Published online Aug 21, 2016. doi: 10.3748/wjg.v22.i31.7030
Peer-review started: March 26, 2016
First decision: May 12, 2016
Revised: June 10, 2016
Accepted: July 6, 2016
Article in press: July 6, 2016
Published online: August 21, 2016
Hepatitis E was identified as an epidemic of non-A, non-B hepatitis from Kashmir, India in 1978. Hepatitis E virus (HEV), the etiological agent is the sole member of family Hepeviridae. The virus has marked heterogeneity and infects many animals like bats, camel, chicken, deer, boar, mongoose, pigs, rats, rabbit and cutthroat trout. Hepatitis E is a disease with a major global impact and has two distinct epidemiological patterns. Hepatitis E is an imperative health issue in developing nations, transmitted through sullied water and happens most every now in young adults. The disease is particularly severe during pregnancy and in people with underlying liver cirrhosis. Autochthonous hepatitis E is increasingly recognized in developed countries. The virus infects domestic pigs, wild boar and Sika deer in these countries. HEV infections in humans occur by eating the undercooked game flesh, raw liver from supermarkets and Figatelli sausages. Blood transfusion-associated HEV infections occur in many countries and screening of donors for HEV RNA is under consideration. Hepatitis E causes a number of extrahepatic diseases, including a wide spectrum of neurological syndromes. HEV genotype 3 causes prolonged viremia, chronic hepatitis, liver fibrosis and cirrhosis in organ transplant patients. The virus is amenable to ribavirin monotherapy and most patients clear the virus in a few weeks. Hepatitis E vaccine -239, marketed in China, has shown high efficacy with sustained protection for over four years.
Core tip: Discovery of hepatitis E came to lime light when 1978-Kashmir epidemic of hepatitis was investigated. Hepatitis E is being recognized as a clinical entity of reemerging importance. Hepatitis E virus has marked heterogeneity and infects many animals like bats, camel, chicken, deer, boar, mongoose, pigs, rats, rabbit and cutthroat trout. Originally reported as a major health problem in poor-resource countries, hepatitis E is now recognized as an important clinical problem in the developed world. Zoonotic foodborne transmission of hepatitis E virus infections has relevance in solid organ transplant population. Major advances have been made in managing chronic hepatitis E. Hepatitis E causes a number of extrahepatic diseases, including a wide spectrum of neurological syndromes. Hepatitis E vaccine -239, marketed in China, has shown high efficacy with sustained protection for over four years.
- Citation: Khuroo MS, Khuroo MS, Khuroo NS. Hepatitis E: Discovery, global impact, control and cure. World J Gastroenterol 2016; 22(31): 7030-7045
- URL: https://www.wjgnet.com/1007-9327/full/v22/i31/7030.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i31.7030
Hepatitis E was identified as an epidemic of non-A, non-B hepatitis (NANBH) from Kashmir, India in 1978[1]. In the last 36 years since the discovery of the disease, major advances have occurred in relation to its causative agent, the host range in the animal kingdom, epidemiology and modes of spread[2]. Hepatitis E virus (HEV) infections are ubiquitous in developing countries as a cause of epidemic and endemic acute hepatitis[3]. However, the disease is now encountered in developed countries as well[4]. Zoonotic foodborne transmission of HEV infection has a particular relevance to the organ transplant population[5]. HEV infections cause a number of non-hepatic manifestations, which include a wide spectrum of neurological syndromes[6,7]. Transfusion-associated HEV infections are reported with increasing frequencies and screening of donors for HEV RNA is being considered[8-10]. Major advances have been made in understanding chronic hepatitis E[11] and the commercial availability of hepatitis E vaccine (HEV-239) in China promises control of epidemic and sporadic hepatitis E[12]. This article shall review the knowledge about the discovery, global impact, control and cure of hepatitis E.
Discovery of hepatitis E came to lime light when 1978-Kashmir epidemic of hepatitis was investigated[1]. This region where the disease occurred did suffer very hard weather conditions, lacked basic health care delivery system and the region was devoid of high tech cutting edge investigative facilities. Events leading to the discovery unravel a remarkable story of human interest, with its complexities, missteps, successes and failures (http://www.drkhuroo.in)[13]. In November 1978, Dr. Mohammad S Khuroo investigated an epidemic of jaundice in and around a town 50 km from Srinagar, Kashmir, India. The epidemic had hit two hundred villages with a population of 600000, caused around 52000 patients with icteric disease and 1700 fatalities[3]. Pregnant women were more affected and many had been reported dead. An ingenious house-to-house investigation of this and subsequent epidemics and sporadic disease was performed over a 14-year period. This led us to the discovery of an enterically transmitted NANBH, which had triggered repeated epidemics in Kashmir[2,14]. The disease affected young adults within the age group of 15 to 45 years. One remarkable discovery during this study was the relationship of this disease with pregnancy[15-17]. The agent was transmitted vertically with high fetal and perinatal mortality[18,19]. The disease was a self-limiting and did not cause chronic viremia, chronic hepatitis and cirrhosis[20]. The loss of IgG antibodies occurred in over half of the population in a period of over 14 years[21]. It was postulated that the disease was caused by yet unrecognized human hepatitis virus[1]. Simultaneously, a sporadic disease identical in epidemiology and clinical features to epidemic disease and causing around half of acute hepatitis in the population was recognized[16,22].
An outbreak of NANBH was reported in Russian military personal posted in Afghanistan[23]. The epidemic had similar epidemiological features as seen in the 1978-Kashmir epidemic. Dr. Mikhail S Balayan, in a self-experimentation, ingested pooled stool extracts from 9 such patients[23]. On 36th d after ingestion of the stool extracts, he developed severe acute hepatitis with jaundice and elevated liver tests. Stool samples of 28th d, 43th d and 45th d showed virus-like-particles (VLP) on immune electron microscopy. A number of primates were experimentally infected with the putative agent and the physicochemical properties of the agent were studied[24].
The virus could not be cloned for a number of years due to low yield of VLP in the infectious material. This was overcome by the basic perception that expansive amounts of VLP’s were gotten from bile collected from infected primates. A partial cloning of the virus was done by Reyes et al[25] (1990). Subsequent to this, Tam et al[26] (1991) sequenced the full-length HEV genome of 7.2 kb and a diagnostic assay based on enzyme immunoassay was established.
HEV was originally thought to resemble HAV and included in Picornaviridae family[27]. Once morphological features and genome organization of HEV were available, it is surmised that HEV is similar to noroviruses and placed in family Caliciviridae, under hepevirus genus. Before long it was built up that HEV has a significant sequence difference from caliciviruses and was removed from Caliciviridae and included in the “Hepatitis E-like viruses”[28]. Subsequently, based on molecular analysis, all 4 recognized genotypes HEV-1 to HEV-4 were classified as sole agents of genus Hepevirus in the Hepeviridae family[29]. Avian HEV was included in the unassigned genus. This classification received a setback as a number of HEV closely related to human HEV infecting rabbit and wild boar and those more divergent HEV infecting rats, ferrets, bats and cutthroat trout were identified. Recently, based on phylogenetical features and HEV hosts, the ICTV Hepeviridae study group gave a new classification for the family Hepeviridae[30,31]. All HEV’s have been placed under Hepeviridae family and further classified under 2 genera namely Orthohepevirus, which included isolates from mammals and chicken and Piscihepevirus which included Cutthroat trout isolates. Orthohepevirus includes species A, B, C and D, while Piscihepevirus include species A only. Orthohepevirus A species included isolates from man, pigs, wild boar, rabbit, deer, mongoose and camel. This species encompassed several HEV genotypes infecting man alone (genotype HEV-1 and HEV-2); pigs and humans (genotype HEV-3 and HEV-4), wild boar (genotype HEV-5 and HEV-6) and dromedaries (genotype HEV-7). Orthohepevirus B contain viruses found in chickens and includes all three genotypes: avian HEV-1 (isolates from chicken in Australia), avian HEV-2 (isolates from chicken in United States and Canada) and avian HEV-3 (isolates from chicken in Europe and China). Orthohepevirus C includes 2 sequences namely HEV-C1 infecting rats and HEV-C2 infecting ferrets. Orthohepevirus D includes a single sequence infecting bat. Recently, partial sequences of other potential members of Hepeviridae family have been described[32-34]. The moose virus has been assigned to Orthohepevirus A, mink HEV to HEV-C2 and rat HEV to HEV-C1. All known cutthroat trout virus isolates have been included in Piscihepevirus A species within the genus Piscihepevirus. As sequences studied from the vast majority of the cutthroat trout infection isolates are just 262 nucleotides long, there are insufficient data as of now to portray outline criteria for species inside this genus.
The virion is a naked particle, spherical in shape and has icosahedral symmetry. The virus shows surface spikes and indentations. The particle has diameter of 27-30 nm on immune electron microscopy, 32-34 nm after sucrose gradient centrifugation and 38.5-42 nm on cryo-EM analysis[23,35]. Buoyant density of the particles in cesium chloride is around 1.35 g/cm3[36]. The virus is relatively stable in acid and mild alkaline conditions and unaffected by chloroform and ether treatment. HEV-infected pig liver homogenates maintain infectivity if incubated at 56° for 1 h, however boiling or frying for 5 min completely inactivates the virus. Treatment of liver suspensions with Tween-20 (0.05%) and formalin (0.05%) reduced infectivity by 1000-fold. HEV is susceptible to chlorine disinfection and chlorine disinfection of water supplies is recommended during epidemics of disease[37-39].
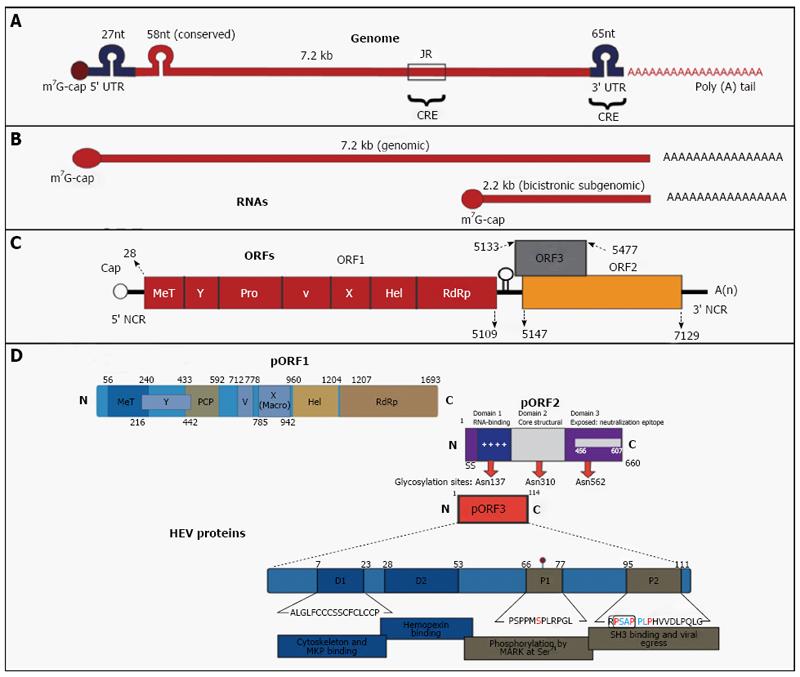
HEV genome consists of single-stranded RNA, has a molecular weight of approximately 7.2 kb with positive-sense polarity and is capped with 7-methylguanine at its 5’-end and poly (A) at its 3’-end (Figure 1). The genome has UTR’s at the 5’ end (27 nucleotides) and at the 3’ end (65 nucleotides) and a conserved stretch (58-nucleotides) near its 5’ end region within open reading frame 1 (ORF1), which fold in to stem-loop and hairpin structures. HEV RNA replicates in to a genomic RNA of 7.2 kb and a bicistronic subgenomic RNA of 2.2 kb. There are 3 ORFs in the genome namely ORF1, ORF2 and ORF3[40,41].
ORF1 of the well-characterized Burmese strain begins after 27 nucleotides of the 5’ end and extends across the 58 nucleotides of the conserved region for 5079 nucleotides before terminating at nucleotide position 5109[42]. ORF1 translates in to a non-structural protein (pORF1) having 1693 amino acids (aa). ORF1 domains along with their aa boundaries based on Burmese isolate are methyltransferase from aa 56-240, a Y domain from aa 216-442, papain-like cysteine protease from aa 433-592, a proline-rich hinge domain V from aa 712-778, a X macrodomain from aa 785-942, an RNA helicase from aa 960-1204 and RNA-dependent RNA polymerase from aa 1207-1693. HEV methyltransferase catalyzes the capping of viral RNA. HEV papain till now has not been associated with any functional activity of the virus. ORF1 contains a functional protease, which may be involved in the polyprotein processing. HEV helicase and RNA-dependent RNA polymerase are needed to replicate the genomic RNA. Recombinant HEV polymerase bind to the genomic RNA at 3’ end. The virion binding is facilitated by stem-loop structures and poly (A) stretch.
ORF2 starts at nucleotide 5147 (38 nucleotides 3’ at the end of ORF1) and develops roughly 1980 nucleotides till nucleotide 7127[43]. ORF2 encodes pORF2, a polyprotein of 660 amino acids, which is involved in the virus assembly and binding and eliciting immune response to produce neutralizing antibodies. The pORF2 has three glycosylation sites at 137, 310 and 562 conserved asparaginase residues. HEV full-length and truncated ORF2 gene expression in baculovirus vectors generate capsid proteins with different molecular weights. Of these, two HEV capsid proteins (VLP/T=1 and VLP-T=3) form virus-like particles (VLP). While VLP/T=1 show no encapsulated RNA, VLP/T=3 contained about 2-kb RNA derived from the ORF2 gene. The buoyant densities of VLP/T=1 and VLP/T=3 are 1.285 g/cm3 and 1.31 g/cm3 in CsCl gradient, respectively, both smaller than the buoyant density of native HEV particles. ORF2 expression in Escherichia coli (E. coli) leads to the formation of three HEV capsid proteins, which are homodimers and include dominant antigenic sites of HEV. HEV p239 self-assemble to empty virus-like particle of 23 nm. Baculovirus/ and E. coli expressed VLP’s are strongly immunogenic and resemble the native virus in their three-dimensional structure. Two VLP’s namely p239 and recombinant Sar 56 kDa have been successfully tried as HEV vaccines. The p239 vaccine is marketed in China as Hecolin[12].
ORF3 is 345 nucleotides in length, begins 23 nucleotides 3’ of the termination of ORF1[40,41]. ORF3 begins after nucleotide 5133 and terminates at nucleotide 5477 and translates in to a phosphoprotein (pORF3) of 114 aa. It has been suggested that pORF3 associates with the cytoskeleton and more specifically with microtubules. It is also implicated in HEV egress from hepatocytes[44]. ORF3 overlaps ORF2 by 328 nucleotides at the 3’ end but does not overlap with ORF1.
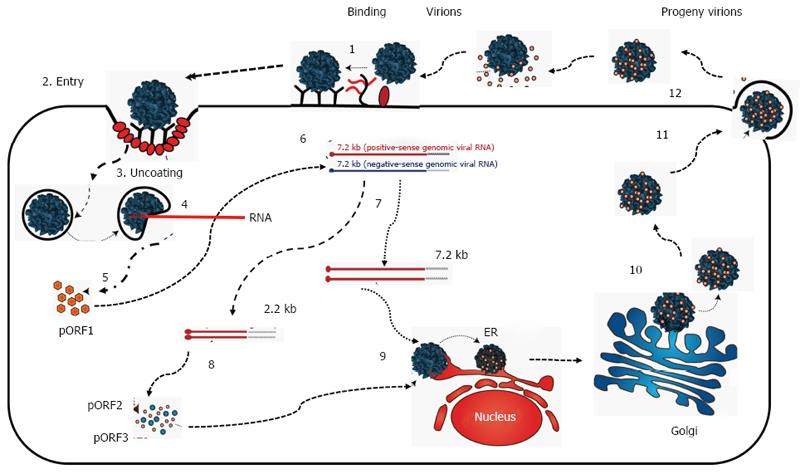
HEV lacks both a proper in vitro culture system and animal model[45] and the life cycle of HEV remains poorly studied (Figure 2). It is assumed that HEV reaches the host through gut epithelial cells; attach to the surface of hepatocytes through heparin sulfate proteoglycans, binds to a receptor and enter the hepatocytes[46]. Once internalized, the virus is uncoated, releases RNA and nonstructural proteins of the virus are translated. Positive sense viral RNA is replicated in to negative sense RNA with help of RNA-dependent RNA polymerase. Negative sense RNA become templates for 7.2 kb positive-sense RNA and 2.2 kb subgenocmic RNA. Subsequent to this, pORF2 and pORF3 are formed with the help of subgenocmic RNA as template. pORF2 protein along with genomic RNA assemble into the new virion while the pORF3 optimizes viral replication. The virion egressed from hepatocytes are coated with pORF3 and lipid layer. Both pORF3 and lipid layer are separated from virion after egress from hepatocytes[47].
Hepatitis E is an ancient ailment. Epidemics of jaundice have been mentioned in medieval writing and in writings generated around 19th century. Till late it was surmised that these epidemics were caused by hepatitis A. Now we know that the disease in reality closely resembled hepatitis E[48]. However, these have not been tested serologically because of non-availability of sera. The most recent epidemic confirmed to be caused by HEV, though retrospectively, was the Delhi epidemic 1955-1956[49]. Recently, the HEV evolutionary history was studied (Figure 3)[50]. It was estimated that the tMRCAs (times-to the most-recent-common-ancestors) for mammalian HEV existed 536 to 1344 years ago. This progenitor develops in to two variants namely anthropotropic varaints, evloving in to HEV-1 and HEV-2 and enzootic variants evolving in to HEV-3 and HEV-4 respectively. The anthropotropic variant is around 367-656 years old and enzootic variant around 417 -656 years old. HEV genotypes existed at various periods namely HEV-1 (87-199 years), HEV-3 (256-342 years) and HEV-4 (131-266 years). HEV from chicken had existed for long in the evolutionary history.
HEV was identified from pigs in the Unites States in 1997[51] and presently known to exist in all the countries of the world[52,53]. HEV infection develops very early and is ubiquitous in pigs from commercial farms. The infection is clinically silent, however, animals do show microscopic evidence of hepatitis. Pigs are infected by either genotype HEV-3 or HEV-4. Both have zoonotic potential and infect humans. HEV-3 has been detected in wild boar from Japan and many European countries[54-56]. HEV prevalence in wild boar was between 12.0% to 42.7% (Ig anti-HEV) and 2.5% to 25% (HEV RNA). HEV-4 and two new HEV genotypes HEV-5 and HEV-6 have been recovered from wild boar. HEV-3 has also been found in the sika/Yezo deer (Cervus nippon) from Japan and red deer (Cervus elaphus) from the Netherlands[57-59]. Mongoose has been observed to be infected with HEV and the full-length genomic arrangement of these confines have been shown to assemble with genotype HEV-3. HEV strains have been identified from rabbits from the United States and China[60,61], included as a distinct isolate of genotype HEV-3 and may be zoonotic. HEV has been discovered from dromedaries from the Middle East and the virus is named DcHEV[33]. About 1.5% of the adult dromedary fecal samples showed presence of DcHEV RNA. A comparative genomic and phylogenetic analyses showed that DcHEV represents a previously unrecognized HEV genotype and designated as HEV-7. Recently, the zoonotic potential of DcHEV has been confirmed in a liver transplant patient from Middle East, who often eat camel meat and drank camel milk[62]. Thus camel-derived food products may be a risk factor for post-transplant hepatitis E. Avian HEV causing BLSD (big liver and spleen disease) was identified in Australia in 1999[63]. Subsequently, avian HEV was identified from chicken in the United States and Canada with HSS (hepatitis-splenomegaly syndrome)[64]. Avian HEV shares approximately 50% to 60% nucleotide sequences with HEV from human and pigs. At present, avian HEV has three isolates namely from the United States, Europe and Australia[53]. HEV infects rats (Rattus norvegicus, Rattus rattus and Sigmodon hispidus) and bandicoots[65,66]. The rat HEV belongs to a separate species under Orthohepevirus C. Ferrets from a number of countries are infected with the virus[67] and shared 72.3% identity with rodent HEV. The ferret HEV genome organization was found to be slightly different from other HEVs and included a putative ORF (ORF4) of 552 nucleotides that overlapped with ORF1. HEV has been detected in bats from Africa, Central America, and Europe[68] and constitute a distinct genus. HEV from cutthroat trout (CTV) has sequence identity of 13% to 27% to human HEV and are classified in genus Piscihepevirus and species Piscihepevirus A in the Hepeviridae family[34].
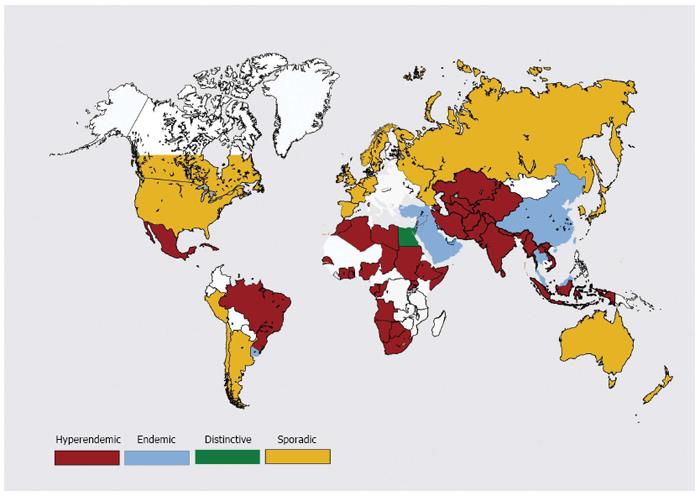
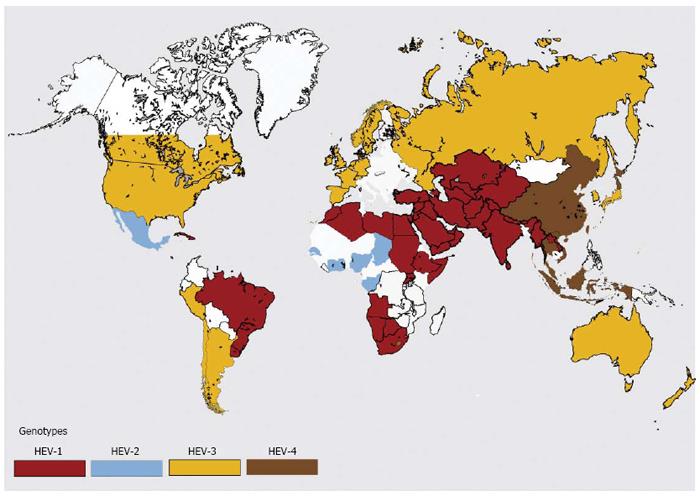
Hepatitis E epidemiology is divided in to four distinct zones (Figures 4 and 5).
Hepatitis E is hyperendemic in many countries located in southern Asia (India, Bangladesh, Bhutan, Nepal, Pakistan and Sri Lanka) , southeast Asia (Burma, Cambodia, Indonesia, Thailand, Vietnam and Laos), central Asia (Kazakhstan, Tajikistan and Uzbekistan); north Africa (Algeria, Morocco, Sudan and Tunisia), east Africa (Kenya, Uganda and Burundi), west Africa (Ivory Coast, Liberia, Nigeria and Mali) and some countries in North America (Mexico)[2]. In these countries, HEV infections present as epidemic and endemic disease. Hyperendemic disease is generally brought about by HEV-1. Be that as it may, the disease is brought on by HEV-2 in Mexico and some African countries.
Hepatitis E is endemic in many countries of Middle East (Turkey, Saudi Arabia, Yemen, Libya, Oman, Bahrain, Iran, Kuwait and the United Arab Emeritus), some regions of Southeast Asia (Singapore) and South America (Brazil, Argentina, Ecuador and Uruguay). Hepatitis E is responsible for more than one-fourth of all cases of acute sporadic hepatitis and fulminant hepatitis. However, epidemics of jaundice caused by HEV infections do not occur in these countries[69].
Hepatitis E epidemiology in Egypt is distinctive and different from other regions of the World[70,71]. Disease occurs at young age and seroprevalence in this community resembles that of HAV. HEV infection in pregnant ladies is either asymptomatic or present as mild disease. HEV infecting Egyptian population is genotype HEV-1, with subtypes not seen in the Asian population.
Autochthonous hepatitis E is increasingly being recognized in developed countries[72]. These infections are caused by HEV-3 and HEV-4[73-77]. Around 50 to 100 cases of hepatitis E are reported each from France, Germany, UK and many other European countries per year. Seroprevalence data show that these reported numbers are grossly lower than the actual disease load. Most HEV infections are unrecognized as these may not be tested or may be asymptomatic or misdiagnosed as drug-induced liver injury (DILI)[78,79].
Hepatitis E is a major global health problem and has infected one-third of the world population. Hepatitis E is a puzzling double-faced disease, with contrasting epidemiological and disease patterns in developing and industrialized countries (Table 1)[80,81].
| Developing countries | Developed countries | |||
| Genotypes | HEV-1 | HEV-2 | HEV-3 | HEV-4 |
| Distribution | Asia, Africa, Latin America | Mexico, West Africa | Worldwide | China, East Asia, Central Europe |
| Disease pattern | Epidemic, Endemic | Autochthonous, sporadic, case-clusters | ||
| Attack rate | About 1 in 2 | 67%-98% asymptomatic | ||
| Seasonality | Yes | No | ||
| Reservoir | Human | Animals (pig, boar, deer) | ||
| Transmission | Water, person-to-person, vertical | Zoonotic-food-borne, vocational, infected water | ||
| Transfusion-associated | Reported | Yes (well-studied) | ||
| Seroprevalence | Low (< 15 yr), rapid increase | Steady increase throughout age groups; varies 7% to 21% | ||
| (15-30 yr), plateau at 30%-40% | ||||
| Seroincidence | 64/1000-yr | 30 (South France), 2 (United Kingdom) | ||
| 7 (United States)/1000-yr | ||||
| Age (yr) | 15-40 | > 50 | ||
| Sex | 2:1 | > 3:1 | ||
| Clinical outcome | Self-limiting in most | Self-limiting in most | ||
| Risk factors | Pregnancy, Cirrhosis | Cirrhosis, LTx, HIV | ||
| Deaths in pregnancy | High (25%) | Not reported | ||
| HEV superinfections | Common, poor outcome | Reported, poor outcome | ||
| Extra-hepatic disease | Yes | Yes | ||
| Chronic infection | Not reported | HEV-3; SOT, HIV, hem NP | ||
| Burden | 3.4 million cases/yr, 70000 deaths, 3000 still births | Unknown | ||
In 2005, HEV-1 and HEV-2 worldwide disease burden were computed in 9 of the 21 GBD regions of 2010, representing 71% world population. It was estimated that 20 million incident infections, 3.4 million cases, 70000 deaths and 3000 stillbirths had occurred in 2005[82]. Several factors need to be considered while evaluating these numbers. Hepatitis E causes repeated epidemics. The disease frequency in these regions is in the range of 6%. The HEV antibodies show a dynamic response, with the loss of antibodies after some time in a sizeable proportion of the population. Recently, the HEV reinfections with altered immune response have been reported[14,21,83]. Thus, these calculated numbers may grossly underestimate the disease load in such countries.
Hepatitis E in resource poor countries causes massive epidemics of jaundice[2,3,84]. These regions have poor socio-economic conditions with drinking water sources which are polluted from sewage[85]. HEV infections cause a huge number of cases and is the reason for significant number of deaths, and represents a health issue in endemic regions. Of major concern is the occurrence of these epidemics on repeated occasions[2]. Over a 4-year period (1978-1982), four epidemics of hepatitis E were encountered in Kashmir, India[3]. The disease affected 52000 people and caused 1700 fatalities. Hepatitis E constitutes around 30% to 70% of sporadic viral hepatitis in endemic areas[16,22]. The disease incidence has been calculated to be around 45/1000 person per year[83] and infects around 2.2 million people per year in India[2]. HEV-related fulminant hepatic failure is a frequent occurrence and constitutes around 43% of all cases of fulminant hepatic failure[17,86]. The mortality in HEV-related liver failure has been reported as 51.9%. One of the most distinctive features of the epidemic and endemic hepatitis E is higher occurrence and mortality of disease in pregnancy[15,16]. Thus, the disease incidence was 8 times higher and acute liver failure occurred 13 times more often in pregnant women than age-matched men and non-pregnant women. The acute liver failure occurred in 44.4% in late pregnancy. Acute liver failure during pregnancy has limited pre-encephalopathy interval, rapid progression, high occurrence of brain edema and coning of the cerebellar tonsils. However, frequent occurrence of disseminated intravascular coagulation (DIC) was distinct feature of this disease. Considering the association of DIC with HEV in pregnant women, it resembles a “Schwartzman-like Phenomenon”. HEV in pregnant women causes substantial fetal and perinatal mortality. There are several studies reporting intrauterine transmission of HEV with high fetal and perinatal mortality[18,19,87]. HEV infection in cirrhotic patients causes rapid deterioration of liver functions and results in high mortality[88-90]. It is estimated that around 21% of cirrhotic patients in India contract HEV superinfection, develop rapidly progressive liver failure, with a 30-d mortality of around 34%[88,89].
Hepatitis E of indigenous origin in industrialized regions of the World is misdiagnosed as drug induced liver injury (DILI)[91]. This has been because of the absence of attention to the disease by clinicians. Most autochthonous HEV disease occurs in older individuals. Such patients have more severe liver disease, with higher hepatic or non-hepatic complications (15%) and acute liver failure (8%-11%)[92]. HEV superinfection in chronic alcoholics and alcoholic chronic liver disease has been reported, culminating in hepatic decompensation, progression of liver disease and death rate approaching to 70%[89,90,93]. Autochthonous HEV infections in industrialized countries does not cause severe disease in pregnant women.
HEV-3 causes prolonged viremia, chronic hepatitis, liver fibrosis and cirrhosis in a subgroup of immunocompromised patients. These include people with solid organ transplant (SOT), subjects who are human immunodeficiency virus (HIV) positive and in those with hematological neoplasms[94,95]. Viremia up to and beyond 3 mo suggests chronic hepatitis E. Patients with SOT in Europe have HEV RNA prevalence of 0.9% to 3.5%. Most of such patients are asymptomatic or have mild liver tests abnormalities and minimal constitutional symptoms. However, in a subgroup of patients, chronic hepatitis E leads to rapidly progressive liver fibrosis and cirrhosis over a period of 2-3 years. Of the 85 SOT patients with HEV-3 infection, 29 patients had self-limiting disease, 56 patients have chronic viremia with mild liver disease and 9 patients presented with liver fibrosis and cirrhosis. Several factors including use of tacrolimus as an immunosuppressant, thrombocytopenia, and low CD4 count as seen in HIV-infected patients were listed as high risk for progression of liver disease and development of liver fibrosis and cirrhosis[96].
Extrahepatic manifestations known to occur with hepatitis E include acute pancreatitis, thrombocytopenia, aplastic anemia, autoimmune thyroiditis, myositis, cryoglobulinemia with skin rashes and glomerulonephritis[97-99]. In addition, neurological manifestations occur in 5% of HEV infections[7,100,101]. The neurological manifestations include Bell’s palsy, encephalitis, brachial neuropathy, peripheral neuropathy and Guillain-Barre syndrome. HEV related neurological disease has been reported from developing and industrialized countries and occurs in both acute and chronic HEV infections. The long-term outcome of neurological syndromes is variable. Pathogenesis of extrahepatic HEV disease is not clear and might be multifactorial. It has been proposed that immune response to HEV may play a part in neurologic disease namely by antiganglioside antibodies through molecular mimicry[102]. Although HEV is a primarily hepatotrophic virus, it has been shown to replicate in extrahepatic sites namely kidneys, small intestine, stomach, spleen, neurological tissues, and placenta[103,104].
There are several mechanisms of transmission of HEV infection.
Hepatitis E in poor resource countries is spread through water contaminated by sewage[2,85]. The way water gets contaminated is different from one epidemic to another; however, it follows the similar mechanism in repeat regional epidemics. Several environmental settings contribute to contamination. These include: (1) heavy monsoon rains; (2) floods causing stagnation and reversal of flow in waterways polluting drinking water sources; (3) seeping pipes supplying drinking water laid down through or crossing across the sewage channels; (4) overcrowding with polluted water sources as in refugee dwellings and fast growing slums; and (5) raw sewage flowing in to open drinking water sources (rivers, streams, unprotected wells). It is imperative to find the mechanism of water contamination in each epidemic so that urgent measures can be taken to block contamination and control the epidemic.
Whether HEV infections are spread from one person to another is controversial?[105-107]. It has been proposed that this form of transmission of HEV infection does not occur as there are no secondary waves of hepatitis following the epidemic[108]. However, epidemics of hepatitis E occur due contamination of water supplies (common source infection) and whole community gets exposed to the virus at one time. In such a situation secondary waves of hepatitis cases caused by person-to-person transmission may not occur. In contrast, epidemics of hepatitis E which lack common source of infection are caused by person-to-person transmission[105]. Sporadic infections are known to spread from one person to another[106]. HEV infection occurred in 18 (29%) of the 62 household contacts to the 13 index cases of sporadic hepatitis E. However, other investigators found insignificant spread from one person to another during sporadic and epidemic hepatitis E[109].
HEV prevalence in several animals in India has been studied. In one such study, HEV infection was ubiquitous in several animals including domestic pig, goat, sheep and buffalo[110]. In another study, 284 domestic pigs from Maharashtra, India were studied. 122 (42.6%) and 13 (4.6%) animals were reactive for IgG antibodies to HEV and HEV RNA respectively[111]. All isolates from domestic pigs in India were of genotype HEV-4. As human HEV infections, both epidemic and sporadic in India are caused uniformly by genotype HEV-1, it is believed that human and pig HEV infections are unrelated and zoonotic transmission plays an insignificant role in human infections in India[111].
In industrialized countries, HEV-3 and HEV-4 is spread through foodborne zoonotic transmission[112,113]. Wild boar, Sika deer and domestic pigs cross transmit HEV, and eating parboiled flesh or liver (a treat in such countries) could be responsible for the outbreaks of hepatitis E. A more common way of HEV spread is through consumption of raw livers from supermarkets or eating Corsican Figatelli sausage. Such livers and sausages are often infected with live HEV. The waste water of domestic pig dung in such countries may pollute waterways and infect those who visit sea beaches or ingest infected mollusks.
In 2004, we reported on transfusion-associated hepatitis E from Kashmir, India[8]. In a case-control study, we detected 13 HEV infections (IgM anti-HEV and HEV RNA) amongst 145 multiply transfused subjects and 2 infections amongst 250 subjects, not transfused. In another study, three post-transfusion HEV infections in 25 susceptible patients were detected and traced to 4 HEV RNA positive donor samples amongst a total of 107 samples. All the 4 donors were asymptomatic and all transfusion-associated infections in Kashmir were caused by genotype HEV-1. Subsequent to this report, a Japanese patient was found to have developed transfusion-associated HEV infection and authors reported a complete donor-patient sequence homology[114]. Several cases and case series of transfusion-associated HEV infections were reported from several countries[115,116]. In a recent study form United Kingdom, 18 (42%) of the 42 HEV-positive transfusion recipients had contracted HEV-3 infection. Of these, 3 had short lasting viremia, while 10 developed prolonged and persistent infection[117].
In developing countries, short lasting viremia often occurs in healthy donors[9,118,119]. Presently, information on HEV RNA status in potential blood donors has been reported from many countries[79,120-122]. Viremia was reported to occur in healthy donors in all countries and varied from 1 out of 672 donations (Germany) to 1 out of 8416 donations (Austria). Duration of viremia extends up to 45 d and the infectious dose of HEV is as low as its detection by RT-PCR. Based on these data, it was estimated that around 80000-100000 transfusion associated HEV infections had occurred in England in 2013. Of the 7.4 million blood products administered in Germany per year, 1600 to 5900 transfusion-associated HEV infections had occurred. Blood or blood products are often required for several clinical conditions in whom hepatitis E runs a more severe course or leads to chronic hepatitis and cirrhosis. These include pregnancy, liver disease, SOT, HIV positive and hematological neoplasm. In view of the above data there is need to conduct screening of blood donors in countries with high HEV prevalence.
HEV can be transmitted vertically to fetus and infant from the infected mothers. We studied 8 mothers who had HEV infection for evidence of vertical transmission. Six babies had contracted vertically transmitted HEV infection[18]. In another study, 26 pregnant women with HEV infection were studied for pattern of vertically-transmitted HEV infection[19]. Five mothers had died prior to delivery, 2 aborted the fetuses, 4 delivered premature babies and 15 had completed pregnancy with normal deliveries. Out of the 19 babies evaluated, 12 were reactive for IgM anti-HEV and 10 showed viremia. In all 15 (78.9%) had evidence of intrauterine HEV infection. HEV infection in babies presented as icteric hepatitis in 7, anicteric hepatitis in 5 and jaundice alone in 3. Six newborns died with liver failure and one due prematurity. Nine babies who survived had short-lasting viremia and none had evidence of chronic hepatitis or cirrhosis. Subsequent to these reports, a number of reports have documented intrauterine transmission of HEV infection with high maternal and fetal mortality[87,123]. Recently HEV replication in placenta was found to occur and correlated with fetal and maternal mortality in acute liver failure[103]. We evaluated outcome of 36 pregnant women with hepatitis E and the occurrence and severity of vertically transmitted HEV infection in fetuses/neonates and found relationship between severity of disease in fetus with severity and outcome of disease in the mother and postulated that acute liver failure in pregnant women may be an example of mirror syndrome akin to acute fatty liver of pregnancy[124].
Serological analysis for HEV infection has been problematic (Table 2)[125,126]. Several issues need to be addressed while evaluating these tests. Some tests have problems while applying to different genotypes. Others perform poorly in immunocompromised persons. Cross reactions with other viral infections have been reported. Several assays available have been developed and evaluated by sera from patients with recent infections. These assays often have poor performance in sensitivity and specificity. Assays developed and evaluated against WHO reference reagents give more predictable results. Assays developed on either “indirect” ELISA or class capture-ELISA technique also give better results[126]. Amongst these, 2 assays for IgM anti-HEV marketed by the Beijing Wantai Biological Pharmacy (Wantai Rapid test) and Genelabs Diagnostics, Singapore (AssureTM) have high sensitivity and specificity. In routine clinical practice, acute HEV infection in immunocompetent patients can predictably be diagnosed by IgM anti-HEV. Around 90% patients are reactive for IgM anti-HEV at 2 wk of infection and stays so for up to 5 mo[127]. In patients with immune deficiency disease, additional testing for HEV RNA is recommended, in view of poor IgM response in this population.
| Test | Method | Uses | Comments |
| IgM anti-HEV | ELISA | Acute infection | Assays vary in performance, issue of genotype applicability, poor performance in immune disorders, cross-reactive with other viral infections |
| ICT (POCT) | |||
| IgG anti-HEV | ELISA | Seroprevalence | Assays vary in performance |
| ICT (POCT) | Acute infection | ||
| Natural protection | |||
| Vaccine efficacy | |||
| HEV RNA | NAT | Acute infection | Viremia short-lasting, in-house assays vary in performance, advantage immune disorders |
| Confirm chronicity | |||
| Anti-viral response | |||
| Donor screening | |||
| HEV antigen | EIA | Acute infection | 81% concordance with HEV RNA |
IgG anti-HEV testing is useful in seroprevalence studies. Rising IgG titers may help in diagnosis of HEV infection in situations with poor IgM anti-HEV response[128]. Testing for IgG anti-HEV titers is essential for determining effectiveness of HEV vaccine. Antibody titers of 2.5 WHO units/mL following vaccination or acute HEV infection are protective.
Testing for HEV RNA is useful in several situations which include: (1) donor screening; (2) diagnosis of HEV infections in patients with poor IgM response; (3) diagnosis of chronic HEV infection; and (4) evaluating response to antiviral drug therapy[129]. In-house assays for HEV RNA detection may have limitations and needs to be standardized with WHO standard (genotype HEV-3a). Conventionally HEV RNA is detected in blood and other body fluids by RTPCR and using primers from conserved segments of HEV. Another assay, the loop-mediated isothermal amplification (LAMP) employs single-tube, one-step amplification of HEV RNA. The test is quick, reliable and needs no special equipment[130].
Prevention and control of epidemics of hepatitis E in poor resource countries is a challenging task[85]. Management should be predominantly preventing and focusing on the supply of clean potable drinking water, adequate sanitation and proper sewage disposal and personal hygienic practices. During travel to countries with high prevalence of HEV infections, one should desist from use of contaminated drinks and beverages and eating of uncooked shellfish. “Clean India Campaign”, a Government of India Campaign 2014 is a USD10 billion project and is meant to clean the environs, construct toilets in homes, societies and schools and execute basic hygienic practices including proper hand hygiene. It is planned that around 1.2 billion Indians shall have access to public latrines in next 5 years[85]. Numerous endemic areas lack proper healthcare amenities, disease surveillance means, health education and recognizing sick patients for referral and these activities need to be instituted[131]. Chlorination of drinking water supplies is frequently being practiced to control epidemics and does help. Administration of IgG from Indian source has not succeeded in control of epidemics[106]. Availability of hyperimmune HEV globulin and a cheap, effective and safe HEV vaccine would help to prevent and help to arrest the spread of the epidemics[132].
Acute hepatitis E in immunocompetent individuals usually only need symptomatic treatment, due short lasting viremia. Ribavirin therapy for 3 wk in patients with severe hepatitis E causes rapid improvement of liver enzymes and functions[133]. Despite the fact that ribavirin is a teratogenic drug, the dangers of untreated HEV to the mother and embryo are high, and trials of drug therapy may be advantageous in such patients. SOT patients should avoid eating uncooked game meat or domestic pig meat and liver and Figatelli sausage. In contrast, all these food items need to be heated to inactivate the virus[38].
An algorithm for treatment of HEV-3 infection in SOT patients has been developed (Table 3)[134]. Reduction in immunosuppression clears the virus in a significant proportion of patients. Calcineurin inhibitors (cyclosporine and tacrolimus) and mTOR inhibitors enhance HEV replication and may cause the increment and persistence of HEV RNA[135-138]. In contrast, mycophenolic acid (including prodrug mycophenolate mofetil) inhibits HEV replication and may help with HEV clearance in chronic hepatitis E. Ribavirin is the drug of choice for patients with persistent viremia for 3 mo[138]. PEGylated IFN can be used in patients who have had liver transplants and not in patients with other solid organ transplants[139]. Sofosbuvir, a nucleotide analog inhibitor of hepatitis C infection polymerase, additionally hinders HEV replication in vitro by inhibiting the viral RNA-dependent RNA polymerase[140]. The role of Sofosbuvir in acute and prolonged HEV infection needs closer examination. Some patients may develop liver fibrosis, cirrhosis and liver failure and are candidates for liver transplantation.
| Class | Drug | Effect on HEV replication | Clinical use |
| Calcineurin inhibitors | Cyclosporine, tacrolimus | Stimulates HEV replication with increase in HEV load and promotes HEV persistence | Reduce dose |
| mTOR inhibitors | Rapamycin, everolimus | Stimulates HEV replication with increase in HEV load | Reduce dose |
| Antimetabolite immunosuppressant | Mycophenolate mofetil | Inhibits HEV replication and helps HEV clearance | Continue the drug |
| Guanosine analog | Ribavirin | Inhibits HEV replication and causes HEV clearance | Primary drug for therapy |
| Cytokines | Pegylated interferon α | Inhibits HEV replication and causes HEV clearance | Indicated if Ribavirin therapy fails |
| Nucleotide analog | Sofosbuvir | Inhibits HEV replication in vitro | Unclear, clinical trials indicated |
HEV-239 is made of VLP, expressed in E. coli (368-606 aa of ORF2) from HEV-1 Chinese strain and has 2 epitopes (533 to 552 aa) and incites an incredible T cell-dependent antibody response[12,141]. The vaccine has effectively completed trials in China and is marketed (Hecolin) in 30-lg doses for 3 dose regimen (0, 1 and 6-mo). At 4.5 years, vaccine offers efficacy was 86.8%. The vaccinated patients have protective antibody levels in 87% as against 9% in the control group[142]. The vaccine has been shown to give cross-protective efficacy between genotype HEV-1 (the vaccine product) to HEV-4 (infection prevalent in area of study).
For the global launch of HEV-239 vaccine, we need safety data in children, elderly patients, patients with chronic liver disease, SOT, HIV and those with immune disorders[143]. Data regarding safety of HEV-239 in pregnant women needs to be extended. Vaccine safety when used concomitantly with other vaccine needs consideration. Post-marketing and cost-effectively studies can be conducted once a vaccine is available in other countries. HEV-239 has been found highly effective in population where HEV-4 is prevalent with low endemicity at HEV attack rate of 0.03%. It is important to determine efficacy of vaccine in the Indian subcontinent where HEV-1 infection is prevalent with very high endemicity at an attack rate of 7.36%. Also vaccine efficacy studies in regions where genotype HEV-3 is prevalent need to be done[132].
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: India
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chuang WL, Gallegos-Orozco JF, Kaplan DE S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Khuroo MS. Study of an epidemic of non-A, non-B hepatitis. Possibility of another human hepatitis virus distinct from post-transfusion non-A, non-B type. Am J Med. 1980;68:818-824. [PubMed] [Cited in This Article: ] |
| 2. | Khuroo MS. Discovery of hepatitis E: the epidemic non-A, non-B hepatitis 30 years down the memory lane. Virus Res. 2011;161:3-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 3. | Khuroo MS. Hepatitis E: the enterically transmitted non-A, non-B hepatitis. Indian J Gastroenterol. 1991;10:96-100. [PubMed] [Cited in This Article: ] |
| 4. | Purcell RH, Emerson SU. Hepatitis E: an emerging awareness of an old disease. J Hepatol. 2008;48:494-503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 508] [Cited by in F6Publishing: 524] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 5. | Zhou X, de Man RA, de Knegt RJ, Metselaar HJ, Peppelenbosch MP, Pan Q. Epidemiology and management of chronic hepatitis E infection in solid organ transplantation: a comprehensive literature review. Rev Med Virol. 2013;23:295-304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Pischke S, Behrendt P, Manns MP, Wedemeyer H. HEV-associated cryoglobulinaemia and extrahepatic manifestations of hepatitis E. Lancet Infect Dis. 2014;14:678-679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Deroux A, Brion JP, Hyerle L, Belbezier A, Vaillant M, Mosnier E, Larrat S, Morand P, Pavese P. Association between hepatitis E and neurological disorders: two case studies and literature review. J Clin Virol. 2014;60:60-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Khuroo MS, Kamili S, Yattoo GN. Hepatitis E virus infection may be transmitted through blood transfusions in an endemic area. J Gastroenterol Hepatol. 2004;19:778-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 137] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Arankalle VA, Chobe LP. Hepatitis E virus: can it be transmitted parenterally? J Viral Hepat. 1999;6:161-164. [PubMed] [Cited in This Article: ] |
| 10. | Bajpai M, Gupta E. Transfusion-transmitted hepatitis E: is screening warranted? Indian J Med Microbiol. 2011;29:353-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Kamar N, Rostaing L, Izopet J. Hepatitis E virus infection in immunosuppressed patients: natural history and therapy. Semin Liver Dis. 2013;33:62-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Zhu FC, Zhang J, Zhang XF, Zhou C, Wang ZZ, Huang SJ, Wang H, Yang CL, Jiang HM, Cai JP. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet. 2010;376:895-902. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 527] [Cited by in F6Publishing: 486] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 13. | Khuroo MS. Discovery of hepatitis E virus-the untold story. JK-Practitioner. 2004;11:291-294. [Cited in This Article: ] |
| 14. | Khuroo MS, Khuroo MS. Seroepidemiology of a second epidemic of hepatitis E in a population that had recorded first epidemic 30 years before and has been under surveillance since then. Hepatol Int. 2010;4:494-499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Khuroo MS, Teli MR, Skidmore S, Sofi MA, Khuroo MI. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70:252-255. [PubMed] [Cited in This Article: ] |
| 16. | Khuroo MS, Kamili S. Aetiology, clinical course and outcome of sporadic acute viral hepatitis in pregnancy. J Viral Hepat. 2003;10:61-69. [PubMed] [Cited in This Article: ] |
| 17. | Khuroo MS, Kamili S. Aetiology and prognostic factors in acute liver failure in India. J Viral Hepat. 2003;10:224-231. [PubMed] [Cited in This Article: ] |
| 18. | Khuroo MS, Kamili S, Jameel S. Vertical transmission of hepatitis E virus. Lancet. 1995;345:1025-1026. [PubMed] [Cited in This Article: ] |
| 19. | Khuroo MS, Kamili S, Khuroo MS. Clinical course and duration of viremia in vertically transmitted hepatitis E virus (HEV) infection in babies born to HEV-infected mothers. J Viral Hepat. 2009;16:519-523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 20. | Khuroo MS, Saleem M, Teli MR, Sofi MA. Failure to detect chronic liver disease after epidemic non-A, non-B hepatitis. Lancet. 1980;2:97-98. [PubMed] [Cited in This Article: ] |
| 21. | Khuroo MS, Kamili S, Dar MY, Moecklii R, Jameel S. Hepatitis E and long-term antibody status. Lancet. 1993;341:1355. [PubMed] [Cited in This Article: ] |
| 22. | Khuroo MS, Duermeyer W, Zargar SA, Ahanger MA, Shah MA. Acute sporadic non-A, non-B hepatitis in India. Am J Epidemiol. 1983;118:360-364. [PubMed] [Cited in This Article: ] |
| 23. | Balayan MS, Andjaparidze AG, Savinskaya SS, Ketiladze ES, Braginsky DM, Savinov AP, Poleschuk VF. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology. 1983;20:23-31. [PubMed] [Cited in This Article: ] |
| 24. | Bradley DW, Purdy MA, Reyes GR. Hepatitis E virus genome. Molecular features, expression of immunoreactive proteins and sequence divergence. J Hepatol. 1991;13 Suppl 4:S152-S154. [PubMed] [Cited in This Article: ] |
| 25. | Reyes GR, Purdy MA, Kim JP, Luk KC, Young LM, Fry KE, Bradley DW. Isolation of a cDNA from the virus responsible for enterically transmitted non-A, non-B hepatitis. Science. 1990;247:1335-1339. [PubMed] [Cited in This Article: ] |
| 26. | Tam AW, Smith MM, Guerra ME, Huang CC, Bradley DW, Fry KE, Reyes GR. Hepatitis E virus (HEV): molecular cloning and sequencing of the full-length viral genome. Virology. 1991;185:120-131. [PubMed] [Cited in This Article: ] |
| 27. | Sreenivasan MA, Arankalle VA, Sehgal A, Pavri KM. Non-A, non-B epidemic hepatitis: visualization of virus-like particles in the stool by immune electron microscopy. J Gen Virol. 1984;65:1005-1007. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | International Committee on Taxonomy of Viruses. Hepatitis E virus. 2004. Available from: http://wwwictvonlineorg/. [Cited in This Article: ] |
| 29. | International Committee on Taxonomy of Viruses. Hepatitis E virus. 2009. Available from: http://wwwictvonlineorg/. [Cited in This Article: ] |
| 30. | International Committee on Taxonomy of Viruses. Hepatitis E virus. 2015. Available from: http://wwwictvonlineorg/. [Cited in This Article: ] |
| 31. | Smith DB, Simmonds P; International Committee on Taxonomy of Viruses Hepeviridae Study Group; Jameel S, Emerson SU, Harrison TJ, Meng XJ, Okamoto H, Van der Poel WH, Purdy MA. Consensus proposals for classification of the family Hepeviridae. J Gen Virol. 2014;95:2223-2232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 482] [Cited by in F6Publishing: 473] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 32. | Lin J, Norder H, Uhlhorn H, Belák S, Widén F. Novel hepatitis E like virus found in Swedish moose. J Gen Virol. 2014;95:557-570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Woo PC, Lau SK, Teng JL, Tsang AK, Joseph M, Wong EY, Tang Y, Sivakumar S, Xie J, Bai R. New hepatitis E virus genotype in camels, the Middle East. Emerg Infect Dis. 2014;20:1044-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 191] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 34. | Batts W, Yun S, Hedrick R, Winton J. A novel member of the family Hepeviridae from cutthroat trout (Oncorhynchus clarkii). Virus Res. 2011;158:116-123. [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 35. | Bradley DW, Balayan MS. Virus of enterically transmitted non-A, non-B hepatitis. Lancet. 1988;1:819. [PubMed] [Cited in This Article: ] |
| 36. | Bradley DW. Hepatitis E virus: a brief review of the biology, molecular virology, and immunology of a novel virus. J Hepatol. 1995;22:140-145. [PubMed] [Cited in This Article: ] |
| 37. | Emerson SU, Arankalle VA, Purcell RH. Thermal stability of hepatitis E virus. J Infect Dis. 2005;192:930-933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 163] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 38. | Girones R, Carratalà A, Calgua B, Calvo M, Rodriguez-Manzano J, Emerson S. Chlorine inactivation of hepatitis E virus and human adenovirus 2 in water. J Water Health. 2014;12:436-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | El-Senousy WM, El-Gamal MS, Mousa AAE, El-Hawary SE, Kamel MM, Fathi MN, El-Mahdy EM. Effect of Chlorine on Noroviruses, Rotaviruses and Hepatitis E Virus in Drinking Water. WASJ. 2014;32:2206-2212. [DOI] [Cited in This Article: ] |
| 40. | Panda SK, Thakral D, Rehman S. Hepatitis E virus. Rev Med Virol. 2007;17:151-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 181] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 41. | Chandra V, Taneja S, Kalia M, Jameel S. Molecular biology and pathogenesis of hepatitis E virus. J Biosci. 2008;33:451-464. [PubMed] [Cited in This Article: ] |
| 42. | Ropp SL, Tam AW, Beames B, Purdy M, Frey TK. Expression of the hepatitis E virus ORF1. Arch Virol. 2000;145:1321-1337. [PubMed] [Cited in This Article: ] |
| 43. | Zhou YH, Purcell RH, Emerson SU. A truncated ORF2 protein contains the most immunogenic site on ORF2: antibody responses to non-vaccine sequences following challenge of vaccinated and non-vaccinated macaques with hepatitis E virus. Vaccine. 2005;23:3157-3165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Zafrullah M, Ozdener MH, Panda SK, Jameel S. The ORF3 protein of hepatitis E virus is a phosphoprotein that associates with the cytoskeleton. J Virol. 1997;71:9045-9053. [PubMed] [Cited in This Article: ] |
| 45. | Reyes GR, Huang CC, Tam AW, Purdy MA. Molecular organization and replication of hepatitis E virus (HEV). Arch Virol Suppl. 1993;7:15-25. [PubMed] [Cited in This Article: ] |
| 46. | Cao D, Meng XJ. Molecular biology and replication of hepatitis E virus. Emerg Microbes Infect. 2012;1:e17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 47. | Yamada K, Takahashi M, Hoshino Y, Takahashi H, Ichiyama K, Tanaka T, Okamoto H. Construction of an infectious cDNA clone of hepatitis E virus strain JE03-1760F that can propagate efficiently in cultured cells. J Gen Virol. 2009;90:457-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 48. | Lee CA, Thomas HC. Classic Papers in Viral Hepatitis. London, England: Science Press 1988; . [Cited in This Article: ] |
| 49. | Wong DC, Purcell RH, Sreenivasan MA, Prasad SR, Pavri KM. Epidemic and endemic hepatitis in India: evidence for a non-A, non-B hepatitis virus aetiology. Lancet. 1980;2:876-879. [PubMed] [Cited in This Article: ] |
| 50. | Purdy MA, Khudyakov YE. Evolutionary history and population dynamics of hepatitis E virus. PLoS One. 2010;5:e14376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 51. | Meng XJ, Purcell RH, Halbur PG, Lehman JR, Webb DM, Tsareva TS, Haynes JS, Thacker BJ, Emerson SU. A novel virus in swine is closely related to the human hepatitis E virus. Proc Natl Acad Sci USA. 1997;94:9860-9865. [PubMed] [Cited in This Article: ] |
| 52. | Meng XJ. From barnyard to food table: the omnipresence of hepatitis E virus and risk for zoonotic infection and food safety. Virus Res. 2011;161:23-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 232] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 53. | Pavio N, Meng XJ, Renou C. Zoonotic hepatitis E: animal reservoirs and emerging risks. Vet Res. 2010;41:46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 259] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 54. | Takahashi M, Nishizawa T, Sato H, Sato Y, Jirintai S, Okamoto H. Analysis of the full-length genome of a hepatitis E virus isolate obtained from a wild boar in Japan that is classifiable into a novel genotype. J Gen Virol. 2011;92:902-908. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 186] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 55. | Widén F, Sundqvist L, Matyi-Toth A, Metreveli G, Belák S, Hallgren G, Norder H. Molecular epidemiology of hepatitis E virus in humans, pigs and wild boars in Sweden. Epidemiol Infect. 2011;139:361-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 56. | Wiratsudakul A, Sariya L, Prompiram P, Tantawet S, Suraruangchai D, Sedwisai P, Sangkachai N, Suksai P, Ratanakorn P. Detection and phylogenetic characterization of hepatitis E virus genotype 3 in a captive wild boar in Thailand. J Zoo Wildl Med. 2012;43:640-644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 57. | Yu C, Zimmerman C, Stone R, Engle RE, Elkins W, Nardone GA, Emerson SU, Purcell RH. Using improved technology for filter paper-based blood collection to survey wild Sika deer for antibodies to hepatitis E virus. J Virol Methods. 2007;142:143-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 58. | Rutjes SA, Lodder-Verschoor F, Lodder WJ, van der Giessen J, Reesink H, Bouwknegt M, de Roda Husman AM. Seroprevalence and molecular detection of hepatitis E virus in wild boar and red deer in The Netherlands. J Virol Methods. 2010;168:197-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 59. | Tomiyama D, Inoue E, Osawa Y, Okazaki K. Serological evidence of infection with hepatitis E virus among wild Yezo-deer, Cervus nippon yesoensis, in Hokkaido, Japan. J Viral Hepat. 2009;16:524-528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 60. | Zhao C, Ma Z, Harrison TJ, Feng R, Zhang C, Qiao Z, Fan J, Ma H, Li M, Song A. A novel genotype of hepatitis E virus prevalent among farmed rabbits in China. J Med Virol. 2009;81:1371-1379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 263] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 61. | Lhomme S, Dubois M, Abravanel F, Top S, Bertagnoli S, Guerin JL, Izopet J. Risk of zoonotic transmission of HEV from rabbits. J Clin Virol. 2013;58:357-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 62. | Lee GH, Tan BH, Chi-Yuan Teo E, Lim SG, Dan YY, Wee A, Aw PP, Zhu Y, Hibberd ML, Tan CK. Chronic Infection With Camelid Hepatitis E Virus in a Liver Transplant Recipient Who Regularly Consumes Camel Meat and Milk. Gastroenterology. 2016;150:355-357.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 329] [Cited by in F6Publishing: 357] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 63. | Payne CJ, Ellis TM, Plant SL, Gregory AR, Wilcox GE. Sequence data suggests big liver and spleen disease virus (BLSV) is genetically related to hepatitis E virus. Vet Microbiol. 1999;68:119-125. [PubMed] [Cited in This Article: ] |
| 64. | Huang FF, Pierson FW, Toth TE, Meng XJ. Construction and characterization of infectious cDNA clones of a chicken strain of hepatitis E virus (HEV), avian HEV. J Gen Virol. 2005;86:2585-2593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 65. | Widén F, Ayral F, Artois M, Olofson AS, Lin J. PCR detection and analysis of potentially zoonotic Hepatitis E virus in French rats. Virol J. 2014;11:90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 66. | Purcell RH, Engle RE, Rood MP, Kabrane-Lazizi Y, Nguyen HT, Govindarajan S, St Claire M, Emerson SU. Hepatitis E virus in rats, Los Angeles, California, USA. Emerg Infect Dis. 2011;17:2216-2222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 67. | Raj VS, Smits SL, Pas SD, Provacia LB, Moorman-Roest H, Osterhaus AD, Haagmans BL. Novel hepatitis E virus in ferrets, the Netherlands. Emerg Infect Dis. 2012;18:1369-1370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 68. | Drexler JF, Seelen A, Corman VM, Fumie Tateno A, Cottontail V, Melim Zerbinati R, Gloza-Rausch F, Klose SM, Adu-Sarkodie Y, Oppong SK. Bats worldwide carry hepatitis E virus-related viruses that form a putative novel genus within the family Hepeviridae. J Virol. 2012;86:9134-9147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 192] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 69. | Ghabrah TM, Stickland GT, Tsarev S, Yarbough P, Farci P, Engle R, Emerson S, Purcell R. Acute viral hepatitis in Saudi Arabia: seroepidemiological analysis, risk factors, clinical manifestations, and evidence for a sixth hepatitis agent. Clin Infect Dis. 1995;21:621-627. [PubMed] [Cited in This Article: ] |
| 70. | Darwish MA, Faris R, Clemens JD, Rao MR, Edelman R. High seroprevalence of hepatitis A, B, C, and E viruses in residents in an Egyptian village in The Nile Delta: a pilot study. Am J Trop Med Hyg. 1996;54:554-558. [PubMed] [Cited in This Article: ] |
| 71. | Amer AF, Zaki SA, Nagati AM, Darwish MA. Hepatitis E antibodies in Egyptian adolescent females: their prevalence and possible relevance. J Egypt Public Health Assoc. 1996;71:273-284. [PubMed] [Cited in This Article: ] |
| 72. | Wedemeyer H, Pischke S, Manns MP. Pathogenesis and treatment of hepatitis e virus infection. Gastroenterology. 2012;142:1388-1397.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 230] [Cited by in F6Publishing: 203] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 73. | Kamar N, Izopet J, Rostaing L. Hepatitis E virus infection. Curr Opin Gastroenterol. 2013;29:271-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 74. | Dalton HR, Thurairajah PH, Fellows HJ, Hussaini HS, Mitchell J, Bendall R, Banks M, Ijaz S, Teo CG, Levine DF. Autochthonous hepatitis E in southwest England. J Viral Hepat. 2007;14:304-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 75. | Wenzel JJ, Preiss J, Schemmerer M, Huber B, Plentz A, Jilg W. Detection of hepatitis E virus (HEV) from porcine livers in Southeastern Germany and high sequence homology to human HEV isolates. J Clin Virol. 2011;52:50-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 76. | Mansuy JM, Peron JM, Abravanel F, Poirson H, Dubois M, Miedouge M, Vischi F, Alric L, Vinel JP, Izopet J. Hepatitis E in the south west of France in individuals who have never visited an endemic area. J Med Virol. 2004;74:419-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 201] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 77. | Yatsuhashi H. Epidemiological and clinical features of hepatitis E in Japan. J Gastroenterol. 2004;39:702-703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 78. | Kuniholm MH, Purcell RH, McQuillan GM, Engle RE, Wasley A, Nelson KE. Epidemiology of hepatitis E virus in the United States: results from the Third National Health and Nutrition Examination Survey, 1988-1994. J Infect Dis. 2009;200:48-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 227] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 79. | Juhl D, Baylis SA, Blümel J, Görg S, Hennig H. Seroprevalence and incidence of hepatitis E virus infection in German blood donors. Transfusion. 2014;54:49-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 80. | Romanò L, Paladini S, Zanetti AR. Hepatitis E: a puzzling double-faced disease. Ann Ig. 2013;25:169-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 81. | Teshale EH, Hu DJ, Holmberg SD. The two faces of hepatitis E virus. Clin Infect Dis. 2010;51:328-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 82. | Rein DB, Stevens GA, Theaker J, Wittenborn JS, Wiersma ST. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology. 2012;55:988-997. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 480] [Cited by in F6Publishing: 460] [Article Influence: 38.3] [Reference Citation Analysis (1)] |
| 83. | Labrique AB, Zaman K, Hossain Z, Saha P, Yunus M, Hossain A, Ticehurst JR, Nelson KE. Epidemiology and risk factors of incident hepatitis E virus infections in rural Bangladesh. Am J Epidemiol. 2010;172:952-961. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 84. | Khuroo MS. Hepatitis E virus: Another addition to the existing alphabet of human hepatitis viruses. Ann Saudi Med. 1996;16:308-319. [PubMed] [Cited in This Article: ] |
| 85. | Khuroo MS. Sanitation and sewage disposal in India. 2014. Available from: http://www.researchgate.net/publication/269411584. [Cited in This Article: ] |
| 86. | Khuroo MS. Acute liver failure in India. Hepatology. 1997;26:244-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 87. | Kumar RM, Uduman S, Rana S, Kochiyil JK, Usmani A, Thomas L. Sero-prevalence and mother-to-infant transmission of hepatitis E virus among pregnant women in the United Arab Emirates. Eur J Obstet Gynecol Reprod Biol. 2001;100:9-15. [PubMed] [Cited in This Article: ] |
| 88. | Ramachandran J, Eapen CE, Kang G, Abraham P, Hubert DD, Kurian G, Hephzibah J, Mukhopadhya A, Chandy GM. Hepatitis E superinfection produces severe decompensation in patients with chronic liver disease. J Gastroenterol Hepatol. 2004;19:134-138. [PubMed] [Cited in This Article: ] |
| 89. | Kumar A, Aggarwal R, Naik SR, Saraswat V, Ghoshal UC, Naik S. Hepatitis E virus is responsible for decompensation of chronic liver disease in an endemic region. Indian J Gastroenterol. 2004;23:59-62. [PubMed] [Cited in This Article: ] |
| 90. | Hamid SS, Atiq M, Shehzad F, Yasmeen A, Nissa T, Salam A, Siddiqui A, Jafri W. Hepatitis E virus superinfection in patients with chronic liver disease. Hepatology. 2002;36:474-478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 169] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 91. | Dalton HR, Fellows HJ, Stableforth W, Joseph M, Thurairajah PH, Warshow U, Hazeldine S, Remnarace R, Ijaz S, Hussaini SH. The role of hepatitis E virus testing in drug-induced liver injury. Aliment Pharmacol Ther. 2007;26:1429-1435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 92. | Péron JM, Bureau C, Poirson H, Mansuy JM, Alric L, Selves J, Dupuis E, Izopet J, Vinel JP. Fulminant liver failure from acute autochthonous hepatitis E in France: description of seven patients with acute hepatitis E and encephalopathy. J Viral Hepat. 2007;14:298-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 176] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 93. | Radha Krishna Y, Saraswat VA, Das K, Himanshu G, Yachha SK, Aggarwal R, Choudhuri G. Clinical features and predictors of outcome in acute hepatitis A and hepatitis E virus hepatitis on cirrhosis. Liver Int. 2009;29:392-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 94. | Kamar N, Selves J, Mansuy JM, Ouezzani L, Péron JM, Guitard J, Cointault O, Esposito L, Abravanel F, Danjoux M. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;358:811-817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1005] [Cited by in F6Publishing: 923] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 95. | Fujiwara S, Yokokawa Y, Morino K, Hayasaka K, Kawabata M, Shimizu T. Chronic hepatitis E: a review of the literature. J Viral Hepat. 2014;21:78-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 96. | Behrendt P, Steinmann E, Manns MP, Wedemeyer H. The impact of hepatitis E in the liver transplant setting. J Hepatol. 2014;61:1418-1429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 97. | Haffar S, Bazerbachi F, Lake JR. HEV-associated cryoglobulinaemia and extrahepatic manifestations of hepatitis E. Lancet Infect Dis. 2015;15:268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 98. | Mishra A, Saigal S, Gupta R, Sarin SK. Acute pancreatitis associated with viral hepatitis: a report of six cases with review of literature. Am J Gastroenterol. 1999;94:2292-2295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 99. | Aggarwal R. Clinical presentation of hepatitis E. Virus Res. 2011;161:15-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 100. | Scharn N, Ganzenmueller T, Wenzel JJ, Dengler R, Heim A, Wegner F. Guillain-Barré syndrome associated with autochthonous infection by hepatitis E virus subgenotype 3c. Infection. 2014;42:171-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 101. | Kamar N, Abravanel F, Lhomme S, Rostaing L, Izopet J. Hepatitis E virus: chronic infection, extra-hepatic manifestations, and treatment. Clin Res Hepatol Gastroenterol. 2015;39:20-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 102. | Cheung MC, Maguire J, Carey I, Wendon J, Agarwal K. Review of the neurological manifestations of hepatitis E infection. Ann Hepatol. 2012;11:618-622. [PubMed] [Cited in This Article: ] |
| 103. | Bose PD, Das BC, Hazam RK, Kumar A, Medhi S, Kar P. Evidence of extrahepatic replication of hepatitis E virus in human placenta. J Gen Virol. 2014;95:1266-1271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 104. | Woolson KL, Forbes A, Vine L, Beynon L, McElhinney L, Panayi V, Hunter JG, Madden RG, Glasgow T, Kotecha A. Extra-hepatic manifestations of autochthonous hepatitis E infection. Aliment Pharmacol Ther. 2014;40:1282-1291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 105. | Teshale EH, Grytdal SP, Howard C, Barry V, Kamili S, Drobeniuc J, Hill VR, Okware S, Hu DJ, Holmberg SD. Evidence of person-to-person transmission of hepatitis E virus during a large outbreak in Northern Uganda. Clin Infect Dis. 2010;50:1006-1010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 106. | Khuroo MS, Dar MY. Hepatitis E: evidence for person-to-person transmission and inability of low dose immune serum globulin from an Indian source to prevent it. Indian J Gastroenterol. 1992;11:113-116. [PubMed] [Cited in This Article: ] |
| 107. | Aggarwal R, Naik SR. Hepatitis E: does person-to-person spread occur? Indian J Gastroenterol. 1992;11:109-112. [PubMed] [Cited in This Article: ] |
| 108. | Naik SR, Aggarwal R, Salunke PN, Mehrotra NN. A large waterborne viral hepatitis E epidemic in Kanpur, India. Bull World Health Organ. 1992;70:597-604. [PubMed] [Cited in This Article: ] |
| 109. | Aggarwal R. Hepatitis E virus and person-to-person transmission. Clin Infect Dis. 2010;51:477-478; author reply 478-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 110. | Aggarwal R. Hepatitis E: Historical, contemporary and future perspectives. J Gastroenterol Hepatol. 2011;26 Suppl 1:72-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 111. | Arankalle VA, Chobe LP, Joshi MV, Chadha MS, Kundu B, Walimbe AM. Human and swine hepatitis E viruses from Western India belong to different genotypes. J Hepatol. 2002;36:417-425. [PubMed] [Cited in This Article: ] |
| 112. | Yugo DM, Meng XJ. Hepatitis E virus: foodborne, waterborne and zoonotic transmission. Int J Environ Res Public Health. 2013;10:4507-4533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 170] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 113. | Izopet J, Kamar N. [Hepatitis E: from zoonotic transmission to chronic infection in immunosuppressed patients]. Med Sci (Paris). 2008;24:1023-1025. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 114. | Matsubayashi K, Nagaoka Y, Sakata H, Sato S, Fukai K, Kato T, Takahashi K, Mishiro S, Imai M, Takeda N. Transfusion-transmitted hepatitis E caused by apparently indigenous hepatitis E virus strain in Hokkaido, Japan. Transfusion. 2004;44:934-940. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 217] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 115. | Yamamoto T, Suzuki H, Toyota T, Takahashi M, Okamoto H. Three male patients with sporadic acute hepatitis E in Sendai, Japan, who were domestically infected with hepatitis E virus of genotype III or IV. J Gastroenterol. 2004;39:292-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 116. | Matsubayashi K, Kang JH, Sakata H, Takahashi K, Shindo M, Kato M, Sato S, Kato T, Nishimori H, Tsuji K. A case of transfusion-transmitted hepatitis E caused by blood from a donor infected with hepatitis E virus via zoonotic food-borne route. Transfusion. 2008;48:1368-1375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 219] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 117. | Hewitt PE, Ijaz S, Brailsford SR, Brett R, Dicks S, Haywood B, Kennedy IT, Kitchen A, Patel P, Poh J. Hepatitis E virus in blood components: a prevalence and transmission study in southeast England. Lancet. 2014;384:1766-1773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 399] [Cited by in F6Publishing: 379] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 118. | Aggarwal R. Hepatitis E: is it a blood-borne pathogen? J Gastroenterol Hepatol. 2004;19:729-731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 119. | Arankalle VA, Chobe LP. Retrospective analysis of blood transfusion recipients: evidence for post-transfusion hepatitis E. Vox Sang. 2000;79:72-74. [PubMed] [Cited in This Article: ] |
| 120. | Fukuda S, Sunaga J, Saito N, Fujimura K, Itoh Y, Sasaki M, Tsuda F, Takahashi M, Nishizawa T, Okamoto H. Prevalence of antibodies to hepatitis E virus among Japanese blood donors: identification of three blood donors infected with a genotype 3 hepatitis E virus. J Med Virol. 2004;73:554-561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 121. | Vollmer T, Diekmann J, Johne R, Eberhardt M, Knabbe C, Dreier J. Novel approach for detection of hepatitis E virus infection in German blood donors. J Clin Microbiol. 2012;50:2708-2713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 122. | Holm DK, Moessner BK, Engle RE, Zaaijer HL, Georgsen J, Purcell RH, Christensen PB. Declining prevalence of hepatitis E antibodies among Danish blood donors. Transfusion. 2015;55:1662-1667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 123. | Singh S, Mohanty A, Joshi YK, Deka D, Mohanty S, Panda SK. Mother-to-child transmission of hepatitis E virus infection. Indian J Pediatr. 2003;70:37-39. [PubMed] [Cited in This Article: ] |
| 124. | Khuroo MS. Association of severity of hepatitis E virus infection in the mother and vertically transmitted infection in the fetus. JK-Practitioner. 2006;13:70-74. [Cited in This Article: ] |
| 125. | Khudyakov Y, Kamili S. Serological diagnostics of hepatitis E virus infection. Virus Res. 2011;161:84-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 126. | Zhou H, Jiang CW, Li LP, Zhao CY, Wang YC, Xu YW, Chen XF. [Comparison of the reliability of two ELISA kits for detecting IgM antibody against hepatitis E virus]. Zhonghua Yu Fang Yi Xue Za Zhi. 2008;42:667-671. [PubMed] [Cited in This Article: ] |
| 127. | Pas SD, Streefkerk RH, Pronk M, de Man RA, Beersma MF, Osterhaus AD, van der Eijk AA. Diagnostic performance of selected commercial HEV IgM and IgG ELISAs for immunocompromised and immunocompetent patients. J Clin Virol. 2013;58:629-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 128. | Aggarwal R. Diagnosis of hepatitis E. Nat Rev Gastroenterol Hepatol. 2013;10:24-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 129. | Zhao ZY, Ruan B, Shao H, Chen ZJ, Liu SL. Detection of hepatitis E virus RNA in sera of patients with hepatitis E by polymerase chain reaction. Hepatobiliary Pancreat Dis Int. 2007;6:38-42. [PubMed] [Cited in This Article: ] |
| 130. | Zhang LQ, Zhao FR, Liu ZG, Kong WL, Wang H, Ouyang Y, Liang HB, Zhang CY, Qi HT, Huang CL. Simple and rapid detection of swine hepatitis E virus by reverse transcription loop-mediated isothermal amplification. Arch Virol. 2012;157:2383-2388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 131. | World Health Organization. Hepatitis E: Fact sheet. 2015. Available from: http://wwwwhoint/mediacentre/factsheets/fs280/en/. [Cited in This Article: ] |
| 132. | Hepatitis E vaccine: WHO position paper, May 2015. Wkly Epidemiol Rec. 2015;90:185-200. [PubMed] [Cited in This Article: ] |
| 133. | Gerolami R, Borentain P, Raissouni F, Motte A, Solas C, Colson P. Treatment of severe acute hepatitis E by ribavirin. J Clin Virol. 2011;52:60-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 134. | Schildgen O, Müller A, Simon A. Chronic hepatitis E and organ transplants. N Engl J Med. 2008;358:2521-2522; author reply 2522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 135. | Kamar N, Garrouste C, Haagsma EB, Garrigue V, Pischke S, Chauvet C, Dumortier J, Cannesson A, Cassuto-Viguier E, Thervet E. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology. 2011;140:1481-1489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 475] [Cited by in F6Publishing: 454] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 136. | Wang Y, Zhou X, Debing Y, Chen K, Van Der Laan LJ, Neyts J, Janssen HL, Metselaar HJ, Peppelenbosch MP, Pan Q. Calcineurin inhibitors stimulate and mycophenolic acid inhibits replication of hepatitis E virus. Gastroenterology. 2014;146:1775-1783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 137. | Zhou X, Wang Y, Metselaar HJ, Janssen HL, Peppelenbosch MP, Pan Q. Rapamycin and everolimus facilitate hepatitis E virus replication: revealing a basal defense mechanism of PI3K-PKB-mTOR pathway. J Hepatol. 2014;61:746-754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 138. | Pischke S, Hardtke S, Bode U, Birkner S, Chatzikyrkou C, Kauffmann W, Bara CL, Gottlieb J, Wenzel J, Manns MP. Ribavirin treatment of acute and chronic hepatitis E: a single-centre experience. Liver Int. 2013;33:722-726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 139. | Kamar N, Rostaing L, Abravanel F, Garrouste C, Esposito L, Cardeau-Desangles I, Mansuy JM, Selves J, Peron JM, Otal P. Pegylated interferon-alpha for treating chronic hepatitis E virus infection after liver transplantation. Clin Infect Dis. 2010;50:e30-e33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 141] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 140. | Dao Thi VL, Debing Y, Wu X, Rice CM, Neyts J, Moradpour D, Gouttenoire J. Sofosbuvir Inhibits Hepatitis E Virus Replication In Vitro and Results in an Additive Effect When Combined With Ribavirin. Gastroenterology. 2016;150:82-85.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 141] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 141. | Kamili S. Toward the development of a hepatitis E vaccine. Virus Res. 2011;161:93-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 142. | Zhang J, Zhang XF, Huang SJ, Wu T, Hu YM, Wang ZZ, Wang H, Jiang HM, Wang YJ, Yan Q. Long-term efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372:914-922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 233] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 143. | Khuroo MS, Khuroo MS. Hepatitis E: an emerging global disease - from discovery towards control and cure. J Viral Hepat. 2016;23:68-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |