Published online May 21, 2016. doi: 10.3748/wjg.v22.i19.4707
Peer-review started: January 20, 2016
First decision: February 18, 2016
Revised: March 2, 2016
Accepted: March 14, 2016
Article in press: March 14, 2016
Published online: May 21, 2016
AIM: To explore the feasibility and safety of laparoscopic colonic anastomosis using a degradable stent in a porcine model.
METHODS: Twenty Bama mini-pigs were randomly assigned to a stent group (n = 10) and control group (hand-sewn anastomosis, n = 10). The anastomotic completion and operation times were recorded, along with histological examination, postoperative general condition, complications, mortality, bursting pressure, and the average anastomotic circumference (AC).
RESULTS: All pigs survived postoperatively except for one in the stent group that died from ileus at 11 wk postoperatively. The operation and anastomotic completion times of the stent group were significantly shorter than those of the control group (P = 0.004 and P = 0.001, respectively). There were no significant differences in bursting pressure between the groups (P = 0.751). No obvious difference was found between the AC and normal circumference in the stent group, but AC was significantly less than normal circumference in the control group (P = 0.047, P < 0.05). No intestinal leakage and luminal stenosis occurred in the stent group. Histological examination revealed that the stent group presented with lower general inflammation and better healing.
CONCLUSION: Laparoscopic colonic anastomosis with a degradable stent is a simple, rapid, and safe procedure in this porcine model.
Core tip: We explored the feasibility and safety of laparoscopic colonic anastomosis using a degradable stent in a porcine model. Twenty Bama mini-pigs were randomly assigned to a stent group and hand-sewn anastomosis group. The operation and anastomotic completion times of the stent group were significantly shorter than those of the control group. There was no significant difference between the anastomotic and normal circumference in the stent group. No intestinal leakage and luminal stenosis occurred in the stent group. Histological examination of anastomoses revealed that the stent group presented with less general inflammation and better healing than the control group.
- Citation: Ma L, Cai XJ, Wang HH, Yu YL, Huang DY, Ge GJ, Hu HY, Yu SC. Laparoscopic colonic anastomosis using a degradable stent in a porcine model. World J Gastroenterol 2016; 22(19): 4707-4715
- URL: https://www.wjgnet.com/1007-9327/full/v22/i19/4707.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i19.4707
There is substantial evidence that laparoscopic surgery plays a pivotal role in abdominal surgical disease[1,2]. Laparoscopic intestinal anastomosis is a basic technique in gastrointestinal procedures. The hand-sewn method has been the most widely accepted procedure for intestinal anastomosis for the past 160 years. Although surgical skills have been significantly improved and developed, hand-sewn anastomoses are still complicated and time-consuming[3]. An effective alternative technique is stapled anastomosis. Although this is relatively convenient and time-saving, stapler devices are more expensive than the hand-sewn method and have a higher risk of anastomotic stenosis[4,5]. Besides, the foreign materials in anastomosis may induce chronic inflammation[6,7], which could slow down the process of healing or lead to secondary leakage and stenosis[8,9]. In addition, intestinal staplers cannot be used for hemostasis, and suturing after stapling may be required[10]. Another feasible technique is sutureless anastomosis, such as compression rings, tissue glue, and laser anastomosis, which have been in use since Murphy’s button in 1892[10-13]. However, sutureless anastomosis is less applied clinically due to some safety issues, such as weak anastomotic strength, necrosis, and stricture. Therefore, an ideal laparoscopic surgical procedure that can achieve the desired results is urgently needed.
In our previous experiments, we have exhibited the feasibility and safety of colonic anastomosis and primary repair of colonic perforation using a degradable stent in a porcine model undergoing open surgery[14-16]. In this study, we further explored the application of this stent in laparoscopic colonic anastomosis using a degradable stent.
Twenty healthy experimental Bama mini-pigs of either sex, weighing approximately 30 kg, were purchased from Shanghai Multi-Bio-Sci-Tech Co. Ltd., China. Animal were raised separately in clean cages at the Experimental Animal Center of Zhejiang University and provided with a liquid diet for 5 d before surgery. They were then were starved for 12 h and fed with 5% magnesium sulfate to clean the colonic lumen. Cefazolin sodium was administered intramuscularly for preoperative antibiotic prophylaxis. All the protocols were approved by the Experimental Animal Ethics Committee of Zhejiang University.
The stent was developed by the Institute of Polymer Science of Zhejiang University (Figure 1). The material properties were demonstrated in our previous studies[14-16]. The stent is synthesized from 1,3-propanediol, 1,2-propanediol, and sebacic acid and decomposes finally to CO2 and water. In vivo, we found that the stents were damage free on day 10 and were degraded and broken on day 28. The stent was approved by the State Food and Drug Administration of China for its biocompatible qualities (No. G20090993)[16].
Twenty pigs were randomized and assigned to a degradable stent group (n = 10) and control group (hand-sewn anastomosis, n = 10). The pigs in each group were divided evenly into two subgroups according to the time of sacrifice (2 wk and 3 mo postoperatively), and each subgroup included five pigs. A laparoscopic colonic anastomosis with a degradable stent was performed in the stent group, and laparoscopic hand-sewn anastomosis was performed in the control group.
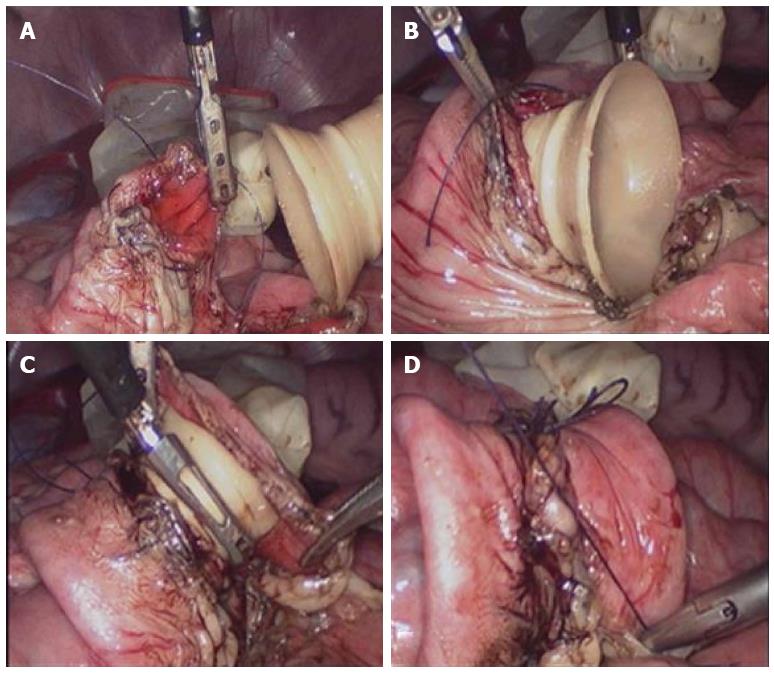
The animals were anesthetized by intramuscular injection of midazolam (total 5 mg, Jiangsu Enhua Pharmaceutical Group, China) and inhalation anesthesia with isoflurane, with subsequent intubation and mechanical ventilation. The animals were in the supine position on a disposable disinfectant towel. Laparotomy was performed via left lower quadrant incision (about 2.5 cm), and pneumoperitoneum was established using CO2 to create a sufficient operating space in the abdominal cavity. The appropriate intestinal segment was selected, and then the colon and partial mesentery was dissociated. The tissue with bad blood supply was cut, and the colonic contents were removed. The colonic lumen was cleaned, and the incisal edge was disinfected. At 0.5 cm from both ends of the bowel transection, a purse-string suture using 3-0 absorbable thread sutures was performed circularly around the intestine at the seromuscular layer of the colon (Figure 2A). One end of the stent was embedded into the intestinal cavity, and the purse string was tightened, knotted, and fixed (Figure 2B). Afterwards, the other end of the stent was placed into the contralateral intestinal cavity, and the purse string was tightened, knotted, and fixed (Figure 2C). Finally, both sides of the purse-string were knotted and fixed, and another one or two stitches were added intermittently, if necessary, to avoid intestinal volvulus (Figure 2D). No abdominal drain was placed, and the incisions were closed. Pigs in the control group received a hand-sewn, end-to-end colonic anastomosis. The procedure was performed as previously described[15].
After the operation, pigs were given free access to water for 24 h and returned to a fluid diet 24 h later, with normal diet being resumed on postoperative day 7. The anastomotic completion and operation times of each group were recorded as well as postoperative general condition, complications, and mortality. Five pigs in each group were sacrificed 2 wk postoperatively to evaluate the bursting pressure, and the rest was sacrificed 3 mo postoperatively to assess the average anastomotic circumference (AC) and healing of the anastomosis. All the procedures were conducted by the same operators. The circumference was approximately 10 cm above and below the anastomotic stoma, and the narrowest circumference was measured. After sacrifice, an approximately 5-cm anastomotic segment was resected for histological examination, including hematoxylin-eosin (HE), Masson’s trichrome stain, and immunohistochemical staining, performed by the Pathology Department of Sir Run Run Shaw Hospital, Hangzhou, China. The samples were fixed, embedded, and sliced into 5-μm thick sections. Immunohistochemical staining was performed with mouse anti-porcine anti-α-smooth muscle actin (SMA) antibody (1:300; Abcam, Cambridge, United Kingdom), anti-basic fibroblast growth factor (b-FGF) antibody (1:300, Santa Cruz Biotechnology, Dallas, TX, United States), or anti-transforming growth factor (TGF)-β1 antibody (1:300, Santa Cruz Biotechnology). The second antibody was performed using rabbit anti-mouse (1:1000, Zhongshan Goldenbridge Biotechnology Co. Ltd., Beijing, China).
Continuous variables, presented as mean ± SD, were compared using Student’s t test. Statistical analysis was performed by using SPSS version 18.0 (SPSS Inc., Chicago, IL, United States).
All procedures were carried out according to the Institutional Animal Care and Use Committee Guide of Center for Drug Safety Evaluation and Research of Zhejiang University with the reference number: IACUC-13001.
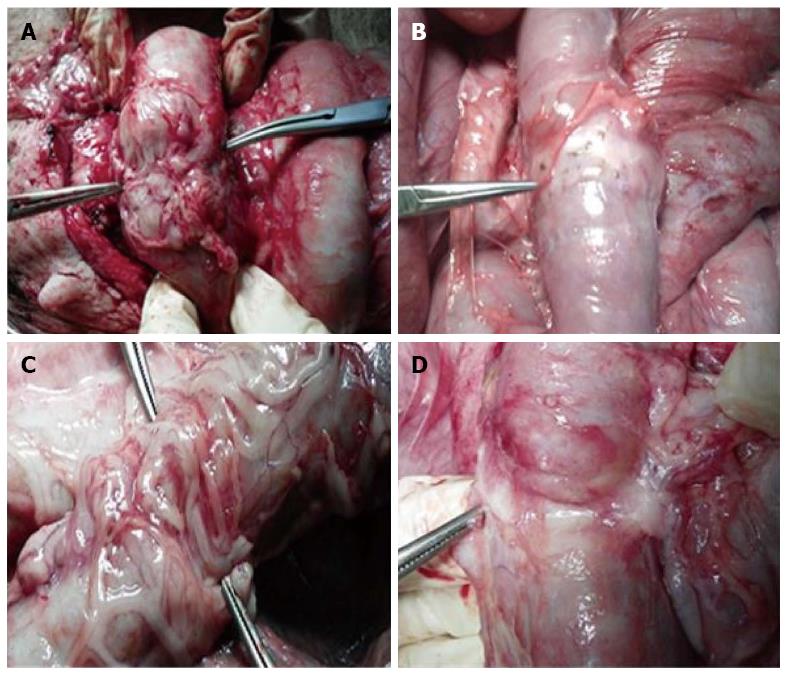
General observation of anastomosis is shown in Figure 3. One pig in the stent group died from ileus at postoperative week 11. Autopsy found that the ileus was located below the anastomosis, and the cause of death was congenital intestinal valvular disease. The other animals all survived.
As shown in Table 1, the mean operation time of the stent group was significantly shorter than that of the control group (P = 0.004, P < 0.01). Within the stent group, there were significant differences between the two subgroups (P = 0.010). The operation time in the 2 wk and 3 mo subgroups was 86.6 ± 10.9 min and 56.6 ± 16.6, respectively. However, no significant differences were found in the two subgroups of the control group (P = 0.426). The mean anastomotic completion time of the stent and control groups was 33.1 ± 18.5 min and 65.5 ± 19.9 min, respectively. There was a significant difference between the two groups (P = 0.001). In the stent group, the anastomotic completion time of the 2 wk subgroup was less than that of the 3 mo subgroup (P = 0.016). In addition, there were no significant differences in the two subgroups of the control group (P = 0.204).
| Groups | Subgroups | Operation time(min) | Completion time (min) | Bursting pressure (cmH2O) | Anastomotic circumference (cm) | Normal circumference (cm) |
| Stent group | 71.6 ± 20.6 | 33.1 ± 18.5 | - | - | - | |
| Week 2 | 56.6 ± 16.6 | 23.6 ± 14.8 | 108.0 ± 34.9 | - | ||
| Month 3 | 86.6 ± 10.9 | 42.6 ± 18.0 | - | 7.7 ± 0.4 | 7.7 ± 0.2 | |
| Control group | 108.3 ± 27.8 | 65.5 ± 19.9 | - | - | - | |
| Week 2 | 100.8 ± 34.1 | 57.2 ± 24.3 | 97.5 ± 60.2 | - | - | |
| Month 3 | 115.8 ± 20.8 | 73.8 ± 11.5 | - | 7.1 ± 1.1 | 8.3 ± 1.1 |
The bursting pressure of the 2 wk subgroup in the stent and control groups was 108.0 ± 34.9 and 97.5 ± 60.2 cm H2O, respectively, and there were no significant differences in the two subgroups (P = 0.751). The normal intestinal circumference and AC of the 3 mo subgroups in the stent and the control groups were 7.7 ± 0.2, 7.7 ± 0.4, 8.3 ± 1.1, and 7.1 ± 1.1 cm, respectively. There were no significant differences between the normal intestinal circumference and the AC in the stent group (P = 0.344). However, the AC was lower than the normal intestinal circumference in the control group (P = 0.047).
No intestinal leakage or luminal stenosis occurred in the stent group; while leakage appeared in three pigs in the 2 wk subgroup of the control group, and two pigs achieved healing. Bowel tapering was found in two pigs in the 3 mo subgroup of the control group. No stents were dislocated in the 2 wk subgroups of both groups, but the stents presented with partial breakages because the material properties were fragile. All the stents were absorbed in anastomotic stoma at 3 mo postoperatively.
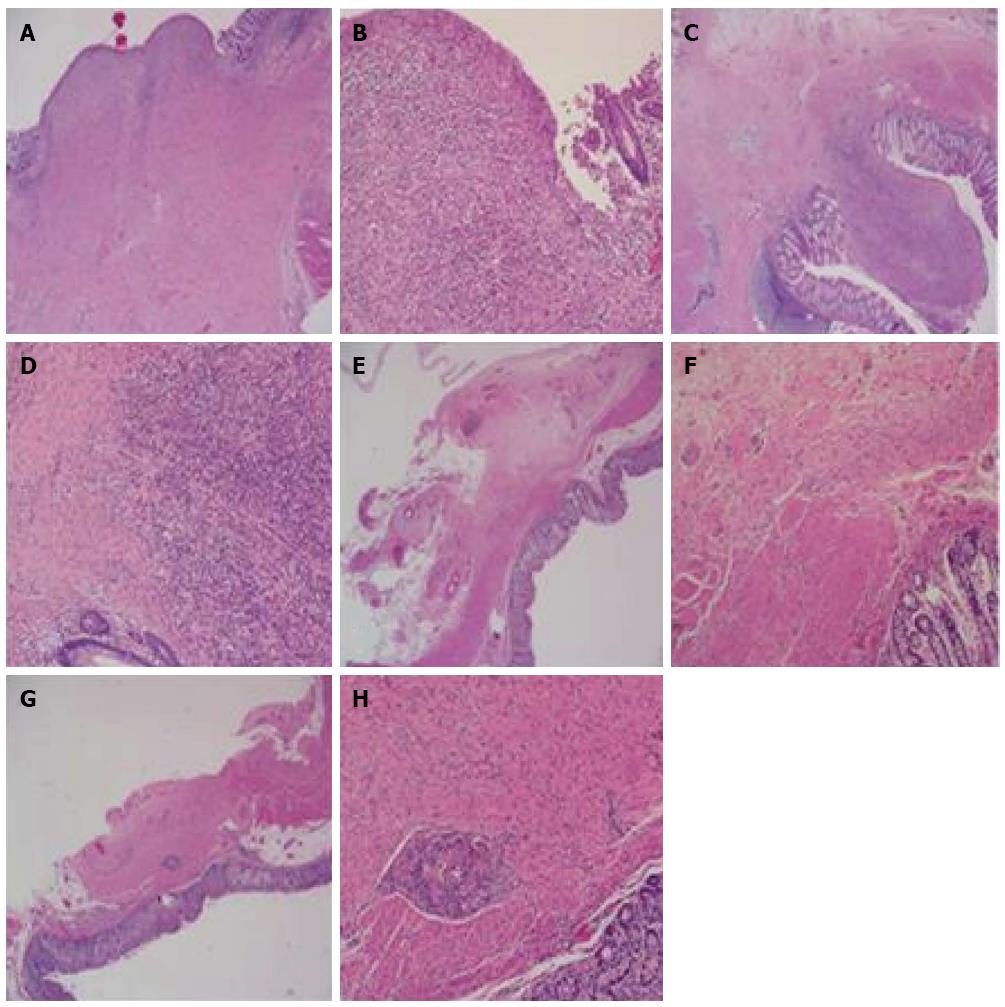
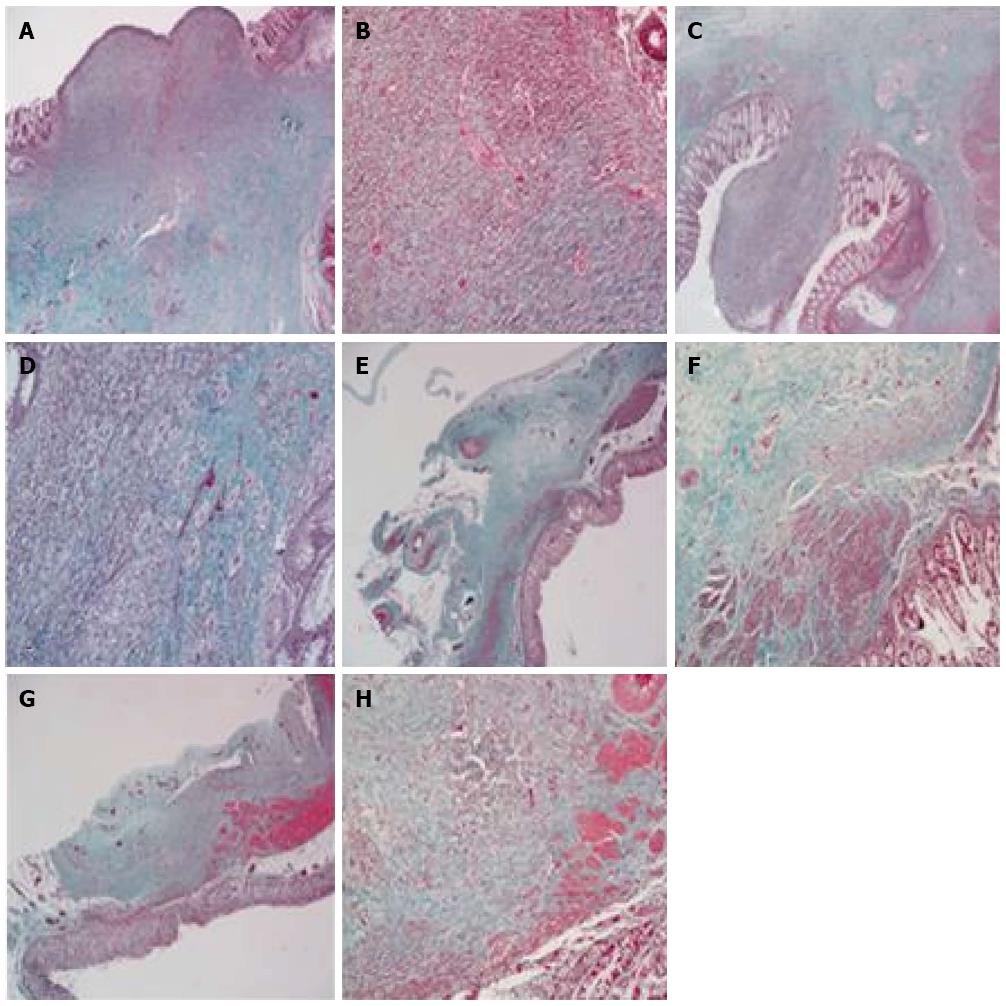
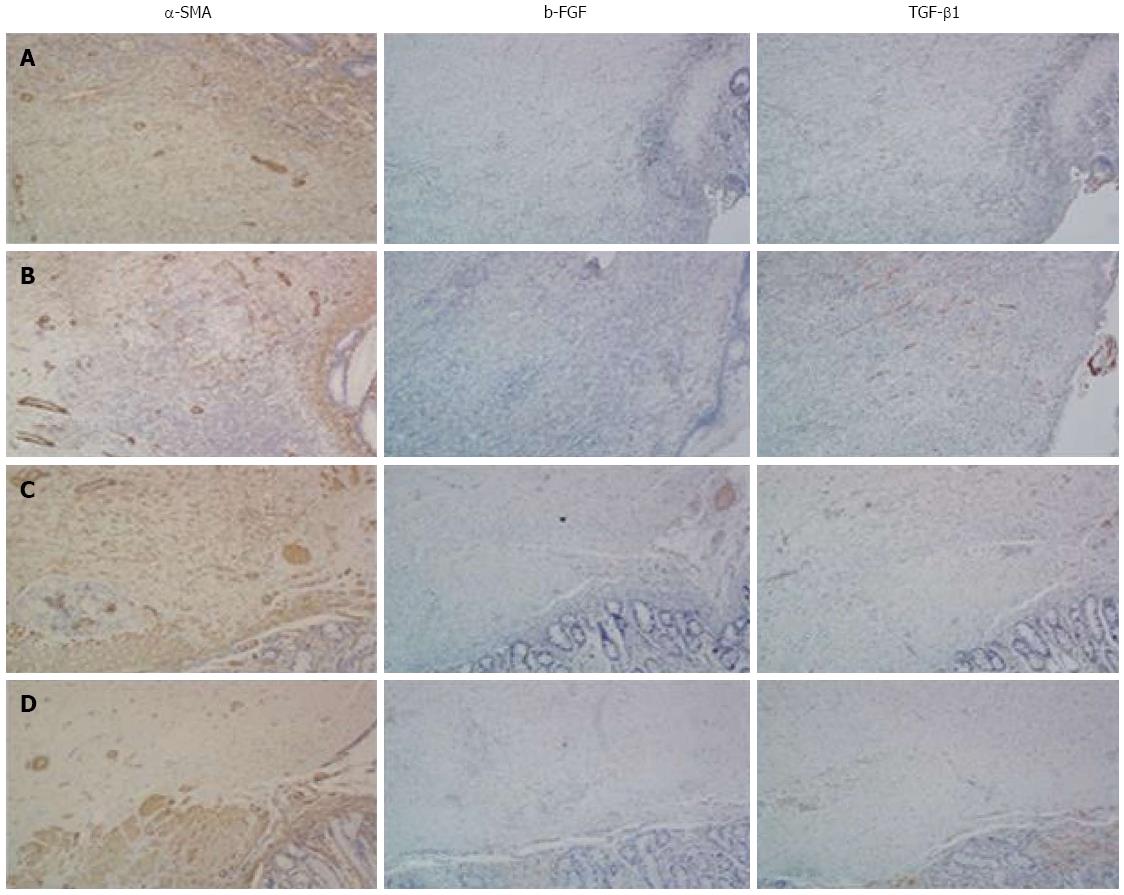
HE staining at postoperative week 2 in both groups showed that the anastomotic mucosa was absent, and granulation tissue formed with infiltration of a large number of lymphocytes, plasma cells, and neutrophils. Fibrous tissue hyperplasia, collagen deposition, and muscularis propria interruption were present. However, the degree of inflammatory infiltration in the stent group was lower than in the control group (Figure 4A-D). Collagen deposition was similar in the two groups (Figure 5A-D). Immunohistochemical staining demonstrated that α-SMA had higher immunostaining intensity and wider range than b-FGF and TGF-β1 and no significant differences were found between the two groups (Figure 6A and B).
HE staining at postoperative month 3 showed that colonic mucosa was present at the site of anastomosis, along with fibrous tissue hyperplasia and collagen deposition that extended through the adventitial layer. Smooth muscle bundles were also interleaved between the mucosa and adventitial layer, fusing with the muscularis mucosa. However, submucosa was absent at the site of the anastomosis. The amount of smooth muscle tissue in the stent group was higher than in the control group (Figure 4E-H). There was a large amount of collagen deposition and scar tissue formation and no obvious differences were found between the two groups (Figure 5E-H). Immunohistochemical staining showed that the intensity of α-SMA staining decreased compared with that at postoperative week 2, but the range was still extensive. Positive staining smooth muscle cells presented with brown color and were arranged in a fascicular pattern. The immunostaining intensity and range of b-FGF and TGF-β1 were lower than those at week 2 (Figure 6).
In the present study, the safety and feasibility of laparoscopic colonic anastomosis using a degradable stent was investigated by comparing with hand-sewn anastomosis. The operation and anastomotic completion times using a degradable stent were significantly shorter than those for the hand-sewn method, while no significant differences were found in bursting pressure. Besides, no obvious significance was found between the AC and normal circumference in the stent group, whereas the AC was significantly less than the normal circumference in the control group. Severe complications, such as intestinal leakage and luminal stenosis, were also less frequent than those with the hand-sewn method. Therefore, we conclude that the simple surgical procedure with a degradable stent was a simple, rapid, feasible, and safe procedure in this porcine model.
The methods for intestinal anastomosis are numerous, and each has its advantages and disadvantages. Extracorporeal anastomosis is simple but is highly invasive. Therefore, totally laparoscopic procedures have been gradually paid more attention. Laparoscopic stapled anastomosis may elicit local inflammation caused by a foreign body reaction and result in leakage and stenosis[17]. The biodegradable compression ring (BAR Valtrac) is hard to perform and is not suitable for laparoscopic operation. Another novel compression anastomosis clip (Hand CAC 30), which is made of a shape-memory alloy of nickel-titanium, is suitable for laparoscopic operation[18-20]. However, CAC is metallic, which cannot be degraded and removal and discharge are difficult[21]. Therefore, it is essential to develop an ideal surgical procedure that is easy to perform. In the present study, we introduced a novel degradable stent that was synthesized from 1,3-propanediol, 1,2-propanediol and sebacic acid, which can decompose finally to CO2 and water. Like the biodegradable anastomosis, the degradable stent leaves no residual foreign body in the anastomosis and can isolate the intestinal contents from the anastomotic stoma, subsequently reducing the chance of infection. In addition, the appropriate stent diameter can be selected, which may avoid anastomotic stenosis and achieve the ideal effect.
Twenty healthy experimental Bama mini-pigs were used in our study. Only one pig died from ileus caused by congenital intestinal valvular disease. Hence, the death was not directly related to the experimental method. The operation and anastomotic completion times in the stent group were significantly shorter than in the control group, indicating that the stent procedure was easier and simpler to perform compared with the hand-sewn method. In the stent group, the operation time in the 3 mo subgroup declined 34.65% more than in the 2 wk subgroup, while the anastomotic completion time declined by 44.6% in both subgroups. The anastomotic completion time for the last four pigs in the stent group was 18, 15, 18, and 17 min, respectively. This was superior to the hand-sewn method (24.5 ± 11.3 min) and close to the stapled intestinal anastomosis (mean 15.5 ± 7.8 min)[22]. The results indicated the potential of the laparoscopic colonic anastomosis stent method. No significant differences were found in bursting pressure between the groups. Bursting pressure is one of the most reliable parameters for measuring the quality of the healing process. The healing process in the stent and control groups was uneventful. There were no significant differences between the average AC and normal circumference in the stent group at 3 mo postoperatively, which showed that no luminal stenosis occurred in the presence of the stents. However, the average AC of the control group at 3 mo postoperatively was less than the normal average circumference, which may be the reason for bowel tapering in two pigs.
Histological examination is the gold standard for confirming anastomotic healing. We found that the mucosa of the anastomosis was absent at postoperative week 2, and granulation tissue formed with a large amount of inflammatory cell infiltration. In addition, fibrous tissue hyperplasia and collagen deposition were noted, but the smooth muscle layer was not formed. The degree of inflammatory infiltration in the stent group was lower than in the control group. The mucosa had grown completely, and smooth muscle was already formed at 3 mo postoperatively. The amount of smooth muscle in the stent group was more than in the control group, and foreign body granuloma was noted in the control group. The results indicated that the healing was similar in the two groups, but the inflammatory reaction was lighter and the growth of intestinal smooth muscle was better in the stent group than in the control group. Therefore, the stent method had some advantages. No foreign body, lower tension, and isolation from intestinal contents might have contributed to the satisfactory results in the stent group.
However, there were some limitations in our study. The sample of animals was small, and the experimental animals were Bama mini-pigs. Although the animals have similar physiological features to humans and comparable operating procedures, the healing ability and anti-infective activity in the animals might not be consistent with those of humans. Hence, further research should be done before the procedure is applied clinically.
In conclusion, laparoscopic colonic anastomosis with degradable stents could be a potential alternative procedure for intestinal anastomosis.
Laparoscopic intestinal anastomosis is a basic technique in gastrointestinal procedures. Although surgical skills have been significantly improved and developed, hand-sewn anastomoses are still complicated and time-consuming. Therefore, an ideal laparoscopic surgical procedure that can achieve the desired results is urgently needed.
Many procedures have been developed, including stapling devices, compression rings, tissue glue, and laser anastomosis. However, stapler devices are expensive and require introduction of a permanent foreign body. Sutureless anastomosis has weak anastomotic strength, necrosis, and stricture. Tissue glue and laser anastomosis are not used clinically because of weak anastomotic strength.
The authors developed a degradable stent and have exhibited its feasibility and safety in colonic anastomosis and primary repair of colonic perforation in a porcine model undergoing open surgery. With this simple technique, suturing time was greatly decreased, the anastomosis was free of compressive pressure, and the damage to the submucosal vascular plexus and mesenteric vessels were minimized. In this study, the application of this stent in laparoscopic colonic anastomosis was explored.
Laparoscopic colonic anastomosis with degradable stents could be a potential alternative procedure for intestinal anastomosis. Further research should be done before the procedure is applied clinically.
Anastomotic circumference: The circumference of the anastomosis. Bursting pressure: the maximum pressure the segment resisted or the pressure at the moment the first leakage.
This is a nice and novel study with good practical value. The novel technique described has potential applications in the future.
P- Reviewer: Garg P, Singhal S S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Ma S
| 1. | Hamad MA, Mentges B, Buess G. Laparoscopic sutured anastomosis of the bowel. Surg Endosc. 2003;17:1840-1844. [PubMed] [Cited in This Article: ] |
| 2. | Karamanakos SN, Sdralis E, Panagiotopoulos S, Kehagias I. Laparoscopy in the emergency setting: a retrospective review of 540 patients with acute abdominal pain. Surg Laparosc Endosc Percutan Tech. 2010;20:119-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Fan C, Ma J, Zhang HK, Gao R, Li JH, Yu L, Wu Z, Lv Y. Sutureless intestinal anastomosis with a novel device of magnetic compression anastomosis. Chin Med Sci J. 2011;26:182-189. [PubMed] [Cited in This Article: ] |
| 4. | Fok M, Ah-Chong AK, Cheng SW, Wong J. Comparison of a single layer continuous hand-sewn method and circular stapling in 580 oesophageal anastomoses. Br J Surg. 1991;78:342-345. [PubMed] [Cited in This Article: ] |
| 5. | Cohen SM, Clem MF, Wexner SD, Jagelman DG. An initial comparative study of two techniques of laparoscopic colonic anastomosis and mesenteric defect closure. Surg Endosc. 1994;8:130-134. [PubMed] [Cited in This Article: ] |
| 6. | Buchberg BS, Masoomi H, Bergman H, Mills SD, Stamos MJ. The use of a compression device as an alternative to hand-sewn and stapled colorectal anastomoses: is three a crowd? J Gastrointest Surg. 2011;15:304-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Lim CB, Goldin RD, Darzi A, Hanna GB. Characterization of materials eliciting foreign body reaction in stapled human gastrointestinal anastomoses. Br J Surg. 2008;95:1044-1050. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Warschkow R, Steffen T, Thierbach J, Bruckner T, Lange J, Tarantino I. Risk factors for anastomotic leakage after rectal cancer resection and reconstruction with colorectostomy. A retrospective study with bootstrap analysis. Ann Surg Oncol. 2011;18:2772-2782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Rullier E, Laurent C, Garrelon JL, Michel P, Saric J, Parneix M. Risk factors for anastomotic leakage after resection of rectal cancer. Br J Surg. 1998;85:355-358. [PubMed] [Cited in This Article: ] |
| 10. | McKinley AJ, Krukowski M. Intestinal anastomoses. Surgery (Oxford). 2006;24:224-228. [Cited in This Article: ] |
| 11. | Khromov Y, Pliakos I, Ibrahim M, Zbar AP, Sayfan J, Papavramidis TS. A prospective multi-institutional study assessing clinical outcome with the NiTi compression anastomosis ring (Biodynamix ColonRingTM) in elective colorectal anastomoses. Hepatogastroenterology. 2013;60:522-527. [PubMed] [Cited in This Article: ] |
| 12. | Avgoustou C, Penlidis P, Tsakpini A, Sioros C, Giannousis D. Compression anastomoses in colon and rectal surgery with the NiTi ColonRing™. Tech Coloproctol. 2012;16:29-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Kuramoto S, Ryan PJ, Kawahara M, Masaki Y. Experimental laser anastomosis of the colon. Long-term results and histologic findings after laser closure of colotomies. Dis Colon Rectum. 1994;37:1198-1204. [PubMed] [Cited in This Article: ] |
| 14. | Wang Y, Cai X, Jin R, Liang Y, Huang D, Peng S. Experimental study of primary repair of colonic leakage with a degradable stent in a porcine model. J Gastrointest Surg. 2011;15:1995-2000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Wang Y, Cai X, Cai H, Liang Y, Huang D, Liang X. Experimental study of colonic anastomosis with a degradable stent in a porcine model. Am J Surg. 2010;199:833-839. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Liu K, Yu H, Zhang M, Yu Y, Wang Y, Cai X. Sutureless primary repair of colonic perforation with a degradable stent in a porcine model of fecal peritonitis. Int J Colorectal Dis. 2012;27:1607-1617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Dziki AJ, Duncan MD, Harmon JW, Saini N, Malthaner RA, Trad KS, Fernicola MT, Hakki F, Ugarte RM. Advantages of handsewn over stapled bowel anastomosis. Dis Colon Rectum. 1991;34:442-448. [PubMed] [Cited in This Article: ] |
| 18. | Lee HY, Woo JH, Park SY, Kang NW, Park KJ, Choi HJ. Intestinal Anastomosis by Use of a Memory-shaped Compression Anastomosis Clip (Hand CAC 30): Early Clinical Experience. J Korean Soc Coloproctol. 2012;28:83-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Nudelman I, Fuko V, Waserberg N, Niv Y, Rubin M, Szold A, Lelcuk S. Colonic anastomosis performed with a memory-shaped device. Am J Surg. 2005;190:434-438. [PubMed] [Cited in This Article: ] |
| 20. | Nudelman I, Fuko V, Rubin M, Lelcuk S. A nickel-titanium memory-shape device for colonic anastomosis in laparoscopic surgery. Surg Endosc. 2004;18:1085-1089. [PubMed] [Cited in This Article: ] |
| 21. | Kaidar-Person O, Rosenthal RJ, Wexner SD, Szomstein S, Person B. Compression anastomosis: history and clinical considerations. Am J Surg. 2008;195:818-826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Erestam S, Erichsen A, Derwinger K, Kodeda K. A survey of surgeons’ perception and awareness of intraoperative time utilization. Patient Saf Surg. 2014;8:30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |