Published online Dec 28, 2015. doi: 10.3748/wjg.v21.i48.13418
Peer-review started: July 2, 2015
First decision: July 20, 2015
Revised: August 11, 2015
Accepted: September 28, 2015
Article in press: September 30, 2015
Published online: December 28, 2015
Although the incidence of hepatolithiasis is decreasing as the pattern of gallstone disease changes in Asia, the prevalence of hepatolithiasis is persistently high, especially in Far Eastern countries. Hepatolithiasis is an established risk factor for cholangiocarcinoma (CCA), and chronic proliferative inflammation may be involved in biliary carcinogenesis and in inducing the upregulation of cell-proliferating factors. With the use of advanced imaging modalities, there has been much improvement in the management of hepatolithiasis and the diagnosis of hepatolithiasis-associated CCA (HL-CCA). However, there are many problems in managing the strictures in hepatolithiasis and differentiating them from infiltrating types of CCA. Surgical resection is recommended in cases of single lobe hepatolithiasis with atrophy, uncontrolled stricture, symptom duration of more than 10 years, and long history of biliary-enteric anastomosis. Even after resection, patients should be followed with caution for development of HL-CCA, because HL-CCA is an independent prognostic factor for survival. It is not yet clear whether hepatic resection can reduce the occurrence of subsequent HL-CCA. Furthermore, there are no consistent findings regarding prediction of subsequent HL-CCA in patients with hepatolithiasis. In the management of hepatolithiasis, important factors are the reduction of recurrence of cholangitis and suspicion of unrecognized HL-CCA.
Core tip: In this study, we review recent studies on hepatolithiasis and discuss hepatolithiasis-associated cholangiocarcinoma (HL-CCA). Management of hepatolithiasis requires proper treatment to reduce recurrence and achieve early detection of HL-CCA. It is not clear whether hepatic resection can reduce the occurrence of HL-CCA, and there is no surveillance tool to predict subsequent occurrence. Patients should be followed after treatment because there are no effective measures to prevent HL-CCA and premalignant lesions.
- Citation: Kim HJ, Kim JS, Joo MK, Lee BJ, Kim JH, Yeon JE, Park JJ, Byun KS, Bak YT. Hepatolithiasis and intrahepatic cholangiocarcinoma: A review. World J Gastroenterol 2015; 21(48): 13418-13431
- URL: https://www.wjgnet.com/1007-9327/full/v21/i48/13418.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i48.13418
Gallstone disease is common in Western and Asian countries. One type of gallstone disease, hepatolithiasis, is characterized by the presence of stones within the intrahepatic bile ducts proximal to the right and left hepatic ducts. Hepatolithiasis is rare in Western countries, and the incidence in East Asian, such as Taiwan, China, Hong Kong, South Korea, and Japan, is higher[1-4].
Hepatolithiasis is benign in nature, but the prognosis is poor due to an association with recurrent cholangitis, biliary strictures, liver abscesses, and atrophy or cirrhosis of the affected liver; however, there have been advances in the management of the stones associated with hepatolithiasis[5-7]. Hepatolithiasis is also a known risk factor for intrahepatic cholangiocarcinoma (CCA)[8,9]. Although mortality due to cholangitis from hepatolithiasis is very low, the occurrence of hepatolithiasis-associated CCA (HL-CCA) is a prognostic factor for poor outcome[10-12]. Early diagnosis of HL-CCA is still challenging even though there have been advances in diagnostic modalities and various efforts to identify it in early stages[13].
Here, we review the epidemiology, pathogenesis, diagnosis, and management of hepatolithiasis and HL-CCA. This review also summarizes the predictive factors for HL-CCA.
The incidence of hepatolithiasis varies, but it is fairly common in Asian, such as China, Taiwan, Hong Kong, Korea, and Japan, with incidence rates ranging from 2% to 25%[1-4]. In Western countries hepatolithiasis is rare, with incidences reported between 0.6% and 1.3%[4,14].
The mechanism of intrahepatic stone formation is not well understood, but malnutrition and low socio-economic status have been suggested to be causatively related to hepatolithiasis. According to epidemiologic surveys, the pattern of gallstone disease is changing, and the incidence of hepatolithiasis is decreasing as people in Eastern countries adopt a Westernized diet. A national survey conducted in Japan reported that the relative proportion of hepatolithiasis was 4.1% in the years from 1970-1977, 3.0% in the years from 1975-1984, 2.3% in the years from 1985-1988, 2.2% in the years from 1989-1992, and 1.7% in the years from 1993-1995[15]. The apparent decrease in the incidence of hepatolithiasis has contributed to this chronological shift, but it may be partly caused by the increase in gallbladder stones due to westernized diet[16]. In Taiwan, the relative proportion of hepatolithiasis slightly decreased from 21.3% in 1981 to 18.7% in 1989[1]. In South Korea, the pattern of gallstone disease has become similar to that seen in Western countries, except for a high prevalence of hepatolithiasis. The proportion of gallbladder stones has gradually increased from 80% to 85% and that of common bile duct stones has decreased from 34% to 19%; however, that of hepatolithiasis remains unchanged, at 11%-15% over a 20 year period from 1980 to 2000[2,3].
Parasitic infestation has often been thought to be a major cause of hepatolithiasis and infestation, as parasites have been detected in up to 30% of patients with hepatolithiasis[17]. In Eastern countries, the persistent prevalence of hepatolithiasis in Korea and the relatively high prevalence in Taiwan may be due to cultural trends of ingesting raw freshwater fish infected with Clonorchis sinensis (C. sinensis). Clonorchiasis is endemic in the Far East, but heavily endemic areas within individual countries are geographically distributed in parallel with the population of the snail that is an intermediate host for this parasite[18]. Nationwide surveys in Korea of intestinal parasitic infections revealed no drop in the average prevalence of C. sinensis infection over time (i.e., 2.6% in 1981 and 2.4% in 2004)[19], and C. sinensis infection remains common in Korea. In 1984, the prevalence was reported to be 1.5% in Taiwan[20]. In Japan, the clonorchiasis rate has markedly decreased in parallel with the decreased number of host snails, which is due to various causes, including water pollution[18].
Hepatolithiasis is an established risk factor for CCA, similar to clonorchiasis, especially in Asian countries[8,9,21-24]. The association between hepatolithiasis and CCA has been well-documented, and many studies on HL-CCA have been published[25-31]. Cases of HL-CCA are not rare, especially in areas with a high prevalence of hepatolithiasis. The overall incidence of HL-CCA was reported to be 5%-13%[12,32,33]. HL-CCA can be detected at any stage, during evaluation, treatment, or follow up of hepatolithiasis. Detection of concomitant HL-CCA during treatment of hepatolithiasis was reported in 12% of patients in Japan, 5% in Taiwan, 9% in South Korea, and 10% in Hong Kong[12,30,32,34-38] (Table 1). HL-CCA can also develop during follow up for hepatolithiasis. Subsequent HL-CCA following hepatolithiasis has been reported to be 1.6%-9.9% in several studies[6,12,30,32,33,36,38-40]. These studies are summarized in Table 2.
| Ref. | Year | Nation/region | Case (n) | Incidence |
| Zhu et al[34] | 2014 | China | 2056 | 107 (5.2) |
| Lin et al[32] | 2013 | Taiwan | 211 | 10 (4.7) |
| Tabrizian et al[35] | 2012 | Italy | 30 | 7 (23.3) |
| Cheon et al[30] | 2009 | South Korea | 90 | 8 (9.0) |
| Uenishi et al[12] | 2009 | Japan | 86 | 10 (11.6) |
| Lee et al[36] | 2007 | Taiwan | 123 | 4 (3.3) |
| Catena et al[37] | 2006 | Italy | 17 | 2 (11.7) |
| Chen et al[38] | 2004 | Hong Kong | 103 | 10 (9.7) |
| Ref. | Year | Nation/region | Cases (n) | Incidence of cholangiocarcinoma | ||
| Total | Stone removal | |||||
| Complete | Residual | |||||
| Kim et al[33] | 2015 | South Korea | 236 | 6.8% | 3.3% | 10.4% |
| Tsuyuguchi et al[39] | 2014 | Japan | 121 | 9.9% | 9.1% | 10.4% |
| Lin et al[32] | 2013 | Taiwan | 197 | 6.1% | 4.9% | 11.8% |
| Park et al[6] | 2013 | South Korea | 85 | 2.4% | ||
| Cheon et al[30] | 2009 | South Korea | 225 | 4.9% | 4.0% | 8.0% |
| Uenishi et al[12] | 2009 | Japan | 76 | 2.6% | ||
| Lee et al[36] | 2007 | Taiwan | 123 | 1.6% | ||
| Chen et al[38] | 2004 | Hong Kong | 91 | 3.3% | ||
| Huang et al[42] | 2003 | Taiwan | 209 | 2.4% | 0.7% | 6.6% |
The mechanism of intrahepatic stone formation has not yet been fully described; but the presence of brown pigment stones as well as cholesterol stones suggests a complex pathogenesis[41-44]. Factors that may contribute to development of these stones include the precipitation of calcium bilirubinate, the solubility of cholesterol in hepatic bile, gene mutations, and ethnic differences[45-51].
A low fat and low protein diet may increase bile stasis and bacterial infection through relaxation of the sphincter of Oddi and decreased release of cholecystokinin[52,53]. Association with bacterial infection has been implicated in stone formation. Bacteria, such as Escherichia coli, and those belonging to the Clostridium and Bacteroides genera are frequently isolated from the bile of patients with hepatolithiasis. The possible route is ascending infection through the sphincter of Oddi, bacteriobilia through the portal venous system, or transient infection with bile stasis[54,55].
Recent experiments suggest that enhanced inflammatory cytokine-induced phospholipase A2, cyclooxygenase-2 (COX-2) and COX-2-derived prostaglandin E2 (PGE2) synthesis in the bile ducts are related to the initiation and propagation of inflammatory changes in hepatolithiasis[56,57].
In terms of the biliary mucin molecules, an increase in acidic mucins, such as sulfomucins and sialomucins, in hepatolithiasis reduces pH in the bile and leads to precipitation of calcium bilirubinate in the bile ducts[56]. The abundance of secretory-type mucins (MUC2, MUC3, MUC5AC, MUC5B, and MUC6) was shown to be significantly higher in the bile ducts of hepatolithiasis patients compared to controls, and gel-forming mucins of MUC2 and 5AC were thought to be more important for the pathogenesis[58,59]. Excessive amounts of mucin secreted into the ducts may provide a microenvironment that initiates a nidus for stones by trapping calcium salts and lipids, which may also cause stones to expand by altering biliary flow in the bile ducts[60,61].
Known risk factors for CCA are primary sclerosing cholangitis, Caroli’s disease, congenital choledochal cysts, parasite infections (C. sinensis, Opisthorchis viverrini), hepatolithiasis, and toxins[8,9,19]. Considerable progress has been made in understanding the pathogenesis of CCA[23,62]. It has been proposed to be a multi-step process, involving hyperplasia, dysplasia, and adenocarcinoma in situ to invasive adenocarcinoma[63]. Although the process of carcinogenesis from hepatolithiasis is not fully understood, chronic proliferative cholangitis plays a role in biliary carcinogenesis[27]. Recurrent cholangitis, biliary stricture, bile stasis, and chronic bacterial infection are common problems in hepatolithiasis patients, even after multimodal treatment. These recurrent or chronic inflammatory events cause prolonged inflammation of the bile duct epithelium and can lead to the development of CCA[27,62].
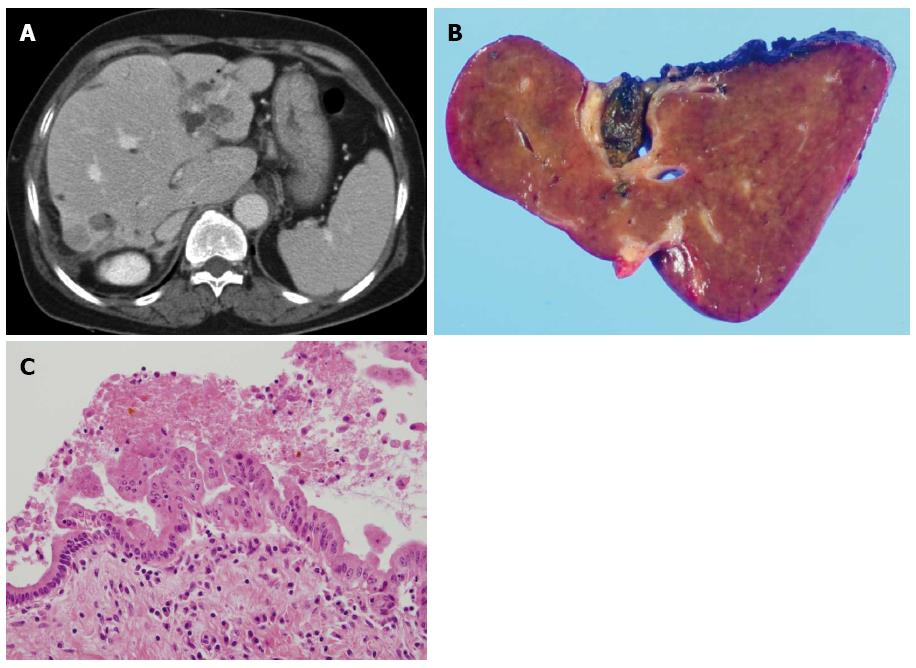
The main morphologic feature of stone-containing bile ducts in hepatolithiasis is chronic proliferative cholangitis and peribiliary glands proliferation, in which the epithelial lining becomes hyperplastic[43]. Chronic inflammation can cause epithelial cell proliferation, and this may increase the rate of cellular DNA synthesis and the subsequent production of mutagens coupled with a compromised cellular repair function[64-66]. If these processes are sustained for a long period of time, they may cause the multiple molecular changes necessary to trigger the development of CCA. During histologic exam by choledochoscopy using percutaneous transhepatic cholangioscopic lithotripsy (PTCSL), atypical epithelial hyperplasia and dysplasia are frequently recognized[27]. Chen et al[67] reported that intraductal papillary neoplasia was found in 30% of patients with hepatolithiasis and displayed a histologic spectrum from papillary growth with dysplasia to carcinoma. Biliary carcinogenesis associated with hepatolithiasis is thought to be present as precancerous lesions. Intraductal papillary neoplasm of the bile duct (IPNB) and biliary intraepithelial neoplasia (BilIN) are known as precancerous lesions of biliary tract carcinomas[68].
Similar to hepatolithiasis and clonorchiasis, IPNB has mainly been reported in Far Eastern countries. IPNB, including carcinoma and precursor lesions, is known to transform from low-grade dysplasia to invasive carcinoma[69]. BilIN is a flat or micropapillary dysplastic epithelium in the bile duct and classified as BilIN-1, BilIN-2, and BilIN-3[70]. It is frequently found in the surgical margin of resection specimens of CCA and is also reported in patients with hepatolithiasis[71,72]. Both BilIN and IPNB can be seen at the same time in patients with hepatolithiasis, unlike primary sclerosing cholangitis and parasitic infections, which are only associated with BilIN[70,72] (Figures 1 and 2). A study of BilIN reported that metaplastic changes were more frequently observed in BilIN-2/3 than BilIN-1, and gastric type foveolar metaplasia was the most frequently observed change[70]. In immunohistochemical studies of IPNB, aberrant expression of cytokeratin 20, MUC2, and MUC5AC was frequently shown[73,74]. Another immunohistochemical study showed that decreased expression of β-catenin and E-cadherin occurred early in the carcinogenesis of both BilIN and IPNB[75].
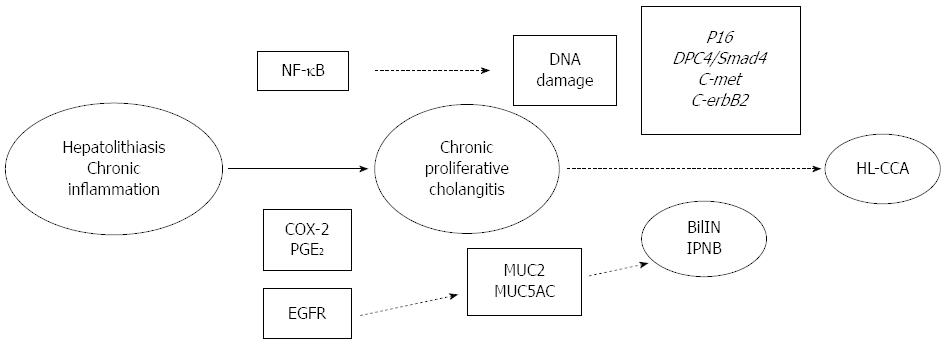
Recent studies have elucidated the molecular mechanism of HL-CCA, which involves epidermal growth factor receptor (EGFR), nuclear factor kappa-B (NF-κB), COX-2, PGE2, p16, c-met, and deleted in pancreatic cancer 4 (DPC4)/signaling effectors mothers against decapentaplegic protein 4 (Smad4)[76,77]. Increased expression of c-erbB2 and EGFR in both hepatolithiasis and intrahepatic CCA has been reported[78,79]. Zhou et al[80] reported that NF-κB and EGFR were more highly expressed in HL-CCA than in patients with hepatolithiasis. These findings demonstrate that prolonged inflammation due to the presence of stones could induce upregulation of these cell-proliferating factors.
COX-2 overexpression correlated with the carcinogenesis of intrahepatic CCA, and it occurred during the early stages of cholangiocarcinogenesis[81,82]. Endo et al[83] reported that ErbB2 and COX-2 were overexpressed in the hyperplastic bile ducts of patients with hepatolithiasis.
PGE2 is known to be increased by upregulated COX activity in inflammatory sites. Shoda et al[57] reported that the synthesis of PGE2 is significantly higher in affected bile ducts with hepatolithiasis, and it can mediate morphologic changes of the intrahepatic bile duct, such as dilatation, stricture, and periductal fibrosis.
It is well known that c-met plays a role in the carcinogenesis of CCA. The c-met gene, a proto-oncogene, encodes the membranous tyrosine kinase receptor for hepatocyte growth factor[84]. Terada et al[85] reported that c-met protein was overexpressed in proliferated biliary cells of hepatolithiasis and in neoplastic biliary epithelium of intrahepatic CCA.
The cyclin-dependent kinase inhibitor p16 is known as a tumor suppressor gene, and p16 promoter hypermethylation is known to occur in various cancers, including CCA[86]. Ishikawa et al[87] reported that inactivation of p16 occurred frequently and at an early stage of IPNB with hepatolithiasis. Sasaki et al[88] reported that p16 expression was decreased from BilIN-2,3 to cholangiocarcinogenesis in patients with hepatolithiasis.
In a study of the DPC4/Smad4 gene, another tumor suppressor gene, Lee et al[89] reported that inactivation of DPC4/Smad4 occurred in both CCA and stone-containing bile ducts, and it was especially pronounced in the dysplastic epithelium of stone-containing bile ducts.
Diagnosis of hepatolithiasis is performed mainly through radiologic examinations, and often incidentally without symptoms or laboratory abnormalities. If symptoms occur, they may include fever, fatigue, abdominal pain, and jaundice. Laboratory tests can show leukocytosis, neutrophilia, and abnormal liver biochemistry of an obstructive pattern with raised serum alkaline phosphate and bilirubin.
A hepatolithiasis research group in Japan classified the severity of hepatolithiasis: grade 1 as no symptoms, grade 2 as having abdominal pain, grade 3 as transient jaundice or cholangitis, and grade 4 as continuous jaundice, sepsis, or concurrent CCA. They reported that more than half of 473 new hepatolithiasis cases were classified as grade 3 or 4[90]. Another recent study of 68 hepatolithiasis patients used a new clinical classification system: type 1 primary type (no previous biliary tract surgery), type 2 inflammatory type (previous biliary tract surgery and cholangitis), type 3 mass-forming type (complicated by hepatic mass-forming lesion), and type 4 terminal type (with secondary biliary cirrhosis and resultant portal hypertension). The authors reported that the incidence of new cases of types 1-4 was 50.1%, 36.8%, 10.3%, and 2.8%, respectively[91].
Abdominal ultrasound (US) and computed tomography (CT) are the primary imaging modalities for hepatolithiasis. The advantages of US are that it is non-invasive, safe, and easily available (even bedside), and can detect dilatations of the biliary tract and stones, as shown by echogenic spots with an acoustic shadow. However, the detectability of stones is dependent on the stone size, shadowing characteristics, echogenicity, and the location in the hepatic lobe[7,90]. Multidetector CT (MDCT) has advanced the diagnosis of hepatolithiasis. MD-CT is very useful for detecting dilated bile ducts, stricture of the bile duct, and calcification stones in the bile duct[7,92]. Therefore, recently, US and CT have become the first choice for examining patients suspected of having hepatolithiasis.
Magnetic resonance imaging (MRI) and magnetic resonance cholangiopancreatography (MRCP) can provide realistic bile duct images and can detect stones without exposing patients to radiation[93,94]. The T1-weighted (T1-W) sequence is helpful for depicting stones and demonstrating parenchymal complications, such as an abscess[95]. However, the cost is high, and its spatial resolution is not perfect. MRI and MRCP are usually performed as ancillary investigations to US and CT.
The clinical manifestations of intrahepatic CCA are nonspecific, although there may be abdominal pain, cachexia, fatigue, and night sweats[96]. Serum CA 19-9 is known as a tumor biomarker for CCA, but it may be normal or increased in benign diseases, such as bacterial cholangitis or choledocholithiasis[96,97].
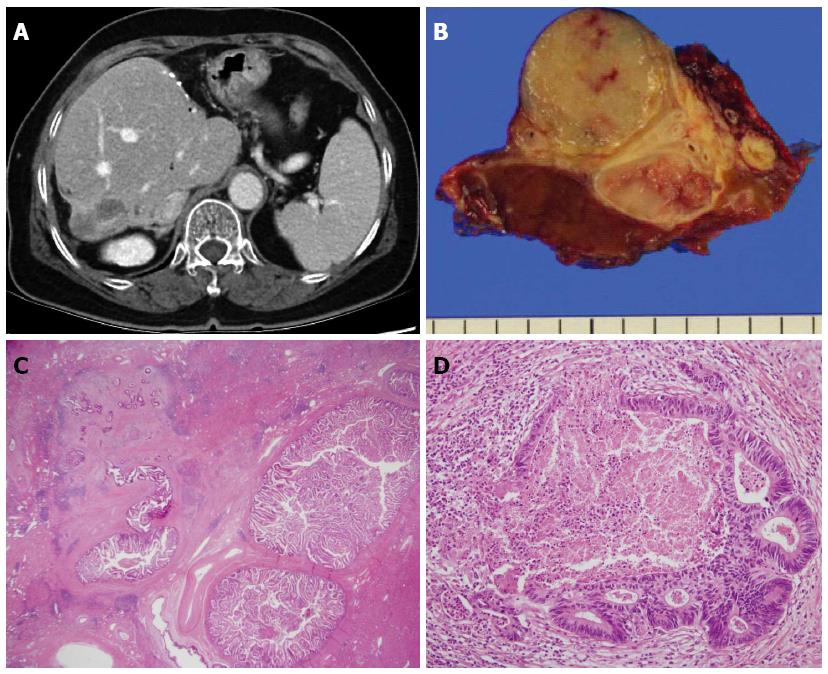
Macroscopically, intrahepatic CCA is classified as a mass-forming, periductal-infiltrating, or intraductal-growing carcinoma, where the mass-forming type is most common[98]. MDCT is used to evaluate the location of the tumor and its relationship with adjacent vascularity[99]. Pre-contrast and multiphase CT is useful for the detection and differentiation of an intraductal stone from an intraductal tumor because hypovascular tumors with abundant fibrous stroma show progressive uptake of contrast during the venous phase[100]. Park et al[101] suggested the following as specific CT findings for HL-CCA: the presence of periductal soft-tissue density, higher enhancement of the duct than the adjacent bile duct on portal venous phase images, ductal wall thickening or enhancement, portal vein obliteration, and lymph node enlargement.
On MRI, CCA is visible as a hypointense mass on T1-W images and a central hypointensity with irregular hyperintense areas on T2-weighted (T2-W) images[102]. MR imaging with MR cholangiography is superior to CT for the assessment of intraductal lesions, intrahepatic metastasis, and presence of satellite lesions[103]. However, CT may be better for the assessment of vascular encasement, identification of extrahepatic metastasis, and determination of resectability[104].
Positron emission tomography (PET) scan is another useful imaging modality, but the accuracy varies depending on morphologic type[105]. The sensitivity of PET for detection is reported to be 85% for the mass-forming type but 18% for the infiltrating type[105]. Mass forming type intrahepatic CCA shows ring-shaped fluorodeoxyglucose uptake (due to desmoplastic lesions within the tumor) and neovascularity at the periphery, but this finding can be shown in any lesion with central necrosis[103]. Thus, PET scanning is more helpful for detection of distant metastases[106,107].
In cases of HL-CCA, there are no specific symptoms other than the clinical manifestation of hepatolithiasis. Laboratory tests can show increased alkaline phosphatase, bilirubin, and CA 19-9[108].
Therefore, detection of CCA in hepatolithiasis is dependent on imaging modalities, such as US, CT, and MRI. However, there are many limitations in differentiating CCA from fibrosis in hepatolithiasis. It is difficult to differentiate strictures, infiltrating types of CCA, mass-forming CCA, and inflammatory pseudo-tumor because prolonged affected liver segments often become fibrotic and scarred[90,109].
The primary goals of treatment for hepatolithiasis are complete stone removal and the prevention of recurrent cholangitis. Current treatment methods are non-surgical treatments, such as percutaneous transhepatic cholangioscopic lithotripsy (PTCSL), and surgical treatment, such as hepatic resection. PTCSL has been frequently used with a fair success rate[110-113]. The rate of complete stone removal was reported to be similarly high in both treatments, but recurrent cholangitis is quite common in PTCSL[6,11,111]. Generally, hepatic resection has been considered the definite treatment for hepatolithiasis, because it could effectively reduce recurrent cholangitis or stone formation[114-116]. The reported overall success rate of hepatic resection is 95%-98%, and the rates of residual stone and stone recurrence are 15.6% and 7.8%-13.9%, respectively, upon long-term follow-up[5,12,115,116].
However, the incidence of post-hepatectomy infection was 23.8% higher in hepatolithiasis than in other hepatic malignancies[117]. Additionally, operative intervention is sometimes not acceptable for patients with risky co-morbidities or stones distributed in multiple segments of both hepatic lobes[118,119]. Thus, a tailored multidisciplinary approach combining both approaches is more reasonable.
Meanwhile there are many hepatolithiasis cases with strictures that make it difficult to manage and differentiate CCA. Thus, patients should be screened for concomitant HL-CCA, even though the incidence is low. Choledochoscopy may be indicated in these cases.
Known risk factors for concomitant HL-CCA are older age, bile duct stenosis (stricture), liver atrophy, elevated CA 19-9, left side stone location, residual stone, recurrence of stone, and choledochoenterostomy[120-124] Neither symptoms (abdominal pain, fever, jaundice, and nausea) nor the location of stones (intrahepatic duct only, both intra- and extra-hepatic ducts, right lobe, left lobe, or both lobes) is a significant risk factor[122,125].
In a comparative study of concomitant HL-CCA and hepatolithiasis only, Kim et al[120] reported that concomitant HL-CCA should be suspected in patients with a longstanding duration of hepatolithiasis accompanied by weight loss, high levels of serum alkaline phosphatase, low levels of serum albumin, high levels of serum CEA, hepatolithiasis located in either the right or both lobes of the liver, and age > 40 years. Liu et al[121] reported that symptom duration of more than 10 years is the most powerful risk factor for ICC in patients with hepatolithiasis, and Lee et al[126] reported an association between localized stricture and long-term history (over 10 years) of hepatolithiasis. Additionally, it is suggested that HL-CCA may occur in cases of atypical clinical manifestation of liver abscess, biliary tract infection that is difficult to control, relapsing infection, and abscesses in a more consolidated area[34].
In a Japanese multicenter study, Suzuki et al[122] reported that the predictive risk factors were a history of choledocoenterostomy (OR = 3.718) and liver atrophy (OR = 4.424). Association of CCA occurrence with previous surgical biliary-enteric anastomosis may be due to chronic inflammation caused by reflux of bowel contents late after choledocoenterostomy[123,124].
Hepatic resection can eliminate both stones and surrounding ductal changes, such as strictures, fibrosis, and micro abscesses. In a long-term follow-up study, operative treatment was reported to reduce recurrence[30,36].
In patients with hepatolithiasis, treatment-related difficulties include the high rates of residual stones and a high recurrence rate even after complete stone removal, especially in patients with biliary strictures[111,112]. Biliary strictures often lead to bile stasis, cholangitis, and stone formation, thus causing stone recurrence. A multivariate logistic regression analysis study reported that risk factors for incomplete stone removal are intrahepatic strictures, nonoperative treatment, and bilateral stones[113]. Thus, hepatectomy is recommended in cases of single lobe hepatolithiasis, atrophy of the affected liver, and stricture[37,118,119].
Furthermore, resection is considered when differential diagnosis is difficult, because detection of HL-CCA is very difficult even during surgical operations, and accurate diagnosis can be proven only through resection. Catena et al[37] reported that the rate of unrecognized CCA was quite high at 11.7% and that it might be underestimated.
Although mortality due to cholangitis is low, the development of HL-CCA is known to be an independent prognostic factor for survival[34,127]. Hepatolithiasis is a significant risk factor for CCA, and the OR was 40 in a Korean report[128].
Theoretically, hepatectomy for treatment of hepatolithiasis has another advantage for eliminating the risk of developing HL-CCA besides complete removal of stones. In general, hepatectomy seems to reduce the risk of developing of CCA. A cohort study in Japan reported that hepatectomy significantly reduced the risk of developing CCA[15]. A Western study by Tabrizian et al[35] reported a high rate (23.3%) of concomitant HL-CCA and excellent long-term results with hepatectomy.
It is not clear whether hepatic resection can reduce the occurrence of HL-CCA. During the follow-up period, the incidence of HL-CCA showed no significant difference between patients with hepatolithiasis with or without previous hepatic resection[30,33]. It is difficult to conclude that hepatic resection definitely prevents the development of HL-CCA in patients with hepatolithiasis.
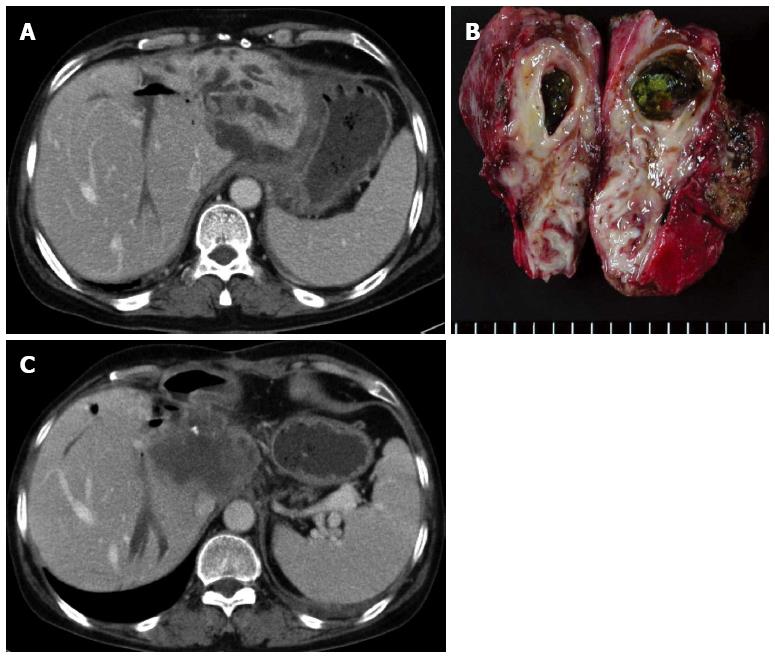
Meanwhile, incomplete resection is also a problem. Survival outcomes are good only in cases with safe surgical margins, even in incidental HL-CCA found in post-operative pathology[32]. In addition, HL-CCA could develop in the hepatic lobe adjacent to the resection margin (Figures 3 and 4). It is possible that the undetected CCA was present in the remnant liver[32,33]. Therefore, aggressive resection, including neighboring segments, is crucial to achieve sufficient hepatic volume.
The secondary goal of treatment for hepatolithiasis is to prevent the progression of the disease to cirrhosis or cancer. HL-CCA can develop even after complete stone removal through PTCSL or initial hepatectomy[33,129]. Development of HL-CCA is an independent predictor for survival in patients who have undergone hepatic resection with hepatolithiasis[12,38]. Early detection of HL-CCA is very important in follow-up after treatment of hepatolithiasis. However, to date, there is no effective measure to predict subsequent HL-CCA in patients with hepatolithiasis.
Known predictive factors for subsequent HL-CCA after treatment are old age, bile duct stricture, bilioenteric anastomosis, stone recurrence, stones in both hepatic lobes, and no hepatic resection[33,42,122].
Kim et al[33] reported that age, gender, CA 19-9, stone location, bile duct stenosis, liver atrophy, stone recurrence, residual stone, and hepatic resection were not significant predictive factors by multivariate analysis. Cheon et al[30] reported that there was no significant risk factor for CCA during the follow-up period.
Huang et al[42] and Lin et al[32] reported that the subsequent HL-CCA incidence was significantly lower after complete stone removal than in cases of residual stones (0.7% vs 6.6% and 4.9% vs 11.8%, respectively). Jo et al[108] reported that incomplete removal of stones was a risk factor for subsequent HL-CCA, but complete stone removal was not significant as a good prognostic factor. Furthermore, there are reports that the incidence of subsequent HL-CCA is not significantly different between groups: Kim et al[33] reported 3.3% vs 10.4% (P = 0.263), Tsuyuguchi et al[39] reported 9.1% vs 10.4% (P = 0.554), and Cheon et al[30] reported 4% vs 8% (P = 0.06).
The risk of subsequent HL-CCA was increased in bilateral hepatolithiasis[118,119]. Lin et al[32] reported that the incidence of concomitant HL-CCA and subsequent HL-CCA was similar in patients with unilateral hepatolithiasis (4.8% and 4.5%, respectively), but the incidence of subsequent HL-CCA (12.2%) was higher than concomitant HL-CCA (4.7%) in patients with bilateral hepatolithiasis.
Most subsequent HL-CCA has been reported to occur within the same hepatic lobe where treatment was performed[130]. However, Cheon et al[30] reported the tumor occurrence in the contralateral hepatic lobe in six out of 11 cases, and Kim et al[33] reported it in three out of 12 cases. It is possible that chronic inflammatory conditions play a role in the development of CCA arising from the bile duct without stones[77].
Suzuki et al[122] reported that risk factors for HL-CCA were choledochoenterostomy (OR = 3.718), biliary stricture (HR = 4.615), and stone recurrence (HR = 6.264)[122]. Bettschart et al[123] reported that patients who underwent bilioenteric anastomosis due to hepatolithiasis should be followed.
Su et al[11] reported that the survival of patients with HL-CCA is worse than that of patients with only CCA. In a study of 66 patients with HL-CCA, radical resection was possible in only 38 patients[131].
In contrast, there are studies that HL-CCA is not inferior in resectability and survival. Chen et al[132] showed a higher rate of resectability for HL-CCA than for CCC without HL (31.1% vs 26.8%). Lee et al[133] showed a resectability rate of 52.6% in HL-CCA and 39.2% in CCC only, and Guglielmi et al[134] also reported a higher rate of resectability in HL-CCA than CCC alone (91.3% vs 68.8%). A possible explanation is that the presence of symptoms due to hepatolithiasis could lead to an earlier diagnosis of CCA[132].
However, despite a higher rate of resectability, there were no differences in overall survival between patients with HL-CCA and patients with CCC alone[133,134]. In 23 patients who underwent resection for HL-CCC, Han et al[135] reported that the overall cumulative survival rates were 43.8%, 13.0%, and 4.3% at 1, 3, and 5 years, respectively, even though they were higher at 88.9%, 33.3%, and 11.1%, respectively, in patients with curative resection.
A recent interesting study showed that palliative surgery resulted in a gain in survival. Li et al[131] reported that the overall survival rates were 58.3%, 31.7%, and 11.7% at 1, 3, and 5 years, respectively. In the radical resection group, survival rates were 71.1%, 39.4% and 15.8%, respectively, 42.9%, 28.6% and 7.1%, respectively, in the palliative resection group, and 25%, 0%, and 0%, respectively, in the group of abdominal exploration (P < 0.001). Zhang et al[136] suggested three reasons for performing palliative resection even in patients classified as Stage IV. First, the indication for operation was not only due to the tumor, but also due to hepatolithiasis, which frequently causes biliary tract infections. Second, significant differences in the median overall survival were found between R1, R2, and non-resection-treated patients (18.2, 14.2, and 9.1 mo, respectively). Third, adjuvant therapy did not significantly prolong the survival. Considering these results, aggressive resection should be performed in practice to prolong survival even when curative treatment cannot be accomplished for patients with advanced HL-CCA.
Subsequent HL-CCA diagnosed after treatment of hepatolithiasis has a worse prognosis than concomitant HL-CCA. Lin et al[32] reported that most patients who developed subsequent HL-CCA after hepatectomy were not eligible for a second hepatectomy due to its locally advanced state, peritoneal seeding, distant metastasis, or insufficient remnant liver volume. Overall, mortality and disease-related mortality of subsequent HL-CCA is significantly higher than that for concomitant HL-CCA. Recently, Tsuyuguchi et al[39] and Kim et al[33] reported similar poor results (Table 3).
| Case | Sex/age | Location of stone | Treatment method | Residual stone | Time of CC (mo) | Location of CC | Stage |
| 1 | M/65 | Lt | ERCP | Yes | 14 | Lt | IVA |
| 2 | M/69 | Lt | PTCSL | Yes | 15 | Lt | III |
| 3 | M/72 | Lt | PTCSL | Yes | 17 | Lt | IVA |
| 4 | M/66 | Lt | ERCP | Yes | 21 | Lt | III |
| 5 | M/51 | Lt | PTCSL | Yes | 13 | Lt | IVA |
| 6 | M/63 | Lt | PTCSL | No | 53 | Lt | IVA |
| 7 | F/71 | Rt | ERCP | Yes | 79 | Both | III |
| 8 | F/54 | Both | PTCSL | Yes | 10 | Lt | IVA |
| 9 | F/56 | Rt | PTCSL | No | 111 | Rt | IVA |
| 10 | F/65 | Both | PTCSL | Yes | 72 | Lt | IVA |
| 11 | M/60 | Both | Lt. hemi-hepatectomy | Yes | 102 | Rt | III |
| 12 | F/67 | Both | Lt. lobectomy | Yes | 55 | Rt | IVA |
| 13 | F/51 | Lt | Lt. hemi- hepatectomy | No | 109 | Lt | II |
| 14 | M/52 | Both | Lt. lobectomy | Yes | 28 | Rt | IVA |
| 151 | F/47 | Lt | Lt. hemi-hepatectomy | No | 14 | Caudate | III |
| 16 | F/56 | Both | Lt. lobectomy | Yes | 81 | Rt | IVB |
Although there have been many advances in the management of hepatolithiasis, there are no consistent results regarding HL-CCA until now. Important factors to consider are the proper treatment to reduce recurrence and early detection of HL-CCA. Patients should be followed after treatment because there is no effective prevention of HL-CCA or premalignant lesions.
P- Reviewer: Liu Y, Zhou YM S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Liu XM
| 1. | Su CH, Lui WY, P’eng FK. Relative prevalence of gallstone diseases in Taiwan. A nationwide cooperative study. Dig Dis Sci. 1992;37:764-768. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 66] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Kim MH, Lim BC, Myung SJ, Lee SK, Ohrr HC, Kim YT, Roe IH, Kim JH, Chung JB, Kim CD, Shim CS, Yun YB, Min YI, Yang US, Kang JK. Epidemiological study on Korean gallstone disease: a nationwide cooperative study. Dig Dis Sci. 1999;44:1674-1683. [PubMed] [Cited in This Article: ] |
| 3. | Park YH, Park SJ, Jang JY, Ahn YJ, Park YC, Yoon YB, Kim SW. Changing patterns of gallstone disease in Korea. World J Surg. 2004;28:206-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Tazuma S. Gallstone disease: Epidemiology, pathogenesis, and classification of biliary stones (common bile duct and intrahepatic). Best Pract Res Clin Gastroenterol. 2006;20:1075-1083. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 252] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 5. | Uchiyama K, Kawai M, Ueno M, Ozawa S, Tani M, Yamaue H. Reducing residual and recurrent stones by hepatectomy for hepatolithiasis. J Gastrointest Surg. 2007;11:626-630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Park JS, Jeong S, Lee DH, Bang BW, Lee JI, Lee JW, Kwon KS, Kim HK, Shin YW, Kim YS. Risk factors for long-term outcomes after initial treatment in hepatolithiasis. J Korean Med Sci. 2013;28:1627-1631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Tsui WM, Chan YK, Wong CT, Lo YF, Yeung YW, Lee YW. Hepatolithiasis and the syndrome of recurrent pyogenic cholangitis: clinical, radiologic, and pathologic features. Semin Liver Dis. 2011;31:33-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:512-522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 427] [Cited by in F6Publishing: 457] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 9. | Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54:173-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 594] [Cited by in F6Publishing: 614] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 10. | Liu CL, Fan ST, Wong J. Primary biliary stones: diagnosis and management. World J Surg. 1998;22:1162-1166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Su CH, Shyr YM, Lui WY, P’Eng FK. Hepatolithiasis associated with cholangiocarcinoma. Br J Surg. 1997;84:969-973. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 95] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Uenishi T, Hamba H, Takemura S, Oba K, Ogawa M, Yamamoto T, Tanaka S, Kubo S. Outcomes of hepatic resection for hepatolithiasis. Am J Surg. 2009;198:199-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Joo I, Lee JM. Imaging bile duct tumors: pathologic concepts, classification, and early tumor detection. Abdom Imaging. 2013;38:1334-1350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Pausawasdi A, Watanapa P. Hepatolithiasis: epidemiology and classification. Hepatogastroenterology. 1997;44:314-316. [PubMed] [Cited in This Article: ] |
| 15. | Suzuki Y, Mori T, Yokoyama M, Nakazato T, Abe N, Nakanuma Y, Tsubouchi H, Sugiyama M. Hepatolithiasis: analysis of Japanese nationwide surveys over a period of 40 years. J Hepatobiliary Pancreat Sci. 2014;21:617-622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Nakayama F, Koga A, Ichimiya H, Todo S, Shen K, Guo RX, Zeng XJ, Zhang ZH. Hepatolithiasis in East Asia: comparison between Japan and China. J Gastroenterol Hepatol. 1991;6:155-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Fung J. Liver fluke infestation and cholangio-hepatitis. Br J Surg. 1961;48:404-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Rim HJ. Clonorchiasis: an update. J Helminthol. 2005;79:269-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 188] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 19. | Oh JK, Lim MK, Yun EH, Cho H, Park EY, Choi MH, Shin HR, Hong ST. Control of clonorchiasis in Korea: effectiveness of health education for community leaders and individuals in an endemic area. Trop Med Int Health. 2014;19:1096-1104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Cross JH. Changing patterns of some trematode infections in Asia. Arzneimittelforschung. 1984;34:1224-1226. [PubMed] [Cited in This Article: ] |
| 21. | Zhang GW, Lin JH, Qian JP, Zhou J. Identification of risk and prognostic factors for patients with clonorchiasis-associated intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2014;21:3628-3637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Choi BI, Han JK, Hong ST, Lee KH. Clonorchiasis and cholangiocarcinoma: etiologic relationship and imaging diagnosis. Clin Microbiol Rev. 2004;17:540-542, table of contents. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 157] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 23. | Khan SA, Toledano MB, Taylor-Robinson SD. Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. HPB (Oxford). 2008;10:77-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 318] [Cited by in F6Publishing: 321] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 24. | Plentz RR, Malek NP. Clinical presentation, risk factors and staging systems of cholangiocarcinoma. Best Pract Res Clin Gastroenterol. 2015;29:245-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Chen MF, Jan YY, Wang CS, Hwang TL, Jeng LB, Chen SC, Chen TJ. A reappraisal of cholangiocarcinoma in patient with hepatolithiasis. Cancer. 1993;71:2461-2465. [PubMed] [Cited in This Article: ] |
| 26. | Koga A, Ichimiya H, Yamaguchi K, Miyazaki K, Nakayama F. Hepatolithiasis associated with cholangiocarcinoma. Possible etiologic significance. Cancer. 1985;55:2826-2829. [PubMed] [Cited in This Article: ] |
| 27. | Nakanuma Y, Terada T, Tanaka Y, Ohta G. Are hepatolithiasis and cholangiocarcinoma aetiologically related? A morphological study of 12 cases of hepatolithiasis associated with cholangiocarcinoma. Virchows Arch A Pathol Anat Histopathol. 1985;406:45-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 88] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Chen MF, Jan YY, Wang CS, Jeng LB, Hwang TL, Chen SC. Intrahepatic stones associated with cholangiocarcinoma. Am J Gastroenterol. 1989;84:391-395. [PubMed] [Cited in This Article: ] |
| 29. | Sheen-Chen SM, Chou FF, Eng HL. Intrahepatic cholangiocarcinoma in hepatolithiasis: A frequently overlooked disease. J Surg Oncol. 1991;47:131-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 58] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Cheon YK, Cho YD, Moon JH, Lee JS, Shim CS. Evaluation of long-term results and recurrent factors after operative and nonoperative treatment for hepatolithiasis. Surgery. 2009;146:843-853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 31. | Falchuk KR, Lesser PB, Galdabini JJ, Isselbacher KJ. Cholangiocarcinoma as related to chronic intrahepatic cholangitis and hepatolithiasis. Case report and review of the literature. Am J Gastroenterol. 1976;66:57-61. [PubMed] [Cited in This Article: ] |
| 32. | Lin CC, Lin PY, Chen YL. Comparison of concomitant and subsequent cholangiocarcinomas associated with hepatolithiasis: Clinical implications. World J Gastroenterol. 2013;19:375-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 16] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Kim HJ, Kim JS, Suh SJ, Lee BJ, Park JJ, Lee HS, Kim CD, Bak YT. Cholangiocarcinoma Risk as Long-term Outcome After Hepatic Resection in the Hepatolithiasis Patients. World J Surg. 2015;39:1537-1542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Zhu QD, Zhou MT, Zhou QQ, Shi HQ, Zhang QY, Yu ZP. Diagnosis and surgical treatment of intrahepatic hepatolithiasis combined with cholangiocarcinoma. World J Surg. 2014;38:2097-2104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Tabrizian P, Jibara G, Shrager B, Schwartz ME, Roayaie S. Hepatic resection for primary hepatolithiasis: a single-center Western experience. J Am Coll Surg. 2012;215:622-626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Lee TY, Chen YL, Chang HC, Chan CP, Kuo SJ. Outcomes of hepatectomy for hepatolithiasis. World J Surg. 2007;31:479-482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 37. | Catena M, Aldrighetti L, Finazzi R, Arzu G, Arru M, Pulitanò C, Ferla G. Treatment of non-endemic hepatolithiasis in a Western country. The role of hepatic resection. Ann R Coll Surg Engl. 2006;88:383-389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | Chen DW, Tung-Ping Poon R, Liu CL, Fan ST, Wong J. Immediate and long-term outcomes of hepatectomy for hepatolithiasis. Surgery. 2004;135:386-393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 110] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 39. | Tsuyuguchi T, Miyakawa K, Sugiyama H, Sakai Y, Nishikawa T, Sakamoto D, Nakamura M, Yasui S, Mikata R, Yokosuka O. Ten-year long-term results after non-surgical management of hepatolithiasis, including cases with choledochoenterostomy. J Hepatobiliary Pancreat Sci. 2014;21:795-800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Liu Z, Tian F, Feng X, He Y, Jiang P, Li J, Guo F, Zhao X, Chang H, Wang S. LPS increases MUC5AC by TACE/TGF-α/EGFR pathway in human intrahepatic biliary epithelial cell. Biomed Res Int. 2013;2013:165715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 41. | Wu SD, Uchiyama K, Fan Y. The role and mechanism of fatty acids in gallstones. Hepatobiliary Pancreat Dis Int. 2007;6:399-401. [PubMed] [Cited in This Article: ] |
| 42. | Huang MH, Chen CH, Yang JC, Yang CC, Yeh YH, Chou DA, Mo LR, Yueh SK, Nien CK. Long-term outcome of percutaneous transhepatic cholangioscopic lithotomy for hepatolithiasis. Am J Gastroenterol. 2003;98:2655-2662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 115] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 43. | Yamashita N, Yanagisawa J, Nakayama F. Composition of intrahepatic calculi. Etiological significance. Dig Dis Sci. 1988;33:449-453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Shoda J, Tanaka N, Matsuzaki Y, Honda A, Osuga T, Shigematsu S, Miyazaki H. Microanalysis of bile acid composition in intrahepatic calculi and its etiological significance. Gastroenterology. 1991;101:821-830. [PubMed] [Cited in This Article: ] |
| 45. | Shoda J, Tanaka N, He BF, Matsuzaki Y, Osuga T, Miyazaki H. Alterations of bile acid composition in gallstones, bile, and liver of patients with hepatolithiasis, and their etiological significance. Dig Dis Sci. 1993;38:2130-2141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 46. | Strichartz SD, Abedin MZ, Ippoliti AF, Derezin M, Roslyn JJ. Intrahepatic cholesterol stones: a rationale for dissolution therapy. Gastroenterology. 1991;100:228-232. [PubMed] [Cited in This Article: ] |
| 47. | Shoda J, Oda K, Suzuki H, Sugiyama Y, Ito K, Cohen DE, Feng L, Kamiya J, Nimura Y, Miyazaki H. Etiologic significance of defects in cholesterol, phospholipid, and bile acid metabolism in the liver of patients with intrahepatic calculi. Hepatology. 2001;33:1194-1205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 48. | Smith AJ, de Vree JM, Ottenhoff R, Oude Elferink RP, Schinkel AH, Borst P. Hepatocyte-specific expression of the human MDR3 P-glycoprotein gene restores the biliary phosphatidylcholine excretion absent in Mdr2 (-/-) mice. Hepatology. 1998;28:530-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 104] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Cohen DE, Leonard MR, Carey MC. In vitro evidence that phospholipid secretion into bile may be coordinated intracellularly by the combined actions of bile salts and the specific phosphatidylcholine transfer protein of liver. Biochemistry. 1994;33:9975-9980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 50. | Mauad TH, van Nieuwkerk CM, Dingemans KP, Smit JJ, Schinkel AH, Notenboom RG, van den Bergh Weerman MA, Verkruisen RP, Groen AK, Oude Elferink RP. Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am J Pathol. 1994;145:1237-1245. [PubMed] [Cited in This Article: ] |
| 51. | de Vree JM, Jacquemin E, Sturm E, Cresteil D, Bosma PJ, Aten J, Deleuze JF, Desrochers M, Burdelski M, Bernard O. Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc Natl Acad Sci USA. 1998;95:282-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 543] [Cited by in F6Publishing: 443] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 52. | Malagelada JR, Go VL, DiMagno EP, Summerskill WH. Interactions between intraluminal bile acids and digestive products on pancreatic and gallbladder function. J Clin Invest. 1973;52:2160-2165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 77] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Ashkin JR, Lyon DT, Shull SD, Wagner CI, Soloway RD. Factors affecting delivery of bile to the duodenum in man. Gastroenterology. 1978;74:560-565. [PubMed] [Cited in This Article: ] |
| 54. | Tabata M, Nakayama F. Bacteriology of hepatolithiasis. Prog Clin Biol Res. 1984;152:163-174. [PubMed] [Cited in This Article: ] |
| 55. | Tabata M, Nakayama F. Bacteria and gallstones. Etiological significance. Dig Dis Sci. 1981;26:218-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 121] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 56. | Shoda J, Kano M, Asano T, Irimura T, Ueda T, Iwasaki R, Furukawa M, Kamiya J, Nimura Y, Todoroki T. Secretory low-molecular-weight phospholipases A2 and their specific receptor in bile ducts of patients with intrahepatic calculi: factors of chronic proliferative cholangitis. Hepatology. 1999;29:1026-1036. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 57. | Shoda J, Ueda T, Kawamoto T, Todoroki T, Asano T, Sugimoto Y, Ichikawa A, Maruyama T, Nimura Y, Tanaka N. Prostaglandin E receptors in bile ducts of hepatolithiasis patients and the pathobiological significance for cholangitis. Clin Gastroenterol Hepatol. 2003;1:285-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 58. | Yang L, Junmin S, Hong Y, Shuodong W. PGE(2) induces MUC2 and MUC5AC expression in human intrahepatic biliary epithelial cells via EP4/p38MAPK activation. Ann Hepatol. 2013;12:479-486. [PubMed] [Cited in This Article: ] |
| 59. | Nakanuma Y, Yamaguchi K, Ohta G, Terada T. Pathologic features of hepatolithiasis in Japan. Hum Pathol. 1988;19:1181-1186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 103] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 60. | Forstner JF, Forstner GG. Calcium binding to intestinal goblet cell mucin. Biochim Biophys Acta. 1975;386:283-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 61. | Woodward H, Horsey B, Bhavanandan VP, Davidson EA. Isolation, purification, and properties of respiratory mucus glycoproteins. Biochemistry. 1982;21:694-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 88] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 62. | Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145:1215-1229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 787] [Cited by in F6Publishing: 856] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 63. | Terada T, Nakanuma Y. Cell kinetic analyses and expression of carcinoembryonic antigen, carbohydrate antigen 19-9 and DU-PAN-2 in hyperplastic, pre-neoplastic and neoplastic lesions of intrahepatic bile ducts in livers with hepatoliths. Virchows Arch A Pathol Anat Histopathol. 1992;420:327-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 64. | Dobrovolskaia MA, Kozlov SV. Inflammation and cancer: when NF-kappaB amalgamates the perilous partnership. Curr Cancer Drug Targets. 2005;5:325-344. [PubMed] [Cited in This Article: ] |
| 65. | Mariani F, Sena P, Roncucci L. Inflammatory pathways in the early steps of colorectal cancer development. World J Gastroenterol. 2014;20:9716-9731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 90] [Cited by in F6Publishing: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 66. | Chisari FV, Klopchin K, Moriyama T, Pasquinelli C, Dunsford HA, Sell S, Pinkert CA, Brinster RL, Palmiter RD. Molecular pathogenesis of hepatocellular carcinoma in hepatitis B virus transgenic mice. Cell. 1989;59:1145-1156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 535] [Cited by in F6Publishing: 502] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 67. | Chen TC, Nakanuma Y, Zen Y, Chen MF, Jan YY, Yeh TS, Chiu CT, Kuo TT, Kamiya J, Oda K. Intraductal papillary neoplasia of the liver associated with hepatolithiasis. Hepatology. 2001;34:651-658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 213] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 68. | Aishima S, Kubo Y, Tanaka Y, Oda Y. Histological features of precancerous and early cancerous lesions of biliary tract carcinoma. J Hepatobiliary Pancreat Sci. 2014;21:448-452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 69. | Wan XS, Xu YY, Qian JY, Yang XB, Wang AQ, He L, Zhao HT, Sang XT. Intraductal papillary neoplasm of the bile duct. World J Gastroenterol. 2013;19:8595-8604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 108] [Cited by in F6Publishing: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 70. | Sato Y, Sasaki M, Harada K, Aishima S, Fukusato T, Ojima H, Kanai Y, Kage M, Nakanuma Y, Tsubouchi H. Pathological diagnosis of flat epithelial lesions of the biliary tract with emphasis on biliary intraepithelial neoplasia. J Gastroenterol. 2014;49:64-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 71. | Aishima S, Kuroda Y, Nishihara Y, Iguchi T, Taguchi K, Taketomi A, Maehara Y, Tsuneyoshi M. Proposal of progression model for intrahepatic cholangiocarcinoma: clinicopathologic differences between hilar type and peripheral type. Am J Surg Pathol. 2007;31:1059-1067. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 72. | Zen Y, Sasaki M, Fujii T, Chen TC, Chen MF, Yeh TS, Jan YY, Huang SF, Nimura Y, Nakanuma Y. Different expression patterns of mucin core proteins and cytokeratins during intrahepatic cholangiocarcinogenesis from biliary intraepithelial neoplasia and intraductal papillary neoplasm of the bile duct--an immunohistochemical study of 110 cases of hepatolithiasis. J Hepatol. 2006;44:350-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 202] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 73. | Nakanuma Y, Sasaki M, Ishikawa A, Tsui W, Chen TC, Huang SF. Biliary papillary neoplasm of the liver. Histol Histopathol. 2002;17:851-861. [PubMed] [Cited in This Article: ] |
| 74. | Shimonishi T, Zen Y, Chen TC, Chen MF, Jan YY, Yeh TS, Nimura Y, Nakanuma Y. Increasing expression of gastrointestinal phenotypes and p53 along with histologic progression of intraductal papillary neoplasia of the liver. Hum Pathol. 2002;33:503-511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 75. | Itatsu K, Zen Y, Ohira S, Ishikawa A, Sato Y, Harada K, Ikeda H, Sasaki M, Nimura Y, Nakanuma Y. Immunohistochemical analysis of the progression of flat and papillary preneoplastic lesions in intrahepatic cholangiocarcinogenesis in hepatolithiasis. Liver Int. 2007;27:1174-1184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 76. | Kuroki T, Tajima Y, Kanematsu T. Hepatolithiasis and intrahepatic cholangiocarcinoma: carcinogenesis based on molecular mechanisms. J Hepatobiliary Pancreat Surg. 2005;12:463-466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 77. | Nakanuma Y, Harada K, Ishikawa A, Zen Y, Sasaki M. Anatomic and molecular pathology of intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2003;10:265-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 78. | Aishima SI, Taguchi KI, Sugimachi K, Shimada M, Sugimachi K, Tsuneyoshi M. c-erbB-2 and c-Met expression relates to cholangiocarcinogenesis and progression of intrahepatic cholangiocarcinoma. Histopathology. 2002;40:269-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 79. | Kim HJ, Kim JS, Kang CD, Lee SJ, Kim JY, Yeon JE, Park JJ, Shim JJ, Byun KS, Bak YT. [Expression of epidermal growth factor receptor, ErbB2 and matrix metalloproteinase-9 in hepatolithiasis and cholangiocarcinoma]. Korean J Gastroenterol. 2005;45:52-59. [PubMed] [Cited in This Article: ] |
| 80. | Zhou Q, Gong Y, Huang F, Lin Q, Zeng B, Li Z, Chen R. Expression levels and significance of nuclear factor-κB and epidermal growth factor receptor in hepatolithiasis associated with intrahepatic cholangiocarcinoma. Dig Surg. 2013;30:309-316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 81. | Chariyalertsak S, Sirikulchayanonta V, Mayer D, Kopp-Schneider A, Fürstenberger G, Marks F, Müller-Decker K. Aberrant cyclooxygenase isozyme expression in human intrahepatic cholangiocarcinoma. Gut. 2001;48:80-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 82. | Hayashi N, Yamamoto H, Hiraoka N, Dono K, Ito Y, Okami J, Kondo M, Nagano H, Umeshita K, Sakon M. Differential expression of cyclooxygenase-2 (COX-2) in human bile duct epithelial cells and bile duct neoplasm. Hepatology. 2001;34:638-650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 108] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 83. | Endo K, Yoon BI, Pairojkul C, Demetris AJ, Sirica AE. ERBB-2 overexpression and cyclooxygenase-2 up-regulation in human cholangiocarcinoma and risk conditions. Hepatology. 2002;36:439-450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 165] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 84. | Varnholt H, Asayama Y, Aishima S, Taguchi K, Sugimachi K, Tsuneyoshi M. C-met and hepatocyte growth factor expression in combined hepatocellular and cholangiocarcinoma. Oncol Rep. 2002;9:35-41. [PubMed] [Cited in This Article: ] |
| 85. | Terada T, Nakanuma Y, Sirica AE. Immunohistochemical demonstration of MET overexpression in human intrahepatic cholangiocarcinoma and in hepatolithiasis. Hum Pathol. 1998;29:175-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 115] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 86. | Liggett WH, Sidransky D. Role of the p16 tumor suppressor gene in cancer. J Clin Oncol. 1998;16:1197-1206. [PubMed] [Cited in This Article: ] |
| 87. | Ishikawa A, Sasaki M, Sato Y, Ohira S, Chen MF, Huang SF, Oda K, Nimura Y, Nakanuma Y. Frequent p16ink4a inactivation is an early and frequent event of intraductal papillary neoplasm of the liver arising in hepatolithiasis. Hum Pathol. 2004;35:1505-1514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 88. | Sasaki M, Yamaguchi J, Itatsu K, Ikeda H, Nakanuma Y. Over-expression of polycomb group protein EZH2 relates to decreased expression of p16 INK4a in cholangiocarcinogenesis in hepatolithiasis. J Pathol. 2008;215:175-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 89. | Lee KT, Chang WT, Wang SN, Chuang SC, Chai CY, Hu SW. Expression of DPC4/Smad4 gene in stone-containing intrahepatic bile duct. J Surg Oncol. 2006;94:338-343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 90. | Mori T, Sugiyama M, Atomi Y. Gallstone disease: Management of intrahepatic stones. Best Pract Res Clin Gastroenterol. 2006;20:1117-1137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 81] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 91. | Liu FB, Yu XJ, Wang GB, Zhao YJ, Xie K, Huang F, Cheng JM, Wu XR, Liang CJ, Geng XP. Preliminary study of a new pathological evolution-based clinical hepatolithiasis classification. World J Gastroenterol. 2015;21:2169-2177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 92. | Lee JW, Han JK, Kim TK, Kim YH, Choi BI, Han MC, Suh KS, Kim SW. CT features of intraductal intrahepatic cholangiocarcinoma. AJR Am J Roentgenol. 2000;175:721-725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 93. | Park DH, Kim MH, Lee SS, Lee SK, Kim KP, Han JM, Kim SY, Song MH, Seo DW, Kim AY. Accuracy of magnetic resonance cholangiopancreatography for locating hepatolithiasis and detecting accompanying biliary strictures. Endoscopy. 2004;36:987-992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 94. | Kubo S, Hamba H, Hirohashi K, Kinoshita H, Lee KC, Yamazaki O, Nishio H, Yamada R. Magnetic resonance cholangiography in hepatolithiasis. Am J Gastroenterol. 1997;92:629-632. [PubMed] [Cited in This Article: ] |
| 95. | Wani NA, Robbani I, Kosar T. MRI of oriental cholangiohepatitis. Clin Radiol. 2011;66:158-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 96. | Khan SA, Davidson BR, Goldin R, Pereira SP, Rosenberg WM, Taylor-Robinson SD, Thillainayagam AV, Thomas HC, Thursz MR, Wasan H. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut. 2002;51 Suppl 6:VI1-VI9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 314] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 97. | Dodson RM, Weiss MJ, Cosgrove D, Herman JM, Kamel I, Anders R, Geschwind JF, Pawlik TM. Intrahepatic cholangiocarcinoma: management options and emerging therapies. J Am Coll Surg. 2013;217:736-750.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 98. | Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J Hepatobiliary Pancreat Surg. 2003;10:288-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 310] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 99. | Choi JY, Kim MJ, Lee JM, Kim KW, Lee JY, Han JK, Choi BI. Hilar cholangiocarcinoma: role of preoperative imaging with sonography, MDCT, MRI, and direct cholangiography. AJR Am J Roentgenol. 2008;191:1448-1457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 100. | Jung AY, Lee JM, Choi SH, Kim SH, Lee JY, Kim SW, Han JK, Choi BI. CT features of an intraductal polypoid mass: Differentiation between hepatocellular carcinoma with bile duct tumor invasion and intraductal papillary cholangiocarcinoma. J Comput Assist Tomogr. 2006;30:173-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 101. | Park HS, Lee JM, Kim SH, Jeong JY, Kim YJ, Lee KH, Choi SH, Han JK, Choi BI. CT Differentiation of cholangiocarcinoma from periductal fibrosis in patients with hepatolithiasis. AJR Am J Roentgenol. 2006;187:445-453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 102. | Manfredi R, Barbaro B, Masselli G, Vecchioli A, Marano P. Magnetic resonance imaging of cholangiocarcinoma. Semin Liver Dis. 2004;24:155-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 103. | Sainani NI, Catalano OA, Holalkere NS, Zhu AX, Hahn PF, Sahani DV. Cholangiocarcinoma: current and novel imaging techniques. Radiographics. 2008;28:1263-1287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 104. | Razumilava N, Gores GJ. Classification, diagnosis, and management of cholangiocarcinoma. Clin Gastroenterol Hepatol. 2013;11:13-21.e1; quiz e3-e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 191] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 105. | Anderson CD, Rice MH, Pinson CW, Chapman WC, Chari RS, Delbeke D. Fluorodeoxyglucose PET imaging in the evaluation of gallbladder carcinoma and cholangiocarcinoma. J Gastrointest Surg. 2004;8:90-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 285] [Cited by in F6Publishing: 294] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 106. | Lan BY, Kwee SA, Wong LL. Positron emission tomography in hepatobiliary and pancreatic malignancies: a review. Am J Surg. 2012;204:232-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 107. | Vilgrain V. Staging cholangiocarcinoma by imaging studies. HPB (Oxford). 2008;10:106-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 108. | Jo JH, Chung MJ, Park JY, Bang S, Park SW, Kim KS, Lee WJ, Song SY, Chung JB. High serum CA19-9 levels are associated with an increased risk of cholangiocarcinoma in patients with intrahepatic duct stones: a case-control study. Surg Endosc. 2013;27:4210-4216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 109. | Menias CO, Surabhi VR, Prasad SR, Wang HL, Narra VR, Chintapalli KN. Mimics of cholangiocarcinoma: spectrum of disease. Radiographics. 2008;28:1115-1129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 110. | Cai X, Wang Y, Yu H, Liang X, Peng S. Laparoscopic hepatectomy for hepatolithiasis: a feasibility and safety study in 29 patients. Surg Endosc. 2007;21:1074-1078. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 111. | Otani K, Shimizu S, Chijiiwa K, Ogawa T, Morisaki T, Sugitani A, Yamaguchi K, Tanaka M. Comparison of treatments for hepatolithiasis: hepatic resection versus cholangioscopic lithotomy. J Am Coll Surg. 1999;189:177-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 88] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 112. | Fan ST, Choi TK, Lo CM, Mok FP, Lai EC, Wong J. Treatment of hepatolithiasis: improvement of result by a systematic approach. Surgery. 1991;109:474-480. [PubMed] [Cited in This Article: ] |
| 113. | Lee SK, Seo DW, Myung SJ, Park ET, Lim BC, Kim HJ, Yoo KS, Park HJ, Joo YH, Kim MH. Percutaneous transhepatic cholangioscopic treatment for hepatolithiasis: an evaluation of long-term results and risk factors for recurrence. Gastrointest Endosc. 2001;53:318-323. [PubMed] [DOI] [Cited in This Article: ] |
| 114. | Cheung MT, Kwok PC. Liver resection for intrahepatic stones. Arch Surg. 2005;140:993-997. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 115. | Lee KF, Chong CN, Ng D, Cheung YS, Ng W, Wong J, Lai P. Outcome of surgical treatment for recurrent pyogenic cholangitis: a single-centre study. HPB (Oxford). 2009;11:75-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 116. | Sakpal SV, Babel N, Chamberlain RS. Surgical management of hepatolithiasis. HPB (Oxford). 2009;11:194-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 117. | Uchiyama K, Ueno M, Ozawa S, Kiriyama S, Kawai M, Hirono S, Tani M, Yamaue H. Risk factors for postoperative infectious complications after hepatectomy. J Hepatobiliary Pancreat Sci. 2011;18:67-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 118. | Yang T, Lau WY, Lai EC, Yang LQ, Zhang J, Yang GS, Lu JH, Wu MC. Hepatectomy for bilateral primary hepatolithiasis: a cohort study. Ann Surg. 2010;251:84-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 119. | Li SQ, Liang LJ, Peng BG, Hua YP, Lv MD, Fu SJ, Chen D. Outcomes of liver resection for intrahepatic stones: a comparative study of unilateral versus bilateral disease. Ann Surg. 2012;255:946-953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 120. | Kim YT, Byun JS, Kim J, Jang YH, Lee WJ, Ryu JK, Kim SW, Yoon YB, Kim CY. Factors predicting concurrent cholangiocarcinomas associated with hepatolithiasis. Hepatogastroenterology. 2003;50:8-12. [PubMed] [Cited in This Article: ] |
| 121. | Liu ZY, Zhou YM, Shi LH, Yin ZF. Risk factors of intrahepatic cholangiocarcinoma in patients with hepatolithiasis: a case-control study. Hepatobiliary Pancreat Dis Int. 2011;10:626-631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 122. | Suzuki Y, Mori T, Abe N, Sugiyama M, Atomi Y. Predictive factors for cholangiocarcinoma associated with hepatolithiasis determined on the basis of Japanese Multicenter study. Hepatol Res. 2012;42:166-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 123. | Bettschart V, Clayton RA, Parks RW, Garden OJ, Bellamy CO. Cholangiocarcinoma arising after biliary-enteric drainage procedures for benign disease. Gut. 2002;51:128-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 124. | Zhang XJ, Jiang Y, Wang X, Tian FZ, Lv LZ. Comparatively lower postoperative hepatolithiasis risk with hepaticocholedochostomy versus hepaticojejunostomy. Hepatobiliary Pancreat Dis Int. 2010;9:38-43. [PubMed] [Cited in This Article: ] |
| 125. | Hur H, Park IY, Sung GY, Lee DS, Kim W, Won JM. Intrahepatic cholangiocarcinoma associated with intrahepatic duct stones. Asian J Surg. 2009;32:7-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 126. | Lee JY, Kim JS, Moon JM, Lim SA, Chung W, Lim EH, Lee BJ, Park JJ, Bak YT. Incidence of Cholangiocarcinoma with or without Previous Resection of Liver for Hepatolithiasis. Gut Liver. 2013;7:475-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 127. | Chijiiwa K, Yamashita H, Yoshida J, Kuroki S, Tanaka M. Current management and long-term prognosis of hepatolithiasis. Arch Surg. 1995;130:194-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 64] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 128. | Lee BS, Park EC, Park SW, Nam CM, Roh J. Hepatitis B virus infection, diabetes mellitus, and their synergism for cholangiocarcinoma development: a case-control study in Korea. World J Gastroenterol. 2015;21:502-510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 30] [Cited by in F6Publishing: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 129. | Chijiiwa K, Ichimiya H, Kuroki S, Koga A, Nakayama F. Late development of cholangiocarcinoma after the treatment of hepatolithiasis. Surg Gynecol Obstet. 1993;177:279-282. [PubMed] [Cited in This Article: ] |
| 130. | Kusano T, Isa T, Ohtsubo M, Yasaka T, Furukawa M. Natural progression of untreated hepatolithiasis that shows no clinical signs at its initial presentation. J Clin Gastroenterol. 2001;33:114-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 131. | Li HY, Zhou SJ, Li M, Xiong D, Singh A, Guo QX, Liu CA, Gong JP. Diagnosis and cure experience of hepatolithiasis-associated intrahepatic cholangiocarcinoma in 66 patients. Asian Pac J Cancer Prev. 2012;13:725-729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 132. | Chen MF, Jan YY, Hwang TL, Jeng LB, Yeh TS. Impact of concomitant hepatolithiasis on patients with peripheral cholangiocarcinoma. Dig Dis Sci. 2000;45:312-316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 133. | Lee CC, Wu CY, Chen GH. What is the impact of coexistence of hepatolithiasis on cholangiocarcinoma? J Gastroenterol Hepatol. 2002;17:1015-1020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 134. | Guglielmi A, Ruzzenente A, Valdegamberi A, Bagante F, Conci S, Pinna AD, Ercolani G, Giuliante F, Capussotti L, Aldrighetti L. Hepatolithiasis-associated cholangiocarcinoma: results from a multi-institutional national database on a case series of 23 patients. Eur J Surg Oncol. 2014;40:567-575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 135. | Han SL, Zhou HZ, Cheng J, Lan SH, Zhang PC, Chen ZJ, Zeng QQ. Diagnosis and surgical treatment of intrahepatic hepatolithiasis associated cholangiocarcinoma. Asian J Surg. 2009;32:1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 136. | Zhang GW, Lin JH, Qian JP, Zhou J. Identification of prognostic factors and the impact of palliative resection on survival of patients with stage IV hepatolithiasis-associated intrahepatic cholangiocarcinoma. J Surg Oncol. 2014;109:494-499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |