Published online Apr 7, 2016. doi: 10.3748/wjg.v22.i13.3573
Peer-review started: November 2, 2015
First decision: November 27, 2015
Revised: December 14, 2015
Accepted: January 11, 2016
Article in press: January 11, 2016
Published online: April 7, 2016
AIM: To investigate the regulatory effect of Vδ1 T cells and the antitumor activity of Vδ2 T cells in rectal cancer.
METHODS: Peripheral blood, tumor tissues and para-carcinoma tissues from 20 rectal cancer patients were collected. Naïve CD4 T cells from the peripheral blood of rectal cancer patients were purified by negative selection using a Naive CD4+ T Cell Isolation Kit II (Miltenyi Biotec). Tumor tissues and para-carcinoma tissues were minced into small pieces and digested in a triple enzyme mixture containing collagenase type IV, hyaluronidase, and deoxyribonuclease for 2 h at room temperature. After digestion, the cells were washed twice in RPMI1640 and cultured in RPMI1640 containing 10% human serum supplemented with L-glutamine and 2-mercaptoethanol and 1000 U/mL of IL-2 for the generation of T cells. Vδ1 T cells and Vδ2 T cells from tumor tissues and para-carcinoma tissues were expanded by anti-TCR γδ antibodies. The inhibitory effects of Vδ1 T cells on naïve CD4 T cells were analyzed using the CFSE method. The cytotoxicity of Vδ2 T cells on rectal cancer lines was determined by the LDH method.
RESULTS: The percentage of Vδ1 T cells in rectal tumor tissues from rectal cancer patients was significantly increased, and positively correlated with the T stage. The percentage of Vδ2 T cells in rectal tumor tissues from rectal cancer patients was significantly decreased, and negatively correlated with the T stage. After culture for 14 d with 1 μg/mL anti-TCR γδ antibodies, the percentage of Vδ1 T cells from para-carcinoma tissues was 21.45% ± 4.64%, and the percentage of Vδ2 T cells was 38.64% ± 8.05%. After culture for 14 d, the percentage of Vδ1 T cells from rectal cancer tissues was 67.45% ± 11.75% and the percentage of Vδ2 T cells was 8.94% ± 2.85%. Tumor-infiltrating Vδ1 T cells had strong inhibitory effects, and tumor-infiltrating Vδ2 T cells showed strong cytolytic activity. The inhibitory effects of Vδ1 T cells from para-carcinoma tissues and from rectal cancer tissue were not significantly different. In addition, the cytolytic activities of Vδ2 T cells from para-carcinoma tissues and from rectal cancer tissues were not significantly different.
CONCLUSION: A percentage imbalance in Vδ1 and Vδ2 T cells in rectal cancer patients may contribute to the development of rectal cancer.
Core tip: The percentage of tumor-infiltrating Vδ1 T cells in rectal cancer patients increased when T stage increased, whereas the percentage of tumor-infiltrating Vδ2 T cells in rectal cancer patients decreased as T stage increased. Vδ1 T cells from rectal cancer tissues had strong regulatory effects, and in rectal cancer tissues the main infiltrating γδ T cells were Vδ1 T cells. Although Vδ2 T cells from rectal cancer tissues have strong cytotoxic effects, there was little infiltration of Vδ2 T cells in rectal cancer tissues. Thus, an immunosuppressant microenvironment was formed in rectal cancer tissues, which may limit antitumor immunity and allow tumors in rectal cancer patients to evade immune surveillance.
- Citation: Rong L, Li K, Li R, Liu HM, Sun R, Liu XY. Analysis of tumor-infiltrating gamma delta T cells in rectal cancer. World J Gastroenterol 2016; 22(13): 3573-3580
- URL: https://www.wjgnet.com/1007-9327/full/v22/i13/3573.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i13.3573
T cells can be divided into two major subsets according to their expression of rearranged adaptive T cell receptors (TCRs, γδ T cells and αβ T cells)[1]. γδ T cells, which represent a small subset (1%-10%) of CD3+ cells[2], can be divided into two subsets: Vδ1 T cells in the epithelial-associated lymphoid tissue and Vδ2 T cells in the peripheral blood[3,4]. They differ in their cytokine production and receptor expression, Vδ2 T cells being more inflammatory[5], and Vδ1 T cells having more of a regulatory phenotype[6]. It has been demonstrated that Vδ1 T cells express Foxp3, and their number is substantially decreased in the peripheral blood of patients with new-onset systemic lupus erythematosus (SLE)[6]. Vδ2 T cells have predominantly been investigated in the context of tumor immunosurveillance and host defense against viral invasion[7-10].
Rectal cancer is one of the most common causes of cancer deaths worldwide[11]. In recent years, combined chemoradiotherapy followed by total mesorectal excision (TME) has become the standard treatment for patients with locally advanced rectal cancer[12]. Local excision is often considered a curative treatment alternative to TME in early rectal cancer[13], however, one of the limitations of this approach is that it is impossible to determine the pN-category[13]. Lymph node involvement in rectal cancer is known to correlate with T stage[14,15]. There is increasing evidence that immune-profiling may help to predict clinical outcomes in rectal cancer, possibly more reliably than TNM classification or grading. There is evidence that a low number of tumor-infiltrating lymphocytes (TILs) predicts lymph node involvement in melanoma, gastric cancer, breast cancer, and cervical cancer[13,16-18]. The precise role of γδ T cells in the development of rectal cancer remains elusive.
In this study, we found that the percentage of Vδ1 T cells in rectal tumor tissues from rectal cancer patients was significantly increased, whereas the percentage of Vδ2 T cells in these tissues was significantly decreased. The percentages of Vδ1 and Vδ2 T cells correlated with the T stage of rectal cancer patients. To obtain Vδ1 and Vδ2 T cells, tumor tissues and para-carcinoma tissues were minced into small pieces, digested with a triple enzyme mixture containing collagenase type IV, hyaluronidase, and deoxyribonuclease and stimulated by anti-TCR γδ antibodies. After 14 d culture, the percentage of Vδ1 T cells from para-carcinoma tissues was 21.45% ± 4.64% and the percentage of Vδ2 T cells was 38.64% ± 8.05%. The percentage of Vδ1 T cells from rectal cancer tissues was 67.45% ± 11.75% and the percentage of Vδ2 T cells was 8.94% ± 2.85%. Functional assays demonstrated that tumor-infiltrating Vδ1 T cells in rectal cancer patients have strong inhibitory effects, and tumor-infiltrating Vδ2 T cells displayed strong cytolytic activity. The inhibitory effects of Vδ1 T cells and the cytolytic activity of Vδ2 T cells from para-carcinoma tissues and from rectal cancer tissues were not significantly different. Collectively, these data suggest that an imbalance in the Vδ1 and Vδ2 T cell percentages creates an immunosuppressant microenvironment in rectal cancer tissues, which may allow tumors to limit antitumor immunity and evade immune surveillance in rectal cancer patients.
Twenty patients with rectal cancer were enrolled in this study. The study was approved by the Fifth Affiliated Hospital of Xinjiang Medical University (Xinjiang, China) and written informed consent was obtained from each participating patient. Peripheral blood, tumor tissues, and para-carcinoma tissues were collected from the patients.
RPMI-1640 medium and fetal bovine serum (FBS) were obtained from Gibco; FITC-conjugated anti-human TCRγδ (IMMU510) was purchased from Beckman Coulter Immunotech; APC-conjugated anti-human CD3 (HIT3a) and FITC-conjugated anti-human TCR Vδ2 (B6) were purchased from Biolegend; FITC-conjugated anti-human TCR Vδ1 (TS8.2) was obtained from Pierce; a CellTrace™ CFSE Cell Proliferation Kit was purchased from Invitrogen; the CytoTox 96® Non-Radioactive Cytotoxicity Assay was purchased from Promega; interleukin 2 was purchased from Read United Cross Pharmaceutical Co., Ltd.
To expand Vδ1 T cells and Vδ2 T cells in vitro, tumor tissues and para-carcinoma tissues were minced into small pieces and then digested with a triple enzyme mixture containing collagenase type IV, hyaluronidase, and deoxyribonuclease for 2 h at room temperature. After digestion, the cells were washed twice in RPMI-1640 and cultured in RPMI-1640 medium with 10% FBS and 200 IU/mL interleukin 2 in 24-well culture plates coated with 1 μg/mL anti-pan-TCRγδ mAb. The HR8348 (human rectal carcinoma) cell line was obtained from the Cell Culture Center, Institute of Basic Medicine, Chinese Academy of Medical Sciences. HR8348 cells were cultured in complete RPMI-1640 medium with 10% FBS.
Cells were washed with PBS containing 1% bovine serum albumin (BSA) and incubated with surface-staining antibodies for 30 min at 4 °C. The cells were then washed and resuspended in PBS. Cytometry data were acquired using a BD Accuri C6 flow cytometer (Becton Dickinson). Data analysis was carried out with FlowJo software (Tree Star Inc.).
Naïve CD4 T cells were labeled with CFSE and used as the responder cells. The cells were cultured with Vδ1 T cells in the dark at a ratio of 1:2. After 5 d in culture, the cells were collected and washed twice with PBS containing 1% BSA. The cells were analyzed using a BD Accuri C6 flow cytometer (Becton Dickinson). Data analysis was performed using FlowJo software (Tree Star Inc.).
To determine specific cytotoxicity, we used the CytoTox 96® Non-Radioactive Cytotoxicity Assay (Promega) based on the calorimetric detection of the released enzyme lactate dehydrogenase (LDH). HR8348 and Vδ2 T cells were co-cultured at the ratios of 10:1, 20:1 and 30:1. Assays were performed in triplicate. After 6 h at 37 °C, 50 μL supernatant was assayed for LDH activity following the manufacturer’s protocol. Controls for spontaneous LDH release in effector and target cells, as well as target maximum release, were prepared. The percentage of cytotoxicity was calculated as follows:
%Cytotoxicity = ([Experimental - Effector spontaneous - Target spontaneous]/[Target maximum - Target spontaneous]) × 100
The results are expressed as mean ± SD. Data were analyzed by t-test or one-way analysis of variance (ANOVA) (SPSS version 16.0), followed by Tukey-Kramer multiple comparisons. In all analyses, the minimum acceptable level of significance was P < 0.05.
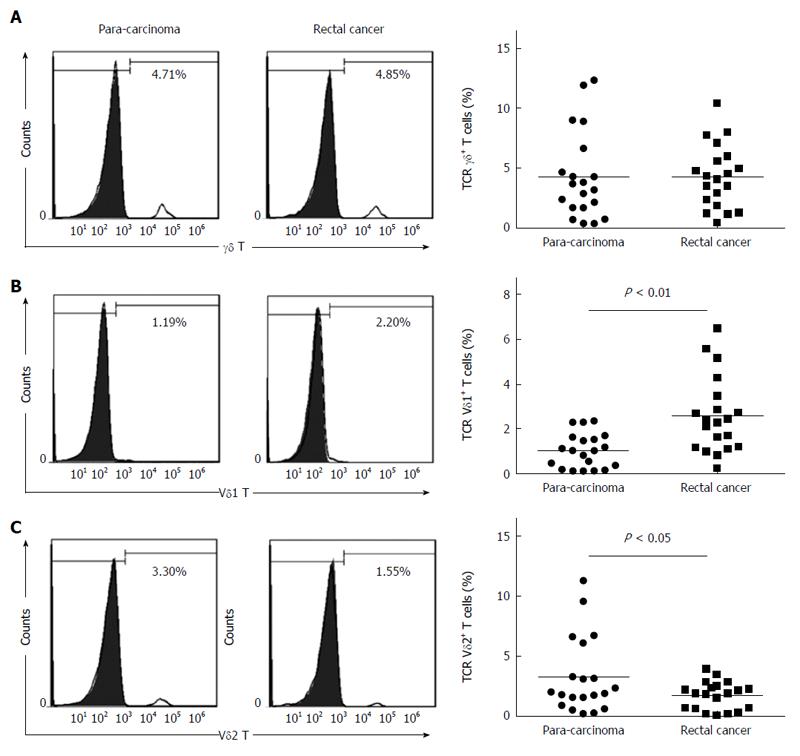
We first compared the percentages of total γδ T cells and the Vδ1 and Vδ2 T subsets in tumor tissues and para-carcinoma tissues from rectal cancer patients. There was no significant difference in the percentage of total γδ T cells in the tumor tissues and para-carcinoma tissues of rectal cancer patients (4.32% ± 0.026% vs 4.30% ± 0.037%, P > 0.05) (Figure 1A). The percentage of Vδ1 T cells in tumor tissues was significantly greater than in para-carcinoma tissues (2.58% ± 0.017% vs 1.03% ± 0.008%, P < 0.01) (Figure 1B), and the percentage of Vδ2 T cells was significantly lower in tumor tissue than in para-carcinoma tissue (1.75% ± 0.012% vs 3.27% ± 0.032%, P < 0.05) (Figure 1C).
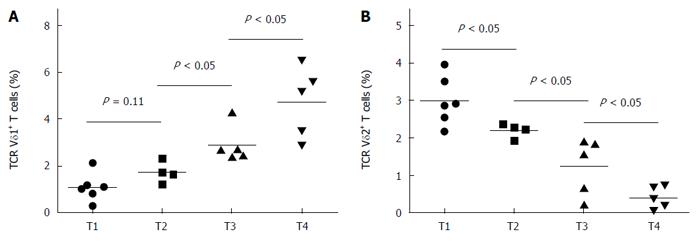
The percentage of peripheral Vδ1 T cells in rectal cancer patients increased as T stage increased (Figure 2A), whereas the percentage of peripheral Vδ2 T cells decreased as T stage increased (Figure 2B). However, there was no significant correlation between N category or M category and the percentage of Vδ1 or Vδ2 T cells (data not shown).
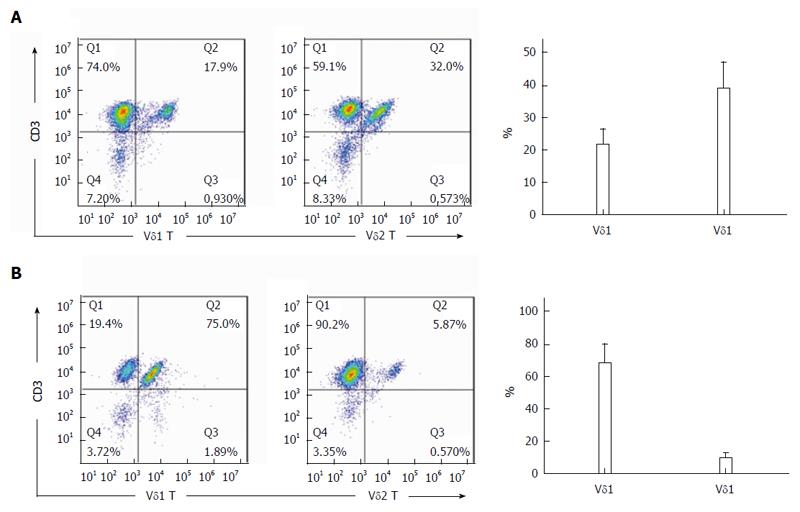
After culture in RPMI-1640 medium containing 10% FBS in 24-well culture plates coated with 1 μg/mL anti-TCR γδ antibody for 14 d, the percentage of Vδ1 T cells from para-carcinoma tissues was 21.45% ± 4.64%, and the percentage of Vδ2 T cells was 38.64% ± 8.05% (Figure 3A). After culture for 14 d, the percentage of Vδ1 T cells from rectal cancer tissues was 67.45% ± 11.75%, and the percentage of Vδ2 T cells was 8.94% ± 2.85% (Figure 3B).
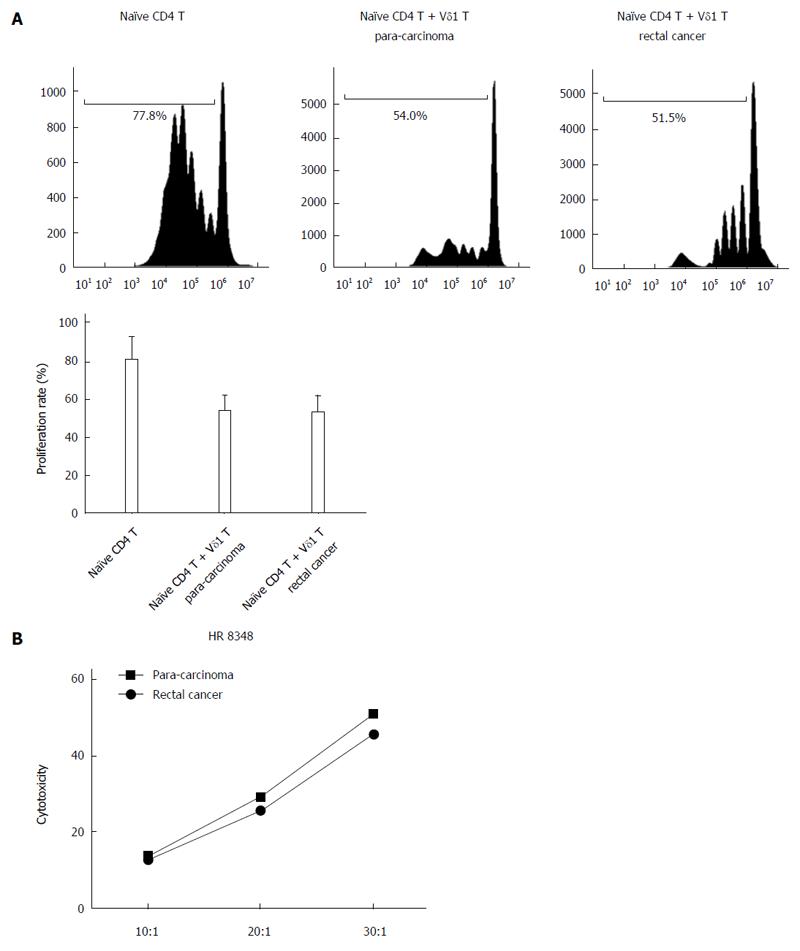
The proliferation rate of naïve CD4T cells from the blood of rectal cancer patients was 80.23% ± 11.86%; when co-cultured with Vδ1 T cells from para-carcinoma tissues, the proliferation rate was 53.45% ± 7.95%, and when co-cultured with Vδ1 T cells from rectal cancer tissues, the proliferation rate was 52.53% ± 8.52% (Figure 4A). The inhibitory effects of Vδ1 T cells from para-carcinoma tissues and from rectal cancer tissues were not significantly different (Figure 4B). In addition, the cytolytic activities of Vδ2 T cells from para-carcinoma tissues and from rectal cancer tissues were not significantly different (Figure 4C).
The major finding of this study is that the percentage of Vδ1 T cells in the rectal tumor tissues of rectal cancer patients significantly increased, and the percentage of Vδ2 T cells in the rectal tumor tissues of rectal cancer patients significantly decreased, when compared with para-carcinoma tissues. γδ T cells can be divided into two subsets: Vδ1 T cells found in the epithelial-associated lymphoid tissue and Vδ2 T cells in the peripheral blood. Our results showed that after culture for 14 d with 1 μg/mL anti-TCR γδ antibody, the major subset from para-carcinoma tissues was Vδ1 T cells, and the major subset from rectal cancer tissues was Vδ2 T cells. Tumor-infiltrating Vδ1 T cells had strong inhibitory effects, and tumor-infiltrating Vδ2 T cells showed strong cytolytic activity. Although there were no significant differences in the cytolytic activities of Vδ2 T cells from para-carcinoma tissues and from rectal cancer tissues, the predominant subset in rectal cancer tissues was Vδ1 T cells. Thus, tumors may limit antitumor immunity and evade immune surveillance in rectal cancer patients by forming an immunosuppressant microenvironment.
The MHC-independent antigen recognition and strong cytotoxicity to tumor cells make γδ T cells attractive candidate effector cells for cancer immunotherapy[19-25]. Administration of Vδ2 T cells at suitable intervals after chemotherapy and zoledronate may substantially increase antitumor activity in a range of malignancies[26], whereas tumor-infiltrating Vδ1 T cells mainly have an immunosuppressive function and promote cancer development[27,28]. The infiltration of γδ T cells in cancer tissues has been reported in some tumors[29-32]. However, to date, there has been no research on the percentages of Vδ1 and Vδ2 T cells in rectal cancer tissues. In this study, we used the FACS method to analyze the percentage of Vδ1 T cells and Vδ2 T cells in tumor tissues and para-carcinoma tissues from 20 rectal cancer patients. The results showed that the percentage of Vδ1 T cells in the rectal tumor tissues of these patients was significantly increased and positively correlated with the T stage, whereas the percentage of Vδ2 T cells in the rectal tumor tissues was significantly decreased and negatively correlated with the T stage.
We also discovered that after culture for 14 d with 1 μg/mL anti-TCR γδ antibody, the major subset of γδ T cells from para-carcinoma tissues was Vδ1, and the major subset from rectal cancer tissues was Vδ2. This result is consistent with the predominant subset in rectal cancer tissues being Vδ1 T cells, and the predominant subset in para-carcinoma tissues being Vδ2 T cells, and with the findings of a previous study which showed that the major γδ T cells infiltrating breast cancer tissues were Vδ1 T cells[28]. Vδ2 T cells have a more inflammatory phenotype; Vδ1 T cells have a more regulatory phenotype and have been shown to express Foxp3[6]. Vδ2 T cells have a major cytotoxicity function, and have predominantly been investigated in tumor immunosurveillance and host defense against viral invasion[7-10]. Our results demonstrate that tumor-infiltrating Vδ1 T cells have strong inhibitory effects, and tumor-infiltrating Vδ2 T cells have strong cytolytic activity, consistent with previous studies on the function of Vδ1 T cells and Vδ2 T cells.
The findings in this study suggest that a percentage imbalance in Vδ1 and Vδ2 T cells creates an immunosuppressant microenvironment in rectal cancer tissues, which may enable tumors to limit antitumor immunity and evade immune surveillance in rectal cancer patients. This is the first report on the percentages of Vδ1 and Vδ2 T cells in rectal cancer tissues. We demonstrate that an imbalance in Vδ1 and Vδ2 T cell percentages in cancer tissues may facilitate the development of rectal cancer. The results of this study provide a new insight into immunotherapy for rectal cancer.
γδT cells can be divided into two subsets: Vδ1 T cells and Vδ2 T cells. Vδ1 T cells, which have been shown to express Foxp3, have a regulatory function. Vδ2 T cells have largely been investigated in the context of tumor immunosurveillance and host defense against viral invasion. An imbalance of Vδ1 and Vδ2 T cell percentages in cancer tissues may facilitate the development of rectal cancer.
γδT cells have been shown to be useful in cancer immunotherapy. They can be divided into two subsets: Vδ1 and Vδ2. Administration of Vδ2 T cells at suitable intervals after chemotherapy and zoledronate may substantially increase antitumor activity in a range of malignancies, whereas tumor-infiltrating Vδ1 T cells mainly have an immune repression function and promote cancer development. However, there has been no research on the percentages of Vδ1 and Vδ2 T cells in rectal cancer tissues.
This is the first study to report the percentages of Vδ1 and Vδ2 T cells in rectal cancer tissues. The authors demonstrate that an imbalance in the percentages of Vδ1 and Vδ2 T cells in cancer tissues may facilitate the development of rectal cancer.
This study provides new insight into immunotherapy for rectal cancer.
This study is meaningful and the findings that the imbalance of Vδ1 and Vδ2 T cell percentages in rectal cancer tissues are interesting, and provide new insight into immunotherapy for rectal cancer.
P- Reviewer: Yoshimatsu K S- Editor: Ma YJ L- Editor: Webster JR E- Editor: Liu XM
| 1. | Haas W, Pereira P, Tonegawa S. Gamma/delta cells. Annu Rev Immunol. 1993;11:637-685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 555] [Cited by in F6Publishing: 607] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 2. | Kabelitz D, Wesch D, He W. Perspectives of gammadelta T cells in tumor immunology. Cancer Res. 2007;67:5-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 207] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 3. | Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975-1026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 857] [Cited by in F6Publishing: 820] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 4. | Triebel F, Hercend T. Subpopulations of human peripheral T gamma delta lymphocytes. Immunol Today. 1989;10:186-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 134] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Kamath AB, Wang L, Das H, Li L, Reinhold VN, Bukowski JF. Antigens in tea-beverage prime human Vgamma 2Vdelta 2 T cells in vitro and in vivo for memory and nonmemory antibacterial cytokine responses. Proc Natl Acad Sci USA. 2003;100:6009-6014. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Li X, Kang N, Zhang X, Dong X, Wei W, Cui L, Ba D, He W. Generation of human regulatory gammadelta T cells by TCRgammadelta stimulation in the presence of TGF-beta and their involvement in the pathogenesis of systemic lupus erythematosus. J Immunol. 2011;186:6693-6700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Harly C, Peyrat MA, Netzer S, Déchanet-Merville J, Bonneville M, Scotet E. Up-regulation of cytolytic functions of human Vδ2-γ T lymphocytes through engagement of ILT2 expressed by tumor target cells. Blood. 2011;117:2864-2873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Kabelitz D, Kalyan S, Oberg HH, Wesch D. Human Vδ2 versus non-Vδ2 γδ T cells in antitumor immunity. Oncoimmunology. 2013;2:e23304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Zhao H, Xi X, Cui L, He W. CDR3δ -grafted γ9δ2T cells mediate effective antitumor reactivity. Cell Mol Immunol. 2012;9:147-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Meraviglia S, Eberl M, Vermijlen D, Todaro M, Buccheri S, Cicero G, La Mendola C, Guggino G, D’Asaro M, Orlando V. In vivo manipulation of Vgamma9Vdelta2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin Exp Immunol. 2010;161:290-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 216] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 11. | Zitt M, DeVries A, Thaler J, Kafka-Ritsch R, Eisterer W, Lukas P, Öfner D. Long-term surveillance of locally advanced rectal cancer patients with neoadjuvant chemoradiation and aggressive surgical treatment of recurrent disease: a consecutive single-centre experience. Int J Colorectal Dis. 2015;30:1705-1714. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Tada N, Kawai K, Tsuno NH, Ishihara S, Yamaguchi H, Sunami E, Kitayama J, Oba K, Watanabe T. Prediction of the preoperative chemoradiotherapy response for rectal cancer by peripheral blood lymphocyte subsets. World J Surg Oncol. 2015;13:30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Däster S, Eppenberger-Castori S, Hirt C, Zlobec I, Delko T, Nebiker CA, Soysal SD, Amicarella F, Iezzi G, Sconocchia G. High frequency of CD8 positive lymphocyte infiltration correlates with lack of lymph node involvement in early rectal cancer. Dis Markers. 2014;2014:792183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Kim E, Hwang JM, Garcia-Aguilar J. Local excision for rectal carcinoma. Clin Colorectal Cancer. 2008;7:376-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Sitzler PJ, Seow-Choen F, Ho YH, Leong AP. Lymph node involvement and tumor depth in rectal cancers: an analysis of 805 patients. Dis Colon Rectum. 1997;40:1472-1476. [PubMed] [Cited in This Article: ] |
| 16. | Taylor RC, Patel A, Panageas KS, Busam KJ, Brady MS. Tumor-infiltrating lymphocytes predict sentinel lymph node positivity in patients with cutaneous melanoma. J Clin Oncol. 2007;25:869-875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 231] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 17. | Lee HE, Chae SW, Lee YJ, Kim MA, Lee HS, Lee BL, Kim WH. Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br J Cancer. 2008;99:1704-1711. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 232] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 18. | Sheu BC, Kuo WH, Chen RJ, Huang SC, Chang KJ, Chow SN. Clinical significance of tumor-infiltrating lymphocytes in neoplastic progression and lymph node metastasis of human breast cancer. Breast. 2008;17:604-610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Moser B, Eberl M. γδ T-APCs: a novel tool for immunotherapy? Cell Mol Life Sci. 2011;68:2443-2452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Bhat J, Kabelitz D. γδ T cells and epigenetic drugs: A useful merger in cancer immunotherapy? Oncoimmunology. 2015;4:e1006088. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Caccamo N, Meraviglia S, Cicero G, Gulotta G, Moschella F, Cordova A, Gulotta E, Salerno A, Dieli F. Aminobisphosphonates as new weapons for gammadelta T Cell-based immunotherapy of cancer. Curr Med Chem. 2008;15:1147-1153. [PubMed] [Cited in This Article: ] |
| 22. | Bryant NL, Gillespie GY, Lopez RD, Markert JM, Cloud GA, Langford CP, Arnouk H, Su Y, Haines HL, Suarez-Cuervo C. Preclinical evaluation of ex vivo expanded/activated γδ T cells for immunotherapy of glioblastoma multiforme. J Neurooncol. 2011;101:179-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Chiplunkar S, Dhar S, Wesch D, Kabelitz D. gammadelta T cells in cancer immunotherapy: current status and future prospects. Immunotherapy. 2009;1:663-678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Gertner-Dardenne J, Fauriat C, Vey N, Olive D. Immunotherapy of acute myeloid leukemia based on γδ T cells. Oncoimmunology. 2012;1:1614-1616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Kang N, Zhou J, Zhang T, Wang L, Lu F, Cui Y, Cui L, He W. Adoptive immunotherapy of lung cancer with immobilized anti-TCRgammadelta antibody-expanded human gammadelta T-cells in peripheral blood. Cancer Biol Ther. 2009;8:1540-1549. [PubMed] [Cited in This Article: ] |
| 26. | Mattarollo SR, Kenna T, Nieda M, Nicol AJ. Chemotherapy and zoledronate sensitize solid tumour cells to Vgamma9Vdelta2 T cell cytotoxicity. Cancer Immunol Immunother. 2007;56:1285-1297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 27. | Mao Y, Yin S, Zhang J, Hu Y, Huang B, Cui L, Kang N, He W. A new effect of IL-4 on human γδ T cells: promoting regulatory Vδ1 T cells via IL-10 production and inhibiting function of Vδ2 T cells. Cell Mol Immunol. 2015;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Peng G, Wang HY, Peng W, Kiniwa Y, Seo KH, Wang RF. Tumor-infiltrating gammadelta T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity. 2007;27:334-348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 302] [Cited by in F6Publishing: 311] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 29. | Hidalgo JV, Bronsert P, Orlowska-Volk M, Díaz LB, Stickeler E, Werner M, Schmitt-Graeff A, Kayser G, Malkovsky M, Fisch P. Histological Analysis of γδ T Lymphocytes Infiltrating Human Triple-Negative Breast Carcinomas. Front Immunol. 2014;5:632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Lo Presti E, Dieli F, Meraviglia S. Tumor-Infiltrating γδ T Lymphocytes: Pathogenic Role, Clinical Significance, and Differential Programing in the Tumor Microenvironment. Front Immunol. 2014;5:607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 31. | Lança T, Silva-Santos B. Recruitment of γδ T lymphocytes to tumors: A new role for the pleiotropic chemokine CCL2. Oncoimmunology. 2013;2:e25461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Donia M, Ellebaek E, Andersen MH, Straten PT, Svane IM. Analysis of Vδ1 T cells in clinical grade melanoma-infiltrating lymphocytes. Oncoimmunology. 2012;1:1297-1304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |