Published online Mar 28, 2016. doi: 10.3748/wjg.v22.i12.3471
Peer-review started: December 22, 2015
First decision: January 13, 2016
Revised: January 30, 2016
Accepted: March 1, 2016
Article in press: March 2, 2016
Published online: March 28, 2016
AIM: To construct a global “metabolic phenotype” of pancreatic ductal adenocarcinoma (PDAC) reflecting tumour-related metabolic enzyme expression.
METHODS: A systematic review of the literature was performed using OvidSP and PubMed databases using keywords “pancreatic cancer” and individual glycolytic and mitochondrial oxidative phosphorylation (MOP) enzymes. Both human and animal studies investigating the oncological effect of enzyme expression changes and inhibitors in both an in vitro and in vivo setting were included in the review. Data reporting changes in enzyme expression and the effects on PDAC cells, such as survival and metastatic potential, were extracted to construct a metabolic phenotype.
RESULTS: Seven hundred and ten papers were initially retrieved, and were screened to meet the review inclusion criteria. 107 unique articles were identified as reporting data involving glycolytic enzymes, and 28 articles involving MOP enzymes in PDAC. Data extraction followed a pre-defined protocol. There is consistent over-expression of glycolytic enzymes and lactate dehydrogenase in keeping with the Warburg effect to facilitate rapid adenosine-triphosphate production from glycolysis. Certain isoforms of these enzymes were over-expressed specifically in PDAC. Altering expression levels of HK, PGI, FBA, enolase, PK-M2 and LDA-A with metabolic inhibitors have shown a favourable effect on PDAC, thus identifying these as potential therapeutic targets. However, the Warburg effect on MOP enzymes is less clear, with different expression levels at different points in the Krebs cycle resulting in a fundamental change of metabolite levels, suggesting that other essential anabolic pathways are being stimulated.
CONCLUSION: Further characterisation of the PDAC metabolic phenotype is necessary as currently there are few clinical studies and no successful clinical trials targeting metabolic enzymes.
Core tip: Our systematic review constructs a global “metabolic phenotype” of pancreatic ductal adenocarcinoma (PDAC) reflecting tumour-related metabolic enzyme expression. We show that the Warburg effect is consistently demonstrated, with the over-expression of glycolytic enzymes and lacate dehydrogenase to facilitate rapid adenosine-triphosphate (ATP) production from glycolysis. We also show that the Warburg effect on mitochrondial oxidative phosphorylation in PDAC is more varied and not solely focused on ATP production, but also to stimulate other anabolic pathways for the purposes of tumourigencity. The metabolic phenotype provides an overview essential to elucidating the pathological changes that occur in PDAC.
- Citation: Chan AK, Bruce JI, Siriwardena AK. Glucose metabolic phenotype of pancreatic cancer. World J Gastroenterol 2016; 22(12): 3471-3485
- URL: https://www.wjgnet.com/1007-9327/full/v22/i12/3471.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i12.3471
Pancreatic cancer (PC), typically ductal adenocarcinoma (PDAC), is the 13th most common cancer[1] contributing to 250000 deaths annually worldwide and accounting for 3.6% of cancer deaths and 0.5% of all deaths[2]. Despite advances in diagnostic technology and treatment modalities, there has been no significant improvement over the last three decades, with five-year survival remaining below 7%. Recent progress in genetics has led to a renewed interest in the Warburg Effect described in 1956 by German physiologist Otto Warburg[3] who postulated that carcinogenesis was the result of “irreversible injuring of respiration”. Eukaryotic cells utilise glycolysis to derive energy, where glucose is broken down over a series of enzymatic steps to produce adenosine-triphosphate (ATP) and pyruvate. In aerobic respiration, pyruvate is oxidised in the Krebs cycle to produce ATP and NADH (nicotinamide adenine dinucleotide hydride). Mitochondrial oxidative phosphorylation (MOP) then occurs via a series of redox reactions to generate more ATP from NADH. Overall, between 30 and 36 ATP are generated from 1 molecule of glucose. In the absence of an adequate oxygen supply, anaerobic fermentation occurs, reducing pyruvate to lactate and converting NADH into NAD+ (nicotinamide adenine dinucleotide) for use in further glycolysis reactions. The energy released per glucose molecule in anaerobic respiration is only 2 ATP; per mole, this is 18-fold less than aerobic respiration but at a much faster rate of several hundred times[4]. The ratio of MOP and anaerobic fermentation is reduced in cancer cells[5-7], such as the Henrietta Lacks (HeLa) cervical cancer cell line where approximately 80% of glucose uptake undergoes glycolysis, and only 5% enters the Krebs cycle[8]. Warburg proposed that this “morphological inferiority” would change highly differentiated cells into undifferentiated cells that can divide, grow and lead to cancer.
Hypoxia is one stress factor in the tumour microenvironment that is thought to lead to this switch[9]. Hypoxia-inducible factor 1 (HIF-1) is an important regulator of cellular oxygen homeostasis[10], but is also up-regulated in many cancers, including pancreatic, gastric, lung, breast and hepatic cancers[11-14]. HIF-1 up-regulates most glycolytic enzymes, including hexokinase II, the first enzyme in the glycolysis pathway[15], and reduces MOP by up-regulating pyruvate dehydrogenase kinase I, responsible for inactivating the pyruvate dehydrogenase complex that subsequently stops pyruvate decarboxylation for entry into the Krebs cycle[16]. HIF-1 also up-regulates other genes including vascular endothelial growth factor (VEGF, a known promoter of tumour angiogenesis[17]) and the glucose transporter protein, Glut-1, facilitating glucose influx[11].
The Warburg Effect is likely a result of mutations in oncogenes and tumour suppressor genes with several pathways contributing to this “metabolic switch”[18]. This study undertakes a systematic literature review of changes in enzyme expression and the resulting metabolite levels in both the glycolytic and MOP pathways in PDAC in order to construct a ‘metabolic phenotype’ of this disease. New potential therapeutic targets can be identified within this phenotype for further study as novel treatments for PDAC.
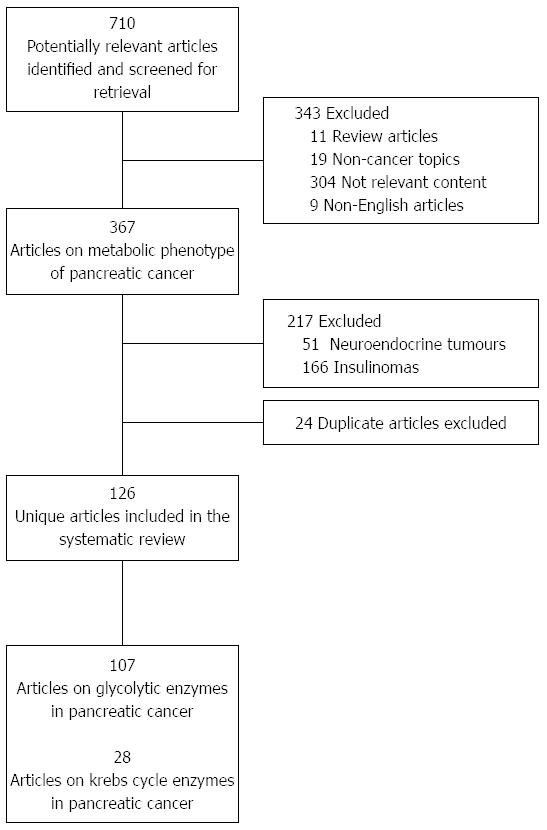
A systematic review of the literature was performed using OvidSP and the PubMed database. Search terms for individual glycolytic enzymes (hexokinase, phosphoglucose isomerase, phosphofructokinase, aldolase, triosephosphate isomerase, glyceraldehyde-3-phosphate dehydrogenase, phosphoglycerate kinase, phosphoglycerate mutase, enolase, pyruvate kinase and lactate dehydrogenase) and Krebs cycle enzymes (pyruvate dehydrogenase, pyruvate carboxylase, citrate synthase, aconitase, isocitrate dehydrogenase, α-ketoglutarate dehydrogenase, succinyl-CoA synthase, succinic dehydrogenase, fumarase and malate dehydrogenase) were combined with key words “PC” and the Boolean “AND” operator (e.g., “Hexokinase and PC”). Human and animal in vivo studies, as well as in vitro studies involving cell lines, were included. The initial search yielded 710 results, and after excluding review articles, non-cancer articles and those with non-relevant content, 367 articles were analysed. A further 217 articles describing pancreatic cancers of histology other than PDAC, such as carcinoid and other neuroendocrine tumours were excluded. Finally, duplicate articles (24) were identified and excluded. One hundred and twenty-six publications were identified as meeting the inclusion criteria of this systematic review looking at the metabolic phenotype of PDAC (Figure 1). Of these, 107 unique articles describe glycolytic enzymes (Table 1) and 28 unique articles describe the MOP pathway (Table 2) in PDAC.
| Glycolytic enzyme | In vitro studies | In vivo/clinical studies | ||
| Ref. | Summary | Ref. | Summary | |
| Hexokinase (HK) | [23-30,32,33,153-155] | Induced by hypoxia. Higher expression in PDAC than acinar cells. Suppresses mitochondrial ATP production | [31,153,156,157] | HK-2 expression suggests an unfavourable clinical outcome |
| Phosphoglucose isomerase (PGI) | [29,38-40,158] | Stimulates cell migration and metastatic potential. Induced by hypoxia. β-1 integrins are stimulated by PGI | [39] | Over-expression contributes to a more aggressive PDAC phenotype |
| Phosphofructokinase (PFK) | [11,48] | Upregulated in PDAC epithelia. | ||
| Aldolase | [49,52,54] | Overexpressed in PDAC. Induced by hypoxia. Delays apoptosis. | [52,53] | Highly expressed in PDAC where HIF-1α is constitutively expressed. Inhibition prolongs survival |
| Triosephosphate isomerase (TPI) | [55,56] | Overexpressed in PDAC | ||
| Glyceradehyde-3-Phosphate Dehydrogenase (G3PD) | [27,50,55,57,58,61,159] | Overexpressed in PDAC. Increases PDAC metabolic activity, and disrupts downstream apoptotic capases | [50,58] | Increased expression. Possible biomarker candidate. |
| Phosphoglycerate kinase (PK) | [62-66,160] | Overexpressed in PDAC. Angiogenesis promoter | [63] | No significant increase in expression in a murine model, but fivefold increase in activity. |
| Phosphoglycerate mutase (PGM) | [67,68] | M-isoform is under-expressed and B-isoform is over-expressed in murine models | ||
| Enolase | [55-58,69-83] | Overexpressed in PDAC. Promotes cell migration and metastasis. Biomarker candidate | [58,69,76,77,161-163] | Increased expression. Induced antibodies to enolase-alpha correlates to a better outcome |
| Pyruvate kinase (PK) | [108,109,117,121,122,157,164-166] | Overexpressed in PDAC. Dimeric form favours synthesis; Tetrameric form favours energy production; PK-M2 used as a tumour marker | [110,112,115,119,120,157,167,168] | M2 isoform levels correlate to tumour metastasis. HK-2 and M2 expression indicates an unfavourable clinical outcome |
| Lactate dehydrogenase (LDH) | [28,89,123-138,164,169-179] | Overexpressed in PDAC. Down regulated by graviola | [153,180-183] | Down regulated by graviola to reduce tumorigenicity and metastasis |
| Glycolytic Enzyme | In vitro/In vivo/clinical studies | |
| Ref. | Summary | |
| Pyruvate dehydrogenase | [30,109,131,132,140,154,164,170,171,173,177,179,180,184-188] | Inhibition of pyruvate dehydrogenase kinase (the activity of which is regulated by pyruvate dehydrogenase) stimulates the Krebs cycle and reverses the Warburg effect. |
| Pyruvate carboxylase | [141] | Over expressed in human and murine PDAC |
| Citrate synthase | [67,124,179] | Over expressed in PDAC. Activity of citrate synthase also higher in PDAC compared to normal tissue |
| Aconitase | [67] | Mitochrondrial isoform under-expressed |
| Isocitrate dehydrogenase | [67,158,189,190] | Regulated by HuR (an RNA-binding protein) in PDAC. |
| a-ketoglutarate dehydrogenase | [67,187] | Marginally increased in murine PDAC |
| Succinyl-CoA synthetase | [67] | Reduced expression |
| Succinic dehydrogenase | [67,128] | Over expressed in human and murine PDAC |
| Fumarase | [30] | HIF-1α increases fumarate by inhibiting distal mitochondrial metabolisms |
| Malate dehydrogenase | [50,64,67] | Over expressed in human and murine PDAC. Possible candidate biomarker |
Studies describing the biochemical mechanisms involved in the physiological, onco-pathological or manipulation of metabolic pathways and/or individual metabolic enzymes in PDAC were included. For quality assurance, information was extracted following a predefined protocol from the text of each article, including changes in expression of metabolic enzymes and/or in the metabolic pathways and their overall effect on normal and PDAC phenotype. The effect on cell viability and growth was also noted, particularly if a metabolic inhibitor was used, as was evidence implicating known oncogenic pathways. The data were collated (Tables 1 and 2) using Microsoft Excel (Microsoft, Richmond, United States) for analysis. A PDAC “metabolic phenotype” was constructed using these data (Tables 3-5), and the results presented as the hexose and triose stage of glycolysis, anaerobic fermentation and aerobic respiration (Krebs Cycle).
| Glycolytic enzyme | Encoding gene | Change in PDAC | Implicated pathways | Known inhibitors | Inhibitor effect in PDAC |
| Hexokinase | HIF | 2-Deoxy-D-glucose | |||
| Hexokinase I | 10q22 | Up | mTor | ||
| Hexokinase II | 2p13 | Up | K-ras | 3-BP[21]; Lonidamine, Everolimus | Reduced PDAC survival/induces PDAC necrosis |
| Hexokinase III | 5q35.2 | - | Akt | ||
| Glucokinase | 7p15.3-p15.1 | ||||
| Phosphoglucose Isomerase (also known as Autocrine Motility Factor) | 19q13.1 | Up | HIF; Apoptosis | Insulin-like growth factor binding protein-3; Herceptin; 3-(5'-hydroxymethyl-2'-furyl)-1-benzyl indazole | Reduces overall cell viability and increases apoptosis rates |
| Phosphofructokinase | HIF | Aurintricarboxylic acid[191] | Inhibits fatty acid synthesis in rat hepatocytes | ||
| PFK-M (muscle type) | 12q13.3 | Down | |||
| PFK-L (liver type) | 21q22.3 | Up | |||
| PFK-P (platelet type) | 10p15.3-p15.2 | Up | |||
| Aldolase | HIF | 3-fluro-D-glucose; 4-fluro-D-glucose | |||
| Aldolase-A | 16p11.2 | Up | 3-[2-hydroxyethyl(methyl)amino]-2-quinoxalinecarbonitrile 1,4-dioxide | Reduces PDAC proliferation and tumour volume | |
| Aldolase-B | 5q22 | Down | |||
| Aldolase-C | 17cen-q12 | Up | |||
| Triose Phosphate Isomerase | 12p13 | Up | 2-phosphoglycolate; D-glycerol-1-phosphate[192] | ||
| Glyceraldehyde Phosphate Dehydrogenase | HIF; p53 | Iodoacetate[27]; gossypol[21] | Reduces cell survival and induces necrosis. No effect on K-ras | ||
| GAPDHS | 12p13 | Up | |||
| GAPDHS (testes-specific) | 19q13.12 | ||||
| Phosphoglycerate Kinase | HIF | 1,3-bisphosphoglycerate[193] | |||
| PGK1 | Xq13.3 | Up | |||
| PGK2 | 6p12.3 | ? | |||
| Phosphoglycerate Mutase | p53 | Inositol hexakisphosphate[194] | |||
| PGM-B | 1p31 | Up | |||
| PGM-M | 4p14 | Down | |||
| Enolase | c-Myc | Sodium fluoride[21]; D-tartonate; 3-aminoenolpyruvate 2-phosphate[195] | |||
| ENO1 (alpha) | 1p36.2 | Up | |||
| ENO2 (gamma, neuronal) | 12.p13 | Up | |||
| ENO3 (beta, muscle) | 17pter-p11 | - | |||
| Pyruvate Kinase | Tyrosine kinase | ||||
| Isoform PK-M1 (liver/RBC) | 1q21 | - | Akt/c-Myc | ||
| Isoform PK-M2 (muscle) | 15q22 | Up | L-phospholactate; M2-PK-binding peptide aptamers[196] | Anti-cancer effects in animal models | |
| Dimeric form | |||||
| Tetrameric form |
| Glycolytic enzyme | Encoding gene | Change in PDAC | Implicated pathways | Known inhibitors | Inhibitor effect in PDAC |
| Lactate dehydrogenase | Oxamate | Inhibits PDAC growth | |||
| Lactate dehydrogenase A | 11p15.4 | Up | c-Myc; mTor | Aryl-substituted N-hydroxyindole-2-carboxylates; Everolimus (by blocking the mTor pathway[28]) | Inhibits PDAC growth |
| Lactate dehydrogenase B | 1p12.2-p12.1 | Up | |||
| Lactate dehydrogenase C | 11p15.1 | - |
| Glycolytic Enzyme | Encoding gene | Change in PDAC | Implicated pathways | Known inhibitors | Inhibitor effect in PDAC |
| Pyruvate dehydrogenase complex | 11p13 | HIF-1 | Dichloroacetate (inhibits PDC regulator, pyruvate dehydrogenase kinase) | Stimulates Krebs Cycle and reverses Warburg effect; Reduces PDAC proliferation and viability | |
| Pyruvate dehydrogenase | Stimulator: Lipoic acid | Reduces cancer cell viability | |||
| Pyruvate dehydrogenase α | Xp22.1 | Down | |||
| Pyruvate dehydrogenase β | 3p21.1-p14.2 | Down | |||
| Pyruvate carboxylase | 11q13.4-q13.5 | Up/down1 | Avidin | ||
| Citrate synthase | 12q13.2 | Up | Succinyl-CoA | ||
| Aconitase | Flouroacetate | ||||
| Aconitase 1 (soluble) | 9p21.1 | ||||
| Aconitase 2 (mitochondrial) | 22q13.2 | Down | |||
| Isocitrate dehydrogenase | |||||
| ICD (soluble) | 2q33.3 | ||||
| ICD (mitochondrial) | 15q26.1 | Down | |||
| Oxoglurate (α-ketoglutarate) Dehydrogenase | 7p14-p13 | Up | |||
| Succinyl-CoA synthetase | Down | ||||
| Succinic dehydrogenase | Up/down1 | ||||
| Fumarase | 1q42.1 | D-malic, trans-aconitic, citrate, glycine | |||
| Malate dehydrogenase | |||||
| MD (soluble) | 2p13.3 | ||||
| MD (mitochondrial) | 7cen-q22 | Up |
Hexokinase: Hexokinase (HK) is the first enzyme in glycolysis, and exists as 4 isoforms (HKI - III, and glucokinase). It phosphorylates glucose into glucose-6-phosphate (G6P), and represents the rate-limiting step in glycolysis[19]. G6P is transported into mitochondria and immediately used for ATP production via glycolysis, or for nucleic acid synthesis via the pentose-phosphate shunt[20]. Three HK isoforms (I, II and III) are competitively inhibited by 2-Deoxy-D-glucose, whereas 3-bromopyruvate (3-BP) selectively inhibits HK-II[21].
Several studies have shown HK to be over-expressed in PDAC[22,23] with expression levels varying by tumour histology. Ductal tumours, for example, take up more glucose and express HK-I and II more than acinar variants[24]. Direct inhibition with 3-BP reduces cell survival and increase necrosis in PDAC[25,26] and Panc-1 cell lines[27]. Treatment of Panc-1 with 3-BP also affects cell signalling, significantly reducing the expression of the GTPase signal transduction KRas (Kirsten rat sarcoma) pathway, as well as the Akt (protein kinase B) and mTOR (mammalian target of rapamycin) pathways, to induce cell necrosis[27]. Inhibiting the mTOR pathway in Panc-1 using everolimus reduces the expression of HK-II and glycolysis, which inhibits cell proliferation and induces apoptosis[28].
The up-regulation of HK is partly due to the HIF-1 pathway in hypoxic conditions[29]. An increase in extracellular glucose is responsible for increasing HIF-1 expression (and subsequently intracellular ATP) whilst inhibiting mitochondrial activity in MiaPaCa2 cells[30]. An interaction between insulin and HIF-1 has also been suggested[31], with type 2 diabetes mellitus associated with the development of PDAC in patients who have a particular HK II (genotype R844K) or glucokinase (ICS1+9652C>T) variant[32,33]. Clinically, a high level of HK-II expression is associated with longer survival, with different variants of HK II (such as N692N) associated with differing clinical outcomes[33].
Phosphoglucose isomerase: Phosphoglucose isomerase (PGI) reversibly catalyses glucose-6-phosphate to fructose-6-phosphate. Previously identified as the autocrine motility factor (AMF)[34], PGI binds to the gp78/AMFR receptor[35] to stimulate cell migration and metastasis[36]. Under hypoxic conditions, PGI expression is regulated in part by the HIF pathway[29,37] and has been found to be over-expressed in the Capan-2 cell line. PGI increases the metastatic potential of PDAC[38] and MiaPaCa-2 cells transfected with PGI grow more aggressively with an increase in tumour mass[39]. Down-regulation of E-cadherin expression - a protein involved in cell adhesion - also occurs. HIF-1 expression in PDAC can be inhibited by 3-(5’-hydroxymethyl-2’-furyl)-1-benzyl indazole, which subsequently reduces PGI mRNA expression; this has the effect of reducing overall cell viability and increasing apoptosis rates[40,41]. Herceptin has been shown to inhibit the expression of PGI and potentiate the effects of other PGI inhibitors[42]. Beta-1 integrins (receptors that facilitate binding between neighbouring cells) are stimulated by PGI, up-regulating cell adhesion, invasion and metastasis possibly by a signalling pathway involving protein kinase C[43]. Beta-1 integrins have been shown to be highly expressed in several PDAC cell lines[44] which may explain its high metastatic potential. PGI has also been shown to regulate the expression of apoptotic protease activating factor 1 (Apaf-1) and caspase-9 genes involved in apoptosis[45].
Phosphofructokinase: Phosphofructokinase (PFK) phosphorylates fructose-6-phosphate into fructose 1,6-bisphosphate[19]. It has been referred to as the “pacemaker of carbohydrate metabolism” as it also regulates a wide range of sugars, including fructose and galactose that can feed into glycolysis[21]. The activity of PFK is regulated by fructose-2,6-bisphosphate, which itself is regulated by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatases, encoded by 4 genes (PFKFB1-4)[46]. PFKFB expression (particularly isoenzyme 3 which exhibits the highest kinase/bisphosphatase activity) is altered in lung, gastric and pancreatic cancers[11,47,48]. Hypoxia also up-regulates the expression of PFKFB-3 and -4 in Panc-1 cells via the HIF-1α dependent pathway[11].
Fructose bisphosphate aldolase: Fructose bisphosphate aldolase (FBA) splits fructose 1,6-biphosphate into glyceraldehyde-3-phosphate (G3P) and dihydroxyacetone phosphate. Three different isoenzymes exist (A, B and C) and are encoded by 3 different genes[21]. FBA-A is overexpressed in PDAC and pancreatic cystadenoma[49], and proteome analysis of murine PDAC has also shown an overexpression of FBA-C[50]. Overexpression of FBA delays apoptosis, as does the addition of the end product G3P, by suppressing caspase-3 activity[51]. Under hypoxic conditions, PDAC cells expressing HIF-1 also express FBA-A and Glut-1 (glucose transporter 1) more highly, making the cell more resistant to apoptosis[52]. The hypoxic cytotoxin 3-[2-hydroxyethyl(methyl)amino]-2-quinoxalinecarbonitrile 1,4-dioxide inhibits the expression of FBA-A together with HIF-1, which subsequently reduces the proliferation of PDAC cells in vivo and reduced tumour volumes in models of in vitro murine cancer[53]. Suppression of HIF-1 also reduces the overexpression of FBA-A in PDAC and subsequently reduces in vivo tumorigenicity[54].
Triose phosphate isomerase: Triose phosphate isomerase (TPI) reversibly isomerises dihydroxyacetone into G3P. TPI is overexpressed in pancreatic cancer in several studies[55,56], but no correlation, however, is found between TPI-1 expression and tumour staging.
Glyceraldehyde phosphate dehydrogenase: Glyceraldehyde phosphate dehydrogenase (GAPDH) reversibly catalyses G3P into 1,3-biphosphoglycerate, and is over-expressed in PDAC[50,55,57,58], as well as other adenocarcinomas such as prostate[59] and breast[60]. GAPDH and Glut transporter over-expression is thought to be partially responsible for an increase in PDAC metabolic capacity[61]. Inhibition with iodoacetate on Panc-1 cells reduces survival and induces necrosis[27] but interestingly, did not significantly reduce the induction of signalling pathways K-ras, Akt and mTOR, as seen with 3-BP.
Phosphoglycerate kinase: Phosphoglycerate kinase (PGK) reversibly dephosphorylates 1,3-biphosphoglycerate into 3-phosphoglycerate, and is over-expressed in human PDAC[62]. In contrast, a study involving hamster PDAC cell lines demonstrated no overall increase in expression levels, but did show a significant fivefold increase in PGK activity[63]. Clinically, its use as a diagnostic marker has been suggested due to its high antibody reactivity and high accuracy in distinguishing between cancer and non-cancer[64-66].
Phosphoglycerate mutase (PGM) reversibly catalyses 3-phosphoglycerate into 2-phosphoglycerate, and exists as two isoenzymes (M and B). Expression levels of PGM-M have been found to be under-expressed, and PGM-B over-expressed, in rat PDAC[67]. PGM expression is regulated by p53[68].
Enolase reversibly catalyses 2-phosphoglycerate into phosphoenolpyuvate, and is over-expressed in PDAC, particularly α-enolase[55-58,69-72]. It is also seen in pancreatic atypical hyperplasia[73] and intraepithelial neoplasia[74]. Its presence on the PDAC cell surface allows the promotion of cell migration and metastasis, but also induces a strong T-cell response leading it to be recognised as a tumour antigen[69,75,76]. As such, an increase in circulating enolase autoantibodies has been observed[76]. Animal studies have also shown good humoral and cellular immune responses against PDAC following inoculation with an α-enolase-encoded plasmid[77]. The resulting IgG response resulted in slower tumour progression and significantly improved survival. Several case reports have also clearly shown an over-expression of γ-enolase in serous microcystic adenomas[78,79], and solid-papillary[80], solid-pseudopapillary[81-86], solid-cystic papillary[87-102], and serous cystic[103,104] pancreatic tumours as well as PDAC[71].
Pyruvate kinase (PK) is the last enzymatic step in glycolysis, dephosphorylating phosphoenolpyruvate into pyruvate and converting ADP to ATP. Pyruvate then fuels the Krebs cycle. PK exists as 2 isoforms (M1 and M2); PK-M2 expressed exclusively in cancer cells (favouring anaerobic respiration) whereas the PK-M1 is predominately expressed in normal cells (favouring increased oxidative phosphorylation)[50,105,106]. Expression of the oncogenic tyrosine kinase receptor pathways inactivate PK to disrupt the pathway between glycolysis and MOP to perpetuate the Warburg effect[107]. Furthermore, two forms of PK-M2 have been identified - a tetrameric form favouring ATP production and a dimeric form that channels glucose into synthesis. The hypoxic and acidified tumour microenvironment staved of glucose favours the dimeric form of PK-M2[108]. Inhibition of the Akt/c-Myc (myelocytomatosis) pathway inhibits the activity of PK-M2 to down-regulate glycolysis[109]. TLN-232/CAP-232 (amino acid peptides targeting M2-PK) has also been shown to have anti-cancer effects in animal models, and clinical trials are underway to ascertain its effectiveness in pancreatic cancer[21].
Several studies have shown an increase in serum PK-M2 in patients with metastatic PDAC[110], and other pancreatic conditions such as chronic pancreatitis[111]. Its presence has also led suggestions that it should be used as a tumour marker for diagnosis, prognosis[112] and surveillance[113,114] in both pancreatic and other gastrointestinal cancers[115-117], particularly in combination with another marker, such as Ca19-9[118] or CEA[119], to increase its specificity value[120]. Surprisingly, PK-M2 over-expression is not found immunohistochemically in premalignant or PDAC tissue samples[64,121]. It is also weakly expressed in some cell lines such as SK-PC-1[122].
Lactate dehydrogenase (LDH) reversibly catalyses pyruvate to lactate, and is a target gene of the c-Myc regulator[123]. There are five isoforms, with LDH-A being the primary and over-expressed isoform in PDAC[124-128], including cell lines Capan-1[129] and SW-1990[130]. Mass spectrometry studies on LDH from PDAC have shown differential methylation to the LDH from normal ductal cells[131]. PDAC LDH-A acetylation, which normally inhibits LDH-A and prepares it for lysosomal degradation is also reduced[132]. Forced expression and inhibition of LDH-A increases and reduces the rate of growth respectively[133]. The activity of LDH-A and subsequent lactate production can be inhibited by blocking the mTor pathway using everolimus. The transcription factor Forkhead box protein M1 (FOXM1) over-expresses LDH-A to increase tumorigenicity[134].
Other inhibitors of LDH-A, such as derivatives of aryl-substituted N-hydroxyindole-2-carboxylates, have also been shown to inhibit the growth of PDAC[135]. Oxidative stress and a subsequent reduction in ATP may be responsible for this inhibition[136]. Lactate in the PDAC microenvironment has also been shown to be immunosuppressive by directly inhibiting Natural Killer cells and preventing an innate response to tumour cells[137]. Clinically, a raised serum LDH levels can also be a poor prognostic indicator in PDAC[138].
The pyruvate dehydrogenase complex (PDC) consists of pyruvate dehydrogenase (PDH), dihydrolipoyl transacetylase and dihydrolipoyl dehydrogenase, and is the first enzymatic reaction that converts pyruvate into acetyl Co-A for the Krebs cycle. PDC itself is regulated and inhibited by pyruvate dehydrogenase kinase (PDK). Three isoenzymatic forms of PDK have been found in eukaryotic cells, with PDK2 being the major form that regulates PDC[139]. Inhibition of PDK by dichloroacetate in Panc-1 cells has been shown to stimulate metabolism via the Krebs cycle and away from glycolysis, with the effect of reducing Panc-1 proliferation and viability[140].
Expression levels of other Krebs cycle enzymes are also changed in PDAC (Table 5). Malate dehydrogenase has been shown to be over-expressed in both human[64] and animal PDAC[50] cell lines, as has succinic dehydrogenase in mice PDAC[128], and pyruvate carboxylase[141]. The activity of citrate synthase was found to be higher in PDAC tissue than in normal pancreatic tissue by up to 20%[67]. The expression levels of mitochondrial citrate synthase and α-ketoglutarate dehydrogenase were found to be marginally increased in recent rat PDAC mRNA microarray studies by Yabushita et al[67]; pyruvate carboxylase, aconitase, isocitrate dehydrogenase, succinic dehydrogenase, succinic-CoA ligase and malate dehydrogenase expression were found to be reduced. Transcriptomic profiling from the same dataset also showed an increase in anaerobic glycolysis and nucleotide degradation and a reduction in Krebs cycle activity.
There is evidence that the mitochondrial oxidative phosphorylation (MOP) pathway can be used as a therapeutic target[142,143]. In glucose-limiting conditions, MOP inhibitors have been shown to be cytotoxic to Panc-1 cells. Momose et al[144] report that treatment with efrapeptin F (a fungal toxin that acts as a potent ATPase inhibitor) was cytotoxic to Panc-1 cells if they were cultured in nutrient-deficient media and, importantly, in glucose limiting conditions. Panc-1 cells grown in nutrient-deficient media were found to have reduced levels of ATP, and were sensitive to MOP inhibitors, suggesting that mitochondrial oxidative phosphorylation contributes to intracellular ATP production.
This detailed overview demonstrates that the metabolic phenotype of pancreatic cells is altered in PDAC where the Warburg effect increases glucose utilisation to fuel the pathological metabolic requirements of neoplastic cells. Enzymes at almost every stage of glycolysis are over-expressed. The rate at which glucose is transported into cells is also increased, and it has long been known that an over-expression of glucose transporters (particularly subtypes Glut-1 and Glut-3 in PDAC[145]) is associated with cancer progression and poor tumour prognosis[145-148]. Glut-1 expression in pancreas neoplasia correlates to tumour size and histological grading, from low grade PanIN 1A dysplastic lesions which are devoid of Glut-1, to PDAC where most cases showed some degree of GLUT-1 expression[149]. Serous cystadenomas consistently exhibit Glut-1 expression[150]. With such differences in expression between normal and cancerous tissue, the Glut family represents a potential therapeutic target. 2-Deoxyglucose, a metabolic inhibitor of Glut, hexokinase and phosphoglucose isomerase, inhibits growth by as much as 59% in Panc-1 cells and over 95% in MIAPaCa-2 after 2 d of treatment[151]. A similar but less profound effect was seen when the cells were treated with oxamate, a competitive metabolic inhibitor of lactate dehydrogenase at the final step of glycolysis. The reduction of cell proliferation is more profound under hypoxic conditions, where Glut-1 expression levels were increased and were more sensitive to inhibition.
The enzyme changes in metabolic phenotype of PDAC is complex, with variable changes in expression levels. Altering expression levels of HK, PGI, FBA, enolase, PK-M2 and LDA-A with metabolic inhibitors have shown a favourable effect on PDAC, thus identifying these as potential therapeutic targets.
To date, there are no clinical trials involving metabolic inhibitors and PDAC. There is, however, progress in using metabolic inhibitors in cell types other than PDAC which show a translation to in vivo treatment. Ko et al[148] have reported the use of 3-BP as an anticancer drug tested on the highly glycolytic hepatocellular cancer cell line AS-30D, where it was shown to rapidly and completely deplete intracellular ATP levels whilst not affecting levels in normal hepatocytes. Cell viability was ultimately affected, dropping to 10% with the ATP depletion. The in vivo effects of 3-BP in an animal model confirmed this anti-cancer effect. More recently, Ko et al[152] published a case study of a 16 year old male with primary hepatocellular cancer treated with 3-BP. The patient responded to treatment with tumour destruction (confirmed by computed tomography) and went on to survive 2 years. Clearly, the use of metabolic inhibitors as a treatment for cancer is at an early stage. By “blocking” glycolysis, there will be a global and unpredictable effect on all cellular functions, and present as an obstacle when translating in vitro cell line research into clinical practice. By using a metabolic phenotype to identify pathologically over-expressed enzymes, a more targeting approach can differentiate a patient’s normal enzymes to that of PDAC.
In summary, the Warburg Effect has been long been recognised to occur in cancer cells, and describes a “metabolic switch” in the way cells use glucose to produce ATP. This overview highlights the extensive changes that in PDAC to produce a distinct metabolic phenotype. This phenotype has potential clinical correlates in that distinct components may be amenable to therapeutic manipulation.
Pancreatic cancer, typically ductal adenocarcinoma (PDAC), is an insidious cancer with poor outcomes that has seen no significant improvement over the last three decades. Recent progress in genetics has lead to a renewed interest in the Warburg effect, which describes the pathological switch from mitochondrial phosphorylation to glycolysis for rapid ATP production, thus presenting new potential therapeutic targets. The primary aim of this review is to provide a comprehensive overview of the metabolic phenotype of PDAC, particularly where metabolic inhibitors have shown a favourable response. The second aim is to identify steps in the metabolic pathways where there is little or no research evidence to show its oncological importance.
The Warburg effect was described in 1956 by German physiologist Otto Warburg who postulated that carcinogenesis was the result of “irreversible injuring of respiration”. Data from high throughput techniques, such as genomic and metabolomic studies, have shown the Warburg effect is much more complex and extends to biomass synthesis for tumour proliferation.
There have been numerous studies looking at individual metabolic enzymes and the effect of inhibiting these enzymes on tumour cell physiology. Retrieved manuscripts concerning the metabolic enzyme and their role in PDAC were reviewed by the authors, and the data extracted to construct an overall “metabolic phenotype”.
The metabolic phenotype of PDAC illustrates the role of individual enzymes, and the known effects on PDAC. Inhibiting certain enzymes - hexokinase, phosphoglucose isomerase, aldolase, pyruvate kinase and lactate dehydrogenase - has been shown to interfere with PDAC cell function. The review also highlights areas (and thus unidentified therapeutic targets) in the pathways, particularly in mitochondrial oxidative phosphorylation, where there is little research into the effects of metabolic inhibition.
The Warburg effect describes the pathological switch from mitochondrial phosphorylation to glycolysis for rapid ATP production. The change in enzyme expression at certain points of these pathways facilitating the Warburg effect may be potentially exploitable as a therapeutic target.
Simply “blocking” major pathways such as glycolysis in an in vitro cell line environment may yield successful in reducing cell growth or tumourigenicity, but would also have a non-specific effect on normal cells leading to as yet unknown toxic effects. This presents a challenge of using such broad inhibitors in an in vivo or clinical setting. A detailed metabolic phenotype is therefore useful in identifying and targeting specific oncological changes and so would theoretically have less effect on normal cells.
P- Reviewer: Chen RF, Kang CM, Korkeila E, Nagaya M, Nakano H S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Boyle P, Levin B. World Cancer Report 2008. Lyon: IARC Press 2009; . [Cited in This Article: ] |
| 2. | WHO. The global burden of disease: 2004 update [Internet]. 2008 [cited 2016-01-28]. Available from: http://www.who.int/healthinfo/global_burden_disease/2004_report_update/en/. [Cited in This Article: ] |
| 3. | Warburg O. On the origin of cancer cells. Science. 1956;123:309-314. [PubMed] [Cited in This Article: ] |
| 4. | Bartrons R, Caro J. Hypoxia, glucose metabolism and the Warburg’s effect. J Bioenerg Biomembr. 2007;39:223-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 5. | Balinsky D, Cayanis E, Geddes EW, Bersohn I. Activities and isoenzyme patterns of some enzymes of glucose metabolism in human primary malignant hepatoma. Cancer Res. 1973;33:249-255. [PubMed] [Cited in This Article: ] |
| 6. | Hammond KD, Balinsky D. Isozyme studies of several enzymes of carbohydrate metabolism in human adult and fetal tissues, tumor tissues, and cell cultures. Cancer Res. 1978;38:1323-1328. [PubMed] [Cited in This Article: ] |
| 7. | Taketa K, Shimamura J, Ueda M, Shimada Y, Kosaka K. Profiles of carbohydrate-metabolizing enzymes in human hepatocellular carcinomas and preneoplastic livers. Cancer Res. 1988;48:467-474. [PubMed] [Cited in This Article: ] |
| 8. | Reitzer LJ, Wice BM, Kennell D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J Biol Chem. 1979;254:2669-2676. [PubMed] [Cited in This Article: ] |
| 9. | Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer. 2008;8:705-713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1203] [Cited by in F6Publishing: 1242] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 10. | Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149-162. [PubMed] [Cited in This Article: ] |
| 11. | Bobarykina AY, Minchenko DO, Opentanova IL, Moenner M, Caro J, Esumi H, Minchenko OH. Hypoxic regulation of PFKFB-3 and PFKFB-4 gene expression in gastric and pancreatic cancer cell lines and expression of PFKFB genes in gastric cancers. Acta Biochim Pol. 2006;53:789-799. [PubMed] [Cited in This Article: ] |
| 12. | Riddle SR, Ahmad A, Ahmad S, Deeb SS, Malkki M, Schneider BK, Allen CB, White CW. Hypoxia induces hexokinase II gene expression in human lung cell line A549. Am J Physiol Lung Cell Mol Physiol. 2000;278:L407-L416. [PubMed] [Cited in This Article: ] |
| 13. | Yasuda S, Arii S, Mori A, Isobe N, Yang W, Oe H, Fujimoto A, Yonenaga Y, Sakashita H, Imamura M. Hexokinase II and VEGF expression in liver tumors: correlation with hypoxia-inducible factor 1 alpha and its significance. J Hepatol. 2004;40:117-123. [PubMed] [Cited in This Article: ] |
| 14. | Isidoro A, Casado E, Redondo A, Acebo P, Espinosa E, Alonso AM, Cejas P, Hardisson D, Fresno Vara JA, Belda-Iniesta C. Breast carcinomas fulfill the Warburg hypothesis and provide metabolic markers of cancer prognosis. Carcinogenesis. 2005;26:2095-2104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Kim JW, Gao P, Liu YC, Semenza GL, Dang CV. Hypoxia-inducible factor 1 and dysregulated c-Myc cooperatively induce vascular endothelial growth factor and metabolic switches hexokinase 2 and pyruvate dehydrogenase kinase 1. Mol Cell Biol. 2007;27:7381-7393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 432] [Cited by in F6Publishing: 475] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 16. | Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1567] [Cited by in F6Publishing: 1608] [Article Influence: 89.3] [Reference Citation Analysis (0)] |
| 17. | Shinkaruk S, Bayle M, Laïn G, Déléris G. Vascular endothelial cell growth factor (VEGF), an emerging target for cancer chemotherapy. Curr Med Chem Anticancer Agents. 2003;3:95-117. [PubMed] [Cited in This Article: ] |
| 18. | Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science. 2010;330:1340-1344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 876] [Cited by in F6Publishing: 905] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 19. | Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633-4646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 997] [Cited by in F6Publishing: 1018] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 20. | Deer EL, González-Hernández J, Coursen JD, Shea JE, Ngatia J, Scaife CL, Firpo MA, Mulvihill SJ. Phenotype and genotype of pancreatic cancer cell lines. Pancreas. 2010;39:425-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 618] [Cited by in F6Publishing: 665] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 21. | Scatena R, Bottoni P, Pontoglio A, Mastrototaro L, Giardina B. Glycolytic enzyme inhibitors in cancer treatment. Expert Opin Investig Drugs. 2008;17:1533-1545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 22. | von Forstner C, Egberts JH, Ammerpohl O, Niedzielska D, Buchert R, Mikecz P, Schumacher U, Peldschus K, Adam G, Pilarsky C. Gene expression patterns and tumor uptake of 18F-FDG, 18F-FLT, and 18F-FEC in PET/MRI of an orthotopic mouse xenotransplantation model of pancreatic cancer. J Nucl Med. 2008;49:1362-1370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Natsuizaka M, Ozasa M, Darmanin S, Miyamoto M, Kondo S, Kamada S, Shindoh M, Higashino F, Suhara W, Koide H. Synergistic up-regulation of Hexokinase-2, glucose transporters and angiogenic factors in pancreatic cancer cells by glucose deprivation and hypoxia. Exp Cell Res. 2007;313:3337-3348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Abasolo I, Pujal J, Rabanal RM, Serafin A, Navarro P, Millán O, Real FX. FDG PET imaging of Ela1-myc mice reveals major biological differences between pancreatic acinar and ductal tumours. Eur J Nucl Med Mol Imaging. 2009;36:1156-1166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Cao X, Bloomston M, Zhang T, Frankel WL, Jia G, Wang B, Hall NC, Koch RM, Cheng H, Knopp MV. Synergistic antipancreatic tumor effect by simultaneously targeting hypoxic cancer cells with HSP90 inhibitor and glycolysis inhibitor. Clin Cancer Res. 2008;14:1831-1839. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Cao X, Jia G, Zhang T, Yang M, Wang B, Wassenaar PA, Cheng H, Knopp MV, Sun D. Non-invasive MRI tumor imaging and synergistic anticancer effect of HSP90 inhibitor and glycolysis inhibitor in RIP1-Tag2 transgenic pancreatic tumor model. Cancer Chemother Pharmacol. 2008;62:985-994. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Bhardwaj V, Rizvi N, Lai MB, Lai JC, Bhushan A. Glycolytic enzyme inhibitors affect pancreatic cancer survival by modulating its signaling and energetics. Anticancer Res. 2010;30:743-749. [PubMed] [Cited in This Article: ] |
| 28. | Liu L, Gong L, Zhang Y, Li N. Glycolysis in Panc-1 human pancreatic cancer cells is inhibited by everolimus. Exp Ther Med. 2013;5:338-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Yoon DY, Buchler P, Saarikoski ST, Hines OJ, Reber HA, Hankinson O. Identification of genes differentially induced by hypoxia in pancreatic cancer cells. Biochem Biophys Res Commun. 2001;288:882-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Liu Z, Jia X, Duan Y, Xiao H, Sundqvist KG, Permert J, Wang F. Excess glucose induces hypoxia-inducible factor-1α in pancreatic cancer cells and stimulates glucose metabolism and cell migration. Cancer Biol Ther. 2013;14:428-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Wang F, Li SS, Segersvärd R, Strömmer L, Sundqvist KG, Holgersson J, Permert J. Hypoxia inducible factor-1 mediates effects of insulin on pancreatic cancer cells and disturbs host energy homeostasis. Am J Pathol. 2007;170:469-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Dong X, Li Y, Chang P, Tang H, Hess KR, Abbruzzese JL, Li D. Glucose metabolism gene variants modulate the risk of pancreatic cancer. Cancer Prev Res (Phila). 2011;4:758-766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Dong X, Tang H, Hess KR, Abbruzzese JL, Li D. Glucose metabolism gene polymorphisms and clinical outcome in pancreatic cancer. Cancer. 2011;117:480-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Watanabe H, Takehana K, Date M, Shinozaki T, Raz A. Tumor cell autocrine motility factor is the neuroleukin/phosphohexose isomerase polypeptide. Cancer Res. 1996;56:2960-2963. [PubMed] [Cited in This Article: ] |
| 35. | Fairbank M, St-Pierre P, Nabi IR. The complex biology of autocrine motility factor/phosphoglucose isomerase (AMF/PGI) and its receptor, the gp78/AMFR E3 ubiquitin ligase. Mol Biosyst. 2009;5:793-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Liotta LA, Mandler R, Murano G, Katz DA, Gordon RK, Chiang PK, Schiffmann E. Tumor cell autocrine motility factor. Proc Natl Acad Sci USA. 1986;83:3302-3306. [PubMed] [Cited in This Article: ] |
| 37. | Funasaka T, Yanagawa T, Hogan V, Raz A. Regulation of phosphoglucose isomerase/autocrine motility factor expression by hypoxia. FASEB J. 2005;19:1422-1430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 38. | Niizeki H, Kobayashi M, Horiuchi I, Akakura N, Chen J, Wang J, Hamada JI, Seth P, Katoh H, Watanabe H. Hypoxia enhances the expression of autocrine motility factor and the motility of human pancreatic cancer cells. Br J Cancer. 2002;86:1914-1919. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Tsutsumi S, Yanagawa T, Shimura T, Kuwano H, Raz A. Autocrine motility factor signaling enhances pancreatic cancer metastasis. Clin Cancer Res. 2004;10:7775-7784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 40. | Zhao Q, Du J, Gu H, Teng X, Zhang Q, Qin H, Liu N. Effects of YC-1 on hypoxia-inducible factor 1-driven transcription activity, cell proliferative vitality, and apoptosis in hypoxic human pancreatic cancer cells. Pancreas. 2007;34:242-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Du J, Zhao Q, Gu H, Teng XL, Qin H, Liu NZ. [Inhibitory effect of YC-1 on induction of VEGF and GPI genes in hypoxic human pancreatic cancer cells]. Zhonghua Zhong Liu Zazhi. 2006;28:486-489. [PubMed] [Cited in This Article: ] |
| 42. | Talukder AH, Bagheri-Yarmand R, Williams RR, Ragoussis J, Kumar R, Raz A. Antihuman epidermal growth factor receptor 2 antibody herceptin inhibits autocrine motility factor (AMF) expression and potentiates antitumor effects of AMF inhibitors. Clin Cancer Res. 2002;8:3285-3289. [PubMed] [Cited in This Article: ] |
| 43. | Timar J, Trikha M, Szekeres K, Bazaz R, Tovari J, Silletti S, Raz A, Honn KV. Autocrine motility factor signals integrin-mediated metastatic melanoma cell adhesion and invasion. Cancer Res. 1996;56:1902-1908. [PubMed] [Cited in This Article: ] |
| 44. | Arao S, Masumoto A, Otsuki M. Beta1 integrins play an essential role in adhesion and invasion of pancreatic carcinoma cells. Pancreas. 2000;20:129-137. [PubMed] [Cited in This Article: ] |
| 45. | Haga A, Funasaka T, Niinaka Y, Raz A, Nagase H. Autocrine motility factor signaling induces tumor apoptotic resistance by regulations Apaf-1 and Caspase-9 apoptosome expression. Int J Cancer. 2003;107:707-714. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 46. | Chesney J. 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase and tumor cell glycolysis. Curr Opin Clin Nutr Metab Care. 2006;9:535-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 47. | Minchenko OH, Ogura T, Opentanova IL, Minchenko DO, Ochiai A, Caro J, Komisarenko SV, Esumi H. 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene family overexpression in human lung tumor. Ukr Biokhim Zh (1999). 2005;77:46-50. [PubMed] [Cited in This Article: ] |
| 48. | Badea L, Herlea V, Dima SO, Dumitrascu T, Popescu I. Combined gene expression analysis of whole-tissue and microdissected pancreatic ductal adenocarcinoma identifies genes specifically overexpressed in tumor epithelia. Hepatogastroenterology. 2008;55:2016-2027. [PubMed] [Cited in This Article: ] |
| 49. | Cui Y, Tian M, Zong M, Teng M, Chen Y, Lu J, Jiang J, Liu X, Han J. Proteomic analysis of pancreatic ductal adenocarcinoma compared with normal adjacent pancreatic tissue and pancreatic benign cystadenoma. Pancreatology. 2009;9:89-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 50. | Menon R, Zhang Q, Zhang Y, Fermin D, Bardeesy N, DePinho RA, Lu C, Hanash SM, Omenn GS, States DJ. Identification of novel alternative splice isoforms of circulating proteins in a mouse model of human pancreatic cancer. Cancer Res. 2009;69:300-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 51. | Jang M, Kang HJ, Lee SY, Chung SJ, Kang S, Chi SW, Cho S, Lee SC, Lee CK, Park BC. Glyceraldehyde-3-phosphate, a glycolytic intermediate, plays a key role in controlling cell fate via inhibition of caspase activity. Mol Cells. 2009;28:559-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 52. | Akakura N, Kobayashi M, Horiuchi I, Suzuki A, Wang J, Chen J, Niizeki H, Kawamura Ki M, Asaka M. Constitutive expression of hypoxia-inducible factor-1alpha renders pancreatic cancer cells resistant to apoptosis induced by hypoxia and nutrient deprivation. Cancer Res. 2001;61:6548-6554. [PubMed] [Cited in This Article: ] |
| 53. | Miyake K, Nishioka M, Imura S, Batmunkh E, Uto Y, Nagasawa H, Hori H, Shimada M. The novel hypoxic cytotoxin, TX-2098 has antitumor effect in pancreatic cancer; possible mechanism through inhibiting VEGF and hypoxia inducible factor-1α targeted gene expression. Exp Cell Res. 2012;318:1554-1563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 54. | Chen J, Zhao S, Nakada K, Kuge Y, Tamaki N, Okada F, Wang J, Shindo M, Higashino F, Takeda K. Dominant-negative hypoxia-inducible factor-1 alpha reduces tumorigenicity of pancreatic cancer cells through the suppression of glucose metabolism. Am J Pathol. 2003;162:1283-1291. [PubMed] [Cited in This Article: ] |
| 55. | Mikuriya K, Kuramitsu Y, Ryozawa S, Fujimoto M, Mori S, Oka M, Hamano K, Okita K, Sakaida I, Nakamura K. Expression of glycolytic enzymes is increased in pancreatic cancerous tissues as evidenced by proteomic profiling by two-dimensional electrophoresis and liquid chromatography-mass spectrometry/mass spectrometry. Int J Oncol. 2007;30:849-855. [PubMed] [Cited in This Article: ] |
| 56. | Mori-Iwamoto S, Kuramitsu Y, Ryozawa S, Mikuria K, Fujimoto M, Maehara S, Maehara Y, Okita K, Nakamura K, Sakaida I. Proteomics finding heat shock protein 27 as a biomarker for resistance of pancreatic cancer cells to gemcitabine. Int J Oncol. 2007;31:1345-1350. [PubMed] [Cited in This Article: ] |
| 57. | Wang Y, Kuramitsu Y, Ueno T, Suzuki N, Yoshino S, Iizuka N, Zhang X, Akada J, Oka M, Nakamura K. Proteomic differential display identifies upregulated vinculin as a possible biomarker of pancreatic cancer. Oncol Rep. 2012;28:1845-1850. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Schek N, Hall BL, Finn OJ. Increased glyceraldehyde-3-phosphate dehydrogenase gene expression in human pancreatic adenocarcinoma. Cancer Res. 1988;48:6354-6359. [PubMed] [Cited in This Article: ] |
| 59. | Epner DE, Coffey DS. There are multiple forms of glyceraldehyde-3-phosphate dehydrogenase in prostate cancer cells and normal prostate tissue. Prostate. 1996;28:372-378. [PubMed] [DOI] [Cited in This Article: ] |
| 60. | Révillion F, Pawlowski V, Hornez L, Peyrat JP. Glyceraldehyde-3-phosphate dehydrogenase gene expression in human breast cancer. Eur J Cancer. 2000;36:1038-1042. [PubMed] [Cited in This Article: ] |
| 61. | Persons DA, Schek N, Hall BL, Finn OJ. Increased expression of glycolysis-associated genes in oncogene-transformed and growth-accelerated states. Mol Carcinog. 1989;2:88-94. [PubMed] [Cited in This Article: ] |
| 62. | Hwang TL, Liang Y, Chien KY, Yu JS. Overexpression and elevated serum levels of phosphoglycerate kinase 1 in pancreatic ductal adenocarcinoma. Proteomics. 2006;6:2259-2272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 63. | Kumble KD, Hirota M, Pour PM, Vishwanatha JK. Enhanced levels of annexins in pancreatic carcinoma cells of Syrian hamsters and their intrapancreatic allografts. Cancer Res. 1992;52:163-167. [PubMed] [Cited in This Article: ] |
| 64. | Li C, Kim HY, Vuong H, Patwa T, Pal M, Brand RE, Simeone DM, Lubman DM. The identification of auto-antibodies in pancreatic cancer patient sera using a naturally fractionated Panc-1 cell line. Cancer Biomark. 2010;7:25-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 65. | Patwa TH, Li C, Poisson LM, Kim HY, Pal M, Ghosh D, Simeone DM, Lubman DM. The identification of phosphoglycerate kinase-1 and histone H4 autoantibodies in pancreatic cancer patient serum using a natural protein microarray. Electrophoresis. 2009;30:2215-2226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 66. | Zhao W, Pao S, Malik F, Soh J, Fernandez S, Chirico WJ. A sandwich ELISA for phosphoglycerate kinase. J Immunoassay Immunochem. 2008;29:220-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 67. | Yabushita S, Fukamachi K, Tanaka H, Fukuda T, Sumida K, Deguchi Y, Mikata K, Nishioka K, Kawamura S, Uwagawa S. Metabolomic and transcriptomic profiling of human K-ras oncogene transgenic rats with pancreatic ductal adenocarcinomas. Carcinogenesis. 2013;34:1251-1259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 68. | Kondoh H, Lleonart ME, Bernard D, Gil J. Protection from oxidative stress by enhanced glycolysis; a possible mechanism of cellular immortalization. Histol Histopathol. 2007;22:85-90. [PubMed] [Cited in This Article: ] |
| 69. | Cappello P, Tomaino B, Chiarle R, Ceruti P, Novarino A, Castagnoli C, Migliorini P, Perconti G, Giallongo A, Milella M. An integrated humoral and cellular response is elicited in pancreatic cancer by alpha-enolase, a novel pancreatic ductal adenocarcinoma-associated antigen. Int J Cancer. 2009;125:639-648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 70. | Takikita M, Altekruse S, Lynch CF, Goodman MT, Hernandez BY, Green M, Cozen W, Cockburn M, Sibug Saber M, Topor M. Associations between selected biomarkers and prognosis in a population-based pancreatic cancer tissue microarray. Cancer Res. 2009;69:2950-2955. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 71. | Chung JC, Oh MJ, Choi SH, Bae CD. Proteomic analysis to identify biomarker proteins in pancreatic ductal adenocarcinoma. ANZ J Surg. 2008;78:245-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 72. | Shen J, Person MD, Zhu J, Abbruzzese JL, Li D. Protein expression profiles in pancreatic adenocarcinoma compared with normal pancreatic tissue and tissue affected by pancreatitis as detected by two-dimensional gel electrophoresis and mass spectrometry. Cancer Res. 2004;64:9018-9026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 262] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 73. | Kitahashi T, Yoshimoto M, Imai T. Novel immunohistochemical marker, integrin α(V)β(3), for BOP-induced early lesions in hamster pancreatic ductal carcinogenesis. Oncol Lett. 2011;2:229-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 74. | Wang L, Liu HL, Li Y, Yuan P. Proteomic analysis of pancreatic intraepithelial neoplasia and pancreatic carcinoma in rat models. World J Gastroenterol. 2011;17:1434-1441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 75. | Amedei A, Niccolai E, Benagiano M, Della Bella C, Cianchi F, Bechi P, Taddei A, Bencini L, Farsi M, Cappello P. Ex vivo analysis of pancreatic cancer-infiltrating T lymphocytes reveals that ENO-specific Tregs accumulate in tumor tissue and inhibit Th1/Th17 effector cell functions. Cancer Immunol Immunother. 2013;62:1249-1260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 76. | Tomaino B, Cappello P, Capello M, Fredolini C, Sperduti I, Migliorini P, Salacone P, Novarino A, Giacobino A, Ciuffreda L. Circulating autoantibodies to phosphorylated α-enolase are a hallmark of pancreatic cancer. J Proteome Res. 2011;10:105-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 77. | Cappello P, Rolla S, Chiarle R, Principe M, Cavallo F, Perconti G, Feo S, Giovarelli M, Novelli F. Vaccination with ENO1 DNA prolongs survival of genetically engineered mice with pancreatic cancer. Gastroenterology. 2013;144:1098-1106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 78. | Marsh WL, Colonna J, Yearsley M, Bloomston M, Frankel WL. Calponin is expressed in serous cystadenomas of the pancreas but not in adenocarcinomas or endocrine tumors. Appl Immunohistochem Mol Morphol. 2009;17:216-219. [PubMed] [Cited in This Article: ] |
| 79. | Akiyama T, Sadahira Y, Irei I, Nishimura H, Hida AI, Notohara K, Hamazaki S. Pancreatic serous microcystic adenoma with extensive oncocytic change. Pathol Int. 2009;59:102-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 80. | Lieber MR, Lack EE, Roberts JR, Merino MJ, Patterson K, Restrepo C, Solomon D, Chandra R, Triche TJ. Solid and papillary epithelial neoplasm of the pancreas. An ultrastructural and immunocytochemical study of six cases. Am J Surg Pathol. 1987;11:85-93. [PubMed] [Cited in This Article: ] |
| 81. | Yuan CH, Xiu DR, Shi XY, Ma ZL, Li ZF, Tao M, Jia YM, Xiong JW, Zhang TL. [Clinicopathologic features, diagnosis and treatment with solid-pseudopapillary tumor of the pancreas: a report of 33 cases]. Zhonghua Wai Ke Zazhi. 2012;50:11-14. [PubMed] [Cited in This Article: ] |
| 82. | Yang B, Tan YS, Ji Y, Liu T, Zeng HY. [Study on clinicopathologic features and metastasizing potential of solid pseudopapillary tumor of pancreas]. Zhonghua Bing Li Xue Zazhi. 2010;39:25-30. [PubMed] [Cited in This Article: ] |
| 83. | Dubova EA, Shchegolev AI, Mishnev OD, Egorov VI. [Solid pseudopapillary tumor of the pancreas]. Arkh Patol. 2008;70:49-52. [PubMed] [Cited in This Article: ] |
| 84. | Santini D, Poli F, Lega S. Solid-papillary tumors of the pancreas: histopathology. JOP. 2006;7:131-136. [PubMed] [Cited in This Article: ] |
| 85. | Bhanot P, Nealon WH, Walser EM, Bhutani MS, Tang WW, Logroño R. Clinical, imaging, and cytopathological features of solid pseudopapillary tumor of the pancreas: a clinicopathologic study of three cases and review of the literature. Diagn Cytopathol. 2005;33:421-428. [PubMed] [Cited in This Article: ] |
| 86. | Daum O, Sima R, Mukensnabl P, Vanecek T, Brouckova M, Benes Z, Michal M. Pigmented solid-pseudopapillary neoplasm of the pancreas. Pathol Int. 2005;55:280-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 87. | Zhang KR, Jia HM, Shu H, Li XY. Solid cystic papillary tumor of pancreas in eight children. Chin Med Sci J. 2007;22:54-57. [PubMed] [Cited in This Article: ] |
| 88. | Zeytunlu M, Firat O, Nart D, Coker A, Yüzer Y, Tekeşin O, Ozütemiz O, Killi R. Solid and cystic papillary neoplasms of the pancreas: report of four cases. Turk J Gastroenterol. 2004;15:178-182. [PubMed] [Cited in This Article: ] |
| 89. | Zhu X, He L, Zeng J. [Solid and cystic tumor of pancreas, analysis of 14 pediatric cases]. Zhonghua Yi Xue Zazhi. 2002;82:1180-1182. [PubMed] [Cited in This Article: ] |
| 90. | Bahri I, Njim L, Khabir A, Mahmoudi H, Ghorbel A, Zakhama A, Jlidi R. [Solid cystic papillary tumor of the pancreas]. Ann Chir. 2001;126:899-902. [PubMed] [Cited in This Article: ] |
| 91. | Zhou H, Cheng W, Lam KY, Chan GC, Khong PL, Tam PK. Solid-cystic papillary tumor of the pancreas in children. Pediatr Surg Int. 2001;17:614-620. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 92. | Petrella T, Rat P, Lizard G, Dusserre-Guion L, Poulard G, Michiels R. [Papillary and cystic tumor of the pancreas. Histological, immunohistochemical and flow cytometric study]. Gastroenterol Clin Biol. 1994;18:1021-1027. [PubMed] [Cited in This Article: ] |
| 93. | Jørgensen LJ, Hansen AB, Burcharth F, Philipsen E, Horn T. Solid and papillary neoplasm of the pancreas. Ultrastruct Pathol. 1992;16:659-666. [PubMed] [Cited in This Article: ] |
| 94. | Pettinato G, Manivel JC, Ravetto C, Terracciano LM, Gould EW, di Tuoro A, Jaszcz W, Albores-Saavedra J. Papillary cystic tumor of the pancreas. A clinicopathologic study of 20 cases with cytologic, immunohistochemical, ultrastructural, and flow cytometric observations, and a review of the literature. Am J Clin Pathol. 1992;98:478-488. [PubMed] [Cited in This Article: ] |
| 95. | Yamaguchi K, Miyagahara T, Tsuneyoshi M, Enjoji M, Horie A, Nakayama I, Tsuda N, Fujii H, Takahara O. Papillary cystic tumor of the pancreas: an immunohistochemical and ultrastructural study of 14 patients. Jpn J Clin Oncol. 1989;19:102-111. [PubMed] [Cited in This Article: ] |
| 96. | Miettinen M, Partanen S, Fräki O, Kivilaakso E. Papillary cystic tumor of the pancreas. An analysis of cellular differentiation by electron microscopy and immunohistochemistry. Am J Surg Pathol. 1987;11:855-865. [PubMed] [Cited in This Article: ] |
| 97. | Kamisawa T, Fukayama M, Koike M, Tabata I, Okamoto A. So-called “papillary and cystic neoplasm of the pancreas.” An immunohistochemical and ultrastructural study. Acta Pathol Jpn. 1987;37:785-794. [PubMed] [Cited in This Article: ] |
| 98. | Kobayashi T, Kimura T, Takabayashi N, Sugimura H. Two synchronous solid and cystic tumors of the pancreas. J Gastroenterol. 1998;33:439-442. [PubMed] [Cited in This Article: ] |
| 99. | Tsunoda T, Eto T, Tsurifune T, Tokunaga S, Ishii T, Motojima K, Matsumoto T, Segawa T, Ura K, Fukui H. Solid and cystic tumor of the pancreas in an adult male. Acta Pathol Jpn. 1991;41:763-770. [PubMed] [Cited in This Article: ] |
| 100. | Stömmer P, Kraus J, Stolte M, Giedl J. Solid and cystic pancreatic tumors. Clinical, histochemical, and electron microscopic features in ten cases. Cancer. 1991;67:1635-1641. [PubMed] [Cited in This Article: ] |
| 101. | Morohoshi T, Kanda M, Horie A, Chott A, Dreyer T, Klöppel G, Heitz PU. Immunocytochemical markers of uncommon pancreatic tumors. Acinar cell carcinoma, pancreatoblastoma, and solid cystic (papillary-cystic) tumor. Cancer. 1987;59:739-747. [PubMed] [Cited in This Article: ] |
| 102. | Chott A, Klöppel G, Buxbaum P, Heitz PU. Neuron specific enolase demonstration in the diagnosis of a solid-cystic (papillary cystic) tumour of the pancreas. Virchows Arch A Pathol Anat Histopathol. 1987;410:397-402. [PubMed] [Cited in This Article: ] |
| 103. | Reese SA, Traverso LW, Jacobs TW, Longnecker DS. Solid serous adenoma of the pancreas: a rare variant within the family of pancreatic serous cystic neoplasms. Pancreas. 2006;33:96-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 104. | Kosmahl M, Wagner J, Peters K, Sipos B, Klöppel G. Serous cystic neoplasms of the pancreas: an immunohistochemical analysis revealing alpha-inhibin, neuron-specific enolase, and MUC6 as new markers. Am J Surg Pathol. 2004;28:339-346. [PubMed] [Cited in This Article: ] |
| 105. | Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2001] [Cited by in F6Publishing: 2052] [Article Influence: 128.3] [Reference Citation Analysis (0)] |
| 106. | Roda O, Chiva C, Espuña G, Gabius HJ, Real FX, Navarro P, Andreu D. A proteomic approach to the identification of new tPA receptors in pancreatic cancer cells. Proteomics. 2006;6 Suppl 1:S36-S41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 107. | Israël M, Schwartz L. The metabolic advantage of tumor cells. Mol Cancer. 2011;10:70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 108. | Kumar Y, Mazurek S, Yang S, Failing K, Winslet M, Fuller B, Davidson BR. In vivo factors influencing tumour M2-pyruvate kinase level in human pancreatic cancer cell lines. Tumour Biol. 2010;31:69-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 109. | Dando I, Donadelli M, Costanzo C, Dalla Pozza E, D’Alessandro A, Zolla L, Palmieri M. Cannabinoids inhibit energetic metabolism and induce AMPK-dependent autophagy in pancreatic cancer cells. Cell Death Dis. 2013;4:e664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 110. | Cerwenka H, Aigner R, Bacher H, Werkgartner G, el-Shabrawi A, Quehenberger F, Mischinger HJ. TUM2-PK (pyruvate kinase type tumor M2), CA19-9 and CEA in patients with benign, malignant and metastasizing pancreatic lesions. Anticancer Res. 1999;19:849-851. [PubMed] [Cited in This Article: ] |
| 111. | Novotný I, Dítĕ P, Dastych M, Záková A, Trna J, Novotná H, Nechutová H. Tumor marker M2-pyruvate-kinase in differential diagnosis of chronic pancreatitis and pancreatic cancer. Hepatogastroenterology. 2008;55:1475-1477. [PubMed] [Cited in This Article: ] |
| 112. | Goonetilleke KS, Mason JM, Siriwardana P, King NK, France MW, Siriwardena AK. Diagnostic and prognostic value of plasma tumor M2 pyruvate kinase in periampullary cancer: evidence for a novel biological marker of adverse prognosis. Pancreas. 2007;34:318-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 113. | Hardt PD, Ewald N. Tumor M2 pyruvate kinase: a tumor marker and its clinical application in gastrointestinal malignancy. Expert Rev Mol Diagn. 2008;8:579-585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 114. | Kumar Y, Gurusamy K, Pamecha V, Davidson BR. Tumor M2-pyruvate kinase as tumor marker in exocrine pancreatic cancer a meta-analysis. Pancreas. 2007;35:114-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 115. | Schneider J, Schulze G. Comparison of tumor M2-pyruvate kinase (tumor M2-PK), carcinoembryonic antigen (CEA), carbohydrate antigens CA 19-9 and CA 72-4 in the diagnosis of gastrointestinal cancer. Anticancer Res. 2003;23:5089-5093. [PubMed] [Cited in This Article: ] |
| 116. | Hardt PD, Ngoumou BK, Rupp J, Schnell-Kretschmer H, Kloer HU. Tumor M2-pyruvate kinase: a promising tumor marker in the diagnosis of gastro-intestinal cancer. Anticancer Res. 2000;20:4965-4968. [PubMed] [Cited in This Article: ] |
| 117. | Oremek GM, Eigenbrodt E, Rädle J, Zeuzem S, Seiffert UB. Value of the serum levels of the tumor marker TUM2-PK in pancreatic cancer. Anticancer Res. 1997;17:3031-3033. [PubMed] [Cited in This Article: ] |
| 118. | Hathurusinghe HR, Goonetilleke KS, Siriwardena AK. Current status of tumor M2 pyruvate kinase (tumor M2-PK) as a biomarker of gastrointestinal malignancy. Ann Surg Oncol. 2007;14:2714-2720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 119. | Schulze G. The tumor marker tumor M2-PK: an application in the diagnosis of gastrointestinal cancer. Anticancer Res. 2000;20:4961-4964. [PubMed] [Cited in This Article: ] |
| 120. | Ventrucci M, Cipolla A, Racchini C, Casadei R, Simoni P, Gullo L. Tumor M2-pyruvate kinase, a new metabolic marker for pancreatic cancer. Dig Dis Sci. 2004;49:1149-1155. [PubMed] [Cited in This Article: ] |
| 121. | Aloysius MM, Zaitoun AM, Bates TE, Albasri A, Ilyas M, Rowlands BJ, Lobo DN. Complete absence of M2-pyruvate kinase expression in benign pancreatic ductal epithelium and pancreaticobiliary and duodenal neoplasia. BMC Cancer. 2009;9:327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 122. | Paciucci R, Berrozpe G, Torà M, Navarro E, García de Herreros A, Real FX. Isolation of tissue-type plasminogen activator, cathepsin H, and non-specific cross-reacting antigen from SK-PC-1 pancreas cancer cells using subtractive hybridization. FEBS Lett. 1996;385:72-76. [PubMed] [Cited in This Article: ] |
| 123. | Shim H, Dolde C, Lewis BC, Wu CS, Dang G, Jungmann RA, Dalla-Favera R, Dang CV. c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc Natl Acad Sci USA. 1997;94:6658-6663. [PubMed] [Cited in This Article: ] |
| 124. | Schlichtholz B, Turyn J, Goyke E, Biernacki M, Jaskiewicz K, Sledzinski Z, Swierczynski J. Enhanced citrate synthase activity in human pancreatic cancer. Pancreas. 2005;30:99-104. [PubMed] [Cited in This Article: ] |
| 125. | Kyriazis AA, Kyriazis AP, Sternberg CN, Sloane NH, Loveless JD. Morphological, biological, biochemical, and karyotypic characteristics of human pancreatic ductal adenocarcinoma Capan-2 in tissue culture and the nude mouse. Cancer Res. 1986;46:5810-5815. [PubMed] [Cited in This Article: ] |
| 126. | Zelinsky-Papez K, Carter TH, Zimmerman JA. Isolation and characterization of chemically transformed pancreatic acinar cell lines from young and old mice. In Vitro Cell Dev Biol. 1987;23:118-122. [PubMed] [Cited in This Article: ] |
| 127. | Kobari M, Hisano H, Matsuno S, Sato T, Kan M, Tachibana T. Establishment of six human pancreatic cancer cell lines and their sensitivities to anti-tumor drugs. Tohoku J Exp Med. 1986;150:231-248. [PubMed] [Cited in This Article: ] |
| 128. | Obara T, Denda A, Murata Y, Makino T, Yokose Y, Katsuragi M, Konishi Y, Ueda N, Namiki M. Enzyme histochemical studies on transplantable pancreatic adenocarcinomas in Syrian golden hamsters. Exp Pathol. 1984;26:205-211. [PubMed] [Cited in This Article: ] |
| 129. | Kyriazis AP, Kyriazis AA, Scarpelli DG, Fogh J, Rao MS, Lepera R. Human pancreatic adenocarcinoma line Capan-1 in tissue culture and the nude mouse: morphologic, biologic, and biochemical characteristics. Am J Pathol. 1982;106:250-260. [PubMed] [Cited in This Article: ] |
| 130. | Kyriazis AP, McCombs WB, Sandberg AA, Kyriazis AA, Sloane NH, Lepera R. Establishment and characterization of human pancreatic adenocarcinoma cell line SW-1990 in tissue culture and the nude mouse. Cancer Res. 1983;43:4393-4401. [PubMed] [Cited in This Article: ] |
| 131. | Zhou W, Capello M, Fredolini C, Racanicchi L, Piemonti L, Liotta LA, Novelli F, Petricoin EF. MS analysis reveals O-methylation of L-lactate dehydrogenase from pancreatic ductal adenocarcinoma cells. Electrophoresis. 2012;33:1850-1854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 132. | Zhao D, Zou SW, Liu Y, Zhou X, Mo Y, Wang P, Xu YH, Dong B, Xiong Y, Lei QY. Lysine-5 acetylation negatively regulates lactate dehydrogenase A and is decreased in pancreatic cancer. Cancer Cell. 2013;23:464-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 231] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 133. | Rong Y, Wu W, Ni X, Kuang T, Jin D, Wang D, Lou W. Lactate dehydrogenase A is overexpressed in pancreatic cancer and promotes the growth of pancreatic cancer cells. Tumour Biol. 2013;34:1523-1530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 134. | Cui J, Shi M, Xie D, Wei D, Jia Z, Zheng S, Gao Y, Huang S, Xie K. FOXM1 promotes the warburg effect and pancreatic cancer progression via transactivation of LDHA expression. Clin Cancer Res. 2014;20:2595-2606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 170] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 135. | Granchi C, Roy S, De Simone A, Salvetti I, Tuccinardi T, Martinelli A, Macchia M, Lanza M, Betti L, Giannaccini G. N-Hydroxyindole-based inhibitors of lactate dehydrogenase against cancer cell proliferation. Eur J Med Chem. 2011;46:5398-5407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 136. | Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL, Dang CV. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci USA. 2010;107:2037-2042. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 893] [Cited by in F6Publishing: 1023] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 137. | Husain Z, Huang Y, Seth P, Sukhatme VP. Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J Immunol. 2013;191:1486-1495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 400] [Cited by in F6Publishing: 497] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 138. | Tas F, Aykan F, Alici S, Kaytan E, Aydiner A, Topuz E. Prognostic factors in pancreatic carcinoma: serum LDH levels predict survival in metastatic disease. Am J Clin Oncol. 2001;24:547-550. [PubMed] [Cited in This Article: ] |
| 139. | Gudi R, Bowker-Kinley MM, Kedishvili NY, Zhao Y, Popov KM. Diversity of the pyruvate dehydrogenase kinase gene family in humans. J Biol Chem. 1995;270:28989-28994. [PubMed] [Cited in This Article: ] |
| 140. | Anderson KM, Jajeh J, Guinan P, Rubenstein M. In vitro effects of dichloroacetate and CO2 on hypoxic HeLa cells. Anticancer Res. 2009;29:4579-4588. [PubMed] [Cited in This Article: ] |
| 141. | Sato T, Kashima K, Gamachi A, Daa T, Nakayama I, Yokoyama S. Immunohistochemical localization of pyruvate carboxylase and carbamyl-phosphate synthetase I in normal and neoplastic human pancreatic tissues. Pancreas. 2002;25:130-135. [PubMed] [Cited in This Article: ] |
| 142. | Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nat Rev Drug Discov. 2010;9:447-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1164] [Cited by in F6Publishing: 1183] [Article Influence: 84.5] [Reference Citation Analysis (0)] |
| 143. | Rodríguez-Enríquez S, Gallardo-Pérez JC, Marín-Hernández A, Aguilar-Ponce JL, Mandujano-Tinoco EA, Meneses A, Moreno-Sánchez R. Oxidative phosphorylation as a target to arrest malignant neoplasias. Curr Med Chem. 2011;18:3156-3167. [PubMed] [Cited in This Article: ] |
| 144. | Momose I, Ohba S, Tatsuda D, Kawada M, Masuda T, Tsujiuchi G, Yamori T, Esumi H, Ikeda D. Mitochondrial inhibitors show preferential cytotoxicity to human pancreatic cancer PANC-1 cells under glucose-deprived conditions. Biochem Biophys Res Commun. 2010;392:460-466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 145. | Yamamoto T, Seino Y, Fukumoto H, Koh G, Yano H, Inagaki N, Yamada Y, Inoue K, Manabe T, Imura H. Over-expression of facilitative glucose transporter genes in human cancer. Biochem Biophys Res Commun. 1990;170:223-230. [PubMed] [Cited in This Article: ] |
| 146. | Macheda ML, Rogers S, Best JD. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol. 2005;202:654-662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 812] [Cited by in F6Publishing: 840] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 147. | Kunkel M, Reichert TE, Benz P, Lehr HA, Jeong JH, Wieand S, Bartenstein P, Wagner W, Whiteside TL. Overexpression of Glut-1 and increased glucose metabolism in tumors are associated with a poor prognosis in patients with oral squamous cell carcinoma. Cancer. 2003;97:1015-1024. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 270] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 148. | Ko YH, Smith BL, Wang Y, Pomper MG, Rini DA, Torbenson MS, Hullihen J, Pedersen PL. Advanced cancers: eradication in all cases using 3-bromopyruvate therapy to deplete ATP. Biochem Biophys Res Commun. 2004;324:269-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 286] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 149. | Basturk O, Singh R, Kaygusuz E, Balci S, Dursun N, Culhaci N, Adsay NV. GLUT-1 expression in pancreatic neoplasia: implications in pathogenesis, diagnosis, and prognosis. Pancreas. 2011;40:187-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 150. | Reid MD, Choi H, Balci S, Akkas G, Adsay V. Serous cystic neoplasms of the pancreas: clinicopathologic and molecular characteristics. Semin Diagn Pathol. 2014;31:475-483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 151. | Maher JC, Savaraj N, Priebe W, Liu H, Lampidis TJ. Differential sensitivity to 2-deoxy-D-glucose between two pancreatic cell lines correlates with GLUT-1 expression. Pancreas. 2005;30:e34-e39. [PubMed] [Cited in This Article: ] |
| 152. | Ko YH, Verhoeven HA, Lee MJ, Corbin DJ, Vogl TJ, Pedersen PL. A translational study „case report“ on the small molecule „energy blocker“ 3-bromopyruvate (3BP) as a potent anticancer agent: from bench side to bedside. J Bioenerg Biomembr. 2012;44:163-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 153. | Torres MP, Rachagani S, Purohit V, Pandey P, Joshi S, Moore ED, Johansson SL, Singh PK, Ganti AK, Batra SK. Graviola: a novel promising natural-derived drug that inhibits tumorigenicity and metastasis of pancreatic cancer cells in vitro and in vivo through altering cell metabolism. Cancer Lett. 2012;323:29-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 154. | Xiao H, Li S, Zhang D, Liu T, Yu M, Wang F. Separate and concurrent use of 2-deoxy-D-glucose and 3-bromopyruvate in pancreatic cancer cells. Oncol Rep. 2013;29:329-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 155. | Zhang D, Cui L, Li SS, Wang F. Insulin and hypoxia-inducible factor-1 cooperate in pancreatic cancer cells to increase cell viability. Oncol Lett. 2015;10:1545-1550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 156. | Lyshchik A, Higashi T, Hara T, Nakamoto Y, Fujimoto K, Doi R, Imamura M, Saga T, Togashi K. Expression of glucose transporter-1, hexokinase-II, proliferating cell nuclear antigen and survival of patients with pancreatic cancer. Cancer Invest. 2007;25:154-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 157. | Ogawa H, Nagano H, Konno M, Eguchi H, Koseki J, Kawamoto K, Nishida N, Colvin H, Tomokuni A, Tomimaru Y. The combination of the expression of hexokinase 2 and pyruvate kinase M2 is a prognostic marker in patients with pancreatic cancer. Mol Clin Oncol. 2015;3:563-571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 158. | Burkhart RA, Pineda DM, Chand SN, Romeo C, Londin ER, Karoly ED, Cozzitorto JA, Rigoutsos I, Yeo CJ, Brody JR. HuR is a post-transcriptional regulator of core metabolic enzymes in pancreatic cancer. RNA Biol. 2013;10:1312-1323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 159. | Dando I, Fiorini C, Pozza ED, Padroni C, Costanzo C, Palmieri M, Donadelli M. UCP2 inhibition triggers ROS-dependent nuclear translocation of GAPDH and autophagic cell death in pancreatic adenocarcinoma cells. Biochim Biophys Acta. 2013;1833:672-679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 160. | Lam W, Bussom S, Cheng YC. Effect of hypoxia on the expression of phosphoglycerate kinase and antitumor activity of troxacitabine and gemcitabine in non-small cell lung carcinoma. Mol Cancer Ther. 2009;8:415-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 161. | Cappello P, Novelli F. A self antigen reopens the games in pancreatic cancer. Oncoimmunology. 2013;2:e24384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 162. | Capello M, Caorsi C, Bogantes Hernandez PJ, Dametto E, Bertinetto FE, Magistroni P, Rendine S, Amoroso A, Novelli F. Phosphorylated alpha-enolase induces autoantibodies in HLA-DR8 pancreatic cancer patients and triggers HLA-DR8 restricted T-cell activation. Immunol Lett. 2015;167:11-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 163. | Principe M, Ceruti P, Shih NY, Chattaragada MS, Rolla S, Conti L, Bestagno M, Zentilin L, Yang SH, Migliorini P. Targeting of surface alpha-enolase inhibits the invasiveness of pancreatic cancer cells. Oncotarget. 2015;6:11098-11113. [PubMed] [Cited in This Article: ] |
| 164. | Zhang J, Gao Q, Zhou Y, Dier U, Hempel N, Hochwald SN. Focal adhesion kinase-promoted tumor glucose metabolism is associated with a shift of mitochondrial respiration to glycolysis. Oncogene. 2015;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 165. | Kim DJ, Park YS, Kang MG, You YM, Jung Y, Koo H, Kim JA, Kim MJ, Hong SM, Lee KB. Pyruvate kinase isoenzyme M2 is a therapeutic target of gemcitabine-resistant pancreatic cancer cells. Exp Cell Res. 2015;336:119-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 166. | Pandita A, Kumar B, Manvati S, Vaishnavi S, Singh SK, Bamezai RN. Synergistic combination of gemcitabine and dietary molecule induces apoptosis in pancreatic cancer cells and down regulates PKM2 expression. PLoS One. 2014;9:e107154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 167. | Joergensen MT, Heegaard NH, Schaffalitzky de Muckadell OB. Comparison of plasma Tu-M2-PK and CA19-9 in pancreatic cancer. Pancreas. 2010;39:243-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 168. | Zhou W, Capello M, Fredolini C, Racanicchi L, Dugnani E, Piemonti L, Liotta LA, Novelli F, Petricoin EF. Mass spectrometric analysis reveals O-methylation of pyruvate kinase from pancreatic cancer cells. Anal Bioanal Chem. 2013;405:4937-4943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 169. | Kyriazis AP, Kyriazis AA. Morphologic, biologic and biochemical characteristics of three human pancreatic ductal adenocarcinomas established as xenotransplants in the nude mouse. In Vivo. 1990;4:137-143. [PubMed] [Cited in This Article: ] |
| 170. | Cui J, Quan M, Jiang W, Hu H, Jiao F, Li N, Jin Z, Wang L, Wang Y, Wang L. Suppressed expression of LDHB promotes pancreatic cancer progression via inducing glycolytic phenotype. Med Oncol. 2015;32:143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 171. | He TL, Zhang YJ, Jiang H, Li XH, Zhu H, Zheng KL. The c-Myc-LDHA axis positively regulates aerobic glycolysis and promotes tumor progression in pancreatic cancer. Med Oncol. 2015;32:187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 172. | Huang CJ, Severin E, Blum M. Flow-cytometric determination of dehydrogenase activities in primary human gastrointestinal tumor cell lines. Anal Cell Pathol. 1994;6:93-103. [PubMed] [Cited in This Article: ] |
| 173. | Lu QY, Zhang L, Yee JK, Go VW, Lee WN. Metabolic Consequences of LDHA inhibition by Epigallocatechin Gallate and Oxamate in MIA PaCa-2 Pancreatic Cancer Cells. Metabolomics. 2015;11:71-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 174. | Maftouh M, Avan A, Sciarrillo R, Granchi C, Leon LG, Rani R, Funel N, Smid K, Honeywell R, Boggi U. Synergistic interaction of novel lactate dehydrogenase inhibitors with gemcitabine against pancreatic cancer cells in hypoxia. Br J Cancer. 2014;110:172-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 122] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 175. | Rajeshkumar NV, Dutta P, Yabuuchi S, de Wilde RF, Martinez GV, Le A, Kamphorst JJ, Rabinowitz JD, Jain SK, Hidalgo M. Therapeutic Targeting of the Warburg Effect in Pancreatic Cancer Relies on an Absence of p53 Function. Cancer Res. 2015;75:3355-3364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 176. | Saito S, Taguchi K, Nishimura N, Watanabe A, Ogoshi K, Niwa M, Furukawa T, Takahashi M. Clinical usefulness of computer-assisted diagnosis using combination assay of tumor markers for pancreatic carcinoma. Cancer. 1993;72:381-388. [PubMed] [Cited in This Article: ] |
| 177. | Isayev O, Rausch V, Bauer N, Liu L, Fan P, Zhang Y, Gladkich J, Nwaeburu CC, Mattern J, Mollenhauer M. Inhibition of glucose turnover by 3-bromopyruvate counteracts pancreatic cancer stem cell features and sensitizes cells to gemcitabine. Oncotarget. 2014;5:5177-5189. [PubMed] [Cited in This Article: ] |
| 178. | Shi M, Cui J, Du J, Wei D, Jia Z, Zhang J, Zhu Z, Gao Y, Xie K. A novel KLF4/LDHA signaling pathway regulates aerobic glycolysis in and progression of pancreatic cancer. Clin Cancer Res. 2014;20:4370-4380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 179. | He G, Jiang Y, Zhang B, Wu G. The effect of HIF-1α on glucose metabolism, growth and apoptosis of pancreatic cancerous cells. Asia Pac J Clin Nutr. 2014;23:174-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 30] [Reference Citation Analysis (0)] |
| 180. | Bardawill C, Chang C. Serum Lactic Dehydrogenase, Leucine Aminopeptidase and 5-Nucleotidase Activities: Observation in Patients with Carcinoma of the Pancreas and Hepatobiliary Disease. Can Med Assoc J. 1963;89:755-761. [PubMed] [Cited in This Article: ] |
| 181. | Fujita F, Fujita M, Nakano Y, Hayata S, Taguchi T. [Characteristics of lactate dehydrogenase (LDH) isozyme in human cancers transplanted into nude mice and its application to the evaluation of experimental chemotherapy]. Gan To Kagaku Ryoho. 1984;11:2212-2220. [PubMed] [Cited in This Article: ] |
| 182. | Haas M, Heinemann V, Kullmann F, Laubender RP, Klose C, Bruns CJ, Holdenrieder S, Modest DP, Schulz C, Boeck S. Prognostic value of CA 19-9, CEA, CRP, LDH and bilirubin levels in locally advanced and metastatic pancreatic cancer: results from a multicenter, pooled analysis of patients receiving palliative chemotherapy. J Cancer Res Clin Oncol. 2013;139:681-689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 183. | Haas M, Laubender RP, Stieber P, Holdenrieder S, Bruns CJ, Wilkowski R, Mansmann U, Heinemann V, Boeck S. Prognostic relevance of CA 19-9, CEA, CRP, and LDH kinetics in patients treated with palliative second-line therapy for advanced pancreatic cancer. Tumour Biol. 2010;31:351-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 184. | Ristorcelli E, Beraud E, Verrando P, Villard C, Lafitte D, Sbarra V, Lombardo D, Verine A. Human tumor nanoparticles induce apoptosis of pancreatic cancer cells. FASEB J. 2008;22:3358-3369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 185. | Schwartz L, Abolhassani M, Guais A, Sanders E, Steyaert JM, Campion F, Israël M. A combination of alpha lipoic acid and calcium hydroxycitrate is efficient against mouse cancer models: preliminary results. Oncol Rep. 2010;23:1407-1416. [PubMed] [Cited in This Article: ] |
| 186. | Guais A, Baronzio G, Sanders E, Campion F, Mainini C, Fiorentini G, Montagnani F, Behzadi M, Schwartz L, Abolhassani M. Adding a combination of hydroxycitrate and lipoic acid (METABLOC™) to chemotherapy improves effectiveness against tumor development: experimental results and case report. Invest New Drugs. 2012;30:200-211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 187. | Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, Perera RM, Ferrone CR, Mullarky E, Shyh-Chang N. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496:101-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1227] [Cited by in F6Publishing: 1400] [Article Influence: 127.3] [Reference Citation Analysis (0)] |
| 188. | Zachar Z, Marecek J, Maturo C, Gupta S, Stuart SD, Howell K, Schauble A, Lem J, Piramzadian A, Karnik S. Non-redox-active lipoate derivates disrupt cancer cell mitochondrial metabolism and are potent anticancer agents in vivo. J Mol Med (Berl). 2011;89:1137-1148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 189. | Basso D, Millino C, Greco E, Romualdi C, Fogar P, Valerio A, Bellin M, Zambon CF, Navaglia F, Dussini N. Altered glucose metabolism and proteolysis in pancreatic cancer cell conditioned myoblasts: searching for a gene expression pattern with a microarray analysis of 5000 skeletal muscle genes. Gut. 2004;53:1159-1166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 190. | Borger DR, Tanabe KK, Fan KC, Lopez HU, Fantin VR, Straley KS, Schenkein DP, Hezel AF, Ancukiewicz M, Liebman HM. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist. 2012;17:72-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 515] [Cited by in F6Publishing: 552] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 191. | McCune SA, Foe LG, Kemp RG, Jurin RR. Aurintricarboxylic acid is a potent inhibitor of phosphofructokinase. Biochem J. 1989;259:925-927. [PubMed] [Cited in This Article: ] |
| 192. | Lolis E, Petsko GA. Crystallographic analysis of the complex between triosephosphate isomerase and 2-phosphoglycolate at 2.5-A resolution: implications for catalysis. Biochemistry. 1990;29:6619-6625. [PubMed] [Cited in This Article: ] |
| 193. | Caplan NA, Pogson CI, Hayes DJ, Blackburn GM. Novel bisphosphonate inhibitors of phosphoglycerate kinase. Bioorg Med Chem Lett. 1998;8:515-520. [PubMed] [Cited in This Article: ] |
| 194. | Rigden DJ, Walter RA, Phillips SE, Fothergill-Gilmore LA. Polyanionic inhibitors of phosphoglycerate mutase: combined structural and biochemical analysis. J Mol Biol. 1999;289:691-699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 195. | Spring TG, Wold F. Studies on two high-affinity enolase inhibitors. Chemical characterization. Biochemistry. 1971;10:4649-4654. [PubMed] [Cited in This Article: ] |
| 196. | Spoden GA, Mazurek S, Morandell D, Bacher N, Ausserlechner MJ, Jansen-Dürr P, Eigenbrodt E, Zwerschke W. Isotype-specific inhibitors of the glycolytic key regulator pyruvate kinase subtype M2 moderately decelerate tumor cell proliferation. Int J Cancer. 2008;123:312-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |