Published online Nov 21, 2015. doi: 10.3748/wjg.v21.i43.12410
Peer-review started: March 23, 2015
First decision: June 19, 2015
Revised: June 30, 2015
Accepted: September 2, 2015
Article in press: September 2, 2015
Published online: November 21, 2015
AIM: To investigate the impact of systemic inflammation-based prognostic markers on overall survival in relapsed/refractory metastatic colorectal cancer (mCRC) patients.
METHODS: To investigate prognostic markers in mCRC patients, this study was performed with patients who have experienced relapsed/refractory mCRC with standard chemotherapy or were inapplicable to conventional treatment modality because of poor performance status, age, or comorbidity. We reviewed the medical records of 177 mCRC patients managed with Korean Medicine (KM) treatment modality using an anticancer agent of Rhus verniciflua Stokes extract from June 2006 to April 2013. The clinicopathologic characteristics, laboratory test, the systemic inflammation markers including the modified Glasgow prognostic score (mGPS), neutrophil lymphocyte ratio (NLR), platelet lymphocyte ratio (PLR), lymphocyte monocyte ratio (LMR), and prognostic nutritional index (PNI) were analyzed. The overall survival of patients was calculated with the Kaplan-Meier method and the statistical significance was compared using with the log-rank test. To compare the impact of systemic inflammation based markers, the hazard ratio (HR) of mGPS, NLR, PLR, LMR, and PNI for overall survival were evaluated with the Cox proportional hazards regression.
RESULTS: The majority of mCRC patients had relapsed/refractory to standard chemotherapy; 128 patients (72.3%) had undergone more than second line chemotherapy, and the median time from diagnosis of mCRC to initiation of KM was 9.4 mo. The median overall survival of enrolled patients was 8.3 mo. On univariate analyses, the inflammation markers of higher mGPS (P < 0.001), NLR ≥ 5 (P < 0.001), PLR > 300 (P = 0.004), LMR ≤ 3.4 (P < 0.001), and PNI ≤ 45.3 (P = 0.001) were significantly associated with decreased survival time. On stepwise multivariate proportional hazards model, mGPS at 2 vs 0 (HR = 3.212, 95%CI: 1.437-7.716, P = 0.004), and LMR ≤ 3.4 (HR = 1.658, 95%CI: 1.092-2.518, P = 0.018) as independent predictors associated with poor overall survival along with carbohydrate antigen 19-9 (HR = 1.482, 95%CI: 1.007-2.182, P = 0.046), AST ≥ 40 (HR = 2.377, 95%CI: 1.359-4.155, P = 0.002), and the treatment duration for KM less than 2.9 mo (HR = 1.718, 95%CI: 1.160-2.543, P = 0.007).
CONCLUSION: These results indicate that the inflammatory markers, mGPS and LMR are independent prognostic factors for predicting overall survival in relapsed/refractory mCRC patients.
Core tip: This is a retrospective study to compare the systemic inflammation based prognostic markers in relapsed or refractory metastatic colorectal cancer (mCRC) patients. We reviewed the medical records of 177 mCRC patients and analyzed the impact of systemic inflammation markers including modified Glasgow prognostic score (mGPS), neutrophil lymphocyte ratio, platelet lymphocyte ratio, lymphocyte monocyte ratio (LMR), and prognostic nutritional index on survival time. The mGPS and LMR were the best inflammatory markers for predicting overall survival time in relapsed or refractory mCRC patients.
- Citation: Song A, Eo W, Lee S. Comparison of selected inflammation-based prognostic markers in relapsed or refractory metastatic colorectal cancer patients. World J Gastroenterol 2015; 21(43): 12410-12420
- URL: https://www.wjgnet.com/1007-9327/full/v21/i43/12410.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i43.12410
Colorectal cancer is the third most common cancer in South Korea, and it is usually diagnosed at an advanced stage[1]. The precise prediction of survival in relapsed/refractory metastatic colorectal cancer (mCRC) patients is important to determine proper management modalities in clinical practice. Over the last couple of decades, there has been a focus on identifying host-related factors, which associated with cancer outcomes. And they have proven to be potential determinants to predict prognosis, guide decisions related to treatment modality, and evaluate treatment efficacy[2]. Studies on inflammation markers and host inflammatory responses in cancer have been actively carried out since 2000[3,4].
Systemic inflammation is known to elevate the C-reactive protein (CRP) and change the relative proportion of white blood cells, raising the neutrophil count and lowering the lymphocyte count[5,6]. Reflecting the distinct feature of inflammation and cancer, several indices such as the Glasgow prognostic score (GPS), neutrophil lymphocyte ratio (NLR), platelet lymphocyte ratio (PLR), lymphocyte monocyte ratio (LMR), and Onodera’s prognostic nutritional index (PNI) have been consistently studied for potential application in cancer prognosis[7-9].
Based on several studies of GPS, hypoalbuminemia without CRP elevation was found to show no significance as a prognostic factor. Thus, recent research has used modified GPS (mGPS) as a potential prognostic factor[10]. The mGPS and NLR were studied as independent prognostic factors in advanced colorectal cancer and PLR in pancreatic cancer[11-14]. There have also been studies showing a correlation between LMR and prognoses of Hodgkin’s lymphoma, and PNI and prognoses of gastrointestinal malignancy[8,15,16].
So far, most studies have focused on the efficacy of inflammatory markers for newly diagnosed advanced cancer patients who were naïve to cancer treatment, and inflammation markers have been used as prognostic markers for the initial treatment, which could be surgery or chemotherapy. However, systemic inflammation is rare in newly diagnosed patients with early conditions of advanced cancers. To enhance the clinical meaning of inflammatory markers as prognostic markers of survival, it is necessary to evaluate far-advanced patients, who relapse after undergoing standard treatments and who show cancer-related symptoms.
Therefore, the aim of this study was to identify the clinical efficacy of systemic inflammation as a prognostic factor to predict overall survival (OS) in mCRC patients who relapsed from standard therapy and to identify which markers from among mGPS, NLR, PLR, LMR, and PNI are useful for identifying patients who should receive palliative care.
This study was performed with review of medical records and it was approved by the Institutional Review Board of Kyung Hee University Hospital at Gangdong (KHNMC-OH-IRB 2013-010).
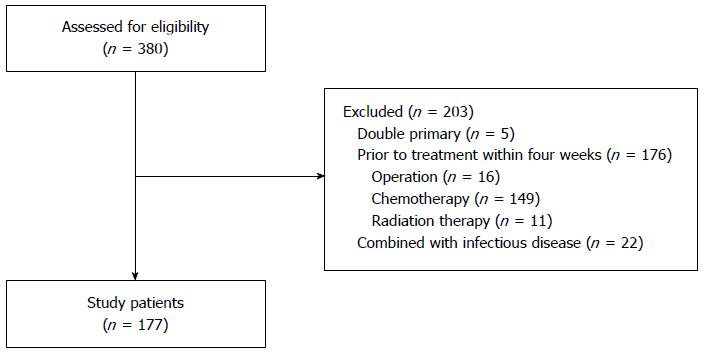
We reviewed the medical records of mCRC patients aged over 20 years who were examined by laboratory tests from June 2006 to April 2013. From 380 cases, we excluded patients with double primary cancers (n = 5), and patients who underwent surgery (n = 16), chemotherapy (n = 149), or radiotherapy (n = 11) within four weeks of the initial laboratory examination to avoid the possible influence of treatment modality on inflammation markers. Also, we excluded cases (n = 22) that showed comorbidity with specific signs and symptoms of inflammation at the initial examination. Finally, 177 patients with mCRC were included in the study (Figure 1).
OS was defined as the time from the first day of treatment to the time of death from any cause. When the patient was lost to follow-up or the death was not recorded, the patient was censored. The survival time of censored patients was defined by the period from the initiation of treatment to the last day of follow-up or to September 29, 2013, the date on which the survival was investigated.
Before initiation of treatment, patients were examined for complete blood count with differential and blood chemistry tests including CRP, albumin, tumor markers, and other laboratory tests.
The mGPS was evaluated with CRP and albumin, and it was based on the previous study demonstrating that hypoalbuminemia without elevated CRP has no significant relationship with OS[10]. Patients with CRP greater than 10 mg/L and albumin lower than 3.5 g/dL were assigned a score of 2. Patients with only CRP elevation were assigned a score of 1. Patients with a normal value for both CRP and albumin or only low albumin levels were assigned a score of 0. NLR was defined as the absolute neutrophil to absolute lymphocyte ratio, and PLR was defined as the platelet to absolute lymphocyte ratio. NLR was categorized into two groups based on the cut-off points (≥ 5 or < 5), and PLR was classified into three groups ( < 150, 150-300, > 300)[9]. LMR was defined as the absolute lymphocyte to absolute monocyte ratio, and PNI was comprised of albumin and lymphocytes. There was no validated cut-off point for the LMR and PNI, so cut-off points of LMR and PNI in this study were presented by the receiver operating characteristic (ROC) curve and the area under the ROC curve (AUC).
The applied treatment modality for relapsed/refractory mCRC patients who had no choice of conventional standard treatment was Korean Medicine (KM). Herbal medication and acupuncture were the major modality for patients underwent KM treatment. The Rhus verniciflua stokes (RVS) extract was used as a main anticancer agent. RVS extract was originated from the lacquer tree, which grows in East Asia. RVS has been shown to have an anti-proliferative effect, apoptotic activity, an anti-angiogenic effect, and an anti-tumor migration effect[17-19]. RVS was extracted by the standardized method with water at 95 °C, then concentrated and lyophilized in powdered form. After being depleted of toxic allergens, the extract was examined for quality with concentrations of the main compounds. Each capsule contained 500 mg of RVS extract, and patients were typically prescribed 1500 mg of RVS extract daily.
The clinicopathologic features, laboratory tests, and systemic inflammation markers were recorded for the potential prognostic factors. OS was calculated with the Kaplan-Meier method and the statistical significance was compared using the log-rank test. The prognostic factors for survival were identified with the proportional hazards regression. Univariate analysis was performed with each potential prognostic factor and stepwise multivariate proportional hazards models were analyzed with statistically significant factors with P value less than 0.05 from univariate analysis for predicting survival. For the precision of model prediction without the complication caused by overlapped factors from univariate analysis, the factors with high priority were entered for multivariate analysis removing the factors with low priority.
The ROC curve analysis was used to determine the optimal cut-off values of the hemoglobin (Hb), neutrophil%, lymphocyte%, monocyte%, absolute neutrophil count (ANC), absolute lymphocyte count (ALC), absolute monocyte count (AMC), LMR, and PNI. The optimal cut-off values of each factor indicated the discrimination on survival by the maximum joint of sensitivity and specificity. All statistical analyses were conducted using MedCalc Statistical Software (version 12.7.5; MedCalc Software, Ostend, Belgium) and SPSS (version 18.0; SPSS Inc., Chicago, IL, United States). A P value less than 0.05 was considered statistically significant.
The clinicopathological and biochemical characteristics of enrolled patients are summarized in Table 1. Of the 177 colorectal cancer patients, 94 were female (53.1%), the median age was 52 (range, 25-81), and the majority of patients (59.3%) had an ECOG performance status of 0 or 1. There were 125 (70.6%) colon cancer cases and 52 (29.4%) rectal cancer cases, and most patients had liver metastases (61.0%). In total, 171 patients (96.6%) had at least one prior treatment, and 128 patients (72.3%) had experienced more than second line chemotherapy. The median time of KM treatment initiation was 9.4 mo after the diagnosis of mCRC (range, 0.1-81.0 mo).
| Variable | n | % | |
| Clinicopathological factors | |||
| Age (yr) | < 65/≥ 65 | 123/54 | 69.5%/30.5% |
| Sex | Male/female | 83/94 | 46.9%/53.1% |
| ECOG-PS | 0-1/2-4 | 105/72 | 59.3%/40.7% |
| Tumor site | Colon/rectum | 125/52 | 70.6%/29.4% |
| Liver metastasis | No/yes | 69/108 | 39.0%/61.0% |
| Prior surgery | No/yes | 33/144 | 18.6%/81.4% |
| Prior chemotherapy | None/1st line/2nd line/≥ 3rd line | 21/28/54/74 | 11.9%/15.8%/30.5%/41.8% |
| Prior radiotherapy | No/yes | 126/51 | 71.2%/28.8% |
| BMI (kg/m2) | < 18.5/18.5-22.9/≥ 23 | 22/88/64 | 12.6%/50.6%/36.8% |
| KM treatment duration (mo) | < 2.9/≥ 2.9 | 89/88 | 50.3%/49.7% |
| Laboratory factors | |||
| CEA (ng/mL) | ≤ 5/> 5 | 31/140 | 18.1%/81.9% |
| CA19-9 (U/mL) | ≤ 27/> 27 | 59/78 | 43.1%/56.9% |
| Hb (g/dL) | > 12.1/ ≤ 12.1 | 95/82 | 53.7%/46.3% |
| AST (IU/L) | < 40/≥ 40 | 147/30 | 83.1%/16.9% |
| ALT (IU/L) | < 40/≥ 40 | 157/20 | 88.7%/11.3% |
| eGFR (mL/min) | ≥ 60/< 60 | 139/36 | 79.4%/20.6% |
| albumin (g/dL) | < 3.5/≥ 3.5 | 13/164 | 7.3%/92.7% |
| CRP (mg/L) | < 10.0/≥ 10.0 | 114/63 | 64.4%/35.6% |
| PLT (× 103/μL) | < 400/≥ 400 | 161/16 | 62.7%/37.3% |
| WBC (× 103/μL) | < 10.0/≥ 10.0 | 161/16 | 91.0%/9.0% |
| Neutrophil (%) | ≤ 68.2/> 68.2 | 92/85 | 52.0%/48.0% |
| Lymphocyte (%) | ≤ 24.3/> 24.3 | 111/66 | 62.7%/37.3% |
| Monocyte (%) | ≤ 6.6/> 6.6 | 59/118 | 33.3%/66.7% |
| ANC (cells/μL) | ≤ 4505.7/> 4505.7 | 104/73 | 58.8%/41.2% |
| ALC (cells/μL) | ≤ 1651.3/> 1651.3 | 123/54 | 69.5%/30.5% |
| AMC (cells/μL) | ≤ 460.8/> 460.8 | 83/94 | 46.9%/53.1% |
| mGPS | 0/1/2 | 114/52/11 | 64.4%/29.4%/6.2% |
| NLR | < 5/≥ 5 | 144/33 | 81.4%/18.6% |
| PLR | < 150/150-300/> 300 | 73/78/26 | 41.2%/44.1%/14.7% |
| LMR | ≤ 3.4/> 3.4 | 113/64 | 63.8%/36.1% |
| PNI | ≤ 45.3/> 45.3 | 52/125 | 29.4%/70.6% |
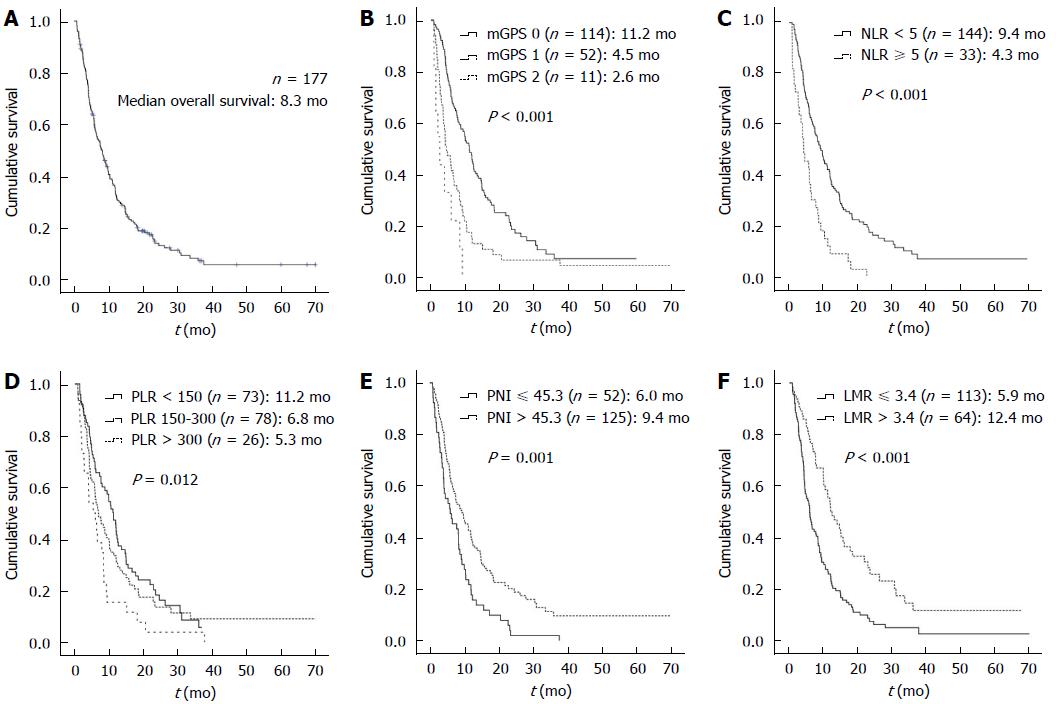
The median OS was 8.3 mo (range, 0.8-70.0 mo) for all patients (Figure 2). The median follow-up period of patients was 3.1 mo (range, 0.1-33.3 mo), and the median treatment duration of KM was 2.9 mo (range, 0.1-33.3 mo).
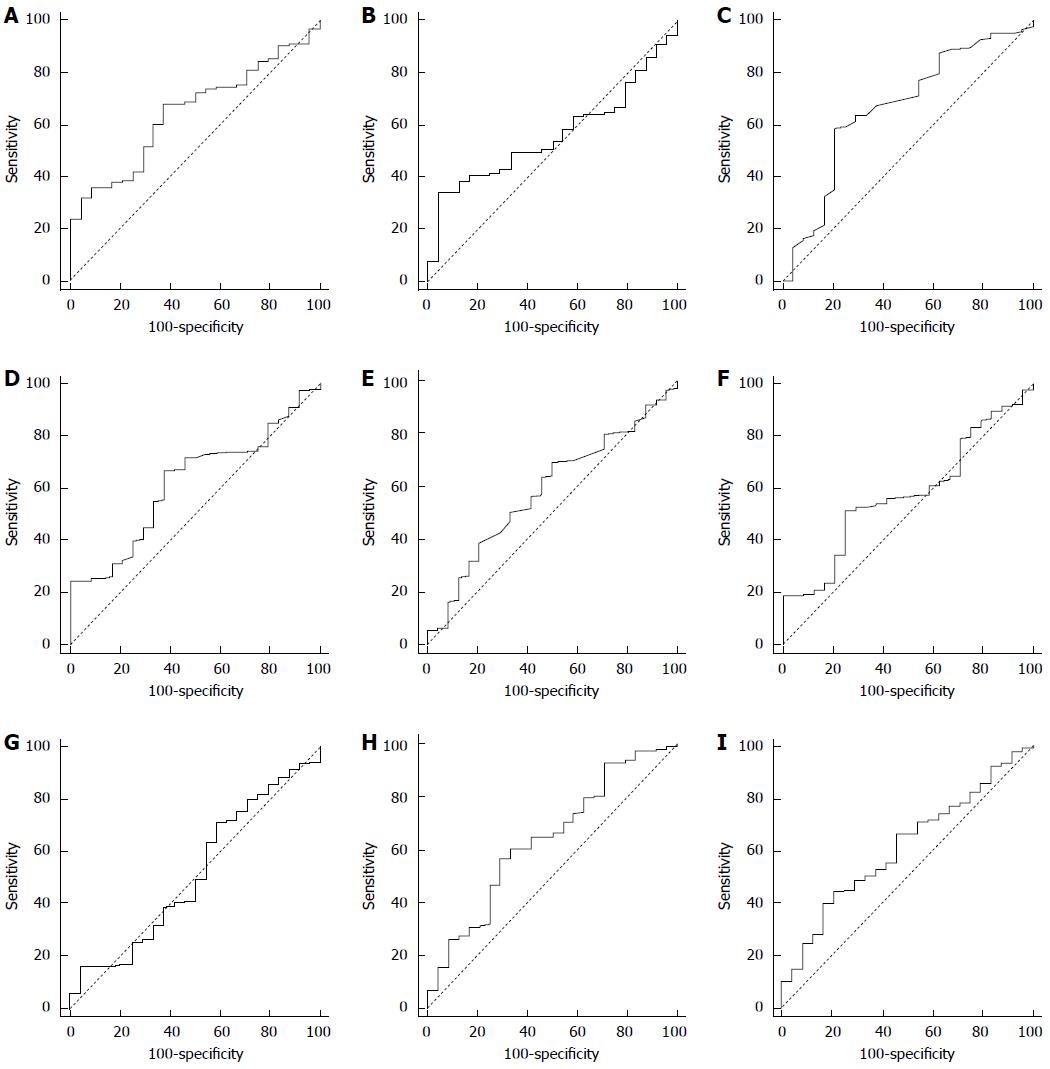
The optimal cut-off values, AUCs, and significances of Hb, neutrophil%, lymphocyte%, monocyte%, ANC, ALC, AMC, LMR, and PNI analyzed by ROC curve are shown in Figure 3. The ROC curves demonstrated that LMR of 3.4 was the optimal cut-off for predicting OS (sensitivity = 68, specificity = 62.5, AUC = 0.647, P = 0.005) and PNI of 45.3 was the optimal cut-off for predicting OS (sensitivity = 34.0, specificity = 95.8, AUC = 0.559, P = 0.256).
The univariate analysis of the proportional hazards regression was performed to determine predictable factors for multivariable analyses, adjusting for other factors to influence survival. Among the clinicopathological factors, poor ECOG performance status and presence of liver metastasis showed a relationship with poor survival. Regarding biochemical factors, several lab tests showed survival impact, such as tumor markers of carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9), liver enzymes of aspartate aminotransferase (AST) and alanine aminotransferase (ALT), albumin, C-reactive protein (CRP), Hb, neutrophil %, lymphocyte %, ANC, and AMC and treatment duration for KM. The survival of patients was significantly affected by the level of systemic inflammation markers of mGPS, NLR, PLR, and LMR, and the nutrition marker of PNI (Table 2). On mGPS, survival time and hazard were incrementally influenced in accordance with the level of mGPS. The median OS of mGPS 0, mGPS 1, and mGPS 2 was 11.2 mo, 4.5 mo, and 2.6 mo, respectively (P < 0.001). The hazard of mGPS 1 (HR = 1.998, 95%CI: 1.403-2.844) and mGPS 2 (HR = 4.741, 95%CI: 2.418-9.297) increased level-dependently (P < 0.001).
| Variable | Hazard ratio (95%CI) | P value | |
| Clinicopathological factors | |||
| Age (yr) | < 65 vs≥ 65 | 1.295 (0.922-1.821) | 0.136 |
| Sex | Female vs male | 0.897 (0.653-1.233) | 0.504 |
| ECOG-PS | 0-1 vs 2-4 | 1.528 (1.107-2.109) | 0.010 |
| Tumor site | Colon vs rectum | 0.823 (0.575-1.177) | 0.286 |
| Liver metastasis | No vs yes | 1.469 (1.056-2.044) | 0.023 |
| Prior surgery | No vs yes | 0.797 (0.525-1.211) | 0.288 |
| Prior chemotherapy | None vs 1st or 2nd line | 0.670 (0.392-1.145) | 0.143 |
| None vs≥ 3rd line | 0.897 (0.526-1.529) | 0.689 | |
| Prior radiotherapy | No vs yes | 0.991 (0.698-1.409) | 0.962 |
| BMI (kg/m2) | < 18.5 vs 18.5-22.9 | 0.697 (0.425-1.144) | 0.154 |
| < 18.5 vs≥ 23 | 0.700 (0.420-1.169) | 0.173 | |
| KM treatment duration (mo) | ≥ 2.9 vs < 2.9 | 1.966 (1.425-2.711) | < 0.001 |
| Laboratory factors | |||
| CEA (ng/mL) | ≤ 5 vs > 5 | 1.576 (1.016-2.444) | 0.042 |
| CA19-9 (U/mL) | ≤ 27 vs > 27 | 1.654 (1.139-2.402) | 0.008 |
| Hb (g/dL) | > 12.1 vs ≤ 12.1 | 1.721 (1.246-2.378) | 0.001 |
| AST (IU/L) | < 40 vs≥ 40 | 2.547 (1.678-3.867) | < 0.001 |
| ALT (IU/L) | < 40 vs≥ 40 | 1.845 (1.139-2.991) | 0.013 |
| eGFR (mL/min) | ≥ 60 vs < 60 | 0.942 (0.634-1.399) | 0.766 |
| albumin (g/dL) | ≥ 3.5 vs < 3.5 | 2.202 (1.214-3.993) | 0.009 |
| CRP (mg/L) | < 10.0 vs≥ 10.0 | 2.202 (1.577-3.076) | < 0.001 |
| PLT (× 103/μL) | < 400 vs≥ 400 | 0.618 (0.367-1.042) | 0.071 |
| WBC (× 103/μL) | < 10.0 vs≥ 10.0 | 1.595 (0.945-2.691) | 0.080 |
| Neutrophil (%) | ≤ 68.2 vs > 68.2 | 1.576 (1.146-2.168) | 0.005 |
| Lymphocyte (%) | > 24.3 vs ≤ 24.3 | 1.923 (1.369-2.700) | < 0.001 |
| Monocyte (%) | ≤ 6.6 vs > 6.6 | 1.316 (0.931-1.860) | 0.120 |
| ANC (cells/μL) | ≤ 4505.7 vs > 4505.7 | 1.832 (1.328-2.527) | < 0.001 |
| ALC (cells/μL) | ≤ 1651.3 vs > 1651.3 | 0.779 (0.549-1.107) | 0.164 |
| AMC (cells/μL) | ≤ 460.8 vs > 460.8 | 1.830 (1.325-2.527) | < 0.001 |
| Systemic inflammation markers | |||
| mGPS | 0 vs 1 | 1.998 (1.403-2.844) | < 0.001 |
| 0 vs 2 | 4.741 (2.418-9.297) | < 0.001 | |
| NLR | < 5 vs≥ 5 | 2.388 (1.609-3.545) | < 0.001 |
| PLR | < 150 vs 150-300 | 1.204 (0.849-1.708) | 0.297 |
| < 150 vs > 300 | 1.989 (1.253-3.157) | 0.004 | |
| LMR | > 3.4 vs ≤ 3.4 | 2.045 (1.450-2.884) | < 0.001 |
| PNI | > 45.3 vs ≤ 45.3 | 1.785 (1.271-2.507) | 0.001 |
The median OS of patients with low NLR (NLR < 5) was 9.4 mo, and the high NLR (NLR ≥ 5) was 4.3 mo (P < 0.001), and high NLR (NLR ≥ 5) reduced the survival period (HR = 2.388, 95%CI: 1.609-3.545, P < 0.001). The median OS of patients with low PLR (PLR < 150), medium PLR (PLR between 150 and 300), and high PLR (PLR > 300) was 11.2 mo, 6.8 mo, and 5.3 mo respectively (P = 0.012), and high PLR significantly resulted in poor survival (HR = 1.989, 95%CI: 1.253-3.157, P = 0.004), whereas the medium PLR showed no significant influence on survival. The median OS of patients with low LMR (LMR ≤ 3.4) and high LMR (LMR > 3.4) was 5.9 mo and 12.4 mo respectively (P < 0.001), and the low LMR group was associated with shortened survival (HR = 2.045, 95%CI: 1.450-2.884, P < 0.001). The median OS of patients with low PNI (PNI ≤ 45.3), and high PNI (PNI > 45.3) was 6.0 mo and 9.4 mo respectively (P = 0.001), and low PNI was highly associated with a short survival time (HR = 1.785, 95%CI: 1.271-2.507, P = 0.001) (Figure 2).
Multivariate analysis using the Cox proportional hazards models with stepwise selection process was performed to identify the independent predictors of survival. The models were analyzed with all significant factors removing overlapped factors. The key potential predictors of hypothesis of this study, mGPS, NLR, PLR, LMR, and PNI of the systemic inflammation markers were calculated with absolute counts of white blood cells and platelet, CRP, and albumin, which was described with detailed formulas in the method section. After adjusting for confounders, the predictors of survival in the stepwise multivariate proportional hazards model were LMR, mGPS, CA19-9, AST, and treatment duration (Table 3). Among the systemic inflammatory markers, mGPS and LMR were independently associated with survival time in relapsed/refractory mCRC patients. The risk of death was remarkably increased according to the level of mGPS score (P = 0.017), the hazard ratio of mGPS 2 (HR = 3.212, 95%CI: 1.437-7.176, P = 0.004) were increased compared with mGPS 0. The patients with low LMR level showed significantly increased risk of death than patients with high LMR level (HR = 1.658, 95%CI: 1.092-2.518, P = 0.018). Of the biochemical markers, the level of CA19-9 and AST were independent predictors for survival. The high level of CA19-9 (CA19-9 > 27) was associated with increased risk of death (HR = 1.482, 95%CI: 1.007-2.182, P = 0.046), and the high level of AST (AST ≥ 40) was significantly associated with poor survival time (HR = 2.377, 95%CI: 1.359-4.155, P = 0.002). Besides, treatment duration less than 2.9 mo was significantly associated with shortened survival time (HR = 1.718, 95%CI: 1.160-2.534, P = 0.007).
| Variable | Hazard ratio (95%CI) | P value | |
| mGPS | 0.017 | ||
| 0 vs 1 | 1.135 (0.717-1.797) | 0.588 | |
| 0 vs 2 | 3.212 (1.437-7.716) | 0.004 | |
| LMR | > 3.4 vs ≤ 3.4 | 1.658 (1.092-2.518) | 0.018 |
| CA19-9 (U/mL) | ≤ 27 vs > 27 | 1.482 (1.007-2.182) | 0.046 |
| AST (IU/L) | < 40 vs≥ 40 | 2.377 (1.359-4.155) | 0.002 |
| KM treatment duration (mo) | ≥ 2.9 vs < 2.9 | 1.718 (1.160-2.543) | 0.007 |
The systemic inflammation response of the host is one of the representative biomarkers of host-related factors that predict the prognosis of cancer. We investigated the relationship between systemic inflammation markers of GPS, NLR, PLR, LMR, and OS in relapsed/refractory mCRC patients. Besides systemic inflammation markers, the clinicopathologic indexes of the performance status, tumor markers, presence of liver metastasis, and nutritional status were also evaluated to determine their impact on the survival time of mCRC patients.
The mGPS is an inflammation marker comprised of CRP and albumin. A previous study reported that mGPS increases with weight loss and low performance status in colon and lung cancer patients, which seemed to be associated with survival[20]. Several studies compared mGPS 0 with mGPS 1-2 or mGPS 0-1 with mGPS 2 for statistical significance[21,22], but mGPS was compared without further grouping in our study. The levels of mGPS were identified as independent prognostic factors for survival. Specifically, patients with a score of mGPS 2 were significantly associated with poor survival, and this result was due to the fact that albumin was one of the highly associated nutritional markers. A high mGPS score before initiation of treatment can predict a poor survival outcome, which may influence the choice of an appropriate treatment modality.
In the tumor microenvironment, it is known that neutrophils affect proliferation, angiogenesis, and metastases by interacting with tumor cells producing cytokines and chemokines[23,24]. Also, there has been a study reporting that elevated neutrophil and monocyte counts are associated with poor survival in metastatic melanoma[25]. Lymphocytes play a key role in human immunity. Lymphopenia is frequently observed in advanced cancer patients, and the decrease in lymphocyte count is strongly associated with a poor prognosis of progression-free survival and OS in advanced cancer patients[26]. Our study demonstrated that an increased percentage of neutrophils and monocytes and a decreased percentage of lymphocytes were related to a short OS. LMR has been studied in Hodgkin’s lymphoma patients, and this study indicated that it is a possible prognostic factor along with International Prognostic Score[7]. In this study, LMR was found to be an independent prognostic factor for mCRC. Chua et al[11] reported that NLR before chemotherapy is an independent prognostic factor of survival in mCRC, but in this study NLR showed an impact on survival only with univariate analysis. PLR was also assessed for its relationship with survival, and it did not show any impact on survival as shown in the previous study[27].
We also evaluated whether PNI could be an effective variable for survival in mCRC patients. Until recently, PNI has been studied for gastric cancer patients who underwent a gastrectomy, and the OS of those patients was mostly dependent on the nutritional status and the immune system[8,15]. Few studies have researched the significance of PNI as a prognostic factor for survival in mCRC.
The efficacy of CA19-9 as a prognostic factor of survival in the previous studies has been reported with diverse outcomes according to the cut-off value of the study[22,28]. The optimal cut-off value of CA19-9 of this study was 27 U/mL by the reference value, and it was an independent prognostic marker for survival.
To evaluate the relationship between survival and hepatic or renal function, AST, ALT, and eGFR were also analyzed with the proportional hazards regression. Fahmueller et al[29] reported that elevated AST and CRP before selective internal radiation therapy in CRC patients with liver metastases were prognostic factors for survival, and concluded that liver tissue damage before treatment and treatment-induced ischemia resulted in poor survival of CRC patients. In this study liver metastasis was shown in 108 patients and the elevations of AST and ALT were observed in 30 and 20 patients, respectively. The liver metastasis and elevated levels of AST and ALT from univariate analysis showed strongly associated with the short survival time (Table 2).
Based on the results of univariate analyses, the factors that showed a significant effect on OS were ECOG performance status of patients, the presence of liver metastasis, tumor markers CEA and CA19-9, the Hb, AST, and ALT in serum, and the nutrition index of PNI, as well as measured systemic inflammation markers of mGPS, NLR, PLR, and LMR. Interestingly all the systemic inflammation markers were revealed to have significant associations on survival time in univariate analysis. On the basis of this result, we could verify that the survival time of mCRC patients was strongly associated with systemic inflammation.
On multivariate analysis using Cox proportional hazards model in forward and backward stepwise manners, the same predictors from analysis results were obtained that mGPS and LMR of systemic inflammation markers, CA19-9, AST, and treatment duration were independent prognostic predictors for survival time. The prognostic factors to predict survival time in relapsed/refractory mCRC patients were mGPS and LMR to reflect the systemic inflammatory condition of host, CA19-9 of colorectal cancer marker, AST of liver enzyme to indicate the hepatic function. Regarding the treatment duration of KM, the prolongation of survival was seemed to be associated with the length of treated time.
Most of studies on mCRC patients have focused on the prognosis of initial treatment after diagnosis. The standard regimen of chemotherapy on mCRC is FOLFOX, FOLFIRI, CapeOX, and FOLFOXIRI with the addition of biologic agents such as bevacizumab, cetuximab, and panitumumab in the case of patients who were appropriate for intensive care[30]. Cancer patients often consider another approach for management after experiencing a relapse in spite of conventional treatment. Patients who can’t undergo radical treatment because of age, performance status, and/or comorbidities also consider cancer management based on traditional medicine in East Asia. In this study, we aimed to focus on relapsed/refractory mCRC patients. The majority, 72.3% of enrolled patients, had received our management after undergoing second- or third-line chemotherapy. It took 9.4 mo to initiate our management after diagnosis of mCRC, demonstrating the status of enrolled patients. Therefore, this study result could be a guide for mCRC patients who relapsed with standard therapy.
Cancer management in East Asian Medicine is focused on host-based treatment to strengthen energy through Qi and Blood, which is the same viewpoint of palliative care, an approach to improve the quality of life in physical, psychosocial, and spiritual ways through the relief of symptoms, pain, and other problems[31].
The anticancer agent of this study, RVS, has been traditionally used since the 15th century in Korea for the treatment of abdominal masses because of its reported ability to break up hardness. There has been evidence for an anticancer effect of RVS based on reported anti-proliferative and apoptotic activity in various cancer cells of lymphoma, osteosarcoma, breast cancer, and hepatoma[17,32-36]. Furthermore, RVS inhibited the cancer cell migration mediated by matrix metalloproteinases, especially MMP-2 and MMP-9, in human fibrosarcoma cells[19]. RVS has also been shown to have antitumor activities involving inhibition of proliferation and migration of human umbilical vein endothelial cells induced by VEGF[17]. RVS has been widely used for advanced cancer patients who do not have any further choice in the conventional standard therapies. The previous study on RVS in mCRC patients reported a median OS of 10.9 mo in 2009[37].
This study is limited by the retrospective review of medical records. Another limitation was the relatively short duration of median treatment and follow-up periods. However, considering that enrolled patients had relapsed mCRC after undergoing second line chemotherapy in 30.5% of cases and third line chemotherapy in 41.8% of cases, the treatment duration of 3 mo based on clinical practice was hardly regarded as short term. We identified useful prognostic factors at the initial treatment point. With regard to far-advanced mCRC patients, evaluating repeated points of inflammation markers during management could lead to more confident results[11,38].
In the management of cancer patients, determination of prognosis could be continuously required to make decisions when undergoing adjuvant therapy, to consider the adverse events of chemotherapy, to apply optimal palliative care, and to determine the best time to initiate supportive care. Especially host-based studies of cancer are becoming a more important issue in various points of cancer management, and research on host-based factors at various cancer will develop to prolong survival and to improve quality of life in cancer treatment. The results of this study could be applied to clinical judgments regarding survival prognosis for relapsed/refractory mCRC patients.
The systemic inflammatory response has been recognized to associate with the progression in cancer patients. In clinical practice C-reactive protein and white blood cell counts has been measured for the assessment of systemic inflammation.
Recently, the identification of prognostic biomarkers to predict overall survival and benefit of therapeutic modality is one of hot issues in cancer practice because the prediction on prognosis for survival enables to decide the treatment goal and to determine the treatment modality.
The value of this study was to compare all the available systemic inflammation markers, modified glasgow prognostic score (mGPS), neutrophil lymphocyte ratio, platelet lymphocyte ratio, lymphocyte monocyte ratio, and prognostic nutritional index in metastatic colorectal cancer (mCRC) patients. Among available markers, the mGPS and lymphocyte monocyte ratio were the strongest prognostic factors to predict overall survival in mCRC patients. Especially, the prediction on survival in relapsed or refractory mCRC patients is important issue to determine the cancer treatment modality. There was none study to compare the systemic inflammation markers for relapsed or refractory mCRC patients.
Based on the result of this study, lymphocyte monocyte ratio (LMR) was the powerful prognostic factors than well-known other systemic inflammation markers. LMR showed consistent significance on the prediction for survival after adjusting other markers. Calculating just with the counts of lymphocyte and monocyte, LMR could be easily applied for the survival predictor in clinical practice. The level of mGPS is also a significant predictor of overall survival, especially mGPS 2 indicates the poor survival outcome.
Song et al reported, in 177 mCRC patients, the correlation between systemic inflammation-based prognostic markers and overall survival. The authors demonstrated the prognosis value of modified Glasgow Prognostic Score, Lymphocyte Monocyte ratio, carbohydrate antigen 19-9, and aspartate aminotransferase in stepwise modeling of multivariate proportional hazards regression. Patients were mostly refractory mCRC. The strength of this study is to have excluded patients with chemotherapy within 4 wk what could skew the biological results. The results can help decision making in refractory mCRC and selected patients for other chemotherapy regimen or best supportive care. The results are well analyzed and described. All these prognostic markers can be easy incorporated in everyday practice work.
P- Reviewer: Cvetanovic A, Tougeron D S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Jung KW, Won YJ, Kong HJ, Oh CM, Lee DH, Lee JS. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2011. Cancer Res Treat. 2014;46:109-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 302] [Cited by in F6Publishing: 311] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 2. | Goodwin PJ, Meyerhardt JA, Hursting SD. Host factors and cancer outcome. J Clin Oncol. 2010;28:4019-4021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10123] [Cited by in F6Publishing: 10557] [Article Influence: 479.9] [Reference Citation Analysis (0)] |
| 4. | Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073-1081. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1802] [Cited by in F6Publishing: 1950] [Article Influence: 130.0] [Reference Citation Analysis (0)] |
| 5. | Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4404] [Cited by in F6Publishing: 4370] [Article Influence: 174.8] [Reference Citation Analysis (0)] |
| 6. | Kitayama J, Yasuda K, Kawai K, Sunami E, Nagawa H. Circulating lymphocyte number has a positive association with tumor response in neoadjuvant chemoradiotherapy for advanced rectal cancer. Radiat Oncol. 2010;5:47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Koh YW, Kang HJ, Park C, Yoon DH, Kim S, Suh C, Go H, Kim JE, Kim CW, Huh J. The ratio of the absolute lymphocyte count to the absolute monocyte count is associated with prognosis in Hodgkin’s lymphoma: correlation with tumor-associated macrophages. Oncologist. 2012;17:871-880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 8. | Nozoe T, Ninomiya M, Maeda T, Matsukuma A, Nakashima H, Ezaki T. Prognostic nutritional index: a tool to predict the biological aggressiveness of gastric carcinoma. Surg Today. 2010;40:440-443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 171] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 9. | Proctor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD, O’Reilly DS, Foulis AK, Horgan PG, McMillan DC. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer. 2011;47:2633-2641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 483] [Cited by in F6Publishing: 570] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 10. | McMillan DC, Crozier JE, Canna K, Angerson WJ, McArdle CS. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int J Colorectal Dis. 2007;22:881-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 340] [Cited by in F6Publishing: 377] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 11. | Chua W, Charles KA, Baracos VE, Clarke SJ. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer. 2011;104:1288-1295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 335] [Cited by in F6Publishing: 353] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 12. | Elahi MM, McMillan DC, McArdle CS, Angerson WJ, Sattar N. Score based on hypoalbuminemia and elevated C-reactive protein predicts survival in patients with advanced gastrointestinal cancer. Nutr Cancer. 2004;48:171-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Sharma R, Zucknick M, London R, Kacevska M, Liddle C, Clarke SJ. Systemic inflammatory response predicts prognosis in patients with advanced-stage colorectal cancer. Clin Colorectal Cancer. 2008;7:331-337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Smith RA, Bosonnet L, Raraty M, Sutton R, Neoptolemos JP, Campbell F, Ghaneh P. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg. 2009;197:466-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 327] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 15. | Nozoe T, Kohno M, Iguchi T, Mori E, Maeda T, Matsukuma A, Ezaki T. The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg Today. 2012;42:532-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 163] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 16. | Porrata LF, Ristow K, Colgan JP, Habermann TM, Witzig TE, Inwards DJ, Ansell SM, Micallef IN, Johnston PB, Nowakowski GS. Peripheral blood lymphocyte/monocyte ratio at diagnosis and survival in classical Hodgkin’s lymphoma. Haematologica. 2012;97:262-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 155] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 17. | Choi WC, Lee JH, Lee EO, Yoon SW, Ahn KS, Kim SH. Study on antiangiogenic and antitumor activities of processed Rhus verniciflua Stokes extract. J of Kor Oriental Physiology & Pathology. 2006;20:825-829. [Cited in This Article: ] |
| 18. | Kim JH, Kim HP, Jung CH, Hong MH, Hong MC, Bae HS, Lee SD, Park SY, Park JH, Ko SG. Inhibition of cell cycle progression via p27Kip1 upregulation and apoptosis induction by an ethanol extract of Rhus verniciflua Stokes in AGS gastric cancer cells. Int J Mol Med. 2006;18:201-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Park JH, Moon G. Effect of allergen removed Rhus verniciflua extract on inhibition of tumor metastasis. J of Kor Traditional Oncology. 2010;15:47-61. [Cited in This Article: ] |
| 20. | Brown DJ, Milroy R, Preston T, McMillan DC. The relationship between an inflammation-based prognostic score (Glasgow Prognostic Score) and changes in serum biochemical variables in patients with advanced lung and gastrointestinal cancer. J Clin Pathol. 2007;60:705-708. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Inoue Y, Iwata T, Okugawa Y, Kawamoto A, Hiro J, Toiyama Y, Tanaka K, Uchida K, Mohri Y, Miki C. Prognostic significance of a systemic inflammatory response in patients undergoing multimodality therapy for advanced colorectal cancer. Oncology. 2013;84:100-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Kishiki T, Masaki T, Matsuoka H, Kobayashi T, Suzuki Y, Abe N, Mori T, Sugiyama M. Modified Glasgow prognostic score in patients with incurable stage IV colorectal cancer. Am J Surg. 2013;206:234-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Pekarek LA, Starr BA, Toledano AY, Schreiber H. Inhibition of tumor growth by elimination of granulocytes. J Exp Med. 1995;181:435-440. [PubMed] [Cited in This Article: ] |
| 24. | Ji H, Houghton AM, Mariani TJ, Perera S, Kim CB, Padera R, Tonon G, McNamara K, Marconcini LA, Hezel A. K-ras activation generates an inflammatory response in lung tumors. Oncogene. 2006;25:2105-2112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 212] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 25. | Schmidt H, Bastholt L, Geertsen P, Christensen IJ, Larsen S, Gehl J, von der Maase H. Elevated neutrophil and monocyte counts in peripheral blood are associated with poor survival in patients with metastatic melanoma: a prognostic model. Br J Cancer. 2005;93:273-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 230] [Cited by in F6Publishing: 238] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 26. | Ray-Coquard I, Cropet C, Van Glabbeke M, Sebban C, Le Cesne A, Judson I, Tredan O, Verweij J, Biron P, Labidi I. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009;69:5383-5391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 443] [Cited by in F6Publishing: 544] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 27. | He W, Yin C, Guo G, Jiang C, Wang F, Qiu H, Chen X, Rong R, Zhang B, Xia L. Initial neutrophil lymphocyte ratio is superior to platelet lymphocyte ratio as an adverse prognostic and predictive factor in metastatic colorectal cancer. Med Oncol. 2013;30:439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 144] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 28. | Furukawa K, Shiba H, Haruki K, Fujiwara Y, Iida T, Mitsuyama Y, Ogawa M, Ishida Y, Misawa T, Yanaga K. The Glasgow prognostic score is valuable for colorectal cancer with both synchronous and metachronous unresectable liver metastases. Oncol Lett. 2012;4:324-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Fahmueller YN, Nagel D, Hoffmann RT, Tatsch K, Jakobs T, Stieber P, Holdenrieder S. Predictive and prognostic value of circulating nucleosomes and serum biomarkers in patients with metastasized colorectal cancer undergoing Selective Internal Radiation Therapy. BMC Cancer. 2012;12:5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 30. | National Cancer Care Network. Clinical Practice Guidelines in Oncology. Colon Cancer ver3. 2014; Available from: http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. [Cited in This Article: ] |
| 31. | WHO Definition of Palliative Care. Available from: http://www.who.int/cancer/palliative/definition/en/. [Cited in This Article: ] |
| 32. | Lee JC, Kim J, Jang YS. Ethanol-eluted extract of Rhus verniciflua stokes inhibits cell growth and induces apoptosis in human lymphoma cells. J Biochem Mol Biol. 2003;36:337-343. [PubMed] [Cited in This Article: ] |
| 33. | Kook SH, Son YO, Chung SW, Lee SA, Kim JG, Jeon YM, Lee JC. Caspase-independent death of human osteosarcoma cells by flavonoids is driven by p53-mediated mitochondrial stress and nuclear translocation of AIF and endonuclease G. Apoptosis. 2007;12:1289-1298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Jang HS, Kook SH, Son YO, Kim JG, Jeon YM, Jang YS, Choi KC, Kim J, Han SK, Lee KY. Flavonoids purified from Rhus verniciflua Stokes actively inhibit cell growth and induce apoptosis in human osteosarcoma cells. Biochim Biophys Acta. 2005;1726:309-316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 35. | Samoszuk M, Tan J, Chorn G. The chalcone butein from Rhus verniciflua Stokes inhibits clonogenic growth of human breast cancer cells co-cultured with fibroblasts. BMC Complement Altern Med. 2005;5:5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Son YO, Lee KY, Lee JC, Jang HS, Kim JG, Jeon YM, Jang YS. Selective antiproliferative and apoptotic effects of flavonoids purified from Rhus verniciflua Stokes on normal versus transformed hepatic cell lines. Toxicol Lett. 2005;155:115-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 37. | Lee SH, Choi WC, Yoon SW. Impact of standardized Rhus verniciflua stokes extract as complementary therapy on metastatic colorectal cancer: a Korean single-center experience. Integr Cancer Ther. 2009;8:148-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Lee Y, Kim SH, Han JY, Kim HT, Yun T, Lee JS. Early neutrophil-to-lymphocyte ratio reduction as a surrogate marker of prognosis in never smokers with advanced lung adenocarcinoma receiving gefitinib or standard chemotherapy as first-line therapy. J Cancer Res Clin Oncol. 2012;138:2009-2016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |