a fibrotic pancreas is also hyperechogenic[60]. Therefore, it has been suggested that ultrasonography (US) shouldn’t be used as a screening tool for pancreatic fat content, and that computed tomography (CT) or magnetic resonance imaging (MRI) should be the second step to confirm the diagnosis[9,60].
CT: CT imaging is widely used to study all abdominal organs. A fatty pancreas will be hypodense in hounsfield units (HU) compared to the spleen[39,67-69]. In severe fatty replacement, the attenuation can be compared with the adjacent retroperitoneal fat[42]. When the condition is severe, differentiation between lipomatosis and pancreatic agenesis can be made by evaluating the ductal system, which will be present in fatty replacement and absent in agenesis[44]. Unenhanced CT should be preferred, as the normal pancreatic parenchyma between fatty areas could exhibit contrast enhancement, and focal fatty replacement could simulate a true mass[70].
To validate CT for the diagnosis of pancreatic steatosis, Saisho et al[30] showed that the fat/parenchyma ratio calculated from CT is analogue to histological evaluation. Moreover, in 2014, Kim et al[39] found that pancreatic fat fraction in histological evaluation was significantly correlated with the difference between pancreatic and splenic attenuation (P < 0.01) and the pancreas-to-spleen attenuation ratio (P < 0.01).
The use of ionizing radiation limits CT as a research method, but recent evidence suggests that preoperative CT evaluation of pancreatic fat may be of importance in predicting the clinical outcome in pancreatic surgery, or as a prognostic marker for pancreatic adenocarcinoma[67,68,71].
MRI: MRI is sensible, noninvasive and safe. For those reasons, it’s currently the most used method to study fat content of the pancreas, especially in prospective studies. Single-voxel magnetic resonance spectroscopy (MRS) is considered almost equivalent to histology and biochemical measurements, and therefore is currently the criterion standard for the determination of pancreatic lipomatosis[72-79].
There are several methods to measure pancreatic fat fraction (PFF) in the pancreas using MRI.
The most used method utilize the frequency shift between the water and the fat resonances to generate in-phase and opposed-phase images, in which the signal of the water and fat net magnetization vectors are at a maximum or a minimum[80].
The Dixon method consist of a post-processing of the in-phase and opposed-phase spin echo images that uses the chemical shift difference between protons in water and fat, leading to water-selective and fat-selective images[81]. However, the results can be affected by T1 and T2 relaxation effects[82]. The novel and fast two-point mDixon exhibits a good correlation with MRS for assessment of PFF, with limited sensitivity for assessing lower fat content[76].
Spectral-spatial excitation technique combine chemical shift selectivity with simultaneous slice-selective excitation in gradient-echo imaging sequences. Apparently, this method is as good as in-phase/opposed-phase imaging on determinate the PFF[80], and is particularly good for determining small amounts of fat[83].
A recently developed method is the three dimensional iterative decomposition with echo asymmetry and least squares estimation (IDEAL), which produces separated fat and water images, optimal in signal-noise ratio. Hu et al[73] reported that this method may be even superior to MRS in the measurement of PFF.
Finally, the newest automated intra-subject registration-based segmentation is potentially suitable for the quantification of abdominal and organ fat and achieves comparable quantitative endpoints with respect to manual segmentation[84].
Only few epidemiologic studies have been performed to assess the prevalence of pancreatic steatosis. The estimated prevalence is between 16% and 35% in Asian populations[64,85,86].
In 2016, Pham et al[87] published the first study on which the prevalence of pancreatic steatosis was assessed in a pediatric population, which was 10%. However, this result may not be extended in general pediatric population, as it was assessed on hospitalized patients.
However, even considering the limits of those studies, all of them suggest a large prevalence in general population.
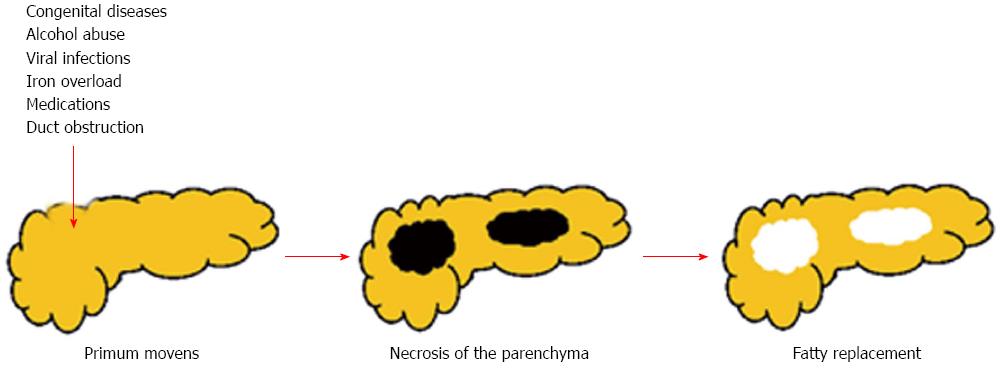
There are at least two mechanisms that can lead to a pancreatic fat accumulation[9]: (1) death of acinar cells and replacement by adipocytes - “fatty replacement” (Figure 4); and (2) fat accumulation associated with obesity and type 2 diabetes mellitus - “fatty infiltration” or “NAFPD”.
Theoretically, any noxa strong enough to cause necrosis of the acinar cells can lead to fatty replacement[9]. Despite that, only little evidence can be found in literature.
Congenital diseases: Cystic fibrosis (CF) or mucoviscidosis is an autosomal recessive disorder. It is caused by the presence of mutations in both copies of the gene for the protein cystic fibrosis transmembrane conductance regulator (CFTR), involved in the production of pancreatic juice. CF results in a more dense pancreatic secretion, which eventually leads to pancreatic damage and replacement with adipocytes[88-101].
Shwachman-Diamond syndrome or Shwachman-Bodian-Diamond syndrome (SBDS) is a rare autosomal recessive disorder characterized by exocrine pancreatic insufficiency with fatty replacement, bone marrow dysfunction, skeletal abnormalities, and short stature. The gene mutated in this syndrome is called SBDS, and its function is probably involved in RNA metabolism or ribosome assembly, although it’s uncertain. Therefore, the pathogenesis of pancreatic damage is still unclear[51,102-108].
Johanson-Blizzard syndrome (JBS) is caused by mutations in the UBR1 gene, which encodes one of several ubiquitin ligase enzymes of the N-end rule pathway. It is associated with developmental errors, impaired apoptosis, and both prenatal and chronic inflammatory damage, necrosis and fibrosis of the pancreatic acini. Pancreatic exocrine insufficiency in JBS can additionally stem from congenital replacement of the acini with fatty tissue[109-114].
Finally, heterozygous carboxyl-ester-lipase mutations are associated with fatty replacement of the pancreas and maturity onset diabetes of the young, probably due to a protein misfolding[55,115].
Those genetic conditions can also be associated with exocrine pancreatic insufficiency (EPI), as a result of the destruction of exocrine pancreatic parenchyma.
Alcohol abuse: It has been suggested that alcohol abuse may be associated with abnormal mitochondrial function, which may account for the fat accumulation observed in pancreatic acinar cells[116]. However, evidence don’t support this theory, and it is more probable that alcohol abuse leads to pancreatic lipomatosis via acute and/or chronic pancreatitis and/or the upregulation of transcription factors involved in the synthesis of cholesterol and triglycerides[12,13,57,59,116-118]. Also, alcoholism is associated with malnutrition, which is also a cause of pancreatic steatosis[13].
Viral infections: Viral infections with Reovirus, may lead to duct obstruction and therefore necrosis of the parenchyma and subsequent substitution with fatty tissue[9,49,119]. In support of this pathogenic pathway, pancreatic duct legation actually leads to fatty replacement[120,121].
Iron overload: The most important causes of iron overload are hereditary hemochromatosis, a genetic disorder, and transfusional iron overload, which can result from repeated blood transfusions. Iron mainly accumulates in reticuloendothelial system, liver, heart, and endocrine or exocrine glands, pancreas included. When the pancreas is involved, iron leads to oxidative stress of acinar and islet cells, apoptosis and substitution with adipocytes. This has been described in patients with transfusion-dependent diseases as myelodysplastic syndrome and cooley’s anemia (β-thalassemia major)[122-125].
Medications: Although it’s theoretically valid, evidence that medications can cause pancreatic tissue necrosis and subsequent substitution with fatty tissue is scarce and mainly based on case reports or animal models. drugs reported are corticosteroids, gemcitabine, rosiglitazone and, more recently, octreotide[17,49,126-130].
Chronic liver disease: Yoshimura et al[131] and Sasaki et al[132] suggested that lipomatous pseudohypertrophy of the pancreas might be caused by chronic advanced hepatic lesions, which lead to ductal obstruction. Thus now, only case reports of patients with chronic hepatitis B and liver cirrhosis support this hypothesis[49,132,133].
Stronger evidence exists that pancreatic steatosis is associated with NAFLD[33,56,57,59,64,75,134-137]. However, the pathogenesis of this association is more likely due to metabolic pathways than pancreatic injury, and it will be discussed in the appropriate section.
Malnutrition: Malnutrition, as seen in alcoholism, kwashiorkor and AIDS is associated with changing in pancreatic structure, including pancreatic lipomatosis[13,29,138]. However, the lack of evidence makes the pathogenesis still unclear.
Pancreatitis: It is theoretically possible that necrotizing pancreatitis leads to fatty replacement, but, to our knowledge, this association has never been reported. In contrast, Recurrent Acute Pancreatitis (RAP) may lead to reduction of the parenchymal mass and substitution with adipocytes[139-141]. Moreover, an increased number of intrapancreatic adipocytes can be observed in pancreata of lean patients with nonhereditary or hereditary chronic pancreatitis[142-144].
Moreover, NAFPD has been associated with an increased risk of developing severe acute pancreatitis, as discussed in the appropriate section.
The most important risk factor for developing NAFPD is obesity. This relation was suggested in the first study by Ogilvie and has been widely confirmed[145]. Experimental models report that maternal obesity and postnatal obesogenic diets can result in a NAFPD by inducing an endoplasmic reticulum imbalance and alteration in circadian metabolic patterns[25,146]. In addition to obesity, studies on sufficiently wide populations (> 1000 subjects) suggest also increasing age, male sex, hypertension, dyslipidemia, alcohol consumption, low serum lipase activity as important risk factors, although data must be considered still insufficient[30,64,86,147]. T2DM and NAFLD are often reported as risk factor. However, data are sometimes contrasting, as further discussed. Wong et al[148] found also a positive correlation between fatty pancreas and hyperferritinemia.
Recently, different ethnicity has been suggested as an independent risk factor of developing pancreatic steatosis. Hispanics and Caucasians showed an increased risk to develop pancreatic fat infiltration than the African Americans[149,150]. More important, PFF may predict the outcome of insulin resistance in African Americans, but didn’t show the same accuracy for Hispanics[151]. However, more research is needed.
The relation with age and low serum lipase activity is probably due to a fatty degeneration of the pancreas, which may be considered paraphysiological[147,152].
How obesity leads to ectopic fat (EF) accumulation is not clear yet. Some individuals are more susceptible to accumulate EF for Body Mass Index (BMI). Although it may seem paradoxical, it has been suggested that those patients may have an impaired subcutaneous fat storage capacity, which leads to visceral and ectopic fat accumulation. An extreme example of this phenomenon is Lipodystropy, also known as Berardinelli-Seip syndrome (BSS). Patients with BSS have a scarce subcutaneous fat storage, but a greater amount of visceral and ectopic fat. On the other hand, several studies prove that healthy individuals with high subcutaneous fat content have low levels of visceral and ectopic fat. Subcutaneous fat may even have a protective action regarding ectopic fact accumulation[153]. In an experimental model, transplantation of normal adipose tissue in the subcutaneous region of lipoatrophic mice, removes their excess of EF and insulin resistance[154].
More efforts have been made to explain ectopic fat accumulation in the liver (NAFLD). It has been hypothesized that insulin resistance facilitates the transport of free fatty acids (FFA) from adipose tissue to the liver, and their storage in hepatocytes. Steatosis occurs when the rate of import and fatty acid synthesis exceed the rate of export and catabolism[155].
An interesting finding of a prospective study on 293 patients[56] is that about 68% of cases with fatty pancreas concurrently had fatty liver, but most subjects (97%) with fatty liver had fatty pancreas. Although the positive predictive value of fatty liver in fatty pancreas was 69.4%, the negative predictive value of fatty liver in normal pancreas was 96.4%. Our group is currently involved in a retrospective CT-based study which preliminary results on 47 patients lead to the same results (Catanzaro R, Cuffari B, Palmucci S et al, unpublished). This implies that fatty pancreas could be used as the initial indicator of EF deposition.
As NAFLD and NAFPD are often associated, one could assume that they could share a common pathway[33,56,57,59,64,75,134-137].
Actually, important information must be taken in account: (1) some studies have found no correlation between NAFLD and NAFPD[80,156]; (2) hepatic fat is mainly intracellular, while NAFPD is a consequence of adipocytes infiltration[5,16]; (3) when patients are treated with bariatric surgery, fat loss in the liver and the pancreas seem to be independent, suggesting tissue-specific mobilization of these ectopic fat stores[77]; and (4) when corrected for BMI, the association between hepatic and pancreatic steatosis can’t be found anymore[33,134,137].
It could be correct to assert that, according with current evidence, the association between NAFLD and NAFPD is a consequence of obesity only. Therefore, hypothesis used to explain hepatic steatosis may not be suitable for NAFPD. However, it is possible that pancreatic and hepatic steatosis affect each other and more research should focus on the different pathways that lead to one or the other condition[135,157].
In conclusion, the pathogenesis of NAFPD is still unknown, and no satisfactory theories have been proposed yet. Therefore, more research will be needed.
About 347 million people worldwide have T2DM, and numbers are increasing[158]. The WHO projects that diabetes will be the seventh leading cause of death in 2030[159].
Since T2DM is increasing problem worldwide, and pancreatic islets have a key role in the metabolism of glucose, one of the main issues in NAFPD research is whether or not pancreatic steatosis is a risk factor for T2DM.
In vitro and animal studies suggest that pancreatic lipomatosis may contribute to β-cell lipotoxicity and lipoapoptosis, with consequent loss of function[160-162]. However, data on humans are inconsistent.
Using proton MRS and oral glucose tolerance tests, Tushuizen et al[163] found that pancreatic fat correlated negatively with β-cell function parameters in nondiabetic subjects. Heni et al[156] found the same association in patients with impaired glucose metabolism and, in a stepwise multivariate regression analysis, pancreatic fat resulted a stronger determinant of impaired insulin secretion than visceral fat. More recently, Della Corte et al[157] found a positive correlation between NAFPD and homeostatic model assessment - insulin resistance (HOMA-IR) in a pediatric population with NAFLD. In contrast with these results, two studies performed using the gold standard hyperglycemic clamp, found no relation between pancreatic fat content and β-cell function in subjects with impaired glucose metabolism[74,164].
Data about patient with full-blown T2DM are even more challenging. Tushuizen et al[163], found no association between pancreatic fat and β-cell dysfunction in diabetic patients. That may suggest that once diabetes occurs, other factors cause further β-cell impairment. However, they found that diabetic subjects had a significantly higher pancreatic fat content than nondiabetic, association confirmed by Lingvay et al[72].
Saisho et al[30], using computed tomography on 1721 nondiabetic and 165 subjects with T2DM, observed that pancreatic fat was not significantly increased in T2DM.
Wang et al[64], in 2014, studying a cohort of 8097 subjects, found that the fatty pancreas group had an increased risk of diabetes (OR = 1.593) than non-fatty pancreas group (P < 0.001).
Finally, a recent study[165] found a significant higher average fat content in the pancreas of patients with newly diagnosed T2DM compared with healthy controls.
Summarizing, three hypotheses can be made about β-cell dysfunction in pancreatic steatosis: (1) the increased amount of triglycerides in pancreatic β-cells can lead to their dysfunction, probably with a mechanism of lipotoxicity, at least in subjects with an already impaired glucose metabolism; (2) intrapancreatic adipocytes may have a negative paracrine effect on β-cells; and (3) NAFPD and T2DM are just consequences of obesity.
According to current evidence, the majority of the authors support the last theory, but more research should focus on this topic and meta-analysis will be required[145].
The MetS is a major and increasing clinical and social issue worldwide, as a result of changing in lifestyle which include high-caloric and high-fat diet and decreasing physical activity. MetS is associated with a 5-fold increase risk of T2DM, 2-fold risk of developing cardiovascular disease, 2- to 4-fold increased risk of stroke, 3- to 4-fold increased risk of myocardial infarction, and 2-fold mortality caused by coronary events[3].
NAFLD is considered the hepatic manifestation of MetS. High levels of FFA and insulin resistance are considered key pathogenic factors in the development of fat accumulation in the liver[3].
There is an increasing evidence of association between NAFPD and all the components of the MetS in animal models and humans[20,56,59,62,148,166].
Weather pancreatic steatosis is a key organ in the development of the MetS or just a marker or that condition (mediated by general obesity), we believe that the assessment of pancreatic fat infiltration will have an increasing role in the clinical management of the syndrome.
As discussed, MetS itself is associated with an increased incidence of cardiovascular diseases. Recently, one study[167] found that pancreatic steatosis may be an independent risk factor on the development of carotid atherosclerosis in non-obese subjects with T2DM. Therefore, it could be a marker of a higher risk of cardiovascular disease, especially in non-obese subjects.
Obesity and the MetS are associated with the incidence and severity of acute pancreatitis[168-171]. Thus now, this association was explained by the fact that both obesity and the MetS are linked with other well-known risk factors for acute pancreatitis, such as gallstones, alcohol abuse, cancer, hypertriglyceridemia, use of medications, moreover, as already pointed out, the MetS may be associated with a pro-inflammatory state which may exacerbate inflammation after the trigger is pulled[172].
There is speculation that pancreatic steatosis may have a key role in the pathogenesis of pancreatitis in obese patients. In analogy with NAFLD leading to non-alcoholic steato-hepatitis (NASH), the term non-alcoholic steato-pancreatitis (NASP) has been proposed[48].
The existence of NASP has not been proved yet, but there is biological plausibility, and evidence is increasing. Adipocytes may generate a local and systemic pro-inflammatory state by producing pro-inflammatory adipokines and cytokines, such as leptin, interleukin 1β and tumor necrosis factor, or toxic fatty acids that may make the pancreas more susceptible to inflammation[28,157,173].
Pokhrel et al[174] found that increased pancreatic fat on MRI was not an independent predictor of post-ERCP pancreatitis, however, data is scarce and more research will be required.
An excellent review by Acharya et al[175] points out that intra-pancreatic fat have a role in the severity of acute pancreatitis: lipases released by acinar cells after the first injury, cause a local and systemic lipolysis, which results in increasing of FFAs, especially unsaturated fatty acids (UFAs). The spread of UFAs in the pancreatic parenchyma has a direct toxic effect on acinar cells (lipolytic flux), causing acinar necrosis. In post-mortem studies, more severe necrosis of the parenchyma occurred closer to necrotizing fat (peri-fat acinar necrosis: PFAN)[143,176]. In 2011, Smits and van Geenen[9] published preliminary results of a CT-based study that show a significant relationship between pancreatic steatosis and severity of pancreatitis (P < 0.03). The use of lipases inhibitors (Orlistat) to prevent conversion of mild into acute pancreatitis may be therefore justified and studies are evaluating its efficacy, with encouraging results[177].
As already discussed, RAP can cause chronic pancreatitis and morphological changes, which include fatty replacement. However, no evidence exists that NAFPD can cause chronic pancreatitis.
Chronic liver inflammation as seen in NASH, leads to necrosis of hepatocytes and liver fibrosis. Theoretically, in the pancreas, NASP could determinate the same changes, but data are inconsistent.
Matsuda et al[26], found that in Zucker Diabetic Fatty rats fed with a chronic high-fat diet, fat accumulates in pancreatic acinar cells, and this fatty change seems to be related to subsequent pancreatic fibrosis and acinar cell injury. However, in Ossabaw swines, another animal model for the MetS, pancreatic steatosis was not associated with other histological abnormalities[20].
In humans, patients with chronic pancreatitis have both an increased amount of pancreatic fat and fibrosis, however, van Geenen et al[35]. found no relationship between pancreatic fibrosis and NAFPD (P = 0.916) in a study over 900 autopsies, and Mathur et al[31] found that pancreatic fat correlated even negatively with fibrosis (P < 0.001).
Therefore, according to current evidence, fatty replacement and pancreatic fibrosis seem to be both independent consequences of chronic inflammation in patients with chronic pancreatitis.
Several studies show that obesity is one of the leading risk factors for pancreatic cancer (PC)[178-184]. However, the pathophysiological pattern of this association is not clear yet. Several mechanisms have been discussed[172]: Evidence exists that the increase of certain hormones in obese patients, such as insulin, adipokines and resistine and systemic oxidative stress may have a role in the development of pancreatic adenocarcinoma[172,185-188].
NAFPD has been independently associated with an increased risk to develop PC[38]. A recent study[40] observed a correlation between pancreatic intraepithelial neoplasia (PanIN) and extra- (P < 0.01) and intralobular (P < 0.0001) pancreatic fat. No clear metabolic pathways have been identified to explain this association, but speculation is possible.
The increased numbers of adipocytes in NAFPD could promote cancerogenesis indirectly by NASP, as occurs in the liver[9].
Persistent organic pollutants (POPs) are lipophilic toxics able to bio-accumulate in fatty rich tissues of animals, especially those higher in the food chain, humans included. Evidence exists that accumulation of POPs in adipose tissue may be associated with PC occurrence, and it is possible that a higher concentration of pancreatic fat, and consequently of POPs, can partially explain the linkage between NAFPD and PC[172].
Along with an increased risk to develop PC, patients with increased pancreatic fat have a poorer outcome than those who develop cancer in a lean pancreas. In particular, NAFPD promotes dissemination and lethality of PC[32,67]. Mathur et al[32] suggested that “pancreatic steatosis alters the tumor microenvironment, enhances tumor spread, and contributes to the early demise of patients with pancreatic adenocarcinoma”.
In addition, pancreaticoduodenectomy in patients with fatty pancreas is associated with an increased risk of intraoperative blood loss and post-operative pancreatic fistula, which contributes with the poor outcome in those patients[31,34,37,50,68,71,189-191]. Therefore, assessment of pancreatic steatosis by preoperative CT or MRI or intraoperative histological evaluation on the frozen sections may have a role, in the future, in the prognostic evaluation of patients with PC[37,67,68,71,189,190].
The first pancreatic transplant was performed in 1966 in the United States[192]. It is a very effective and yet the only definitive option for curing insulin-dependent diabetics. However, the spreading of this technique is restricted by the significant rate of surgical complications resulting in graft failure/loss and recipient morbidity and mortality.
Increasing obesity and age, of both recipient and donor, increase the risk of technical complications like graft pancreatitis, graft thrombosis, intra-abdominal infections, gangrene and pancreatic fistula. However, if the transplantation is successful, there is not an increased risk of allograft failure[193,194]. Older patients with higher BMI are more likely to have steatosis, and that could explain the association.
Certain transplant surgeons do not use pancreas that are infiltrated by fat on inspection, since the procedure is technically more difficult when using a fatty pancreas, but a more objective measurement could avoid to waste organs that would be suitable for transplant. Verma et al[11] propose the IDEAL scanning on the sole organ as a possible solution. Furthermore, “defatting” of the organ is possible and a successful case has been reported in 2004[195].
In 1996, Gullo et al[196] first reported a benign syndrome characterized by increased levels of serum amylase, pancreatic isoamylase, lipase and trypsin. This condition is nowadays called Gullo’s syndrome and has been better characterized[196,197].
Cavallini et al[198], in a US based study, found that HP was related with hyperamylasemia. However, Gullo et al[54] found no correlation in a MRI based study. More recently, Wu et al[62] found that amylase levels in patients with pancreatic lipomatosis were even lower than controls.
In theory, pancreatic steatosis can lead to EPI with different mechanisms: (1) fat droplet accumulation in acinar cells and consequent lipotoxicity; (2) negative paracrine effect mediated by adipocytes; and (3) death of acinar cells as cause of both EPI and fatty replacement.
However, the exocrine function in patients with NAFPD has never been extensively investigated. Therefore, data are mainly based on case reports of extreme cases of complete fatty replacement of pancreas with fatty tissue[36,52,199-201].
In one recent study[202], fecal level of pancreatic elastase (PE-1) was evaluated in patients with T2DM. EPI was more frequent in patients with high HbA1c, but did not correlate with pancreatic steatosis.
Since NAFPD has been only recently extensively studied and its clinical significance is not clear, no clinical trials have validated any medications yet. Anyway, evidence exist that it is reversible.
Pancreatic fat can be reduced by weight loss, with or without bariatric surgery, and that is associated with improvement of insulin sensibility[203].
Moreover, specific treatment showed efficacy in vitro or on murine models: (1) Troglitazone[204]; (2) combination of Telmisartan and Sitagliptin[21]; and (3) Berberine and Cinnamic Acid, as components in Jiaotai Pill, a traditional Chinese medication[27,205].
We hope that increasing evidence on the clinical significance of pancreatic steatosis will support further research.
Pancreatic steatosis, which comprehends fatty replacement and fatty infiltration of the pancreas, is a very common condition, easily diagnosable but often neglected by physicians and researchers.
Its clinical significance ranges widely in Internal Medicine and Surgery, and more correlations may be found in future. Nevertheless, evidence is not exhaustive and the pathophysiology is yet unknown.
We believe that, according to current literature, pancreatic steatosis should have stronger consideration in clinical practice, in particular: (1) as an early marker of ectopic fat accumulation and insulin resistance in the MetS; (2) in the differential diagnosis with pancreatic fibrosis, especially when the pancreas is observed with US or EUS; (3) as a prognostic and/or predictive marker for acute pancreatitis and PC; (4) in pre-operatory evaluation before pancreaticoduodenectomy or pancreatic transplant; and (5) as a possible cause of unexplained exocrine pancreatic insufficiency.
More research, in future, should focus on the clinical consequences of pancreatic steatosis, in order to understand its impact on human health, its pathophysiology and eventually support clinical trials.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Antonini F, Peng SY S- Editor: Qi Y L- Editor: A E- Editor: Ma S
| 1. | World Health Organization. Obesity and overweight. Fact sheet n° 311, 2011-01, cited 2016-06-03. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/. [Cited in This Article: ] |
| 2. | Alberti KG, Zimmet P, Shaw J; IDF Epidemiology Task Force Consensus Group. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366:1059-1062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5130] [Cited by in F6Publishing: 5057] [Article Influence: 266.2] [Reference Citation Analysis (0)] |
| 3. | Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1063] [Cited by in F6Publishing: 1052] [Article Influence: 105.2] [Reference Citation Analysis (0)] |
| 4. | Bobulescu IA, Lotan Y, Zhang J, Rosenthal TR, Rogers JT, Adams-Huet B, Sakhaee K, Moe OW. Triglycerides in the human kidney cortex: relationship with body size. PLoS One. 2014;9:e101285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Feldman M, Friedman LS, Brandt LJ. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. Philadelphia: Elsevier 2016; 1428-1441. [Cited in This Article: ] |
| 6. | Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2:901-910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 764] [Cited by in F6Publishing: 809] [Article Influence: 80.9] [Reference Citation Analysis (0)] |
| 7. | Ogilvie R. The island of Langerhans in 19 cases of obesity. J Pathol. 1933;37:473-481. [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 148] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 8. | Olsen TS. Lipomatosis of the pancreas in autopsy material and its relation to age and overweight. Acta Pathol Microbiol Scand A. 1978;86A:367-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 50] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Smits MM, van Geenen EJ. The clinical significance of pancreatic steatosis. Nat Rev Gastroenterol Hepatol. 2011;8:169-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 189] [Article Influence: 14.5] [Reference Citation Analysis (2)] |
| 10. | Altinel D, Basturk O, Sarmiento JM, Martin D, Jacobs MJ, Kooby DA, Adsay NV. Lipomatous pseudohypertrophy of the pancreas: a clinicopathologically distinct entity. Pancreas. 2010;39:392-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Verma AR, Papalois V. Evaluating steatosis in pancreatic transplant. Exp Clin Transplant. 2011;9:159-164. [PubMed] [Cited in This Article: ] |
| 12. | Wilson JS, Colley PW, Sosula L, Pirola RC, Chapman BA, Somer JB. Alcohol causes a fatty pancreas. A rat model of ethanol-induced pancreatic steatosis. Alcohol Clin Exp Res. 1982;6:117-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | López JM, Bombi JA, Valderrama R, Giménez A, Parés A, Caballería J, Imperial S, Navarro S. Effects of prolonged ethanol intake and malnutrition on rat pancreas. Gut. 1996;38:285-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Kelley LC, Harmon BG, McCaskey PC. A retrospective study of pancreatic tumors in slaughter cattle. Vet Pathol. 1996;33:398-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Larsen MO, Juhl CB, Pørksen N, Gotfredsen CF, Carr RD, Ribel U, Wilken M, Rolin B. Beta-cell function and islet morphology in normal, obese, and obese beta-cell mass-reduced Göttingen minipigs. Am J Physiol Endocrinol Metab. 2005;288:E412-E421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Pinnick KE, Collins SC, Londos C, Gauguier D, Clark A, Fielding BA. Pancreatic ectopic fat is characterized by adipocyte infiltration and altered lipid composition. Obesity (Silver Spring). 2008;16:522-530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 17. | Fernandes-Santos C, Evangelista Carneiro R, de Souza Mendonca L, Barbosa Aguila M, Mandarim-de-Lacerda CA. Rosiglitazone aggravates nonalcoholic Fatty pancreatic disease in C57BL/6 mice fed high-fat and high-sucrose diet. Pancreas. 2009;38:e80-e86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Lee Y, Lingvay I, Szczepaniak LS, Ravazzola M, Orci L, Unger RH. Pancreatic steatosis: harbinger of type 2 diabetes in obese rodents. Int J Obes (Lond). 2010;34:396-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Oben JA, Patel T, Mouralidarane A, Samuelsson AM, Matthews P, Pombo J, Morgan M, McKee C, Soeda J, Novelli M. Maternal obesity programmes offspring development of non-alcoholic fatty pancreas disease. Biochem Biophys Res Commun. 2010;394:24-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Fullenkamp AM, Bell LN, Robbins RD, Lee L, Saxena R, Alloosh M, Klaunig JE, Mirmira RG, Sturek M, Chalasani N. Effect of different obesogenic diets on pancreatic histology in Ossabaw miniature swine. Pancreas. 2011;40:438-443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Souza-Mello V, Gregório BM, Relvas-Lucas B, da Silva Faria T, Aguila MB, Mandarim-de-Lacerda CA. Pancreatic ultrastructural enhancement due to telmisartan plus sitagliptin treatment in diet-induced obese C57BL/6 mice. Pancreas. 2011;40:715-722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 22. | Hori M, Kitahashi T, Imai T, Ishigamori R, Takasu S, Mutoh M, Sugimura T, Wakabayashi K, Takahashi M. Enhancement of carcinogenesis and fatty infiltration in the pancreas in N-nitrosobis(2-oxopropyl)amine-treated hamsters by high-fat diet. Pancreas. 2011;40:1234-1240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Grippo PJ, Fitchev PS, Bentrem DJ, Melstrom LG, Dangi-Garimella S, Krantz SB, Heiferman MJ, Chung C, Adrian K, Cornwell ML. Concurrent PEDF deficiency and Kras mutation induce invasive pancreatic cancer and adipose-rich stroma in mice. Gut. 2012;61:1454-1464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Gotoh K, Inoue M, Shiraishi K, Masaki T, Chiba S, Mitsutomi K, Shimasaki T, Ando H, Fujiwara K, Katsuragi I. Spleen-derived interleukin-10 downregulates the severity of high-fat diet-induced non-alcoholic fatty pancreas disease. PLoS One. 2012;7:e53154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Carter R, Mouralidarane A, Soeda J, Ray S, Pombo J, Saraswati R, Novelli M, Fusai G, Rappa F, Saracino C. Non-alcoholic fatty pancreas disease pathogenesis: a role for developmental programming and altered circadian rhythms. PLoS One. 2014;9:e89505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Matsuda A, Makino N, Tozawa T, Shirahata N, Honda T, Ikeda Y, Sato H, Ito M, Kakizaki Y, Akamatsu M. Pancreatic fat accumulation, fibrosis, and acinar cell injury in the Zucker diabetic fatty rat fed a chronic high-fat diet. Pancreas. 2014;43:735-743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Zou X, Liu DL, Lu FE, Dong H, Xu LJ, Luo YH, Wang KF. [Effect of jiaotai pill on pancreatic fat accumulation and islet cell apoptosis in rats with type 2 diabetes]. Zhongguo Zhong Yao Zazhi. 2014;39:2106-2111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Zyromski NJ, Mathur A, Pitt HA, Lu D, Gripe JT, Walker JJ, Yancey K, Wade TE, Swartz-Basile DA. A murine model of obesity implicates the adipokine milieu in the pathogenesis of severe acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2008;295:G552-G558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Chehter EZ, Longo MA, Laudanna AA, Duarte MI. Involvement of the pancreas in AIDS: a prospective study of 109 post-mortems. AIDS. 2000;14:1879-1886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Saisho Y, Butler AE, Meier JJ, Monchamp T, Allen-Auerbach M, Rizza RA, Butler PC. Pancreas volumes in humans from birth to age one hundred taking into account sex, obesity, and presence of type-2 diabetes. Clin Anat. 2007;20:933-942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 314] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 31. | Mathur A, Pitt HA, Marine M, Saxena R, Schmidt CM, Howard TJ, Nakeeb A, Zyromski NJ, Lillemoe KD. Fatty pancreas: a factor in postoperative pancreatic fistula. Ann Surg. 2007;246:1058-1064. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 264] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 32. | Mathur A, Zyromski NJ, Pitt HA, Al-Azzawi H, Walker JJ, Saxena R, Lillemoe KD. Pancreatic steatosis promotes dissemination and lethality of pancreatic cancer. J Am Coll Surg. 2009;208:989-994; discussion 994-996. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 33. | van Geenen EJ, Smits MM, Schreuder TC, van der Peet DL, Bloemena E, Mulder CJ. Nonalcoholic fatty liver disease is related to nonalcoholic fatty pancreas disease. Pancreas. 2010;39:1185-1190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 34. | Gaujoux S, Cortes A, Couvelard A, Noullet S, Clavel L, Rebours V, Lévy P, Sauvanet A, Ruszniewski P, Belghiti J. Fatty pancreas and increased body mass index are risk factors of pancreatic fistula after pancreaticoduodenectomy. Surgery. 2010;148:15-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 264] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 35. | van Geenen EJ, Smits MM, Schreuder TC, van der Peet DL, Bloemena E, Mulder CJ. Smoking is related to pancreatic fibrosis in humans. Am J Gastroenterol. 2011;106:1161-1166; quiz 1167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Khan NA, Amin MS, Islam MZ. Pancreatic lipomatosis with massive steatorrhea. Mymensingh Med J. 2011;20:712-714. [PubMed] [Cited in This Article: ] |
| 37. | Belyaev O, Munding J, Herzog T, Suelberg D, Tannapfel A, Schmidt WE, Mueller CA, Uhl W. Histomorphological features of the pancreatic remnant as independent risk factors for postoperative pancreatic fistula: a matched-pairs analysis. Pancreatology. 2011;11:516-524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Hori M, Takahashi M, Hiraoka N, Yamaji T, Mutoh M, Ishigamori R, Furuta K, Okusaka T, Shimada K, Kosuge T. Association of pancreatic Fatty infiltration with pancreatic ductal adenocarcinoma. Clin Transl Gastroenterol. 2014;5:e53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 39. | Kim SY, Kim H, Cho JY, Lim S, Cha K, Lee KH, Kim YH, Kim JH, Yoon YS, Han HS. Quantitative assessment of pancreatic fat by using unenhanced CT: pathologic correlation and clinical implications. Radiology. 2014;271:104-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 40. | Rebours V, Gaujoux S, d’Assignies G, Sauvanet A, Ruszniewski P, Lévy P, Paradis V, Bedossa P, Couvelard A. Obesity and Fatty Pancreatic Infiltration Are Risk Factors for Pancreatic Precancerous Lesions (PanIN). Clin Cancer Res. 2015;21:3522-3528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 142] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 41. | Matsumoto S, Mori H, Miyake H, Takaki H, Maeda T, Yamada Y, Oga M. Uneven fatty replacement of the pancreas: evaluation with CT. Radiology. 1995;194:453-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 108] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 42. | Itai Y, Saida Y, Kurosaki Y, Kurosaki A, Fujimoto T. Focal fatty masses of the pancreas. Acta Radiol. 1995;36:178-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 43. | Anand R, Narula MK, Chaudhary V, Agrawal R. Total pancreatic lipomatosis with malabsorption syndrome. Indian J Endocrinol Metab. 2011;15:51-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Mortelé KJ, Rocha TC, Streeter JL, Taylor AJ. Multimodality imaging of pancreatic and biliary congenital anomalies. Radiographics. 2006;26:715-731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 225] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 45. | Yan MX, Li YQ, Meng M, Ren HB, Kou Y. Long-term high-fat diet induces pancreatic injuries via pancreatic microcirculatory disturbances and oxidative stress in rats with hyperlipidemia. Biochem Biophys Res Commun. 2006;347:192-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Moffitt JH, Fielding BA, Evershed R, Berstan R, Currie JM, Clark A. Adverse physicochemical properties of tripalmitin in beta cells lead to morphological changes and lipotoxicity in vitro. Diabetologia. 2005;48:1819-1829. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 47. | Cnop M, Hannaert JC, Hoorens A, Eizirik DL, Pipeleers DG. Inverse relationship between cytotoxicity of free fatty acids in pancreatic islet cells and cellular triglyceride accumulation. Diabetes. 2001;50:1771-1777. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 409] [Cited by in F6Publishing: 403] [Article Influence: 17.5] [Reference Citation Analysis (1)] |
| 48. | Mathur A, Marine M, Lu D, Swartz-Basile DA, Saxena R, Zyromski NJ, Pitt HA. Nonalcoholic fatty pancreas disease. HPB (Oxford). 2007;9:312-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 134] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 49. | Walters MN. Adipose atrophy of the exocrine pancreas. J Pathol Bacteriol. 1966;92:547-557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Rosso E, Casnedi S, Pessaux P, Oussoultzoglou E, Panaro F, Mahfud M, Jaeck D, Bachellier P. The role of “fatty pancreas” and of BMI in the occurrence of pancreatic fistula after pancreaticoduodenectomy. J Gastrointest Surg. 2009;13:1845-1851. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 51. | Robberecht E, Nachtegaele P, Van Rattinghe R, Afschrift M, Kunnen M, Verhaaren R. Pancreatic lipomatosis in the Shwachman-Diamond syndrome. Identification by sonography and CT-scan. Pediatr Radiol. 1985;15:348-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 52. | So CB, Cooperberg PL, Gibney RG, Bogoch A. Sonographic findings in pancreatic lipomatosis. AJR Am J Roentgenol. 1987;149:67-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 53. | MacMaster SA, Cummings TM. Computed tomography and ultrasonography findings for an adult with Shwachman syndrome and pancreatic lipomatosis. Can Assoc Radiol J. 1993;44:301-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 54. | Gullo L, Salizzoni E, Serra C, Calculli L, Bastagli L, Migliori M. Can pancreatic steatosis explain the finding of pancreatic hyperenzymemia in subjects with dyslipidemia? Pancreas. 2006;33:351-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 55. | Raeder H, Haldorsen IS, Ersland L, Grüner R, Taxt T, Søvik O, Molven A, Njølstad PR. Pancreatic lipomatosis is a structural marker in nondiabetic children with mutations in carboxyl-ester lipase. Diabetes. 2007;56:444-449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 56. | Lee JS, Kim SH, Jun DW, Han JH, Jang EC, Park JY, Son BK, Kim SH, Jo YJ, Park YS. Clinical implications of fatty pancreas: correlations between fatty pancreas and metabolic syndrome. World J Gastroenterol. 2009;15:1869-1875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 175] [Cited by in F6Publishing: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 57. | Al-Haddad M, Khashab M, Zyromski N, Pungpapong S, Wallace MB, Scolapio J, Woodward T, Noh K, Raimondo M. Risk factors for hyperechogenic pancreas on endoscopic ultrasound: a case-control study. Pancreas. 2009;38:672-675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 58. | Choi CW, Kim GH, Kang DH, Kim HW, Kim DU, Heo J, Song GA, Park DY, Kim S. Associated factors for a hyperechogenic pancreas on endoscopic ultrasound. World J Gastroenterol. 2010;16:4329-4334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 55] [Cited by in F6Publishing: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 59. | Sepe PS, Ohri A, Sanaka S, Berzin TM, Sekhon S, Bennett G, Mehta G, Chuttani R, Kane R, Pleskow D. A prospective evaluation of fatty pancreas by using EUS. Gastrointest Endosc. 2011;73:987-993. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 60. | Ustundag Y, Ceylan G, Hekimoglu K. Pancreatic hyperechogenicity on endoscopic ultrasound examination. World J Gastroenterol. 2011;17:2061-2062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 7] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 61. | Ou HY, Wang CY, Yang YC, Chen MF, Chang CJ. The association between nonalcoholic fatty pancreas disease and diabetes. PLoS One. 2013;8:e62561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 62. | Wu WC, Wang CY. Association between non-alcoholic fatty pancreatic disease (NAFPD) and the metabolic syndrome: case-control retrospective study. Cardiovasc Diabetol. 2013;12:77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 63. | Sosa-Valencia L, Liu H, Ramírez J, Rodriguez-Wulff E, Rodríguez-Morales AJ. [Risk factors for hyperechogenic páncreas in ecoendoscopy: study of cases and controls]. Rev Gastroenterol Peru. 2013;33:131-137. [PubMed] [Cited in This Article: ] |
| 64. | Wang CY, Ou HY, Chen MF, Chang TC, Chang CJ. Enigmatic ectopic fat: prevalence of nonalcoholic fatty pancreas disease and its associated factors in a Chinese population. J Am Heart Assoc. 2014;3:e000297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 65. | Uygun A, Kadayifci A, Demirci H, Saglam M, Sakin YS, Ozturk K, Polat Z, Karslioglu Y, Bolu E. The effect of fatty pancreas on serum glucose parameters in patients with nonalcoholic steatohepatitis. Eur J Intern Med. 2015;26:37-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 66. | Jeong HT, Lee MS, Kim MJ. Quantitative analysis of pancreatic echogenicity on transabdominal sonography: correlations with metabolic syndrome. J Clin Ultrasound. 2015;43:98-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 67. | Mathur A, Hernandez J, Shaheen F, Shroff M, Dahal S, Morton C, Farrior T, Kedar R, Rosemurgy A. Preoperative computed tomography measurements of pancreatic steatosis and visceral fat: prognostic markers for dissemination and lethality of pancreatic adenocarcinoma. HPB (Oxford). 2011;13:404-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 68. | Tranchart H, Gaujoux S, Rebours V, Vullierme MP, Dokmak S, Levy P, Couvelard A, Belghiti J, Sauvanet A. Preoperative CT scan helps to predict the occurrence of severe pancreatic fistula after pancreaticoduodenectomy. Ann Surg. 2012;256:139-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 69. | Lim S, Bae JH, Chun EJ, Kim H, Kim SY, Kim KM, Choi SH, Park KS, Florez JC, Jang HC. Differences in pancreatic volume, fat content, and fat density measured by multidetector-row computed tomography according to the duration of diabetes. Acta Diabetol. 2014;51:739-748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 70. | Kim HJ, Byun JH, Park SH, Shin YM, Kim PN, Ha HK, Lee MG. Focal fatty replacement of the pancreas: usefulness of chemical shift MRI. AJR Am J Roentgenol. 2007;188:429-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 71. | Roberts KJ, Storey R, Hodson J, Smith AM, Morris-Stiff G. Pre-operative prediction of pancreatic fistula: is it possible? Pancreatology. 2013;13:423-428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 72. | Lingvay I, Esser V, Legendre JL, Price AL, Wertz KM, Adams-Huet B, Zhang S, Unger RH, Szczepaniak LS. Noninvasive quantification of pancreatic fat in humans. J Clin Endocrinol Metab. 2009;94:4070-4076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 147] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 73. | Hu HH, Kim HW, Nayak KS, Goran MI. Comparison of fat-water MRI and single-voxel MRS in the assessment of hepatic and pancreatic fat fractions in humans. Obesity (Silver Spring). 2010;18:841-847. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 74. | van der Zijl NJ, Goossens GH, Moors CC, van Raalte DH, Muskiet MH, Pouwels PJ, Blaak EE, Diamant M. Ectopic fat storage in the pancreas, liver, and abdominal fat depots: impact on β-cell function in individuals with impaired glucose metabolism. J Clin Endocrinol Metab. 2011;96:459-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 75. | Hannukainen JC, Borra R, Linderborg K, Kallio H, Kiss J, Lepomäki V, Kalliokoski KK, Kujala UM, Kaprio J, Heinonen OJ. Liver and pancreatic fat content and metabolism in healthy monozygotic twins with discordant physical activity. J Hepatol. 2011;54:545-552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 76. | Livingstone RS, Begovatz P, Kahl S, Nowotny B, Strassburger K, Giani G, Bunke J, Roden M, Hwang JH. Initial clinical application of modified Dixon with flexible echo times: hepatic and pancreatic fat assessments in comparison with (1)H MRS. MAGMA. 2014;27:397-405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 77. | Gaborit B, Abdesselam I, Kober F, Jacquier A, Ronsin O, Emungania O, Lesavre N, Alessi MC, Martin JC, Bernard M. Ectopic fat storage in the pancreas using 1H-MRS: importance of diabetic status and modulation with bariatric surgery-induced weight loss. Int J Obes (Lond). 2015;39:480-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 78. | Ma J, Song Z, Yan F. Detection of hepatic and pancreatic fat infiltration in type II diabetes mellitus patients with IDEAL-Quant using 3.0T MR: comparison with single-voxel proton spectroscopy. Chin Med J (Engl). 2014;127:3548-3552. [PubMed] [Cited in This Article: ] |
| 79. | Begovatz P, Koliaki C, Weber K, Strassburger K, Nowotny B, Nowotny P, Müssig K, Bunke J, Pacini G, Szendrödi J. Pancreatic adipose tissue infiltration, parenchymal steatosis and beta cell function in humans. Diabetologia. 2015;58:1646-1655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 80. | Schwenzer NF, Machann J, Martirosian P, Stefan N, Schraml C, Fritsche A, Claussen CD, Schick F. Quantification of pancreatic lipomatosis and liver steatosis by MRI: comparison of in/opposed-phase and spectral-spatial excitation techniques. Invest Radiol. 2008;43:330-337. [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 81. | Kovanlikaya A, Mittelman SD, Ward A, Geffner ME, Dorey F, Gilsanz V. Obesity and fat quantification in lean tissues using three-point Dixon MR imaging. Pediatr Radiol. 2005;35:601-607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 82. | Hussain HK, Chenevert TL, Londy FJ, Gulani V, Swanson SD, McKenna BJ, Appelman HD, Adusumilli S, Greenson JK, Conjeevaram HS. Hepatic fat fraction: MR imaging for quantitative measurement and display--early experience. Radiology. 2005;237:1048-1055. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 237] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 83. | Schick F, Machann J, Brechtel K, Strempfer A, Klumpp B, Stein DT, Jacob S. MRI of muscular fat. Magn Reson Med. 2002;47:720-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 84. | Joshi AA, Hu HH, Leahy RM, Goran MI, Nayak KS. Automatic intra-subject registration-based segmentation of abdominal fat from water-fat MRI. J Magn Reson Imaging. 2013;37:423-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 85. | Lesmana CR, Pakasi LS, Inggriani S, Aidawati ML, Lesmana LA. Prevalence of Non-Alcoholic Fatty Pancreas Disease (NAFPD) and its risk factors among adult medical check-up patients in a private hospital: a large cross sectional study. BMC Gastroenterol. 2015;15:174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 86. | Zhou J, Li ML, Zhang DD, Lin HY, Dai XH, Sun XL, Li JT, Song LY, Peng H, Wen MM. The correlation between pancreatic steatosis and metabolic syndrome in a Chinese population. Pancreatology. 2016;16:578-583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 87. | Pham YH, Bingham BA, Bell CS, Greenfield SA, John SD, Robinson LH, Eissa MA. Prevalence of Pancreatic Steatosis at a Pediatric Tertiary Care Center. South Med J. 2016;109:196-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 88. | Jones JS. Adult cystic fibrosis (mucoviscidosis). Fatty replacement of the pancreas in a woman aged 47 years. Br J Dis Chest. 1970;64:25-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 89. | Daneman A, Gaskin K, Martin DJ, Cutz E. Pancreatic changes in cystic fibrosis: CT and sonographic appearances. AJR Am J Roentgenol. 1983;141:653-655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 90. | Fiel SB, Friedman AC, Caroline DF, Radecki PD, Faerber E, Grumbach K. Magnetic resonance imaging in young adults with cystic fibrosis. Chest. 1987;91:181-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 91. | Tham RT, Heyerman HG, Falke TH, Zwinderman AH, Bloem JL, Bakker W, Lamers CB. Cystic fibrosis: MR imaging of the pancreas. Radiology. 1991;179:183-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 51] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 92. | Lugo-Olivieri CH, Soyer PA, Fishman EK. Cystic fibrosis: spectrum of thoracic and abdominal CT findings in the adult patient. Clin Imaging. 1998;22:346-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 93. | Soyer P, Spelle L, Pelage JP, Dufresne AC, Rondeau Y, Gouhiri M, Scherrer A, Rymer R. Cystic fibrosis in adolescents and adults: fatty replacement of the pancreas--CT evaluation and functional correlation. Radiology. 1999;210:611-615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 94. | Feigelson J, Pécau Y, Poquet M, Terdjman P, Carrère J, Chazalette JP, Ferec C. Imaging changes in the pancreas in cystic fibrosis: a retrospective evaluation of 55 cases seen over a period of 9 years. J Pediatr Gastroenterol Nutr. 2000;30:145-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 95. | King LJ, Scurr ED, Murugan N, Williams SG, Westaby D, Healy JC. Hepatobiliary and pancreatic manifestations of cystic fibrosis: MR imaging appearances. Radiographics. 2000;20:767-777. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 96. | Carucci LR, Jacobs JE. Focal fatty sparing of the pancreatic head in cystic fibrosis: CT findings. Abdom Imaging. 2003;28:853-855. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 97. | Sodhi KS, Thapa BR, Khandelwal S, Suri S. Pancreatic lipomatosis in an infant with cystic fibrosis. Pediatr Radiol. 2005;35:1157-1158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 98. | Robertson MB, Choe KA, Joseph PM. Review of the abdominal manifestations of cystic fibrosis in the adult patient. Radiographics. 2006;26:679-690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 99. | Tsushima Y, Matsumoto M, Inaba S, Watanabe M, Ohno Y. A Japanese adult case of cystic fibrosis causing bone demineralization. Radiat Med. 1992;10:157-162. [PubMed] [Cited in This Article: ] |
| 100. | Murayama S, Robinson AE, Mulvihill DM, Goyco PG, Beckerman RC, Hines MR, Stallworth JM. MR imaging of pancreas in cystic fibrosis. Pediatr Radiol. 1990;20:536-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 101. | Keyzer C, Knoop C, Van Wettere M, Dehu M, Gosset N, De Maertelaer V, Gevenois PA. Cystic fibrosis: unenhanced CT description of the appendix in asymptomatic adults. AJR Am J Roentgenol. 2014;202:759-764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 102. | Kurdziel JC, Dondelinger R. Fatty infiltration of the pancreas in Shwachman’s syndrome: computed tomography demonstration. Eur J Radiol. 1984;4:202-204. [PubMed] [Cited in This Article: ] |
| 103. | Lacaille F, Mani TM, Brunelle F, Lallemand D, Schmitz J. Magnetic resonance imaging for diagnosis of Shwachman’s syndrome. J Pediatr Gastroenterol Nutr. 1996;23:599-603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 104. | Cubuk M, Arslan G, Ceken K, Ozkaynak C, Lüyleci E. Schwachman-Diamond syndrome. A case report. Acta Radiol. 2000;41:627-628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 105. | Belkind-Gerson J, Ontiveros-Nevares P, Ocampo-Roosens V, Sandoval-Juárez D. Shwachman-Diamond syndrome in a Mexican family. Arch Med Res. 2001;32:318-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 106. | Lee JH, Bae SH, Yu JJ, Lee R, Yun YM, Song EY. A case of Shwachman-Diamond syndrome confirmed with genetic analysis in a Korean child. J Korean Med Sci. 2008;23:142-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 107. | Ruggiero A, Molinari F, Coccia P, Attinà G, Maurizi P, Riccardi R, Bonomo L. MRI findings in Shwachman diamond syndrome. Pediatr Blood Cancer. 2008;50:352-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 108. | Myers KC, Bolyard AA, Otto B, Wong TE, Jones AT, Harris RE, Davies SM, Dale DC, Shimamura A. Variable clinical presentation of Shwachman-Diamond syndrome: update from the North American Shwachman-Diamond Syndrome Registry. J Pediatr. 2014;164:866-870. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 109. | Daentl DL, Frías JL, Gilbert EF, Opitz JM. The Johanson-Blizzard syndrome: case report and autopsy findings. Am J Med Genet. 1979;3:129-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 110. | Jones NL, Hofley PM, Durie PR. Pathophysiology of the pancreatic defect in Johanson-Blizzard syndrome: a disorder of acinar development. J Pediatr. 1994;125:406-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 111. | Maunoury V, Nieuwarts S, Ferri J, Ernst O. [Pancreatic lipomatosis revealing Johanson-Blizzard syndrome]. Gastroenterol Clin Biol. 1999;23:1099-1101. [PubMed] [Cited in This Article: ] |
| 112. | Takahashi T, Fujishima M, Tsuchida S, Enoki M, Takada G. Johanson-blizzard syndrome: loss of glucagon secretion response to insulin-induced hypoglycemia. J Pediatr Endocrinol Metab. 2004;17:1141-1144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 113. | Zenker M, Mayerle J, Reis A, Lerch MM. Genetic basis and pancreatic biology of Johanson-Blizzard syndrome. Endocrinol Metab Clin North Am. 2006;35:243-253, vii-viii. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 114. | Alkhouri N, Kaplan B, Kay M, Shealy A, Crowe C, Bauhuber S, Zenker M. Johanson-Blizzard syndrome with mild phenotypic features confirmed by UBR1 gene testing. World J Gastroenterol. 2008;14:6863-6866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 18] [Cited by in F6Publishing: 15] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 115. | Johansson BB, Torsvik J, Bjørkhaug L, Vesterhus M, Ragvin A, Tjora E, Fjeld K, Hoem D, Johansson S, Ræder H. Diabetes and pancreatic exocrine dysfunction due to mutations in the carboxyl ester lipase gene-maturity onset diabetes of the young (CEL-MODY): a protein misfolding disease. J Biol Chem. 2011;286:34593-34605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 116. | Wilson JS, Korsten MA, Leo MA, Lieber CS. New technique for the isolation of functional rat pancreatic mitochondria and its application to models of pancreatic injury. J Lab Clin Med. 1986;107:51-58. [PubMed] [Cited in This Article: ] |
| 117. | Wilson JS, Somer JB, Pirola RC. Chronic ethanol feeding causes accumulation of serum cholesterol in rat pancreas. Exp Mol Pathol. 1984;41:289-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 118. | Yang W, Gao J, Tai Y, Chen M, Huang L, Wen S, Huang Z, Liu R, Li J, Tang C. Betaine Attenuates Alcohol-Induced Pancreatic Steatosis. Pancreas. 2016;45:836-845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 119. | Walters MN, Leak PJ, Joske RA, Stanley NF, Perret dh. Murine Infection with Reovirus. 3. Pathology of Infection with Types 1 and 2. Br J Exp Pathol. 1965;46:200-212. [PubMed] [Cited in This Article: ] |
| 120. | Watanabe S, Abe K, Anbo Y, Katoh H. Changes in the mouse exocrine pancreas after pancreatic duct ligation: a qualitative and quantitative histological study. Arch Histol Cytol. 1995;58:365-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 67] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 121. | Kimura W. Histological study on pathogenesis of sites of isolated islets of Langerhans and their course to the terminal state. Am J Gastroenterol. 1989;84:517-522. [PubMed] [Cited in This Article: ] |
| 122. | Volk BW, Wellman KF. The Diabetic Pancreas. 1st ed. New York: Plenum Press 1977; 317-324. [DOI] [Cited in This Article: ] |
| 123. | Midiri M, Lo Casto A, Sparacia G, D’Angelo P, Malizia R, Finazzo M, Montalto G, Solbiati L, Lagalla R, De Maria M. MR imaging of pancreatic changes in patients with transfusion-dependent beta-thalassemia major. AJR Am J Roentgenol. 1999;173:187-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 124. | Papakonstantinou O, Ladis V, Kostaridou S, Maris T, Berdousi H, Kattamis C, Gourtsoyiannis N. The pancreas in beta-thalassemia major: MR imaging features and correlation with iron stores and glucose disturbances. Eur Radiol. 2007;17:1535-1543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 50] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 125. | Lin WC, Chen JH, Lin CH, Shen WC. Rapidly progressive pancreatic lipomatosis in a young adult patient with transfusion-dependent myelodysplastic syndrome. J Formos Med Assoc. 2007;106:676-679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 126. | Patel S, Bellon EM, Haaga J, Park CH. Fat replacement of the exocrine pancreas. AJR Am J Roentgenol. 1980;135:843-845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 54] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 127. | Kim KH, Kim CD, Ryu HS, Hyun JH, Chung JP, Chung JB, Kang JK, Chi HS, Park JJ. Endoscopic retrograde pancreatographic findings of pancreatic lipomatosis. J Korean Med Sci. 1999;14:578-581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 128. | Park CM, Han JK, Kim TK, Choi BI. Fat replacement with absence of acinar and ductal structure in the pancreatic body and tail. J Comput Assist Tomogr. 2000;24:893-895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 129. | Makay O, Kazimi M, Aydin U, Nart D, Yilmaz F, Zeytunlu M, Goker E, Coker A. Fat replacement of the malignant pancreatic tissue after neoadjuvant therapy. Int J Clin Oncol. 2010;15:88-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 130. | Yu T, Liu R, Li M, Li X, Qiang O, Huang W, Tang C. [Effects of octreotide on fatty infiltration of the pancreas in high-fat diet induced obesity rats]. Wei Sheng Yan Jiu. 2014;43:186-192. [PubMed] [Cited in This Article: ] |
| 131. | Yoshimura N, Hayashi S, Fukushima Y. Diffuse Mallory bodies in the liver, diffuse Lewy bodies in the brain and diffuse fat replacement (lipomatous pseudohypertrophy) of the pancreas in a patient with juvenile Parkinson’s disease. Acta Pathol Jpn. 1992;42:826-831. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 132. | Sasaki M, Nakanuma Y, Ando H. Lipomatous pseudohypertrophy of the pancreas in a patient with cirrhosis due to chronic hepatitis B. Pathol Int. 1998;48:566-568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 133. | Kuroda N, Okada M, Toi M, Hiroi M, Enzan H. Lipomatous pseudohypertrophy of the pancreas: further evidence of advanced hepatic lesion as the pathogenesis. Pathol Int. 2003;53:98-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 134. | Targher G, Rossi AP, Zamboni GA, Fantin F, Antonioli A, Corzato F, Bambace C, Pozzi Mucelli R, Zamboni M. Pancreatic fat accumulation and its relationship with liver fat content and other fat depots in obese individuals. J Endocrinol Invest. 2012;35:748-753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 11] [Reference Citation Analysis (0)] |
| 135. | Patel NS, Peterson MR, Brenner DA, Heba E, Sirlin C, Loomba R. Association between novel MRI-estimated pancreatic fat and liver histology-determined steatosis and fibrosis in non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2013;37:630-639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 136. | Patel NS, Peterson MR, Lin GY, Feldstein A, Schnabl B, Bettencourt R, Seki E, Sirlin CB, Loomba R. Insulin Resistance Increases MRI-Estimated Pancreatic Fat in Nonalcoholic Fatty Liver Disease and Normal Controls. Gastroenterol Res Pract. 2013;2013:498296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 137. | Pacifico L, Di Martino M, Anania C, Andreoli GM, Bezzi M, Catalano C, Chiesa C. Pancreatic fat and β-cell function in overweight/obese children with nonalcoholic fatty liver disease. World J Gastroenterol. 2015;21:4688-4695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 29] [Cited by in F6Publishing: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 138. | Diamond I, Vallbona C. Kwashiorkor in a North American white male. Pediatrics. 1960;25:248-257. [PubMed] [Cited in This Article: ] |
| 139. | Klöppel G, Maillet B. Chronic pancreatitis: evolution of the disease. Hepatogastroenterology. 1991;38:408-412. [PubMed] [Cited in This Article: ] |
| 140. | Klöppel G, Maillet B. The morphological basis for the evolution of acute pancreatitis into chronic pancreatitis. Virchows Arch A Pathol Anat Histopathol. 1992;420:1-4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 127] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 141. | Suda K, Takase M, Takei K, Kumasaka T, Suzuki F. Histopathologic and immunohistochemical studies on the mechanism of interlobular fibrosis of the pancreas. Arch Pathol Lab Med. 2000;124:1302-1305. [PubMed] [Cited in This Article: ] |
| 142. | Gaiser S, Daniluk J, Liu Y, Tsou L, Chu J, Lee W, Longnecker DS, Logsdon CD, Ji B. Intracellular activation of trypsinogen in transgenic mice induces acute but not chronic pancreatitis. Gut. 2011;60:1379-1388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 143. | Acharya C, Cline RA, Jaligama D, Noel P, Delany JP, Bae K, Furlan A, Baty CJ, Karlsson JM, Rosario BL. Fibrosis reduces severity of acute-on-chronic pancreatitis in humans. Gastroenterology. 2013;145:466-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 144. | Singhi AD, Pai RK, Kant JA, Bartholow TL, Zeh HJ, Lee KK, Wijkstrom M, Yadav D, Bottino R, Brand RE. The histopathology of PRSS1 hereditary pancreatitis. Am J Surg Pathol. 2014;38:346-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 145. | Pezzilli R, Calculli L. Pancreatic steatosis: Is it related to either obesity or diabetes mellitus? World J Diabetes. 2014;5:415-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 48] [Cited by in F6Publishing: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 146. | Soeda J, Mouralidarane A, Cordero P, Li J, Nguyen V, Carter R, Kapur SR, Pombo J, Poston L, Taylor PD. Maternal obesity alters endoplasmic reticulum homeostasis in offspring pancreas. J Physiol Biochem. 2016;72:281-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 147. | Kühn JP, Berthold F, Mayerle J, Völzke H, Reeder SB, Rathmann W, Lerch MM, Hosten N, Hegenscheid K, Meffert PJ. Pancreatic Steatosis Demonstrated at MR Imaging in the General Population: Clinical Relevance. Radiology. 2015;276:129-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 148. | Wong VW, Wong GL, Yeung DK, Abrigo JM, Kong AP, Chan RS, Chim AM, Shen J, Ho CS, Woo J. Fatty pancreas, insulin resistance, and β-cell function: a population study using fat-water magnetic resonance imaging. Am J Gastroenterol. 2014;109:589-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 176] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 149. | Lê KA, Ventura EE, Fisher JQ, Davis JN, Weigensberg MJ, Punyanitya M, Hu HH, Nayak KS, Goran MI. Ethnic differences in pancreatic fat accumulation and its relationship with other fat depots and inflammatory markers. Diabetes Care. 2011;34:485-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 150. | Szczepaniak LS, Victor RG, Mathur R, Nelson MD, Szczepaniak EW, Tyer N, Chen I, Unger RH, Bergman RN, Lingvay I. Pancreatic steatosis and its relationship to β-cell dysfunction in humans: racial and ethnic variations. Diabetes Care. 2012;35:2377-2383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 151. | Toledo-Corral CM, Alderete TL, Hu HH, Nayak K, Esplana S, Liu T, Goran MI, Weigensberg MJ. Ectopic fat deposition in prediabetic overweight and obese minority adolescents. J Clin Endocrinol Metab. 2013;98:1115-1121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 152. | Chantarojanasiri T, Hirooka Y, Ratanachu-Ek T, Kawashima H, Ohno E, Goto H. Evolution of pancreas in aging: degenerative variation or early changes of disease? J Med Ultrason (2001). 2015;42:177-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 153. | Gyllenhammer LE, Alderete TL, Toledo-Corral CM, Weigensberg M, Goran MI. Saturation of subcutaneous adipose tissue expansion and accumulation of ectopic fat associated with metabolic dysfunction during late and post-pubertal growth. Int J Obes (Lond). 2016;40:601-606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 154. | Sattar N, Gill JM. Type 2 diabetes as a disease of ectopic fat? BMC Med. 2014;12:123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 127] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 155. | Papandreou D, Rousso I, Mavromichalis I. Update on non-alcoholic fatty liver disease in children. Clin Nutr. 2007;26:409-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 156. | Heni M, Machann J, Staiger H, Schwenzer NF, Peter A, Schick F, Claussen CD, Stefan N, Häring HU, Fritsche A. Pancreatic fat is negatively associated with insulin secretion in individuals with impaired fasting glucose and/or impaired glucose tolerance: a nuclear magnetic resonance study. Diabetes Metab Res Rev. 2010;26:200-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 191] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 157. | Della Corte C, Mosca A, Majo F, Lucidi V, Panera N, Giglioni E, Monti L, Stronati L, Alisi A, Nobili V. Nonalcoholic fatty pancreas disease and Nonalcoholic fatty liver disease: more than ectopic fat. Clin Endocrinol (Oxf). 2015;83:656-662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 158. | Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011;378:31-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2472] [Cited by in F6Publishing: 2384] [Article Influence: 183.4] [Reference Citation Analysis (0)] |
| 159. | Alwan A. Global status report on noncommunicable diseases 2010. 1st ed. Geneva: WHO Press 2011; 1-176 Available from: http://www.who.int/nmh/publications/ncd_report2010/en/. [Cited in This Article: ] |
| 160. | van Raalte DH, van der Zijl NJ, Diamant M. Pancreatic steatosis in humans: cause or marker of lipotoxicity? Curr Opin Clin Nutr Metab Care. 2010;13:478-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 161. | Lee Y, Hirose H, Ohneda M, Johnson JH, McGarry JD, Unger RH. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships. Proc Natl Acad Sci USA. 1994;91:10878-10882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 590] [Cited by in F6Publishing: 595] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 162. | Zhao ZZ, Xin LL, Xia JH, Yang SL, Chen YX, Li K. Long-term High-fat High-sucrose Diet Promotes Enlarged Islets and β-Cell Damage by Oxidative Stress in Bama Minipigs. Pancreas. 2015;44:888-895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 163. | Tushuizen ME, Bunck MC, Pouwels PJ, Bontemps S, van Waesberghe JH, Schindhelm RK, Mari A, Heine RJ, Diamant M. Pancreatic fat content and beta-cell function in men with and without type 2 diabetes. Diabetes Care. 2007;30:2916-2921. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 287] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 164. | Chen DL, Liess C, Poljak A, Xu A, Zhang J, Thoma C, Trenell M, Milner B, Jenkins AB, Chisholm DJ. Phenotypic Characterization of Insulin-Resistant and Insulin-Sensitive Obesity. J Clin Endocrinol Metab. 2015;100:4082-4091. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 165. | Chai J, Liu P, Jin E, Su T, Zhang J, Shi K, Hong XU, Yin J, Yu H. MRI chemical shift imaging of the fat content of the pancreas and liver of patients with type 2 diabetes mellitus. Exp Ther Med. 2016;11:476-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 166. | Maggio AB, Mueller P, Wacker J, Viallon M, Belli DC, Beghetti M, Farpour-Lambert NJ, McLin VA. Increased pancreatic fat fraction is present in obese adolescents with metabolic syndrome. J Pediatr Gastroenterol Nutr. 2012;54:720-726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 167. | Kim MK, Chun HJ, Park JH, Yeo DM, Baek KH, Song KH, Chung DJ, Kwon HS. The association between ectopic fat in the pancreas and subclinical atherosclerosis in type 2 diabetes. Diabetes Res Clin Pract. 2014;106:590-596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 168. | Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640-1645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8720] [Cited by in F6Publishing: 9580] [Article Influence: 638.7] [Reference Citation Analysis (0)] |
| 169. | Sadr-Azodi O, Orsini N, Andrén-Sandberg Å, Wolk A. Abdominal and total adiposity and the risk of acute pancreatitis: a population-based prospective cohort study. Am J Gastroenterol. 2013;108:133-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 71] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 170. | Taguchi M, Kubo T, Yamamoto M, Muramatsu K, Yasunaga H, Horiguchi H, Fujimori K, Matsuda S, Fushimi K, Harada M. Body mass index influences the outcome of acute pancreatitis: an analysis based on the Japanese administrative database. Pancreas. 2014;43:863-866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 171. | Blomgren KB, Sundström A, Steineck G, Wiholm BE. Obesity and treatment of diabetes with glyburide may both be risk factors for acute pancreatitis. Diabetes Care. 2002;25:298-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 172. | Di Ciaula A, Portincasa P. Fat, epigenome and pancreatic diseases. Interplay and common pathways from a toxic and obesogenic environment. Eur J Intern Med. 2014;25:865-873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 173. | Frossard JL, Lescuyer P, Pastor CM. Experimental evidence of obesity as a risk factor for severe acute pancreatitis. World J Gastroenterol. 2009;15:5260-5265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 59] [Cited by in F6Publishing: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 174. | Pokhrel B, Choi EK, Khalid O, Sandrasegaran K, Fogel EL, McHenry L, Sherman S, Watkins J, Cote GA, Pitt HA. Increased fat in pancreas not associated with risk of pancreatitis post-endoscopic retrograde cholangiopancreatography. Clin Exp Gastroenterol. 2014;7:199-204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 175. | Acharya C, Navina S, Singh VP. Role of pancreatic fat in the outcomes of pancreatitis. Pancreatology. 2014;14:403-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 176. | Navina S, Acharya C, DeLany JP, Orlichenko LS, Baty CJ, Shiva SS, Durgampudi C, Karlsson JM, Lee K, Bae KT. Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Sci Transl Med. 2011;3:107ra110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 275] [Article Influence: 22.9] [Reference Citation Analysis (1)] |
| 177. | Patel K, Trivedi RN, Durgampudi C, Noel P, Cline RA, DeLany JP, Navina S, Singh VP. Lipolysis of visceral adipocyte triglyceride by pancreatic lipases converts mild acute pancreatitis to severe pancreatitis independent of necrosis and inflammation. Am J Pathol. 2015;185:808-819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 178. | Stolzenberg-Solomon RZ, Adams K, Leitzmann M, Schairer C, Michaud DS, Hollenbeck A, Schatzkin A, Silverman DT. Adiposity, physical activity, and pancreatic cancer in the National Institutes of Health-AARP Diet and Health Cohort. Am J Epidemiol. 2008;167:586-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 179. | Arslan AA, Helzlsouer KJ, Kooperberg C, Shu XO, Steplowski E, Bueno-de-Mesquita HB, Fuchs CS, Gross MD, Jacobs EJ, Lacroix AZ. Anthropometric measures, body mass index, and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan). Arch Intern Med. 2010;170:791-802. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 255] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 180. | Aune D, Greenwood DC, Chan DS, Vieira R, Vieira AR, Navarro Rosenblatt DA, Cade JE, Burley VJ, Norat T. Body mass index, abdominal fatness and pancreatic cancer risk: a systematic review and non-linear dose-response meta-analysis of prospective studies. Ann Oncol. 2012;23:843-852. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 305] [Cited by in F6Publishing: 307] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 181. | Li D, Morris JS, Liu J, Hassan MM, Day RS, Bondy ML, Abbruzzese JL. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA. 2009;301:2553-2562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 298] [Cited by in F6Publishing: 296] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 182. | O’Rorke MA, Cantwell MM, Cardwell CR, Mulholland HG, Murray LJ. Can physical activity modulate pancreatic cancer risk? a systematic review and meta-analysis. Int J Cancer. 2010;126:2957-2968. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 183. | Luo J, Margolis KL, Adami HO, LaCroix A, Ye W. Obesity and risk of pancreatic cancer among postmenopausal women: the Women’s Health Initiative (United States). Br J Cancer. 2008;99:527-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 184. | Michaud DS, Giovannucci E, Willett WC, Colditz GA, Stampfer MJ, Fuchs CS. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA. 2001;286:921-929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 435] [Cited by in F6Publishing: 400] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 185. | Renehan AG, Roberts DL, Dive C. Obesity and cancer: pathophysiological and biological mechanisms. Arch Physiol Biochem. 2008;114:71-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in F6Publishing: 281] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 186. | Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med. 2010;61:301-316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 427] [Cited by in F6Publishing: 411] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 187. | Jiang CY, Wang W, Yin YL, Yuan ZR, Wang LB. Expression of the adipocytokine resistin and its association with the clinicopathological features and prognosis of pancreatic ductal adenocarcinoma. Oncol Lett. 2012;4:960-964. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 188. | Matsuda M, Shimomura I. Increased oxidative stress in obesity: implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes Res Clin Pract. 2013;7:e330-e341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 375] [Cited by in F6Publishing: 399] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 189. | Lee SE, Jang JY, Lim CS, Kang MJ, Kim SH, Kim MA, Kim SW. Measurement of pancreatic fat by magnetic resonance imaging: predicting the occurrence of pancreatic fistula after pancreatoduodenectomy. Ann Surg. 2010;251:932-936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 190. | Yoon JH, Lee JM, Lee KB, Kim SW, Kang MJ, Jang JY, Kannengiesser S, Han JK, Choi BI. Pancreatic Steatosis and Fibrosis: Quantitative Assessment with Preoperative Multiparametric MR Imaging. Radiology. 2016;279:140-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 191. | Nanashima A, Abo T, Hamasaki K, Wakata K, Kunizaki M, Nakao K, Tanaka K, Fukuda D, Nagasaki T, Tou K. Predictive parameters of intraoperative blood loss in patients who underwent pancreatectomy. Hepatogastroenterology. 2013;60:1217-1221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 192. | Kelly WD, Lillehei RC, Merkel FK, Idezuki Y, Goetz FC. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery. 1967;61:827-837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 193. | Hanish SI, Petersen RP, Collins BH, Tuttle-Newhall J, Marroquin CE, Kuo PC, Butterly DW, Smith SR, Desai DM. Obesity predicts increased overall complications following pancreas transplantation. Transplant Proc. 2005;37:3564-3566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 194. | Humar A, Ramcharan T, Kandaswamy R, Gruessner RW, Gruessner AG, Sutherland DE. The impact of donor obesity on outcomes after cadaver pancreas transplants. Am J Transplant. 2004;4:605-610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 195. | Nghiem DD, Olson PR, Ormond D. The “fatty pancreas allograft”: anatomopathologic findings and clinical experience. Transplant Proc. 2004;36:1045-1047. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 196. | Gullo L, Pezzilli R, Tomassetti P. Unusual association of macroamylasemia and hyperlipasemia: report of two cases. Am J Gastroenterol. 1996;91:2441-2442. [PubMed] [Cited in This Article: ] |
| 197. | Catanzaro R, Italia A. [Pancreatic hyperenzymemia: new advances in the field of clinical-diagnostic approach, with particular attention about Gullo’s syndrome]. Minerva Med. 2012;103:393-412. [PubMed] [Cited in This Article: ] |
| 198. | Cavallini G, Frulloni L, Vaona B, Di Francesco V, Bovo P. Is hyperamylasemia related to dyslipidemia? Gastroenterology. 1997;112:1058-1059. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 199. | Lozano M, Navarro S, Pérez-Ayuso R, Llach J, Ayuso C, Guevara MC, Ros E. Lipomatosis of the pancreas: an unusual cause of massive steatorrhea. Pancreas. 1988;3:580-582. [PubMed] [Cited in This Article: ] |
| 200. | Aubert A, Gornet JM, Hammel P, Lévy P, O’Toole D, Ruszniewski P, Modigliani R, Lémann M. [Diffuse primary fat replacement of the pancreas: an unusual cause of steatorrhea]. Gastroenterol Clin Biol. 2007;31:303-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 201. | Ambesh P, Lal H. Pancreatic Lipomatosis: Complete Replacement of Pancreas by Fat. J Clin Diagn Res. 2015;9:OL01. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 202. | Terzin V, Várkonyi T, Szabolcs A, Lengyel C, Takács T, Zsóri G, Stájer A, Palkó A, Wittmann T, Pálinkás A. Prevalence of exocrine pancreatic insufficiency in type 2 diabetes mellitus with poor glycemic control. Pancreatology. 2014;14:356-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 203. | Honka H, Koffert J, Hannukainen JC, Tuulari JJ, Karlsson HK, Immonen H, Oikonen V, Tolvanen T, Soinio M, Salminen P. The effects of bariatric surgery on pancreatic lipid metabolism and blood flow. J Clin Endocrinol Metab. 2015;100:2015-2023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 204. | Jia DM, Fukumitsu KI, Tabaru A, Akiyama T, Otsuki M. Troglitazone stimulates pancreatic growth in congenitally CCK-A receptor-deficient OLETF rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1332-R1340. [PubMed] [Cited in This Article: ] |
| 205. | Zhao L, Jiang SJ, Lu FE, Xu LJ, Zou X, Wang KF, Dong H. Effects of berberine and cinnamic acid on palmitic acid-induced intracellular triglyceride accumulation in NIT-1 pancreatic β cells. Chin J Integr Med. 2016;22:496-502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |