Published online Jul 14, 2016. doi: 10.3748/wjg.v22.i26.6008
Peer-review started: March 22, 2016
First decision: April 14, 2016
Revised: May 1, 2016
Accepted: May 21, 2016
Article in press: May 23, 2016
Published online: July 14, 2016
Severe chronic liver disease (CLD) may result from portal hypertension, hepatocellular failure or the combination of both. Some of these patients may develop pulmonary complications independent from any pulmonary pathology that they may have. Among them the hepatopulmonary syndrome (HPS), portopulmonary hypertension (PPH) and hepatic hydrothorax (HH) are described in detail in this literature review. HPS is encountered in approximately 15% to 30% of the patients and its presence is associated with increase in mortality and also requires liver transplantation in many cases. PPH has been reported among 4%-8% of the patient with CLD who have undergone liver transplantation. The HH is another entity, which has the prevalence rate of 5% to 6% and is associated in the absence of cardiopulmonary disease. These clinical syndromes occur in similar pathophysiologic environments. Most treatment modalities work as temporizing measures. The ultimate treatment of choice is liver transplant. This clinical review provides basic concepts; pathophysiology and clinical presentation that will allow the clinician to better understand these potentially life-threatening complications. This article will review up-to-date information on the pathophysiology, clinical features and the treatment of the pulmonary complications among liver disease patients.
Core tip: Pulmonary complications are found in some patients with liver disease. The hepatopulmonary syndrome is found in 15% to 30% and, its presence, increases mortality and risk of requiring liver transplantation. Portopulmonary hypertension has been reported to be present in 4% to 8% in patients whom have undergone liver transplant evaluation. Hepatic hydrothorax, with a prevalence of 5%-6% in these patients, is suspected when a patient develops pleural effusions without presence of cardiopulmonary disease. All of these entities can only be solved with successful liver transplant.
- Citation: Surani SR, Mendez Y, Anjum H, Varon J. Pulmonary complications of hepatic diseases. World J Gastroenterol 2016; 22(26): 6008-6015
- URL: https://www.wjgnet.com/1007-9327/full/v22/i26/6008.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i26.6008
Patients with chronic liver disease (CLD) can develop extra-hepatic pulmonary complications[1]. Among them, three life-threatening conditions are of concern. The hepatopulmonary syndrome (HPS), in which there is vasodilatation of the microvascular vessels of the lungs, with or without the presence of hypoxemia in patients where other cardiopulmonary conditions has been excluded[2]. Portopulmonary hypertension (PPH) results from arterial vasoconstriction linked to remodeling of the vascularity of the lung due to prolonged portal hypertension, which causes pulmonary arterial hypertension[3]. This entity is rare, but when present is seen in females and patients with autoimmune hepatitis. Lastly, hepatic hydrothorax (HH) is a more common clinical entity that is suspected when a pleural effusion is present in patients with liver disease in the absence of cardiopulmonary conditions[4].
This article reviews existing and up-to-date information on the epidemiology, pathophysiology, clinical manifestations, diagnosis and treatment options for these patients.
Cirrhosis is the final pathway of CLD. In the United States, this condition has a prevalence of approximately of 0.27%[5]. The prevalence is higher among non-Hispanic blacks, and Mexican Americans[5]. Despite advances in its management, it has a reported mortality of 26.4% per-2-year interval compared with 8.4% in propensity matched-controls and the highest mortality in Latin America is found in Mexico[5,6]. The HPS is found in approximately 15% to 30% of patients who has cirrhosis. HPS is infrequent among smokers[7,8]. On the other hand, PPH has an estimated prevalence of 2% to 5% of patients with portal hypertension, and 4% to 6% in patients that are evaluated for liver transplant[9]. The prevalence of HH, in patients with cirrhosis is approximately 4% to 6%[10].
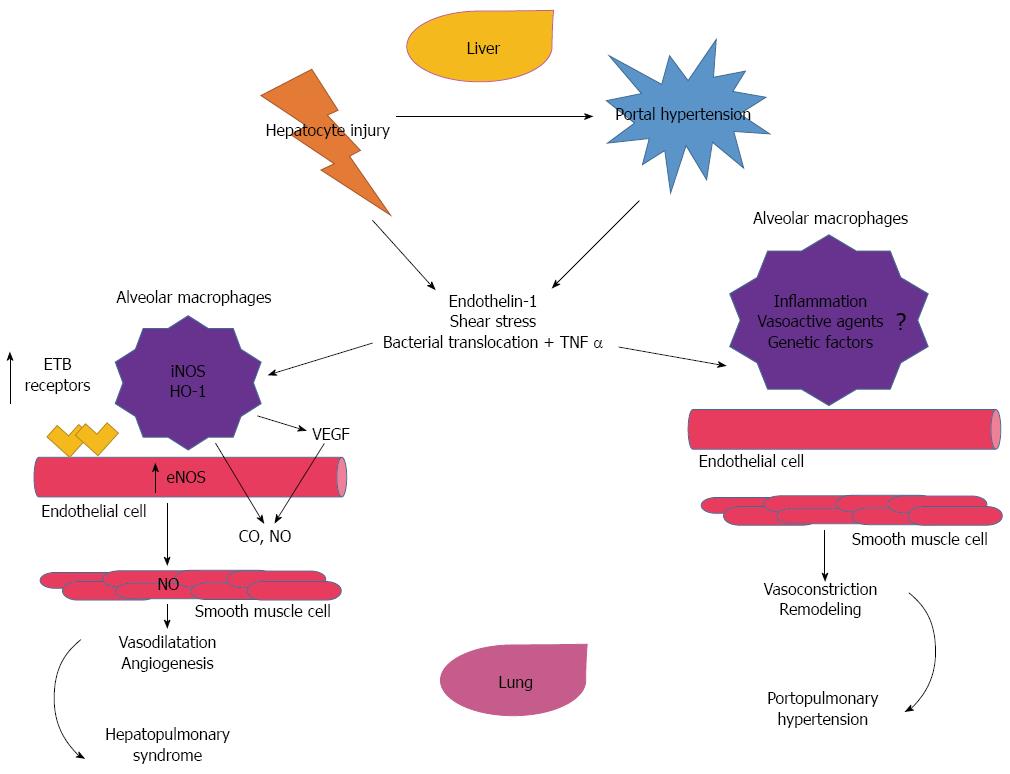
In the HPS, there is excessive vasodilatation of pre-capillary and post-capillary vasculature, resulting in impaired oxygenation of venous blood as it passes through the lung, is the primary pathological insult[11]. Several human studies have demonstrated the increase in nitric oxide production among these patients[12,13]. This is thought to be related to shear stress and the production of endothelin-1 and tumor necrosis alpha (TNFα) in the liver, which in turn, activates the endothelial nitric oxide synthase (eNOS) and inducible nitric oxide synthase (iNOS) in the lungs[14]. Both, eNOS and iNOS, contribute to the monocyte accumulation of the Beta-Endothelin (ETβ) receptor overexpression, in the pulmonary vascular endothelium[15-17]. Other factors that can contribute to this monocyte accumulation include bacterial translocation and endotoxemia[18]. The endothelial activation of the fractalkaline chemokine (CX3CL1) in the lungs is a common pathway as it pertains to monocyte adherence in the pulmonary microcirculation[17,19]. It has been postulated that this activation is part of the pathway of angiogenesis[20]. In addition, another factor in activating eNOS and iNOS, is the increased carbon monoxide (CO) production in monocytes[17,21]. Once these processes occur, the monocytes start to bind growing factors, such as the vasculo-endothelial growth factor-A (VEGF-A), causing angiogenesis and activating angiogenic signaling pathways[17,20,22]. The more angiogenesis, the more intravascular monocytes, and finally an excessive vasodilatation (Figure 1).
PPH is defined in patients with CLD as pulmonary arterial hypertension with mean pulmonary artery pressure (MPAP) > 25 mmHg at rest or MPAP > 30 mmHg with exercise and pulmonary capillary wedge pressure (PAOP) of < 15 mmHg and pulmonary vascular resistance > 240 dynes·s·cm-5)[9,17,23]. The pathogenesis is not fully understood, as this entity has a low prevalence[24,25]. Some authors describe the histopathology of PPH identical to that of idiopathic pulmonary arterial hypertension[3,26]. In both instances, there is vascular injury caused by shear stress and vasoactive mediators (Endothelin-1, prostacyclin and thromboxane)[9,17,27]. Moreover, these promote inflammatory processes with establishment of plexogenic arteriopathy, which advances to concentric intimal fibrosis, and smooth muscle hyperplasia and hypertrophy[28]. Patients with autoimmune liver disease can develop PPH, but its relationship is not clear, and it is possible that these diseases should be regarded as systemic multi-organ manifestations[29-31].
The most accepted pathogenesis for HH is the direct passage of ascitic fluid from the peritoneal cavity to the pleural space due to defects in the diaphragm[4]. This is possible with a “valvular” mechanism, when the negative intra thoracic pressure favors the transfer of fluid across the defects[30]. Some risk factors for HH include anatomic thinning and separation of tightly drawn collagenous fibers in the tendinous portion of the diaphragm (includes congenital factors), cirrhotic cachexia secondary to protein malnutrition, an increased in the intra abdominal pressure due to ascites that causes the peritoneum lining to evaginates, which results in formation of pleuroperitoneal blebs that are likely to rupture with unidirectional migration of ascitic fluid into the pleural cavity[10,30,32]. Most of these defects measure less than 1 centimeter in diameter[30]. Most of the effusions are right sided, in close to 85% of patients with HH, due to the fact that the tendinous portion of the diaphragm predominates[32,33]. Bilateral effusions occur in only 2% of these patients[34]. Other theories involves: the azygous vein, which increases it pressure and flow, leading to subsequent leakage of plasma; the movement of peritoneal fluid to the pleural space via transdiaphragmatic lymphatics; the leakage of the thoracic duct; and the decrease of the colloid-osmotic pressure due to hypoalbuminemia[35-37]. Occasionally, some patients with CLD without ascites, may develop HH, as a result of the one way or unidirectional flow of the ascitic fluid into the pleural space, exceeding the capacity of the pleura to resorb ascites[38].
In patients with CLD, the pulmonary complications may be subtle or life-threatening[1,39]. These patients may have concurrent pulmonary conditions, such as chronic pulmonary obstructive disease.
Most patients are asymptomatic. However, 18% of HHS patients may have a clinical presentation that starts with an insidious onset of dyspnea during the early stages of the syndrome[2,40]. Platypnea, which is worsening dyspnea during standing, or orthodeoxia (defined as hypoxemia that is exacerbated in the upright position), are seen in symptomatic patients with HPS[2,11,12]. The cutoff value for orthodeoxia is defined as decrease in PaO2 of 5% or 4 mmHg from the supine position[17,41]. This hypoxemia is caused due to vasodilatation at the lower lobes of the lungs and an increased shunting through these regions when the patient is in the upright position[42]. Significant desaturaion during sleep may occur in these patients, even if the daytime hypoxemia is moderate[11,43]. Patients with severe HHS, may display digital clubbing as well as cyanosis[11,44].
Most patients complain of is dyspnea, that may be accompanied with orthopnea, fatigue, syncope, chest pain (that patients refer as oppressive), and lightheadedness[9,45,46]. On physical examination, a tricuspid regurgitation murmur, with a pronounced P2 sound can be heard. In addition, increased jugular venous pressure, peripheral edema and ascites[9,47,48]. PPH is classified based on the MPAP values into mild (25-35 mmHg), moderate (35-50 mmHg), and severe (> 50 mmHg)[46,49].
When a patient with CLD develops a pleural effusion, we must suspect HH, even if ascites is absent[38,50,51]. As noted, these patients could be totally asymptomatic or can exhibit symptoms of dyspnea on exertion, dry and non-productive cough, chest discomfort, hypoxemia and even respiratory failure[37,52,53]. Relatively small amounts of fluid in the thoracic cavity (e.g., 1-2 L) can cause symptoms. Occasionally, patients with HH may have life-threatening complications such as acute tension hydrothorax (TH). In patients with TH, severe dyspnea and hypotension are seen[52,53]. This process occurs rapidly, usually over the course of an hour, and be secondary to sudden pleuroperitoneal bleb[50,54,55]. Spontaneous bacterial empyema (SBE), which is under-recognized because it is rarely described, may occur in 2% of cirrhotic patients, and 13%-16% among cirrhotic patients with hepatic hydrothorax[10,34,56]. SBE may be confused with pleural empyema, as there is no evidence of abscess formation in the chest cavity and is commonly related to pneumonia[10,56,57]. This clinical entity has a mortality rate of 20% to 38% and rapid diagnosis is essential for appropriate and timely initiation of treatment[56,58].
The criterion for the diagnosis of HPS requires the presence of gas exchange abnormalities (due to intrapulmonary vasodilatation), and this can be measured with arterial blood gases, calculation of Alveolar-arterial oxygen gradient (abnormal if > 15-20 mmHg, on room air; age corrected) with or without hypoxemia (PaO2). All of this is measured in the sitting position[11,26]. In addition, contrast-enhanced echocardiography can be used to screen for intrapulmonary vasodilatation[59]. When intrapulmonary shunting is present, the left ventricle gets opacified with the contrast at least 3 heartbeats after the right ventricle (delayed shunting)[60].
Once the diagnosis of HHS is made it is important to classify its severity depending on the changes in partial pressure of oxygen, into: mild with PaO2 > 80 mmHg, moderate > 60 mmHg to < 80 mmHg, severe > 50 mmHg to < 60 mmHg or very severe < 50 mmHg[12]. Pulmonary angiography may assist in the diagnosis and two patterns are commonly seen: First pattern seen has a finely diffuse, spidery arterial vascular abnormalities due to diffuse spongy appearance, and the second one has discrete, localized arteriovenous communications[61]. As this is an invasive procedure, this is not commonly performed. A computed tomography (CT) of the chest can be useful, but it is less sensitive[1,62].
Transthoracic echocardiography (TTE) has been deemed as one of the most practical method to detect PPH[45]. This diagnostic technique attempts to identify the tricuspid regurgitant peak velocity, which helps in estimating the right atrial pressure by inferior vena cava changes on inspiration, and by using the modified Bernoulli equation, estimates the right ventricular systolic pressure in over 80% of patients with portal hypertension[3]. It has a high sensitivity and specificity if the pressure of the right ventricle is > 40 mmHg. In that case the sensitivity in the diagnosis of PPH is 100% and the specificity is 82%[63]. The American Association for the Study of Liver Disease recommends TTE to detect pulmonary hypertension in all the patients that are considered for liver transplant in the United States[45]. These patients may present right-sided systolic dysfunction and finally cor pulmonale.
A simple chest X-ray can be used to confirm pleural effusions, and a thoracentesis can then be performed to confirm the presence of peritoneal fluid[10]. In the later test cell count, gram stain, culture, protein, albumin, lactate dehydrogenase and bilirubin are commonly analyzed. The pleural fluid composition in hepatic hydrothorax is usually transudative, however, due to a difference in the water reabsorptive capacity between the pleural space and peritoneal cavity, it has a higher protein concentration than ascitic fluid. If excessive diuresis is used, this fluid can be exudative[38]. In these patients, a CT of the chest can be helpful in differentiating and eliminating the pulmonary or pleural pathologies of left-sided pleural effusions[55]. Ultrasonography and magnetic resonance can be utilized to visualize diaphragmatic defects[32,50].
To date, there is no effective medical therapy for this clinical condition. Spontaneous resolution is quite rare. In uncontrolled clinical trials, the use of beta-blockers, steroids, cyclophosphamide and/or nitric oxide have shown no mortality benefit[2,12,23,26]. As the mortality rate is quite high in these patients, the only chance for clinical improvement is undergoing liver transplant. This therapeutic intervention is successful in up to 85% of the patients with HHS that undergo this life-saving technique[11]. Priority of transplant is given to patients with HHS that also present hypoxemia (PaO2 < 60 mmHg)[64].
The primary goal in these patients is to reduce the obstruction of pulmonary artery blood flow, in an attempt to improve hemodynamics[49-51]. This is accomplished by reducing the MPAP, and the pulmonary vascular resistance[45]. A secondary goal is to normalize the right ventricular pressure[3,25,29]. A variety of medications have been tried in these patients targeting pulmonary arterial hypertension. Among them, endothelin receptor antagonist, phosphodiesterase type-5 inhibitors, prostanoids, and combination therapy have been utilized[65,66]. Sildenafil as a single agent or in combination with prostacyclins is commonly used (Table 1)[49,67]. These agents are usually used to improve symptoms, prior to liver transplantation[63]. Failure to reduce the MPAP to < 50 mmHg, is considered a contraindication for transplant[68].
| Classification | Name | Mechanism of action |
| Endothelin receptor antagonist | Bosentan | Dual ETA and ETB receptor subtypes antagonist. Specifically, inhibition of ET-1 receptors |
| Ambrisentan | Highly selective ETA receptor inhibition | |
| Macitentan | High affinity ETA than ETB antagonist. | |
| Phosphodiesterase 5 inhibitors | Sildenafil | High selectivity for PD5 vs PD2, 3 and 4. |
| Tadalafil | High selectivity for PD5 compared with PD1, 4, 7 and 10. | |
| Prostanoids | Epoprostenol | Synthetic prostacyclin with potent effects of vasodilatation and platelet aggregator inhibitor. |
| Treprostinil | Long acting tricyclic benzindene synthetic analogue of prostacyclin. Vasodilator and inhibits platelet inhibition. |
Over the past two decades, transjugular intrahepatic portosystemic shunting (TIPS), has been used as a treatment for uncontrolled gastrointestinal bleeding, refractory ascites or hydrothorax in patients with CLD. It can temporarily increase the MPAP, carbon monoxide and PVR and clinicians must be vigilant of these changes[69]. A contraindication to this procedure is a right ventricular systolic pressure > 50 mmHg, as well as an abnormal right ventricular size and function[25,49,68]. The pathophysiology, comparing the HPS and PPH is shown in Figure 1. Table 2 illustrates the pathophysiology, clinical features, diagnoses and treatment between these two diseases entity.
| Hepatopulmonary syndrome | Portopulmonary hypertension | |
| Pathophysiology | Severe vasodilatation | Severe vasoconstriction |
| Production of endothelin-1 and tumor necrosis alpha and eNOS and iNOS | Concentric intimal fibrosis, and smooth muscle hyperplasia and hypertrophy | |
| Increase of CO | Endothelin-1, prostacyclin and thromboxane | |
| Vasculoendothelial growth factor-A | ||
| Angiogenesis | ||
| Clinical features | Most patients are asymptomatic | Dyspnea |
| Dyspnea | Orthopnea | |
| Platypnea | Fatigue | |
| Orthodeoxia | Syncope | |
| Significant sleep-time oxygen desaturation | Chest pain | |
| Lightheadedness | ||
| Tricuspid regurgitation murmur, with a pronounced P2 sound | ||
| Increased jugular venous pressure | ||
| Peripheral edema | ||
| Ascites | ||
| Diagnosis | Corrected alveolar-arterial oxygen gradient (Abnormal if > 15-20 mmHg) with or without hypoxemia (PaO2), all in sitting position | Transthoracic echocardiography. |
| Contrast-enhanced echocardiography | ||
| Degree of severity: Alveolar-Arterial oxygen gradient > 15 mmHg,mild with PaO2 > 80 mmHg, moderate > 60 mmHg to < 80 mmHg, severe > 50 mmHg to < 60 mmHg or very severe < 50 mmHg | ||
| Pulmonary angiography | ||
| Treatment | Liver transplant | Endothelin receptor antagonist, phosphodiesterase type-5inhibitors, prostanoids, and combination therapy |
| Sildenafil alone or combined with prostacyclins | ||
| Transjugular intrahepatic portosystemic shunting | ||
| Liver transplant |
Once the HH is managed emergently, these patients must be evaluated for liver transplant[51]. The primary treatment objective is to reduce the ascitic fluid accumulation and to relieve symptoms. In addition, preventing complications is paramount in these patients[4,30,37]. Sodium restriction is the first-line treatment, as well as gentle diuresis (e.g., spironolactone at a dose of 50-100 up to 400 mg per day)[34,37,70]. Before using a second diuretic, the dose of spironolactone must be increased gradually. A low-sodium diet, with 70-90 mmol per day, and weight loss of 0.5 kg per day in patients without edema, and 1.0 kg per day in those with edema is an initial goal of therapy. Thoracentesis is helpful for immediate symptomatic relief, and it is indicated for large pleural effusions (1.5-2.0 L) and for those with refractory hydrothorax and patients who are not candidates for TIPS[70,71]. As noted above, TIPS could be used to decompress the portal tract and decrease the portal venous pressure and can help in decreasing the rate of ascites formation. This intervention partially resolves the pathogenesis of ascites formation and it is a better alternative than that repeated thoracentesis[72]. In these individuals, the indications for TIPS are the patients with refractory ascites, failure to respond to adequate diuretic therapy, and frequent thoracentesis (> 1 in a period of 2-3 wk)[73]. TIPS can be done safely in patients < 65 years of age with a serum bilirubin < 3 mg/dL, ALT < 100 IU/L and a Child-Pugh score < 10[31]. Other contraindications in the context of HH include hepatic encephalopathy, pulmonary hypertension and an ejection fraction < 60%[74].
The pulmonary complications seen in patients with CLD frequently require liver transplant evaluation. The HPS is more frequent than PPH hypertension and HH, and the best chance for improved life-quality in these patients is liver transplant. PPH can be confused with idiopathic pulmonary hypertension. TIPS can be used in some of these patients. Of all the complications reviewed in this manuscript, HH has the best outcomes.
Manuscript source: Invited manuscript
P- Reviewer: Zaky A S- Editor: Gong ZM L- Editor: A E- Editor: Zhang DN
| 1. | Fallon MB, Abrams GA. Pulmonary dysfunction in chronic liver disease. Hepatology. 2000;32:859-865. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 163] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 2. | Krowka MJ, Cortese DA. Hepatopulmonary syndrome. Current concepts in diagnostic and therapeutic considerations. Chest. 1994;105:1528-1537. [PubMed] [Cited in This Article: ] |
| 3. | Montani D, Günther S, Dorfmüller P, Perros F, Girerd B, Garcia G, Jaïs X, Savale L, Artaud-Macari E, Price LC. Pulmonary arterial hypertension. Orphanet J Rare Dis. 2013;8:97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 188] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 4. | Cardenas A, Kelleher T, Chopra S. Review article: hepatic hydrothorax. Aliment Pharmacol Ther. 2004;20:271-279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Scaglione S, Kliethermes S, Cao G, Shoham D, Durazo R, Luke A, Volk ML. The Epidemiology of Cirrhosis in the United States: A Population-based Study. J Clin Gastroenterol. 2015;49:690-696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 416] [Cited by in F6Publishing: 456] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 6. | Mokdad AA, Lopez AD, Shahraz S, Lozano R, Mokdad AH, Stanaway J, Murray CJ, Naghavi M. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med. 2014;12:145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 623] [Cited by in F6Publishing: 672] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 7. | Arguedas MR, Singh H, Faulk DK, Fallon MB. Utility of pulse oximetry screening for hepatopulmonary syndrome. Clin Gastroenterol Hepatol. 2007;5:749-754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Fallon MB, Krowka MJ, Brown RS, Trotter JF, Zacks S, Roberts KE, Shah VH, Kaplowitz N, Forman L, Wille K. Impact of hepatopulmonary syndrome on quality of life and survival in liver transplant candidates. Gastroenterology. 2008;135:1168-1175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 207] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 9. | Hoeper MM, Krowka MJ, Strassburg CP. Portopulmonary hypertension and hepatopulmonary syndrome. Lancet. 2004;363:1461-1468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 344] [Cited by in F6Publishing: 271] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 10. | Norvell JP, Spivey JR. Hepatic hydrothorax. Clin Liver Dis. 2014;18:439-449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Lv Y, Fan D. Hepatopulmonary Syndrome. Dig Dis Sci. 2015;60:1914-1923. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Rodríguez-Roisin R, Krowka MJ. Hepatopulmonary syndrome--a liver-induced lung vascular disorder. N Engl J Med. 2008;358:2378-2387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 483] [Cited by in F6Publishing: 391] [Article Influence: 24.4] [Reference Citation Analysis (1)] |
| 13. | Katsuta Y, Zhang XJ, Ohsuga M, Akimoto T, Komeichi H, Shimizu S, Kato Y, Miyamoto A, Satomura K, Takano T. Arterial hypoxemia and intrapulmonary vasodilatation in rat models of portal hypertension. J Gastroenterol. 2005;40:811-819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Rolla G. Hepatopulmonary syndrome: role of nitric oxide and clinical aspects. Dig Liver Dis. 2004;36:303-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Luo B, Abrams GA, Fallon MB. Endothelin-1 in the rat bile duct ligation model of hepatopulmonary syndrome: correlation with pulmonary dysfunction. J Hepatol. 1998;29:571-578. [PubMed] [Cited in This Article: ] |
| 16. | Schroeder RA, Ewing CA, Sitzmann JV, Kuo PC. Pulmonary expression of iNOS and HO-1 protein is upregulated in a rat model of prehepatic portal hypertension. Dig Dis Sci. 2000;45:2405-2410. [PubMed] [Cited in This Article: ] |
| 17. | Kochar R, Tanikella R, Fallon MB. Serial pulse oximetry in hepatopulmonary syndrome. Dig Dis Sci. 2011;56:1862-1868. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Sztrymf B, Libert JM, Mougeot C, Lebrec D, Mazmanian M, Humbert M, Herve P. Cirrhotic rats with bacterial translocation have higher incidence and severity of hepatopulmonary syndrome. J Gastroenterol Hepatol. 2005;20:1538-1544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Zhang J, Yang W, Luo B, Hu B, Maheshwari A, Fallon MB. The role of CX3CL1/CX3CR1 in pulmonary angiogenesis and intravascular monocyte accumulation in rat experimental hepatopulmonary syndrome. J Hepatol. 2012;57:752-758. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 20. | Zhang J, Luo B, Tang L, Wang Y, Stockard CR, Kadish I, Van Groen T, Grizzle WE, Ponnazhagan S, Fallon MB. Pulmonary angiogenesis in a rat model of hepatopulmonary syndrome. Gastroenterology. 2009;136:1070-1080. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 21. | Schenk P, Madl C, Rezaie-Majd S, Lehr S, Müller C. Methylene blue improves the hepatopulmonary syndrome. Ann Intern Med. 2000;133:701-706. [PubMed] [Cited in This Article: ] |
| 22. | Thenappan T, Goel A, Marsboom G, Fang YH, Toth PT, Zhang HJ, Kajimoto H, Hong Z, Paul J, Wietholt C. A central role for CD68(+) macrophages in hepatopulmonary syndrome. Reversal by macrophage depletion. Am J Respir Crit Care Med. 2011;183:1080-1091. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 23. | Hervé P, Lebrec D, Brenot F, Simonneau G, Humbert M, Sitbon O, Duroux P. Pulmonary vascular disorders in portal hypertension. Eur Respir J. 1998;11:1153-1166. [PubMed] [Cited in This Article: ] |
| 24. | Singh C, Sager JS. Pulmonary complications of cirrhosis. Med Clin North Am. 2009;93:871-873, viii. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Benjaminov FS, Prentice M, Sniderman KW, Siu S, Liu P, Wong F. Portopulmonary hypertension in decompensated cirrhosis with refractory ascites. Gut. 2003;52:1355-1362. [PubMed] [Cited in This Article: ] |
| 26. | Rodríguez-Roisin R, Krowka MJ, Hervé P, Fallon MB. Pulmonary-Hepatic vascular Disorders (PHD). Eur Respir J. 2004;24:861-880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 564] [Cited by in F6Publishing: 600] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 27. | Tsiakalos A, Hatzis G, Moyssakis I, Karatzaferis A, Ziakas PD, Tzelepis GE. Portopulmonary hypertension and serum endothelin levels in hospitalized patients with cirrhosis. Hepatobiliary Pancreat Dis Int. 2011;10:393-398. [PubMed] [Cited in This Article: ] |
| 28. | Edwards BS, Weir EK, Edwards WD, Ludwig J, Dykoski RK, Edwards JE. Coexistent pulmonary and portal hypertension: morphologic and clinical features. J Am Coll Cardiol. 1987;10:1233-1238. [PubMed] [Cited in This Article: ] |
| 29. | Hira HS, Gupta M, Tyagi SK. Portopulmonary hypertension in a patient of autoimmune hepatitis. Indian J Chest Dis Allied Sci. 2005;47:127-130. [PubMed] [Cited in This Article: ] |
| 30. | Baikati K, Le DL, Jabbour II, Singhal S, Anand S. Hepatic hydrothorax. Am J Ther. 2014;21:43-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Abe K, Takahashi A, Sato Y, Okai K, Katsushima F, Monoe K, Kanno Y, Saito H, Ohira H. Case of idiopathic portal hypertension complicated with autoimmune hepatitis. Hepatol Res. 2013;43:984-990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Lazaridis KN, Frank JW, Krowka MJ, Kamath PS. Hepatic hydrothorax: pathogenesis, diagnosis, and management. Am J Med. 1999;107:262-267. [PubMed] [Cited in This Article: ] |
| 33. | Roussos A, Philippou N, Mantzaris GJ, Gourgouliannis KI. Hepatic hydrothorax: pathophysiology diagnosis and management. J Gastroenterol Hepatol. 2007;22:1388-1393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Garcia N, Mihas AA. Hepatic hydrothorax: pathophysiology, diagnosis, and management. J Clin Gastroenterol. 2004;38:52-58. [PubMed] [Cited in This Article: ] |
| 35. | Gur C, Ilan Y, Shibolet O. Hepatic hydrothorax--pathophysiology, diagnosis and treatment--review of the literature. Liver Int. 2004;24:281-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Dumont AE, Mulholland JH. Flow rate and composition of thoracic-duct lymph in patients with cirrhosis. N Engl J Med. 1960;263:471-474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 148] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Milanez de Campos JR, Andrade Filho LO, de Campos Werebe E, Pandulo FL, Filomeno LT, Jatene FB. Hepatic hydrothorax. Semin Respir Crit Care Med. 2001;22:665-674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Sukcharoen K, Dixon S, Mangat K, Stanton A. Hepatic hydrothorax in the absence of ascites. BMJ Case Rep. 2013;2013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Yeshua H, Blendis LM, Oren R. Pulmonary manifestations of liver diseases. Semin Cardiothorac Vasc Anesth. 2009;13:60-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Aboussouan LS, Stoller JK. The hepatopulmonary syndrome. Baillieres Best Pract Res Clin Gastroenterol. 2000;14:1033-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Gómez FP, Martínez-Pallí G, Barberà JA, Roca J, Navasa M, Rodríguez-Roisin R. Gas exchange mechanism of orthodeoxia in hepatopulmonary syndrome. Hepatology. 2004;40:660-666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 42. | Fritz JS, Fallon MB, Kawut SM. Pulmonary vascular complications of liver disease. Am J Respir Crit Care Med. 2013;187:133-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 43. | Palma DT, Philips GM, Arguedas MR, Harding SM, Fallon MB. Oxygen desaturation during sleep in hepatopulmonary syndrome. Hepatology. 2008;47:1257-1263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Krowka MJ. Hepatopulmonary syndrome versus portopulmonary hypertension: distinctions and dilemmas. Hepatology. 1997;25:1282-1284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 97] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 45. | Kuo PC, Plotkin JS, Johnson LB, Howell CD, Laurin JM, Bartlett ST, Rubin LJ. Distinctive clinical features of portopulmonary hypertension. Chest. 1997;112:980-986. [PubMed] [Cited in This Article: ] |
| 46. | Budhiraja R, Hassoun PM. Portopulmonary hypertension: a tale of two circulations. Chest. 2003;123:562-576. [PubMed] [Cited in This Article: ] |
| 47. | Robalino BD, Moodie DS. Association between primary pulmonary hypertension and portal hypertension: analysis of its pathophysiology and clinical, laboratory and hemodynamic manifestations. J Am Coll Cardiol. 1991;17:492-498. [PubMed] [Cited in This Article: ] |
| 48. | Naeije R. Hepatopulmonary syndrome and portopulmonary hypertension. Swiss Med Wkly. 2003;133:163-169. [PubMed] [Cited in This Article: ] |
| 49. | Halank M, Ewert R, Seyfarth HJ, Hoeffken G. Portopulmonary hypertension. J Gastroenterol. 2006;41:837-847. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Cárdenas A, Arroyo V. Management of ascites and hepatic hydrothorax. Best Pract Res Clin Gastroenterol. 2007;21:55-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 51. | Endo K, Iida T, Yagi S, Yoshizawa A, Fujimoto Y, Ogawa K, Ogura Y, Mori A, Kaido T, Uemoto S. Impact of preoperative uncontrollable hepatic hydrothorax and massive ascites in adult liver transplantation. Surg Today. 2014;44:2293-2299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Kim YS, Susanto I, Lazar CA, Zarrinpar A, Eshaghian P, Smith MI, Busuttil R, Wang TS. Pneumothorax ex-vacuo or “trapped lung” in the setting of hepatic hydrothorax. BMC Pulm Med. 2012;12:78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 53. | Krok KL. Hepatic hydrothorax: current concepts. Clin Liver Disease. 2014;4:35-37. [Cited in This Article: ] |
| 54. | Okuyama T, Kimura M, Uchida J, Nishino K, Kumagai T, Fujiwara A, Higashiyama M, Imamura F. Porous diaphragm syndrome with repeated rapid accumulation of pleural effusion. Intern Med. 2014;53:1075-1077. [PubMed] [Cited in This Article: ] |
| 55. | Leung AN, Müller NL, Miller RR. CT in differential diagnosis of diffuse pleural disease. AJR Am J Roentgenol. 1990;154:487-492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 357] [Cited by in F6Publishing: 283] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 56. | Chen SC, Shih CM, Tseng GC, Cheng WE, Chiou J, Hsiao M, Kuo ML, Su JL, Chen CY. Vascular endothelial growth factor C as a predictor of early recurrence and poor prognosis of resected stage I non-small cell lung cancer. Ann Acad Med Singapore. 2011;40:319-324. [PubMed] [Cited in This Article: ] |
| 57. | Gurung P, Goldblatt M, Huggins JT, Doelken P, Nietert PJ, Sahn SA. Pleural fluid analysis and radiographic, sonographic, and echocardiographic characteristics of hepatic hydrothorax. Chest. 2011;140:448-453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 58. | Tu CY, Chen CH. Spontaneous bacterial empyema. Curr Opin Pulm Med. 2012;18:355-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 59. | Rollán MJ, Muñoz AC, Pérez T, Bratos JL. Value of contrast echocardiography for the diagnosis of hepatopulmonary syndrome. Eur J Echocardiogr. 2007;8:408-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 60. | Mimidis KP, Vassilakos PI, Mastorakou AN, Spiropoulos KV, Lambropoulou-Karatza CA, Thomopoulos KC, Tepetes KN, Nikolopoulou VN. Evaluation of contrast echocardiography and lung perfusion scan in detecting intrapulmonary vascular dilatation in normoxemic patients with early liver cirrhosis. Hepatogastroenterology. 1998;45:2303-2307. [PubMed] [Cited in This Article: ] |
| 61. | Saad NE, Lee DE, Waldman DL, Saad WE. Pulmonary arterial coil embolization for the management of persistent type I hepatopulmonary syndrome after liver transplantation. J Vasc Interv Radiol. 2007;18:1576-1580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 62. | Helmy AM, Awadallah MF. Study of pulmonary dysfunctions in liver cirrhosis. Egypt J Chest Dis Tuber. 2014;63:1079-1085. [Cited in This Article: ] |
| 63. | Bozbas SS, Bozbas H. Portopulmonary hypertension in liver transplant candidates. World J Gastroenterol. 2016;22:2024-2029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 24] [Cited by in F6Publishing: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 64. | Swanson KL, Wiesner RH, Krowka MJ. Natural history of hepatopulmonary syndrome: Impact of liver transplantation. Hepatology. 2005;41:1122-1129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 326] [Cited by in F6Publishing: 339] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 65. | Liberal R, Grant CR, Baptista R, Macedo G. “Porto-pulmonary hypertension: a comprehensive review”. Clin Res Hepatol Gastroenterol. 2015;39:157-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 66. | Porres-Aguilar M, Mukherjee D. Portopulmonary hypertension: an update. Respirology. 2015;20:235-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 67. | Wang YW, Lin HC, Yang YY, Hou MC, Lee SD. Sildenafil decreased pulmonary arterial pressure but may have exacerbated portal hypertension in a patient with cirrhosis and portopulmonary hypertension. J Gastroenterol. 2006;41:593-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 68. | Golbin JM, Krowka MJ. Portopulmonary hypertension. Clin Chest Med. 2007;28:203-218, ix. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 69. | Lin YH, Cai ZS, Jiang Y, Lü LZ, Zhang XJ, Cai QC. Perioperative risk factors for pulmonary complications after liver transplantation. J Int Med Res. 2010;38:1845-1855. [PubMed] [Cited in This Article: ] |
| 70. | Morrow CS, Kantor M, Armen RN. Hepatic hydrothorax. Ann Intern Med. 1958;49:193-203. [PubMed] [Cited in This Article: ] |
| 71. | Van der Linden P, Le Moine O, Ghysels M, Ortinez M, Devière J. Pulmonary hypertension after transjugular intrahepatic portosystemic shunt: effects on right ventricular function. Hepatology. 1996;23:982-987. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 72. | Freedman AM, Sanyal AJ, Tisnado J, Cole PE, Shiffman ML, Luketic VA, Purdum PP, Darcy MD, Posner MP. Complications of transjugular intrahepatic portosystemic shunt: a comprehensive review. Radiographics. 1993;13:1185-1210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 234] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 73. | Ong JP, Sands M, Younossi ZM. Transjugular intrahepatic portosystemic shunts (TIPS): a decade later. J Clin Gastroenterol. 2000;30:14-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 74. | Senousy BE, Draganov PV. Evaluation and management of patients with refractory ascites. World J Gastroenterol. 2009;15:67-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 38] [Cited by in F6Publishing: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |