Published online May 28, 2016. doi: 10.3748/wjg.v22.i20.4860
Peer-review started: November 25, 2015
First decision: January 13, 2016
Revised: January 27, 2016
Accepted: February 20, 2016
Article in press: February 22, 2016
Published online: May 28, 2016
AIM: To determine gastric emptying, blood pressure, mesenteric artery blood flow, and blood glucose responses to oral glucose in Parkinson’s disease.
METHODS: Twenty-one subjects (13 M, 8 F; age 64.2 ± 1.6 years) with mild to moderate Parkinson’s disease (Hoehn and Yahr score 1.4 ± 0.1, duration of known disease 6.3 ± 0.9 years) consumed a 75 g glucose drink, labelled with 20 MBq 99mTc-calcium phytate. Gastric emptying was quantified with scintigraphy, blood pressure and heart rate with an automated device, superior mesenteric artery blood flow by Doppler ultrasonography and blood glucose by glucometer for 180 min. Autonomic nerve function was evaluated with cardiovascular reflex tests and upper gastrointestinal symptoms by questionnaire.
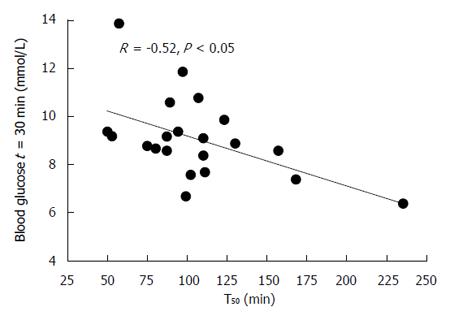
RESULTS: The mean gastric half-emptying time was 106 ± 9.1 min, gastric emptying was abnormally delayed in 3 subjects (14%). Systolic and diastolic blood pressure fell (P < 0.001) and mesenteric blood flow and blood glucose (P < 0.001 for both) increased, following the drink. Three subjects (14%) had definite autonomic neuropathy and 8 (38%) had postprandial hypotension. There were no significant relationships between changes in blood pressure, heart rate or mesenteric artery blood flow with gastric emptying. Gastric emptying was related to the score for autonomic nerve function (R = 0.55, P < 0.01). There was an inverse relationship between the blood glucose at t = 30 min (R = -0.52, P < 0.05), while the blood glucose at t = 180 min was related directly (R = 0.49, P < 0.05), with gastric emptying.
CONCLUSION: In mild to moderate Parkinson’s disease, gastric emptying is related to autonomic dysfunction and a determinant of the glycaemic response to oral glucose.
Core tip: We measured gastric emptying, blood pressure and blood glucose responses to a glucose drink in 21 patients with mild-to-moderate Parkinson’s disease. Gastric emptying was shown to be abnormally delayed 3 patients and 40% had postprandial hypotension - a fall in systolic blood pressure > 20 mmHg after the glucose drink. We demonstrated relationships between gastric emptying and autonomic dysfunction, so that slower gastric emptying was associated with greater autonomic dysfunction, as well as relationships between the blood glucose response with gastric emptying.
- Citation: Trahair LG, Kimber TE, Flabouris K, Horowitz M, Jones KL. Gastric emptying, postprandial blood pressure, glycaemia and splanchnic flow in Parkinson’s disease. World J Gastroenterol 2016; 22(20): 4860-4867
- URL: https://www.wjgnet.com/1007-9327/full/v22/i20/4860.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i20.4860
While gastrointestinal dysfunction occurs frequently in Parkinson’s disease (PD)[1,2], the prevalence of abnormally delayed gastric emptying (GE) remains uncertain because of substantial variations in both the cohorts studied and the methodology used to quantify GE. Delayed GE has been associated with upper gastrointestinal and motor symptoms, as well as impaired absorption of dopaminergic therapy[3,4].
There is little or no information about the potential impact of GE in two other areas: postprandial blood pressure (BP) and glycaemia. Postprandial hypotension (PPH), a fall in systolic BP of ≥ 20 mmHg within 2 h of a meal[5], was reported for the first time in 1977 in a patient with PD[6] and is a clinically important disorder, predisposing to syncope and falls and being associated with increased mortality[7]. PPH may occur frequently in PD, but information is limited[8]. It has also been suggested that PPH represents an “early” marker of autonomic dysfunction in PD[8,9]. Our studies have established that GE is pivotal to the regulation of postprandial BP- in healthy older subjects and patients with type 2 diabetes, the magnitude of the hypotensive response is greater when GE is relatively faster[10]. When glucose is infused intraduodenally in healthy older subjects at 1, 2 or 3 kcal/min, there is a substantial fall in systolic BP in response to the 2 and 3 kcal/min, but not the 1 kcal/min, load[11]. In contrast to the effect of GE, gastric distension attenuates the fall in BP[12], and consumption of water has been advocated as a treatment for PPH[7]. Only one study has evaluated the impact of GE on BP in PD and found no relationship in a cohort of 12 patients with mild to moderate disease[13]; BP was not a primary outcome in this study. The hypotensive response to a meal may relate to splanchnic blood pooling, as assessed by measurement of superior mesenteric artery (SMA) blood flow using Doppler ultrasound[14].
GE is an important determinant of postprandial glycaemia, which is a major contributor to “overall” glycaemic control in diabetes, as assessed by glycated hemoglobin[15]. Accordingly, in health[16], subjects with impaired glucose tolerance[16,17] and type 2 diabetes[17], when GE is faster, there is a greater initial glycaemic response. While diabetes per se does not appear to increase the propensity to PD[18], type 2 diabetes may be associated with greater impairments in postural stability and gait[19]. PD is associated with impaired insulin signalling in the brain[20] and drugs developed for the management of diabetes, particularly glucagon-like peptide-1 agonists, may have efficacy in treatment[21]. There is no information about the impact of GE on postprandial glycaemia in PD.
The primary aims of this study were to quantify the GE, BP, SMA flow and blood glucose responses to oral glucose in mild to moderate PD and evaluate the relationships of changes in BP and glycaemia with the rate of GE. We hypothesised that there would be a high prevalence of delayed GE, that consumption of glucose would result in a fall in BP and rises in both SMA flow and blood glucose, and that these responses would be related to GE.
Twenty one subjects with mild to moderate PD were recruited through advertisements placed in a local Parkinson’s newsletter, and outpatient referral by a neurologist (TK). Mild to moderate PD was defined as a score ≤ 2.5 on the modified Hoehn and Yahr scale[22]. Subjects who were unable to move independently, or who had a history of falls, gastrointestinal disease (unrelated to Parkinson’s), diabetes, significant respiratory or cardiac disease, alcohol abuse or epilepsy, were excluded. 13 males and 8 females, age 64.2 ± 1.6 years (range: 51-77 years), body mass index (BMI) 25.2 ± 0.8 kg/m2 (range: 20.3-34.5 kg/m2) and known duration of PD 6.3 ± 0.9 years (range: 1-16 years), were studied. Two patients were receiving antihypertensive drugs, which were withdrawn for 24 h before the study day. Details of anti-Parkinsonian medication are summarised in Table 1. Four subjects had received deep brain stimulation for the management of their PD.
| Drug | Patients |
| Pramipexole | 11 (52) |
| Levodopa | 9 (43) |
| Levodopa and Carbidopa | 8 (38) |
| Levodopa, Carbidopa and Entacapone | 4 (19) |
| Rasagiline | 3 (14) |
| Amantadine | 1 (5) |
| Apomorphone | 1 (5) |
| Pregabalin | 1 (5) |
| Selegiline | 1 (5) |
At an initial screening visit 6-65 d before the study day, a medical history and staging of Parkinson’s symptoms on the modified Hoehn and Yahr scale were performed by a neurologist (TK)[22] and a questionnaire to asses symptoms referrable to delayed GE completed[23,24].
On the study day, subjects attended the Department of Nuclear Medicine, Positron Emission Tomography and Bone Densitometry at the Royal Adelaide Hospital at 0830h after an overnight fast from solids (14 h) and liquids (12 h). Where possible, subjects were asked to withhold the morning dose of anti-Parkinsonian medication. On arrival, the subject was seated in front of a gamma camera and an IV cannula inserted into the left antecubital vein for blood sampling. An automated cuff was placed around the upper right arm to measure BP and HR. The subject was then allowed to “rest” for approximately 15 min[11]. At t = -3 min, the subject consumed a drink comprising 75 g glucose and 5 g 3-O-Methyl-D-gluco-pyranose (3-OMG) (Carbosynth, Berkshire, United Kingdom) dissolved in water (total drink volume 300 mL), labelled with 20 MBq 99mTc-calcium phytate (Radpharm Scientific, Belconnen, ACT, Australia) within 3 min. GE, BP, SMA blood flow and blood glucose were measured for 180 min following the drink. At t = 180 min, the IV cannula was removed and the subject given a meal. Evaluation of autonomic function, using standardised cardiovascular reflex tests[25], was then performed, prior to the subject leaving the laboratory.
The protocol was approved by the Research Ethics Committee of the Royal Adelaide Hospital, and each subject provided written, informed consent prior to their inclusion. All experiments were carried out in accordance with the Declaration of Helsinki.
Gastric emptying: Radioisotopic data was acquired for 180 min following consumption of the drink (60 s frames between t = 0-60 min, then 180 s frames from t = 60-180 min), where t = 0 was the time of completion of the drink. Data were corrected for subject movement, radionuclide decay and γ-ray attenuation[26]. A region-of-interest was drawn around the total stomach and gastric emptying curves (expressed as percentage retention over time) derived. The amount of the drink remaining in the total stomach at 15 min intervals between t = 0-180 min, as well as the 50% gastric emptying time (T50)[26], were calculated. The normal range for the T50 of this drink is 43-157 min, based on data in 21 healthy subjects (age 64.8 ± 1.8 years), matched for age (i.e., within 2 years) to each subject with PD[17]. GE was considered to be abnormally fast or slow when the T50 was above, or below, this normal range.
Blood pressure and heart rate: BP and HR were measured using an automated BP monitor (DINAMAP ProCare 100, GE Medical Systems, Milwaukee, WI, United States), every 3 min during the “rest” period, and from t = 0-180 min. Baseline BP was calculated as an average of the three measurements obtained immediately prior to the consumption of the drink (i.e., t = -9, t = 6 and t = -3 min)[11]. Maximum changes in BP and HR were calculated as the greatest change that occurred from baseline. Subjects were categorised according to the maximum fall in systolic BP following the drink, i.e., those in which the fall was ≤ 10 mmHg, > 10 mmHg but < 20 mmHg and ≥ 20 mmHg. PPH was defined as a sustained (> 10 min) fall in systolic BP of ≥ 20 mmHg[5].
Superior mesenteric artery blood flow: SMA flow was measured using a LogiqTM e ultrasound system (GE Healthcare Technologies, Sydney, NSW, Australia) and a 3.5C broad spectrum 2.5-4 MHz convex linear array transducer. Measurements were obtained immediately prior to the consumption of the drink (t = -3 min), every 15 min between t = 0-60 min, and then at t = 90 min, 120 min and 180 min. Blood flow (mL/min) was calculated automatically using the formula: π× r2× TAMV × 60, where R = the radius of the SMA and TAMV is the time-averaged mean velocity[14]. In all subjects two measurements were acquired by the same, experienced investigator (LT) at each time point.
Blood glucose: Venous blood was sampled immediately prior to the consumption of the drink (t = -3 min), every 15 min between t = 0-60 min and then at t = 90 min, 120 min and 180 min. Blood glucose (mmol/L) was determined immediately using a portable glucometer (Medisense Companion 2 m, Medisense Inc. Waltham, MA, United States). Results were classified, according to World Health Organisation criteria, as normal glucose tolerance (NGT) (fasting blood glucose < 6.1 mmol/L, and 2 h < 7.8 mmol/L), impaired fasting glucose (IFG) (fasting blood glucose < 7.0 mmol/L, but > 6.1 mmol/L), impaired glucose tolerance (IGT) (2 h blood glucose < 11.1 mmol/L, but > 7.8 mmol/L), or diabetes (fasting blood glucose ≥ 7.0 mmol/L and/or 2 h blood glucose ≥ 11.1 mmol/L)[27].
Upper gastrointestinal symptoms: Upper gastrointestinal symptoms assessed at the screening visit by questionnaire[23], included anorexia, nausea, early satiety, bloating, vomiting, abdominal pain, dysphagia, heart burn and acid regurgitation. Each was scored as: 0 = none, 1 = mild, 2 = moderate or 3 = severe, for a maximum score of 27[23].
Cardiovascular autonomic nerve function: Autonomic nerve function (ANF) was assessed using standardised cardiovascular reflex tests[25]. Parasympathetic function was evaluated by the variation (R-R interval) of the heart rate during deep breathing and the response to standing (“30:15” ratio). Sympathetic function was assessed by the fall in systolic BP in response to standing. Each of the results was scored according to age-adjusted predefined criteria as 0 = normal, 1 = borderline and 2 = abnormal for a total maximum score of 6. A score ≥ 3 was considered to indicate definite autonomic dysfunction[25,28]. Orthostatic hypotension (OH) was defined as a sustained reduction in systolic BP of > 20 mmHg within 3 min of standing[29].
Statistical analysis: BP and HR were assessed as changes from baseline, whereas GE, SMA flow and blood glucose were analysed as absolute values. The maximum changes from baseline in BP, HR and blood glucose were also calculated. Areas under the curve (AUCs) were calculated for BP, HR, SMA flow and blood glucose using the trapezoidal rule. Changes in each variable over time were evaluated with ANOVA. Pearson’s correlation was used to evaluate relationships between variables. Relationships of BP, upper gastrointestinal symptoms and glycaemia with GE were assessed using the GE T50, given the observed overall linear pattern. A P value < 0.05 was considered significant in all analyses. The number of subjects included was based on power calculations derived from our previous study[10]. The statistical analysis was supervised and reviewed by a professional biostatistician. Data are presented as mean ± SE.
The studies were well tolerated and no adverse events were reported. The mean Hoehn and Yahr score was 1.4 ± 0.1 (range: 1-2.5) and duration of known PD 6.3 ± 0.9 years (range: 1-16 years). Three subjects were unwilling, or unable to withhold their morning anti-Parkinson medications because of the risk of significant motor dysfunction. Three subjects had definite autonomic neuropathy, in 10 subjects the score was ≥ 2; the mean ANF score was 1.8 ± 0.3 (range: 0-5); 5 subjects had OH. Eight subjects had PPH. In another 8, the maximum fall was > 10 mmHg but < 20 mmHg and in 5 subjects the fall was < 10 mmHg. Four of the 5 subjects with OH also had PPH. The mean score for upper gastrointestinal symptoms was 1.5 ± 0.4 (range: 0-5).
Gastric emptying of the drink approximated an overall linear pattern. The T50 was 106 ± 9.1 min. In three subjects, GE (T50) was abnormally slow; no subject had abnormally rapid GE.
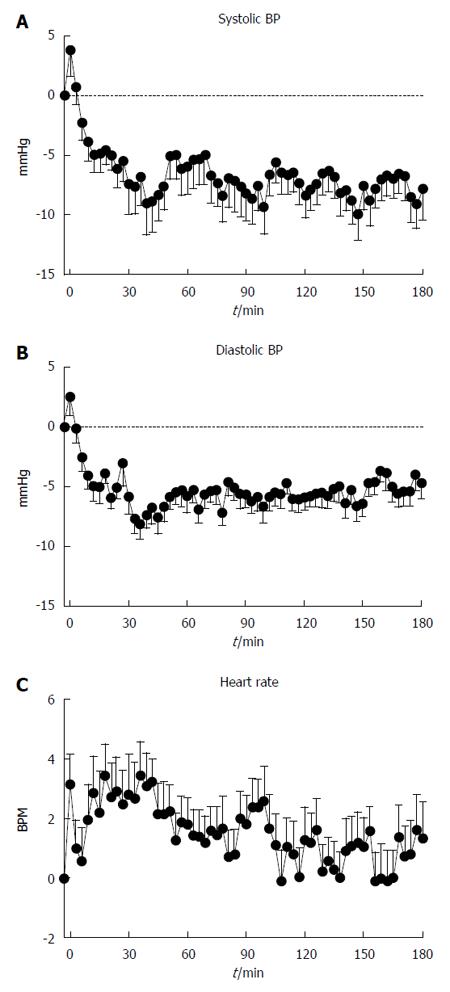
Baseline systolic BP was 116.9 ± 2.4 mmHg. Following the drink, there was a transient modest rise, followed by a fall, in systolic BP (P < 0.001, Figure 1A), which was sustained until the end of the study. The maximum fall was -18.6 ± 2.0 mmHg, occurring at t = 76.5 ± 12.8 min.
Baseline diastolic BP was 69.1 ± 1.6 mmHg. Following the drink, there was a transient initial rise, and then a fall, in diastolic BP (P < 0.001, Figure 1B), with a nadir between t = 30-45 min, which was sustained until the end of the study. The maximum fall in diastolic BP was -15.6 ± 0.9 mmHg, occurring at t = 85.9 ± 11.6 min.
Baseline heart rate was 69.5 ± 2.1 BPM. Following the drink, there was an increase in heart rate (P < 0.001, Figure 1C), which had returned to baseline by approximately t = 60 min. The maximum increase in HR was 9.5 ± 0.7 BPM occurring at 75.6 ± 13.3 min.
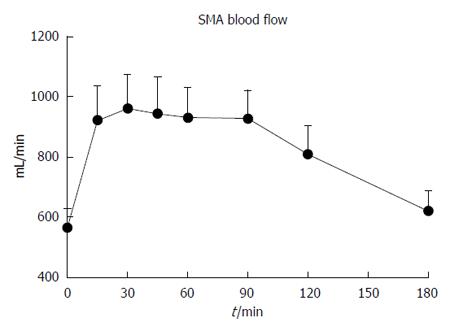
Baseline SMA flow was 565.0 ± 62.5 mL/min. Following the drink, there was a prompt increase in SMA flow (P < 0.001, Figure 2), which had returned to baseline by t = 180 min. The maximum SMA flow was 1208.8 ± 123.0 mL/min, occurring at t = 54.8 ± 8.1 min.
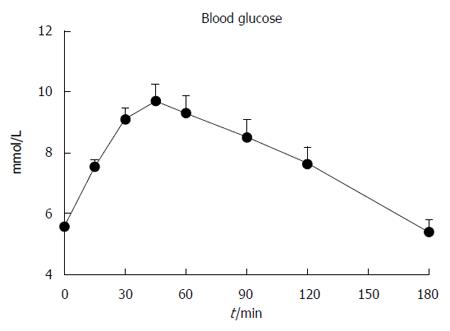
Baseline blood glucose was 5.6 ± 0.1 mmol/L. Following the drink, there was an increase in blood glucose (P < 0.001, Figure 3), which had returned to baseline by t = 180 min. The maximum blood glucose was 10.2 ± 0.5 mmol/L, occurring at 48.9 ± 4.0 min. Five subjects had IGT, 2 had both IFG and IGT and 1 had “marginal” diabetes (fasting and 2 h blood glucose of 7.1 mmol/L and 11.1 mmol/L, respectively).
There were no significant relationships between the changes in systolic BP, diastolic BP, HR or SMA flow at any time point (absolute values and AUCs). The T50 was related directly to the ANF score (R = 0.55, P < 0.01, Figure 4). Upper gastrointestinal symptoms were also related to the score for ANF (R = 0.45, P < 0.05), but not GE.
There was an inverse relationship between the blood glucose at t = 30 min (R = -0.52, P < 0.05, Figure 5), while the blood glucose at t = 180 min (but not 120 min) was related directly (R = 0.49, P < 0.05) to the T50.
There were no significant relationships between T50, Hoehn and Yahr score, duration of disease, or age.
Our study has quantified the GE, BP, SMA and glycaemic responses to oral glucose in mild to moderate PD. In the majority of patients, oral glucose induced a significant fall in systolic BP; i.e., in 16 of 21 patients (76%), this fall was > 10 mmHg and 8 (38%) had PPH. GE of glucose was abnormally delayed in 3 patients (14%), a prevalence lower than we anticipated and slower in those patients with cardiovascular autonomic neuropathy, and gastric emptying was not accelerated in any subject. There was, however, no relationship between the magnitude of the fall in BP with GE. A relationship between the initial glycaemic response to glucose with GE, comparable to that observed in subjects without PD, was demonstrated.
The outcome of studies relating to the prevalence of disordered GE in PD is inconsistent. We measured GE using the “gold-standard” technique of scintigraphy and, while a liquid, rather than a solid, “meal” was used, the precision of solid and high-nutrient liquid meals in the diagnosis of delayed GE appears comparable[30]. It should, however, be recognised that our definition of delayed GE - a T50 that was greater than the range observed in healthy subjects, was deliberately stringent, so that more modest gastric motor function cannot be excluded. The observations of a relationship between GE and the severity of autonomic dysfunction and the high prevalence of autonomic dysfunction are not surprising. The pathophysiology of disordered GE in PD is heterogeneous - alpha-synuclein aggregation, abnormalities in the dorsal motor nucleus of the vagus and enteric nervous system, and drugs such as L-dopa may all be important[2]. As with previous studies, there was no significant relationship between GE and the duration of PD[31]. Patients had mild upper gastrointestinal symptoms, possibly in part because the majority were studied off dopaminergic therapy, although symptoms were more common in patients with impaired ANF.
The high prevalence of PPH is comparable to that reported previously - 8 subjects had PPH and the fall in systolic BP was ≥ 10 mmHg in 16 of 21 (76%) subjects[8]. That the latter may have adverse consequences, even in apparently “asymptomatic” patients[32], dictates the need for greater recognition. We did not observe a relationship between the magnitude of the fall in BP and GE, for which there are a number of potential explanations. Baseline systolic BP was in most cases “normal”, which is predictive of a smaller postprandial fall[33]. We have demonstrated in healthy older subjects that the relationship between the fall in BP and the rate of duodenal glucose delivery is non-linear, so that a “threshold” between 1-2 kcal/min must be exceeded to elicit a hypotensive response[11]. In the current study, based on the T50, GE was ≥ 2 kcal/min in only 4 subjects. Hence, it would be appropriate to re-evaluate this hypothesis further in a larger group of patients. The current study certainly does not exclude the possibility that PD patients with relatively more rapid GE are at increased risk for PPH.
There was an approximate doubling in SMA flow following the glucose drink, as anticipated. In healthy subjects and patients with autonomic failure[34], comparable increases in SMA flow have been observed, but a reduction in BP was only evident in patients with autonomic failure, probably reflecting inadequate sympathetic compensation[34]. The absence of a relationship between BP and SMA flow, may reflect the relatively narrow distribution of the rises in SMA flow, and modest size of the cohort. OH is a frequent manifestation of autonomic involvement in PD and a concordance of PPH and OH in PD has been reported[8], and supported by our study.
The relationship between the initial glycaemic response to the drink and the rate of GE in PD is consistent with observations in health[16], impaired glucose tolerance[16,17], and type 2 diabetes[17] as well as the effect of delayed GE on the absorption of L-dopa in PD[2]. It is now recognised that postprandial glycaemic excursions are a major determinant of overall glycaemic control in type 2 diabetes, assuming increasing importance as glycated hemoglobin normalises[15]. Eight of our 21 subjects (38%) had either impaired glucose tolerance (7 subjects) or “marginal” diabetes (1 subject); that the blood glucose level at 180 min, but not 120 min, was inversely, rather than directly, related to GE, presumably reflects higher insulin levels achieved earlier, associated with insulin resistance[16]. In healthy subjects an inverse relationship is evidence at 120 min after a 75 g oral glucose load[17]. The recognition that GE is a determinant of glycaemia in PD is not surprising, but potentially important- slower GE, including that induced by dopaminergic therapy, would potentially be advantageous in optimising glycaemic control in type 2 patients with PD. Interestingly, GLP-1 agonists, such as exenatide BD which are undergoing evaluation of their efficacy in the management of PD[21], diminish postprandial glycaemic excursions primarily by slowing GE[35].
In interpreting our observations, it should be recognised that 3 subjects did not withdraw their medication, which may represent a cofounder. We also did not include a control (water) drink because of potential ethical concerns. A normal range for GE allowed the prevalence of disordered GE in PD to be determined - a formal control group was not included because the focus of the present study was on relationships between variables within the Parkinson’s group. As discussed, one subject had diabetes, based on fasting and 2 h blood glucose, but these levels were only marginally above the diagnostic cut-offs and this subject was not excluded.
In conclusion, in this unselected population of patients with mild to moderate PD, GE was delayed in only a minority, oral glucose induced a substantial reduction in BP, as well as rises in SMA flow and blood glucose, and GE was an important determinant of the glycaemic, but not the BP, response.
Delayed gastric emptying is recognised as a sequela of Parkinson’s disease (PD), but its prevalence remains uncertain and the potential impact on both postprandial blood pressure and glycaemia have not been evaluated. Postprandial hypotension is known to occur frequently in PD and may be influenced by changes in superior mesenteric artery blood flow.
Delayed gastric emptying in PD is associated with fluctuations in motor response, upper gastrointestinal symptoms and impaired absorption of dopaminergic therapy. The pathophysiology of postprandial hypotension is poorly defined, but the magnitude of the fall in blood pressure is known to be dependent on the rate of gastric emptying as well as changes in superior mesenteric artery blood flow.
The outcome of studies investigating the prevalence of disordered gastric emptying in PD have been inconsistent, at least in part reflecting variations in the cohorts studied and methodology employed to measure gastric emptying. The authors have measured gastric emptying using the “gold-standard” technique of scintigraphy in a well defined cohort of patients with PD to address these limitations. Furthermore, no study in PD has assessed changes in blood pressure and superior mesenteric artery blood flow following oral glucose.
The recognition that gastric emptying is a determinant of glycaemia is of importance to the management of glycaemic control in patients with PD who have type 2 diabetes. The authors identified a high prevalence of postprandial hypotension in this population, and given the substantial adverse sequale associated with this condition, this represents an important consideration in the management of PD with likely autonomic involvement.
Postprandial hypotension, a fall in systolic blood pressure > 20 mmHg occurring within two hours of a “meal”.
This is an excellent manuscript which comprehensively describes a very well-conducted investigation that is clinically relevant.
P- Reviewer: Marin T S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2003;2:107-116. [PubMed] [Cited in This Article: ] |
| 2. | Marrinan S, Emmanuel AV, Burn DJ. Delayed gastric emptying in Parkinson’s disease. Mov Disord. 2014;29:23-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 3. | Goetze O, Nikodem AB, Wiezcorek J, Banasch M, Przuntek H, Mueller T, Schmidt WE, Woitalla D. Predictors of gastric emptying in Parkinson’s disease. Neurogastroenterol Motil. 2006;18:369-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 114] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Doi H, Sakakibara R, Sato M, Masaka T, Kishi M, Tateno A, Tateno F, Tsuyusaki Y, Takahashi O. Plasma levodopa peak delay and impaired gastric emptying in Parkinson’s disease. J Neurol Sci. 2012;319:86-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 5. | Jansen RW, Lipsitz LA. Postprandial hypotension: epidemiology, pathophysiology, and clinical management. Ann Intern Med. 1995;122:286-295. [PubMed] [Cited in This Article: ] |
| 6. | Seyer-Hansen K. Postprandial hypotension. Br Med J. 1977;2:1262. [PubMed] [Cited in This Article: ] |
| 7. | Trahair LG, Horowitz M, Jones KL. Postprandial hypotension: a systematic review. J Am Med Dir Assoc. 2014;15:394-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 8. | Loew F, Gauthey L, Koerffy A, Herrmann FR, Estade M, Michel JP, Vallotton MB. Postprandial hypotension and orthostatic blood pressure responses in elderly Parkinson’s disease patients. J Hypertens. 1995;13:1291-1297. [PubMed] [Cited in This Article: ] |
| 9. | Meco G, Pratesi L, Bonifati V. Cardiovascular reflexes and autonomic dysfunction in Parkinson’s disease. J Neurol. 1991;238:195-199. [PubMed] [Cited in This Article: ] |
| 10. | Jones KL, Tonkin A, Horowitz M, Wishart JM, Carney BI, Guha S, Green L. Rate of gastric emptying is a determinant of postprandial hypotension in non-insulin-dependent diabetes mellitus. Clin Sci (Lond). 1998;94:65-70. [PubMed] [Cited in This Article: ] |
| 11. | Vanis L, Gentilcore D, Rayner CK, Wishart JM, Horowitz M, Feinle-Bisset C, Jones KL. Effects of small intestinal glucose load on blood pressure, splanchnic blood flow, glycemia, and GLP-1 release in healthy older subjects. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1524-R1531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Vanis L, Gentilcore D, Hausken T, Pilichiewicz AN, Lange K, Rayner CK, Feinle-Bisset C, Meyer JH, Horowitz M, Jones KL. Effects of gastric distension on blood pressure and superior mesenteric artery blood flow responses to intraduodenal glucose in healthy older subjects. Am J Physiol Regul Integr Comp Physiol. 2010;299:R960-R967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Thomaides T, Karapanayiotides T, Zoukos Y, Haeropoulos C, Kerezoudi E, Demacopoulos N, Floodas G, Papageorgiou E, Armakola F, Thomopoulos Y. Gastric emptying after semi-solid food in multiple system atrophy and Parkinson disease. J Neurol. 2005;252:1055-1059. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Perko MJ. Duplex ultrasound for assessment of superior mesenteric artery blood flow. Eur J Vasc Endovasc Surg. 2001;21:106-117. [PubMed] [Cited in This Article: ] |
| 15. | Monami M, Lamanna C, Lambertucci L, Longo R, Cocca C, Addante F, Lotti E, Masotti G, Marchionni N, Mannucci E. Fasting and post-prandial glycemia and their correlation with glycated hemoglobin in Type 2 diabetes. J Endocrinol Invest. 2006;29:619-624. [PubMed] [Cited in This Article: ] |
| 16. | Trahair LG, Horowitz M, Marathe CS, Lange K, Standfield S, Rayner CK, Jones KL. Impact of gastric emptying to the glycemic and insulinemic responses to a 75-g oral glucose load in older subjects with normal and impaired glucose tolerance. Physiol Rep. 2014;2:pii e12204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Marathe CS, Horowitz M, Trahair LG, Wishart JM, Bound M, Lange K, Rayner CK, Jones KL. Relationships of Early And Late Glycemic Responses With Gastric Emptying During An Oral Glucose Tolerance Test. J Clin Endocrinol Metab. 2015;100:3565-3571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Lu L, Fu DL, Li HQ, Liu AJ, Li JH, Zheng GQ. Diabetes and risk of Parkinson’s disease: an updated meta-analysis of case-control studies. PLoS One. 2014;9:e85781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 19. | Kotagal V, Albin RL, Müller ML, Koeppe RA, Frey KA, Bohnen NI. Diabetes is associated with postural instability and gait difficulty in Parkinson disease. Parkinsonism Relat Disord. 2013;19:522-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Lima MM, Targa AD, Noseda AC, Rodrigues LS, Delattre AM, dos Santos FV, Fortes MH, Maturana MJ, Ferraz AC. Does Parkinson’s disease and type-2 diabetes mellitus present common pathophysiological mechanisms and treatments? CNS Neurol Disord Drug Targets. 2014;13:418-428. [PubMed] [Cited in This Article: ] |
| 21. | Hölscher C. Drugs developed for treatment of diabetes show protective effects in Alzheimer’s and Parkinson’s diseases. Sheng Li Xue Bao. 2014;66:497-510. [PubMed] [Cited in This Article: ] |
| 22. | Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, Giladi N, Holloway RG, Moore CG, Wenning GK. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord. 2004;19:1020-1028. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1264] [Cited by in F6Publishing: 1424] [Article Influence: 71.2] [Reference Citation Analysis (0)] |
| 23. | Jones KL, Horowitz M, Carney BI, Wishart JM, Guha S, Green L. Gastric emptying in early noninsulin-dependent diabetes mellitus. J Nucl Med. 1996;37:1643-1648. [PubMed] [Cited in This Article: ] |
| 24. | Horowitz M, Maddox AF, Wishart JM, Harding PE, Chatterton BE, Shearman DJ. Relationships between oesophageal transit and solid and liquid gastric emptying in diabetes mellitus. Eur J Nucl Med. 1991;18:229-234. [PubMed] [Cited in This Article: ] |
| 25. | Piha SJ. Cardiovascular autonomic reflex tests: normal responses and age-related reference values. Clin Physiol. 1991;11:277-290. [PubMed] [Cited in This Article: ] |
| 26. | Gentilcore D, Bryant B, Wishart JM, Morris HA, Horowitz M, Jones KL. Acarbose attenuates the hypotensive response to sucrose and slows gastric emptying in the elderly. Am J Med. 2005;118:1289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539-553. [PubMed] [Cited in This Article: ] |
| 28. | Ewing DJ, Clarke BF. Diagnosis and management of diabetic autonomic neuropathy. Br Med J (Clin Res Ed). 1982;285:916-918. [PubMed] [Cited in This Article: ] |
| 29. | Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton Neurosci. 2011;161:46-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 385] [Cited by in F6Publishing: 324] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 30. | Phillips LK, Rayner CK, Jones KL, Horowitz M. Measurement of gastric emptying in diabetes. J Diabetes Complications. 2014;28:894-903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Hardoff R, Sula M, Tamir A, Soil A, Front A, Badarna S, Honigman S, Giladi N. Gastric emptying time and gastric motility in patients with Parkinson’s disease. Mov Disord. 2001;16:1041-1047. [PubMed] [Cited in This Article: ] |
| 32. | Kohara K, Jiang Y, Igase M, Takata Y, Fukuoka T, Okura T, Kitami Y, Hiwada K. Postprandial hypotension is associated with asymptomatic cerebrovascular damage in essential hypertensive patients. Hypertension. 1999;33:565-568. [PubMed] [Cited in This Article: ] |
| 33. | Masuo K, Mikami H, Habara N, Ogihara T. Orthostatic and postprandial blood pressure reduction in patients with essential hypertension. Clin Exp Pharmacol Physiol. 1991;18:155-161. [PubMed] [Cited in This Article: ] |
| 34. | Kooner JS, Raimbach S, Watson L, Bannister R, Peart S, Mathias CJ. Relationship between splanchnic vasodilation and postprandial hypotension in patients with primary autonomic failure. J Hypertens Suppl. 1989;7:S40-S41. [PubMed] [Cited in This Article: ] |
| 35. | Nauck MA, Niedereichholz U, Ettler R, Holst JJ, Orskov C, Ritzel R, Schmiegel WH. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol. 1997;273:E981-E988. [PubMed] [Cited in This Article: ] |