Published online Jan 7, 2016. doi: 10.3748/wjg.v22.i1.37
Peer-review started: April 24, 2015
First decision: June 2, 2015
Revised: June 25, 2015
Accepted: September 2, 2015
Article in press: September 2, 2015
Published online: January 7, 2016
Excessive consumption of alcoholic beverages is a serious cause of liver disease worldwide. The metabolism of ethanol generates reactive oxygen species, which play a significant role in the deterioration of alcoholic liver disease (ALD). Antioxidant phytochemicals, such as polyphenols, regulate the expression of ALD-associated proteins and peptides, namely, catalase, superoxide dismutase, glutathione, glutathione peroxidase, and glutathione reductase. These plant antioxidants have electrophilic activity and may induce antioxidant enzymes via the Kelch-like ECH-associated protein 1-NF-E2-related factor-2 pathway and antioxidant responsive elements. Furthermore, these antioxidants are reported to alleviate cell injury caused by oxidants or inflammatory cytokines. These phenomena are likely induced via the regulation of mitogen-activating protein kinase (MAPK) pathways by plant antioxidants, similar to preconditioning in ischemia-reperfusion models. Although the relationship between plant antioxidants and ALD has not been adequately investigated, plant antioxidants may be preventive for ALD because of their electrophilic and regulatory activities in the MAPK pathway.
Core tip: The metabolic process of ethanol generates reactive oxygen species, which play a significant role in the deterioration of alcoholic liver disease (ALD). Antioxidant phytochemicals, such as polyphenols, upregulate the expression of antioxidant enzymes and peptides via the Kelch-like ECH-associated protein 1-NF-E2-related factor-2 pathway, which leads to antioxidant responsive elements in animal models. Furthermore, these antioxidants alleviate cell injury caused by oxidants or inflammatory cytokines via impairment of hyperactivation of mitogen-activating protein kinase pathways, similar to preconditioning in ischemia-reperfusion models. Although the relationship between plant antioxidants and ALD has not been adequately investigated, plant antioxidants may be preventive for ALD.
- Citation: Han KH, Hashimoto N, Fukushima M. Relationships among alcoholic liver disease, antioxidants, and antioxidant enzymes. World J Gastroenterol 2016; 22(1): 37-49
- URL: https://www.wjgnet.com/1007-9327/full/v22/i1/37.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i1.37
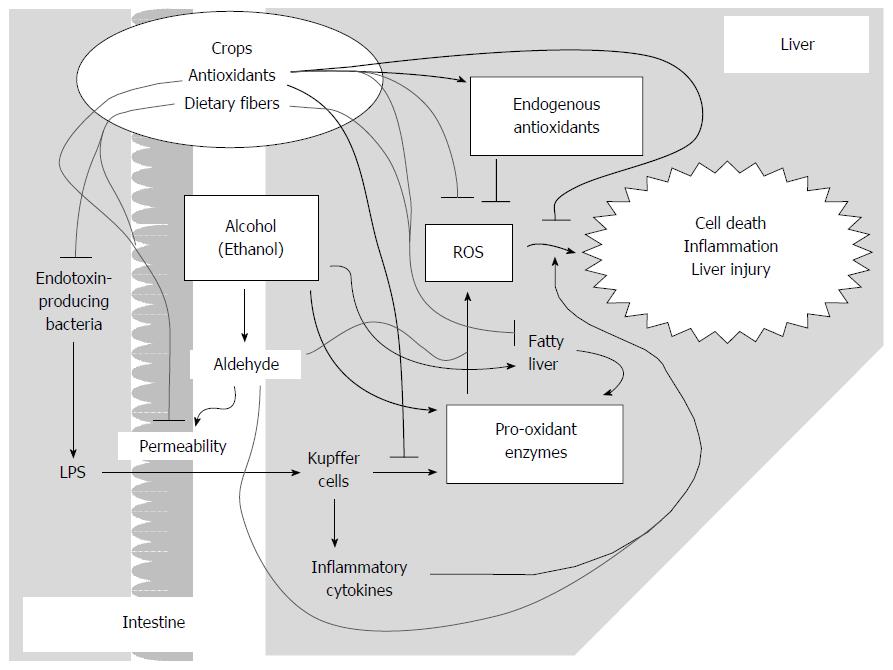
Humans are surrounded by many chemicals, including nutrients, phytochemicals, food additives, pharmaceuticals, and drugs. Although the intestine and liver absorb and metabolize many types of chemicals[1] for utilization or detoxification[2], some become more toxic once metabolized[3]. Ethanol, which is a component of alcoholic beverages, is one of the most common and abundant chemicals in daily life. Consuming ethanol can be relaxing and provides other benefits, but excessive drinking can be harmful physically and mentally and may decrease quality of life. Moderate consumption of alcohol has been shown to reduce the risks of cardiovascular disease[4] and non-alcohol fatty liver disease[5]. With moderate intake, most ethanol is oxidized by alcohol dehydrogenase and catabolized to acetaldehyde, which is subsequently catabolized to acetate via aldehyde dehydrogenase in the mitochondria. However, with binge drinking, ethanol is predominately metabolized to acetaldehyde via cytochrome P450, family 2, subfamily E, polypeptide 1 (CYP2E1), which comprises a microsomal ethanol-oxidizing system[6] that is involved in the generation of reactive oxygen species (ROS)[7-9]. Despite much evidence demonstrating a role for CYP2E1 in alcoholic liver disease (ALD), several of our studies have demonstrated that consumption of ethanol-containing diets significantly increased hepatic CYP2E1 levels without significantly affecting plasma alanine aminotransferase (ALT) activity (unpublished data). These findings support the existence of a potent endogenous antioxidant system that can prevent potential damage via the excessive expression of CYP2E1[10].
Binge drinking may cause liver injury, as demonstrated by increased blood levels of ALT, aspartate aminotransferase (AST), and/or lactate dehydrogenase (LDH)[11-14] and lipid accumulation in the liver-alcoholic fatty liver[12,13,15,16]. Hepatic functions are gradually lost with the progression of ALD[11], which is one of the most critical causes of cirrhosis[11,17]. Three mechanisms have been proposed to cause alcoholic liver injury: (1) acetaldehyde toxicity[18]; (2) metabolic generation of ROS or exposure to oxidative stress[10,19-21]; and (3) provocation of an immune response that causes oxidative stress in hepatocytes[13,22-24]. ALD patients appear to exhibit oxidative stress[11]; thus, increasing defense activities against this stress is important in the prevention of ALD.
In mammals, ROS is scavenged by antioxidant enzymes, such as superoxide dismutase (SOD) and catalase, and antioxidant substances, such as vitamins and glutathione (GSH) in collaboration with glutathione peroxidase (GPx) and glutathione reductase (GR)[25]. In previous studies, the induction and/or restoration of these substances and enzymes, which are reduced by ethanol administration, appeared to ameliorate ALD[12,13,23,26]. Some vitamins exhibit antioxidant activity and are reduced in the ALD model[27-29]. They are also deficient in ALD patients, although if present in sufficient quantities, may contribute to the prevention of oxidative stress[30]. Vitamin E is not only a lipophilic antioxidant but also may improve lipid metabolism via interaction with lipid accumulation-related proteins, namely patatin-like phospholipase domain containing 3 (PNPLA3) and microsomal triglyceride transfer protein[31]. However, several clinical studies have identified only partial effects of vitamin E in ALD[32,33]. Therefore, the induction of antioxidant enzymes may be more effective than vitamin supplementation in the prevention of ALD.
A trend in gastronomic culture is the exclusion of low molecular weight phytochemicals during plant breeding or processing because of their toxicity, taste, or deteriorating color. However, phytochemicals have recently received attention for their physiological activities in mammals. Many types of phytochemicals abundant in fruit and vegetables are known to have antioxidant activity. Although research efforts have focused on phenolic compounds due to their direct scavenging activity of ROS[34,35], their direct activity towards endogenous ROS appears limited in mammals because of their relatively low concentrations in the bloodstream[2,36,37]. However, many types of polyphenols, non-phenolic phytochemicals, and antioxidant-rich plant fractions have recently been reported to elicit an antioxidant defense system against liver damage induced by ethanol[34,35,38,39], other chemicals[40-43], or abnormal metabolism[21,44] to reduce oxidative stress and cell death[34,42,43,45] and to improve lipid metabolism[12,16,44,46] in various organs. In addition, some phytochemicals change both phase I and phase II enzymes of drug metabolism, including CYP2E1[7,13,16,47]. Recent reports indicated that some polyphenols can improve epithelial cell junctions[48-51], indicating a role for the hepatic immune response. These findings suggest that phytochemicals could potentially have a comprehensive preventive effect on ALD. However, the physiological activities of phytochemicals in the prevention of ALD have not been well recognized.
In this review, we discuss the physiological activities of phytochemicals and the mechanisms for cell injury, the regulation of antioxidant and pro-oxidant enzyme expression, and concomitant intestinal permeability. Herein, “antioxidants” are defined as the phytochemicals that elicit or enhance the antioxidant defense system, regardless of their radical scavenging activity. Because information regarding the effects of antioxidants in ALD patients or animal models is insufficient for discussion, various oxidative stress models in animals and cells are included. In particular, the mechanisms of non-alcoholic fatty liver disease (NAFLD) may comprise, in part, the mechanisms of ALD because these two diseases likely share many common pathways[31].
As a cause of oxidative stress, ROS are generated by pro-oxidant enzymes, such as CYP2E1 in hepatocytes[7,52,53] and NADPH oxidase (NOX) in Kupffer cells (liver-dwelling macrophages)[25]. In addition, populations of intestinal bacteria that comprise the intestinal environment have been suggested to be involved in ALD via stimulation of the immune system. For example, lipopolysaccharides (LPS) derived from intestinal bacteria[15,24,54] activate NOXs and produce inflammatory cytokines[55-58] in macrophages. Acetaldehyde increases the permeability of LPS between intestinal epithelial cells[15,59,60], which is also involved in the deterioration of ALD. Dietary polyunsaturated fatty acids are also thought to enhance oxidative stress[15,29] and are a source of prostaglandins[61]. In a previous study, ethanol administration increased the plasma prostaglandin E2 level[62], and some prostaglandins are thought to cause inflammation in NAFLD[61,63]. These data suggest that prostaglandins enhance deterioration of ALD; however, the influence of antioxidants on prostaglandins will not be detailed here.
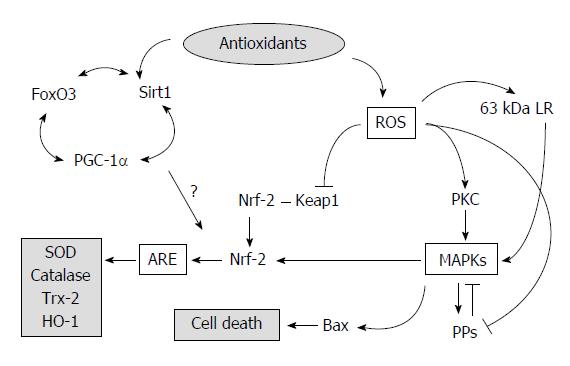
As shown in Figure 1, oxidative stress stimulates intracellular events via the mitogen-activating protein kinase (MAPK)[64] pathway, as initiated by the activation of protein kinase C (PKC)[30,65,66] or the degradation of protein phosphatases (PPs)[67]. These signals activate the Kelch-like ECH-associated protein 1 (Keap1)-NF-E2-related factor-2 (Nrf2) pathway, which leads to antioxidant responsive element (ARE)[45,68-70]. However, MAPK hyperactivation also leads to cell death via activation of the Bax/Bcl-2 pathway[71,72]. In addition, antioxidant enzymes have been reported to be induced via several intracellular pathways, such as the Keap1-Nrf2-ARE pathway[45,69,70,73] and the Sirt1 (sirtuin-1)-FoxO3 (forkhead winged-helix box class O3 transcription factor)-PGC-1a (PPARγ coactivator-1a) pathway[45,68]. The regulation of Sirt1 and Nrf2 levels has also been reported[45], which implies cross-talk between both pathways, whereas the activation of Sirt1 and resveratrol, an activator of Sirt1, have been reported to inhibit the DNA-binding activity of Nrf2 via deacetylation in vitro[74]. Taken together, substances that deactivate or normalize MAPKs and/or activate ARE or Sirt1[45,75] are potential candidates for the prevention of ALD, but the mechanisms are unknown.
In mammals, SOD generates hydrogen peroxide, which is catabolized to a hydroxyl radical by catalase and detoxified by GSH in collaboration with GPx[25]. The oxidized glutathione form is recruited to GSH by GR with NAD(P)H[76]. Heme oxygenase-1 (HO-1) contributes to the antioxidant system because of the production of bilirubin as a redox substance.
It has been suggested that the hepatic catalase level is negatively associated with the severity of alcoholic liver injury[10] and that SODs scavenge hydroxyl peroxides generated in the cytosol and mitochondria, thereby terminating autoxidation. Thus, catalase and SODs are essential for the antioxidant system. There are three isozymes of SOD in the cytosol, mitochondria, and extracellular matrix: CuZn-SOD, Mn-SOD, and extracellular SOD. SOD levels have been shown to be regulated by MAPK activity[77]. GSH is not an enzyme but a redox tripeptide that acts as a proton donor. GSH levels, GPx content, and/or GR content were reduced in rats fed ethanol diets and, in some cases, ALD animals[16,23,62] or under other oxidative conditions[3,78]. The FoxO transcriptional factor is involved in GPx and Sirt1 protein expression[79]. These findings indicate that in addition to catalase and SOD, GSH is essential for reducing hepatic oxidative stress.
Under oxidative conditions, HO-1 appears to be rapidly induced via the Keap1-Nrf2 pathway[45,69,80,81]. This enzyme may also be involved in the immune response[55]. Furthermore, in ALD model animals, HO-1 levels have been reported to be reduced[13,16,82]. Adiponectin has received recent attention because of its anti-inflammatory functions via Sirt1 activation, HO-1 induction, and NOX suppression in Kupffer cells[55]. However, the blood concentration of this adipokine was higher in ALD patients compared with controls[83] or equal to the controls in ALD animals[84], which suggests that adiponectin may be less effective against ALD than antioxidants.
Thioredoxin (Trx) is a ubiquitous scavenger of oxidative species. Endogenous Trx is reported to be reduced by ethanol ingestion; however, the levels can be restored by supplementation with exogenous Trx, which has been demonstrated to ameliorate the symptoms of ALD[84]. Because Trx is a peptide, it must be digested in the digestive system, indicating that it is difficult for exogenous Trx to directly scavenge hepatic ROS.
In microsomes, CYP2E1 is a phase I enzyme of drug metabolism that adds a hydroxyl residue to chemicals to increase hydrophilia and may generate ROS[7-9]. Chronic ingestion of ethanol and other small chemicals increase hepatic CYP2E1. CYP2E1 induction has also been demonstrated in animals with NAFLD[52,85] and hepatic insufficiency. Insulin signaling may suppress CYP2E1 expression[53] via the Akt pathway but not the MAPK pathway[86], with subsequent expression of certain microRNAs[87].
Macrophage-like cells, including Kupffer cells, express NOXs and generate ROS with the consumption of NAD(P)H[24] to eliminate xenobiotics[25]. Many isoforms of NOXs have been identified, and NOX-2 is uniquely expressed in phagocytes. NOX expression was regulated via the Keap1-Nrf2 pathway in a mouse glial-neural co-cultured system[88] in which NOX-2 predominantly caused oxidative stress. In ALD animals, NOX-2 in Kupffer cells was activated by LPS[55]. In addition, Kupffer cells produce inflammatory cytokines[13,24,55], such as tumor necrosis factor alpha (TNF-α) and interleukin-6. Thus, the reduction of NOXs and inflammatory cytokines are important for ALD.
Given the gut-liver axis in ALD, intestinal conditions play a considerable role in ALD severity, particularly conditions mediated by LPS[15,60]. In the large intestine in humans (or the cecum in animals), an enormous number of intestinal bacteria live and ferment undigested food matter, flaked epithelial cells, and digestive fluid[25]; some of these species generate LPS, which provokes the host’s immune system[15]. Small amounts of LPS can pass through gaps in the epithelial cells into the intestine. Ethanol or its metabolites are reported to widen this gap[15,59]. Therefore, improving intercellular junctions or reducing LPS-producing bacteria may have a partial preventive effect on ALD[15].
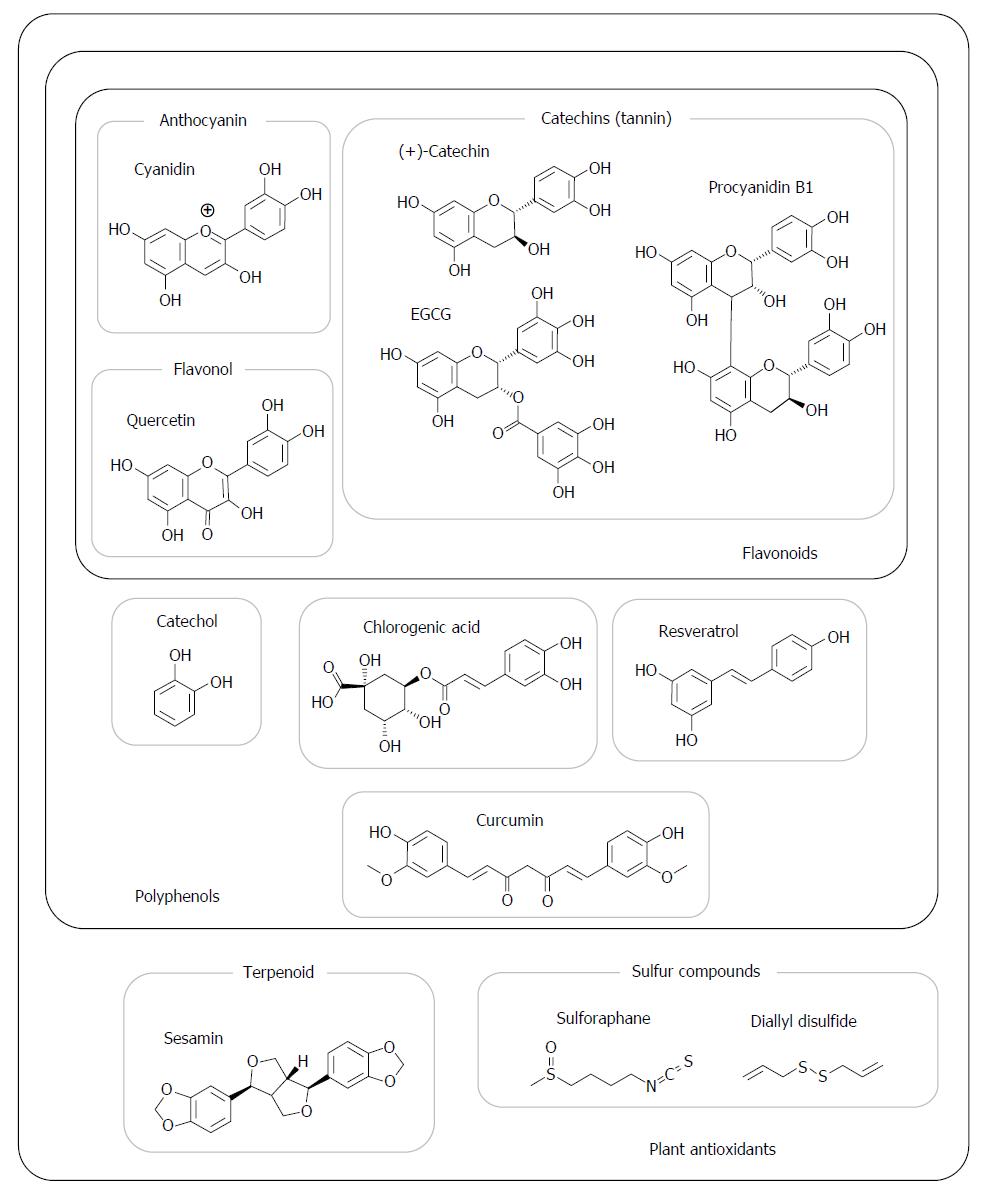
Figure 2 shows the structures of representative antioxidants abundant in fruit and vegetables. Polyphenol is a generic name for compounds that have a mono- or polycyclic structure with hydroxyl residues. Flavonoids, including anthocyanins, catechins, and flavonols, form one of the largest groups of polyphenols. Anthocyanins have a red, purple, or blue color in grapes[42], berries[34], seed coats[89], and root crops[37,77]. Catechins include epicatechin, epigallocatechin, and epigallocatechin galate (EGCG) and are sometimes referred to as “tannins”[35]. Proanthocyanidins are polymers of catechins (but not anthocyanin despite the similarity in names); they are categorized as catechins and are widely abundant in crops, particularly tea[27,90], apples[91], and grapes[92]. Quercetin, kaempferol, and isorhamnetin belong to the flavonol group and are ubiquitous in plants. Narirutin and hesperidin belong to the flavanone group and are abundant in the albedo of citrus peel[14,23]. Resveratrol is categorized as a stilbenoid, a phytoalexin, and is present in wine[93] and grapes[42]; it has recently received substantial attention for its physiological functions. Chlorogenic acid is a caffeic acid derivative and one of the most widely consumed polyphenols because of its abundance in coffee and other plants. Alkaloids, such as berberine[46], are also included in the polyphenol group. Curcumin, a curcuminoid present in turmeric, has a yellow color and also belongs to polyphenols.
Lignans, a terpenoid whose metabolites exert estrogenic activity in the lumen, as well as isoflavones and coumestans possess antioxidant activity. Sulfide and thiocyanate compounds are present in garlic[12,82], onions[47], and Brassicaceae plants[16] and are reported to be chemopreventive.
In animal models, quercetin ameliorated lipid metabolism and ethanol-induced liver damage by inducing antioxidant enzymes, increasing GSH levels, and reducing CYP2E1 activity[20,39]. Quercetin also inhibited the activity and expression of CYP2E1 in human hepatocytes[20,94], which was consistent with in vivo findings. In non-alcoholic steatohepatitis animals, quercetin ingestion increased hepatic catalase, SOD, GPx, and GR activities and the GSH level[21] and reduced hepatic lipid accumulation and CYP2E1 expression[21,85]. A computer simulation predicted the involvement of quercetin in PGC1a and PNPLA3[31]. Furthermore, hyperoside (quercetin-3-O-galactoside) has been reported to increase cell viability and HO-1 activity via MAPKs and ARE[95] in L-02 cells.
Pigments from grapes[42], colored potatoes[77], and black soybean seed coats[89] that contain abundant anthocyanin have been reported to induce antioxidant enzymes via the alteration of MAPK activities in cells in other oxidative conditions. An anthocyanin fraction from bilberries appears effective in improving lipid metabolism via the AMP-activated protein kinase pathway[96]; however, its involvement in ALD has not been assessed. Alcohol-free red wine increased the blood antioxidant capacity in a human study[97], which suggests a preventive function of the polyphenol fraction in red wine against ALD. However, other studies have demonstrated that alcohol-free red wine worked with ochratoxin A to increase the intercellular permeability in Caco-2/TC7 cells[98], and alcohol-containing red wine increased hepatic and renal CYP2E1 expression in rats, whereas ethanol did not[99]. Malvidin, an anthocyanin in red wine, has been reported to attenuate MAPK activity, which was promoted by LPS[64], and to enhance PP activity in RAW 264.7 macrophage cells. An anthocyanin-rich extract from colored potato increased Mn-SOD expression via extracellular signal regulated kinase (ERK) activation in HepG2 cells[77]. It has also been reported that an ethanol-induced acute gastric lesion was prevented by the ingestion of strawberry extract rich in anthocyanin prior to ethanol treatment via the induction of gastric antioxidant enzymes[34].
In animal studies, catechin- and tannin-rich extracts from pecan nut shells improved ALD symptoms by restoring antioxidant enzymes[35,38]. A tea extract rich in catechins reduced CYP2E1 expression and hepatic lesion via paracetamol injection[92], and a diet that contained EGCG improved hepatic injury; although there was no reduction in hepatic CYP2E1 levels[100]. In a clinical study, EGCG-rich green tea and its extract also increased the blood GSH level[90]. The ingestion of green tea extract also restored antioxidant activity in the brain that had been decreased by ethanol and aging[28]. Furthermore, catechins have been reported to suppress the expression of NOX and inflammatory cytokines in macrophages[56], dendrocytes[57], and human cerebral microvascular endothelial cells (hCMEC)[101] as well as restore antioxidant enzymes in human neuroblastoma cells[102]. Catechins have both antioxidant and pro-oxidant activities. They have recently been reported to stimulate the 63 kDa laminin receptor[56,57,101,103], which ROS may initiate[104], and consequently to calm over-activation of the immune system via the inactivation of the Toll-like receptor (TLR) 2 and 4 pathways. TLR 4, in particular, plays a central role in Kupffer cell stimulation with LPS and the induction of ALD deterioration[57]. Dietary catechins may thus contribute to the impairment of ROS generation via LPS and the prevention of ALD.
Citrus flavonoids, narirutin, and glycosylated citrus flavonoids also improved ALD and reduced inflammatory cytokine levels[14,23].
Resveratrol (Figure 2) restores or induces antioxidant enzymes in ALD model rats[93], lung fibroblasts[105], and rats with spontaneous hypertension[75] and diabetes[44,73] via the activation of sirtuins in some cases. In vitro, resveratrol stimulated HO-1 induction via the MAPK-Nrf2 pathway in PC12 cells[81]. Thus, red wine consumption is likely to be superior to other alcoholic beverages in the prevention of ALD. Resveratrol concentrations in wine may be insufficient to prevent ALD; however, it may be responsible for the “French paradox”[106]. Resveratrol has been reported to activate monocytes and produce inflammatory cytokines in vitro, which indicates that provoking the immune system with resveratrol may not prevent the deterioration of ALD[107]. Thus, excessive red wine consumption should not be recommended. Polydatin, a resveratrol glycoside, stimulates Sirt1 and Nrf2 and induces antioxidant enzymes in glomerular cells[45].
Chlorogenic acid (Figure 2) and caffeic acid restored the hepatic activity of SOD and GPx and hepatic injuries promoted by methamphetamine injection for 7 d[43].
Honokiol, identified in Magnolia officinalis[19], improved ALD, restored the hepatic GSH content and SOD activity, and reduced inflammatory cytokine levels in an ALD animal model[19].
Hispidin, a fungal polyphenol with PKC-inhibitory activity, increased HO-1 and catalase activities in H9c2 cardiomyoblast cells[65].
Berberine is a benzyl isoquinoline alkaloid in the Coptis genus that has been reported to reduce ALD symptoms, increase levels of GSH and PGC1a, and normalize CYP2E1 expression in the livers of animals fed an alcohol-containing diet[46].
The sulfur-containing compounds (Figure 2) diallyl disulfide and garlic oil have been reported to improve alcoholic hepatic injury[12] by increasing HO-1 levels via the Nrf2 pathway and increasing the GSH level in vivo[82] and in vitro[94]. A similar preventive effect has also been identified in diallyl sulfide treatment in astrocytes[30]. Sulforaphane has been reported to act as an inducer of HO-1[16], which suggests that these compounds may be useful in the treatment of ALD. In addition to restoring HO-1 levels, sulforaphane improved hepatic lipid accumulation in ALD animals[16]. The consumption of onion powder, which is rich in sulfide compounds and flavonols, has also been reported to reduce hepatic CYP2E1 levels in normal rats[47].
Oleanolic acid, a triterpenoid, restored antioxidant enzymes and increased nucleic Nrf2 levels and improved ALD[13]. Sesamin (Figure 2) is a well-characterized terpenoid in sesame seeds that may contribute to the reduction of fatty liver by promoting β-oxidation of fatty acids and inducing hepatic aldehyde dehydrogenase[108,109]. Maslinic acid, a triterpenoid rich in basil, brown mustard, and other plants, has been reported to protect hepatic injury via acute ethanol toxicity[62]. These data suggest that some types of terpenoids may improve the symptoms of ALD.
Curcumin (Figure 2), but not resveratrol, has been reported to restore hepatic antioxidant enzymes reduced by aflatoxin in rats[110]. Curcumin also increased antioxidant enzymes as well as Nrf2 and HO-1 levels in quails under heat stress[111].
Mangiferin, identified in mango[112], is a xanthine derivative that has been reported to restore pulmonic and hepatic antioxidant enzyme levels reduced by benzo(a)pyrene in mice[3].
Plant extracts that contain significant amounts of antioxidants also prevent oxidative damage in various other organs. An extract from black tea[27] improved ALD symptoms in rats. The extracts from apples[91], Amorphophallus commutatus[40], cinnamon[113], and hibiscus[22,41] partially normalized hepatic oxidative stress induced by chemical toxins.
Alcoholic fatty liver is a predictive symptom of ALD, and hepatic inflammation is also present in non-alcohol steatohepatitic animals[21,41,52]. Moreover, a computer simulation predicted many common pathways between alcoholic fatty liver and NAFLD that were associated with inflammation, lipid metabolism, and some immunity[31]. These data suggest that a reduction in lipids in the liver may lead to an improvement in liver injuries[16,19,100]. In addition to the induction of antioxidant enzymes, some plant antioxidants have recently been reported to improve lipid metabolism and reduce hepatic lipid accumulation[19,39,46], which may also contribute to the amelioration of ALD.
Antioxidants, such as quercetin, resveratrol, EGCG, and naringenin, prevent the downregulation of junction proteins, namely, Zo-1 and/or Occludins, and consequently enhance intercellular barrier functions in vitro[49] and in vivo[50]. In contrast, EGCG has been reported to disturb the barrier function of hepatic epithelial cells[114] because of ROS-induced ERK activation. In addition to intestinal cell models, cocoa polyphenol extract improved barrier functions disturbed by a high glucose condition in retinal pigment epithelium cells[51]. Cocoa polyphenol extract and resveratrol also attenuated the permeability of renal cell junctions in vitro[48,115], and EGCG increased the adhesion of hCMEC[101]. The tightness of cellular junctions regulated by antioxidants may be involved in the severity of ALD and should be elucidated.
Cellular oxidative stress is caused by many factors, such as exposure to humoral factors[22,75], enzymatic generation of ROS[7-9,24], metabolites of chemicals[41,91,102,116], or the mitochondrial respiratory chain[39]. Two major mechanisms may be proposed for hepatic injury prevention via oxidation: (1) the impairment of oxidative signaling that leads to cell death; and (2) the activation of the Keap1-Nrf2 pathway, which results in the induction of antioxidant enzymes.
As a leading mechanism, “preconditioning” in ischemia-reperfusion models has been proposed to alleviate tissue damage. In ischemia-reperfusion models, excessive ROS are present following reperfusion, whereas slight ischemic-reperfusion pretreatment to tissues or cells alters MAPK activities and interferes with cellular damage[117-119]. It has been reported that ROS stimulate PKC, MAPKs, and subsequent events that lead to cell death[89] or induce an antioxidant system (Figure 1). MAPKs appear to activate both PPs[66,120] and Nrf2[69]. Once activated, PPs may deactivate not only MAPKs but also other phosphorylated proteins related to the MAPK signaling pathways[66], which may lead to a comprehensive impairment of MAPK signaling. Despite their antioxidant activity, polyphenols also have a slight pro-oxidant activity[72,121]. This impact may increase MAPK and PP activity[103] or PP stability[120] prior to crucial oxidative stress by ROS. At minimum, PPs activated by antioxidants may partially inhibit MAPK pathway activation. Following pretreatment with plant antioxidants, the hyperactivation of MAPKs by injuring stimuli appears to decrease[22,41,48,64]. These findings may support the preconditioning hypothesis[1]. Taken together, ROS and/or MAPK are key regulators of both cell injury and antioxidant enzyme induction.
In addition, this mechanism can explain the effects of antioxidants on the barrier functions of epithelial cells. Junction proteins and the intercellular barrier function are disturbed by oxidative stress[48,114]. Antioxidants have been reported to exhibit minimal activity to generate ROS[114,121] and subsequently activate MAPKs, which disturbs barrier function in vitro[114]. However, antioxidant pretreatment may diminish excessive oxidative stress, as previously discussed, which leads to the protection of barrier function[49,50].
It has been suggested that ROS (and electrophilic reagents) directly activate the Keap1-Nrf2 pathway. Keap1 is a sensor of intracellular oxidative stress and couples with Nrf2[122]. Once Keap1 is oxidized, Nrf2 is released, moves to the nuclei, and activates ARE. Regarding the relationship between chemical structures and antioxidant activities, it has been suggested that electrophilic compounds, such as flavonoids, curcumin, and thiocyanate-related compounds, stimulate the Keap1-Nrf2 pathway[122]. Satoh et al[123] proposed the importance of ortho- or para-positions of hydroxyl residues in the benzene structure, which result in hydroquinone and catechol, respectively (Figure 2), because of their electrophilic residue. Some flavonoid compounds have a catechol structure (Figure 2), which indicates an interaction between flavonoids and Keap1. These results may support the hypothesis proposed by Satoh et al[123].
This hypothesis suggests that antioxidants directly activate Keap1. However, some antioxidants appear to induce antioxidant enzymes via MAPK activation despite the upper proteins of Keap1 (Figure 1), as demonstrated with specific inhibitors of MAPKs that diminished the induction[77] or activation of Nrf2[81]. Antioxidants may contribute to the induction of antioxidant enzymes via MAPK pathways rather than through direct activation of Keap1. Moreover, resveratrol has a resorcinol structure rather than a catechol structure. Resorcinol has less electrophilic activity than catechol[123]; however, it appears to stimulate Nrf2[122]. This mechanism must also be elucidated.
In in vivo studies, the ingestion of antioxidants induces (or tends to induce) antioxidant enzymes in the lung[3], thymus[124], brain[28,125], and kidney[45], despite very low concentrations in the bloodstream[2,36,37]. These reports imply that there is an intermediate signal by polyphenols, such as nerve and/or humoral pathways, rather than direct stimulation of cells or organs; they may also be explained by remote ischemic preconditioning[117]. This preconditioning suggests that some types of stimuli can regulate MAPK activities in remote organs.
Even ubiquitous plant antioxidants, such as anthocyanins and flavonols, appear to have many physiological activities, indicating that botanical substances can provoke the antioxidant system. Apart from oxidative stress via lipid accumulation, lipids also appear to be a central cause of ALD. For example, prostaglandins, which are initiated by phospholipase (PL) A2 and activated by cyclooxygenases[61], are involved in inflammatory events, and PNPLA3 has been suggested to have PLA2 activity and to regulate hepatic lipid accumulation[63]. Therefore, the regulation of prostaglandins and/or expression of their related proteins may be critical for the improvement of ALD.
Fruits and vegetables are great sources of antioxidants as well as dietary fibers (DFs)[126], which were once considered to be unwanted materials or non-nutrients. It is now well established that the ingestion of DFs improves lipid metabolism and reduces hepatic lipids[127,128]. Some types of DFs, particularly water-soluble fibers, promote the excretion of lipids into feces and the synthesis of short-chain fatty acids (SCFA) in the intestine[126,129], which are proposed as prebiotics. Oral ingestion of butyrate, a type of SCFA produced from DF, promotes junction protein expression and an increase in intestinal barrier function[130]. These findings also suggest the potential of DFs in the prevention of ALD. Thus, intact fruits and vegetables, including both antioxidants and DF, are worthy of consideration for ALD prevention.
Mammals often intrinsically treat plant chemicals as xenobiotics and have developed metabolic systems against phytochemicals[1]. The human body evolved with environmental factors, including phytochemicals and DFs. The data reviewed here imply the necessity for the unwanted materials to elicit an accomplished defense system, a barrier function in the intestine and a chemical metabolizing system in the intestine, and liver against xenobiotic substances.
However, most of these data are derived from animal and cell studies. In these studies, antioxidants may, in some cases, be overdosed[75], which makes it difficult to justify their effectiveness in humans, particularly ALD patients who may have impaired liver functions[11]. As previously reported, vitamin E supplementation only partially improved ALD[32,33] despite its effectiveness in cell studies. Thus, it is important for future studies to accumulate clinical data regarding the relationships among ALD, antioxidants, and antioxidant enzymes.
In conclusion, plants have a potential role in the prevention of ALD (Figure 3). Although most individuals are aware that abstinence from alcohol is the most effective way to prevent ALD, it is recognized that this is not easy. Therefore, it is important to improve our defense system against ALD. Many types of plant antioxidants with electrophilic activity may activate antioxidant enzymes or peptides under oxidative conditions and alleviate ALD, which may occur via a mechanism that is somewhat similar to preconditioning in ischemia-reperfusion models[117-119]. The antioxidants reviewed here are common in vegetables and fruits, which can be easily consumed. Moreover, plants contain abundant amounts of DF and vitamins. Vitamins are wasted by binge drinking[27,28], and DFs can improve lipid metabolism and intestinal conditions[127,128] in mammals. Therefore, non-processed food materials may have considerable intrinsic potential. Clearly, ALD patients should be administered appropriate medications to facilitate recovery from crucial damage. However, fresh vegetables and fruits may be more effective than processed foods in comprehensively preventing hepatic damage induced by alcohol. Antioxidants commonly taste bitter, and DFs appear to exhibit a bad texture; thus, they have been eliminated from foods over centuries. However, humans have evolved alongside phytochemicals and DFs to overcome these issues. Thus, an approach that elicits the intrinsic potential of the human body to prevent ALD and other lifestyle-related disorders should be reconsidered.
The authors would like to thank Enago (http://www.enago.jp) for the English language review.
P- Reviewer: Pirola CJ S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Wang CH
| 1. | Wu B, Kulkarni K, Basu S, Zhang S, Hu M. First-pass metabolism via UDP-glucuronosyltransferase: a barrier to oral bioavailability of phenolics. J Pharm Sci. 2011;100:3655-3681. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 204] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 2. | Del Rio D, Stalmach A, Calani L, Crozier A. Bioavailability of coffee chlorogenic acids and green tea flavan-3-ols. Nutrients. 2010;2:820-833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Rajendran P, Ekambaram G, Sakthisekaran D. Cytoprotective effect of mangiferin on benzo(a)pyrene-induced lung carcinogenesis in swiss albino mice. Basic Clin Pharmacol Toxicol. 2008;103:137-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Nova E, Baccan GC, Veses A, Zapatera B, Marcos A. Potential health benefits of moderate alcohol consumption: current perspectives in research. Proc Nutr Soc. 2012;71:307-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Sookoian S, Castaño GO, Pirola CJ. Modest alcohol consumption decreases the risk of non-alcoholic fatty liver disease: a meta-analysis of 43 175 individuals. Gut. 2014;63:530-532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 6. | Beier JI, McClain CJ. Mechanisms and cell signaling in alcoholic liver disease. Biol Chem. 2010;391:1249-1264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 7. | Cederbaum AI. Molecular mechanisms of the microsomal mixed function oxidases and biological and pathological implications. Redox Biol. 2015;4:60-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Das S, Seth RK, Kumar A, Kadiiska MB, Michelotti G, Diehl AM, Chatterjee S. Purinergic receptor X7 is a key modulator of metabolic oxidative stress-mediated autophagy and inflammation in experimental nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2013;305:G950-G963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Abdelmegeed MA, Banerjee A, Jang S, Yoo SH, Yun JW, Gonzalez FJ, Keshavarzian A, Song BJ. CYP2E1 potentiates binge alcohol-induced gut leakiness, steatohepatitis, and apoptosis. Free Radic Biol Med. 2013;65:1238-1245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 147] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 10. | Powell CL, Bradford BU, Craig CP, Tsuchiya M, Uehara T, O’Connell TM, Pogribny IP, Melnyk S, Koop DR, Bleyle L. Mechanism for prevention of alcohol-induced liver injury by dietary methyl donors. Toxicol Sci. 2010;115:131-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Chen YL, Chen LJ, Bair MJ, Yao ML, Peng HC, Yang SS, Yang SC. Antioxidative status of patients with alcoholic liver disease in southeastern Taiwan. World J Gastroenterol. 2011;17:1063-1070. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 6] [Reference Citation Analysis (0)] |
| 12. | Raghu R, Liu CT, Tsai MH, Tang X, Kalari KR, Subramanian S, Sheen LY. Transcriptome analysis of garlic-induced hepatoprotection against alcoholic fatty liver. J Agric Food Chem. 2012;60:11104-11119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Liu J, Wang X, Liu R, Liu Y, Zhang T, Fu H, Hai C. Oleanolic acid co-administration alleviates ethanol-induced hepatic injury via Nrf-2 and ethanol-metabolizing modulating in rats. Chem Biol Interact. 2014;221:88-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Park HY, Ha SK, Eom H, Choi I. Narirutin fraction from citrus peels attenuates alcoholic liver disease in mice. Food Chem Toxicol. 2013;55:637-644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Zhong W, Li Q, Xie G, Sun X, Tan X, Sun X, Jia W, Zhou Z. Dietary fat sources differentially modulate intestinal barrier and hepatic inflammation in alcohol-induced liver injury in rats. Am J Physiol Gastrointest Liver Physiol. 2013;305:G919-G932. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Zhou R, Lin J, Wu D. Sulforaphane induces Nrf2 and protects against CYP2E1-dependent binge alcohol-induced liver steatosis. Biochim Biophys Acta. 2014;1840:209-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 17. | Manos MM, Leyden WA, Murphy RC, Terrault NA, Bell BP. Limitations of conventionally derived chronic liver disease mortality rates: Results of a comprehensive assessment. Hepatology. 2008;47:1150-1157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Guo R, Zhong L, Ren J. Overexpression of aldehyde dehydrogenase-2 attenuates chronic alcohol exposure-induced apoptosis, change in Akt and Pim signalling in liver. Clin Exp Pharmacol Physiol. 2009;36:463-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Yin HQ, Kim YC, Chung YS, Kim YC, Shin YK, Lee BH. Honokiol reverses alcoholic fatty liver by inhibiting the maturation of sterol regulatory element binding protein-1c and the expression of its downstream lipogenesis genes. Toxicol Appl Pharmacol. 2009;236:124-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Tang Y, Li Y, Yu H, Gao C, Liu L, Xing M, Liu L, Yao P. Quercetin attenuates chronic ethanol hepatotoxicity: implication of “free” iron uptake and release. Food Chem Toxicol. 2014;67:131-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Surapaneni KM, Jainu M. Comparative effect of pioglitazone, quercetin and hydroxy citric acid on the status of lipid peroxidation and antioxidants in experimental non-alcoholic steatohepatitis. J Physiol Pharmacol. 2014;65:67-74. [PubMed] [Cited in This Article: ] |
| 22. | Kao ES, Hsu JD, Wang CJ, Yang SH, Cheng SY, Lee HJ. Polyphenols extracted from Hibiscus sabdariffa L. inhibited lipopolysaccharide-induced inflammation by improving antioxidative conditions and regulating cyclooxygenase-2 expression. Biosci Biotechnol Biochem. 2009;73:385-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Park HY, Choi HD, Eom H, Choi I. Enzymatic modification enhances the protective activity of citrus flavonoids against alcohol-induced liver disease. Food Chem. 2013;139:231-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Cohen JI, Chen X, Nagy LE. Redox signaling and the innate immune system in alcoholic liver disease. Antioxid Redox Signal. 2011;15:523-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Kalyanaraman B. Teaching the basics of redox biology to medical and graduate students: Oxidants, antioxidants and disease mechanisms. Redox Biol. 2013;1:244-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 314] [Cited by in F6Publishing: 315] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 26. | Hashimoto N, Sekiguchi H, Masunaka A, Saito K, Yamauchi H, Noda T, Han KH, Fukushima M. Hepatic cytochrome P450 2E1 level rather than cecal condition contributes to induction of early stage of the alcoholic liver damage in rats. J Health Sci. 2009;55:356-362. [Cited in This Article: ] |
| 27. | Łuczaj W, Skrzydlewska E. Antioxidant properties of black tea in alcohol intoxication. Food Chem Toxicol. 2004;42:2045-2051. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Skrzydlewska E, Augustyniak A, Michalak K, Farbiszewski R. Green tea supplementation in rats of different ages mitigates ethanol-induced changes in brain antioxidant abilities. Alcohol. 2005;37:89-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Nanji AA. Use of dietary saturated fatty acids and vitamin E in the treatment of alcoholic liver disease. Asia Pac J Clin Nutr. 1997;6:46-48. [PubMed] [Cited in This Article: ] |
| 30. | Jin M, Ande A, Kumar A, Kumar S. Regulation of cytochrome P450 2e1 expression by ethanol: role of oxidative stress-mediated pkc/jnk/sp1 pathway. Cell Death Dis. 2013;4:e554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 31. | Sookoian S, Pirola CJ. The genetic epidemiology of nonalcoholic fatty liver disease: toward a personalized medicine. Clin Liver Dis. 2012;16:467-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Mezey E, Potter JJ, Rennie-Tankersley L, Caballeria J, Pares A. A randomized placebo controlled trial of vitamin E for alcoholic hepatitis. J Hepatol. 2004;40:40-46. [PubMed] [Cited in This Article: ] |
| 33. | Bjelakovic G, Gluud LL, Nikolova D, Bjelakovic M, Nagorni A, Gluud C. Antioxidant supplements for liver diseases. Cochrane Database Syst Rev. 2011;CD007749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Alvarez-Suarez JM, Dekanski D, Ristić S, Radonjić NV, Petronijević ND, Giampieri F, Astolfi P, González-Paramás AM, Santos-Buelga C, Tulipani S. Strawberry polyphenols attenuate ethanol-induced gastric lesions in rats by activation of antioxidant enzymes and attenuation of MDA increase. PLoS One. 2011;6:e25878. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 35. | Müller LG, Pase CS, Reckziegel P, Barcelos RC, Boufleur N, Prado AC, Fett R, Block JM, Pavanato MA, Bauermann LF. Hepatoprotective effects of pecan nut shells on ethanol-induced liver damage. Exp Toxicol Pathol. 2013;65:165-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Czank C, Cassidy A, Zhang Q, Morrison DJ, Preston T, Kroon PA, Botting NP, Kay CD. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: a (13)C-tracer study. Am J Clin Nutr. 2013;97:995-1003. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 439] [Cited by in F6Publishing: 422] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 37. | Suda I, Oki T, Masuda M, Nishiba Y, Furuta S, Matsugano K, Sugita K, Terahara N. Direct absorption of acylated anthocyanin in purple-fleshed sweet potato into rats. J Agric Food Chem. 2002;50:1672-1676. [PubMed] [Cited in This Article: ] |
| 38. | Bharrhan S, Koul A, Chopra K, Rishi P. Catechin suppresses an array of signalling molecules and modulates alcohol-induced endotoxin mediated liver injury in a rat model. PLoS One. 2011;6:e20635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 39. | Tang Y, Gao C, Xing M, Li Y, Zhu L, Wang D, Yang X, Liu L, Yao P. Quercetin prevents ethanol-induced dyslipidemia and mitochondrial oxidative damage. Food Chem Toxicol. 2012;50:1194-1200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 40. | Raj S, Gothandam KM. Hepatoprotective effect of polyphenols rich methanolic extract of Amorphophallus commutatus var. wayanadensis against CCl4 induced hepatic injury in swiss albino mice. Food Chem Toxicol. 2014;67:105-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Lee CH, Kuo CY, Wang CJ, Wang CP, Lee YR, Hung CN, Lee HJ. A polyphenol extract of Hibiscus sabdariffa L. ameliorates acetaminophen-induced hepatic steatosis by attenuating the mitochondrial dysfunction in vivo and in vitro. Biosci Biotechnol Biochem. 2012;76:646-651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Rodrigues AD, Scheffel TB, Scola G, Dos Santos MT, Fank B, Dani C, Vanderlinde R, Henriques JA, Coitinho AS, Salvador M. Purple grape juices prevent pentylenetetrazol-induced oxidative damage in the liver and serum of Wistar rats. Nutr Res. 2013;33:120-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 43. | Koriem KM, Soliman RE. Chlorogenic and caftaric acids in liver toxicity and oxidative stress induced by methamphetamine. J Toxicol. 2014;2014:583494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 44. | Schmatz R, Perreira LB, Stefanello N, Mazzanti C, Spanevello R, Gutierres J, Bagatini M, Martins CC, Abdalla FH, Daci da Silva Serres J. Effects of resveratrol on biomarkers of oxidative stress and on the activity of delta aminolevulinic acid dehydratase in liver and kidney of streptozotocin-induced diabetic rats. Biochimie. 2012;94:374-383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 45. | Huang K, Chen C, Hao J, Huang J, Wang S, Liu P, Huang H. Polydatin promotes Nrf2-ARE anti-oxidative pathway through activating Sirt1 to resist AGEs-induced upregulation of fibronetin and transforming growth factor-β1 in rat glomerular messangial cells. Mol Cell Endocrinol. 2015;399:178-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 46. | Zhang P, Ma D, Wang Y, Zhang M, Qiang X, Liao M, Liu X, Wu H, Zhang Y. Berberine protects liver from ethanol-induced oxidative stress and steatosis in mice. Food Chem Toxicol. 2014;74:225-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 47. | Teyssier C, Amiot MJ, Mondy N, Auger J, Kahane R, Siess MH. Effect of onion consumption by rats on hepatic drug-metabolizing enzymes. Food Chem Toxicol. 2001;39:981-987. [PubMed] [Cited in This Article: ] |
| 48. | Lee DE, Kang NJ, Lee KM, Lee BK, Kim JH, Lee KW, Lee HJ. Cocoa polyphenols attenuate hydrogen peroxide-induced inhibition of gap-junction intercellular communication by blocking phosphorylation of connexin 43 via the MEK/ERK signaling pathway. J Nutr Biochem. 2010;21:680-686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 49. | Carrasco-Pozo C, Morales P, Gotteland M. Polyphenols protect the epithelial barrier function of Caco-2 cells exposed to indomethacin through the modulation of occludin and zonula occludens-1 expression. J Agric Food Chem. 2013;61:5291-5297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 50. | Azuma T, Shigeshiro M, Kodama M, Tanabe S, Suzuki T. Supplemental naringenin prevents intestinal barrier defects and inflammation in colitic mice. J Nutr. 2013;143:827-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 51. | Rosales MA, Silva KC, Duarte DA, Rossato FA, Lopes de Faria JB, Lopes de Faria JM. Endocytosis of tight junctions caveolin nitrosylation dependent is improved by cocoa via opioid receptor on RPE cells in diabetic conditions. Invest Ophthalmol Vis Sci. 2014;55:6090-6100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 52. | Abdelmegeed MA, Banerjee A, Yoo SH, Jang S, Gonzalez FJ, Song BJ. Critical role of cytochrome P450 2E1 (CYP2E1) in the development of high fat-induced non-alcoholic steatohepatitis. J Hepatol. 2012;57:860-866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 184] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 53. | Leung TM, Nieto N. CYP2E1 and oxidant stress in alcoholic and non-alcoholic fatty liver disease. J Hepatol. 2013;58:395-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 311] [Cited by in F6Publishing: 345] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 54. | Minemura M, Shimizu Y. Gut microbiota and liver diseases. World J Gastroenterol. 2015;21:1691-1702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 110] [Cited by in F6Publishing: 103] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 55. | Mandal P, Roychowdhury S, Park PH, Pratt BT, Roger T, Nagy LE. Adiponectin and heme oxygenase-1 suppress TLR4/MyD88-independent signaling in rat Kupffer cells and in mice after chronic ethanol exposure. J Immunol. 2010;185:4928-4937. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 56. | Byun EH, Omura T, Yamada K, Tachibana H. Green tea polyphenol epigallocatechin-3-gallate inhibits TLR2 signaling induced by peptidoglycan through the polyphenol sensing molecule 67-kDa laminin receptor. FEBS Lett. 2011;585:814-820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 57. | Byun EB, Choi HG, Sung NY, Byun EH. Green tea polyphenol epigallocatechin-3-gallate inhibits TLR4 signaling through the 67-kDa laminin receptor on lipopolysaccharide-stimulated dendritic cells. Biochem Biophys Res Commun. 2012;426:480-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 58. | Kwon HJ, Won YS, Park O, Chang B, Duryee MJ, Thiele GE, Matsumoto A, Singh S, Abdelmegeed MA, Song BJ. Aldehyde dehydrogenase 2 deficiency ameliorates alcoholic fatty liver but worsens liver inflammation and fibrosis in mice. Hepatology. 2014;60:146-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 128] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 59. | Elamin E, Jonkers D, Juuti-Uusitalo K, van Ijzendoorn S, Troost F, Duimel H, Broers J, Verheyen F, Dekker J, Masclee A. Effects of ethanol and acetaldehyde on tight junction integrity: in vitro study in a three dimensional intestinal epithelial cell culture model. PLoS One. 2012;7:e35008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 60. | Purohit V, Bode JC, Bode C, Brenner DA, Choudhry MA, Hamilton F, Kang YJ, Keshavarzian A, Rao R, Sartor RB. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol. 2008;42:349-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 232] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 61. | Das UN. A defect in the activities of Δ and Δ desaturases and pro-resolution bioactive lipids in the pathobiology of non-alcoholic fatty liver disease. World J Diabetes. 2011;2:176-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 62. | Yan SL, Yang HT, Lee HL, Yin MC. Protective effects of maslinic acid against alcohol-induced acute liver injury in mice. Food Chem Toxicol. 2014;74:149-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 63. | Sookoian S, Pirola CJ. PNPLA3, the triacylglycerol synthesis/hydrolysis/storage dilemma, and nonalcoholic fatty liver disease. World J Gastroenterol. 2012;18:6018-6026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 43] [Cited by in F6Publishing: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 64. | Bognar E, Sarszegi Z, Szabo A, Debreceni B, Kalman N, Tucsek Z, Sumegi B, Gallyas F. Antioxidant and anti-inflammatory effects in RAW264.7 macrophages of malvidin, a major red wine polyphenol. PLoS One. 2013;8:e65355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 65. | Kim DE, Kim B, Shin HS, Kwon HJ, Park ES. The protective effect of hispidin against hydrogen peroxide-induced apoptosis in H9c2 cardiomyoblast cells through Akt/GSK-3β and ERK1/2 signaling pathway. Exp Cell Res. 2014;327:264-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 66. | Bhalla US, Ram PT, Iyengar R. MAP kinase phosphatase as a locus of flexibility in a mitogen-activated protein kinase signaling network. Science. 2002;297:1018-1023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 500] [Cited by in F6Publishing: 520] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 67. | Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649-661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1403] [Cited by in F6Publishing: 1413] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 68. | Olmos Y, Sánchez-Gómez FJ, Wild B, García-Quintans N, Cabezudo S, Lamas S, Monsalve M. SirT1 regulation of antioxidant genes is dependent on the formation of a FoxO3a/PGC-1α complex. Antioxid Redox Signal. 2013;19:1507-1521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 213] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 69. | Pullikotil P, Chen H, Muniyappa R, Greenberg CC, Yang S, Reiter CE, Lee JW, Chung JH, Quon MJ. Epigallocatechin gallate induces expression of heme oxygenase-1 in endothelial cells via p38 MAPK and Nrf-2 that suppresses proinflammatory actions of TNF-α. J Nutr Biochem. 2012;23:1134-1145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 70. | Angeloni C, Motori E, Fabbri D, Malaguti M, Leoncini E, Lorenzini A, Hrelia S. H2O2 preconditioning modulates phase II enzymes through p38 MAPK and PI3K/Akt activation. Am J Physiol Heart Circ Physiol. 2011;300:H2196-H2205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 71. | Nam TW, Yoo CI, Kim HT, Kwon CH, Park JY, Kim YK. The flavonoid quercetin induces apoptosis and inhibits migration through a MAPK-dependent mechanism in osteoblasts. J Bone Miner Metab. 2008;26:551-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 72. | Li H, Chen J, Xiong C, Wei H, Yin C, Ruan J. Apoptosis Induction by the Total Flavonoids from Arachniodes exilis in HepG2 Cells through Reactive Oxygen Species-Mediated Mitochondrial Dysfunction Involving MAPK Activation. Evid Based Complement Alternat Med. 2014;2014:906941. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 73. | Huang K, Huang J, Xie X, Wang S, Chen C, Shen X, Liu P, Huang H. Sirt1 resists advanced glycation end products-induced expressions of fibronectin and TGF-β1 by activating the Nrf2/ARE pathway in glomerular mesangial cells. Free Radic Biol Med. 2013;65:528-540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 202] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 74. | Kawai Y, Garduño L, Theodore M, Yang J, Arinze IJ. Acetylation-deacetylation of the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) regulates its transcriptional activity and nucleocytoplasmic localization. J Biol Chem. 2011;286:7629-7640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 241] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 75. | Movahed A, Yu L, Thandapilly SJ, Louis XL, Netticadan T. Resveratrol protects adult cardiomyocytes against oxidative stress mediated cell injury. Arch Biochem Biophys. 2012;527:74-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 76. | Chen Y, Dong H, Thompson DC, Shertzer HG, Nebert DW, Vasiliou V. Glutathione defense mechanism in liver injury: insights from animal models. Food Chem Toxicol. 2013;60:38-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 149] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 77. | Hashimoto N, Noda T, Kim SJ, Yamauchi H, Takigawa S, Matsuura-Endo C, Suzuki T, Han KH, Fukushima M. Colored potato extracts induce superoxide dismutase-2 mRNA via ERK1/2 pathway in HepG2 cells. Plant Foods Hum Nutr. 2010;65:266-270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 78. | Sadi G, Bozan D, Yildiz HB. Redox regulation of antioxidant enzymes: post-translational modulation of catalase and glutathione peroxidase activity by resveratrol in diabetic rat liver. Mol Cell Biochem. 2014;393:111-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 79. | Akasaki Y, Alvarez-Garcia O, Saito M, Caramés B, Iwamoto Y, Lotz MK. FoxO transcription factors support oxidative stress resistance in human chondrocytes. Arthritis Rheumatol. 2014;66:3349-3358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 80. | Zhu X, Fan WG, Li DP, Kung H, Lin MC. Heme oxygenase-1 system and gastrointestinal inflammation: a short review. World J Gastroenterol. 2011;17:4283-4288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 53] [Cited by in F6Publishing: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 81. | Chen CY, Jang JH, Li MH, Surh YJ. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochem Biophys Res Commun. 2005;331:993-1000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 334] [Cited by in F6Publishing: 300] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 82. | Zeng T, Zhang CL, Song FY, Zhao XL, Yu LH, Zhu ZP, Xie KQ. The activation of HO-1/Nrf-2 contributes to the protective effects of diallyl disulfide (DADS) against ethanol-induced oxidative stress. Biochim Biophys Acta. 2013;1830:4848-4859. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 83. | Kasztelan-Szczerbinska B, Surdacka A, Slomka M, Rolinski J, Celinski K, Smolen A, Szczerbinski M. Association of serum adiponectin, leptin, and resistin concentrations with the severity of liver dysfunction and the disease complications in alcoholic liver disease. Mediators Inflamm. 2013;2013:148526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 84. | Cohen JI, Roychowdhury S, DiBello PM, Jacobsen DW, Nagy LE. Exogenous thioredoxin prevents ethanol-induced oxidative damage and apoptosis in mouse liver. Hepatology. 2009;49:1709-1717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 85. | Surapaneni KM, Priya VV, Mallika J. Pioglitazone, quercetin and hydroxy citric acid effect on cytochrome P450 2E1 (CYP2E1) enzyme levels in experimentally induced non alcoholic steatohepatitis (NASH). Eur Rev Med Pharmacol Sci. 2014;18:2736-2741. [PubMed] [Cited in This Article: ] |
| 86. | Kim SK, Novak RF. The role of intracellular signaling in insulin-mediated regulation of drug metabolizing enzyme gene and protein expression. Pharmacol Ther. 2007;113:88-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 87. | Shukla U, Tumma N, Gratsch T, Dombkowski A, Novak RF. Insights into insulin-mediated regulation of CYP2E1: miR-132/-212 targeting of CYP2E1 and role of phosphatidylinositol 3-kinase, Akt (protein kinase B), mammalian target of rapamycin signaling in regulating miR-132/-212 and miR-122/-181a expression in primary cultured rat hepatocytes. Drug Metab Dispos. 2013;41:1769-1777. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 88. | Kovac S, Angelova PR, Holmström KM, Zhang Y, Dinkova-Kostova AT, Abramov AY. Nrf2 regulates ROS production by mitochondria and NADPH oxidase. Biochim Biophys Acta. 2015;1850:794-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 344] [Cited by in F6Publishing: 416] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 89. | Kim SM, Chung MJ, Ha TJ, Choi HN, Jang SJ, Kim SO, Chun MH, Do SI, Choo YK, Park YI. Neuroprotective effects of black soybean anthocyanins via inactivation of ASK1-JNK/p38 pathways and mobilization of cellular sialic acids. Life Sci. 2012;90:874-882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 90. | Basu A, Betts NM, Mulugeta A, Tong C, Newman E, Lyons TJ. Green tea supplementation increases glutathione and plasma antioxidant capacity in adults with the metabolic syndrome. Nutr Res. 2013;33:180-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 91. | Kujawska M, Ignatowicz E, Ewertowska M, Markowski J, Jodynis-Liebert J. Cloudy apple juice protects against chemical-induced oxidative stress in rat. Eur J Nutr. 2011;50:53-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 92. | Chen X, Sun CK, Han GZ, Peng JY, Li Y, Liu YX, Lv YY, Liu KX, Zhou Q, Sun HJ. Protective effect of tea polyphenols against paracetamol-induced hepatotoxicity in mice is significantly correlated with cytochrome P450 suppression. World J Gastroenterol. 2009;15:1829-1835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 38] [Cited by in F6Publishing: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 93. | Kasdallah-Grissa A, Mornagui B, Aouani E, Hammami M, El May M, Gharbi N, Kamoun A, El-Fazaâ S. Resveratrol, a red wine polyphenol, attenuates ethanol-induced oxidative stress in rat liver. Life Sci. 2007;80:1033-1039. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 155] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 94. | Yao P, Hao L, Nussler N, Lehmann A, Song F, Zhao J, Neuhaus P, Liu L, Nussler A. The protective role of HO-1 and its generated products (CO, bilirubin, and Fe) in ethanol-induced human hepatocyte damage. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1318-G1323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 95. | Xing HY, Liu Y, Chen JH, Sun FJ, Shi HQ, Xia PY. Hyperoside attenuates hydrogen peroxide-induced L02 cell damage via MAPK-dependent Keap1-Nrf2-ARE signaling pathway. Biochem Biophys Res Commun. 2011;410:759-765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 96. | Takikawa M, Inoue S, Horio F, Tsuda T. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J Nutr. 2010;140:527-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 313] [Cited by in F6Publishing: 317] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 97. | Noguer MA, Cerezo AB, Donoso Navarro E, Garcia-Parrilla MC. Intake of alcohol-free red wine modulates antioxidant enzyme activities in a human intervention study. Pharmacol Res. 2012;65:609-614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 98. | Ranaldi G, Mancini E, Ferruzza S, Sambuy Y, Perozzi G. Effects of red wine on ochratoxin A toxicity in intestinal Caco-2/TC7 cells. Toxicol In Vitro. 2007;21:204-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 99. | Cowpland C, Su GM, Murray M, Puddey IB, Croft KD. Effect of alcohol on cytochrome p450 arachidonic acid metabolism and blood pressure in rats and its modulation by red wine polyphenolics. Clin Exp Pharmacol Physiol. 2006;33:183-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 100. | Yun JW, Kim YK, Lee BS, Kim CW, Hyun JS, Baik JH, Kim JJ, Kim BH. Effect of dietary epigallocatechin-3-gallate on cytochrome P450 2E1-dependent alcoholic liver damage: enhancement of fatty acid oxidation. Biosci Biotechnol Biochem. 2007;71:2999-3006. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 101. | Li J, Ye L, Wang X, Liu J, Wang Y, Zhou Y, Ho W. (-)-Epigallocatechin gallate inhibits endotoxin-induced expression of inflammatory cytokines in human cerebral microvascular endothelial cells. J Neuroinflammation. 2012;9:161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 102. | Lee SJ, Lee KW. Protective effect of (-)-epigallocatechin gallate against advanced glycation endproducts-induced injury in neuronal cells. Biol Pharm Bull. 2007;30:1369-1373. [PubMed] [Cited in This Article: ] |
| 103. | Tsukamoto S, Huang Y, Umeda D, Yamada S, Yamashita S, Kumazoe M, Kim Y, Murata M, Yamada K, Tachibana H. 67-kDa laminin receptor-dependent protein phosphatase 2A (PP2A) activation elicits melanoma-specific antitumor activity overcoming drug resistance. J Biol Chem. 2014;289:32671-32681. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 104. | Gundimeda U, McNeill TH, Fan TK, Deng R, Rayudu D, Chen Z, Cadenas E, Gopalakrishna R. Green tea catechins potentiate the neuritogenic action of brain-derived neurotrophic factor: role of 67-kDa laminin receptor and hydrogen peroxide. Biochem Biophys Res Commun. 2014;445:218-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 105. | Robb EL, Page MM, Wiens BE, Stuart JA. Molecular mechanisms of oxidative stress resistance induced by resveratrol: Specific and progressive induction of MnSOD. Biochem Biophys Res Commun. 2008;367:406-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 106. | Catalgol B, Batirel S, Taga Y, Ozer NK. Resveratrol: French paradox revisited. Front Pharmacol. 2012;3:141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 270] [Cited by in F6Publishing: 277] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 107. | Gualdoni GA, Kovarik JJ, Hofer J, Dose F, Pignitter M, Doberer D, Steinberger P, Somoza V, Wolzt M, Zlabinger GJ. Resveratrol enhances TNF-α production in human monocytes upon bacterial stimulation. Biochim Biophys Acta. 2014;1840:95-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 108. | Kiso Y, Tsuruoka N, Kidokoro A, Matsumoto I, Abe K. Sesamin ingestion regulates the transcription levels of hepatic metabolizing enzymes for alcohol and lipids in rats. Alcohol Clin Exp Res. 2005;29:116S-120S. [PubMed] [Cited in This Article: ] |
| 109. | Tsuruoka N, Kidokoro A, Matsumoto I, Abe K, Kiso Y. Modulating effect of sesamin, a functional lignan in sesame seeds, on the transcription levels of lipid- and alcohol-metabolizing enzymes in rat liver: a DNA microarray study. Biosci Biotechnol Biochem. 2005;69:179-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 110. | El-Agamy DS. Comparative effects of curcumin and resveratrol on aflatoxin B(1)-induced liver injury in rats. Arch Toxicol. 2010;84:389-396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 111. | Sahin K, Orhan C, Tuzcu Z, Tuzcu M, Sahin N. Curcumin ameloriates heat stress via inhibition of oxidative stress and modulation of Nrf2/HO-1 pathway in quail. Food Chem Toxicol. 2012;50:4035-4041. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 112. | Barreto JC, Trevisan MT, Hull WE, Erben G, de Brito ES, Pfundstein B, Würtele G, Spiegelhalder B, Owen RW. Characterization and quantitation of polyphenolic compounds in bark, kernel, leaves, and peel of mango (Mangifera indica L.). J Agric Food Chem. 2008;56:5599-5610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 231] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 113. | Moselhy SS, Ali HK. Hepatoprotective effect of cinnamon extracts against carbon tetrachloride induced oxidative stress and liver injury in rats. Biol Res. 2009;42:93-98. [PubMed] [Cited in This Article: ] |
| 114. | Kang NJ, Lee KM, Kim JH, Lee BK, Kwon JY, Lee KW, Lee HJ. Inhibition of gap junctional intercellular communication by the green tea polyphenol (-)-epigallocatechin gallate in normal rat liver epithelial cells. J Agric Food Chem. 2008;56:10422-10427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 115. | Wen D, Huang X, Zhang M, Zhang L, Chen J, Gu Y, Hao CM. Resveratrol attenuates diabetic nephropathy via modulating angiogenesis. PLoS One. 2013;8:e82336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 116. | Sriram N, Kalayarasan S, Sudhandiran G. Enhancement of antioxidant defense system by epigallocatechin-3-gallate during bleomycin induced experimental pulmonary fibrosis. Biol Pharm Bull. 2008;31:1306-1311. [PubMed] [Cited in This Article: ] |
| 117. | Wang Y, Shen J, Xiong X, Xu Y, Zhang H, Huang C, Tian Y, Jiao C, Wang X, Li X. Remote ischemic preconditioning protects against liver ischemia-reperfusion injury via heme oxygenase-1-induced autophagy. PLoS One. 2014;9:e98834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 118. | Kovalska M, Kovalska L, Mikuskova K, Adamkov M, Tatarkova Z, Lehotsky J. p-ERK involvement in the neuroprotection exerted by ischemic preconditioning in rat hippocampus subjected to four vessel occlusion. J Physiol Pharmacol. 2014;65:767-776. [PubMed] [Cited in This Article: ] |
| 119. | Zhang J, Bian HJ, Li XX, Liu XB, Sun JP, Li N, Zhang Y, Ji XP. ERK-MAPK signaling opposes rho-kinase to reduce cardiomyocyte apoptosis in heart ischemic preconditioning. Mol Med. 2010;16:307-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 120. | Chen P, Li J, Barnes J, Kokkonen GC, Lee JC, Liu Y. Restraint of proinflammatory cytokine biosynthesis by mitogen-activated protein kinase phosphatase-1 in lipopolysaccharide-stimulated macrophages. J Immunol. 2002;169:6408-6416. [PubMed] [Cited in This Article: ] |
| 121. | Kim M, Murakami A, Kawabata K, Ohigashi H. (-)-Epigallocatechin-3-gallate promotes pro-matrix metalloproteinase-7 production via activation of the JNK1/2 pathway in HT-29 human colorectal cancer cells. Carcinogenesis. 2005;26:1553-1562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 122. | Turpaev KT. Keap1-Nrf2 signaling pathway: mechanisms of regulation and role in protection of cells against toxicity caused by xenobiotics and electrophiles. Biochemistry (Mosc). 2013;78:111-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 123. | Satoh T, McKercher SR, Lipton SA. Nrf2/ARE-mediated antioxidant actions of pro-electrophilic drugs. Free Radic Biol Med. 2013;65:645-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 195] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 124. | Ramiro-Puig E, Urpí-Sardà M, Pérez-Cano FJ, Franch A, Castellote C, Andrés-Lacueva C, Izquierdo-Pulido M, Castell M. Cocoa-enriched diet enhances antioxidant enzyme activity and modulates lymphocyte composition in thymus from young rats. J Agric Food Chem. 2007;55:6431-6438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 125. | Anandhan A, Tamilselvam K, Vijayraja D, Ashokkumar N, Rajasankar S, Manivasagam T. Resveratrol attenuates oxidative stress and improves behaviour in 1 -methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) challenged mice. Ann Neurosci. 2010;17:113-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 126. | Hashimoto N, Nakamura Y, Noda T, Han KH, Fukushima M. Effects of feeding potato pulp on cholesterol metabolism and its association with cecal conditions in rats. Plant Foods Hum Nutr. 2011;66:401-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 127. | Jakobsdottir G, Xu J, Molin G, Ahrné S, Nyman M. High-fat diet reduces the formation of butyrate, but increases succinate, inflammation, liver fat and cholesterol in rats, while dietary fibre counteracts these effects. PLoS One. 2013;8:e80476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 225] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 128. | Aprikian O, Duclos V, Guyot S, Besson C, Manach C, Bernalier A, Morand C, Rémésy C, Demigné C. Apple pectin and a polyphenol-rich apple concentrate are more effective together than separately on cecal fermentations and plasma lipids in rats. J Nutr. 2003;133:1860-1865. [PubMed] [Cited in This Article: ] |
| 129. | Han KH, Hayashi N, Hashimoto N, Shimada K, Sekikawa M, Noda T, Fukushima M. Feeding potato flakes affects cecal short-chain fatty acids, microflora and fecal bile acids in rats. Ann Nutr Metab. 2008;52:1-7. [PubMed] [Cited in This Article: ] |
| 130. | Ferreira TM, Leonel AJ, Melo MA, Santos RR, Cara DC, Cardoso VN, Correia MI, Alvarez-Leite JI. Oral supplementation of butyrate reduces mucositis and intestinal permeability associated with 5-Fluorouracil administration. Lipids. 2012;47:669-678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |