Published online May 28, 2016. doi: 10.3748/wjg.v22.i20.4868
Peer-review started: October 12, 2015
First decision: March 7, 2016
Revised: March 20, 2016
Accepted: April 15, 2016
Article in press: April 15, 2016
Published online: May 28, 2016
AIM: To investigate the influence of phosphatidylinositol-3-kinase protein kinase B (PI3K/AKT)-HIF-1α signaling pathway on glycolysis in esophageal carcinoma cells under hypoxia.
METHODS: Esophageal carcinoma cell lines Eca109 and TE13 were cultured under hypoxia environment, and the protein, mRNA and activity levels of hypoxia inducible factor-1 alpha (HIF-1α), glucose transporter 1, hexokinase-II, phosphofructokinase 2 and lactate dehydrogenase-A were determined. Supernatant lactic acid concentrations were also detected. The PI3K/AKT signaling pathway was then inhibited with wortmannin, and the effects of hypoxia on the expression or activities of HIF-1α, associated glycolytic enzymes and lactic acid concentrations were observed. Esophageal carcinoma cells were then transfected with interference plasmid with HIF-1α-targeting siRNA to assess impact of the high expression of HIF-1α on glycolysis.
RESULTS: HIF-1α is highly expressed in the esophageal carcinoma cell lines tested, and with decreasing levels of oxygen, the expression of HIF-1α and the associated glycolytic enzymes and the extracellular lactic acid concentration were enhanced in the esophageal carcinoma cell lines Eca109 and TE13. In both normoxia and hypoxic conditions, the level of glycolytic enzymes and the secretion of lactic acid were both reduced by wortmannin. The expression and activities of glycolytic enzymes and the lactic acid concentration in cells were reduced by inhibiting HIF-1α, especially the decreasing level of glycolysis was significant under hypoxic conditions.
CONCLUSION: The PI3K/AKT pathway and HIF-1α are both involved in the process of glycolysis in esophageal cancer cells.
Core tip: Fluorescence analysis, spectrophotometry, real-time PCR, Western blot and siRNA interference technology were used to investigate the influence of phosphatidylinositol-3-kinase protein kinase B-HIF-1α signaling pathway on glycolysis under hypoxia in esophageal carcinoma cells. The results obtained provide experimental evidence for the mechanism of glycolysis enhanced by hypoxia.
- Citation: Zeng L, Zhou HY, Tang NN, Zhang WF, He GJ, Hao B, Feng YD, Zhu H. Wortmannin influences hypoxia-inducible factor-1 alpha expression and glycolysis in esophageal carcinoma cells. World J Gastroenterol 2016; 22(20): 4868-4880
- URL: https://www.wjgnet.com/1007-9327/full/v22/i20/4868.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i20.4868
Esophageal cancer, as one of the most common malignant tumors in China with a poor prognosis, is characterized by fast invasion and early metastasis with chemotherapy and radiotherapy tolerance. Previous studies have confirmed that tumor hypoxia is an important factor leading to radiotherapy and chemotherapy resistance and that it promotes tumor invasion and metastasis and affects cell energy metabolism[1,2]. Tumor cells always prefer aerobic glycolysis metabolism to obtain energy, and this preference was named Warburg effect[3]. It enhances the ability of tumor cells to metabolize glucose to provide energy sources for their rapid proliferation and growth as well as help with their hypoxia tolerance. Synergies between tumor tissue hypoxia and the Warburg effect may play an important role in the promotion of tumor metastasis and resistance to chemotherapy.
Hypoxia-inducible factor-1 alpha (HIF-1α) is known as a key regulatory factor of tissue adaptation under hypoxia. It is highly expressed in most tumors and metastases and is inseparable from the glycolytic pathway of tumor cells[4]. The PI3K/AKT signaling pathway widely exists in cells, and it is involved in cell growth, proliferation, differentiation and regulatory signal transduction pathways. The pathway is also one of the most closely regulated pathways in cancer cells influencing glucose metabolism, in addition to its participation in the regulation of HIF-1α expression[5-7]. Therefore, we hypothesized that the PI3K/AKT pathway and HIF-1α may play a key role in the synergistic effect of hypoxia and the Warburg effect.
Human esophageal carcinoma cell lines TE13 and Eca109 were purchased from the Shanghai Institute of Biochemistry and Cell Biology (Shanghai, China). Interference plasmid with HIF-1α-targeting siRNA was manufactured by the Kejira Corporation (Shanghai, China). Competent E. coli TOP10 cells were bought from the Bordi Corporation (Nanjing, China). The antibodies for HIF-1α, AKT, glucose transporter-1 (GLUT-1), lactate dehydrogenase-A (LDHA) and the secondary antibodies were obtained from Santa Cruz Biotechnology. The antibodies for HK-II and p-AKT were obtained from Cell Signaling Technology. The GAPDH antibody was purchased from Bioworld. The Takara reverse transcription kit, the SYBR Green quantitative PCR kit, TRIzol and all the primers were obtained from the Shanghai to Betting Biotechnology Co., Ltd. A hypoxic incubator was purchased from Sanyo.
Esophageal carcinoma cell lines TE13 and Eca109 (2 × 105 cells/well) maintained in DMEM with 10% fetal bovine serum were covered with serum-free medium when the cells grew to 60% confluency and starved for 24 h. Three groups of adherent cells in the logarithmic growth phase were placed into the hypoxia incubator (5% CO2, 1% O2 and 94% N2), and the cells were incubated for 6 h, 12 h, 24 h and 48 h. A corresponding blank control was also set up.
Two pairs of HIF-1α-siRNA oligonucleotide fragments were designed and synthesized according to the human HIF-1α gene sequence (GenBank No. NM001530). The sequences were 5’-GATCCCGAGGAAGAACTATGAACATAATTCAAGAGATTATGTTCATAGTTCTTCCTCTTTTTGGAT-3’ (sense strand) and 5’-AGCTATCCAAAAAGAGGAAGAACTATGAACATAATCTCTTGAATTATGTTCATAGTTCTTCCTCGG-3’ (antisense strand) for sequence one, and 5’-GATCCCGACTGATGACCAGCAACTTGATTCAAGAGATCAAGTTGCTGGTCATCAGTCTTTTTGGAT-3’ (sense strand) and 5’-AGCTATCCAAAAAGACTGATGACCAGCAACTTGATCTCTTGAATCAAGTTGCTGGTCATCAGTCGG-3’ (antisense strand) for sequence two.
To construct a plasmid on the basis of the pGCsi vector manual, TOP10 cells were amplified and agarose gel electrophoresis was performed to acquire the plasmids, which were named pGCsi-HIF-1 and pGCsi-HIF-2. They were routinely used to transfect the cell lines Eca109 and TE13. The cell transfection efficiencies were determined based on the green fluorescence as detected by fluorescence microscopy, and finally, the pGCsi-HIF-1 plasmid was selected as the follow-up interference plasmid. The plasmid pGCsi-HIF-1 and its negative control plasmids were transfected. The cell clones were batched and picked after four weeks. The results of RT-PCR and Western blot were combined. The plasmid pGCsi-HIF-1 and corresponding negative control plasmids were named TE13/shRNA, TE13/Neo, Eca109/shRNA and Eca109/Neo.
Wortmannin at an experimental concentration of 2 μmol/L was incubated with the cells in a hypoxia incubator (1% O2) for 12 h and the control group was cultured for the same time under normoxia.
Western blot analysis was performed to detect the protein expression of HIF-1α and the associated glycolysis genes. The proteins were conventionally extracted, transferred to membranes and incubated. The corresponding primary antibody concentrations were as follows: HIF-1α (1:500), HK-II (1:1000), GLUT-1 (1:200), LDHA (1:200) and β-actin (1:4000). The secondary antibodies conjugated with HRP were goat anti-mouse (1:4000), goat anti-rabbit (1:4000) and rabbit anti-goat (1:5000). The signal was developed using ECL chemiluminescence.
To extract and purify the total RNA, TRIzol-blue reagent was used to extract the cells pretreated in each group according to the instructions of the TRIzol Kit. Then, 1 μg of total RNA was reverse transcribed with the RevertAidTM First Strand cDNA Synthesis Kit. The mRNA levels were determined by qRT-PCR using the Bio-Rad MJ Mini Opticon.
The cells (5 × 105 cells/well) were washed twice in PBS and 500 μL of PBA was added after trypsin digestion. Sonication and centrifugation (10000 r/min) for 10 min were used to acquire the supernatant, and then the activities of LDH and HK were detected by colorimetry according to the kit instructions.
Spectrophotometry was performed to detect the concentration of lactic acid in the supernatant of the nutrient solution.
Statistical analyses were performed using SPSS 18.0 software. Gray value analysis was performed with Tanon Gis software, and the ratio of the target band to the internal reference band represented the protein expression levels of the target gene. The differences in the expression of HIF-1α and the associated glycolytic enzymes, the activities of HK and LDH and the lactic acid concentration among the multiple groups were analyzed using an F test, and indicators between the two groups were compared using Student’s t-test or t-test. P < 0.05 was considered statistically significant.
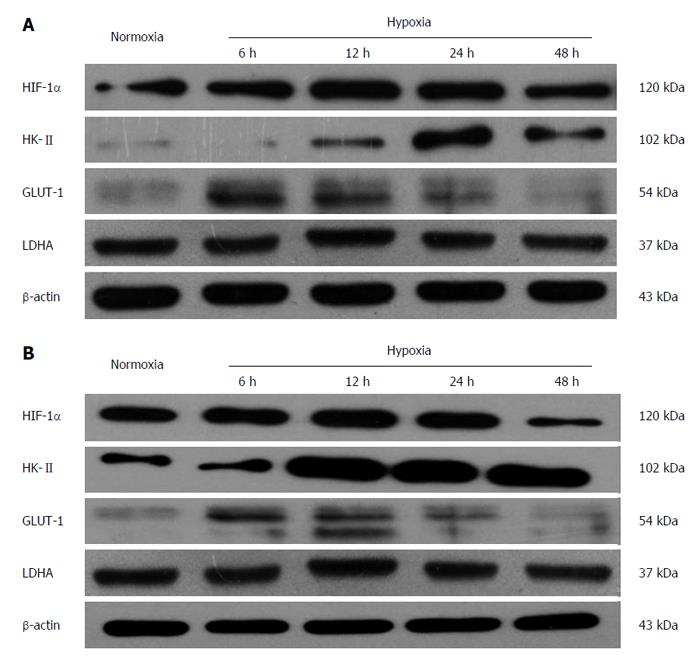
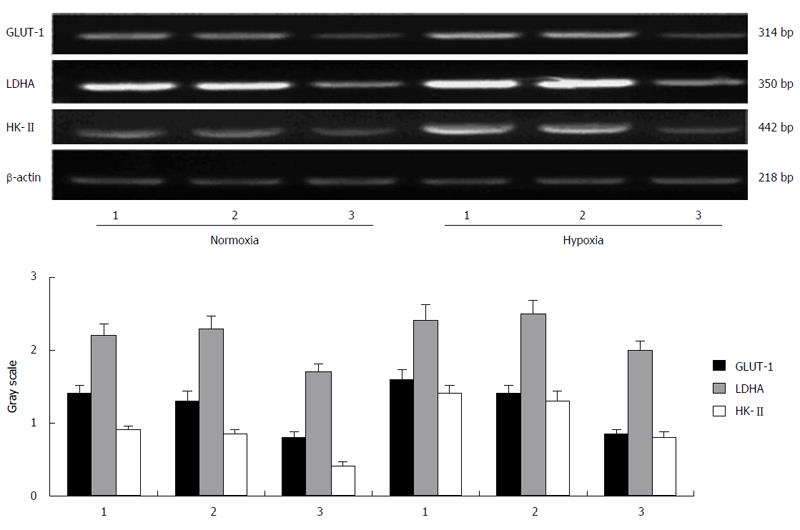
Under hypoxic condition (1% O2), the protein expression of HIF-1α in esophageal carcinoma cell lines increased gradually with time. After 12 h of hypoxia, its expression peaked and then maintained a high level. Then, it began to decrease after 48 h. The protein expression of GLUT-1 and HK-II also elevated gradually after incubation under hypoxic conditions and reached its peak at a time between 12 and 24 h, whereas the protein change of LDHA did not follow a similar trend (Figure 1).
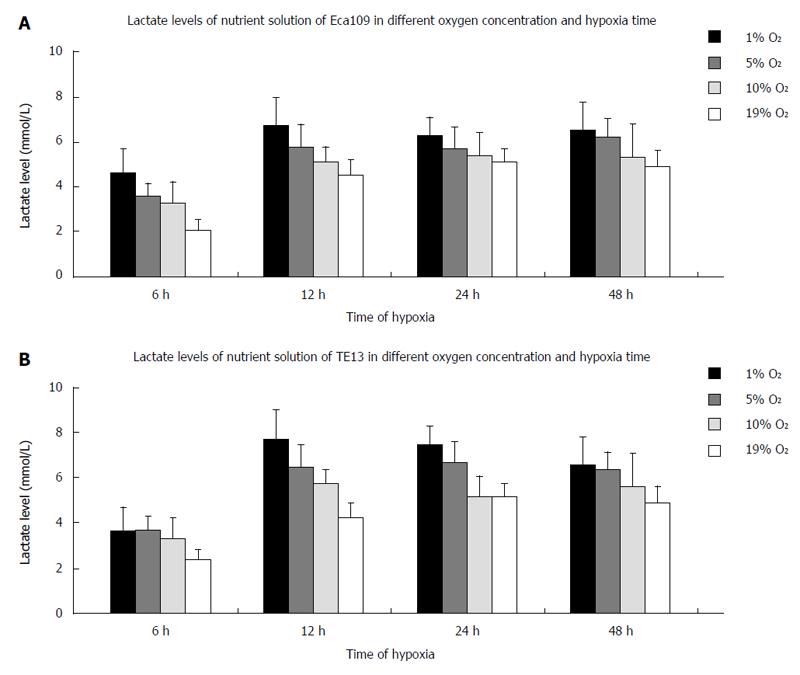
The lactic acid concentration in the supernatant of the cells increased in an upward trend with lower oxygen concentrations. When the culture time for the esophageal carcinoma cell lines (TE13 and Eca109) under hypoxia was extended for 6-48 h, the lactic acid concentration peaked at 12 h and was then maintained at that level or decreased slightly (Figure 2).
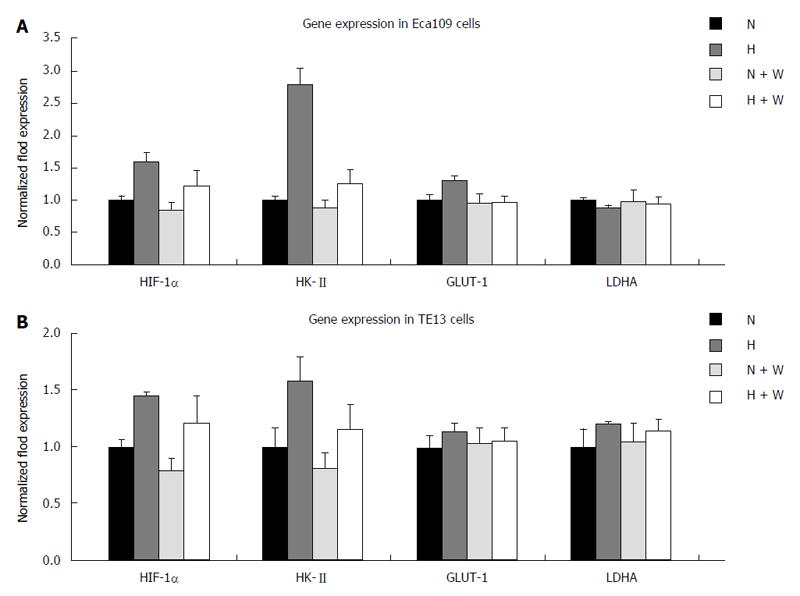
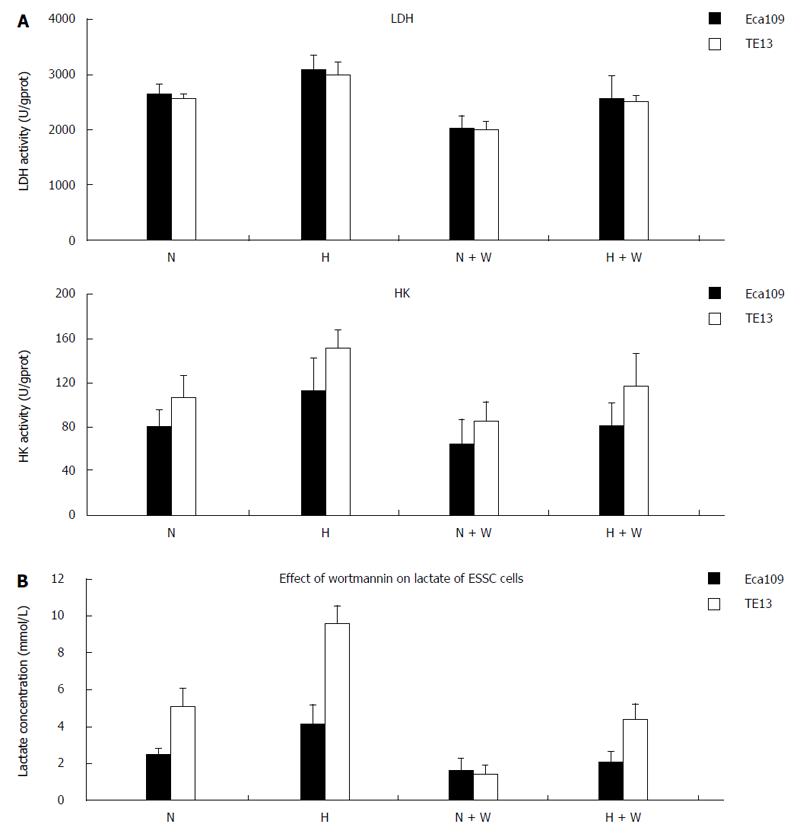
mRNA expression: Compared with a normoxic environment, the mRNA expression of HIF-1α and HK-II under hypoxic conditions increased significantly in the esophageal carcinoma cell lines Eca109 and TE13. In both normoxic and hypoxic conditions, the mRNA expression of the enzymes in the group pretreated with wortmannin decreased slightly compared with that in the group without pretreatment (P < 0.05), but the differences among each group for GLUT-1 and LDHA at the mRNA level were not significant (P > 0.05) (Figure 3).
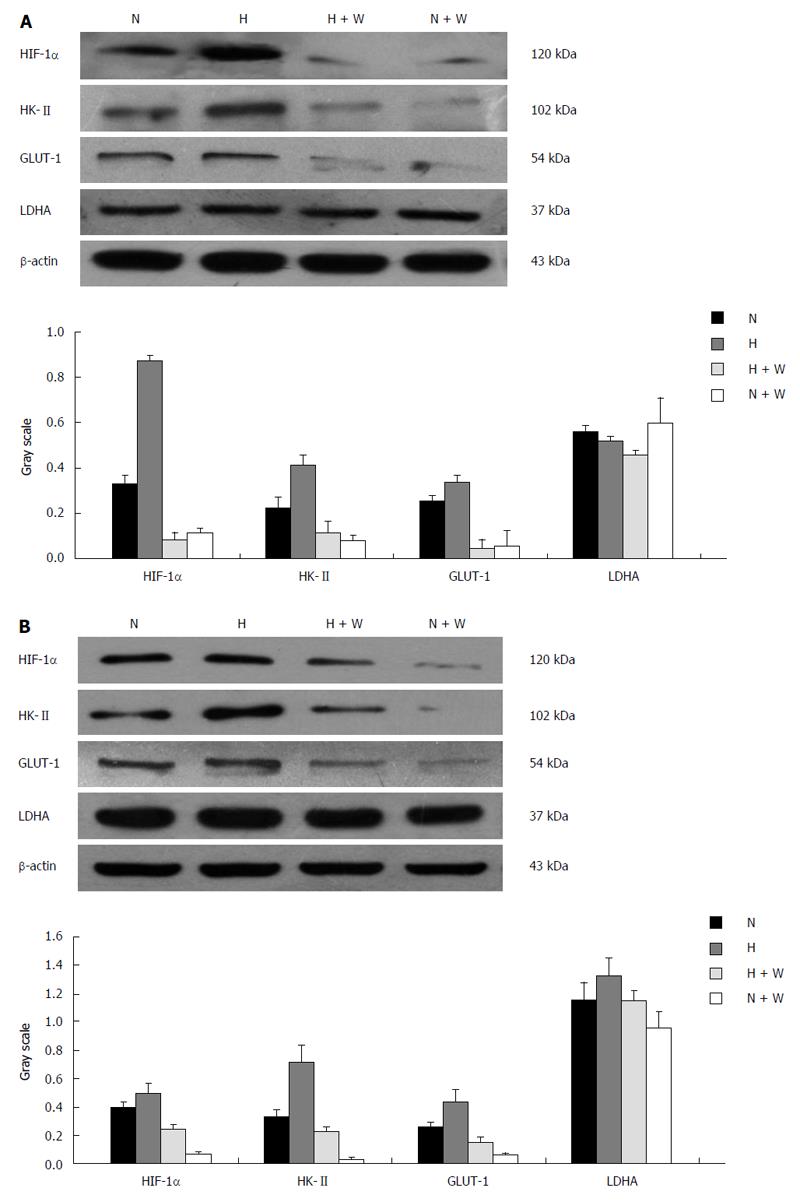
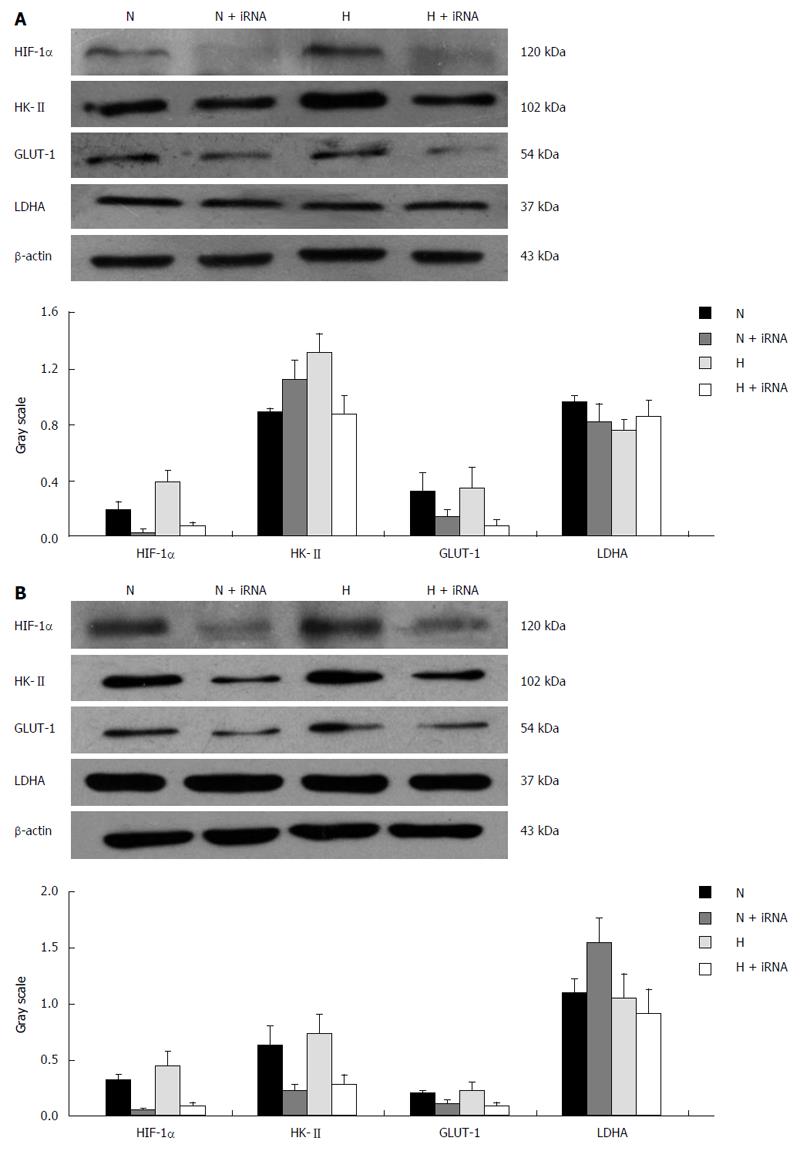
Protein expression: Compared with groups not pretreated with wortmannin, the protein expression of HIF-1α in the pretreated esophageal carcinoma cell lines Eca109 and TE13 incubated under normoxic and hypoxic conditions was inhibited markedly (P < 0.05), and the protein expression of the hypoxic group was higher than that of the normoxic group (P < 0.01). Because the expression of HIF-1α was repressed most when a culture time of 12 h was used, we chose that time for the subsequent experiments.
The protein expression of HK-II and GLUT-1 under normoxia or hypoxia in the groups pretreated with wortmannin was significantly reduced compared with those groups not pretreated (P < 0.05 between each of the groups). In the condition of hypoxia, the protein expression of LDHA among each group increased compared with the normal oxygen environment (P < 0.05). However, the difference between the group pretreated with wortmannin and the group not pretreated was not statistically significant (P > 0.05) (Figure 4).
By detecting the activities of LDH and HK-II in the esophageal carcinoma cell lines Eca109 and TE13 incubated for 12 h under normoxia and hypoxia, we found that the activities of LDH and HK in the groups pretreated with wortmannin all obviously declined compared with those in the group without pretreatment (P < 0.05) (Figure 5A). In the normoxic and hypoxic environments, the lactic acid concentrations in the pretreated group decreased markedly compared with those in the group without pretreatment (P < 0.05) (Figure 5B).
mRNA expression: Compared with the esophageal carcinoma cells of the untransfected group and the empty vector group, the mRNA expression of GLUT-1 and HK-II in the Eca109/siRNA group obviously decreased (P < 0.05) (Figure 6). However, the decline in the mRNA expression of LDHA was too small to be significant. The expression of the associated genes in the untransfected group and the empty vector group under hypoxia were enhanced slightly, and the difference was considered statistically significant (P < 0.05).
Protein expression: Compared with the TE13 and Eca109 groups, the protein expression of HIF-1α in the TE13/siRNA and Eca109/siRNA groups were clearly reduced, and hypoxia could not correct this phenomenon. Regardless of the oxygen concentration used for culturing, the expression of GLUT-1 and HK-II at the protein level in the TE13/siRNA and Eca109/siRNA groups was obviously weaker than that in the control group (P < 0.05). However, the expression of LDHA in the group of TE13 and Eca109 under hypoxia was up-regulated significantly compared with that of the normal oxygen group (P < 0.05), but mildly declined after inhibiting HIF-1α compared to the cells that were not silenced. The results were not statistically significant (P > 0.05) (Figure 7).
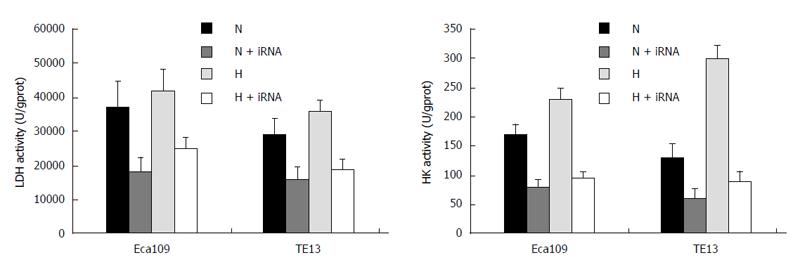
The activities of LDH and HK-II under normoxia or hypoxia in the Eca109/siRNA and TE13/siRNA groups obviously decreased compared with those of the untransfected and empty vector groups (P < 0.05), and the activities of the enzymes under hypoxia increased slightly in the untransfected and empty vector groups (P > 0.05) (Figure 8). Moreover, compared with the untransfected group in the normoxia and hypoxia environments, the lactic acid concentrations in the Eca109/siRNA and TE13/siRNA groups were clearly reduced (P < 0.05) (Table 1).
Hypoxia is one of the basic characteristics of solid tumor microenvironments. On one hand, hypoxia results from the increase in oxygen consumption caused by the rapid proliferation of malignant tumors. On the other hand, the abnormality of the vascular structure and function of tumors leads to a decrease in the blood and oxygen supply, which could also be an important factor further adding to the risk of hypoxia in tumors[8-10]. When tumor size is greater than 1 mm3, there were a considerable number of tumor cells in a hypoxic state. Hypoxia in tumors is not only the consequence of pathophysiology but also an important initiating factor of malignant transformation and even metastasis in the development of tumors. Research has confirmed that hypoxia can promote oncogenes to produce functionally acquired mutations to cause high expression of the gene itself. It has also been found that the corresponding proteins, apart from the tumor suppressor genes, have functionally lacking mutations that down-regulate the expression of genes or weaken the activities of products. In addition, hypoxia can also promote the functionally lacking mutations of the gene associated with DNA repair to improve the mutation frequency of oncogenes and tumor suppressor genes; thus, it is involved in malignant transformations in tumor genesis[11,12]. Furthermore, hypoxia participates in the processes of invasion, metastasis, angiogenesis, immune evasion, and radiotherapy and chemotherapy resistance to affect prognosis[13,14]. Most normal tissues always use aerobic oxidation for energy metabolism in an anaerobic environment, and the glycolytic pathway started to mobilize only when the oxygen supply was insufficient. However, tumor cells prefer glycolysis to gain energy to meet their needs, regardless of the oxygen condition, which is known as the Warburg effect[3]. It is more convenient for tumor cells to gain energy for metabolism and survive under hypoxic conditions, which helps in the acquisition of hypoxia tolerance. The radiotherapy and chemotherapy resistance caused by low metabolic rates during hypoxia helps some tumor cells survive, which could be a source of tumor recurrence[15].
HIF-1 is a transcription factor that widely exists in mammals and humans under hypoxic conditions and is a key player in the body’s ability to adapt to hypoxia by promoting the expression of hypoxia-induced genes to elicit the specific response to hypoxia of tissues. HIF-1 consisted of HIF-1α and HIF-1β subunits. HIF-1α is not only peculiar to HIF-1 but also the oxygen regulator unit. The hypoxia-inducible genes regulated by HIF are involved in angiogenesis, erythropoiesis, energy metabolism, apoptosis, proliferation and other cellular processes. Previous studies have found that the related glycolytic genes regulated by HIF-1 include the GLUT-1, lactate dehydrogenase A (LDHA), phosphofructokinase 2 (PFK2), aldolase A, enolase 1, phosphoglycerate kinase 1 and glyceraldehyde-3-phosphate dehydrogenase coding genes. HIF-1 induced the expression of the above genes to enhance glycolysis to meet the needs of energy metabolism when the process of oxidative phosphorylation was inhibited by the condition of hypoxia[16-18].
The role of external hypoxia in initiating glycolysis and promoting its progression in tumor cells was previously uncertain. Past studies have investigated breast cancer cell lines MDA-mb-4 and MCF-7, which are highly and lowly malignant, respectively, cultured under normal oxygen conditions and found that the expression of HIF-1α and glycolytic enzymes and the level of glycolysis in MDA-mb-4 were greater in strength than those of MCF-7. Moreover, it is interesting that compared with the expression in normoxic environments, the expression of the genes in the breast cancer cell line MCF-7 under hypoxia was markedly elevated. However, there was no obvious change in MDA-mb-435, which suggested that the enhanced glycolysis in tumor cells was not caused by a single factor and that the environment and other factors were also involved in the process[19]. Renal carcinoma cell line RCC4, which has high expression of HIF-1α, was chosen for study because of its lack of the Von Hippel-Lindau gene (vH-L gene), which resulted in the blocked degradation of HIF-1α. In contrast with the level of glycolysis in normoxia and hypoxia conditions, no significant differences between these two conditions were found, but the level of glycolysis under normoxic conditions obviously decreased and increased clearly under hypoxic condition after restoring the regulatory ability of HIF-1α through the transfection of the vH-L gene in the RCC4 cell line[20]. It prompted the idea that the added HIF-1α protein accumulated or abnormally induced in cells might be the main factor in the regulatory process of glycolysis in tumor cells, and blocking HIF-1α might be an effective means of suppressing the glycolytic pathway in tumor cells.
Research has shown that most tumor tissues and their metastases have high expression of HIF-1α, and this is closely related to the glycolytic pathway of tumor cells[4]. We also confirmed the high expression of HIF-1α in esophageal cancer tissues in our previous studies[21,22]. To define the influence of hypoxia on the glycolytic level of esophageal carcinoma cells, we observed the changes in the expression of HIF-1α, the associated glycolytic proteins, such as GLUT-1, HK-II, and LDHA, and the lactic acid content under different oxygen concentrations. The results showed that the expression of the proteins and the lactic acid concentration changed obviously under hypoxic conditions, and this suggests that the external oxygen concentration plays a regulatory role in glycolysis of esophageal carcinoma cells. Although there was a high glycolysis level under normal oxygen pressure in esophageal tumors, the glycolytic level could be further enhanced with a decrease in the external oxygen concentration. The enhanced glycolysis caused by hypoxia and the change in the genetic signaling pathways by itself both evolved to the advantages of the Warburg effect. However, experiments also showed that HIF-1α and the associated glycolytic proteins, such as GLUT-1, HK-II and LDHA, had a certain correlation in the enhanced process of glycolysis induced by hypoxia and played an important role in the pathophysiological mechanisms of promoting glycolysis, which was found to be enhanced in esophageal carcinoma cells. In addition to the involvement of glucose transport and glycolysis, the relevant proteins of glycolysis in tumor cells also included the glycolysis products, such as carbonic anhydrase (CA) and monocarboxylate transporters (MCTs). Studies have already confirmed that the CAIX and MCT4 gene promoter sequences are the binding sites for HIF-1α, which can promote the transmembrane transport of lactic acid and hydrogen ions by enhancing the expression and activity of CAIX and MCT4 and ensure the smooth progress of glycolysis[23-26]. This finding suggests that HIF-1α, as a key factor in the process of regulating tumor glycolysis, may be involved in the enhanced glycolysis process of tumor cells through glucose transport, glycolysis activation and lactic acid transport.
To further confirm the impact of the high expression of HIF-1α on the process of glycolysis in esophageal tumors, we inhibited HIF-1α successfully in the cell lines Eca109 and TE13 using siRNA interference technology to observe the changes in the lactic acid concentration and the expression of the related glycolytic enzymes in the extracellular nutrient solution under different oxygen concentrations. The results showed that the mRNA and protein expression of GLUT-1 and HK-II was down-regulated, and the lactic acid secretion reduced significantly, which was consistent with the trends found when HIF-1α was restrained, and compared with the control group under hypoxia, the expression was still at a low level, although the expression increased mildly when cultured under hypoxia. Thus, we speculate that the regulation of HIF-1α in the glycolysis of esophageal carcinomas depends on the activation of its downstream glycolytic enzymes. However, it is worth noting that the protein expression of LDHA changed slightly when HIF-1α was inhibited, and it also suggests that the regulation of LDHA was not only confined within the role of HIF-1α. When integrated with the literature, the regulation of glucose metabolism in tumors should be the consequence of a combination of multiple factors and multi-links with hypoxia. It also may be that HIF-1α is in a prominent position in the regulatory process, but not all of the glycolysis in esophageal cancers results from this single factor.
The PI3K/AKT signaling pathway is currently attracting much attention in cancer research, and it has been found that PI3K/AKT signaling is disordered in most human tumors and has been closely associated with proliferation, apoptosis, angiogenesis, invasion, metastasis, chemotherapy and radiotherapy resistance. The pathway plays an important role in adapting to the hypoxia environment and participates in the regulation of the HIF-1α singling pathway. A previous study reported that epidermal growth factor (EGF), fibroblast growth factor 2 and insulin-like growth factor 1 could induce the protein expression of HIF-1α by activating the PI3K/AKT signaling pathway and the corresponding tyrosine kinase receptors under normal oxygen pressure[7]. The mechanism of hypoxia tolerance activated by the PI3K/AKT pathway is widespread in mammalian cells, where HIF-1α exerts a critical intermediary role[27].
Based on the speculation that the PI3K/AKT signaling pathway might affect glycolysis in esophageal cancer cells, wortmannin, a specific ATP uncompetitive irreversible inhibitor of PI3K, with a half inhibitory concentration of 0.004 microns, was first extracted from the fungus Penicillium wortmannin in 1957. This compound was used to block the PI3K/AKT signaling pathway in the cell lines Eca109 and TE13. Wortmannin binds to PI3K gamma and causes the irreversible modification of lysine 833, while is the active site of PI3K[28]. Wortmannin at high concentrations also had different inhibitory effects on the PIKK family, such as mTOR, DNA-PK, ATM and ATR[29,30]. The results confirmed that the glycolytic level in esophageal cancer cells declined once the PI3K pathway was inhibited, regardless of whether the external oxygen supply was sufficient or not, and it was accompanied by a decrease in the expression of HIF-1α and the associated glycolytic proteins. This was not corrected effectively by culturing under hypoxia. Moreover, we found that the influence of wortmannin on the protein expression of the enzymes of glycolysis was different; the protein expression of GLUT-1 was down-regulated, whereas the mRNA expression did not change much by using wortmannin, which suggests that the regulatory effect of wortmannin on GLUT-1 might act at the protein level. In addition, the results also showed that the activities of HK-II and LDH in cells and the secretion of lactic acid were restrained by adding wortmannin, and it could be elevated at different levels after treatment with hypoxia. Therefore, we surmise that the impact of the PI3K/AKT signaling pathway on glycolysis in esophageal tumors involves multiple steps and many factors, including the expression of key protein factors and the regulation of activities of different glycolytic enzymes. This suggests that the PI3K/AKT signaling pathway is involved into the process of glycolysis in esophageal carcinoma.
In summary, the level of glycolysis in esophageal carcinomas could be affected by hypoxic environment and is closely associated with the expression of HIF-1α. The PI3K/AKT pathway and HIF-1α are both involved in glycolysis in esophageal cancer cells, and LDHA, HK-II and GLUT-1 might cause downstream effects. The regulation of glycolysis under hypoxic conditions could be achieved by activating the PI3K/AKT pathway through EGF and HIF-1α, and the expression level of glycolysis decreased markedly when the PI3K/AKT pathway was inhibited, which may be of potential therapeutic value in the future. This study will contribute to an effective treatment strategy for tumor cells under hypoxic environments and provide strong evidence and new thoughts on the diagnosis and treatment of cancers. It also provides direction on the development of new antitumor drugs and thus gives more reasonable and individualized therapeutic schedules to cancer patients in the clinic.
Esophageal cancer is the sixth leading cause of cancer-related death in China. Although recent developments in therapeutic strategies have helped cure many patients with early-stage disease, the prognosis of patients with advanced disease and metastasis remains poor.
Tumor hypoxia is an important factor leading to radiotherapy and chemotherapy resistance and promotes tumor invasion and metastasis. It also involves cell energy metabolism. Hypoxia-inducible factor-1 alpha (HIF-1α) is a key regulatory factor of tissue adaptation under hypoxia and is highly expressed in most tumors and metastases. It is also inseparable from the glycolytic pathway of tumor cells. The PI3K/AKT signaling pathway widely exists in cells and is involved in cell growth, proliferation, differentiation and regulatory signal transduction pathways. It is also one of the most closely regulatory pathways of the glucose metabolism of cancer cells besides participating in the regulation of HIF-1α expression.
The authors investigated the signaling pathway of glycolysis from the perspective of hypoxia tolerance in esophageal tumor cells based on HIF-1α and analyzed multiple related glycolytic genes through the PI3K/AKT-HIF-1α pathway to obtain more information regarding the molecular regulatory mechanism of esophageal cancers.
The findings of this study indicated that the PI3K/AKT pathway and HIF-1α are both involved in the process of glycolysis in esophageal cancer cells, and the regulation of glycolysis in the hypoxia condition could be achieved by activating the PI3K/AKT pathway through EGF and HIF-1α. The expression level of glycolytic enzymes decreased markedly when the PI3K/AKT pathway was inhibited.
The study adopted mature and reliable laboratory methods, such as fluorescence analysis, spectrophotometry, real time PCR, Western blot and siRNA interference technology.
Poor prognosis of esophageal cancer is a well-known fact and early diagnosis of this disease is so important. This is a well-written paper.
P- Reviewer: Dinc T, Inamori M S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Ma S
| 1. | Li Y, Fu L, Li JB, Qin Y, Zeng TT, Zhou J, Zeng ZL, Chen J, Cao TT, Ban X. Increased expression of EIF5A2, via hypoxia or gene amplification, contributes to metastasis and angiogenesis of esophageal squamous cell carcinoma. Gastroenterology. 2014;146:1701-1713.e9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 2. | Bayer C, Vaupel P. Acute versus chronic hypoxia in tumors: Controversial data concerning time frames and biological consequences. Strahlenther Onkol. 2012;188:616-627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 3. | Warburg O. On the origin of cancer cells. Science. 1956;123:309-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9117] [Cited by in F6Publishing: 9245] [Article Influence: 136.0] [Reference Citation Analysis (0)] |
| 4. | Lum JJ, Bui T, Gruber M, Gordan JD, DeBerardinis RJ, Covello KL, Simon MC, Thompson CB. The transcription factor HIF-1alpha plays a critical role in the growth factor-dependent regulation of both aerobic and anaerobic glycolysis. Genes Dev. 2007;21:1037-1049. [PubMed] [Cited in This Article: ] |
| 5. | Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 2003;4:257-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 6. | Xia S, Yu SY, Yuan XL, Xu SP. [Effects of hypoxia on expression of P-glycoprotein and multidrug resistance protein in human lung adenocarcinoma A549 cell line]. Zhonghua Yi Xue Zazhi. 2004;84:663-666. [PubMed] [Cited in This Article: ] |
| 7. | Shi YH, Wang YX, Bingle L, Gong LH, Heng WJ, Li Y, Fang WG. In vitro study of HIF-1 activation and VEGF release by bFGF in the T47D breast cancer cell line under normoxic conditions: involvement of PI-3K/Akt and MEK1/ERK pathways. J Pathol. 2005;205:530-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Sutherland RM. Tumor hypoxia and gene expression--implications for malignant progression and therapy. Acta Oncol. 1998;37:567-574. [PubMed] [Cited in This Article: ] |
| 9. | Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249-257. [DOI] [Cited in This Article: ] [Cited by in Crossref: 6437] [Cited by in F6Publishing: 6263] [Article Influence: 261.0] [Reference Citation Analysis (0)] |
| 10. | Dachs GU, Tozer GM. Hypoxia modulated gene expression: angiogenesis, metastasis and therapeutic exploitation. Eur J Cancer. 2000;36:1649-1660. [PubMed] [Cited in This Article: ] |
| 11. | Wykoff CC, Beasley NJ, Watson PH, Turner KJ, Pastorek J, Sibtain A, Wilson GD, Turley H, Talks KL, Maxwell PH. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000;60:7075-7083. [PubMed] [Cited in This Article: ] |
| 12. | Meiron M, Anunu R, Scheinman EJ, Hashmueli S, Levi BZ. New isoforms of VEGF are translated from alternative initiation CUG codons located in its 5’UTR. Biochem Biophys Res Commun. 2001;282:1053-1060. [PubMed] [Cited in This Article: ] |
| 13. | Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8:967-975. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1000] [Cited by in F6Publishing: 999] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 14. | Semenza GL. Molecular mechanisms mediating metastasis of hypoxic breast cancer cells. Trends Mol Med. 2012;18:534-543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 15. | Biaglow JE, Cerniglia G, Tuttle S, Bakanauskas V, Stevens C, McKenna G. Effect of oncogene transformation of rat embryo cells on cellular oxygen consumption and glycolysis. Biochem Biophys Res Commun. 1997;235:739-742. [PubMed] [Cited in This Article: ] |
| 16. | Yu ZT, Zhao HF, Shang XB. [Expression of hypoxia-inducible factor-1alpha and vessel endothelial growth factor in esophageal squamous cell carcinoma and clinico-pathological significance thereof]. Zhonghua Yi Xue Zazhi. 2008;88:2465-2469. [PubMed] [Cited in This Article: ] |
| 17. | Elson DA, Ryan HE, Snow JW, Johnson R, Arbeit JM. Coordinate up-regulation of hypoxia inducible factor (HIF)-1alpha and HIF-1 target genes during multi-stage epidermal carcinogenesis and wound healing. Cancer Res. 2000;60:6189-6195. [PubMed] [Cited in This Article: ] |
| 18. | Ashrafian H. Cancer’s sweet tooth: the Janus effect of glucose metabolism in tumorigenesis. Lancet. 2006;367:618-621. [PubMed] [Cited in This Article: ] |
| 19. | Robey IF, Lien AD, Welsh SJ, Baggett BK, Gillies RJ. Hypoxia-inducible factor-1alpha and the glycolytic phenotype in tumors. Neoplasia. 2005;7:324-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 178] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 20. | Semenza GL. HIF-1 mediates the Warburg effect in clear cell renal carcinoma. J Bioenerg Biomembr. 2007;39:231-234. [PubMed] [Cited in This Article: ] |
| 21. | Du YP, Shi RH, Xiao B, Zhu H. Expression and significance of hypoxia-inducible factor-1 alpha in esophageal squamous cancer cell line EC-109. World Chinese Journal of Digestology. 2006;14:1247-1251. [Cited in This Article: ] |
| 22. | Jing SW, Wang YD, Kuroda M, Su JW, Sun GG, Liu Q, Cheng YJ, Yang CR. HIF-1α contributes to hypoxia-induced invasion and metastasis of esophageal carcinoma via inhibiting E-cadherin and promoting MMP-2 expression. Acta Med Okayama. 2012;66:399-407. [PubMed] [Cited in This Article: ] |
| 23. | Kwon JE, Jung WH, Koo JS. Expression of glycolysis-related proteins in solid papillary carcinoma of the breast according to basement membrane status. Yonsei Med J. 2014;55:576-583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Ullah MS, Davies AJ, Halestrap AP. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J Biol Chem. 2006;281:9030-9037. [PubMed] [Cited in This Article: ] |
| 25. | Rosafio K, Pellerin L. Oxygen tension controls the expression of the monocarboxylate transporter MCT4 in cultured mouse cortical astrocytes via a hypoxia-inducible factor-1α-mediated transcriptional regulation. Glia. 2014;62:477-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Jamali S, Klier M, Ames S, Barros LF, McKenna R, Deitmer JW, Becker HM. Hypoxia-induced carbonic anhydrase IX facilitates lactate flux in human breast cancer cells by non-catalytic function. Sci Rep. 2015;5:13605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 27. | Kitajima Y, Miyazaki K. The Critical Impact of HIF-1a on Gastric Cancer Biology. Cancers (Basel). 2013;5:15-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 28. | Walker EH, Pacold ME, Perisic O, Stephens L, Hawkins PT, Wymann MP, Williams RL. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol Cell. 2000;6:909-919. [PubMed] [Cited in This Article: ] |
| 29. | Brunn GJ, Williams J, Sabers C, Wiederrecht G, Lawrence JC, Abraham RT. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J. 1996;15:5256-5267. [PubMed] [Cited in This Article: ] |
| 30. | Sarkaria JN, Tibbetts RS, Busby EC, Kennedy AP, Hill DE, Abraham RT. Inhibition of phosphoinositide 3-kinase related kinases by the radiosensitizing agent wortmannin. Cancer Res. 1998;58:4375-4382. [PubMed] [Cited in This Article: ] |