Published online Nov 14, 2015. doi: 10.3748/wjg.v21.i42.11984
Peer-review started: May 7, 2015
First decision: June 2, 2015
Revised: July 19, 2015
Accepted: September 14, 2015
Article in press: September 14, 2015
Published online: November 14, 2015
The hepatitis C virus (HCV), first described in 1989, is now a leading cause of liver cirrhosis and hepatocellular carcinoma. With more than 170 million people infected globally, this virus is a major public health issue. The current standard therapy is based on interferon in combination with ribavirin. This costly therapy often fails to completely clear the infection and is associated with adverse side effects. Recent anti-HCV therapies are interferon-free direct-acting antiviral (DAA) regimens for HCV, including simeprevir, sofosbuvir, and ledipasvir, which have effects on non-structural proteins. DAA regimens have several advantages, such as specifically targeting HCV viral replication, accompanied by very high sustained virological response rates and lower side effects like flu-like syndrome. These facts plus the fact that most HCV cases progress to chronic infection suggest the potential need for an efficient HCV vaccine. Different innovative methods, including methods based on peptide, recombinant protein, DNA, vector-based, and virus-like particles, have been introduced for the development of HCV vaccines. An extensive number of studies have been published on these vaccines, and some vaccines were even tested in clinical trials. In the current review, progress in the development of preventive and therapeutic vaccines against the HCV is reviewed in the context of peptide vaccines, recombinant protein vaccines, HCV-like particle, DNA vaccines and viral vectors expressing HCV genes.
Core tip: Chronic hepatitis C virus (HCV) infection occurs in about 75%-90% of acutely infected individuals. It may progress to liver cirrhosis or hepatocellular carcinoma. Despite satisfactory progress in the management and treatment of chronic hepatitis C, it remains one of the most prominent viral infections worldwide. Although no reliable vaccine for it has yet been developed, researchers are trying to design and develop different types of vaccines to prevent HCV infection or to cure the chronic form of the disease. The current article provides an overview of the latest progress in the development of preventive and therapeutic vaccines against HCV infection in the context of peptide vaccines, recombinant protein vaccines, HCV-like particle, DNA vaccines, and viral vectors expressing HCV genes.
- Citation: Ghasemi F, Rostami S, Meshkat Z. Progress in the development of vaccines for hepatitis C virus infection. World J Gastroenterol 2015; 21(42): 11984-12002
- URL: https://www.wjgnet.com/1007-9327/full/v21/i42/11984.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i42.11984
The hepatitis C virus (HCV) is an enveloped, positive-sensed, single-stranded RNA virus belonging to the hepacivirus genus of the Flaviviridae family[1]. Approximately 75% of acute HCV cases develop into chronic HCV infection, of which 3%-11% develop into liver cirrhosis within 20 years. This may eventually lead to liver failure or hepatocellular carcinoma (HCC) and may necessitate a liver transplant[2-4].
HCV is mainly transmitted through blood as in transfusion, injection drug use, organ transplantation, hemodialysis, or accidental exposure; however, unprotected sexual contact and vertical mother-to-child transmissions have also been documented[5,6].
First discovered in 1989[7], it is estimated that 3% of the world’s population is infected with HCV. Infection rates vary from less than 1% to over 10% in different countries[8]. HCV antibody (anti-HCV) testing is used to assess the prevalence of HCV. Studies have reported that countries located in Africa and Asia have the highest anti-HCV rates, whereas industrialized countries, such as those located in North America, Western Europe, and Australia, have lower rates[9-11]. Although the incidence rate of HCV is decreasing in developed countries, mortality due to secondary liver disease from HCV infection is expected to continue to rise over the next 20 years[12]. HCV infection is more common in adult populations (people older than 15 years)[13]. With efficient screening strategies, better treatments, and the use of preventive vaccines, it is estimated that HCV could be eliminated in the next 15 to 20 years[13,14].
HCV strains are categorized into seven genotypes (1-7) based on their phylogenetic similarities and genome sequence. HCV genotype 1 is the most common, accounting for 46.2% of all cases worldwide, followed by genotype 3, which is responsible for 30.1% of all cases[15].
The majority of newly-infected people are asymptomatic or have a mild illness. Therefore, they are normally not aware of their condition[4]. Symptoms are anorexia, nausea, vomiting, abdominal discomfort, and jaundice. HCV rarely causes fulminant hepatic failure[16].
Drug therapy for HCV consists of the administration of interferon alpha (INF-α) and ribavirin and is associated with adverse side effects. A new class of drugs called direct-acting antivirals (DAA) is beginning to be used in combination with INF-α and ribavirin, resulting in an increase in their effectiveness. However, the drawbacks of DAAs are their high cost and more adverse side effects, such as fever, fatigue, chills, and depression[15]. Based on these factors and the fact that only a small percentage of HCV patients can be totally cured[17], the need for an effective HCV vaccine is apparent. Here, we review the obstacles and progress in the development of effective vaccines against HCV.
HCV has an icosahedral capsid that consists of a single-stranded, positive-sensed RNA and is enveloped with E1 and E2 glycoproteins that are highly variable[18]. Scientists have been unable to culture HCV in vitro. This, in turn, makes studying its structure and replication processes difficult, but some progress has been made[19,20]. Virus replication occurs mainly in hepatocytes. It is estimated that each infected cell produces 50 virions daily. The virus also replicates in peripheral blood mononuclear cells (PBMCs) and may be responsible for high levels of immunologic disorders in chronic hepatitis C patients[1].
The genome of HCV contains a long open reading frame (ORF) encoding a 3000 amino acid length polyprotein that is cleaved into structural and non-structural (NS) proteins. A 5′ untranslated region functions as an internal ribosome entry site and permits direct binding of ribosomes to the start codon of the ORF, thus directing translation of ORF[21,22]. The first about 40 nucleotides of the RNA genome may be involved in RNA replication[23]. The 3′ untranslated region is comprised of four elements: (1) a short variable sequence; (2) a poly(U) tract of heterogeneous length; (3) a polypyrimidine stretch mainly containing U and a few Cs; and (4) a highly conserved 98 nucleotide sequence[24,25]. The poly(U) tract and the highly conserved region of 3’ UTR are essential for the infectivity of HCV[26].
Viral structural proteins include core protein (22 kDa), E1 (33 kDa), and E2 (70 kDa) glycoproteins, and the NS proteins NS2, NS3, NS4A, NS5A, and NS5B[27,28]. HCV core is a 22 kD basic protein responsible for forming the capsid structure. It has been implicated in HCV pathogenesis, the development of chronic infection, and immune response modulation. Core is the most conserved protein among various HCV genotypes, and humoral responses against it are the first host responses to develop in an infected patient[29-31]. E1 and E2 play roles in cell entry. E2 glycoprotein interacts with the HCV receptor CD81 in liver cells. Moreover, low density lipoprotein (LDL) receptors, scavenger-receptor class B type 1 (SR-B1), and Claudin-1 act in the process of cell entry[18]. E2 contains three hypervariable regions (HVR) that play roles in cell entry, antibody binding, and outcome of the disease. In the HCV genome, P7 gene encodes the first NS protein and is located after the genes that encode structural proteins. It forms the ion channels that seem to be essential for the efficient assembly, release, and production of HCV[32].
P7 was the first NS protein located after structural proteins. Its function is unknown, but it forms the ion channels that seem to be essential for HCV production[19,29]. NS2 (23 kDa), which is located early after P7, is a membrane-associated protease that can cleave the NS2-3 junction. P7 has been shown to cooperate with other NS proteins and may facilitate viral assembly[29,33]. NS3 (67 kDa) is a serine protease with RNA helicase and nucleoside triphosphatase (NTPase) activities that cleaves HCV polyprotein at various junctions between NS proteins[29,33]. NS3 also regulates NS2 protease activity and inhibits type 1 interferon (IFN) production by blocking Toll-like receptor 3 (TLR3) and retinoic acid-inducible gene 1 (RIG-I) signaling pathways[34,35]. NS4 is further cleaved into NS4A and NS4B. NS4A plays a role in the assembly of the HCV replication complex, viral pathogenesis, modulation of NS3 helicase, and facilitation of NS3 serine protease activities[29,33]. NS4B is responsible for the formation of cellular membranous webs that are the site of genomic replication in HCV. Any allelic variation in its sequence may strongly affect HCV replication[36].
The NS5A protein has RNA-binding activity and directly binds to NS5B, modulating its RNA polymerase activity[29,33,37]. The NS5B protein is an RNA-dependent RNA polymerase that lacks proofreading activity, thus giving rise to a high rate of replication errors and generating significant genetic diversity. This high rate of genetic variability produces a mixture of variants called quasispecies, even in a single infected individual[38].
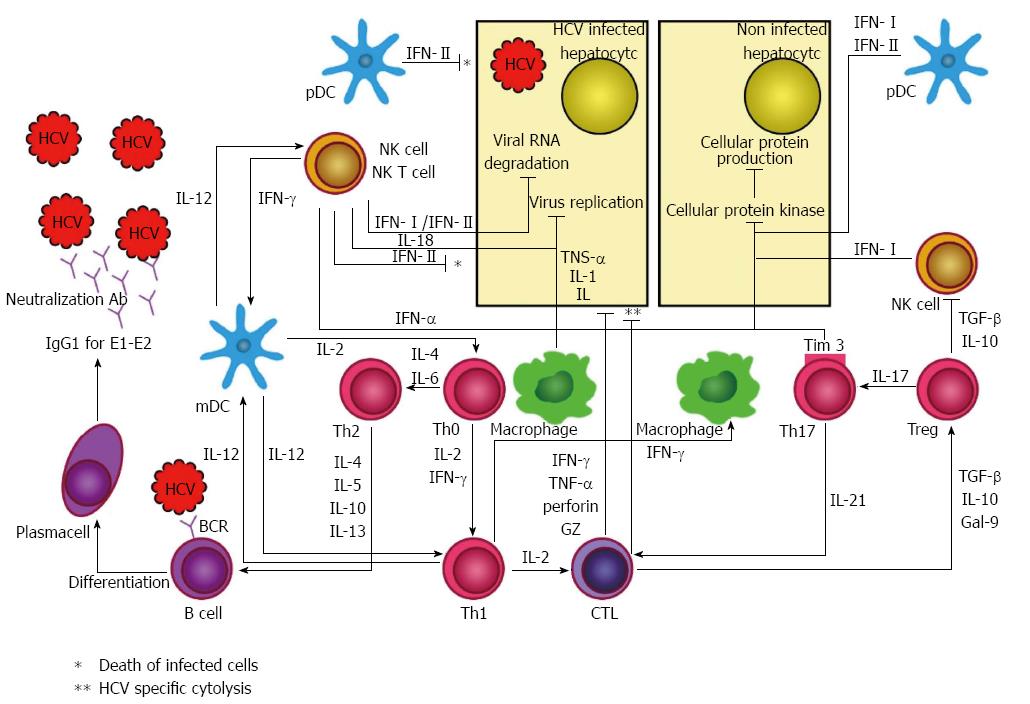
Figure 1 summarizes immune responses against HCV with a focus on both innate and adaptive immune responses. More details about the components and effects of such responses are found in the sections below.
Innate immunity, the first system to respond to HCV infection, contains proinflammatory cytokines and a cellular component. In addition to resisting infection, innate immunity is also involved in provoking adaptive immunity[39]. Three arms of innate immunity that identify HCV infection as a threat are: (1) RIG-I-like receptors (RLRs); (2) TLRs; and (3) nucleotide oligomerization domain (NOD)-like receptors (NLRs)[40]. After HCV recognition, these arms send various downstream signals to induce the production of various cytokines, such as interleukins (IL) and IFNs, which create an antiviral state for uninfected cells, decrease HCV replication in infected cells, and link innate immunity to adaptive immunity[39]. The RIG-I pathway is activated shortly after infection. HCV promotes a structural change in RIG-I and leads to the activation of the transcription factors IFN regulatory factor 3 (IRF3) and nuclear factor kappa B (NFκB), which results in the production of type I and III IFNs[41].
TLRs can sense both viral nucleic acid and protein. TLR3 and 7 have been shown to play roles in intracellular HCV recognition[42]. TLR3 is found in hepatocytes and Kupffer cells and inhibits HCV replication[43,44]. TLR7 resides in plasmacytoid dendritic cells (pDCs) and Kupffer cells, leading to the production of type I and III IFNs and IL-1β and IL-18[45,46].
The NLR pathway results in the activation of inflammasome, a protein complex made of a sensor (NLR protein 3), the adaptor (ASC), and caspase 1 (a cellular protease)[47]. NLRs trigger the secretion of IL-1β and IL-18, which cause hepatic stellate cells to form fibrosis[48].
IFNs-α and β (type I IFNs) bind to similar receptors and activate the janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway, which in turn induces interferon-stimulated genes (ISGs). This pathway amplifies total IFN responses and degrades viral RNA[49]. Type III IFNs include IFN-λ 1, 2, and 3 (IL-29 and IL-28 A and B, respectively). They activate the JAK/STAT pathway and induce ISGs, the same as type I IFNs; however, they bind to different receptors with distinct cellular distribution[50].
The role of IL-18 is still unclear in HCV infection, but it may suppress virus replication and induce the maturation of monocyte-derived dendritic cells (DCs)[51]. pDCs may be the major source of IFN-α production. Myeloid DCs (mDCs) migrate to lymphoid tissue to activate native T cells and bridge innate and adaptive immune responses[45].
Natural killer (NK) and NKT cells also play roles in HCV infection through IFN type II (IFN-γ) production and cytotoxic destruction of infected cells[52,53]. IFN-γ activates Kupffer cells, and they release proinflammatory molecules, such as tumor necrosis factor (TNF)-α, galectin-9, and IL-18[51,54].
Humoral responses to HCV infection include B cell activation and production of antibodies that are primarily low titers of IgG1 and appear late in the course of the disease[55,56]. Antibodies can be detected after 7-10 wk of infection; however, viral RNA can be detected 1-3 wk after infection[57].
Although a few antibodies for E1 glycoprotein as well as NS proteins have been found, the main target for antibodies are the surface E2 glycoprotein[56,58,59]. Antibodies against NS proteins were found earlier in the disease course and with a greater magnitude. Nevertheless, E2 glycoprotein is still considered the main target for antibodies.
Recent studies have suggested that patients infected with HCV show an elevated level of activated B cells, and people with defects in antibodies experience rapid disease progression, emphasizing the role of humoral immunity in HCV[60-62]. Patients with hypogammaglobulinemia could spontaneously resolve acute hepatitis C, suggesting that humoral immunity is not essential for HCV clearance[63]. The activity of humoral immunity may contribute to some of the extra hepatic disorders associated with HCV, such as mixed cryoglobulimia and B-cell non-Hodgkin’s lymphoma, although the mechanisms of these associations are still not well understood[64,65].
Cell immunity for HCV is composed of two arms: CD4+ and CD8+ T cells that are detectable in peripheral blood or the liver several weeks post-infection[66]. These T cells directly kill infected cells or produce soluble factors that clear the virus in a non-cytolytic manner. These cells may also directly damage the liver or attract non-specific inflammatory cells to accelerate liver inflammation[67].
Upon recognition of HCV through the major histocompatibility complex (MHC) class II[68], CD4+ T cells (T helper) mainly produce IFN-γ that correlates with decreased HCV titer and transaminase levels[66]. These cells also produce IL-2 that helps the production of CD8+ (cytotoxic) T cells. Moreover, they can enhance B cell production. T helper actions are essential for spontaneous recovery during acute HCV[69]. It has been shown that T helper responses are not observed in patients developing chronic disease[70].
CD8+ T cells recognize HCV-infected cells through MHC class I and lead to lysis of infected cells[68]. The role of MHC class I in HCV recognition indicates that spontaneous clearance of HCV is related to the presence of some MHC class I molecules, including human leukocyte antigen (HLA)-B*57, HLA-B*27, HLA-A*11, HLA-A*03, and HLA-Cw*01, and to the absence of HLA-Cw*04[71]. The mechanism of the influence of these alleles in the clearance of HCV is unknown; however, some studies suggest that these allelic variants produce binding proteins to immunogenic or functionally conserved T cell epitopes[72]. There is evidence for the binding of some proteins to inhibitory receptors of NK cells. MHC class II genes, such as DQB1*0301 and HLA-DRB1*1101, have also been associated with HCV clearance. Patients who lack efficient cytotoxic T cell response develop persistent hepatitis C infection, but the magnitude of the cytotoxic T cell response is associated with the clinical outcome[73,74].
Studies suggest that the HCV first appeared over 1000 years ago and evolved in different environments into seven distinct genotypes and more than 100 subtypes[15,75,76]. HCV mutates at a rate of nearly one nucleotide per replication cycle. This is due in part to the lack of proofreading activity of NS5B RNA-dependent polymerase that causes an error rate of 10-3 to 10-5 per nucleotide per replication cycle[19]. These frequent mutations, in combination with a short viral half-life and rapid turnover (1010 to 1012 virions per day), lead to a high genetic variability that results in the presence of distinct but closely related HCV variants (known as quasispecies) in one infected individual[38]. E1 and E2 glycoproteins show the highest variability among HCV proteins[75]. It is estimated that HCV is 10 times more variable than the human immunodeficiency virus (HIV), and this clearly poses a significant challenge for successful vaccine development[77].
HCV can escape the immune system by inhibiting NK cells and IFN-α production and by mutating to evade antibody and CD8+ T cell recognition. The mutations in CD8+ T cell epitopes have been carefully described in many chimpanzee[78,79] and human studies[80-82].
During HCV replication, NS3 and NS4A block the signaling pathways of TLR3 and RIG-I, and that ultimately inhibits the production of type I IFN[83-85]. This may lead to a decrease in NK cell activity through a lack of IL-15 production[86,87]. Core protein interferes with TNF-α and lymphotoxin signaling[88], and E1 and E2 glycoproteins build a glycan shield and facilitate cell-to-cell spread[89]. The NS5A protein could stimulate IL-8, inhibit double-stranded RNA-activated protein kinase, and interfere with 2’,5’-oligoadenylate synthetase (2’,5’-OAS), thereby interrupting the signaling of type 1 interferon[90,91]. The overexpression of HCV core protein interacts with the STAT1 SH2 domain and leads to the inhibition of IFN signaling[92,93]. The upregulation of ubiquitin-specific peptidase 18 (USP18) via long-term IFN stimulation suppresses ISG15 activation and JAK/STAT signaling, which can lead to type I IFN stimulation resistance[94,95]. NK and NKT cells are present in liver tissue and produce IFN-γ and other cytokines to prime cellular immune responses[96].
HCV escapes humoral immunity by several mechanisms: (1) HCV binds to very low density lipoprotein, which facilitates its uptake by hepatocytes[97]; (2) three glycans at the CD81 binding site of E2 glycoprotein decrease the immunogenicity of the virus[98]; (3) CD81 and Claudin-1 allow HCV to infect surrounding cells through cell-to-cell contact[99]; and (4) constant mutations in HCV can induce interfering antibodies[100].
Another obstacle in developing an efficient vaccine is the persistence of HCV, mainly because of (1) rapid HCV escape mutations[101]; (2) the secretion of immune regulatory cytokines[102]; (3) T cell exhaustion and depletion[103,104]; and (4) T regulatory cell induction[105]. A further obstacle is the lack of a suitable animal model to study HCV.
HCV polymerase works at a high rate and lacks proof-reading activity, which leads to a rapid viral escape from humoral and cellular immunity[106,107]. Mutations in MHC class I restricted epitopes that are targeted by cytotoxic T cells may lead to persistence[108,109].
IL-10, an immune-regulatory cytokine, increases in chronic hepatitis C. It is produced by T cells as well as monocyte and NK cells. IL-10 not only suppresses IFN-α and IFN-γ production and T cell proliferation but also promotes pDC cell apoptosis[110], resulting in liver damage in patients with chronic HCV infection[104]. Tumor growth factor-β also plays a role in HCV persistence[102].
In T cell exhaustion, T cells are in a state of dysfunction. This phenomenon can develop during many chronic infections like HCV. These cells lose their ability to produce IL-2, which is essential for the production of T cells, and show sustained expression of inhibitory receptors. These changes result in a loss of cytotoxicity and the cytokine production of cellular immunity[111]. Programmed cell death protein-1 (PD-1) plays important roles in CD8+ T cell exhaustion, and blocking PD-1 restores CD8+ T cell responses[112-114]. T lymphocyte antigen 4 [cytotoxic t lymphocyte (CTL)A4] also contributes to T cell exhaustion. Inhibition of both PD-1 and CTLA4 improves the restoration of T cell responses[115]. HCV induces T cell deletion by upregulating the pro-apoptotic molecule Bcl-2-interacting mediator (Bim) and downregulating the induced myeloid leukemia cell differentiation protein (Mcl-1)[104].
Regulatory T cells control the balance between host damage and viral control. These cells can induce immune-tolerance when excessive immune response could be harmful for the host. They inhibit the maturation of antigen presenting cells and the activation of T cells. Thus, they control auto-immunity and immune responses[116]. Although higher T regulatory cell frequency has been observed in chronic HCV compared to self-resolving infection[117-119], a study in chimpanzees showed that HCV infection increases the number of T regulatory cells and their suppressive actions irrespective of the disease outcome[120].
HCV attacks mitochondria in liver cells and induces mitochondrial dysfunction and oxidative stress[121-123], resulting in the induction of cell autophagy and mitophagy, selective elimination of mitochondria, and the induction of mechanisms to eliminate dysfunctional mitochondria[124]. Autophagy proteins act as proviral factors that initiate the translation of HCV RNA in newly-infected cells, and autophagosomes will become sanctuaries for HCV replication away from host immune surveillance, which paves the way for chronic infection and liver failure[125,126]. Kim et al[127] suggested a similar role for mitophagy in the HCV life cycle.
Other than humans, chimpanzees[128] and a non-rodent small mammal named Tupaiabelangeri[129], also known as the tree shrew, are naturally susceptible to HCV infection. In addition to the mentioned animal models, much effort has been made to introduce genetically-manipulated animals, preferably mice or rats, for the study of HCV pathogenesis and vaccine design[130-133]. The results of chimpanzee studies are highly variable and difficult to interpret because of the genetic variability between animals and small sample sizes. Approximately 30%-40% of chimpanzees develop chronic HCV infection, whereas up to 85% of infected humans develop chronic HCV[134]. The high cost of acquiring and maintaining the animals, their limited availability, and ethical considerations are major drawbacks to using chimpanzees as HCV animal models[133]. The infection rate and viremia of tree shrews is low and rarely sustained; however, they can develop chronic hepatitis in some cases[135,136]. The limited availability of tree shrews, their high housing costs, and the lack of tupaia specific reagents (used to assess HCV-host interactions) limit their use for the study of HCV[128].
HCV is a highly complex virus. The study of immune responses was long hampered by the lack of a cell culture system or readily accessible small animal model. HCV can replicate in lymphoid cells or PBMCs but only for limited periods of time and very low viral loads[137]. The invention of HCV pseudo-particles (HCVpp) expressing unmodified E1E2 glycoproteins has provided a way for more thorough studies of HCV-specific antibodies[138], although the structure and neutralization of HCVpp are significantly different from natural HCV[139].
The first successful tissue model of HCV infection was developed in 2005 using the HCV/JFH1 cell culture system[140]. The whole genome of the JFH1 virus was separated from a Japanese patient with fulminant hepatitis; it was then multiplied with PCR, cloned, and named JFH1. The JFH1 virus is of the 2a genotype and has a full-length HCV genome. This culturing system efficiently maintains the replication of HCV in human hepatoma cell line Huh7 and produces fair titers of cell-cultured derived HCV particles (HCVcc) with natural infectious properties. This platform was further broadened to include HCV subtype 1a strain H77-S, which is more commonly associated with HCV complications, such as HCC and cirrhosis, and more resistant to IFN therapy than HCV genotype 2[141].
Any preventive (prophylactic) or curative (therapeutic) immunogen that inhibits or treats the chronic phase of HCV infection can be referred to as an effective HCV vaccine, since HCV persistence results in liver failure and the development of clinical complications[142]. Patients reinfected with HCV demonstrated a significantly increased spontaneous viral clearance rate and broader T-cell responses compared with those who had been affected with primary infections[143]. Differences in viral kinetics and rates of clearance between primary and secondary infections strongly support the significance of memory immune responses in the clearance of natural infection and support the development of therapeutic and prophylactic vaccines[143,144].
Most chronic HCV infections exhibit lower HCV-specific T-cell responses compared with humoral responses. Thus, efforts have focused more on enhancing the T cell rather than humoral responses. In addition to inducing strong and cross-reactive neutralizing antiviral antibodies (humoral immunity), a suitable HCV vaccine should provoke long-lasting cellular immune responses involving helper CD4+and CD8+ T cells (cellular immunity)[31,145]. Although current therapeutic vaccine trials have successfully induced HCV-specific immune responses and transiently reduced viral RNA in subsets of patients, they have not completely cleared HCV infections or consistently reduced viral titers[145-147]. Here, different vaccine development strategies and the clinical trials in which they were tested were reviewed (Table 1).
| Type of vaccine | Lead author | Year | Vaccine | Tested in | Adjuvant | Ref. |
| Recombinant protein | Leroux-Roels | 2004 | Recombinant E1 (T2S-918/InnoVac-C) | Human n = 20 | Alum | [160] |
| Choo | 1994 | Recombinant E1 or E2 | Chimpanzee n = 7 | MF59 | [153] | |
| Frey | 2010 | Recombinant E1 or E2 | Human n = 60 | MF59 | [161] | |
| Drane | 2009 | Recombinant Core | Human n = 30 | ISCOMATRIX | [163] | |
| Verstrepen | 2011 | Recombinant E1 or E2 | Chimpanzee n = 4 | Alum | [162] | |
| Puig | 2004 | Recombinant E1 or E2 | Chimpanzee n = 2 | Oil/water | [155] | |
| Peptide | Firbas | 2006 | 7 HLA-A2 restricted peptides (IC41) | Human n = 128 | Poly-L-arginine | [149] |
| Firbas | 2010 | 7 HLA-A2 restricted peptides (IC41) | Human n = 54 | Poly-L-arginine | [150] | |
| Yutani | 2007 | Peptide vaccine targeting E1, E2, NS3 and NS5A | Human n = 12 | - | [145] | |
| Klade | 2008 | Synthetic peptide vaccine (core, NS3, NS4) (IC41) | Human n = 60 | Poly-L-arginine | [146] | |
| DNA vaccine | Forns | 2000 | DNA vaccine expressing E2 protein | Chimpanzee n = 2 | - | [227] |
| Sallberg | 2009 | NS3/4A expressing plasmid (ChronVac-C) | Human n = 12 | - | [179] | |
| Alvarez-Lajonchere | 2009 | Plasmid (CIGB-230) expressing core, E1, E2 plus recombinant core protein | Human n = 15 | - | [175] | |
| Virally vectored | Rollier | 2007 | DNA/MVA | Chimpanzee n = 4 | - | [201] |
| Folgori | 2006 | Ad6/Ad24 + electroporated DNA | Chimpanzee n = 5 | - | [191] | |
| Fattori | 2006 | Ad6/Ad6/ChAd32 | Rhesus macaque n = 3 | - | [194] | |
| Barnes | 2012 | Ad6/ChAd3 | Human n = 30 | - | [193] | |
| Youn | 2008 | Recombinant vaccinia | Chimpanzee n = 4 | - | [207] | |
| Habersetzer | 2009 | Modified vaccinia Ankara virus expressing HCV NS3-NS5B (TG4040) | Human n = 15 | - | [203] | |
| Others | Elmowalid | 2007 | Virus like particles | Chimpanzee n = 4 | AS01B | [215] |
| Batdelgar | 2008 | V-5 Immunitor–heat- inactivated HCV antigens from HCV infected donors (tablet administered orally) | Human n = 10 | - | [219] |
Peptide vaccines induce HCV specific T cell responses by presenting vaccine peptide to the T-cell receptor via HLA molecules. Therefore, they are HLA-specific and target only a selected subset of epitope sequences within the HCV genome. The high rate of genetic variation in viruses within populations and geographic regions limits the universal usage of these vaccines. They often contain multiple epitopes in order to induce broader T cell responses. Unfortunately, some peptides may induce T regulatory cells or immune tolerance[148].
Intercell (Intercell AG, Vienna, Austria) developed a peptide vaccine, IC41, which consists of five synthetic peptides derived from conserved regions of core, NS3, and NS4 proteins of HCV genotypes 1 and 2 with a poly-L-arginine adjuvant. IC41 was assessed in 128 HLA-A2+ healthy volunteers in a phase I, dose-escalation, placebo-controlled randomized trial where it was proven to be safe and well tolerated. IC41 vaccination induced few interferon-producing cells and dose-dependent T cell immune responses[149]. Klade et al[146] sought to determine whether IC41 is able to induce HCV-specific T-cell responses in chronic hepatitis C patients. In their study, 60 HLA-A2+ chronic HCV patients who failed to respond to or relapsed from conventional therapy were sorted randomly into five groups to receive six doses of IC41 in a double-blind phase II study. T-cell proliferation was recorded in up to 67% of patients, and IFN-γ responses were observed in up to 42% of patients in the IC41 vaccine groups. Moreover, three patients had transient declines of HCV serum RNA. Another randomized trial included 54 healthy subjects receiving either subcutaneous or intradermal IC41 vaccinations weekly or bi-weekly. Results showed that IC41 induced significant immunological responses; adding imiquimod to the formulation did not enhance immunogenicity and was associated with a lower immune response. Furthermore, intradermal injections caused more noticeable reactions, especially erythema and edema[150]. In another clinical trial conducted by Klade et al[151], 50 patients received eight intradermal IC41-plus-imiquimod vaccinations bi-weekly, and 21 patients received 16 subcutaneous vaccinations without imiquimod weekly. The first group showed a statistically significant HCV viral load decline, whereas no effect on HCV viral load was observed in the second group. A team in Japan also worked on HCV peptide vaccines and published their findings in two papers[145]. The first study assessed the functionality of a “personalized” vaccine containing four CD8+ A24 peptides combined with Freund’s adjuvant in 12 HCV patients (genotype 1b) who had previously failed in standard IFN-based therapy. After the first vaccination, the rest of the vaccinations were carried out with only those peptides that produced responses in each participant. Although most patients developed peptide-specific T cell responses after the seventh injection, the authors observed a dose-dependent decrease in serum alanine aminotransferase (ALT) and HCV RNA levels in only five and three patients, respectively[145]. In the second study, another peptide vaccine composed of HCV core region (C35-44) peptides with ISA51, an emulsified incomplete Freund’s adjuvant, was shown to be safe and well tolerated in a phase I trial on 25 HCV non-responder patients. Around 60% of patients showed an increase in peptide-specific T cytotoxic responses. Also, a more than 30% improvement in ALT and a > 1 log reduction in viral load were observed in seven and two patients, respectively[152].
A phase I, dose-escalation, placebo-controlled randomized control trial was performed to assess a virosome-based peptide vaccine containing NS3 peptides in 30 healthy participants; however, no results have been released so far (ClinicalTrials.gov Identifier: NCT00445419).
Recombinant protein vaccines are developed by isolating the gene(s) encoding the corresponding protein and cloning it/them in bacteria, yeast, or mammalian cells. This approach is based on the theory that an efficient number of viral epitopes can induce enough immune responses to develop protective immunity, generally including antibodies and CD4+ T cell responses. Some recombinant proteins are, by themselves, sufficient for developing a strong immune response, whereas others require adjuvants. The advantages of recombinant protein vaccines are that they do not contain the pathogen or its genetic material and no organism culture is required.
In 1994, a recombinant heterodimeric E1E2 vaccine was tested on seven chimpanzees, and sterilizing immunity against a homologous HCV strain was shown in five (Table 1)[153]. This vaccine was further tested against persistent infection by homologous and heterologous HCV strains and offered protection in 10/12 and 8/9 chimpanzees, respectively[154]. Puig et al[155] vaccinated two chimpanzees, one naïve and one recovered from acute HCV infection, with recombinant HCV E1E2 glycoproteins. High antibody titers to E1E2 in addition to strong T cell proliferative responses were observed. After challenge with HCV, viremia was delayed in both vaccinated animals compared to the non-immunized animals. The naïve chimpanzee became persistently infected, despite an initial strong immune responses, and the other chimpanzee had a significantly shorter and milder viremia compared to the re-infection of the non-vaccinated animals. Many other studies evaluating the efficacy of recombinant HCV vaccines in animals, especially chimpanzees, achieved similar results, which paved the way for recombinant HCV vaccine trials in humans (Table 1)[156-159].
The first prophylactic HCV vaccine tested in humans was a C-terminally shortened recombinant E1 protein with aluminum hydroxide (alum) adjuvant named T2S-918/InnoVac-C. This vaccine provoked a higher antibody response against E1 in healthy volunteers than in patients with persistent HCV infection. Studies on this vaccine, however, were ceased in 2007 (Table 1)[160].
Another recombinant E1E2 heterodimer vaccine with MF59C adjuvant was given to 60 healthy subjects in three different doses on day 0 and weeks 4, 24, and 48. Neutralizing antibodies and T cell responses to E1/E2 were observed in all subjects. Moreover, the produced antibodies were shown to block CD81, a major entry molecule for HCV. Although the vaccine was safe and well tolerated, its usage was hampered by technical difficulties in E1E2 protein manufacturing (Table 1)[161].
In 2011, Verstrepen et al[162] vaccinated four chimpanzees with either genotype 1b E1 or E2 recombinant with alum adjuvant and observed antibody responses in all subjects. Only antibodies against E1, however, were shown to neutralize HCV pseudo-particles. Furthermore, only chimpanzees vaccinated with E1 were protected from persistent hepatitis when challenged with a 1b strain of HCV.
Drane et al[163] evaluated a recombinant HCV core protein vaccine with ISCOMATRIX™ adjuvant. After satisfying results were obtained in rhesus macaques, a phase I, placebo-controlled, dose-escalation clinical trial was conducted on 30 healthy volunteers. All participants except one demonstrated antibodies against HCV core protein. Despite the induction of strong CD4+ and CD8+ T cell responses in monkeys, T cell responses were observed in only two subjects who received the highest dose of vaccine. T cell cytokines were detected in all but one of the participants in the highest dose group.
InnoVAC-C, developed by Innogenetics Company (Innogenetics NV, Ghent, Belgium), was the first HCV therapeutic vaccine candidate based on recombinant E1 protein in alum adjuvant. The vaccine was administered to 26 of 35 chronically infected HCV patients (genotype 1) at weeks 0, 4, 8, 12 and 24, and others received a placebo (alum only). Then, 34 subjects received open-label E1 vaccines at weeks 50, 53, 56, 59, 62 and 65. Twenty-four subjects underwent liver biopsies before E1 vaccination and 17 mo later. According to Ishak scores, 9/24 patients (38%) improved two points or more, whereas others remained stable or their condition was exacerbated. The increase in anti-E1 specific antibody levels correlated with the relative decrease in ALT level. A significant de novo E1 specific T cell response was observed in all but three subjects[164]. Finally, studies on this vaccine led to a larger, placebo-controlled trial where 122 chronically HCV infected patients were randomly chosen to receive four courses of six injections over 3 years. Vaccination induced anti-E1 humoral and cellular immune responses, but contrary to earlier findings, the histological progression of liver disease was not halted[165]. The Innogenetics company in Belgium eventually abandoned this HCV vaccine program in 2008, and no further work has been published[166].
Another therapeutic vaccine, GI-5005, is based on recombinant core and NS3 proteins of HCV produced in yeast cells (Saccharomyces cervisiae). Studies of in vitro and in vivo models demonstrated the robust immunogenicity of GI5005. Moreover, GI-5005 was evaluated in a phase I b clinical trial and displayed efficacy in patients with chronic HCV infection[167]. GI5005 was evaluated in combination with the standard therapy [pegylated (PEG)-IFN/ribavirin] in more than 250 chronic HCV-1 patients in a phase II, placebo-controlled trial. Triple therapy was well tolerated with no increase in withdrawals due to adverse effects. Moreover, improved early virological responses were observed in all treated naïve patients as well as an increase in sustained virological response rates in prior non-responder patients[168].
In 2001, the first DNA vaccine was licensed for use to protect horses from West Nile virus[169]. The injection of a plasmid encoding antigenic HCV protein(s) or peptide epitope(s) resulted in protein expression in vivo, leading to both humoral and cellular immune responses in rhesus macaques[170-172]. DNA vaccination against HCV primes periphery T cells, which subsequently enter the liver and help clear the infection. Unfortunately, the initial success with the DNA vaccination in mice did not continue in humans, possibly because as the size of the immunized host increases, the efficacy of DNA uptake and gene expression decreases[173,174]. DNA vaccine includes the nucleotides encoding an antigenic portion of the virus, such as the viral core region or envelope region. The DNA vaccine is taken up by the host cell, transcribed, and translated to yield proteins. These proteins are processed via the endogenous MHC class 1 pathway and promoted via cell-mediated immunity (CMI). The first DNA-based vaccine to reach clinical trial for HCV infection was CIGB-230, which contained a mixture of Core/E1/E2-expressing plasmid with HCV core protein. The phase I trial of this vaccine resulted in neutralizing antibody and HCV core specific T cell responses in the majority of patients. Furthermore, nearly half of the vaccinated individuals developed an improvement in liver histology with a reduction in fibrosis, even though viremia persisted[175]. Another clinical trial for CIGB-230 on 15 chronic HCV patients who were non-responsive to IFN treatment indicated that six patients developed weak de novo neutralizing antibody responses, and only one patient had a drop of > 1 log10 in viral load[176].
Due to the heterogeneity of HCV subtypes, a DNA vaccine including the most conserved regions (NS3 and NS4a) was designed. Through extensive codon modification, the DNA was effectively expressed in vivo and prime T helper cell type 1 (Th1) and CD8+ CTL responses in transgenic mice models[177,178]. This vaccine, named ChronVac-C and generated by the Tripep Company of Sweden, was the second DNA-based HCV vaccine to reach a human trial, and it was given to 12 chronic HCV patients through intramuscular electroporation. Initial results suggested safety and immunogenicity of vaccine. Two out of three patients receiving the highest dose showed a decrease in serum HCV RNA. Also, three patients who were under standard IFN-based therapy had an accelerated viral load clearance. Therefore, a treatment combining ChronVac-c with the standard IFN-based therapy was proposed for chronic HCV patients[179]. This vaccine is now in phase II clinical trials for HCV infection (ClinicalTrials.gov Identifier: NCT01335711).
The nonstructural protein 3 (NS3) of the HCV, an attractive candidate for use in HCV vaccination, plays an important role in the viral life cycle while simultaneously interfering with the host defense system. The serine protease of NS3 cleaves the mitochondrial antiviral signaling proteins, thereby blocking IFN-β production[180-183]. NTPase/RNA helicase activities of NS3 may interfere with cellular RNA helicase-mediated functions, such as DNA replication, RNA transcription, and other cellular phenomena[183,184]. To overcome this challenge, Ratnoglik et al[185] constructed a series of DNA vaccines that express NS3 of HCV but with mutations to the catalytic triad of the serine protease and the NTPase/RNA helicase domain to eliminate the problems mentioned above. Immunization of BALB/c mice with resultant DNA vaccine candidates induced T cell immune responses similar to those induced by the wild type NS3 or NS3/4A complex.
The main idea for vector-based vaccines is the use of manipulated viruses to deliver foreign genetic material to mammalian cells. Viruses can enter cells and express desired genes within the host while becoming non-pathogenic and non-replicative by deleting specific genes. Compared to peptide vaccines, vector-based vaccines introduce a broader range of viral epitopes and are presented through the MHC-I pathway and induce broader CD4+ and CD8+ T cell responses[186]. Adenoviral (Ad) vectors are the most used viral vectors for T-cell priming in non-human primates (NHPs) and humans[187]. Studies of HIV vaccination on rodents and NHPs established the use of Ad vectors as the most efficient approach to prime cytotoxic T cell responses[188]. Ad vaccines can stably express large foreign genes, remain outside the host’s chromosome, and become replication defective by deleting the E1 locus[186,189]. However, one major drawback to using Ad vectors is a pre-existing immunity to them that results in vector clearance prior to induction of the desired immune response. To overcome this problem, rare subtypes, non-human adenoviruses (which are harmless to humans), or adenoviruses with altered surface proteins are used[187].
An Ad6-based vaccine, encoding the NS3, NS4A, NS4B, NS5A, and inactivated NS5B, was capable of inducing strong and specific T-cell responses against NS antigens of HCV in mice and rhesus macaques[190]. Okairos, Inc. (Rome, Italy) further investigated this vaccine by testing it on five chimpanzees. Four chimpanzees showed protective levels after being challenged with a heterologous HCV virus via the induction of cross-reactive cellular responses[191]. After the safety of the Okairos vaccine was confirmed in a phase I study[192], another trial was directed, but no results have been published yet (ClinicalTrials.gov Identifier: NCT01070407).
Based on the strength of this preclinical data, Barnes et al[193] conducted a phase I clinical trial to assess chimpanzee Ad3 (ChAd3) and Ad6 vectors in 36 healthy volunteers and found that both vaccines induced specific T cell responses against multiple HCV proteins. These responses were capable of recognizing heterologous HCV strains (genotypes 1A and 3A). However, the vaccine was not as effective as predicted from the results in rhesus macaques, possibly due to higher levels of cross-reactive antibodies against Ads in humans[193,194].
Another approach in developing vector-based vaccines is the use of modified vaccinia Ankara (MVA) viruses to encode HCV specific genes. They are a highly attenuated poxvirus strain used in several vaccine designs for various conditions, including colorectal cancer, tuberculosis (TB), HIV, and melanoma[195-198]. The MVA genome is incapable of integrating into the host genome, as its life cycle takes place entirely in the host cytoplasm. Its use is more efficient than Ad-based vectors due to minimal pre-existing anti-MVA immunity[199,200].
The Transgene Company vaccinated chimpanzees with a heterologous prime-boost regimen with DNA and MVA encoding core, E1, E2, and NS3 genes (Table 1)[201]. Immunization induced strong HCV specific CD4+ and CD8+ T cell responses. In addition, all animals receiving prime-boost vaccines achieved high HCV-specific antibody titers, whereas those receiving the DNA prime alone did not. When challenged with a heterologous strain of HCV, three out of four chimpanzees developed chronic infection[201].
An MVA-based vaccine encoding HCV NS3, NS4, and NS5B genes was able to induce strong long-lasting cross-reactive HCV specific helper and cytotoxic T cells in HLA-A2.1 and HLA-B7.2 transgenic mice. An additional dose of vaccine given after 6 mo boosted these responses, suggesting that the vaccine can induce effective memory T cells[202]. This vaccine, also called TG4040, was evaluated in a dose-escalation trial of 15 chronically infected HCV patients. Results showed that six patients had a decline in HCV viral load (0.5-1.4 log10) that was associated with a significant CD8+ T-cell response[203]. A phase II trial using the TG4040 vaccine in combination with standard PEG-IFN and ribavirin therapy has been completed, but no results have been published yet (ClinicalTrials.gov Identifier: NCT01055821).
Vector-based vaccines with strategies using neither modified vaccinia Ankara viruses nor adenoviruses are also under development. A study in HLA-A2.1-transgenic mice used the canary pox virus as a viral vector of two plasmids coding the entire HCV genome to boost the primary immunization with HCV DNA vaccine. Two months after a canary pox virus boost, strong cellular immune responses to HCV proteins were observed[204]. Another study evaluated the canary pox virus as a viral vector to boost the primary immunization with DNA vaccine. The results were similar to those previously discussed and revealed the potential for the canary pox virus as a viral vector for HCV vaccination[205]. Youn et al transdermally immunized four chimpanzees with recombinant vaccinia viruses expressing HCV genes developed by Marion Perkus at Virogenetics Inc.[206]. Upon challenge with infective doses of homologous HCV, two control chimpanzees that received only the parental vaccinia virus, developed chronic HCV infections, whereas the immunized animals developed strong IFN-γ-producing T cell responses and moderate T cell proliferative responses[207].
HCV virus-like particles (VLP) are safe and easily manufactured vectors for gene delivery that closely resemble the mature HCV structure. Therefore, it is possible to induce neutralizing antibodies and T cell responses against many epitopes using a single VLP-based vaccine. Their usage has been licensed for other viral infections, such as the hepatitis B virus (HBV) and human papillomavirus (HPV)[208-210]. Baumert et al[211] generated HCV-like particles (HCV-LPs) in insect cells using a recombinant baculovirus containing the complementary DNA for HCV structural proteins. Structural and antigenic compositions of HCV-LPs were similar to the original HCV, and after testing in mice, it was capable of inducing immune responses against various epitopes of HCV[212]. Another study showed the superior immunogenicity of the HCV-LP-based vaccine compared with the DNA vaccine in transgenic HLA2.1 and BLAB/c mice[213]. This vaccination approach was also tested on a NHP model - baboons. All 12 animals developed broad and long-lasting HCV-specific humoral and cellular immune responses. Adjuvants only marginally enhanced the immunogenicity of the HCV-LP based vaccine[214]. Four chimpanzees were also immunized with HCV-LPs, and they all developed HCV-specific T cell and proliferative lymphocyte responses against core, E1, and E2 proteins. After encountering an infectious HCV genotype, one chimpanzee developed transient viremia and the other three exhibited higher levels of viremia, but their viral levels became unquantifiable after 10 wk. Four naïve chimpanzees were also challenged with the same HCV genotype, and three developed persistent infections[215]. No results of human clinical trials testing HCV-like particles have been published to date.
Gowans et al[216] developed a novel peptide delivery system using autologous monocyte-derived DCs. In a phase I dose-escalation clinical trial of HCV patients, DCs were loaded and activated ex vivo with HCV-specific HLA-A2 restricted T cell epitope peptides. All six patients who received the vaccine exhibited weak de novo HCV-specific CD8+ T cell responses. However, the T cell responses were not continuous, and no change was observed in HCV RNA, anti-HCV antibody, or circulating cytokine levels.
An oral immunization method was introduced using attenuated Salmonella typhimurium as a carrier for delivery of HCV DNA to the lymphoid tissue of the gastrointestinal tract. Vaccination of HLA-A2.1 transgenic mice induced T cell specific responses in 86% of mice that continued for a minimum of 10 mo[217]. Another oral delivery approach was developed using attenuated salmonella carrying a plasmid coding HCV core and E2 proteins. Vaccination of BLAB/c mice induced cellular immune responses and antibodies against HCV core and E2 proteins[218]. Batdelger et al[219] evaluated the efficacy of V-5 immunitor tablets, comprising heat-inactivated HCV antigens from HBV and HCV infected donors’ blood in 10 chronically infected HCV patients. All of the analyzed patients receiving a daily dose of this therapeutic vaccine tablet showed decreased liver enzymes after 1 mo. Moreover, no adverse side effects were observed. However, larger scale and longer studies are required to further evaluate this method and its safety and efficacy.
Plant-based vaccines are another novel approach for developing an easily producible and inexpensive HCV vaccine. The tobacco mosaic virus (TMV) was engineered to encode a chimeric protein containing E2 hyper variable region 1 (HVR1) protein and C-terminal of the B subunit of cholera toxin (CTB). Plants infected with this genetically engineered TMV produced the HVR1 peptide fused to the CTB. HVR-1 specific antibodies acquired from HCV infected individuals reacted with plant-derived HVR1/CTB. Moreover, the intranasal immunization of mice with plant-derived HVR1/CTB chimeric protein induced both anti-CTB and anti-HVR1 serum antibodies[220]. Another attempt at plant-based vaccines used an engineered cucumber mosaic virus (CMV) expressing a HCV 27-amino acid synthetic peptide. Rabbits fed engineered CMV-infected lettuce plants exhibited an HCV-specific humoral immune response[221].
Chimeric HBV/HCV vaccines are based on the fact that the HBV surface antigen is a safe VLP-forming delivery system that can carry various antigens, including HCV envelope proteins[222]. In 1999, Wu et al[223] fused truncated HCV core protein (HCc) with the HBV virus and compared its immunogenicity to HCV core protein alone in the sera of HCV-infected patients. The fused antigens exhibited antigenicity to both HBV and HCV, whereas HCc alone resulted in antigenicity to HCV. They tested the chimeric protein in rabbits and mice as well and concluded that chimeric protein is more immunogenic than HCc alone. In another study, HBV core protein (HBc) was fused with HCc or HCV NS3. The purified HBc/HCc and HBc/HCV NS3 were tested in mice. Both induced high humoral and cellular responses to HBV core protein. HBc/HCc led to low antibody and T cell responses to HCV core protein, whereas HBc/HCV NS3 induced higher levels of antibodies and no T cell responses to the NS3 epitope of HCV[224]. Chiron Corp. also invented a chimeric HBV/HCV vaccine using HCV envelope proteins fused with HBsAg[225]. Another chimeric vaccine was developed by replacing the N-terminal transmembrane domain of HBV surface antigen with the transmembrane domain of HCV E1 or E2 glycoproteins from genotype 1a. The chimeric particles were produced in the stably transduced ovary cells of Chinese hamsters and were used to vaccinate New Zealand rabbits. Vaccination resulted in a strong specific humoral response against both HBV and HCV envelope proteins. Induced antibodies were also able to neutralize HCV pseudo particles derived from heterologous HCV 1a, 1b, 2a, and 3 strains. Furthermore, responses against HBV were equivalent to those induced by the conventional HBV vaccine[226,227]. No clinical trials in chimpanzees or humans have evaluated such chimeric vaccines thus far.
This review focused on the development of different types of vaccines against HCV. The arms of the host immune system that are involved in the eradication of the virus were reviewed as well.
Different methods, including peptide, recombinant protein, DNA, vector-based, and VLPs, are currently used to develop and produce HCV vaccines. Peptide vaccines are the leading cause of HCV specific T cell responses. One associated problem is the high rate of genetic variation in viruses within populations and geographic regions. Recombinant protein vaccines are produced by specific gene(s) encoding the immune dominant protein(s). Viral epitopes are the leading cause of immune responses to develop protective immunity, generally including antibodies and CD4+ T cell responses. Vector-based vaccines are manipulated viruses that deliver foreign genetic material to mammalian cells. Viruses can enter cells and express desired genes within the host while becoming non-pathogenic and non-replicative by deleting specific genes. HCV VLPs are safe and easily manufactured vectors for gene delivery that closely resemble the mature HCV structure.
The challenge for the development of an efficient vaccine with relatively low side effects is still yet to be overcome. Although significant steps have been taken in accomplishing this goal, no promising results have been achieved to date. In part, this depends on the current understanding of the interaction of the virus and the host. Therefore, a more thorough understanding of the host immunity in the context of HCV infection is expected to lead to better results. Of course, other areas of biomedical sciences can greatly contribute to these studies. For instance, providing a suitable animal model for preliminary studies of the newly designed vaccines is crucial.
An effective vaccine against HCV may need to target another cellular process for HCV entry and release or may need to target another sequence of the virus genome. However, due to the high HCV mutation rate, that is not necessarily the best option. A balance between its efficiency in targeting a sequence that can inhibit further growth and a sequence that is highly conserved among different HCV genomes must be found. Alternatively, an efficient vaccine may target several cellular and molecular processes simultaneously. Vaccines may also be accompanied by different adjuvants to increase their efficiency rates. Moreover, other approaches may rely on using other targets, e.g., targeting mRNAs by using synthetic interfering RNAs, such as microRNAs and small interfering RNAs.
In addition to attempts to develop vaccines, integrated programs should be carried out to study their effects in individuals; for instance, in which intervals of injection (in case of several infections), in which age group or sex, and with which genetic background, especially regarding HLA types and genotype variations in different positions of cytokine genes, is it more efficient.
Another seemingly important fact is the relative danger of exposure to HCV or its related particles during studies. This requires laboratories with high safety levels, which may not be readily available everywhere, and this can reduce the pace of studies on HCV. It is expected that ongoing studies will eventually lead to the development of more reliable vaccine types, and this, in turn will reduce worldwide incidence and prevalence rates of HCV infection.
P- Reviewer: Stocco G, Tanaka N S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Ma S
| 1. | Shakeri MT, Nomani H, Ghayour Mobarhan M, Sima HR, Gerayli S, Shahbazi S, Rostami S, Meshkat Z. The prevalence of hepatitis C virus in mashhad, iran: a population-based study. Hepat Mon. 2013;13:e7723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13:34-41. [PubMed] [Cited in This Article: ] |
| 3. | Poynard T, Yuen MF, Ratziu V, Lai CL. Viral hepatitis C. Lancet. 2003;362:2095-2100. [PubMed] [Cited in This Article: ] |
| 4. | Somi MH, Etemadi J, Ghojazadeh M, Farhang S, Faramarzi M, Foroutan S, Soleimanpour M. Risk factors of HCV seroconversion in hemodialysis patients in tabriz, iran. Hepat Mon. 2014;14:e17417. [PubMed] [Cited in This Article: ] |
| 5. | Pradat P, Trépo C. HCV: epidemiology, modes of transmission and prevention of spread. Baillieres Best Pract Res Clin Gastroenterol. 2000;14:201-210. [PubMed] [Cited in This Article: ] |
| 6. | Aniszewska M, Kowalik-Mikołajewska B, Pokorska-Lis M, Kalinowska M, Marczyńskai M. [Mother-to-infant HCV transmission. Can we influence the frequency and the course of the infection?]. Przegl Lek. 2010;67:9-12. [PubMed] [Cited in This Article: ] |
| 7. | Farci P. Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome [Science 1989; 244: 359-362]. J Hepatol. 2002;36:582-585. [PubMed] [Cited in This Article: ] |
| 8. | Averhoff FM, Glass N, Holtzman D. Global burden of hepatitis C: considerations for healthcare providers in the United States. Clin Infect Dis. 2012;55 Suppl 1:S10-S15. [PubMed] [Cited in This Article: ] |
| 9. | Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558-567. [PubMed] [Cited in This Article: ] |
| 10. | Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333-1342. [PubMed] [Cited in This Article: ] |
| 11. | Global surveillance and control of hepatitis C. Report of a WHO Consultation organized in collaboration with the Viral Hepatitis Prevention Board, Antwerp, Belgium. J Viral Hepat. 1999;6:35-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Razavi H, Elkhoury AC, Elbasha E, Estes C, Pasini K, Poynard T, Kumar R. Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology. 2013;57:2164-2170. [PubMed] [Cited in This Article: ] |
| 13. | Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45-S57. [PubMed] [Cited in This Article: ] |
| 14. | Wedemeyer H, Duberg AS, Buti M, Rosenberg WM, Frankova S, Esmat G, Örmeci N, Van Vlierberghe H, Gschwantler M, Akarca U. Strategies to manage hepatitis C virus (HCV) disease burden. J Viral Hepat. 2014;21 Suppl 1:60-89. [PubMed] [Cited in This Article: ] |
| 15. | Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, Barnes E. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61:77-87. [PubMed] [Cited in This Article: ] |
| 16. | Marcellin P. Hepatitis C: the clinical spectrum of the disease. J Hepatol. 1999;31 Suppl 1:9-16. [PubMed] [Cited in This Article: ] |
| 17. | Feuerstadt P, Bunim AL, Garcia H, Karlitz JJ, Massoumi H, Thosani AJ, Pellecchia A, Wolkoff AW, Gaglio PJ, Reinus JF. Effectiveness of hepatitis C treatment with pegylated interferon and ribavirin in urban minority patients. Hepatology. 2010;51:1137-1143. [PubMed] [Cited in This Article: ] |
| 18. | Mazumdar B, Banerjee A, Meyer K, Ray R. Hepatitis C virus E1 envelope glycoprotein interacts with apolipoproteins in facilitating entry into hepatocytes. Hepatology. 2011;54:1149-1156. [PubMed] [Cited in This Article: ] |
| 19. | Niu Y, Si Y, Li Y, Chi X, Li X, Liu X, Li D, Cheng M, Fan J, Si S. A novel small-molecule inhibitor of hepatitis C virus replication acts by suppressing signal transducer and activator of transcription 3. J Antimicrob Chemother. 2015;70:2013-2023. [PubMed] [Cited in This Article: ] |
| 20. | Legrand N, Ploss A, Balling R, Becker PD, Borsotti C, Brezillon N, Debarry J, de Jong Y, Deng H, Di Santo JP. Humanized mice for modeling human infectious disease: challenges, progress, and outlook. Cell Host Microbe. 2009;6:5-9. [PubMed] [Cited in This Article: ] |
| 21. | Tsukiyama-Kohara K, Iizuka N, Kohara M, Nomoto A. Internal ribosome entry site within hepatitis C virus RNA. J Virol. 1992;66:1476-1483. [PubMed] [Cited in This Article: ] |
| 22. | Wang C, Sarnow P, Siddiqui A. Translation of human hepatitis C virus RNA in cultured cells is mediated by an internal ribosome-binding mechanism. J Virol. 1993;67:3338-3344. [PubMed] [Cited in This Article: ] |
| 23. | Bartenschlager R, Lohmann V. Replication of hepatitis C virus. J Gen Virol. 2000;81:1631-1648. [PubMed] [Cited in This Article: ] |
| 24. | Kolykhalov AA, Feinstone SM, Rice CM. Identification of a highly conserved sequence element at the 3’ terminus of hepatitis C virus genome RNA. J Virol. 1996;70:3363-3371. [PubMed] [Cited in This Article: ] |
| 25. | Tanaka T, Kato N, Cho MJ, Sugiyama K, Shimotohno K. Structure of the 3’ terminus of the hepatitis C virus genome. J Virol. 1996;70:3307-3312. [PubMed] [Cited in This Article: ] |
| 26. | Yanagi M, St Claire M, Emerson SU, Purcell RH, Bukh J. In vivo analysis of the 3’ untranslated region of the hepatitis C virus after in vitro mutagenesis of an infectious cDNA clone. Proc Natl Acad Sci USA. 1999;96:2291-2295. [PubMed] [Cited in This Article: ] |
| 27. | Scheel TK, Rice CM. Understanding the hepatitis C virus life cycle paves the way for highly effective therapies. Nat Med. 2013;19:837-849. [PubMed] [Cited in This Article: ] |
| 28. | Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nat Rev Microbiol. 2007;5:453-463. [PubMed] [Cited in This Article: ] |
| 29. | Tan SL, editor . Hepatitis C Viruses: Genomes and Molecular Biology. Norfolk (UK): Horizon Bioscience 2006; . [PubMed] [Cited in This Article: ] |
| 30. | McLauchlan J. Properties of the hepatitis C virus core protein: a structural protein that modulates cellular processes. J Viral Hepat. 2000;7:2-14. [PubMed] [Cited in This Article: ] |
| 31. | Roohvand F, Kossari N. Advances in hepatitis C virus vaccines, Part one: Advances in basic knowledge for hepatitis C virus vaccine design. Expert Opin Ther Pat. 2011;21:1811-1830. [PubMed] [Cited in This Article: ] |
| 32. | Vieyres G, Dubuisson J, Patel AH. Characterization of antibody-mediated neutralization directed against the hypervariable region 1 of hepatitis C virus E2 glycoprotein. J Gen Virol. 2011;92:494-506. [PubMed] [Cited in This Article: ] |
| 33. | Schulze zur Wiesch J, Schmitz H, Borowski E, Borowski P. The proteins of the Hepatitis C virus: their features and interactions with intracellular protein phosphorylation. Arch Virol. 2003;148:1247-1267. [PubMed] [Cited in This Article: ] |
| 34. | Ishii S, Koziel MJ. Immune responses during acute and chronic infection with hepatitis C virus. Clin Immunol. 2008;128:133-147. [PubMed] [Cited in This Article: ] |
| 35. | Zhang X, Dou J, Germann MW. Characterization of the cellular immune response in hepatitis C virus infection. Med Res Rev. 2009;29:843-866. [PubMed] [Cited in This Article: ] |
| 36. | Blight KJ. Allelic variation in the hepatitis C virus NS4B protein dramatically influences RNA replication. J Virol. 2007;81:5724-5736. [PubMed] [Cited in This Article: ] |
| 37. | Tellinghuisen TL, Foss KL, Treadaway J. Regulation of hepatitis C virion production via phosphorylation of the NS5A protein. PLoS Pathog. 2008;4:e1000032. [PubMed] [Cited in This Article: ] |
| 38. | Bukh J, Miller RH, Purcell RH. Genetic heterogeneity of hepatitis C virus: quasispecies and genotypes. Semin Liver Dis. 1995;15:41-63. [PubMed] [Cited in This Article: ] |
| 39. | Terilli RR, Cox AL. Immunity and hepatitis C: a review. Curr HIV/AIDS Rep. 2013;10:51-58. [PubMed] [Cited in This Article: ] |
| 40. | Wilkins C, Gale M. Recognition of viruses by cytoplasmic sensors. Curr Opin Immunol. 2010;22:41-47. [PubMed] [Cited in This Article: ] |
| 41. | Horner SM. Activation and evasion of antiviral innate immunity by hepatitis C virus. J Mol Biol. 2014;426:1198-1209. [PubMed] [Cited in This Article: ] |
| 42. | Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008;48:322-335. [PubMed] [Cited in This Article: ] |
| 43. | Li K, Li NL, Wei D, Pfeffer SR, Fan M, Pfeffer LM. Activation of chemokine and inflammatory cytokine response in hepatitis C virus-infected hepatocytes depends on Toll-like receptor 3 sensing of hepatitis C virus double-stranded RNA intermediates. Hepatology. 2012;55:666-675. [PubMed] [Cited in This Article: ] |
| 44. | Wang N, Liang Y, Devaraj S, Wang J, Lemon SM, Li K. Toll-like receptor 3 mediates establishment of an antiviral state against hepatitis C virus in hepatoma cells. J Virol. 2009;83:9824-9834. [PubMed] [Cited in This Article: ] |
| 45. | Takahashi K, Asabe S, Wieland S, Garaigorta U, Gastaminza P, Isogawa M, Chisari FV. Plasmacytoid dendritic cells sense hepatitis C virus-infected cells, produce interferon, and inhibit infection. Proc Natl Acad Sci USA. 2010;107:7431-7436. [PubMed] [Cited in This Article: ] |
| 46. | Negash AA, Ramos HJ, Crochet N, Lau DT, Doehle B, Papic N, Delker DA, Jo J, Bertoletti A, Hagedorn CH. IL-1β production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease. PLoS Pathog. 2013;9:e1003330. [PubMed] [Cited in This Article: ] |
| 47. | Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397-411. [PubMed] [Cited in This Article: ] |
| 48. | Wang Y, Li J, Wang X, Sang M, Ho W. Hepatic stellate cells, liver innate immunity, and hepatitis C virus. J Gastroenterol Hepatol. 2013;28 Suppl 1:112-115. [PubMed] [Cited in This Article: ] |
| 49. | Li K, Lemon SM. Innate immune responses in hepatitis C virus infection. Semin Immunopathol. 2013;35:53-72. [PubMed] [Cited in This Article: ] |
| 50. | Balagopal A, Thomas DL, Thio CL. IL28B and the control of hepatitis C virus infection. Gastroenterology. 2010;139:1865-1876. [PubMed] [Cited in This Article: ] |
| 51. | Mengshol JA, Golden-Mason L, Arikawa T, Smith M, Niki T, McWilliams R, Randall JA, McMahan R, Zimmerman MA, Rangachari M. A crucial role for Kupffer cell-derived galectin-9 in regulation of T cell immunity in hepatitis C infection. PLoS One. 2010;5:e9504. [PubMed] [Cited in This Article: ] |
| 52. | Amadei B, Urbani S, Cazaly A, Fisicaro P, Zerbini A, Ahmed P, Missale G, Ferrari C, Khakoo SI. Activation of natural killer cells during acute infection with hepatitis C virus. Gastroenterology. 2010;138:1536-1545. [PubMed] [Cited in This Article: ] |
| 53. | Zeromski J, Mozer-Lisewska I, Kaczmarek M, Kowala-Piaskowska A, Sikora J. NK cells prevalence, subsets and function in viral hepatitis C. Arch Immunol Ther Exp (Warsz). 2011;59:449-455. [PubMed] [Cited in This Article: ] |
| 54. | An P, Thio CL, Kirk GD, Donfield S, Goedert JJ, Winkler CA. Regulatory polymorphisms in the interleukin-18 promoter are associated with hepatitis C virus clearance. J Infect Dis. 2008;198:1159-1165. [PubMed] [Cited in This Article: ] |
| 55. | Chen M, Sällberg M, Sönnerborg A, Weiland O, Mattsson L, Jin L, Birkett A, Peterson D, Milich DR. Limited humoral immunity in hepatitis C virus infection. Gastroenterology. 1999;116:135-143. [PubMed] [Cited in This Article: ] |
| 56. | Netski DM, Mosbruger T, Depla E, Maertens G, Ray SC, Hamilton RG, Roundtree S, Thomas DL, McKeating J, Cox A. Humoral immune response in acute hepatitis C virus infection. Clin Infect Dis. 2005;41:667-675. [PubMed] [Cited in This Article: ] |
| 57. | Widell A, Elmud H, Persson MH, Jonsson M. Transmission of hepatitis C via both erythrocyte and platelet transfusions from a single donor in serological window-phase of hepatitis C. Vox Sang. 1996;71:55-57. [PubMed] [Cited in This Article: ] |
| 58. | Edwards VC, Tarr AW, Urbanowicz RA, Ball JK. The role of neutralizing antibodies in hepatitis C virus infection. J Gen Virol. 2012;93:1-19. [PubMed] [Cited in This Article: ] |
| 59. | Meunier JC, Russell RS, Goossens V, Priem S, Walter H, Depla E, Union A, Faulk KN, Bukh J, Emerson SU, Purcell RH. Isolation and characterization of broadly neutralizing human monoclonal antibodies to the e1 glycoprotein of hepatitis C virus. J Virol. 2008;82:966-973. [PubMed] [Cited in This Article: ] |
| 60. | Bjøro K, Frøland SS, Yun Z, Samdal HH, Haaland T. Hepatitis C infection in patients with primary hypogammaglobulinemia after treatment with contaminated immune globulin. N Engl J Med. 1994;331:1607-1611. [PubMed] [Cited in This Article: ] |
| 61. | Oliviero B, Cerino A, Varchetta S, Paudice E, Pai S, Ludovisi S, Zaramella M, Michelone G, Pugnale P, Negro F. Enhanced B-cell differentiation and reduced proliferative capacity in chronic hepatitis C and chronic hepatitis B virus infections. J Hepatol. 2011;55:53-60. [PubMed] [Cited in This Article: ] |
| 62. | Rosa D, Saletti G, De Gregorio E, Zorat F, Comar C, D’Oro U, Nuti S, Houghton M, Barnaba V, Pozzato G. Activation of naïve B lymphocytes via CD81, a pathogenetic mechanism for hepatitis C virus-associated B lymphocyte disorders. Proc Natl Acad Sci USA. 2005;102:18544-18549. [PubMed] [Cited in This Article: ] |
| 63. | Razvi S, Schneider L, Jonas MM, Cunningham-Rundles C. Outcome of intravenous immunoglobulin-transmitted hepatitis C virus infection in primary immunodeficiency. Clin Immunol. 2001;101:284-288. [PubMed] [Cited in This Article: ] |
| 64. | Holz LE, Yoon JC, Raghuraman S, Moir S, Sneller MC, Rehermann B. B cell homeostasis in chronic hepatitis C virus-related mixed cryoglobulinemia is maintained through naïve B cell apoptosis. Hepatology. 2012;56:1602-1610. [PubMed] [Cited in This Article: ] |
| 65. | Forghieri F, Luppi M, Barozzi P, Maffei R, Potenza L, Narni F, Marasca R. Pathogenetic mechanisms of hepatitis C virus-induced B-cell lymphomagenesis. Clin Dev Immunol. 2012;2012:807351. [PubMed] [Cited in This Article: ] |
| 66. | Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest. 2009;119:1745-1754. [PubMed] [Cited in This Article: ] |
| 67. | Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol. 2006;1:23-61. [PubMed] [Cited in This Article: ] |
| 68. | Liang TJ, Rehermann B, Seeff LB, Hoofnagle JH. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med. 2000;132:296-305. [PubMed] [Cited in This Article: ] |
| 69. | Smyk-Pearson S, Tester IA, Klarquist J, Palmer BE, Pawlotsky JM, Golden-Mason L, Rosen HR. Spontaneous recovery in acute human hepatitis C virus infection: functional T-cell thresholds and relative importance of CD4 help. J Virol. 2008;82:1827-1837. [PubMed] [Cited in This Article: ] |
| 70. | Urbani S, Amadei B, Fisicaro P, Tola D, Orlandini A, Sacchelli L, Mori C, Missale G, Ferrari C. Outcome of acute hepatitis C is related to virus-specific CD4 function and maturation of antiviral memory CD8 responses. Hepatology. 2006;44:126-139. [PubMed] [Cited in This Article: ] |
| 71. | Ray S, Bailey J, Thomas D. Hepatitis C virus. Fields Virol. 2013;6:795-824. [Cited in This Article: ] |
| 72. | Neumann-Haefelin C, Oniangue-Ndza C, Kuntzen T, Schmidt J, Nitschke K, Sidney J, Caillet-Saguy C, Binder M, Kersting N, Kemper MW. Human leukocyte antigen B27 selects for rare escape mutations that significantly impair hepatitis C virus replication and require compensatory mutations. Hepatology. 2011;54:1157-1166. [PubMed] [Cited in This Article: ] |
| 73. | Cangussu LO, Teixeira R, Campos EF, Rampim GF, Mingoti SA, Martins-Filho OA, Gerbase-DeLima M. HLA class II alleles and chronic hepatitis C virus infection. Scand J Immunol. 2011;74:282-287. [PubMed] [Cited in This Article: ] |
| 74. | Francavilla V, Accapezzato D, De Salvo M, Rawson P, Cosimi O, Lipp M, Cerino A, Cividini A, Mondelli MU, Barnaba V. Subversion of effector CD8+ T cell differentiation in acute hepatitis C virus infection: exploring the immunological mechanisms. Eur J Immunol. 2004;34:427-437. [PubMed] [Cited in This Article: ] |
| 75. | Simmonds P, Bukh J, Combet C, Deléage G, Enomoto N, Feinstone S, Halfon P, Inchauspé G, Kuiken C, Maertens G. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology. 2005;42:962-973. [PubMed] [Cited in This Article: ] |
| 76. | Simmonds P. Viral heterogeneity of the hepatitis C virus. J Hepatol. 1999;31 Suppl 1:54-60. [PubMed] [Cited in This Article: ] |
| 77. | Halliday J, Klenerman P, Barnes E. Vaccination for hepatitis C virus: closing in on an evasive target. Expert Rev Vaccines. 2011;10:659-672. [PubMed] [Cited in This Article: ] |
| 78. | Erickson AL, Kimura Y, Igarashi S, Eichelberger J, Houghton M, Sidney J, McKinney D, Sette A, Hughes AL, Walker CM. The outcome of hepatitis C virus infection is predicted by escape mutations in epitopes targeted by cytotoxic T lymphocytes. Immunity. 2001;15:883-895. [PubMed] [Cited in This Article: ] |
| 79. | Weiner A, Erickson AL, Kansopon J, Crawford K, Muchmore E, Hughes AL, Houghton M, Walker CM. Persistent hepatitis C virus infection in a chimpanzee is associated with emergence of a cytotoxic T lymphocyte escape variant. Proc Natl Acad Sci USA. 1995;92:2755-2759. [PubMed] [Cited in This Article: ] |
| 80. | Bowen DG, Walker CM. Mutational escape from CD8+ T cell immunity: HCV evolution, from chimpanzees to man. J Exp Med. 2005;201:1709-1714. [PubMed] [Cited in This Article: ] |
| 81. | Cox AL, Mosbruger T, Mao Q, Liu Z, Wang XH, Yang HC, Sidney J, Sette A, Pardoll D, Thomas DL. Cellular immune selection with hepatitis C virus persistence in humans. J Exp Med. 2005;201:1741-1752. [PubMed] [Cited in This Article: ] |
| 82. | Thimme R, Binder M, Bartenschlager R. Failure of innate and adaptive immune responses in controlling hepatitis C virus infection. FEMS Microbiol Rev. 2012;36:663-683. [PubMed] [Cited in This Article: ] |
| 83. | Li K, Foy E, Ferreon JC, Nakamura M, Ferreon AC, Ikeda M, Ray SC, Gale M, Lemon SM. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc Natl Acad Sci USA. 2005;102:2992-2997. [PubMed] [Cited in This Article: ] |
| 84. | Ferreon JC, Ferreon AC, Li K, Lemon SM. Molecular determinants of TRIF proteolysis mediated by the hepatitis C virus NS3/4A protease. J Biol Chem. 2005;280:20483-20492. [PubMed] [Cited in This Article: ] |
| 85. | Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, Tschopp J. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167-1172. [PubMed] [Cited in This Article: ] |
| 86. | Meier UC, Owen RE, Taylor E, Worth A, Naoumov N, Willberg C, Tang K, Newton P, Pellegrino P, Williams I. Shared alterations in NK cell frequency, phenotype, and function in chronic human immunodeficiency virus and hepatitis C virus infections. J Virol. 2005;79:12365-12374. [PubMed] [Cited in This Article: ] |
| 87. | Golden-Mason L, Madrigal-Estebas L, McGrath E, Conroy MJ, Ryan EJ, Hegarty JE, O’Farrelly C, Doherty DG. Altered natural killer cell subset distributions in resolved and persistent hepatitis C virus infection following single source exposure. Gut. 2008;57:1121-1128. [PubMed] [Cited in This Article: ] |
| 88. | Park J, Kang W, Ryu SW, Kim WI, Chang DY, Lee DH, Park do Y, Choi YH, Choi K, Shin EC. Hepatitis C virus infection enhances TNFα-induced cell death via suppression of NF-κB. Hepatology. 2012;56:831-840. [PubMed] [Cited in This Article: ] |
| 89. | Helle F, Duverlie G, Dubuisson J. The hepatitis C virus glycan shield and evasion of the humoral immune response. Viruses. 2011;3:1909-1932. [PubMed] [Cited in This Article: ] |
| 90. | Rosen HR. Emerging concepts in immunity to hepatitis C virus infection. J Clin Invest. 2013;123:4121-4130. [PubMed] [Cited in This Article: ] |
| 91. | Polyak SJ, Khabar KS, Paschal DM, Ezelle HJ, Duverlie G, Barber GN, Levy DE, Mukaida N, Gretch DR. Hepatitis C virus nonstructural 5A protein induces interleukin-8, leading to partial inhibition of the interferon-induced antiviral response. J Virol. 2001;75:6095-6106. [PubMed] [Cited in This Article: ] |
| 92. | Bode JG, Ludwig S, Ehrhardt C, Albrecht U, Erhardt A, Schaper F, Heinrich PC, Häussinger D. IFN-alpha antagonistic activity of HCV core protein involves induction of suppressor of cytokine signaling-3. FASEB J. 2003;17:488-490. [PubMed] [Cited in This Article: ] |
| 93. | Lin W, Kim SS, Yeung E, Kamegaya Y, Blackard JT, Kim KA, Holtzman MJ, Chung RT. Hepatitis C virus core protein blocks interferon signaling by interaction with the STAT1 SH2 domain. J Virol. 2006;80:9226-9235. [PubMed] [Cited in This Article: ] |
| 94. | Heim MH. Innate immunity and HCV. J Hepatol. 2013;58:564-574. [PubMed] [Cited in This Article: ] |
| 95. | Honda M, Sakai A, Yamashita T, Nakamoto Y, Mizukoshi E, Sakai Y, Yamashita T, Nakamura M, Shirasaki T, Horimoto K. Hepatic ISG expression is associated with genetic variation in interleukin 28B and the outcome of IFN therapy for chronic hepatitis C. Gastroenterology. 2010;139:499-509. [PubMed] [Cited in This Article: ] |
| 96. | Ahmad A, Alvarez F. Role of NK and NKT cells in the immunopathogenesis of HCV-induced hepatitis. J Leukoc Biol. 2004;76:743-759. [PubMed] [Cited in This Article: ] |
| 97. | Maillard P, Huby T, Andréo U, Moreau M, Chapman J, Budkowska A. The interaction of natural hepatitis C virus with human scavenger receptor SR-BI/Cla1 is mediated by ApoB-containing lipoproteins. FASEB J. 2006;20:735-737. [PubMed] [Cited in This Article: ] |
| 98. | Helle F, Goffard A, Morel V, Duverlie G, McKeating J, Keck ZY, Foung S, Penin F, Dubuisson J, Voisset C. The neutralizing activity of anti-hepatitis C virus antibodies is modulated by specific glycans on the E2 envelope protein. J Virol. 2007;81:8101-8111. [PubMed] [Cited in This Article: ] |
| 99. | Timpe JM, Stamataki Z, Jennings A, Hu K, Farquhar MJ, Harris HJ, Schwarz A, Desombere I, Roels GL, Balfe P. Hepatitis C virus cell-cell transmission in hepatoma cells in the presence of neutralizing antibodies. Hepatology. 2008;47:17-24. [PubMed] [Cited in This Article: ] |
| 100. | Zhang P, Zhong L, Struble EB. Depletion of interfering antibodies in chronic hepatitis C patients and vaccinated chimpanzees reveals broad cross-genotype neutralizing activity. J Infect Dis. 2010;202:862-866. [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 101. | Timm J, Li B, Daniels MG, Bhattacharya T, Reyor LL, Allgaier R, Kuntzen T, Fischer W, Nolan BE, Duncan J. Human leukocyte antigen-associated sequence polymorphisms in hepatitis C virus reveal reproducible immune responses and constraints on viral evolution. Hepatology. 2007;46:339-349. [PubMed] [Cited in This Article: ] |
| 102. | Alatrakchi N, Graham CS, van der Vliet HJ, Sherman KE, Exley MA, Koziel MJ. Hepatitis C virus (HCV)-specific CD8+ cells produce transforming growth factor beta that can suppress HCV-specific T-cell responses. J Virol. 2007;81:5882-5892. [PubMed] [Cited in This Article: ] |
| 103. | Penna A, Pilli M, Zerbini A, Orlandini A, Mezzadri S, Sacchelli L, Missale G, Ferrari C. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology. 2007;45:588-601. [PubMed] [Cited in This Article: ] |
| 104. | Larrubia JR, Lokhande MU, García-Garzón S, Miquel J, González-Praetorius A, Parra-Cid T, Sanz-de-Villalobos E. Persistent hepatitis C virus (HCV) infection impairs HCV-specific cytotoxic T cell reactivity through Mcl-1/Bim imbalance due to CD127 down-regulation. J Viral Hepat. 2013;20:85-94. [PubMed] [Cited in This Article: ] |
| 105. | Sugimoto K, Ikeda F, Stadanlick J, Nunes FA, Alter HJ, Chang KM. Suppression of HCV-specific T cells without differential hierarchy demonstrated ex vivo in persistent HCV infection. Hepatology. 2003;38:1437-1448. [PubMed] [Cited in This Article: ] |
| 106. | Tester I, Smyk-Pearson S, Wang P, Wertheimer A, Yao E, Lewinsohn DM, Tavis JE, Rosen HR. Immune evasion versus recovery after acute hepatitis C virus infection from a shared source. J Exp Med. 2005;201:1725-1731. [PubMed] [Cited in This Article: ] |
| 107. | Chang KM, Rehermann B, McHutchison JG, Pasquinelli C, Southwood S, Sette A, Chisari FV. Immunological significance of cytotoxic T lymphocyte epitope variants in patients chronically infected by the hepatitis C virus. J Clin Invest. 1997;100:2376-2385. [PubMed] [Cited in This Article: ] |
| 108. | Ray SC, Fanning L, Wang XH, Netski DM, Kenny-Walsh E, Thomas DL. Divergent and convergent evolution after a common-source outbreak of hepatitis C virus. J Exp Med. 2005;201:1753-1759. [PubMed] [Cited in This Article: ] |
| 109. | Timm J, Lauer GM, Kavanagh DG, Sheridan I, Kim AY, Lucas M, Pillay T, Ouchi K, Reyor LL, Schulze zur Wiesch J. CD8 epitope escape and reversion in acute HCV infection. J Exp Med. 2004;200:1593-1604. [PubMed] [Cited in This Article: ] |
| 110. | Blackburn SD, Wherry EJ. IL-10, T cell exhaustion and viral persistence. Trends Microbiol. 2007;15:143-146. [PubMed] [Cited in This Article: ] |
| 111. | Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492-499. [PubMed] [Cited in This Article: ] |
| 112. | Larrubia JR, Benito-Martínez S, Miquel J, Calvino M, Sanz-de-Villalobos E, Parra-Cid T. Costimulatory molecule programmed death-1 in the cytotoxic response during chronic hepatitis C. World J Gastroenterol. 2009;15:5129-5140. [PubMed] [Cited in This Article: ] |
| 113. | Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007;81:9249-9258. [PubMed] [Cited in This Article: ] |
| 114. | Nakamoto N, Kaplan DE, Coleclough J, Li Y, Valiga ME, Kaminski M, Shaked A, Olthoff K, Gostick E, Price DA. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology. 2008;134:1927-1937, 1937.e1-2. [PubMed] [Cited in This Article: ] |
| 115. | Nakamoto N, Cho H, Shaked A, Olthoff K, Valiga ME, Kaminski M, Gostick E, Price DA, Freeman GJ, Wherry EJ. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog. 2009;5:e1000313. [PubMed] [Cited in This Article: ] |
| 116. | Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636-645. [PubMed] [Cited in This Article: ] |
| 117. | Boettler T, Spangenberg HC, Neumann-Haefelin C, Panther E, Urbani S, Ferrari C, Blum HE, von Weizsäcker F, Thimme R. T cells with a CD4+CD25+ regulatory phenotype suppress in vitro proliferation of virus-specific CD8+ T cells during chronic hepatitis C virus infection. J Virol. 2005;79:7860-7867. [PubMed] [Cited in This Article: ] |
| 118. | Cabrera R, Tu Z, Xu Y, Firpi RJ, Rosen HR, Liu C, Nelson DR. An immunomodulatory role for CD4(+)CD25(+) regulatory T lymphocytes in hepatitis C virus infection. Hepatology. 2004;40:1062-1071. [PubMed] [Cited in This Article: ] |
| 119. | Rushbrook SM, Ward SM, Unitt E, Vowler SL, Lucas M, Klenerman P, Alexander GJ. Regulatory T cells suppress in vitro proliferation of virus-specific CD8+ T cells during persistent hepatitis C virus infection. J Virol. 2005;79:7852-7859. [PubMed] [Cited in This Article: ] |
| 120. | Manigold T, Shin EC, Mizukoshi E, Mihalik K, Murthy KK, Rice CM, Piccirillo CA, Rehermann B. Foxp3+CD4+CD25+ T cells control virus-specific memory T cells in chimpanzees that recovered from hepatitis C. Blood. 2006;107:4424-4432. [PubMed] [Cited in This Article: ] |
| 121. | Barbaro G, Di Lorenzo G, Asti A, Ribersani M, Belloni G, Grisorio B, Filice G, Barbarini G. Hepatocellular mitochondrial alterations in patients with chronic hepatitis C: ultrastructural and biochemical findings. Am J Gastroenterol. 1999;94:2198-2205. [PubMed] [Cited in This Article: ] |
| 122. | Kageyama F, Kobayashi Y, Kawasaki T, Toyokuni S, Uchida K, Nakamura H. Successful interferon therapy reverses enhanced hepatic iron accumulation and lipid peroxidation in chronic hepatitis C. Am J Gastroenterol. 2000;95:1041-1050. [PubMed] [Cited in This Article: ] |
| 123. | Paradis V, Kollinger M, Fabre M, Holstege A, Poynard T, Bedossa P. In situ detection of lipid peroxidation by-products in chronic liver diseases. Hepatology. 1997;26:135-142. [PubMed] [Cited in This Article: ] |
| 124. | Ruggieri V, Mazzoccoli C, Pazienza V, Andriulli A, Capitanio N, Piccoli C. Hepatitis C virus, mitochondria and auto/mitophagy: exploiting a host defense mechanism. World J Gastroenterol. 2014;20:2624-2633. [PubMed] [Cited in This Article: ] |
| 125. | Dreux M, Chisari FV. Impact of the autophagy machinery on hepatitis C virus infection. Viruses. 2011;3:1342-1357. [PubMed] [Cited in This Article: ] |
| 126. | Dreux M, Gastaminza P, Wieland SF, Chisari FV. The autophagy machinery is required to initiate hepatitis C virus replication. Proc Natl Acad Sci USA. 2009;106:14046-14051. [PubMed] [Cited in This Article: ] |
| 127. | Kim SJ, Syed GH, Siddiqui A. Hepatitis C virus induces the mitochondrial translocation of Parkin and subsequent mitophagy. PLoS Pathog. 2013;9:e1003285. [PubMed] [Cited in This Article: ] |
| 128. | Uchida T, Soe S, Suzuki K, Komatsu K, Shikata T, Iida F, Rikihisa T, Mizuno K. [Epidemic non-A, non-B viral hepatitis--animal model and causative virus]. Nihon Rinsho. 1988;46:2589-2595. [PubMed] [Cited in This Article: ] |
| 129. | Xie ZC, Riezu-Boj JI, Lasarte JJ, Guillen J, Su JH, Civeira MP, Prieto J. Transmission of hepatitis C virus infection to tree shrews. Virology. 1998;244:513-520. [PubMed] [Cited in This Article: ] |
| 130. | Billerbeck E, de Jong Y, Dorner M, de la Fuente C, Ploss A. Animal models for hepatitis C. Curr Top Microbiol Immunol. 2013;369:49-86. [PubMed] [Cited in This Article: ] |
| 131. | Couto LB, Kolykhalov AA. Animal Models for HCV Study. Hepatitis C Viruses: Genomes and Molecular Biology. Norfolk (UK): Horizon Bioscience 2006; Chapter 12. [Cited in This Article: ] |
| 132. | Kremsdorf D, Brezillon N. New animal models for hepatitis C viral infection and pathogenesis studies. World J Gastroenterol. 2007;13:2427-2435. [PubMed] [Cited in This Article: ] |
| 133. | Mailly L, Robinet E, Meuleman P, Baumert TF, Zeisel MB. Hepatitis C virus infection and related liver disease: the quest for the best animal model. Front Microbiol. 2013;4:213. [PubMed] [Cited in This Article: ] |
| 134. | Lanford RE, Bigger C, Bassett S, Klimpel G. The chimpanzee model of hepatitis C virus infections. ILAR J. 2001;42:117-126. [PubMed] [Cited in This Article: ] |
| 135. | Xu X, Chen H, Cao X, Ben K. Efficient infection of tree shrew (Tupaia belangeri) with hepatitis C virus grown in cell culture or from patient plasma. J Gen Virol. 2007;88:2504-2512. [PubMed] [Cited in This Article: ] |
| 136. | Amako Y, Tsukiyama-Kohara K, Katsume A, Hirata Y, Sekiguchi S, Tobita Y, Hayashi Y, Hishima T, Funata N, Yonekawa H. Pathogenesis of hepatitis C virus infection in Tupaia belangeri. J Virol. 2010;84:303-311. [PubMed] [Cited in This Article: ] |
| 137. | García F, García F, Roldán C, López I, Martínez NM, Alvarez M, Bernal MC, Hernández J, Maroto MC. Detection of HCV and GBV-CHGV RNA in peripheral blood mononuclear cells of patients with chronic type C hepatitis. Microbios. 2000;103:7-15. [PubMed] [Cited in This Article: ] |
| 138. | Bartosch B, Dubuisson J, Cosset FL. Infectious hepatitis C virus pseudo-particles containing functional E1-E2 envelope protein complexes. J Exp Med. 2003;197:633-642. [PubMed] [Cited in This Article: ] |
| 139. | Cashman SB, Marsden BD, Dustin LB. The Humoral Immune Response to HCV: Understanding is Key to Vaccine Development. Front Immunol. 2014;5:550. [PubMed] [Cited in This Article: ] |
| 140. | Heller T, Saito S, Auerbach J, Williams T, Moreen TR, Jazwinski A, Cruz B, Jeurkar N, Sapp R, Luo G. An in vitro model of hepatitis C virion production. Proc Natl Acad Sci USA. 2005;102:2579-2583. [PubMed] [Cited in This Article: ] |
| 141. | Yi M, Villanueva RA, Thomas DL, Wakita T, Lemon SM. Production of infectious genotype 1a hepatitis C virus (Hutchinson strain) in cultured human hepatoma cells. Proc Natl Acad Sci USA. 2006;103:2310-2315. [PubMed] [Cited in This Article: ] |
| 142. | Roohvand F, Kossari N. Advances in hepatitis C virus vaccines, part two: advances in hepatitis C virus vaccine formulations and modalities. Expert Opin Ther Pat. 2012;22:391-415. [PubMed] [Cited in This Article: ] |
| 143. | Osburn WO, Fisher BE, Dowd KA, Urban G, Liu L, Ray SC, Thomas DL, Cox AL. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology. 2010;138:315-324. [PubMed] [Cited in This Article: ] |
| 144. | Dahari H, Feinstone SM, Major ME. Meta-analysis of hepatitis C virus vaccine efficacy in chimpanzees indicates an importance for structural proteins. Gastroenterology. 2010;139:965-974. [PubMed] [Cited in This Article: ] |
| 145. | Yutani S, Yamada A, Yoshida K, Takao Y, Tamura M, Komatsu N, Ide T, Tanaka M, Sata M, Itoh K. Phase I clinical study of a personalized peptide vaccination for patients infected with hepatitis C virus (HCV) 1b who failed to respond to interferon-based therapy. Vaccine. 2007;25:7429-7435. [PubMed] [Cited in This Article: ] |
| 146. | Klade CS, Wedemeyer H, Berg T, Hinrichsen H, Cholewinska G, Zeuzem S, Blum H, Buschle M, Jelovcan S, Buerger V. Therapeutic vaccination of chronic hepatitis C nonresponder patients with the peptide vaccine IC41. Gastroenterology. 2008;134:1385-1395. [PubMed] [Cited in This Article: ] |
| 147. | Habersetzer F, Honnet G, Bain C, Maynard-Muet M, Leroy V, Zarski JP, Feray C, Baumert TF, Bronowicki JP, Doffoël M. A poxvirus vaccine is safe, induces T-cell responses, and decreases viral load in patients with chronic hepatitis C. Gastroenterology. 2011;141:890-899.e1-4. [PubMed] [Cited in This Article: ] |
| 148. | Kaneko T, Moriyama T, Udaka K, Hiroishi K, Kita H, Okamoto H, Yagita H, Okumura K, Imawari M. Impaired induction of cytotoxic T lymphocytes by antagonism of a weak agonist borne by a variant hepatitis C virus epitope. Eur J Immunol. 1997;27:1782-1787. [PubMed] [Cited in This Article: ] |
| 149. | Firbas C, Jilma B, Tauber E, Buerger V, Jelovcan S, Lingnau K, Buschle M, Frisch J, Klade CS. Immunogenicity and safety of a novel therapeutic hepatitis C virus (HCV) peptide vaccine: a randomized, placebo controlled trial for dose optimization in 128 healthy subjects. Vaccine. 2006;24:4343-4353. [PubMed] [Cited in This Article: ] |
| 150. | Firbas C, Boehm T, Buerger V, Schuller E, Sabarth N, Jilma B, Klade CS. Immunogenicity and safety of different injection routes and schedules of IC41, a Hepatitis C virus (HCV) peptide vaccine. Vaccine. 2010;28:2397-2407. [PubMed] [Cited in This Article: ] |
| 151. | Klade CS, Schuller E, Boehm T, von Gabain A, Manns MP. Sustained viral load reduction in treatment-naive HCV genotype 1 infected patients after therapeutic peptide vaccination. Vaccine. 2012;30:2943-2950. [PubMed] [Cited in This Article: ] |
| 152. | Yutani S, Komatsu N, Shichijo S, Yoshida K, Takedatsu H, Itou M, Kuromatu R, Ide T, Tanaka M, Sata M. Phase I clinical study of a peptide vaccination for hepatitis C virus-infected patients with different human leukocyte antigen-class I-A alleles. Cancer Sci. 2009;100:1935-1942. [PubMed] [Cited in This Article: ] |
| 153. | Choo QL, Kuo G, Ralston R, Weiner A, Chien D, Van Nest G, Han J, Berger K, Thudium K, Kuo C. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc Natl Acad Sci USA. 1994;91:1294-1298. [PubMed] [Cited in This Article: ] |
| 154. | Houghton M, Abrignani S. Prospects for a vaccine against the hepatitis C virus. Nature. 2005;436:961-966. [PubMed] [Cited in This Article: ] |
| 155. | Puig M, Major ME, Mihalik K, Feinstone SM. Immunization of chimpanzees with an envelope protein-based vaccine enhances specific humoral and cellular immune responses that delay hepatitis C virus infection. Vaccine. 2004;22:991-1000. [PubMed] [Cited in This Article: ] |
| 156. | Rollier C, Depla E, Drexhage JA, Verschoor EJ, Verstrepen BE, Fatmi A, Brinster C, Fournillier A, Whelan JA, Whelan M. Control of heterologous hepatitis C virus infection in chimpanzees is associated with the quality of vaccine-induced peripheral T-helper immune response. J Virol. 2004;78:187-196. [PubMed] [Cited in This Article: ] |
| 157. | Youn JW, Park SH, Lavillette D, Cosset FL, Yang SH, Lee CG, Jin HT, Kim CM, Shata MT, Lee DH. Sustained E2 antibody response correlates with reduced peak viremia after hepatitis C virus infection in the chimpanzee. Hepatology. 2005;42:1429-1436. [PubMed] [Cited in This Article: ] |
| 158. | Puig M, Mihalik K, Tilton JC, Williams O, Merchlinsky M, Connors M, Feinstone SM, Major ME. CD4+ immune escape and subsequent T-cell failure following chimpanzee immunization against hepatitis C virus. Hepatology. 2006;44:736-745. [PubMed] [Cited in This Article: ] |
| 159. | Fuller MJ, Callendret B, Zhu B, Freeman GJ, Hasselschwert DL, Satterfield W, Sharpe AH, Dustin LB, Rice CM, Grakoui A. Immunotherapy of chronic hepatitis C virus infection with antibodies against programmed cell death-1 (PD-1). Proc Natl Acad Sci USA. 2013;110:15001-15006. [PubMed] [Cited in This Article: ] |
| 160. | Leroux-Roels G, Depla E, Hulstaert F, Tobback L, Dincq S, Desmet J, Desombere I, Maertens G. A candidate vaccine based on the hepatitis C E1 protein: tolerability and immunogenicity in healthy volunteers. Vaccine. 2004;22:3080-3086. [PubMed] [Cited in This Article: ] |
| 161. | Frey SE, Houghton M, Coates S, Abrignani S, Chien D, Rosa D, Pileri P, Ray R, Di Bisceglie AM, Rinella P. Safety and immunogenicity of HCV E1E2 vaccine adjuvanted with MF59 administered to healthy adults. Vaccine. 2010;28:6367-6373. [PubMed] [Cited in This Article: ] |
| 162. | Verstrepen BE, Depla E, Rollier CS, Mares G, Drexhage JA, Priem S, Verschoor EJ, Koopman G, Granier C, Dreux M. Clearance of genotype 1b hepatitis C virus in chimpanzees in the presence of vaccine-induced E1-neutralizing antibodies. J Infect Dis. 2011;204:837-844. [PubMed] [Cited in This Article: ] |
| 163. | Drane D, Maraskovsky E, Gibson R, Mitchell S, Barnden M, Moskwa A, Shaw D, Gervase B, Coates S, Houghton M. Priming of CD4+ and CD8+ T cell responses using a HCV core ISCOMATRIX vaccine: a phase I study in healthy volunteers. Hum Vaccin. 2009;5:151-157. [PubMed] [Cited in This Article: ] |
| 164. | Nevens F, Roskams T, Van Vlierberghe H, Horsmans Y, Sprengers D, Elewaut A, Desmet V, Leroux-Roels G, Quinaux E, Depla E. A pilot study of therapeutic vaccination with envelope protein E1 in 35 patients with chronic hepatitis C. Hepatology. 2003;38:1289-1296. [PubMed] [Cited in This Article: ] |
| 165. | Wedemeyer H, Mazur W, Nevens F, Horsmans Y, Adler M, Blum H, Inglot M, Gerken G, Janczewska E, Roskams T. Factors influencing progression of liver fibrosis in patients with chronic hepatitis C: Results of the 3-year T2S-918-HCV study with HCVE1 therapeutic vaccination. J Hepatol. 2008;48:S27-S28. [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 166. | Luo Y, Jiang L, Mao Z. Recent Advances in Clinical Trials of HCV Vaccines. J Applied Virol. 2014;3:10-24. [Cited in This Article: ] |
| 167. | Habersetzer F, Baumert TF, Stoll-Keller F. GI-5005, a yeast vector vaccine expressing an NS3-core fusion protein for chronic HCV infection. Curr Opin Mol Ther. 2009;11:456-462. [PubMed] [Cited in This Article: ] |
| 168. | Pockros P, Jacobson I, Boyer TD. GI-5005 therapeutic vaccine plus pegIFN/ribavirin improves sustained virologic response versus pegIFN/ribavirin in prior non-responders with genotype 1 chronic HCV infection. Hepatology. 2010;52:107A. [Cited in This Article: ] |
| 169. | Davis BS, Chang GJ, Cropp B, Roehrig JT, Martin DA, Mitchell CJ, Bowen R, Bunning ML. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J Virol. 2001;75:4040-4047. [PubMed] [Cited in This Article: ] |
| 170. | Chong SY, Egan MA, Kutzler MA, Megati S, Masood A, Roopchard V, Garcia-Hand D, Montefiori DC, Quiroz J, Rosati M. Comparative ability of plasmid IL-12 and IL-15 to enhance cellular and humoral immune responses elicited by a SIVgag plasmid DNA vaccine and alter disease progression following SHIV(89.6P) challenge in rhesus macaques. Vaccine. 2007;25:4967-4982. [PubMed] [Cited in This Article: ] |
| 171. | Boyer JD, Robinson TM, Kutzler MA, Vansant G, Hokey DA, Kumar S, Parkinson R, Wu L, Sidhu MK, Pavlakis GN. Protection against simian/human immunodeficiency virus (SHIV) 89.6P in macaques after coimmunization with SHIV antigen and IL-15 plasmid. Proc Natl Acad Sci USA. 2007;104:18648-18653. [PubMed] [Cited in This Article: ] |
| 172. | Boyer JD, Robinson TM, Kutzler MA, Parkinson R, Calarota SA, Sidhu MK, Muthumani K, Lewis M, Pavlakis G, Felber B. SIV DNA vaccine co-administered with IL-12 expression plasmid enhances CD8 SIV cellular immune responses in cynomolgus macaques. J Med Primatol. 2005;34:262-270. [PubMed] [Cited in This Article: ] |
| 173. | Wang B, Boyer J, Srikantan V, Coney L, Carrano R, Phan C, Merva M, Dang K, Agadjanan M, Gilbert L. DNA inoculation induces neutralizing immune responses against human immunodeficiency virus type 1 in mice and nonhuman primates. DNA Cell Biol. 1993;12:799-805. [PubMed] [Cited in This Article: ] |
| 174. | Davis HL, Whalen RG, Demeneix BA. Direct gene transfer into skeletal muscle in vivo: factors affecting efficiency of transfer and stability of expression. Hum Gene Ther. 1993;4:151-159. [PubMed] [Cited in This Article: ] |
| 175. | Alvarez-Lajonchere L, Shoukry NH, Grá B, Amador-Cañizares Y, Helle F, Bédard N, Guerra I, Drouin C, Dubuisson J, González-Horta EE. Immunogenicity of CIGB-230, a therapeutic DNA vaccine preparation, in HCV-chronically infected individuals in a Phase I clinical trial. J Viral Hepat. 2009;16:156-167. [PubMed] [Cited in This Article: ] |
| 176. | Castellanos M, Cinza Z, Dorta Z, Veliz G, Vega H, Lorenzo I, Ojeda S, Dueñas-Carrera S, Alvarez-Lajonchere L, Martínez G. Immunization with a DNA vaccine candidate in chronic hepatitis C patients is safe, well tolerated and does not impair immune response induction after anti-hepatitis B vaccination. J Gene Med. 2010;12:107-116. [PubMed] [Cited in This Article: ] |
| 177. | Ahlen G, Nystrom J, Pult I, Frelin L, Hultgren C, Sallberg M. In vivo clearance of hepatitis C virus nonstructural 3/4A-expressing hepatocytes by DNA vaccine-primed cytotoxic T lymphocytes. J Infect Dis. 2005;192:2112-2116. [PubMed] [Cited in This Article: ] |
| 178. | Frelin L, Ahlén G, Alheim M, Weiland O, Barnfield C, Liljeström P, Sällberg M. Codon optimization and mRNA amplification effectively enhances the immunogenicity of the hepatitis C virus nonstructural 3/4A gene. Gene Ther. 2004;11:522-533. [PubMed] [Cited in This Article: ] |
| 179. | Sallberg MM, Frelin L, Diepolder H. A first clinical trial of therapeutic vaccination using naked DNA delivered by in vivo electroporation shows antiviral effects in patients with chronic hepatitis C. J Hepatol. 2009;50:S18-S19. [Cited in This Article: ] |
| 180. | Gale M, Foy EM. Evasion of intracellular host defence by hepatitis C virus. Nature. 2005;436:939-945. [PubMed] [Cited in This Article: ] |
| 181. | Foy E, Li K, Sumpter R, Loo YM, Johnson CL, Wang C, Fish PM, Yoneyama M, Fujita T, Lemon SM. Control of antiviral defenses through hepatitis C virus disruption of retinoic acid-inducible gene-I signaling. Proc Natl Acad Sci USA. 2005;102:2986-2991. [PubMed] [Cited in This Article: ] |
| 182. | Kaukinen P, Sillanpää M, Kotenko S, Lin R, Hiscott J, Melén K, Julkunen I. Hepatitis C virus NS2 and NS3/4A proteins are potent inhibitors of host cell cytokine/chemokine gene expression. Virol J. 2006;3:66. [PubMed] [Cited in This Article: ] |
| 183. | Morikawa K, Lange CM, Gouttenoire J, Meylan E, Brass V, Penin F, Moradpour D. Nonstructural protein 3-4A: the Swiss army knife of hepatitis C virus. J Viral Hepat. 2011;18:305-315. [PubMed] [Cited in This Article: ] |
| 184. | Robert F, Pelletier J. Perturbations of RNA helicases in cancer. Wiley Interdiscip Rev RNA. 2013;4:333-349. [PubMed] [Cited in This Article: ] |
| 185. | Ratnoglik SL, Jiang DP, Aoki C, Sudarmono P, Shoji I, Deng L, Hotta H. Induction of cell-mediated immune responses in mice by DNA vaccines that express hepatitis C virus NS3 mutants lacking serine protease and NTPase/RNA helicase activities. PLoS One. 2014;9:e98877. [PubMed] [Cited in This Article: ] |
| 186. | Bråve A, Ljungberg K, Wahren B, Liu MA. Vaccine delivery methods using viral vectors. Mol Pharm. 2007;4:18-32. [PubMed] [Cited in This Article: ] |
| 187. | Casimiro DR, Chen L, Fu TM, Evans RK, Caulfield MJ, Davies ME, Tang A, Chen M, Huang L, Harris V. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J Virol. 2003;77:6305-6313. [PubMed] [Cited in This Article: ] |
| 188. | Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, Zhang ZQ, Simon AJ, Trigona WL, Dubey SA. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415:331-335. [PubMed] [Cited in This Article: ] |
| 189. | Tatsis N, Ertl HC. Adenoviruses as vaccine vectors. Mol Ther. 2004;10:616-629. [PubMed] [Cited in This Article: ] |
| 190. | Capone S, Meola A, Ercole BB, Vitelli A, Pezzanera M, Ruggeri L, Davies ME, Tafi R, Santini C, Luzzago A. A novel adenovirus type 6 (Ad6)-based hepatitis C virus vector that overcomes preexisting anti-ad5 immunity and induces potent and broad cellular immune responses in rhesus macaques. J Virol. 2006;80:1688-1699. [PubMed] [Cited in This Article: ] |
| 191. | Folgori A, Capone S, Ruggeri L, Meola A, Sporeno E, Ercole BB, Pezzanera M, Tafi R, Arcuri M, Fattori E. A T-cell HCV vaccine eliciting effective immunity against heterologous virus challenge in chimpanzees. Nat Med. 2006;12:190-197. [PubMed] [Cited in This Article: ] |
| 192. | Colloca S, Barnes E, Folgori A, Ammendola V, Capone S, Cirillo A, Siani L, Naddeo M, Grazioli F, Esposito ML. Vaccine vectors derived from a large collection of simian adenoviruses induce potent cellular immunity across multiple species. Sci Transl Med. 2012;4:115ra2. [PubMed] [Cited in This Article: ] |
| 193. | Barnes E, Folgori A, Capone S, Swadling L, Aston S, Kurioka A, Meyer J, Huddart R, Smith K, Townsend R. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci Transl Med. 2012;4:115ra1. [PubMed] [Cited in This Article: ] |
| 194. | Fattori E, Zampaglione I, Arcuri M, Meola A, Ercole BB, Cirillo A, Folgori A, Bett A, Cappelletti M, Sporeno E. Efficient immunization of rhesus macaques with an HCV candidate vaccine by heterologous priming-boosting with novel adenoviral vectors based on different serotypes. Gene Ther. 2006;13:1088-1096. [PubMed] [Cited in This Article: ] |
| 195. | Dangoor A, Lorigan P, Keilholz U, Schadendorf D, Harris A, Ottensmeier C, Smyth J, Hoffmann K, Anderson R, Cripps M. Clinical and immunological responses in metastatic melanoma patients vaccinated with a high-dose poly-epitope vaccine. Cancer Immunol Immunother. 2010;59:863-873. [PubMed] [Cited in This Article: ] |
| 196. | Sandström E, Nilsson C, Hejdeman B, Bråve A, Bratt G, Robb M, Cox J, Vancott T, Marovich M, Stout R. Broad immunogenicity of a multigene, multiclade HIV-1 DNA vaccine boosted with heterologous HIV-1 recombinant modified vaccinia virus Ankara. J Infect Dis. 2008;198:1482-1490. [PubMed] [Cited in This Article: ] |
| 197. | Scriba TJ, Tameris M, Smit E, van der Merwe L, Hughes EJ, Kadira B, Mauff K, Moyo S, Brittain N, Lawrie A. A phase IIa trial of the new tuberculosis vaccine, MVA85A, in HIV- and/or Mycobacterium tuberculosis-infected adults. Am J Respir Crit Care Med. 2012;185:769-778. [PubMed] [Cited in This Article: ] |
| 198. | Harrop R, Connolly N, Redchenko I, Valle J, Saunders M, Ryan MG, Myers KA, Drury N, Kingsman SM, Hawkins RE. Vaccination of colorectal cancer patients with modified vaccinia Ankara delivering the tumor antigen 5T4 (TroVax) induces immune responses which correlate with disease control: a phase I/II trial. Clin Cancer Res. 2006;12:3416-3424. [PubMed] [Cited in This Article: ] |
| 199. | Mayr A, Stickl H, Müller HK, Danner K, Singer H. [The smallpox vaccination strain MVA: marker, genetic structure, experience gained with the parenteral vaccination and behavior in organisms with a debilitated defence mechanism (author’s transl)]. Zentralbl Bakteriol B. 1978;167:375-390. [PubMed] [Cited in This Article: ] |
| 200. | Sutter G, Moss B. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc Natl Acad Sci USA. 1992;89:10847-10851. [PubMed] [Cited in This Article: ] |
| 201. | Rollier CS, Paranhos-Baccala G, Verschoor EJ, Verstrepen BE, Drexhage JA, Fagrouch Z, Berland JL, Komurian-Pradel F, Duverger B, Himoudi N. Vaccine-induced early control of hepatitis C virus infection in chimpanzees fails to impact on hepatic PD-1 and chronicity. Hepatology. 2007;45:602-613. [PubMed] [Cited in This Article: ] |
| 202. | Fournillier A, Gerossier E, Evlashev A, Schmitt D, Simon B, Chatel L, Martin P, Silvestre N, Balloul JM, Barry R. An accelerated vaccine schedule with a poly-antigenic hepatitis C virus MVA-based candidate vaccine induces potent, long lasting and in vivo cross-reactive T cell responses. Vaccine. 2007;25:7339-7353. [PubMed] [Cited in This Article: ] |
| 203. | Habersetzer F, Zarski JP, Leroy V, Maynard-Muet M, Bronowicki JP, Feray C, Hézode C, Fournillier A, Bain C, Inchauspé G. A novel vectorized HCV therapeutic vaccine (TG4040): Results of a phase I study in naive patients chronically infected by HCV. J Hepatol. 2009;50:S18. [DOI] [Cited in This Article: ] |
| 204. | Pancholi P, Perkus M, Tricoche N, Liu Q, Prince AM. DNA immunization with hepatitis C virus (HCV) polycistronic genes or immunization by HCV DNA priming-recombinant canarypox virus boosting induces immune responses and protection from recombinant HCV-vaccinia virus infection in HLA-A2.1-transgenic mice. J Virol. 2003;77:382-390. [PubMed] [Cited in This Article: ] |
| 205. | Pancholi P, Liu Q, Tricoche N, Zhang P, Perkus ME, Prince AM. DNA prime-canarypox boost with polycistronic hepatitis C virus (HCV) genes generates potent immune responses to HCV structural and nonstructural proteins. J Infect Dis. 2000;182:18-27. [PubMed] [Cited in This Article: ] |
| 206. | Perkus ME, Limbach K, Paoletti E. Cloning and expression of foreign genes in vaccinia virus, using a host range selection system. J Virol. 1989;63:3829-3836. [PubMed] [Cited in This Article: ] |
| 207. | Youn JW, Hu YW, Tricoche N, Pfahler W, Shata MT, Dreux M, Cosset FL, Folgori A, Lee DH, Brotman B. Evidence for protection against chronic hepatitis C virus infection in chimpanzees by immunization with replicating recombinant vaccinia virus. J Virol. 2008;82:10896-10905. [PubMed] [Cited in This Article: ] |
| 208. | Kao JH, Chen DS. Global control of hepatitis B virus infection. Lancet Infect Dis. 2002;2:395-403. [PubMed] [Cited in This Article: ] |
| 209. | Papaevangelou G, Dandolos E, Roumeliotou-Karayannis A, Richardson SC. Immunogenicity of recombinant hepatitis B vaccine. Lancet. 1985;1:455-456. [PubMed] [Cited in This Article: ] |
| 210. | Giannini SL, Hanon E, Moris P, Van Mechelen M, Morel S, Dessy F, Fourneau MA, Colau B, Suzich J, Losonksy G. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine. 2006;24:5937-5949. [PubMed] [Cited in This Article: ] |
| 211. | Baumert TF, Ito S, Wong DT, Liang TJ. Hepatitis C virus structural proteins assemble into viruslike particles in insect cells. J Virol. 1998;72:3827-3836. [PubMed] [Cited in This Article: ] |
| 212. | Baumert TF, Vergalla J, Satoi J, Thomson M, Lechmann M, Herion D, Greenberg HB, Ito S, Liang TJ. Hepatitis C virus-like particles synthesized in insect cells as a potential vaccine candidate. Gastroenterology. 1999;117:1397-1407. [PubMed] [Cited in This Article: ] |
| 213. | Murata K, Lechmann M, Qiao M, Gunji T, Alter HJ, Liang TJ. Immunization with hepatitis C virus-like particles protects mice from recombinant hepatitis C virus-vaccinia infection. Proc Natl Acad Sci USA. 2003;100:6753-6758. [PubMed] [Cited in This Article: ] |
| 214. | Jeong SH, Qiao M, Nascimbeni M, Hu Z, Rehermann B, Murthy K, Liang TJ. Immunization with hepatitis C virus-like particles induces humoral and cellular immune responses in nonhuman primates. J Virol. 2004;78:6995-7003. [PubMed] [Cited in This Article: ] |
| 215. | Elmowalid GA, Qiao M, Jeong SH, Borg BB, Baumert TF, Sapp RK, Hu Z, Murthy K, Liang TJ. Immunization with hepatitis C virus-like particles results in control of hepatitis C virus infection in chimpanzees. Proc Natl Acad Sci USA. 2007;104:8427-8432. [PubMed] [Cited in This Article: ] |
| 216. | Gowans EJ, Roberts S, Jones K, Dinatale I, Latour PA, Chua B, Eriksson EM, Chin R, Li S, Wall DM. A phase I clinical trial of dendritic cell immunotherapy in HCV-infected individuals. J Hepatol. 2010;53:599-607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 217. | Wedemeyer H, Gagneten S, Davis A, Bartenschlager R, Feinstone S, Rehermann B. Oral immunization with HCV-NS3-transformed Salmonella: induction of HCV-specific CTL in a transgenic mouse model. Gastroenterology. 2001;121:1158-1166. [PubMed] [Cited in This Article: ] |
| 218. | Cao J, Chen Z, Ren Y, Luo Y, Cao M, Lu W, Zhao P, Qi Z. Oral immunization with attenuated Salmonella carrying a co-expression plasmid encoding the core and E2 proteins of hepatitis C virus capable of inducing cellular immune responses and neutralizing antibodies in mice. Vaccine. 2011;29:3714-3723. [PubMed] [Cited in This Article: ] |
| 219. | Batdelger D, Dandii D, Jirathitikal V, Bourinbaiar AS. Open-label trial of therapeutic immunization with oral V-5 Immunitor (V5) vaccine in patients with chronic hepatitis C. Vaccine. 2008;26:2733-2737. [PubMed] [Cited in This Article: ] |
| 220. | Nemchinov LG, Liang TJ, Rifaat MM, Mazyad HM, Hadidi A, Keith JM. Development of a plant-derived subunit vaccine candidate against hepatitis C virus. Arch Virol. 2000;145:2557-2573. [PubMed] [Cited in This Article: ] |
| 221. | Nuzzaci M, Vitti A, Condelli V, Lanorte MT, Tortorella C, Boscia D, Piazzolla P, Piazzolla G. In vitro stability of Cucumber mosaic virus nanoparticles carrying a Hepatitis C virus-derived epitope under simulated gastrointestinal conditions and in vivo efficacy of an edible vaccine. J Virol Methods. 2010;165:211-215. [PubMed] [Cited in This Article: ] |
| 222. | Patient R, Hourioux C, Vaudin P, Pagès JC, Roingeard P. Chimeric hepatitis B and C viruses envelope proteins can form subviral particles: implications for the design of new vaccine strategies. N Biotechnol. 2009;25:226-234. [PubMed] [Cited in This Article: ] |
| 223. | Wu CL, Leu TS, Chang TT, Shiau AL. Hepatitis C virus core protein fused to hepatitis B virus core antigen for serological diagnosis of both hepatitis C and hepatitis B infections by ELISA. J Med Virol. 1999;57:104-110. [PubMed] [Cited in This Article: ] |
| 224. | Mihailova M, Boos M, Petrovskis I, Ose V, Skrastina D, Fiedler M, Sominskaya I, Ross S, Pumpens P, Roggendorf M. Recombinant virus-like particles as a carrier of B- and T-cell epitopes of hepatitis C virus (HCV). Vaccine. 2006;24:4369-4377. [PubMed] [Cited in This Article: ] |
| 225. | Selby M, Glazer E, Houghton M. HBV/HCV virus-like particle. : Google Patents 2004; . [Cited in This Article: ] |
| 226. | Beaumont E, Patient R, Hourioux C, Dimier-Poisson I, Roingeard P. Chimeric hepatitis B virus/hepatitis C virus envelope proteins elicit broadly neutralizing antibodies and constitute a potential bivalent prophylactic vaccine. Hepatology. 2013;57:1303-1313. [PubMed] [Cited in This Article: ] |
| 227. | Forns X, Payette PJ, Ma X, Satterfield W, Eder G, Mushahwar IK, Govindarajan S, Davis HL, Emerson SU, Purcell RH. Vaccination of chimpanzees with plasmid DNA encoding the hepatitis C virus (HCV) envelope E2 protein modified the infection after challenge with homologous monoclonal HCV. Hepatology. 2000;32:618-625. [PubMed] [Cited in This Article: ] |