Published online Jun 21, 2016. doi: 10.3748/wjg.v22.i23.5353
Peer-review started: March 3, 2016
First decision: April 1, 2016
Revised: April 14, 2016
Accepted: May 4, 2016
Article in press: May 4, 2016
Published online: June 21, 2016
AIM: To investigate the effects of different parameters of gastric electrical stimulation (GES) on interstitial cells of Cajal (ICCs) and changes in the insulin-like growth factor 1 (IGF-1) signal pathway in streptozotocin-induced diabetic rats.
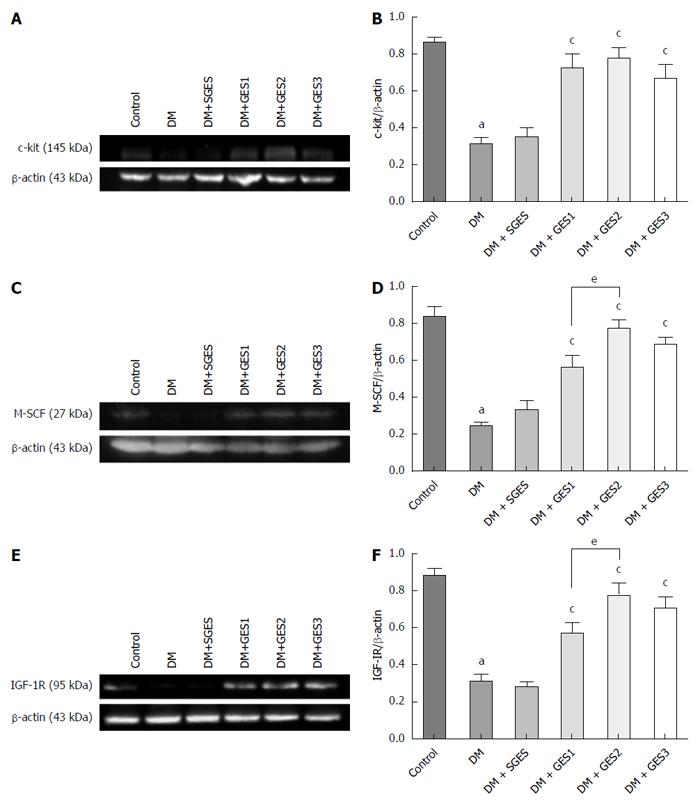
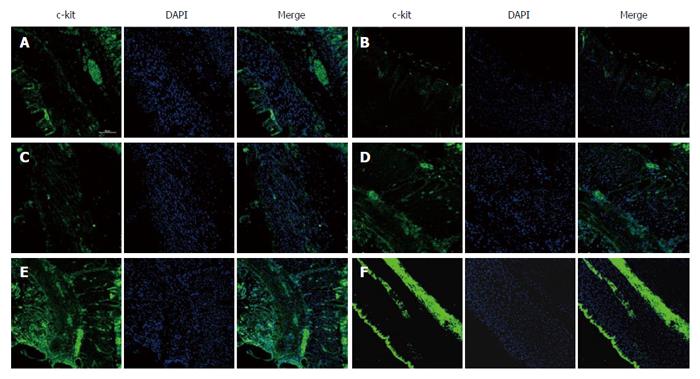
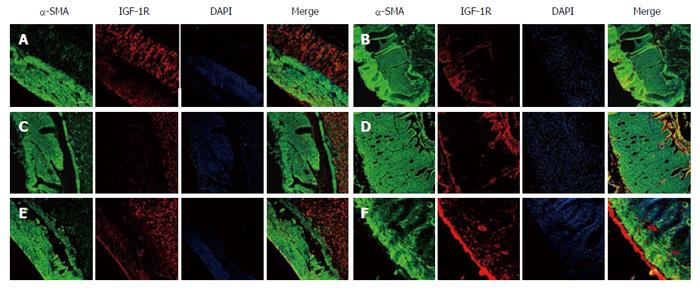
METHODS: Male rats were randomized into control, diabetic (DM), diabetic with sham GES (DM + SGES), diabetic with GES1 (5.5 cpm, 100 ms, 4 mA) (DM + GES1), diabetic with GES2 (5.5 cpm, 300 ms, 4 mA) (DM + GES2) and diabetic with GES3 (5.5 cpm, 550 ms, 2 mA) (DM + GES3) groups. The expression levels of c-kit, M-SCF and IGF-1 receptors were evaluated in the gastric antrum using Western blot analysis. The distribution of ICCs was observed using immunolabeling for c-kit, while smooth muscle cells and IGF-1 receptors were identified using α-SMA and IGF-1R antibodies. Serum level of IGF-1 was tested using enzyme-linked immunosorbent assay.
RESULTS: Gastric emptying was delayed in the DM group but improved in all GES groups, especially in the GES2 group. The expression levels of c-kit, M-SCF and IGF-1R were decreased in the DM group but increased in all GES groups. More ICCs (c-kit+) and smooth muscle cells (α-SMA+/IGF-1R+) were observed in all GES groups than in the DM group. The average level of IGF-1 in the DM group was markedly decreased, but it was up-regulated in all GES groups, especially in the GES2 group.
CONCLUSION: The results suggest that long-pulse GES promotes the regeneration of ICCs. The IGF-1 signaling pathway might be involved in the mechanism underlying this process, which results in improved gastric emptying.
Core tip: Gastric electrical stimulation (GES) regulates gastric motility and protects the interstitial cells of Cajal (ICCs). However, the mechanisms underlying these processes have not been determined. In this study, we report that long-pulse GES with three different parameters protected ICCs and that the insulin-like growth factor 1 signaling pathway is probably involved in this process. One GES parameter (5.5 cpm, 300 ms, 4 mA) showed immense potential as a clinical application for applying long-pulse GES as the optimal parameter.
- Citation: Li H, Chen Y, Liu S, Hou XH. Long-pulse gastric electrical stimulation protects interstitial cells of Cajal in diabetic rats via IGF-1 signaling pathway. World J Gastroenterol 2016; 22(23): 5353-5363
- URL: https://www.wjgnet.com/1007-9327/full/v22/i23/5353.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i23.5353
Gastrointestinal motility disorders (such as early satiety, bloating and vomiting) often plague patients with long-standing diabetes. However, the pathogenesis of these disorders remains unclear, and effective pharmacological management is limited for these symptoms. Fortunately, a few recent non-pharmacological treatments, including gastric electrical stimulation (GES), have renewed the hope for achieving new management strategies, but the mechanisms underlying their efficacy remain unclear.
Recently, a number of studies have indicated that interstitial cells of Cajal (ICCs) may play an important physiological role in coordinating gastric contractile activity and gastric motility[1,2]. C-kit protein is known to localize on the surface of ICCs and to be activated by interactions between kit receptors and stem cell factor (SCF). SCF/c-kit signaling is important for maintaining the ICC phenotype and ICC proliferation, differentiation and survival[3,4]. Furthermore, some studies have suggested that damage to or the loss of ICCs leads to gastrointestinal motility disorders in diabetic models[5-7] and that decreases in the levels of SCF and insulin-like growth factor 1 (IGF-1) are involved in ICC deficiency[8]. According to the results of recent studies, SCF and IGF-1, which are necessary for the differentiation and maintenance of the ICC phenotype, are derived from smooth muscle cells (SMCs), and IGF-1 increases the expression of SCF[9]. Moreover, the IGF-1 receptor (IGF-1R) appears to be lacking in mature ICCs, and IGF-1 is likely to directly interact with receptors on SMCs to enhance SCF synthesis, resulting in a protective effect in ICCs[8]. Based on a recent study, the levels of serum IGF-1 and its binding protein 3 are altered in type 1 diabetes and that circulating IGF-1 and its binding protein 3 control colonic stem cell function and gastrointestinal complications of diabetes[10].
Generally, GES is classified into three categories according to the length of the pulse and the energy that is applied: the short-pulse GES, the long-pulse GES and the dual-pulse GES. The effects of GES varied with the parameters. In refractory gastroparesis patients who cannot undergo drug therapy due to serious side effects or who are unable to undergo surgical treatment, GES is an alternative option. Our previous study indicated that GES might increase the expression of membrane stem cell factor (M-SCF) in streptozotocin (STZ)-induced diabetic rats[11]. However, the mechanism by which GES affects gastric motility and the involvement of IGF-1 signaling remain unknown. The aim of this study was to: (1) systematically assess the effects of applying GES with different parameters on gastric emptying; (2) ascertain the effects of GES on ICCs in diabetic rats; and (3) determine whether the IGF-1 pathway is involved in the regeneration of ICCs.
Adult male Sprague-Dawley rats (250-300 g) obtained from the Experiment Animal Center of Tongji Medical College were used in this study. All appropriate measures were adopted to minimize discomfort or pain to the animals. The rats were housed in standardized laboratory conditions (a 12/12 h light/dark cycle at 22 °C) and allowed free access to standard solid food and sterile water. The rats were formally enrolled after they were adapted to the laboratory conditions for one week. All experiments were reviewed and approved by the Institutional Animal Care and Use Committee of Tongji Medical College.
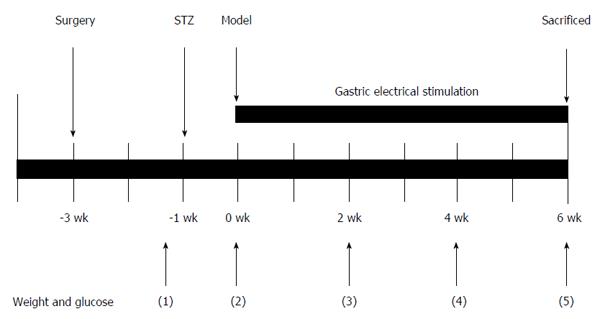
The animals were randomized into one of six equal groups (6 rats/group): control, diabetic (DM), diabetic and sham GES (DM + SGES), diabetic and GES parameter 1 (5.5 cpm, 100 ms, 4 mA) (DM + GES1), diabetic and GES parameter 2 (5.5 cpm, 300 ms, 4 mA) (DM + GES2), and diabetic and GES parameter 3 (5.5 cpm, 550 ms, 2 mA) (DM + GES3). Diabetes was induced using STZ (60 mg/kg, ip, Alexis Biochemical, United States). Rats in the control group were injected with equal volume of diluent. Blood glucose concentrations were measured one week after the injection. The rats were considered diabetic if their blood glucose level increased and was maintained at equal to or more than 16.7 mmol/L. Body weight and blood glucose levels were also tested before the rats were injected and during weeks 0, 2, 4, and 6 after the induction of diabetes. After sequential GES intervention was performed for 6 wk, gastric emptying was measured in all groups. Serum samples were collected for enzyme-linked immunosorbent assay (ELISA), and the rats were sacrificed and the specimens of the antrum were acquired. Each antrum specimen was separated into two pieces. One of these was stored at -80 °C for Western blot analysis, and a relatively large piece was fixed in Zamboni’s fixative [1.5% saturated picric acid solution and 2% paraformaldehyde in 0.1 M phosphate buffer solution (PBS, pH = 7.4)] for the immunofluorescence study.
Rats in the GES and sham GES groups were anesthetized using pentobarbital sodium (30 mg/kg, ip, Sigma, United States). A midline laparotomy was performed, and stimulating electrodes were pierced the subserosa of the stomach and placed on the serosal surface along the greater curvature. The distal pair of electrodes was about 0.5 cm away from the pylorus and the proximal pair was approximately 1.5 cm away from the distal pair. The electrodes of the proximal pair were placed 0.3 cm apart. The wires were tunneled through the anterior abdominal wall and led out of the skin so they could be connected to the stimulator (G6805-2A; Shanghai Huayi Medical Instrument Factory, China). The abdominal wall was closed using a simple interrupted suture. After the rats recovered from the surgery completely (usually 2 wk), they continuously received GES intervention for 30 min/day for 6 wk (Figure 1). The following respective long-pulse GES frequencies, pulse widths and amplitude parameters were used: GES1, 5.5 cycles/min (cpm), 100 ms and 4 mA; GES2, 5.5 cpm, 300 ms and 4 mA; and GES3, 5.5 cpm, 550 ms and 2 mA. These parameters were shown to be effective and representative in previous experiments[11-13]. Rats in the DM + SGES group were connected only to the stimulator and were not stimulated with an electric current.
The test meals, including carboxymethylcellulose (15 mg/mL) and phenol red (0.5 mg/mL), were continually stirred and maintained at 37 °C. After the rats were deprived of food overnight, the animals were fed 2 mL of the test meal using a straight gavage needle. After 30 min, the rats were quickly sacrificed using cervical dislocation. The stomach was acquired after it was ligated at the pylorus and cardia, and then it was opened. The gastric contents were placed into a test tube and then washed using distilled water. A NaOH solution (20 mL, 1 mol/L) was placed in each test tube. Absorbance at 560 nm was read using a spectrophotometer (U-2900, Hitachi, Japan) to determine the quantity of remained phenol red.
Gastric emptying was calculated using the following formula: gastric emptying (%) = 100 × (1-X/Y), where X is the absorbance of phenol red measured at 30 min after the test meal, and Y is the absorbance of phenol red in the control rats that were sacrificed immediately after they were fed the test meal[14].
The gastric antrum tissues were rapidly fixed in Zamboni’s fixative for 24 h at room temperature. They were then dehydrated, embedded in paraffin and cut at a thickness of 5-7 μm. The paraffin sections were deparaffinized and hydrated before antigen retrieval was performed. Endogenous peroxidase was controlled using 3% hydrogen peroxide (H2O2) for 30 min. The sections were then microwaved (750 W) for 5 min, and nonspecific reactions were inhibited using normal goat serum for 20 min. The primary antibodies, including IGF-1R (1:200; Santa Cruz Biotechnology, Inc), c-kit (1:150; Santa Cruz Biotechnology, Inc) and α-SMA (1:500; Santa Cruz Biotechnology, Inc), were added dropwise to the paraffin sections which were placed at 4 °C overnight. The sections were then rinsed three times using PBS (PH 7.4) and incubated in secondary antibodies for 60 min at 37 °C, followed by incubation with DAPI (Sigma, United States) for 30 min. The sections were then counterstained and dehydrated. The specimens were observed using a laser scanning confocal microscope (Olympus, Tokyo, Japan).
The antrum specimens were homogenized in RIPA buffer (Upstate, United States) containing protease inhibitor (Beyotime, China) and incubated for 30 min on ice. The liquid was centrifuged at 12000 g at 4 °C for 8 min, and the supernatant containing the total extracted proteins was then collected. BCA reagent (Pierce, Rockford, IL, United States) was used to analyze the protein concentration of each sample. Total proteins were separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to PVDF membranes (Millipore, United States). The membranes were immersed in 5% skim milk solution for 1 h and then incubated with primary antibodies against c-kit (1:200, Sigma-Aldrich, United States), SCF (1:200, Abcam, United Kingdom), IGF-1R (1:200, Boster, China) or β-actin (1:1000, Beyotime, China) at 37 °C with gentle shaking for 1 h, followed by maintenance at 4 °C overnight. After incubation with secondary antibodies for 1 h, the bands on the PVDF membranes were visualized using enhanced chemiluminescence (Amersham Pharmacia, United States). A densitometry analysis was conducted using AlphaView software.
For this experiment, approximately 2 mL of blood was obtained from each rat to study differences in serum IGF-1 levels. The concentration of IGF-1 was quantified using rat IGF-1 ELISA kits (RayBiotech, United States). A standard curve was established for IGF-1 by testing the standard with a spectrophotometer at 450 nm, and the concentration of IGF-1 in the serum was then determined by comparing the optical densities of the study samples to those of the standard samples.
The gastric antrum was immersed in fixative solution (2.5% glutaraldehyde) for 2 h at 4 °C. Tissue samples (approximately 1 mm × 5 mm) were separated from the gastric antrum and soaked in 1% OsO4 for 60-120 min. They were then rinsed with 0.1 mol/L phosphate buffer and dehydrated in ethanol. The tissue samples were immersed in propylene oxide followed by mixtures of Epon Resin and propylene oxide for 2 h, and then embedded in Epon. An ultramicrotome was used to identify the regions of interest in the study and to section them into ultrathin sections (70 nm). The sections were visualized using a transmission electron microscope (Tecnai G212, FEI, The Netherlands).
The data are presented as the mean ± SEM. One-way analysis of variance was used to evaluate differences among groups. The least significant difference post hoc test was applied to compare differences between groups. A P value < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS 17.0 software.
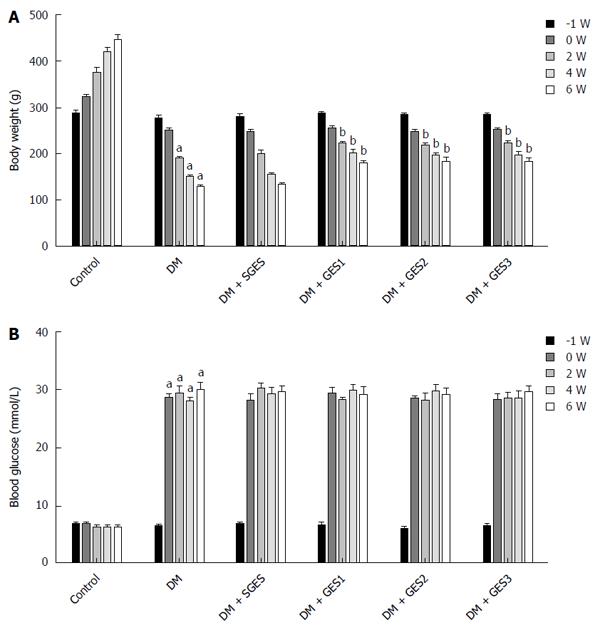
Baseline weight did not markedly differ between the groups (Figure 2A). The weights of the rats in the DM group were markedly lower at the end of weeks 2, 4 and 6 (P = 0.013, P = 0.005 and P < 0.0001, respectively) than the weights of the controls. The weights of the DM + GES1, DM + GES2 and DM + GES3 groups were significantly higher at the 6th week than the weights in the DM group (P = 0.035, 0.028 and 0.031, respectively).
As shown in Figure 2B, baseline blood glucose levels were not significantly different between the groups. After the induction of diabetes, the blood glucose levels were higher in the DM group than in the control group for the remainder of the experiment (P < 0.0001). After long-pulse GES intervention was applied, blood glucose levels were not markedly different between the DM + GES1, DM + GES2 or DM + GES3 group and the DM group (P = 0.332, 0.281 and 0.416, respectively).
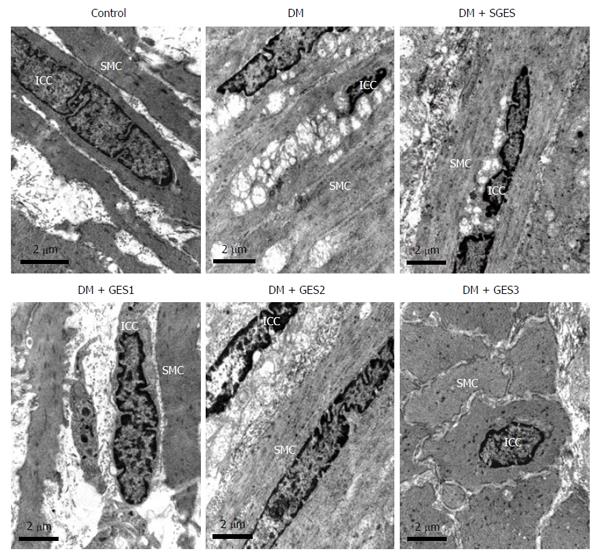
In the control group, ICCs showed higher electron-dense cytoplasm than SMCs and were abundant in the endoplasmic reticulum, mitochondria and basal lamina (Figure 3A). In addition, they showed a close connection with SMCs and enteric nerves. However, in the DM group, ICCs were seriously impaired (Figure 3B): swollen endoplasmic reticulum and mitochondria, extensive vacuolization, cytoplasmic depletion and lamellar bodies were frequently observed in their cell bodies. The intercellular spaces were dilated, gap junctions between ICCs and enteric nerves were reduced and exudation of fibrin was occasionally observed. ICCs in the DM + SGES group displayed ultrastructural changes that were similar to those in the ICCs in the DM group (Figure 3C). However, most of the ICCs in the DM + GES1 (Figure 3D), DM + GES2 (Figure 3E) and DM + GES3 (Figure 3F) groups were repaired, and minor damage (the structure of the cytomembrane was relatively complete, organelles were abundant, there were slight cytoplasmic depletion, slightly swollen endoplasmic reticulum and mitochondria and limited vacuolization, there were few denuded ribosomes and a small number of lysosomes, and there was little heterochromatin.) was occasionally observed.
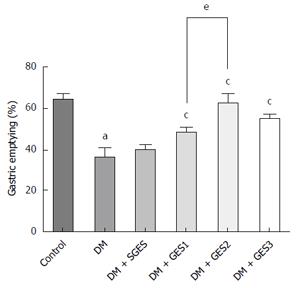
Figure 4 shows the effect of GES on gastric emptying. Gastric emptying in the DM group was slower than that in the control group (P < 0.0001). SGES did not markedly affect gastric emptying compared to the DM group (P = 0.573), but GES1, GES2 and GES3 significantly improved the delayed gastric emptying from 35.89% ± 4.43% to 48.27% ± 2.20%, 61.84% ± 4.87 and 53.34 ± 2.26%, respectively (P = 0.03, 0.0028 and 0.0041, respectively). These improvements were significantly different between the DM + GES1 group and the DM + GES2 group (P = 0.029), indicating that the effect of GES2 on gastric emptying was stronger than that of GES1.
As shown in Figure 5A and B, the expression of c-kit was evaluated in the antrum. The level of c-kit was markedly lower in the DM group than in the control group (P < 0.0001). Conversely, the expression of c-kit was dramatically higher in the DM + GES1, DM + GES2 and DM + GES3 groups than in the DM group (P = 0.0004, P < 0.0001 and P = 0.0027, respectively). The expression of M-SCF (Figure 5C and D) was lower in the DM group than in the control group (P < 0.0001). However, GES1, GES2 and GES3 resulted in a higher level of expression of M-SCF than was observed in the DM group (P = 0.001, P < 0.0001 and P < 0.0001, respectively). The effect of GES2 was stronger than that of GES1 (P = 0.028).
Compared to the control, the expression of IGF-1R (Figure 5E and F) was markedly lower in the DM group (P < 0.0001), whereas the level of IGF-1R in the DM + GES1, DM + GES2 and DM + GES3 groups was significantly higher than the level in the DM group (P = 0.004, P < 0.0001 and P = 0.001, respectively). This effect was stronger in the GES2 group than in the GES1 group (P = 0.039).
Table 1 shows the levels of IGF-1 in the serum in each group. The IGF-1 levels in the Control, DM, DM + SGES, DM + GES1, DM + GES2 and DM + GES3 groups were 281.22 ± 9.75, 31.27 ± 5.61, 29.18 ± 5.02, 90.88 ± 6.74, 121.62 ± 9.97 and 113.97 ± 9.37 ng/mL, respectively. The average IGF-1 level in the DM group was lower than that in the control group (P < 0.0001). However, the IGF-1 level was higher in the DM + GES1, DM + GES2 and DM + GES3 groups than in the DM group (P < 0.0001 for all). The level of IGF-1 was not markedly different between the DM + SGES and the DM groups (P = 0.794).
As shown in Figure 6, c-kit immunoreactivity revealed the distribution of ICCs in the gastric antrum of each group. There were a number of c-kit+ cells in the control group. However, few ICCs were observed in the DM group. Similar results were found in the DM + SGES group, and long-pulse GES interventions markedly increased the expression of c-kit in the DM + GES1, DM + GES2 and DM + GES3 groups.
In Figure 7, α-SMA and IGF-1R immunoreactivity reflects the distribution of smooth muscle cells and IGF-1 receptors, respectively, in the gastric antrum of each group. In the control group, a large number of IGF-1R+ cells was observed in the intramuscular layer, whereas few IGF-1R+ cells were observed in the DM group. Analogously, few IGF-1R+ cells were observed in the DM + SGES group. However, IGF-1R expression was markedly increased in the DM + GES1, DM + GES2 and DM + GES3 groups after treatment with long-pulse GES.
In the current study, applying GES using various settings increased the expression of M-SCF and IGF-1/IGF-1R and improved the regeneration of ICCs in diabetic rats, which ameliorated delayed gastric emptying in these rats.
In recent years, GES has been proposed as a therapeutic option for patients suffering from refractory gastroparesis. Some studies have demonstrated that the effects of GES depend on the GES parameters. Short-pulse GES improved dyspeptic symptoms, while long-pulse GES normalized gastric dysrhythmia and regulated gastric slow waves, and dual-pulse GES reduced dyspeptic symptoms and gastric dysrhythmia[15-20]. Because in some instances, long-pulse GES re-establishes normal slow wave activity and improves gastric emptying, it is usually applied to treat gastroparesis. Based on the results of previous studies, a frequency of 5.5 cpm, a pulse width ranging from 100 ms to 300 ms and an amplitude of 4 mA are frequently used and have been shown to be effective in regulating gastric dysrhythmia and gastric slow waves[21,22]. In this study, we selected representative widths of 100 ms (GES1: 5.5 cpm, 100 ms, and 4 mA) and 300 ms (GES2: 5.5 cpm, 300 ms, and 4 mA) for further study. Additionally, GES3 (5.5 cpm, 550 ms, 2 mA) was also demonstrated to be effective in our previous study[11], and that setting was consequently also used in this study. Bellahsène et al[23] demonstrated that long-pulse GES (7 cpm, 300 ms, 4 mA) accelerated gastric emptying in a canine model of gastroparesis. Moreover, McCallum et al[24] discovered that long-pulse GES (at a frequency that was 10% higher than the intrinsic slow-wave frequency, 300 ms, 4 mA) accelerated gastric emptying in gastroparesis patients. However, Xing et al[20] found that long-pulse GES (6 cpm, 375 ms, 4 mA) did not significantly influence gastric emptying in a canine model. In the present study, delayed gastric emptying was accelerated after GES intervention, especially in the GES2 group (5.5 cpm, 300 ms, 4 mA), which supports the findings of most studies in this field. Our results showed that long-pulse GES improved delayed gastric emptying in diabetic rats, and the effect of GES2 (5.5 cpm, 300 ms, 4 mA) was more pronounced than those of GES1 and GES3. Thus, the GES2 protocol should potentially be applied in the clinic, but further studies are needed to explore this issue.
The mechanisms by which long-pulse GES affects gastric emptying remain unclear. ICCs have been shown to play an important role in the regulation of gastric peristalsis, which significantly affects gastric emptying[1,25]. However, few studies have examined the involvement of ICCs in the effect of long-pulse GES on gastric emptying. We conducted related experiments in which we examined the effect of GES on ICCs. The results revealed that long-pulse GES (5.5 cpm, 550 ms, 2 mA) resulted in ICC remodeling in diabetic rats. Furthermore, our previous study showed that both low- and high-frequency electroacupuncture at ST-36 increased the number of ICCs[14]. In the present study, GES intervention also markedly recovered the ultrastructure of ICCs to a minor injury or normal state[26] and increased the number of ICCs in the antrum in diabetic rats, indicating that long-pulse GES might induce ICC remodeling and further contribute to improved gastric emptying.
The c-kit/SCF pathway is one of the most important regulators of ICCs. As the ligand of c-kit, SCF exists in both a soluble form (S-SCF) and a transmembrane form (M-SCF). M-SCF may play a more important role than S-SCF during the differentiation, survival and maintenance of the ICC phenotype[27]. Thus, we focused on M-SCF in our evaluation of c-kit/SCF signaling and our goal of identifying the mechanisms by which GES results in ICC remodeling. Horváth et al[8] linked reduced SCF to an ICC deficiency in diabetic gastroparesis and found that the level of M-SCF was decreased in the gastric antrum in diabetic mice. Our results also indicate that the expression of M-SCF is markedly decreased in diabetic rats. However, the effects of GES on the expression of M-SCF have rarely been investigated. In a previous study, we showed that both low- and high-frequency electroacupuncture at ST-36 increased the expression of M-SCF in diabetic rats[14]. In the present study, we found that GES treatment also increased the expression of M-SCF, and this increase was stronger when GES2 (5.5 cpm, 300 ms, 4 mA) was applied. Therefore, M-SCF is likely to be involved in ICC remodeling as a result of long-pulse GES.
IGF-1 signaling is one of the main factors that regulate the expression of M-SCF. IGF-1R, which is necessary for the differentiation and maintenance of ICC phenotype, is located on smooth muscle cells but not on ICCs[8,28]. Horváth et al[29] showed the first in vitro evidence that IGF-1 signaling was responsible for maintaining the ICC network. In their subsequent study, they suggested that endogenous IGF-1 expression was decreased in the diabetic group and that adding IGF-1 to the medium improved the expression of M-SCF[9]. The results of the present study show that the expression of IGF-1R in gastric antral SMCs was markedly decreased in the DM group, which supports the findings of previous studies[8,9]. Moreover, serum IGF-1 levels were also decreased in the DM group. However, few studies have examined the effects of GES on IGF-1 and IGF-1R levels or the involvement of IGF-1 signaling in the induction of ICC remodeling by GES. In our study, the levels of both IGF-1 and IGF-1R were significantly increased following GES treatment, which mirrored the changes we observed in M-SCF. Additionally, GES2 (5.5 cpm, 300 ms, 4 mA) was more effective in improving the expression of IGF-1 and IGF-1R. These results indicate that improvements in IGF-1 signaling might be involved in the up-regulation of M-SCF expression and that it might also contribute to ICC remodeling in response to long-pulse GES in diabetic rats.
Xing et al[30] reported that the total area under the curve for blood glucose in healthy dogs was markedly decreased by long-pulse GES (10 cpm, 300 ms, 8 mA). In the present study, GES did not significantly affect blood glucose levels. We speculate that the difference between these effects might be associated with differences in the parameters, animal models and physical states that were used, and that the effect of GES on the blood glucose levels in diabetic models might therefore require further study. Yan et al[31] reported that pulse-train GES (0.3 ms, 3 mA, 20 Hz for 2 s on and 3 s off) reduced body weight in obese rats. Our results show that long-pulse GES treatment markedly increased body weight in diabetic rats. Therefore, the effects of GES on body weight might depend on the parameters and physical states that are used. Broadly, our results suggest that GES increased the body weight but did not significantly affect blood glucose levels in STZ-induced diabetic rats.
In summary, our results reveal that the expression levels of M-SCF, IGF-1 and IGF-1R are significantly lower and that the number of ICCs is markedly decreased in the gastric antrum of diabetic rats. These characteristics likely contribute to delayed gastric emptying. Following long-pulse GES intervention, ICCs remodeled and gastric emptying was improved. The IGF-1 signaling pathway might participate in this process by enhancing the expression of M-SCF on SMCs and then protecting ICCs. Furthermore, the effects of GES2 (5.5 cpm, 300 ms, 4 mA) on the expression of M-SCF and IGF-1/IGF-1R appeared to be stronger than those of GES1 and GES2. Long-pulse GES involves low-frequency/high-energy stimulation; its frequency is slightly higher than the intrinsic slow wave and requires a high amount of energy. This stimulus could directly activate ICCs and/or SMCs without involving cholinergic nerves[21], and the magnitude of energy is probably responsible for the effects of long-pulse GES. In our study, GES2 (5.5 cpm, 300 ms, 4 mA) afforded higher energy than did GES1 (5.5 cpm, 100 ms, 4 mA) and GES3 (5.5 cpm, 550 ms, 2 mA), and this might partly explain why GES2 had a more significant effect on gastric emptying and the IGF-1 signaling pathway. Thus, this parameter should be used in clinical applications involving long-pulse GES. However, further studies into the mechanisms underlying these processes are needed to ensure the safety and clinical efficacy of using long-pulse GES as a treatment for refractory functional gastric disorders.
Gastric electrical stimulation (GES) regulates gastric motility and promotes the renovation of interstitial cells of Cajal (ICCs). However, the mechanisms underlying this effect remain unclear. Previous studies demonstrated that ICCs are damaged and the insulin-like growth factor 1 (IGF-1) pathway is down-regulated in diabetic models. In this study, the authors report that applying long-pulse GES using three different parameters promoted the regeneration of ICCs and that the IGF-1 signaling pathway is likely to be involved in this process.
Experiments have shown that ICCs are damaged in diabetic models. The c-kit/SCF pathway is one of the most important regulators of ICCs. M-stem cell factor (SCF) may play a more important role than S-SCF in the differentiation, survival and maintenance of the ICC phenotype. IGF-1 signaling is one of the main factors that regulate the expression of M-SCF.
This study provides evidence showing that long-pulse GES protects the ICCs in diabetic rats and that the IGF-1 signaling pathway is involved in this effect. The effects of one GES parameter (5.5 cpm, 300 ms, 4 mA) were stronger.
One GES parameter (5.5 cpm, 300 ms, 4 mA) showed greater potential for being used in clinical application involving long-pulse GES, and the IGF-1 signaling pathway may be a possible therapeutic target in gastroparesis.
ICCs, as the gastrointestinal pace-making cells, play an important physiological role in coordinating gastric contractile activity and gastric motility. Long-pulse GES restores injured ICCs. SCF and IGF-1 are regulatory proteins that protect ICCs.
An interesting paper! The paper by Li and colleagues describes the effect of different timing pulse GES (gastric electrical stimulation) on gastric emptying in a rat diabetic model. In particular, the authors demonstrate that long-term pulsing GES protects ICCs located in the gastric antrum and this is associated with an improvement of gastric emptying delay during diabetes. Interestingly, the authors also suggest that the IGF-1 signaling pathway may be involved.
P- Reviewer: Blair PJ, Fiorina P, Kito Y S- Editor: Yu J L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Hirst GD, Edwards FR. Electrical events underlying organized myogenic contractions of the guinea pig stomach. J Physiol. 2006;576:659-665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Wang XY, Lammers WJ, Bercik P, Huizinga JD. Lack of pyloric interstitial cells of Cajal explains distinct peristaltic motor patterns in stomach and small intestine. Am J Physiol Gastrointest Liver Physiol. 2005;289:G539-G549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Torihashi S, Nishi K, Tokutomi Y, Nishi T, Ward S, Sanders KM. Blockade of kit signaling induces transdifferentiation of interstitial cells of cajal to a smooth muscle phenotype. Gastroenterology. 1999;117:140-148. [PubMed] [Cited in This Article: ] |
| 4. | Tong W, Jia H, Zhang L, Li C, Ridolfi TJ, Liu B. Exogenous stem cell factor improves interstitial cells of Cajal restoration after blockade of c-kit signaling pathway. Scand J Gastroenterol. 2010;45:844-851. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Forster J, Damjanov I, Lin Z, Sarosiek I, Wetzel P, McCallum RW. Absence of the interstitial cells of Cajal in patients with gastroparesis and correlation with clinical findings. J Gastrointest Surg. 2005;9:102-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 170] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 6. | Wang XY, Huizinga JD, Diamond J, Liu LW. Loss of intramuscular and submuscular interstitial cells of Cajal and associated enteric nerves is related to decreased gastric emptying in streptozotocin-induced diabetes. Neurogastroenterol Motil. 2009;21:1095-e92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Ordög T, Takayama I, Cheung WK, Ward SM, Sanders KM. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes. 2000;49:1731-1739. [PubMed] [Cited in This Article: ] |
| 8. | Horváth VJ, Vittal H, Lörincz A, Chen H, Almeida-Porada G, Redelman D, Ordög T. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of cajal in murine diabetic gastroparesis. Gastroenterology. 2006;130:759-770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 171] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 9. | Wang Y, Xu XY, Tang YR, Yang WW, Yuan YF, Ning YJ, Yu YJ, Lin L. Effect of endogenous insulin-like growth factor and stem cell factor on diabetic colonic dysmotility. World J Gastroenterol. 2013;19:3324-3331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 12] [Cited by in F6Publishing: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | D’Addio F, La Rosa S, Maestroni A, Jung P, Orsenigo E, Ben Nasr M, Tezza S, Bassi R, Finzi G, Marando A. Circulating IGF-I and IGFBP3 Levels Control Human Colonic Stem Cell Function and Are Disrupted in Diabetic Enteropathy. Cell Stem Cell. 2015;17:486-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Li C, Liu S, Guan Y, Qian W, du F, Hou X. Long pulse gastric electrical stimulation induces regeneration of myenteric plexus synaptic vesicles in diabetic rats. Neurogastroenterol Motil. 2010;22:453-61, e108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Liu J, Qiao X, Micci MA, Pasricha PJ, Chen JD. Improvement of gastric motility with gastric electrical stimulation in STZ-induced diabetic rats. Digestion. 2004;70:159-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Du F, Wang L, Qian W, Liu S. Loss of enteric neurons accompanied by decreased expression of GDNF and PI3K/Akt pathway in diabetic rats. Neurogastroenterol Motil. 2009;21:1229-e114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Chen Y, Xu J, Liu S, Hou X. Electroacupuncture at ST36 increases contraction of the gastric antrum and improves the SCF/c-kit pathway in diabetic rats. Am J Chin Med. 2013;41:1233-1249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Xu X, Qian L, Chen JD. Anti-dysrhythmic effects of long-pulse gastric electrical stimulation in dogs. Digestion. 2004;69:63-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Xing J, Brody F, Rosen M, Chen JD, Soffer E. The effect of gastric electrical stimulation on canine gastric slow waves. Am J Physiol Gastrointest Liver Physiol. 2003;284:G956-G962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Lei Y, Chen JD. Effects of dual pulse gastric electrical stimulation on gastric tone and compliance in dogs. Dig Liver Dis. 2009;41:277-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Xu X, Brining DL, Chen JD. Effects of vasopressin and long pulse-low frequency gastric electrical stimulation on gastric emptying, gastric and intestinal myoelectrical activity and symptoms in dogs. Neurogastroenterol Motil. 2005;17:236-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Chen JD, Qian L, Ouyang H, Yin J. Gastric electrical stimulation with short pulses reduces vomiting but not dysrhythmias in dogs. Gastroenterology. 2003;124:401-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 147] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Xing J, Brody F, Brodsky J, Rosen M, Larive B, Ponsky J, Soffer E. Gastric electrical-stimulation effects on canine gastric emptying, food intake, and body weight. Obes Res. 2003;11:41-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Bortolotti M. Gastric electrical stimulation for gastroparesis: a goal greatly pursued, but not yet attained. World J Gastroenterol. 2011;17:273-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 34] [Cited by in F6Publishing: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Song GQ, Lei Y, Xu X, Chen JD. Gastric electrical stimulation with long pulses in humans and animals: can data obtained in animals be replicated in humans? Neuromodulation. 2010;13:87-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Bellahsène BE, Lind CD, Schirmer BD, Updike OL, McCallum RW. Acceleration of gastric emptying with electrical stimulation in a canine model of gastroparesis. Am J Physiol. 1992;262:G826-G834. [PubMed] [Cited in This Article: ] |
| 24. | McCallum RW, Chen JD, Lin Z, Schirmer BD, Williams RD, Ross RA. Gastric pacing improves emptying and symptoms in patients with gastroparesis. Gastroenterology. 1998;114:456-461. [PubMed] [Cited in This Article: ] |
| 25. | Vittal H, Farrugia G, Gomez G, Pasricha PJ. Mechanisms of disease: the pathological basis of gastroparesis--a review of experimental and clinical studies. Nat Clin Pract Gastroenterol Hepatol. 2007;4:336-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Mitsui R, Komuro T. Distribution and ultrastructure of interstitial cells of Cajal in the gastric antrum of wild-type and Ws/Ws rats. Anat Embryol (Berl). 2003;206:453-460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Rich A, Miller SM, Gibbons SJ, Malysz J, Szurszewski JH, Farrugia G. Local presentation of Steel factor increases expression of c-kit immunoreactive interstitial cells of Cajal in culture. Am J Physiol Gastrointest Liver Physiol. 2003;284:G313-G320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Ward SM, Ordög T, Bayguinov JR, Horowitz B, Epperson A, Shen L, Westphal H, Sanders KM. Development of interstitial cells of Cajal and pacemaking in mice lacking enteric nerves. Gastroenterology. 1999;117:584-594. [PubMed] [Cited in This Article: ] |
| 29. | Horváth VJ, Vittal H, Ordög T. Reduced insulin and IGF-I signaling, not hyperglycemia, underlies the diabetes-associated depletion of interstitial cells of Cajal in the murine stomach. Diabetes. 2005;54:1528-1533. [PubMed] [Cited in This Article: ] |
| 30. | Xing JH, Lei Y, Ancha HR, Harty RF, Chen JD. Effect of acute gastric electrical stimulation on the systemic release of hormones and plasma glucose in dogs. Dig Dis Sci. 2007;52:495-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Yan Y, Xiang XL, Qian W, Xu JY, Hou XH. Changes of neuronal activities after gut electrical stimulation with different parameters and locations in lateral hypothalamus area of obese rats. J Huazhong Univ Sci Technolog Med Sci. 2014;34:510-515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |