Published online Aug 28, 2016. doi: 10.3748/wjg.v22.i32.7373
Peer-review started: March 9, 2016
First decision: March 31, 2016
Revised: April 27, 2016
Accepted: May 23, 2016
Article in press: May 23, 2016
Published online: August 28, 2016
To assess the efficacy of CO2 insufflation for reduction of mediastinal emphysema (ME) immediately after endoscopic submucosal dissection (ESD).
A total of 46 patients who were to undergo esophageal ESD were randomly assigned to receive either CO2 insufflation (CO2 group, n = 24) or air insufflation (Air group, n = 22). Computed tomography (CT) was carried out immediately after ESD and the next morning. Pain and abdominal distention were chronologically recorded using a 100-mm visual analogue scale (VAS). The volume of residual gas in the digestive tract was measured using CT imaging.
The incidence of ME immediately after ESD in the CO2 group was significantly lower than that in the Air group (17% vs 55%, P = 0.012). The incidence of ME the next morning was 8.3% vs 32% respectively (P = 0.066). There were no differences in pain scores or distention scores at any post-procedure time points. The volume of residual gas in the digestive tract immediately after ESD was significantly smaller in the CO2 group than that in the Air group (808 mL vs 1173 mL, P = 0.013).
CO2 insufflation during esophageal ESD significantly reduced postprocedural ME. CO2 insufflation also reduced the volume of residual gas in the digestive tract immediately after ESD, but not the VAS scores of pain and distention.
Core tip: This randomized, double-blind, controlled trial assessed the efficacy of CO2 insufflation for reduction of mediastinal emphysema immediately after endoscopic submucosal dissection (ESD). This study showed that CO2 insufflation during esophageal ESD significantly reduced postprocedural mediastinal emphysema. CO2 insufflation also reduced the volume of residual gas in the digestive tract immediately after ESD, but not the visual analogue scale scores of pain and distention.
- Citation: Maeda Y, Hirasawa D, Fujita N, Ohira T, Harada Y, Yamagata T, Koike Y, Suzuki K. Carbon dioxide insufflation in esophageal endoscopic submucosal dissection reduces mediastinal emphysema: A randomized, double-blind, controlled trial. World J Gastroenterol 2016; 22(32): 7373-7382
- URL: https://www.wjgnet.com/1007-9327/full/v22/i32/7373.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i32.7373
Carbon dioxide (CO2) is rapidly cleared from the gastrointestinal (GI) tract by passive absorption and subsequently exhaled from the lungs. In several studies, CO2 insufflation during diagnostic or therapeutic endoscopy has been shown to be safe and effective in reducing procedure-related pain and discomfort[1-5].
The safety of CO2 insufflation for endoscopic submucosal dissection (ESD) has also been shown in several studies[6-8]. As for esophageal ESD, it is known that mediastinal emphysema (ME) can develop even if no perforation is recognized during or at the end of the procedure because the esophagus has no serosa[9-11]. CO2 insufflation during esophageal ESD is expected to reduce the incidence of ME. We have previously reported the results of a pilot study concerning ME after esophageal ESD with CO2 insufflation[8]. To further assess the efficacy of CO2 insufflation for reduction of post-ESD ME, we conducted a prospective, double-blind, randomized controlled trial, the results of which are reported herein.
This study was a single-center, randomized, double-blind, controlled trial in Japan. This study was approved by the institutional review board of Sendai City Medical Center and met all criteria of the Declaration of Helsinki. The trial was registered with the UMIN Clinical Trials Registry (No. UMIN000006441).
Between February 2011 and May 2012, all consecutive patients undergoing esophageal ESD at the center were screened for recruitment. The inclusion criterion was all consecutive patients undergoing esophageal ESD. The following patients were excluded: those who had severe chronic obstructive pulmonary disease (COPD) resulting in less than 50% of the predicted values of the forced expiratory volume in 1 s (FEV1.0) or less than 70% of FEV1.0/FVC (forced vital capacity)[12], those who had experienced CO2 retention, those who had multiple synchronous esophageal lesions treated at one time, those who were to undergo esophageal ESD under general anesthesia with positive pressure ventilation, and those who refused to participate. All participants provided written informed consent prior to enrollment in the study.
Participants were randomly assigned to either the CO2 insufflation group (CO2 group) or the air insufflation group (Air group). Randomization took place immediately before the ESD procedure. Individual randomization to the two treatment groups (1:1) was performed by using computer-generated random numbers. A sequentially numbered, opaque, sealed envelope containing a random number was opened sequentially by an endoscopy nurse after participant details were written on the envelope. When the number was even, the patient was allocated to the CO2 group and administration of CO2 was started. When the number was odd, the endoscopy nurse pretended to start administration of CO2. Both the CO2 regulator and the air inlet button on the processor were concealed from the endoscopists, so that the patients and the endoscopists were all blind with regard to the type of gas used. The endoscopy nurse was responsible for switching the CO2 device on and off. The CO2 delivery system was set in the endoscopy room and attached to the endoscopic air-water auxiliary system throughout the study period, regardless of its use.
ESD was performed as described by Oyama et al[10], using a HookKnife, GIF-Q260J Gastroscope (Olympus Medical System Corp., Tokyo, Japan) and an electrocautery unit (ICC200; ERBE, Tübingen, Germany). The modes of electric power used were the 50 W auto-cut mode and the 50 W spray-coagulation mode[8,9]. Ten percent glycerin with 0.007% epinephrine was used for local injection into the submucosal layer. The ESD procedures in this study were performed by three endoscopists who had at least 5 years’ experience in endoscopy and experience in more than 20 cases of gastric ESD. The procedures were performed on an inpatient basis.
In the CO2 group, CO2 was administered by using a commercially available CO2 regulation unit (OLYMPUS UCR; Olympus), which was connected to a CO2 bottle. A CO2 nasal sampling set with O2 tubing (CapnoLine H O2; ORIDON MEDICAL 1987 Ltd., Israel) was used to monitor end-tidal CO2 pressure (EtCO2). Standard monitors including electrocardiography, an oscillometric blood pressure cuff and a pulse oximeter were employed.
The sedation technique was standardized for all patients. No premedication was given. Propofol was administered slowly as a drip infusion approximately 10 mg/kg per hour initially, with monitoring of the patient’s level of consciousness and movement. The level of sedation was evaluated following the American Society of Anesthesiologists classification and maintained at a moderate to deep level[13,14]. After achieving a suitable sedation level for ESD, drip infusion (1-5 mg/kg per hour) of propofol using a syringe pump was continued and adjusted to maintain an adequate depth of sedation. An analgesic (pentazocine, 7.5-15 mg) was given intravenously at the beginning of sedation and further injection was performed depending on the patient’s condition. When the combination of propofol and pentazocine could not achieve or maintain an adequate level of sedation, droperidol was added[15].
On the day of ESD and the day after, the patient was kept fasting. An antibiotic (Cefamezin 1 g × 2/d) was administered intravenously for 3 d after the procedure. When a patient suffered from a fever of over 38 °C and/or from post-sternal pain, fasting and administration of antibiotics were prolonged until symptoms improved.
The primary outcome was the incidence of ME evaluated on computed tomography (CT) immediately after ESD. The secondary outcome measurements were as follows: incidence of ME the next morning, severity of pain and bowel distention, volume of residual gas in the GI tract, amount of sedative drugs, procedure time, EtCO2 pressure, oxygen saturation, rate of en-bloc resection and R0-resection, and clinical course.
Low-dose plain CT was carried out immediately after ESD and the next morning. A 64-detector row helical CT (Aquilion 64 TSX-101A; Toshiba Medical Systems Co., Tochigi, Japan) with automatic exposure control (AEC) (Volume EC; Toshiba Medical Systems Co.), which adjusts tube current automatically to achieve consistent image quality and to reduce the radiation dose, was employed[16-18]. To further reduce the radiation dose, targeted SD of CT values in the setting of CT-AEC in this study was set at 30 as a low-dose protocol, which is much higher than that of 7.5 in the standard protocol. All other parameters were the same as those of the standard protocol of CT scanning with a constant voltage of 120 kV. For a CT scan with a scanned length of approximately 400 mm, an effective dose based on the effective weighted CT dose index was expected to be approximately 1.9 mGy in the low-dose technique used in this study, which is much lower than that of 30 mGy in the standard protocol.
Four grades of ME were employed: Grade-0, no ME; Grade-I, bubbles around the esophagus; Grade-II, ME around the thoracic aorta; Grade-III, ME extending around the heart and/or beyond the mediastinum into the neck; and Grade-IV, ME with pneumothorax and/or subcutaneous emphysema (Figure 1)[9].
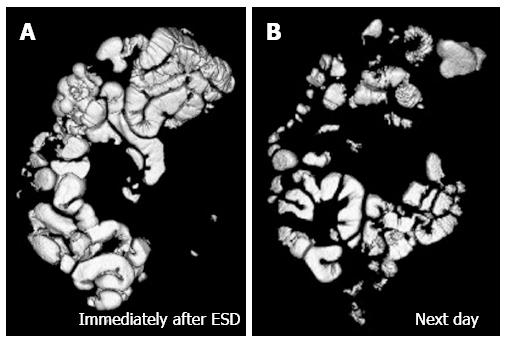
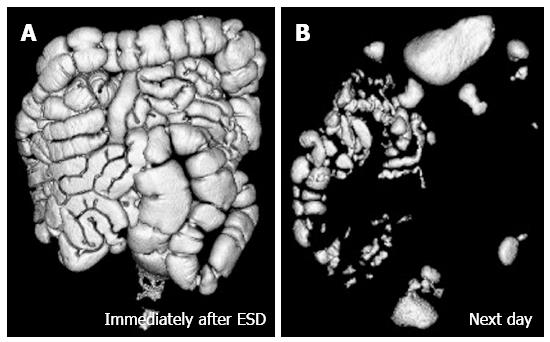
The CT data were transferred to a workstation running a software program (Ziosstation; Ziosoft Inc. Tokyo, Japan) for volume rendering. The volume of residual gas was calculated from the volume-rendering image of the GI tract. Figure 2 shows a rendering image of the residual gas in the GI tract after completion of ESD with CO2 insufflation immediately after ESD (Figure 2A) and the next morning (Figure 2B), the volume of residual gas being 517 mL and 217 mL respectively. The case shown in Figure 3 received air insufflation during ESD, the volume of residual gas being 1638 mL immediately after ESD (Figure 3A) and 224 mL the next morning (Figure 3B).
The degrees of pain and bowel distention were recorded using a 100-mm visual analogue scale (VAS) immediately after the procedure, 1 and 3 h after the procedure and the next morning. The amount of sedative drugs (propofol, pentazocine and droperidol), procedure time, EtCO2 pressure, oxygen saturation, rate of en bloc resection and R0-resection, and clinical courses were recorded.
Sample size was determined by power calculation using Fisher’s exact test. Based on the results of a pilot study[8], the incidence of ME with air insufflation was 63% and that of CO2 was 30%. To detect this difference with a power of 0.7 and alpha of 0.05, 22 patients per group would be required. Assuming dropout, we set our recruitment goal as 46 patients total.
Analyses were performed on an intention-to-treat basis for patients who underwent the treatment. Continuous variables (e.g., VAS) were compared by using the t-test, and categorical variables (e.g., incidence of ME) were compared by using the χ2 test (or Fisher’s exact test, when appropriate). A two-sided P value of < 0.05 was considered statistically significant for all tests.
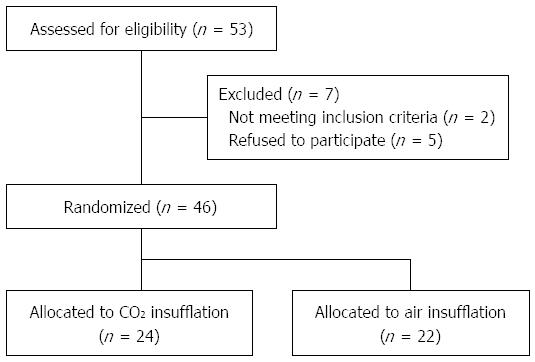
Between February 2011 and May 2012, 53 patients underwent esophageal ESD in our department. Figure 4 shows the flow of these patients. After exclusion of those who were to undergo ESD under general anesthesia with positive pressure ventilation (n = 2) and those who refused to participate (n = 5), a total of 46 patients consented to take part in the trial and were randomized: 24 to receive CO2 insufflation (CO2 group) and 22 to receive air insufflation (Air group).
The demographic data of patients are shown in Table 1; the two groups did not differ at baseline. The mean procedure time was 69.2 min in the CO2 group and 65.0 min in the Air group, with no statistically significant difference (NS).
| CO2 group | Air group | P value | |
| Total No. of patients | 24 | 22 | |
| Sex, M/F | 21/3 | 19/3 | 0.7460 |
| Age (yr, mean ± SD) | 67.5 ± 5.8 | 72.0 ± 7.2 | 0.7718 |
| Location1 | |||
| Cervical esophagus (Ce) | 0 | 0 | 0.6015 |
| Upper thoracic esophagus (Ut) | 2 | 4 | |
| Middle thoracic esophagus (Mt) | 17 | 11 | |
| Lower thoracic esophagus (Lt) | 4 | 4 | |
| Abdominal esophagus (Ae) | 1 | 3 | |
| Histology1 | |||
| Squamous cell carcinoma | 23 | 19 | 0.3364 |
| Barrett’s adenocarcinoma | 1 | 3 | |
| Histological depth1 | |||
| EP | 5 | 6 | 0.1734 |
| LPM | 11 | 9 | |
| MM | 4 | 6 | |
| SM1 | 0 | 1 | |
| SM2 | 4 | 0 | |
| Tumor size (mm, mean ± SD) | 26.6 ± 14.4 | 27.4 ± 22.9 | 0.8955 |
| Resection size (mm, mean ± SD) | 40.0 ± 14.1 | 42.3 ± 21.2 | 0.6620 |
| En-bloc resection | 24 | 22 | - |
| R0 resection | 22 | 20 | 0.9378 |
| HM+ | 1 | 1 | 0.5087 |
| VM+ | 1 | 0 | 0.9649 |
| Ly+ | 0 | 0 | - |
| V+ | 1 | 3 | 0.3364 |
| Procedure time (min, mean ± SD) | 69.2 ± 28.1 | 65.0 ± 39.2 | 0.6847 |
Although most patients in both groups had squamous cell carcinoma, 1 patient in the CO2 group and 3 patients in the Air group had Barrett’s adenocarcinoma.
The average size of the resected specimen was 40.0 mm vs 42.3 mm, respectively (NS). The rate of R0 resection was 92% vs 95%, respectively (NS).
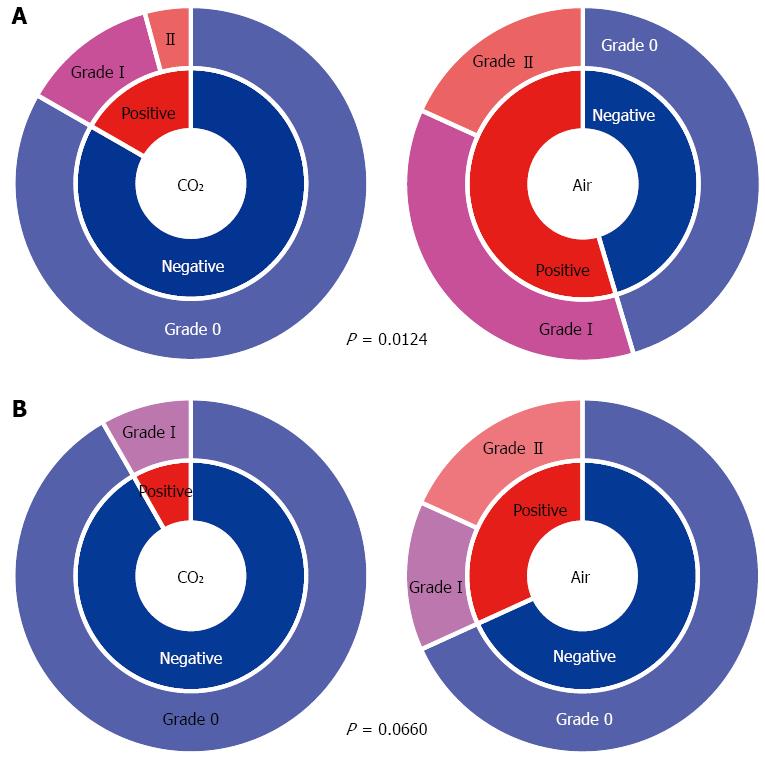
In the CO2 group, the incidence of ME immediately after ESD was significantly less compared with that in the Air group (17% vs 55%, P = 0.012) (Figure 5A). As for the grade of ME immediately after ESD, Grade-I was 13% in the CO2 group vs 36% in the Air group. Grade-II was 4.2% vs 18%, and Grade-III and Grade IV were 0% in both groups. The CO2 group tended to have a lower grade of ME (P = 0.065) (Figure 5A). The incidence of ME the next morning tended to be lower in the CO2 group compared with that in the Air group (8.3% vs 32%, P = 0.066) (Figure 5B). About half of Grade-I ME observed immediately after ESD had disappeared by the next morning (Figure 5).
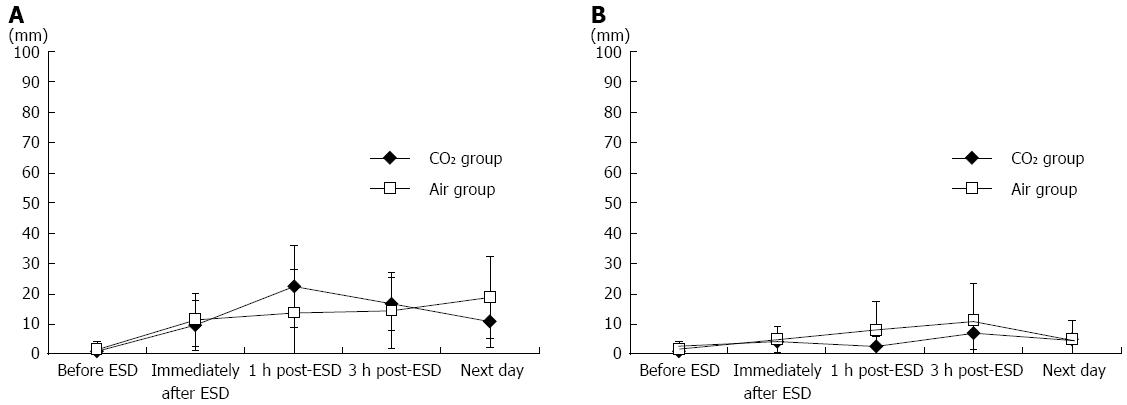
The mean severity of pain on a 100-mm VAS in the CO2 group compared that in the Air group was 9.6 mm vs 11.1 mm immediately after ESD, 22.4 vs 13.9 at 1-h after the procedure, 16.7 vs 14.3 at 3-h after the procedure, and 10.9 vs 18.8 the next morning, showing no difference between the groups (Figure 6A). There were no differences in the mean severity of abdominal distension at any post-procedure point of time either (Figure 6B).
The mean volume of residual gas in the GI tract immediately after ESD was significantly smaller in the CO2 group than that in the Air group (803 mL vs 1173 mL, P = 0.013) (Table 2). On the day after the procedure, gas volume in the GI tract was reduced in both groups without a significant difference between the groups (P = 0.945).
| CO2 group | Air group | P value | |
| Gas volume in the GI tract | |||
| Immediately after ESD (mL) | 803 ± 371 | 1173 ± 580 | 0.0128 |
| Next day (mL) | 300 ± 136 | 304 ± 215 | 0.9449 |
| End-tidal carbon dioxide partial pressure (EtCO2) measurements | |||
| Baseline EtCO2 level (mmHg) | 38.2 ± 3.6 | 38.1 ± 4.1 | 0.7543 |
| Maximum EtCO2 level (mmHg) | 45.9 ± 4.1 | 44.3 ± 6.7 | 0.8562 |
| Oxygen saturation (SpO2) measurements | |||
| Baseline SpO2 level (%) | 98.9 ± 1.3 | 98.4 ± 1.0 | 0.2762 |
| Minimum SpO2 level (%) | 93.7 ± 3.4 | 93.9 ± 2.3 | 0.8198 |
| Sedative drugs | |||
| Propofol dose (mg) | 537 ± 258 | 610 ± 533 | 0.5655 |
| Pentazocine hydrochloride dose (mg) | 27.2 ± 4.5 | 27.1 ± 5.8 | 0.9658 |
| Droperidol used, No. of patients | 5 | 2 | 0.4859 |
| Droperidol dose (mg) | 3.0 ± 1.1 | 2.5 ± 0.0 | 0.5761 |
Concerning maximum EtCO2 pressure levels during ESD, there was no difference between the CO2 and the Air groups (45.9 mmHg vs 44.3 mmHg). Minimum oxygen saturation levels by pulse oximeter (SpO2) did not differ by group either (93.7% vs 93.9%) (Table 2).
The impact of CO2 insufflation on the dosages of sedative drugs administered during the procedures was assessed. The mean dosage of propofol used was 537 mg in the CO2 group and 610 mg in the Air group, with no statistically significant difference (Table 2). The mean dosage of pentazocine did not differ either. The number of the cases using droperidol was 5 in the CO2 group and 2 in the Air group. The mean dosage of droperidol in those cases was 3.0 mg in the CO2 group and 2.5 mg in the Air group (Table 2).
No perforation or postprocedural bleeding was encountered in either of the groups. The incidence of fever of over 38 °C was infrequent and similar in both groups (8.3% vs 9.1%, NS). The mean duration of fever over 38 °C was 1.5 d vs 2.0 d, respectively (NS) (Table 3). The mean duration of fasting did not differ by group. The incidence of adverse events was infrequent and did not differ between the two groups. All cases recovered with conservative treatment such as prolonged fasting and administration of antibiotics (Table 3).
| CO2 group | Air group | P value | |
| Fever ≥ 38 °C | 8.3% | 9.1% | 0.6652 |
| Duration of fever ≥ 38 °C (d) | 1.5 ± 0.7 | 2.0 ± 1.4 | 0.6984 |
| Duration of fasting, d | 2.4 ± 0.8 | 2.1 ± 0.2 | 0.1639 |
| Complications | |||
| Perforation | 0% | 0% | - |
| Post-procedure hemorrhage | 0% | 0% | - |
| Esophageal stricture with dysphagia | 8.3% | 9.1% | 0.6652 |
| Pneumonia | 0% | 0% | - |
| Death | 0% | 0% | - |
CO2 is rapidly absorbed from the GI tract into the bloodstream and subsequently excreted through expiration. The usefulness and safety of CO2 as an alternative to air in patients who undergo diagnostic or therapeutic endoscopy under conscious or intravenously sedated conditions have been demonstrated in several randomized controlled studies[1-6]. No pulmonary complications or CO2 retention have reportedly occurred from CO2 insufflation in patients without some type of pulmonary dysfunction, and no adverse event related to CO2 insufflation developed in the present study either. Neither elevation of EtCO2 levels nor depression of SpO2 levels occurred due to CO2 insufflation, compared with air insufflation. These results indicate that CO2 insufflation is safe to use during esophageal ESD.
ME can develop after esophageal ESD even without perforation because the esophagus has no serosa. In contrast, no free air without perforation after gastric ESD was observed in a previously reported randomized controlled study[6]. During ESD, it is mandatory to maintain an adequate endoscopic view with insufflation of gas to achieve a safe procedure. In cases with exposure of the muscular layer, leakage of the insufflated gas into the mediastinum via the gap of the muscle fibers is considered to be a mechanism for the development of ME during ESD. However, ME can develop even in cases without exposure of the muscular layer[9], indicating that preservation of the submucosa is not a perfect barrier against leakage of insufflated gas. This randomized controlled study demonstrated that CO2 insufflation during esophageal ESD can significantly reduce postprocedural ME as compared with air insufflation. CO2 insufflation may restrain the increase in the inner pressure of the esophagus as a result of rapid absorption into the bloodstream. ME itself may also rapidly disappear because leaking CO2 in the mediastinum is also more quickly absorbed into the bloodstream than air. ME detected by X-ray is not so common though CT immediately after ESD revealed a certain prevalence of post-ESD ME[9-11]. Patients with high-grade ME are more likely to develop severe inflammatory changes and to experience a longer febrile period[9]. CT evaluation of the mediastinum for early recognition of extensive ME will lead to prompt careful observations and timely treatments, resulting in avoidance of severe complications. Meanwhile, low-grade ME is asymptomatic. Evaluation of ME on CT is not always necessary for patients who have undergone esophageal ESD. Based on the results of this study, we now evaluate ME on CT only for suspected cases of severe ME. The incidences of ME in both groups were lower in this study than those in the prior pilot study[8]. Improvement of ESD techniques might decrease the incidence of ME. CO2 insufflation may be more effective for beginners.
The volume of residual gas in the GI tract immediately after ESD was significantly smaller in the CO2 group than in the Air group. The gas volume in the GI tract on the day after the procedure decreased in both groups to about the same level. Scores of pain and distention on 100-mm VAS at any post-procedure points of time were consistently low and similar in both groups. Neither the dosage of sedative drugs required during ESD nor the clinical course differed. These results were similar to those of a trial performed in patients undergoing gastric ESD[6], namely, CO2 insufflation reduces bowel gas volume but not procedure-related pain and discomfort. Although most trials concerning CO2 insufflation during various kinds of endoscopic procedures have demonstrated a reduction of pain and discomfort[2-5], some randomized trials in endoscopic retrograde cholangiopancreatography have reported that CO2 insufflation was not effective in reducing procedure-related pain[20,21], the same as in this trial. Most of the patients in this trial had no pain after the procedure and the mean VAS scores of pain and distension were consistently low not only in the CO2 group but also, unexpectedly, in the Air group. One of the reasons for this result may be that sufficiently deep sedation with propofol and pentazocine during ESD may have provided palliation of pain and discomfort of the patients. The half-life of the sedative drugs used, propofol and pentazocine, is 2.6 and 43.8 min, respectively, though pentazocine is reported to provide analgesia as long as 3 to 4 h[22]. The effectiveness of CO2 insufflation in ESD for the reduction of pain and discomfort remains in question.
Another possible advantage of CO2 insufflation is fewer adverse events. Air insufflation is associated with rare but serious adverse events of endoscopic procedures, such as air embolism and tension pneumothorax[23-27]. As a matter of fact, several fatal air embolisms caused by endoscopic procedures have been reported. CO2 is expected to reduce the incidence and severity of such adverse events because CO2 in the vessels is also more rapidly absorbed into bloodstream than air.
In this study protocol, low-dose CT (approximately 1.9 mGy, which is much lower than 30 mGy in the standard technique) was performed immediately after ESD and the next morning. In view of the inherently high contrast between air and the soft tissue density of body organs, a low-dose protocol was employed for CT, without a loss of diagnostic accuracy. Low-dose protocols for CT have been used in many studies, such as the CT colonography and for detection of occult colonic perforation after colonoscopy[28-30]. Low-dose CT is considered to be a standard technique for the evaluation of ME and measurement of the residual gas in the GI tract.
The present study has some limitations. This trial was conducted at a single center. Clinical significance in consequence of a reduction of ME was not demonstrated. In spite of these limitations, the use of CO2 for insufflation during esophageal ESD is recommended due to the above-mentioned reasons.
In conclusion, insufflation of CO2 during esophageal ESD, as compared with that of air, significantly reduced postprocedural ME. CO2 insufflation can be recommended for esophageal ESD.
Carbon dioxide (CO2) is rapidly cleared from the GI tract by passive absorption and subsequently exhaled from the lungs. In several studies, CO2 insufflation during diagnostic or therapeutic endoscopy has been shown to be safe and effective in reducing procedure-related pain and discomfort. As for esophageal endoscopic submucosal dissection (ESD), it is known that mediastinal emphysema can develop after ESD even without perforation because the esophagus has no serosa. CO2 insufflation during esophageal ESD is expected to reduce the incidence of mediastinal emphysema.
The authors have previously reported the results of a pilot study concerning mediastinal emphysema after esophageal ESD with CO2 insufflation. To further assess the efficacy of CO2 insufflation for reduction of post-ESD mediastinal emphysema, they conducted a prospective, double-blind, randomized controlled trial.
This randomized controlled study demonstrated that CO2 insufflation during esophageal ESD can significantly reduce postprocedural mediastinal emphysema as compared with air insufflation. CO2 insufflation was also shown to reduce the volume of residual gas in the digestive tract immediately after ESD.
Insufflation of CO2 during esophageal ESD, as compared with that of air, significantly reduced postprocedural mediastinal emphysema. CO2 insufflation can be recommended for esophageal ESD.
Mediastinal emphysema sometimes develops following esophageal ESD without perforation because the esophagus has no serosa. In cases with exposure of the muscular layer during ESD, leakage of the insufflated gas into the mediastinum via the gap of the muscle fibers is considered to be a mechanism for the development of mediastinal emphysema. However, mediastinal emphysema can develop even in cases without exposure of the muscular layer, indicating that preservation of the submucosa is not a perfect barrier against leakage of insufflated gas. Mediastinal emphysema detected by X-ray is not so common, although CT immediately after ESD revealed a certain prevalence of post-ESD mediastinal emphysema. Patients with high-grade mediastinal emphysema are more likely to develop severe inflammatory changes and to experience a longer febrile period. Endoscopists should strive to avoid mediastinal emphysema in esophageal ESD.
The work is well-done, well-written, documented and structured. The information included is interesting and the number of cases presented is very valuable. This study provides interesting results on the efficacy of CO2 insufflation for reduction of ME immediately after ESD.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gonzalez ES, Tan YF S- Editor: Qi Y L- Editor: Filipodia E- Editor: Ma S
| 1. | Dellon ES, Hawk JS, Grimm IS, Shaheen NJ. The use of carbon dioxide for insufflation during GI endoscopy: a systematic review. Gastrointest Endosc. 2009;69:843-849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 2. | Sumanac K, Zealley I, Fox BM, Rawlinson J, Salena B, Marshall JK, Stevenson GW, Hunt RH. Minimizing postcolonoscopy abdominal pain by using CO(2) insufflation: a prospective, randomized, double blind, controlled trial evaluating a new commercially available CO(2) delivery system. Gastrointest Endosc. 2002;56:190-194. [PubMed] [Cited in This Article: ] |
| 3. | Bretthauer M, Thiis-Evensen E, Huppertz-Hauss G, Gisselsson L, Grotmol T, Skovlund E, Hoff G. NORCCAP (Norwegian colorectal cancer prevention): a randomised trial to assess the safety and efficacy of carbon dioxide versus air insufflation in colonoscopy. Gut. 2002;50:604-607. [PubMed] [Cited in This Article: ] |
| 4. | Maple JT, Keswani RN, Hovis RM, Saddedin EZ, Jonnalagadda S, Azar RR, Hagen C, Thompson DM, Waldbaum L, Edmundowicz SA. Carbon dioxide insufflation during ERCP for reduction of postprocedure pain: a randomized, double-blind, controlled trial. Gastrointest Endosc. 2009;70:278-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Domagk D, Bretthauer M, Lenz P, Aabakken L, Ullerich H, Maaser C, Domschke W, Kucharzik T. Carbon dioxide insufflation improves intubation depth in double-balloon enteroscopy: a randomized, controlled, double-blind trial. Endoscopy. 2007;39:1064-1067. [PubMed] [Cited in This Article: ] |
| 6. | Maeda Y, Hirasawa D, Fujita N, Obana T, Sugawara T, Ohira T, Harada Y, Yamagata T, Suzuki K, Koike Y. A prospective, randomized, double-blind, controlled trial on the efficacy of carbon dioxide insufflation in gastric endoscopic submucosal dissection. Endoscopy. 2013;45:335-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Saito Y, Uraoka T, Matsuda T, Emura F, Ikehara H, Mashimo Y, Kikuchi T, Kozu T, Saito D. A pilot study to assess the safety and efficacy of carbon dioxide insufflation during colorectal endoscopic submucosal dissection with the patient under conscious sedation. Gastrointest Endosc. 2007;65:537-542. [PubMed] [Cited in This Article: ] |
| 8. | Maeda Y, Hirasawa D, Fujita N, Obana T, Sugawara T, Ohira T, Harada Y, Yamagata T, Suzuki K, Koike Y. A pilot study to assess mediastinal emphysema after esophageal endoscopic submucosal dissection with carbon dioxide insufflation. Endoscopy. 2012;44:565-571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Maeda Y, Hirasawa D, Fujita N, Suzuki T, Obana T, Sugawara T, Ohira T, Harada Y, Noda Y. Mediastinal emphysema after esophageal endoscopic submucosal dissection: its prevalence and clinical significance. Dig Endosc. 2011;23:221-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Oyama T, Tomori A, Hotta K, Morita S, Kominato K, Tanaka M, Miyata Y. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 2005;3:S67-S70. [PubMed] [Cited in This Article: ] |
| 11. | Tamiya Y, Nakahara K, Kominato K, Serikawa O, Watanabe Y, Tateishi H, Takedatsu H, Toyonaga A, Sata M. Pneumomediastinum is a frequent but minor complication during esophageal endoscopic submucosal dissection. Endoscopy. 2010;42:8-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Committee for the Third Edition of the COPD Guidelines of The Japanese Respiratory Society. Guidelines for the Diagnosis and Treatment of COPD (Chronic Obstructive Pulmonary Disease). 3rd ed. 2009;. [Cited in This Article: ] |
| 13. | Faigel DO, Baron TH, Goldstein JL, Hirota WK, Jacobson BC, Johanson JF, Leighton JA, Mallery JS, Peterson KA, Waring JP. Guidelines for the use of deep sedation and anesthesia for GI endoscopy. Gastrointest Endosc. 2002;56:613-617. [PubMed] [Cited in This Article: ] |
| 14. | Training Committee. American Society for Gastrointestinal Endoscopy. Training guideline for use of propofol in gastrointestinal endoscopy. Gastrointest Endosc. 2004;60:167-172. [PubMed] [Cited in This Article: ] |
| 15. | Yamagata T, Hirasawa D, Fujita N, Suzuki T, Obana T, Sugawara T, Ohira T, Harada Y, Maeda Y, Koike Y. Efficacy of propofol sedation for endoscopic submucosal dissection (ESD): assessment with prospective data collection. Intern Med. 2011;50:1455-1460. [PubMed] [Cited in This Article: ] |
| 16. | Lee TY, Chhem RK. Impact of new technologies on dose reduction in CT. Eur J Radiol. 2010;76:28-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Kalra MK, Naz N, Rizzo SM, Blake MA. Computed tomography radiation dose optimization: scanning protocols and clinical applications of automatic exposure control. Curr Probl Diagn Radiol. 2005;34:171-181. [PubMed] [Cited in This Article: ] |
| 18. | Matsumoto K, Ohno Y, Koyama H, Kono A, Inokawa H, Onishi Y, Nogami M, Takenaka D, Araki T, Sugimura K. 3D automatic exposure control for 64-detector row CT: radiation dose reduction in chest phantom study. Eur J Radiol. 2011;77:522-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | The Japan Esophageal Society. Japanese Classification of Esophageal Cacer[article in Japanese with English abstract in part]. Tokyo: Kanehara Shuppan 2008; . [Cited in This Article: ] |
| 20. | Dellon ES, Velayudham A, Clarke BW, Isaacs KL, Gangarosa LM, Galanko JA, Grimm IS. A randomized, controlled, double-blind trial of air insufflation versus carbon dioxide insufflation during ERCP. Gastrointest Endosc. 2010;72:68-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Kuwatani M, Kawakami H, Hayashi T, Ishiwatari H, Kudo T, Yamato H, Ehira N, Haba S, Eto K, Kato M. Carbon dioxide insufflation during endoscopic retrograde cholangiopancreatography reduces bowel gas volume but does not affect visual analogue scale scores of suffering: a prospective, double-blind, randomized, controlled trial. Surg Endosc. 2011;25:3784-3790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Sadove MS, Balagot RC. Pentazocine--A New Nonaddicting Analgesic. A Double-Blind Evaluation in Postoperative Pain. JAMA. 1965;193:887-892. [PubMed] [Cited in This Article: ] |
| 23. | Katzgraber F, Glenewinkel F, Fischler S, Rittner C. Mechanism of fatal air embolism after gastrointestinal endoscopy. Int J Legal Med. 1998;111:154-156. [PubMed] [Cited in This Article: ] |
| 24. | Bisceglia M, Simeone A, Forlano R, Andriulli A, Pilotto A. Fatal systemic venous air embolism during endoscopic retrograde cholangiopancreatography. Adv Anat Pathol. 2009;16:255-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Green BT, Tendler DA. Cerebral air embolism during upper endoscopy: case report and review. Gastrointest Endosc. 2005;61:620-623. [PubMed] [Cited in This Article: ] |
| 26. | Morley AP, Lau JY, Young RJ. Tension pneumothorax complicating a perforation of a duodenal ulcer during ERCP with endoscopic sphincterotomy. Endoscopy. 1997;29:332. [PubMed] [Cited in This Article: ] |
| 27. | Rai A, Iftikhar S. Tension pneumothorax complicating diagnostic upper endoscopy: a case report. Am J Gastroenterol. 1999;94:845-847. [PubMed] [Cited in This Article: ] |
| 28. | van Gelder RE, Venema HW, Florie J, Nio CY, Serlie IW, Schutter MP, van Rijn JC, Vos FM, Glas AS, Bossuyt PM. CT colonography: feasibility of substantial dose reduction--comparison of medium to very low doses in identical patients. Radiology. 2004;232:611-620. [PubMed] [Cited in This Article: ] |
| 29. | Florie J, van Gelder RE, Schutter MP, van Randen A, Venema HW, de Jager S, van der Hulst VP, Prent A, Bipat S, Bossuyt PM. Feasibility study of computed tomography colonography using limited bowel preparation at normal and low-dose levels study. Eur Radiol. 2007;17:3112-3122. [PubMed] [Cited in This Article: ] |
| 30. | Hough DM, Kuntz MA, Fidler JL, Johnson CD, Petersen BT, Kofler JM, Fletcher JG. Detection of occult colonic perforation before CT colonography after incomplete colonoscopy: perforation rate and use of a low-dose diagnostic scan before CO2 insufflation. AJR Am J Roentgenol. 2008;191:1077-1081. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |