Published online Sep 7, 2016. doi: 10.3748/wjg.v22.i33.7604
Peer-review started: May 5, 2016
First decision: May 30, 2016
Revised: June 27, 2016
Accepted: July 31, 2016
Article in press: July 31, 2016
Published online: September 7, 2016
To detect chronic hepatitis B (CHB), chronic hepatitis C (CHC) and human immunodeficiency virus (HIV) infections in dried blood spot (DBS) and compare these samples to venous blood sampling in real-life.
We included prospective patients with known viral infections from drug treatment centers, a prison and outpatient clinics and included blood donors as negative controls. Five drops of finger capillary blood were spotted on filter paper, and a venous blood sample was obtained. The samples were analyzed for HBsAg, anti-HBc, anti-HBs, anti-HCV, and anti-HIV levels as well as subjected to a combined nucleic acid test (NAT) for HBV DNA, HCV RNA and HIV RNA.
Samples from 404 subjects were screened (85 CHB, 116 CHC, 114 HIV and 99 blood donors). DBS had a sensitivity of > 96% and a specificity of > 98% for the detection of all three infections. NAT testing did not improve sensitivity, but correctly classified 95% of the anti-HCV-positive patients with chronic and past infections. Anti-HBc and anti-HBS showed low sensitivity in DBS (68% and 42%).
DBS sampling, combined with an automated analysis system, is a feasible screening method to diagnose chronic viral hepatitis and HIV infections outside of the health care system.
Core tip: This study shows that it is feasible to combine serology and nucleic acid screening for hepatitis B virus (HBV), hepatitis C virus (HCV) and human immunodeficiency virus (HIV) infections in one dried blood sample (DBS) collected in real life and analyzed using a modern laboratory platform. We observed a sensitivity and specificity of > 96% for HBV, HCV, and HIV and correctly classified 95% of all HCV patients into past vs chronic infections compared to simultaneously collected venous blood samples. The study confirms that DBSs are feasible samples in outreach clinics and confirms the high sensitivity and specificity of previous laboratory-based studies.
- Citation: Mössner BK, Staugaard B, Jensen J, Lillevang ST, Christensen PB, Holm DK. Dried blood spots, valid screening for viral hepatitis and human immunodeficiency virus in real-life. World J Gastroenterol 2016; 22(33): 7604-7612
- URL: https://www.wjgnet.com/1007-9327/full/v22/i33/7604.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i33.7604
Chronic viral hepatitis B (CHB) and C (CHC) represent a major health burden worldwide, with approximately 350 million and 170 million people infected, respectively[1]. Persistent infection may progress to end-stage liver disease and hepatocellular carcinoma, and primary liver cancer is now the second leading cause of cancer death worldwide[2].
In Denmark, as in most western countries, drug users have the highest prevalence of chronic hepatitis C[3,4]. Still, a Danish register-based study showed that the majority of drug users(67%) have never been tested[5].
The existing literature indicates a wide range of barriers for testing that are related to both the individual patient (e.g., lack of knowledge, not feeling sick, fear of invasive tests, and distrust of the medical system) and the healthcare provider system (e.g., no access to basic medical care or a lack of specific medical care in substance user treatment centers)[6-11].
In Denmark, chronic Hepatitis B and HIV infections are highly prevalent among men who have sex with men, sex workers and immigrants from highly endemic countries. These groups also have difficulties accessing the existing health system and may have poor knowledge of the diseases and risk factors[12].
A recent Danish study has shown that the large majority of HIV patients are successfully managed in the existing health system; however, new interventions are needed to improve care for immigrants and drug users[13].
Easy access to serological testing for Hepatitis C and non-invasive methods to diagnose liver fibrosis have improved the screening rates among drug users. In the era of the new treatment regimens for hepatitis C with direct-acting antivirals (DAA) with few side effects and a short treatment duration, it has now become feasible to test and treat the drug-user population[14-16].
Dried blood samples on filter paper (DBS) were originally used to screen for neonatal phenylketonuria[17]. Since then, the method has been evaluated and used with increased frequency, e.g., antibody testing for a wide range of infectious diseases, particularly in resource-limited settings[18-21].
Recently, it was shown that DBS can be combined with automatic analysis on a modern analysis platform (e.g., Abbott Architect), allowing high throughput testing at a reasonable price[22,23]. However, in most of these studies, venous whole blood was used for the DBS samples instead of capillary blood, and the performance and feasibility of “real life” DBS testing in this set-up is, to our knowledge, not well described.
The objective of this study was to determine and compare the sensitivity of DBS from capillary blood to whole plasma when the DBS sampling was obtained in real life settings and analyzed at a modern high throughput diagnostic laboratory using an automated analytic platform.
The study was conducted from September 2014 to September 2015. Combined NAT screening for HIV RNA, HBV DNA and HCV RNA was performed using the Procleix Ultrio Elite assay from Grifols (Grifols Diagnostic Solutions, Allschwil, Switzerland), and serological screening for HIV, HBV and HCV was performed using Abbott Architect assays (Abbott Diagnostics, Delkenheim, Germany) .
The study was conducted in the region of southern Denmark.
Patients had a known HBV, HCV or HIV infection, and attended either the Outreach Clinics in the Drug Treatment Center or state prison in Nyborg or the Outpatient Clinic at the Hospital to be eligible for inclusion. Patients were informed about the study at previously planned visits. If they were willing to participate, they were included with due consideration time.
Due to the relatively low prevalence of HIV and HBV infections among Danish drug users and prisoners, paired EDTA plasma samples/DBS were obtained from these patients at the Departments of Infectious Diseases at, Odense University Hospital and Lillebaelt Hospital, Kolding. Samples from Hepatitis C-infected patients were obtained from drug treatment centers and the prison. According to the power estimation, optimally, 100 patients with each infection should be included, corresponding to an overall 30% prevalence of the tested markers, which is comparable to the 30%-40% prevalence of HCV infection in the target drug-using population. In addition, paired EDTA plasma samples/DBS were obtained from 99 blood donors who were negative for all three viruses from the blood bank at Odense University Hospital.
The DBS and EDTA plasma samples were collected simultaneously in most cases, but up to 10 d was allowed between the two samples.
All samples were analyzed at the Department of Clinical Immunology, Odense University Hospital. Five spots on a Whatman® 903 protein saver card (Sigma-Aldrich, Copenhagen, Denmark) were each covered with a large drop of capillary blood (approximately 75 μL) from the fingertip(s) of the patient/donor and subsequently dried at room temperature for at least 24 h and up to 5 d to prepare the DBS.
The approximate volume of 75 μL of whole blood in each dried blood spot was based on preliminary studies where different amounts of exact volumes of whole blood were added to the Whatman® 903 protein saver card. We found that 75 μL was the amount needed to fill a single spot on the Whatman filter paper. In the real-life study, the intention was to fill each of the 5 spots using their visual appearance, thereby approximating a volume of 75 microliters in each spot.
We compared the test results for all viruses in DBS samples that had dried for 24 h or for up to 7 d at room temperature using the NAT test to validate the stability and found no difference (data not shown). Each of the dried blood spots was eluted with 1000 μL of a buffer (PBS/0.05% Tween 20/0.08% sodium azide) overnight at room temperature on a shaker, followed by centrifugation of the eluate for two minutes at 10000 rpm. Except for the discriminatory NAT analysis, all laboratory analyses were performed immediately after centrifugation of the eluates. The average estimated DBS sample dilution was 1:23 compared to plasma.
The DBS eluates and the corresponding plasma samples were tested for the presence of anti-HIV, anti-HCV, hepatitis B surface antigen, anti-HBc and anti-HBs with the Architect HIV Ab/Ag, Architect HCV Ab, Architect HBsAg, Architect HBs Ab and Architect HBc Ab assays using the Architect system (Abbott Diagnostics, Delkenheim, Germany). In addition, all samples were tested with the Procleix Ultrio Elite assay using the Procleix Panther System (Grifols Diagnostic Solutions, Allschwil, Switzerland). The Ultrio Elite assay is a qualitative nucleic acid amplification test (NAT) for the simultaneous detection of HIV-1/2 RNA, HCV RNA and HBV DNA. The detection limits (at the 95% confidence level) for the Ultrio Elite assay are 18.0 IU/mL (HIV-1-RNA), 3.0 IU/mL (HCV-RNA) and 4.3 IU/mL (HBV-DNA), according to the package insert. For samples that were reactive in the initial NAT analysis, an additional discriminatory NAT was performed for HIV-1/2 RNA (Ultrio Elite dHIV), HCV RNA (Ultrio Elite dHCV), and HBV DNA (Ultrio Elite dHBV). The initially reactive samples were stored at -20 °C for 1-5 d before the discriminatory NAT was performed. All plasma samples were analyzed according to the manufacturer’s instructions.
A quantitative nucleic acid amplification test was performed using the COBAS® AmpliPrep/COBAS® TaqMan® 48 system (Roche Diagnostics, Basel, Switzerland) to quantify the viral load in plasma samples that were positive for either HIV RNA, HCV RNA or HBV DNA in the Ultrio Elite assay. The COBAS® AmpliPrep/COBAS® TaqMan® HIV-1 Test, v2.0, COBAS® AmpliPrep/COBAS® TaqMan® HCV Quantitative Test, v2.0 and COBAS® AmpliPrep/COBAS® TaqMan® HBV Test, v2.0 assays were used, and all the quantitative analyses were performed according to the manufacturer’s instructions. The lower detection limit for the Cobas TaqMan assays described above was slightly higher than the NAT according to the package insert: 20 copies/mL (HIV), 15 IU/mL (HCV) and 20 IU/mL (HBV).
The DBS results were compared with plasma, which was used as the gold standard to assess the sensitivity, specificity, positive predictive value, negative predictive value and receiver operating characteristics curve (ROC-curve) of the DBS results. Descriptive statistics were shown as the means, medians and interquartile ranges, with the minimum and maximum values reported, as appropriate. A confidence interval of 95% was used. All data analysis was performed using STATA 14 IC software (Statacorp LP, College Station, TX).
Sample size and power estimation: With an expected prevalence of 33% (100 positive and 200 negative tests for all markers, except the HBs antibody), an observed agreement of 95% between DBS and plasma samples would correspond to 5 false negative and 10 false positive results for each marker. More than 12 false negative and 20 false positive samples would lead to a rejection of the 95% agreement between the venous blood sample and DBS.
Ethical considerations: All patients signed written informed consent before inclusion in the study. The Danish Research Ethics Committee approved the study (Project-ID: S-20140128).
Corresponding plasma samples and DBS were obtained from the 404 subjects included in the study: 114 HIV-infected, 85 hepatitis B-infected, 116 hepatitis C-infected and 99 blood donors. The blood donors were all negative for the 3 infections (Table 1).
| Viral infection | Total, n | Mono- + Co-infected, n | N with quantitative analysis | Median | IQR (25-75) |
| Blood donors | 99 | 0 | NA | ||
| HBV | 85 | 78 + 5 HIV + 2 HCV | 78 | 144 | 19-2780 |
| HCV | 116 | 111 + 3 HIV + 2 HBV | 105 | 225000 | 4300-1110000 |
| HIV | 114 | 106 + 5 HBV + 3 HCV | 112 | 0 | 0-19 |
The overall performance of the serology markers for anti-HCV, anti-HIV and HBsAg had a sensitivity of > 96% and a specificity >99% for all three markers. In contrast, a lower sensitivity for anti-HBc and anti-HBs was observed (Table 2).
| Plasma, n | DBS, n | Sensitivity (%) | Specificity (%) (95%CI) | ROC | PPV | NPV | |||||
| Negative | Positive | Negative | Positive | (95%CI) | |||||||
| Anti-HCV | 288 | 116 | 292 | 112 | 96.6 | (91.4; 99.1) | 100.0 | (98.7; 100) | 0.98 | 100.0% | 98.6% |
| HBsAg | 318 | 86 | 319 | 85 | 96.5 | (90.1; 99.3) | 99.4 | (97.7; 99.9) | 0.98 | 97.6% | 99.1% |
| Anti-HBc | 232 | 172 | 286 | 118 | 68.0 | (60.5; 74.9) | 99.6 | (97.6; 100) | 0.84 | 99.2% | 80.8% |
| Anti-HBs | 246 | 158 | 337 | 67 | 42.4 | (34.6; 50.5) | 100.0 | (98.5; 100) | 0.71 | 100.0% | 73.0% |
| Anti-HIV | 289 | 115 | 290 | 114 | 98.3 | (93.9; 99.8) | 99.7 | (98.1; 100) | 0.99 | 99.1% | 99.3% |
After combining all analyses, we correctly classified 98.0% (396/404) of patients for all 3 infections and 99.3% of all serological tests (1204/1212) using DBS, with 6 false negative (4 anti-HCV, 1 HBsAg and 1 anti-HIV) and 2 false positive (HBsAg) results. One patient with hepatitis B was HBsAg-negative in both plasma and DBS. Thus, the addition of NAT identified 1 (HBV) patient who would have been missed by serological testing alone. However, the overall advantage of adding NAT to the analysis was that 95% of Hepatitis C patients were correctly classified into ongoing vs past infection groups. For HBV and HIV, the classification of viremia by DBS was not reliable because a significant proportion (16% and 98%) of these patients had very low viral loads as result of antiviral treatment.
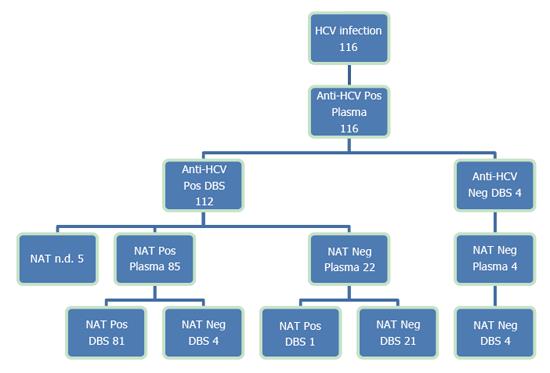
Among the 116 known HCV-infected patients, all were plasma anti-HCV-positive and 112 (96.6%) were also DBS anti-HCV-positive (Figure 1). The four DBS-negative patients were all HCV-RNA-negative in both plasma and DBS, indicating a past infection. Among the plasma anti-HCV-positive patients, 73.3% (85/116) were plasma NAT-positive, indicating an ongoing infection. Of these patients, 95.3% (81/85) were also DBS NAT-positive, and 100% (85/85) of the active infections were anti-HCV-positive in the DBS.
The four patients who were plasma HCV RNA-positive and DBS-negative had a viral load < 100 IU/mL (range 0-95), and two of these were on DAA treatment. One patient was plasma NAT-negative, but weakly DBS-positive, corresponding to a specificity of 99.5% (193 DBS/194 plasma) for the NAT.
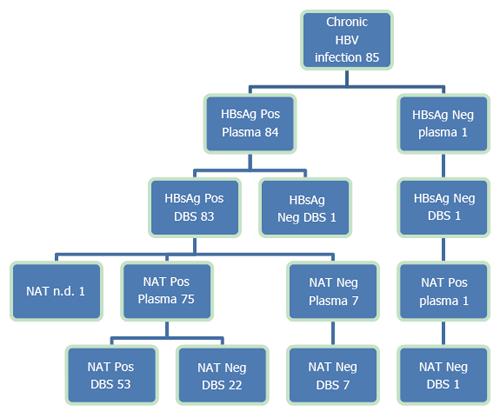
Among the 85 known HBV-infected patients, 83 were both plasma- and DBS-positive for HBsAg, one was only positive in plasma and one was negative in both plasma and DBS (Figure 2). The patient who was HBsAg-positive in plasma and negative in DBS had a quantitative plasma HBsAg level below 175 IU/mL. This patient was under treatment for HBV infection and HBV DNA was positive in both plasma and DBS. The patient who was HBsAg-negative in both plasma and DBS was also under treatment for HBV. HBV DNA was positive in plasma, but below the LLOQ, anti-HBc and anti-HBs were positive in both plasma and DBS, suggesting seroconversion. All 71 untreated CHB patients were HBsAg-positive by DBS.
Two participants without known HBV infections were (weakly) positive for HBsAg in DBS and negative in plasma; one was an HCV-infected patient and one was a blood donor. Both were anti-HBs-positive in plasma and NAT-negative in both plasma and DBS. A duplicate retest of the two DBS samples showed that they were HBsAg-negative; thus, we concluded that the first DBS test was a false positive test.
A low sensitivity for anti-HBc (68%) and anti-HBs (42%) was observed. In 2/85 CHB patients, discrepant results for anti-HBc were observed, with positive plasma and negative DBS (CMIA-plasma 5.2-6.8 vs CMIA-DBS 0.3-0.4); both were HBsAg-positive and HBV DNA-negative.
Among the 91 anti-HBc-positive plasma samples from non-HBV infected patients, discrepant results were found in 53 samples in which the plasma was positive (CMIA median 6.19, range 1.1-10.1) and DBS was negative (CMIA median 0.295, range 0.06-0.99).
Among the 53 discrepant DBS-negative results, one was co-infected with HIV/ HCV, 31 were anti-HIV-positive, 19 were anti-HCV-positive, and 2 were negative for all other serological markers (blood donors).
In twelve out of 85 CHB patients, a discrepant result for the anti-HBs analysis was obtained. Anti-HBs was positive in plasma (1.08-9.44 mIU/mL), but negative in the DBS (0-0.3 mIU/mL). However, all twelve patients were HBsAg-positive in both plasma and DBS.
Among the 145 anti-HBs-positive plasma samples in non-HBV infected patients, discrepant results were observed in 79 patients in which the plasma was positive (median 9.9 mIU/mL, range 1-75) and the DBS was negative (median 0.01 mIU/mL, range 0-0.93). Among the 79 discrepant DBS-negative results, one was co-infected with HIV/ HCV, 43 were anti-HCV-positive, 17 were anti-HIV-positive, and 18 were negative for all other serological markers (blood donors). Vaccination status for Hepatitis B was not recorded in our study, but of the HBV-negative patients, 106 had protective anti-HBs titers (anti-HBs ≥ 10 mIU/mL) in plasma compared to 28 in the DBS.
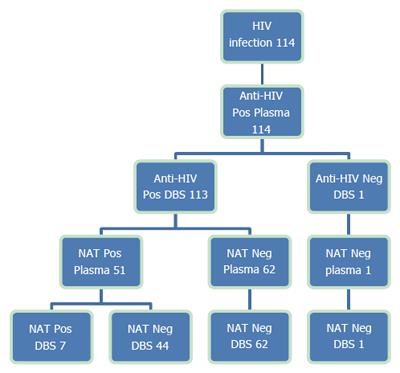
Among the 114 patients with known HIV infections, all were anti-HIV-positive in plasma, and 113 (99.1%) were also positive in the DBS (Figure 3). The one HIV-positive patient who was not detected using the DBS received antiretroviral treatment (ART) and had undetectable levels of the HIV-RNA in both plasma and DBS.
Furthermore, one blood donor was weakly anti-HIV-positive in plasma (1.62/cut-off 1.0), but negative in the anti-HIV confirmatory test. The donor was also DBS anti-HIV-negative and NAT-negative in both plasma and DBS.
Among the 114 anti-HIV-positive patients, 112 were undergoing antiretroviral treatment. HIV-RNA was negative in both plasma and DBS from 54.9% of patients (62/113), 6.2% (7/113) were positive in both tests, and 38.9% (44/113) were HIV-RNA-positive in plasma and negative in DBS. Among these patients, 43 received ART and had the following HIV-RNA levels: 20 patients were below the LLD (lower limit of detection), 15 were below the LLOQ (lower limit of quantification) and 8 patients had quantifiable HIV-RNA levels (range 32-483 copies/mL).
The use of DBS with a high throughput automated platform to screen for viral Hepatitis B, C and HIV showed acceptable agreement between the plasma and DBS tests for anti-HCV, anti-HIV and HBsAg in real-life settings. However, the overall sensitivity of anti-HBc and anti-HBs was low. There are several possible explanations for this observation, including the amount of blood used for the DBS samples was small and the addition of the eluate-buffer further diluted the sample 23-fold. In a study by Lee et al[24], DBS was prepared from venous blood samples, and they noted that the assay titers obtained from the DBS samples were generally lower than the titers obtained from the plasma samples. The authors suggested that different cut-off values should be used to validate tests that were positive in the DBS because the small amount of blood in the DBS led to lower assay titers.
The low sensitivity for the serological markers anti-HBs and anti-HBc in DBS vs plasma observed in our study is in contrast to recent studies using automated platforms[22,23]. This observation may to some extent be caused by the different starting titers of the samples, resulting in different dilution factors by elution. The results are consistent with other studies, where low DBS sensitivities have been reported, ranging between 70% and 100%, with specificities from 94% to 100%[18,25,26]. In the study by Ross, almost perfect sensitivities and specificities for DBS were observed compared to serum samples; however, DBS were prepared by applying 100 μL of whole blood to generate DBS (Whatman filter paper), a procedure that most likely is not possible with DBS in real-world settings, where we estimated the average amount to be 75 μL. However, with this standardized method, allowances could be made for the dilution and a lower cut-off calculated for anti-HBs in DBS samples. When applying this cut-off, the sensitivity rose from 43% to 54% in our study, which was still not acceptable (data not shown).
We speculate that if an indicator for the amount of blood collected on the paper could be developed to ensure that enough blood is present for the analysis (e.g., weight, hemoglobin, visual guidance, etc.), it would also allow the calculation of a quantitative anti-HBs level/mL of serum from DBS, enabling the method to be used in outreach vaccination trials.
A study by Villar showed that a lower sampling volume led to more discordant results and emphasized that even though the sampling is relatively easy, care must be taken to ensure an adequate sample size[27]. We recommend that the visual inspection should be used to confirm that the circles of the filter paper have been appropriately filled with blood when using DBS for routine screening.
In our study population, only moderate agreement was observed for HBV DNA and HIV RNA by NAT, probably because a significant number of the included patients had very low viral loads as a result of antiviral therapy. Several studies have shown decreased sensitivity of DBS compared to plasma in samples with lower viral loads[20,28,29]. In our study, we estimated that the LLD was below 500 copies/mL for HIV-RNA, 100 IU/mL for HCV-RNA and 200 IU/mL for HBV-DNA by DBS testing. This result may be partially explained by the 23-fold dilution of the DBS compared to plasma during preparation. However, this dilution is unlikely to be a significant problem when using DBS as a screening method in an untreated population. In this setting, viral loads below DBS cut-offs are rare, and all these patients will be positive by serology testing.
Our study has several limitations. As it is a real-life study, we did not know the volume of blood applied to the DBS filter paper (and we did not record whether the filter circles were appropriately filled with blood). Therefore, we cannot rule out the possibility that the discrepant results were due to a low sampling volume. Furthermore, due to the low prevalence of HIV and HBV in our target population, a significant number of the HBV- and HIV-infected participants were recruited from the Department of Infectious Diseases and had low or undetectable viral loads as a result of antiviral treatment. However, as a screening tool, DBS will be used among treatment naïve patients, and in this population, DBS had 100% sensitivity, except for HBV patients in the latent phase, who will be detected by HBsAg.
As for the 4 HCV patients who were anti-HCV-positive but HCV RNA false negative in DBS, we suggest that patients who were categorized as having past HCV infection using DBS should have this result confirmed using a venous blood sample.
As the NAT did not improve DBS sensitivity, it would have been more cost-effective to perform DBS testing as a two-step analysis: first screening with anti-HCV, anti-HIV and HBsAg, and then performing a NAT of samples that were positive in serology. Depending on the prevalence of infection, this strategy could save 50%-95% of NATs and still identify > 95% of chronic infections. Some primary advantages of DBS are that screening of all three infections can be performed in one sample; the samples can be collected by on-site personnel, ensuring correct person identification; and the samples may be sent by regular mail to the laboratory. These advantages enable “on-site” testing without the need for specialized personnel, and therefore, the test is both cheaper and more effective than the current methods used to identify patients with chronic viral hepatitis or HIV infections.
In conclusion, the collection of DBS outside of the health care system, combined with an automated laboratory system for analysis, is a feasible method of screening for chronic viral hepatitis and HIV infections among drug users in prisons and other resource-limited settings.
In Danish guidelines, yearly screening in drug users is recommended; however, this test has been difficult to implement using venous blood sampling. Easy access to DBS testing enables large-scale implementation in difficult to reach populations. The identification of the infected patients is the first step to control these infections, and we suggest that national testing strategies should include DBS testing to improve the coverage of clinical care in these populations.
Easy access to serological testing for hepatitis C (HCV) and non-invasive methods to diagnose liver fibrosis have improved the screening rates among drug users. dried blood spot (DBS) testing enables large-scale implementation in difficult to reach populations. Recently, it has been shown that combining DBS with automatic analysis on a modern analysis platform allows high throughput testing at a reasonable price. However, in most of these studies, venous whole blood was used for the DBS instead of capillary blood, and the performance and feasibility of “real-life” DBS testing using this set-up is, to our knowledge, not well described.
Performance of DBS testing in real-life settings, as well as strengths and limitations.
Easy access to DBS testing enables large-scale implementation in difficult to reach populations. The identification of the infected patients is the first step to control these infections. In real-life settings, high sensitivity for detecting HCV, HBV or HIV infections was observed. However, the sensitivities for anti-HBs and anti-HBc were lower than in previous laboratory-based studies, and refinement/further studies are needed before implementing the DBS method as a screening method for vaccination status.
The collection of DBS samples outside of the health care system, combined with an automated laboratory system for analysis, is a feasible method for screening for chronic viral hepatitis and HIV infections in prison populations, drug users and other resource-limited settings.
DBS; Dried blood spot is a method where blood samples are blotted and dried on filter paper, which enables on-site testing without the need for specialized personnel. The dried samples can be sent to the laboratory by mail.
Mössner et al investigates the sensitivity and specificity of a Dried Blood Spot (DBS) screening for HIV, HBV and HCV in patients in drug treatment centers. The authors find that DBS had a high sensitivity > 96% and a high specificity 98% for all three infections; however, the antiHBc and antiHBs showed low sensitivities in DBS (42% and 68%, respectively). Chronic infections such as HIV, HBV and HCV remains major sources of mortality and morbidity. However, due to their mild symptoms during early phases of infection, most of infected people are not aware of infection status. Developing a low-cost medically scalable diagnostic tool for testing chronic infections such as HIV, HBV and HCV, is of great importance and has substantial public health implications. Evaluating the feasibility using DBS as diagnostic tool is especially important in a resource limited settings. Thus, this manuscript addresses an important question, and the results are promising in many ways. It is well written. The methodology and analysis are well documented and the results are analyzed rigorously.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Denmark
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ke RA, Seitz R S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Te HS, Jensen DM. Epidemiology of hepatitis B and C viruses: a global overview. Clin Liver Dis. 2010;14:1-21, vii. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 304] [Cited by in F6Publishing: 319] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 2. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11128] [Cited by in F6Publishing: 11614] [Article Influence: 893.4] [Reference Citation Analysis (4)] |
| 3. | Bruggmann P, Berg T, Øvrehus AL, Moreno C, Brandão Mello CE, Roudot-Thoraval F, Marinho RT, Sherman M, Ryder SD, Sperl J. Historical epidemiology of hepatitis C virus (HCV) in selected countries. J Viral Hepat. 2014;21 Suppl 1:5-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 179] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 4. | Qureshi K, Cowan S. Acute and Chronic Hepatitis C 2007. 2008;. [Cited in This Article: ] |
| 5. | Christensen PB, Hay G, Jepsen P, Omland LH, Just SA, Krarup HB, Weis N, Obel N, Cowan S. Hepatitis C prevalence in Denmark -an estimate based on multiple national registers. BMC Infect Dis. 2012;12:178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Wong VW, Wong GL, Chim AM, Cheng TF, Cheung SW, Lai CM, Szeto KJ, Tsang S, Wu SH, Yan KK. Targeted hepatitis C screening among ex-injection drug users in the community. J Gastroenterol Hepatol. 2014;29:116-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Treloar C, Newland J, Rance J, Hopwood M. Uptake and delivery of hepatitis C treatment in opiate substitution treatment: perceptions of clients and health professionals. J Viral Hepat. 2010;17:839-844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 8. | Swan D, Long J, Carr O, Flanagan J, Irish H, Keating S, Keaveney M, Lambert J, McCormick PA, McKiernan S. Barriers to and facilitators of hepatitis C testing, management, and treatment among current and former injecting drug users: a qualitative exploration. AIDS Patient Care STDS. 2010;24:753-762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 9. | Strauss SM, Astone-Twerell J, Munoz-Plaza CE, Des Jarlais DC, Gwadz M, Hagan H, Osborne A, Rosenblum A. Drug treatment program patients’ hepatitis C virus (HCV) education needs and their use of available HCV education services. BMC Health Serv Res. 2007;7:39. [PubMed] [Cited in This Article: ] |
| 10. | Grebely J, Genoway KA, Raffa JD, Dhadwal G, Rajan T, Showler G, Kalousek K, Duncan F, Tyndall MW, Fraser C. Barriers associated with the treatment of hepatitis C virus infection among illicit drug users. Drug Alcohol Depend. 2008;93:141-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 164] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 11. | Barocas JA, Brennan MB, Hull SJ, Stokes S, Fangman JJ, Westergaard RP. Barriers and facilitators of hepatitis C screening among people who inject drugs: a multi-city, mixed-methods study. Harm Reduct J. 2014;11:1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Hansen N, Hay G, Cowan S, Jepsen P, Bygum Krarup H, Obel N, Weis N, Brehm Christensen P. Hepatitis B prevalence in Denmark - an estimate based on nationwide registers and a national screening programme, as on 31 December 2007. Euro Surveill. 2013;18. [PubMed] [Cited in This Article: ] |
| 13. | Helleberg M, Häggblom A, Sönnerborg A, Obel N. HIV care in the Swedish-Danish HIV cohort 1995-2010, closing the gaps. PLoS One. 2013;8:e72257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Hickman M, McDonald T, Judd A, Nichols T, Hope V, Skidmore S, Parry JV. Increasing the uptake of hepatitis C virus testing among injecting drug users in specialist drug treatment and prison settings by using dried blood spots for diagnostic testing: a cluster randomized controlled trial. J Viral Hepat. 2008;15:250-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | McAllister G, Innes H, Mcleod A, Dillon JF, Hayes PC, Fox R, Barclay ST, Templeton K, Aitken C, Gunson R. Uptake of hepatitis C specialist services and treatment following diagnosis by dried blood spot in Scotland. J Clin Virol. 2014;61:359-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Moessner BK, Jørgensen TR, Skamling M, Vyberg M, Junker P, Pedersen C, Christensen PB. Outreach screening of drug users for cirrhosis with transient elastography. Addiction. 2011;106:970-976. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Guthrie R, Susi A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics. 1963;32:338-343. [PubMed] [Cited in This Article: ] |
| 18. | Brandão CP, Marques BL, Marques VA, Villela-Nogueira CA, Do Ó KM, de Paula MT, Lewis-Ximenez LL, Lampe E, Sá Ferreira JA, Villar LM. Simultaneous detection of hepatitis C virus antigen and antibodies in dried blood spots. J Clin Virol. 2013;57:98-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Castro AC, Borges LG, Souza Rda S, Grudzinski M, D’Azevedo PA. Evaluation of the human immunodeficiency virus type 1 and 2 antibodies detection in dried whole blood spots (DBS) samples. Rev Inst Med Trop Sao Paulo. 2008;50:151-156. [PubMed] [Cited in This Article: ] |
| 20. | Mohamed S, Raimondo A, Pénaranda G, Camus C, Ouzan D, Ravet S, Bourlière M, Khiri H, Dukan P, Olive D. Dried blood spot sampling for hepatitis B virus serology and molecular testing. PLoS One. 2013;8:e61077. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Snijdewind IJ, van Kampen JJ, Fraaij PL, van der Ende ME, Osterhaus AD, Gruters RA. Current and future applications of dried blood spots in viral disease management. Antiviral Res. 2012;93:309-321. [PubMed] [Cited in This Article: ] |
| 22. | Ross RS, Stambouli O, Grüner N, Marcus U, Cai W, Zhang W, Zimmermann R, Roggendorf M. Detection of infections with hepatitis B virus, hepatitis C virus, and human immunodeficiency virus by analyses of dried blood spots--performance characteristics of the ARCHITECT system and two commercial assays for nucleic acid amplification. Virol J. 2013;10:72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 23. | Soulier A, Poiteau L, Rosa I, Hézode C, Roudot-Thoraval F, Pawlotsky JM, Chevaliez S. Dried Blood Spots: A Tool to Ensure Broad Access to Hepatitis C Screening, Diagnosis, and Treatment Monitoring. J Infect Dis. 2016;213:1087-1095. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 24. | Lee CE, Sri Ponnampalavanar S, Syed Omar SF, Mahadeva S, Ong LY, Kamarulzaman A. Evaluation of the dried blood spot (DBS) collection method as a tool for detection of HIV Ag/Ab, HBsAg, anti-HBs and anti-HCV in a Malaysian tertiary referral hospital. Ann Acad Med Singapore. 2011;40:448-453. [PubMed] [Cited in This Article: ] |
| 25. | Dokubo EK, Evans J, Winkelman V, Cyrus S, Tobler LH, Asher A, Briceno A, Page K. Comparison of Hepatitis C Virus RNA and antibody detection in dried blood spots and plasma specimens. J Clin Virol. 2014;59:223-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Tejada-Strop A, Drobeniuc J, Mixson-Hayden T, Forbi JC, Le NT, Li L, Mei J, Terrault N, Kamili S. Disparate detection outcomes for anti-HCV IgG and HCV RNA in dried blood spots. J Virol Methods. 2015;212:66-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Villar LM, de Oliveira JC, Cruz HM, Yoshida CF, Lampe E, Lewis-Ximenez LL. Assessment of dried blood spot samples as a simple method for detection of hepatitis B virus markers. J Med Virol. 2011;83:1522-1529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Bennett S, Gunson RN, McAllister GE, Hutchinson SJ, Goldberg DJ, Cameron SO, Carman WF. Detection of hepatitis C virus RNA in dried blood spots. J Clin Virol. 2012;54:106-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Johannessen A, Garrido C, Zahonero N, Sandvik L, Naman E, Kivuyo SL, Kasubi MJ, Gundersen SG, Bruun JN, de Mendoza C. Dried blood spots perform well in viral load monitoring of patients who receive antiretroviral treatment in rural Tanzania. Clin Infect Dis. 2009;49:976-981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |