Published online May 7, 2016. doi: 10.3748/wjg.v22.i17.4373
Peer-review started: December 28, 2015
First decision: January 28, 2016
Revised: February 11, 2016
Accepted: March 2, 2016
Article in press: March 2, 2016
Published online: May 7, 2016
AIM: To evaluate the usefulness of the functional hepatic resection rate (FHRR) calculated using 3D computed tomography (CT)/99mTc-galactosyl-human serum albumin (GSA) single-photon emission computed tomography (SPECT) fusion imaging for surgical decision making.
METHODS: We enrolled 57 patients who underwent bi- or trisectionectomy at our institution between October 2013 and March 2015. Of these, 26 patients presented with hepatocellular carcinoma, 12 with hilar cholangiocarcinoma, six with intrahepatic cholangiocarcinoma, four with liver metastasis, and nine with other diseases. All patients preoperatively underwent three-phase dynamic multidetector CT and 99mTc-GSA scintigraphy. We compared the parenchymal hepatic resection rate (PHRR) with the FHRR, which was defined as the resection volume counts per total liver volume counts on 3D CT/99mTc-GSA SPECT fusion images.
RESULTS: In total, 50 patients underwent bisectionectomy and seven underwent trisectionectomy. Biliary reconstruction was performed in 15 patients, including hepatopancreatoduodenectomy in two. FHRR and PHRR were 38.6 ± 19.9 and 44.5 ± 16.0, respectively; FHRR was strongly correlated with PHRR. The regression coefficient for FHRR on PHRR was 1.16 (P < 0.0001). The ratio of FHRR to PHRR for patients with preoperative therapies (transcatheter arterial chemoembolization, radiation, radiofrequency ablation, etc.), large tumors with a volume of > 1000 mL, and/or macroscopic vascular invasion was significantly smaller than that for patients without these factors (0.73 ± 0.19 vs 0.82 ± 0.18, P < 0.05). Postoperative hyperbilirubinemia was observed in six patients. Major morbidities (Clavien-Dindo grade ≥ 3) occurred in 17 patients (29.8%). There was no case of surgery-related death.
CONCLUSION: Our results suggest that FHRR is an important deciding factor for major hepatectomy, because FHRR and PHRR may be discrepant owing to insufficient hepatic inflow and congestion in patients with preoperative therapies, macroscopic vascular invasion, and/or a tumor volume of > 1000 mL.
Core tip: We evaluated the usefulness of the functional hepatic resection rate (FHRR) calculated using 3D computed tomography (CT)/99mTc-galactosyl human serum albumin (GSA) single-photon emission computed tomography fusion imaging and found a strong correlation between FHRR and the parenchymal hepatic resection rate (PHRR). However, FHRR and PHRR were discrepant because of insufficient hepatic inflow and congestion in patients with preoperative therapies, macroscopic vascular invasion, or a tumor volume of > 1000 mL.
- Citation: Tsuruga Y, Kamiyama T, Kamachi H, Shimada S, Wakayama K, Orimo T, Kakisaka T, Yokoo H, Taketomi A. Significance of functional hepatic resection rate calculated using 3D CT/99mTc-galactosyl human serum albumin single-photon emission computed tomography fusion imaging. World J Gastroenterol 2016; 22(17): 4373-4379
- URL: https://www.wjgnet.com/1007-9327/full/v22/i17/4373.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i17.4373
The development of postoperative liver failure (PHLF) is a major cause of morbidity and mortality after hepatectomy[1]. The mortality rate after major hepatectomy is reported as 2.0% to 6.8%[1-3]. A smaller remnant liver volume and an impaired hepatic functional capacity increase the risk of PHLF[4]. Preoperative estimation of the hepatic functional reserve is important for major hepatectomy[5]. Although the future remnant hepatic volume can be calculated by computed tomography (CT) volumetry[6], precise estimation of the future liver remnant function is difficult.
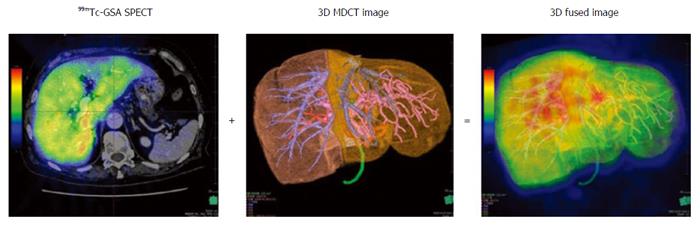
The asialoglycoprotein receptor is only expressed on the sinusoidal surfaces of mammalian hepatocytes. 99mTc-galactosyl human serum albumin (99mTc-GSA) was developed as a liver scintigraphy agent that binds to the asialoglycoprotein receptor on hepatocytes[7]. 99mTc-GSA scintigraphy is frequently used for evaluating the hepatic functional reserve, and several parameters for quantitative determination of hepatic function such as the hepatic uptake ratio to the liver plus heart at 15 min (LHL15)[8] and the maximum removal rate of 99mTc-GSA (Rmax)[9] have been reported. 99mTc-GSA single-photon emission computed tomography (SPECT) can acquire the regional distribution of hepatic function[10]. However, the spatial resolution of 99mTc-GSA SPECT is low. Furthermore, it is difficult to precisely estimate the functional reserve of the future liver remnant in patients requiring complicated resection. CT/99mTc-GSA SPECT fusion imaging has been reported to enable the precise evaluation of hepatic function distribution, given the high spatial resolution provided by CT[11]. Several parameters using CT/99mTc-GSA SPECT fusion imaging such as the preoperative total amount of receptor in the future remnant liver (R0-remnant)[12], the liver uptake value corrected for body surface area (LUV(BSA))[13] and the uptake index (UI)[14] have been reported to be useful for preoperative estimation of remnant hepatic function, but they were considerably complex. We considered hepatic resection rate is more intuitively usable.
In the present study, we calculated the functional hepatic resection rate (FHRR) using 3D CT/99mTc-GSA SPECT fusion imaging for patients undergoing major hepatectomy and determined its usefulness by correlation with the parenchymal hepatic resection rate (PHRR).
Between October 2013 and March 2015, 57 patients who underwent bi- or trisectionectomy at the Department of Gastroenterological Surgery I, Hokkaido University Hospital were enrolled in this study. The baseline characteristics of the patients are shown in Table 1. All patients preoperatively underwent three-phase dynamic multidetector CT and 99mTc-GSA scintigraphy. Preoperative portal vein embolization (PVE) is performed for patients with a PHRR of > 60% at our institution[12]. Accordingly, 13 patients with a mean PHRR of 62.1% ± 7.9% (range, 47.0%-71.3%) underwent preoperative PVE.
| Age mean (range) | 65.7 (34-82) |
| Men/women | 33/24 |
| HBsAg positivity | 10 (1.6) |
| HCV positivity | 7 (1.2) |
| Diagnosis | |
| HCC | 26 (45.6) |
| Hilar cholangiocarcinoma | 12 (21.1) |
| Intrahepatic cholangiocarcinoma | 6 (10.5) |
| Metastatic tumor | 4 (7.0) |
| Hemangioma | 4 (7.0) |
| Others | 5 (8.8) |
| Child-Pugh score (5/6/7/8) | 44/8/3/2 |
| Child-Pugh classification (A/B) | 52/5 |
| ICGR15, mean (range) | 10.8 (1.5-44.3) |
| LHL15, mean (range) | 0.920 (0.712-0.973) |
| Preoperative therapies | 18 (31.6) |
| TACE | 6 (10.5) |
| Radiation | 5 (8.8) |
| RFA | 3 (5.3) |
| Partial resection | 3 (5.3) |
| Chemotherapy | 1 (1.8) |
| Tumor size > 1000 mL | 5 (8.8) |
| Portal vein thrombosis | 8 (14.0) |
| Hepatic vein thrombosis | 3 (5.3) |
| Preoperative biliary drainage | 15 (26.3) |
| Preoperative portal vein embolization | 13 (22.8) |
An algorithm (Hokkaido University Algorithm) incorporating the indocyanine green retention at 15 min and remnant liver volume is generally used to determine the nature of sectionectomy required, e.g., bisectionectomy[15]. In the present study, FHRR and PHRR were used to determine the required procedure.
Three-phase dynamic CT was performed with a 320-row multidetector CT device (Aquilion ONE, Toshiba Medical Systems Co., Otawara, Japan). The obtained Digital Imaging and Communications in Medicine (DICOM) data were imported to the 3D image analysis system[16] (Volume Analyzer SYNAPSE VINCENT; Fuji Film Medical, Tokyo, Japan). 3D images were reconstructed from the DICOM data.
99mTc-GSA scintigraphy was performed separately from CT. Dynamic scanning was initially performed using a large-field view gamma camera (E.CAM; Siemens Japan, Tokyo) in an anterior view equipped with a low-energy high-resolution collimator, with the patient in the supine position after a bolus IV injection of 185 MBq of 99mTc-GSA. Dynamic planar images were obtained for 30 min by 147 serial frames (60 × 1 s, 87 × 20 s), with a matrix size of 128 × 128. Hepatic SPECT images were acquired after the dynamic study. The Digital Imaging and Communications in Medicine (DICOM) data obtained from SPECT were also imported to the SYNAPSE VINCENT and subsequently fused with the 3D CT images (Figure 1). PHRR and FHRR were calculated using the following formula:
Parenchymal hepatic resection rate (PHRR) (%) = liver resection volume/(total liver volume - tumor volume × 100)
Functional hepatic resection rate (FHRR) (%) = resection volume counts/total liver volume counts × 100
Statistical analyses were performed using JMP PRO version 11.2.0 (JMP statistical software; SAS Institute, Cary, NC, USA). The values are expressed as mean ± SDs. The correlation between PHRR and FHRR was assessed using Pearson’s correlation product-moment coefficient. The ratio of FHRR to PHRR was calculated after stratifying the patients according to the presence or absence of preoperative therapies, macroscopic vascular invasion, and a tumor volume of 1000 mL. The ratio was derived by dividing the smaller value (PHRR or FHRR) with the larger value. The Wilcoxon signed rank test was used to compare data between groups.
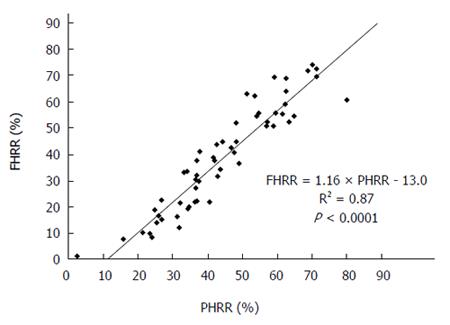
PHRR and FHRR were preoperatively calculated for all patients, and their correlation was determined (Figure 2). The mean PHRR and FHRR values were 44.5% ± 16.0% and 38.6% ± 19.9%, respectively. The FHRR value was approximately 10% smaller than the PHRR value, although there was a strong correlation between the two. The regression coefficient was 1.16 (P < 0.0001).
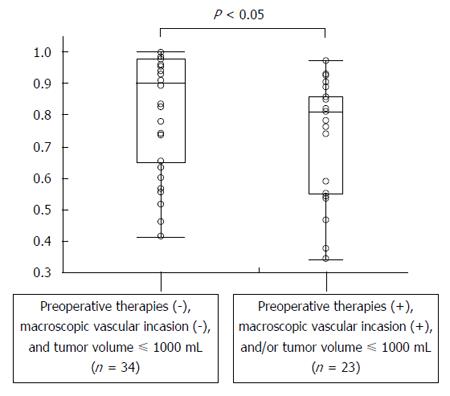
There were 34 patients with preoperative therapies, including transcatheter arterial chemoembolization, radiation, radiofrequency ablation, chemotherapy, and partial resection), macroscopic vascular invasion, and/or a tumor volume of > 1000 mL, while 23 patients did not have any of these factors. The mean ratios for patients with and without these factors were 0.73 ± 0.19 and 0.82 ± 0.18, respectively (Figure 3). Thus, the ratio was significantly smaller for the former than for the latter (P < 0.05).
Among the 57 patients, the most frequently performed procedure was right hepatectomy ± segment I (n = 18, 31.6%; Table 2). Biliary reconstruction was performed for 15 patients, including hepatopancreatoduodenectomy in two. Five patients exhibited Child-Pugh class B cirrhosis. These patients did not have chronic hepatitis, but they exhibited obstructive jaundice and malnutrition. Postoperative hyperbilirubinemia (serum total bilirubin ≥ 5.0 mg/dL) was observed in six patients (10.5%). Five of these six patients underwent biliary reconstruction, and the remaining one exhibited preoperative cholangitis and underwent biliary drainage. PHLF, assessed according to the International Study Group of Liver Surgery definition, occurred in 12 patients (21.0%). Grade C liver failure was diagnosed in a patient who required repeat surgery because of portal vein thrombosis. Major morbidities (Clavien-Dindo grade ≥ 3) occurred in 17 patients (29.8%). Bile leakage was a frequent occurrence (eight of 17 patients; 47.0%). There were no surgery-related deaths.
| Hepatectomy procedure | |
| Right trisectionectomy | 5 (8.8) |
| Extended right hepatectomy | 2 (3.5) |
| Right hepatectomy ± segment I | 18 (31.6) |
| Central bisectionectomy | 5 (8.8) |
| Left hepatectomy ± segment I | 16 (28.1) |
| Extended left hepatectomy ± segment I | 9 (15.8) |
| Left trisectionectomy ± segment I | 2 (3.5) |
| Biliary reconstruction | 15 (26.3) |
| Surgical duration (min), mean ± SD | 432 ± 167 |
| Blood loss, median (g), range | 430 (0-5700) |
| Red blood cell transfusion | 18 (31.6) |
| Postoperative hyperbilirubinemia | 6 (10.5) |
| (T-bil ≥ 5.0 mg/dL) | |
| PHLF ISGLS grade (A/B/C) | 9/2/1 |
| Major morbidity (Clavien-Dindo grade ≥ 3) | 12/5 |
| (IIIa/IIIb) | |
| Surgery-related death | 0 |
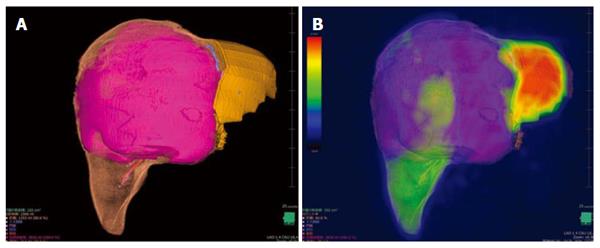
A 34-year-old woman was diagnosed with a giant hemangioma (Figure 4). CT demonstrated that the posterior section was congested because of obstruction of the right hepatic vein by the giant hemangioma. Right trisectionectomy was planned. CT volumetry showed a tumor size of 2818 mL, a future remnant hepatic volume of 313 mL and a PHRR of 80.0%. 3D CT/99mTc-GSA SPECT fusion imaging showed an FHRR of 60.8%. She underwent right trisectionectomy without PVE and recovered without PHLF or any morbidity.
In the present study evaluating the significance of FHRR calculated using 3D CT/99mTc-GSA SPECT fusion imaging, we found a strong correlation between FHRR and PHRR. However, the ratio of FHRR to PHRR was significantly smaller for patients with preoperative therapies, a tumor volume of > 1000 mL, and/or macroscopic vascular invasion than for those without these factors.
99mTc-GSA scintigraphy is a well-accepted modality for assessment of the hepatic functional reserve, along with the indocyanine green clearance test[7,8,17]. We previously reported the conversion formula for 99mTc-GSA scintigraphy data to the indocyanine green retention at 15 min[18]. This is useful for estimating the functional reserve of the whole liver, although the function of the future liver remnant can only be estimated from the volume calculated by CT volumetry. The introduction of 3D CT/99mTc-GSA SPECT fusion imaging has enabled precise estimation of the function of the focal liver lesion[11-14,19,20].
In the present study, PHRR and FHRR were calculated using 3D CT/99mTc-GSA SPECT fusion imaging along the accurate cutting line. FHRR was strongly correlated with PHRR, although they were discrepant for patients with preoperative therapies, macroscopic vascular invasion, and/or a tumor volume of >1000 mL. Theoretically, the discrepancy cannot occur without any deflection in liver function. Akaki et al[10] suspected that the decrease in the portal venous flow caused by the tumor is a major factor for the discrepancy between CT and 99mTc-GSA SPECT data. Mitsumori et al[21] reported that 99mTc-GSA SPECT findings were better correlated with the remnant liver function than CT findings because of the following reasons. First, the degree of hepatic dysfunction in the segment or lobe containing the hepatocellular carcinoma is greater than that in the segment or lobe without cancer. Second, the liver parenchyma surrounding the tumor is damaged by mechanical compression. Third, secondary liver damage may occur because of compression of the vessels and bile ducts by the tumor. We also hypothesized that preoperative therapies such as transcatheter arterial chemoemobolization, radiofrequency ablation, and radiation induce focal liver damage, and that macroscopic vascular invasion and large tumors cause insufficient inflow or congestion. To prove the hypothesis, we compared the ratio of FHRR to PHRR between patients with and without the abovementioned factors and found that it was significantly smaller for patients with these factors. Thus, PHRR and FHRR are likely to be discrepant for patients with these conditions.
Recent advances in surgical techniques and pre- and postoperative care, including the decision criteria for hepatectomy and indications for liver resection, have been applied to extended hepatectomy. However, the famous decision criteria of Makuuchi[22] do not include the future remnant liver volume. A smaller remnant liver volume and an impaired hepatic functional capacity increase the risk of PHLF[4]; therefore, calculation of the future remnant hepatic volume using CT volumetry is important[6]. It was reported that the type of surgery (more than or equal to hemihepatectomy vs less than hemihepatectomy) was associated with in-hospital mortality, and, specifically, patients who underwent less than a hemihepatectomy exhibited a mortality rate of 4.1%, while those who underwent more than or equal to hemihepatectomy exhibited a mortality rate of 6.5%[23]. Therefore, major hepatectomy requires detailed preoperative evaluations and excellent techniques. When FHRR is larger than PHRR, surgical decision making should be more meticulous to prevent PHLF and postoperative morbidities. It was reported that preoperative functional assessment by 99mTc-GSA SPECT is more useful than volumetric assessments by CT for the prediction of surgical outcomes[21,24]. On the other hand, when FHRR is smaller than PHRR, the indications for hepatectomy may be expanded beyond the safe limits. The reported safe limit of PHRR for a normal liver is 75%[25,26]. In the present study, a 34-year-old woman with a giant hemangioma uneventfully underwent right trisectionectomy. Although her PHRR was 80.0%, right trisectionectomy was accomplished without PVE after referring to an FHRR of 60.8%. This was probably because the function of the posterior section may have been deteriorated by congestion caused by obstruction of the right hepatic vein by the giant hemangioma. She recovered without PHLF or any morbidity. Further studies are required to confirm the safety of giving more importance to FHRR over PHRR.
In conclusion, we found an overall strong correlation between FHRR calculated using 3D CT/99mTc-GSA SPECT and PHRR in our study. However, FHRR and PHRR were discrepant for patients with preoperative therapies, macroscopic vascular invasion, and/or a tumor volume of > 1000 mL. These findings suggest that FHRR is useful for accurate estimation of the future liver remnant function and an important deciding factor for major hepatectomy.
Preoperative estimation of the hepatic functional reserve is important for major hepatectomy. Precise estimation of the future liver remnant function is difficult. 99mTc-galactosyl human serum albumin (99mTc-GSA) is frequently used for evaluating the hepatic functional reserve. 99mTc-GSA single-photon emission computed tomography (SPECT) can show the regional distribution of hepatic function. However, it is difficult to precisely estimate the functional reserve of the future liver remnant in patients requiring complicated resection. CT/99mTc-GSA SPECT fusion imaging enables the precise evaluation of hepatic function distribution, given the high spatial resolution provided by CT. In the present study, the authors calculated the functional hepatic resection rate (FHRR) using 3D CT/99mTc-GSA SPECT fusion imaging for patients undergoing major hepatectomy and determined its usefulness by determining its correlation with the parenchymal hepatic resection rate (PHRR).
Several parameters using CT/99mTc-GSA SPECT fusion imaging, such as the preoperative total amount of receptor in the future remnant liver (R0-remnant), the liver uptake value corrected for body surface area [LUV(BSA)], and the uptake index (UI), have been reported to be useful for the preoperative estimation of remnant hepatic function, although they are considerably complex.
In the present study, the authors calculated the FHRR using 3D CT/99mTc-GSA SPECT fusion imaging and determined its usefulness. They considered the hepatic resection rate to be more intuitively useful compared with former reported parameters. A strong correlation between FHRR and PHRR in the present study was found. However, FHRR and PHRR were discrepant for patients with preoperative therapies, macroscopic vascular invasion, and/or a tumor volume of > 1000 mL.
This study suggests that the measurement of FHRR is particularly useful for patients with preoperative therapies, macroscopic vascular invasion, and/or a tumor volume of > 1000 mL.
99mTc-GSA scintigraphy: An image inspection used for evaluating the hepatic functional reserve. 99mTc-GSA SPECT: A procedure to obtain tomographic images of 99mTc-GSA scintigraphy.
The manuscript is well organized and developed. The study is inserted in the wide topic of selection of the patients for hepatic surgery, first of all based on the evaluation of the functions of the remnant liver.
P- Reviewer: Neri V S- Editor: Yu J L- Editor: A E- Editor: Liu XM
| 1. | Reissfelder C, Rahbari NN, Koch M, Kofler B, Sutedja N, Elbers H, Büchler MW, Weitz J. Postoperative course and clinical significance of biochemical blood tests following hepatic resection. Br J Surg. 2011;98:836-844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 2. | Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 800] [Cited by in F6Publishing: 778] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 3. | Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, Abdalla EK, Curley SA, Capussotti L, Clary BM. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854-862; discussion 862-864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 425] [Cited by in F6Publishing: 471] [Article Influence: 27.7] [Reference Citation Analysis (1)] |
| 4. | Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, Corvera C, Weber S, Blumgart LH. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397-406; discussion 406-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1148] [Cited by in F6Publishing: 1030] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 5. | Kim HJ, Kim CY, Park EK, Hur YH, Koh YS, Kim HJ, Cho CK. Volumetric analysis and indocyanine green retention rate at 15 min as predictors of post-hepatectomy liver failure. HPB (Oxford). 2015;17:159-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Simpson AL, Geller DA, Hemming AW, Jarnagin WR, Clements LW, D’Angelica MI, Dumpuri P, Gönen M, Zendejas I, Miga MI. Liver planning software accurately predicts postoperative liver volume and measures early regeneration. J Am Coll Surg. 2014;219:199-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Ha-Kawa SK, Tanaka Y, Hasebe S, Kuniyasu Y, Koizumi K, Ishii Y, Yamamoto K, Kashiwagi T, Ito A, Kudo M. Compartmental analysis of asialoglycoprotein receptor scintigraphy for quantitative measurement of liver function: a multicentre study. Eur J Nucl Med. 1997;24:130-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Kudo M, Todo A, Ikekubo K, Yamamoto K, Vera DR, Stadalnik RC. Quantitative assessment of hepatocellular function through in vivo radioreceptor imaging with technetium 99m galactosyl human serum albumin. Hepatology. 1993;17:814-819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Kwon AH, Matsui Y, Kaibori M, Ha-Kawa SK. Preoperative regional maximal removal rate of technetium-99m-galactosyl human serum albumin (GSA-Rmax) is useful for judging the safety of hepatic resection. Surgery. 2006;140:379-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Akaki S, Okumura Y, Sasai N, Sato S, Tsunoda M, Kuroda M, Kanazawa S, Hiraki Y. Hepatectomy simulation discrepancy between radionuclide receptor imaging and CT volumetry: influence of decreased unilateral portal venous flow. Ann Nucl Med. 2003;17:23-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Beppu T, Hayashi H, Okabe H, Masuda T, Mima K, Otao R, Chikamoto A, Doi K, Ishiko T, Takamori H. Liver functional volumetry for portal vein embolization using a newly developed 99mTc-galactosyl human serum albumin scintigraphy SPECT-computed tomography fusion system. J Gastroenterol. 2011;46:938-943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Yumoto Y, Yagi T, Sato S, Nouso K, Kobayashi Y, Ohmoto M, Yumoto E, Nagaya I, Nakatsukasa H. Preoperative estimation of remnant hepatic function using fusion images obtained by (99m)Tc-labelled galactosyl-human serum albumin liver scintigraphy and computed tomography. Br J Surg. 2010;97:934-944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Yoshida M, Shiraishi S, Sakaguchi F, Utsunomiya D, Tashiro K, Tomiguchi S, Okabe H, Beppu T, Baba H, Yamashita Y. Fused 99m-Tc-GSA SPECT/CT imaging for the preoperative evaluation of postoperative liver function: can the liver uptake index predict postoperative hepatic functional reserve? Jpn J Radiol. 2012;30:255-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Mao Y, Du S, Ba J, Li F, Yang H, Lu X, Sang X, Li S, Che L, Tong J. Using Dynamic 99mT c-GSA SPECT/CT fusion images for hepatectomy planning and postoperative liver failure prediction. Ann Surg Oncol. 2015;22:1301-1307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Kamiyama T, Nakanishi K, Yokoo H, Kamachi H, Tahara M, Yamashita K, Taniguchi M, Shimamura T, Matsushita M, Todo S. Perioperative management of hepatic resection toward zero mortality and morbidity: analysis of 793 consecutive cases in a single institution. J Am Coll Surg. 2010;211:443-449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 16. | Ohshima S. Volume analyzer SYNAPSE VINCENT for liver analysis. J Hepatobiliary Pancreat Sci. 2014;21:235-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 17. | Okabe H, Beppu T, Hayashi H, Mima K, Nakagawa S, Kuroki H, Imai K, Nitta H, Masuda T, Hashimoto D. Rank classification based on the combination of indocyanine green retention rate at 15 min and (99m)Tc-DTPA-galactosyl human serum albumin scintigraphy predicts the safety of hepatic resection. Nucl Med Commun. 2014;35:478-483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Kawamura H, Kamiyama T, Nakagawa T, Nakanishi K, Yokoo H, Tahara M, Kamachi H, Toi H, Matsushita M, Todo S. Preoperative evaluation of hepatic functional reserve by converted ICGR15 calculated from Tc-GSA scintigraphy. J Gastroenterol Hepatol. 2008;23:1235-1241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Sumiyoshi T, Shima Y, Tokorodani R, Okabayashi T, Kozuki A, Hata Y, Noda Y, Murata Y, Nakamura T, Uka K. CT/99mTc-GSA SPECT fusion images demonstrate functional differences between the liver lobes. World J Gastroenterol. 2013;19:3217-3225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Nanashima A, Abo T, Kudo T, Sakamoto I, Hayashi H, Murakami G, Takeshita H, Hidaka S, Kido Y, Nagayasu T. Usefulness of examining hepatic functional volume using technetium-99m galactosyl serum albumin scintigraphy in hepatocellular carcinoma. Nucl Med Commun. 2013;34:478-488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Mitsumori A, Nagaya I, Kimoto S, Akaki S, Togami I, Takeda Y, Joja I, Hiraki Y. Preoperative evaluation of hepatic functional reserve following hepatectomy by technetium-99m galactosyl human serum albumin liver scintigraphy and computed tomography. Eur J Nucl Med. 1998;25:1377-1382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 68] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Makuuchi M, Kosuge T, Takayama T, Yamazaki S, Kakazu T, Miyagawa S, Kawasaki S. Surgery for small liver cancers. Semin Surg Oncol. 1993;9:298-304. [PubMed] [Cited in This Article: ] |
| 23. | Asiyanbola B, Chang D, Gleisner AL, Nathan H, Choti MA, Schulick RD, Pawlik TM. Operative mortality after hepatic resection: are literature-based rates broadly applicable? J Gastrointest Surg. 2008;12:842-851. [PubMed] [Cited in This Article: ] |
| 24. | Hayashi H, Beppu T, Okabe H, Kuroki H, Nakagawa S, Imai K, Nitta H, Chikamoto A, Ishiko T, Baba H. Functional assessment versus conventional volumetric assessment in the prediction of operative outcomes after major hepatectomy. Surgery. 2015;157:20-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 25. | Shoup M, Gonen M, D’Angelica M, Jarnagin WR, DeMatteo RP, Schwartz LH, Tuorto S, Blumgart LH, Fong Y. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7:325-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 350] [Cited by in F6Publishing: 385] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 26. | Vauthey JN, Chaoui A, Do KA, Bilimoria MM, Fenstermacher MJ, Charnsangavej C, Hicks M, Alsfasser G, Lauwers G, Hawkins IF. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127:512-519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 494] [Cited by in F6Publishing: 534] [Article Influence: 22.3] [Reference Citation Analysis (0)] |