Published online Nov 7, 2015. doi: 10.3748/wjg.v21.i41.11854
Peer-review started: February 10, 2015
First decision: April 23, 2015
Revised: June 9, 2015
Accepted: August 31, 2015
Article in press: August 31, 2015
Published online: November 7, 2015
AIM: To make orthotopic colon cancer murine models a more clearly understood subject. The orthotopic tumor models have been found to be more relevant in replicating the human disease process as compared to heterotopic models, many techniques for making orthotopic colorectal murine models have been reported.
METHODS: We evaluated the current literature for various reported orthotopic colon cancer models to understand their techniques, advantages and limitations. An extensive literature review was performed by searching the National Library of Medicine Database (PubMed) using MeSH terms animal model; colon cancer; orthotopic model; murine model. Twenty studies related to colon cancer orthotopic xenograft model were evaluated in detail and discussed here.
RESULTS: The detailed analysis of all relevant reports on orthotopic model showed tumor take rate between 42%-100%. While models using the enema technique and minimally invasive technique have reported development of tumor from mucosa with tumor take rate between 87%-100% with metastasis in 76%-90%.
CONCLUSION: Over the years, the increased understanding of the murine models of human colon cancer has resulted in the development of various models. Each reported model has some limitations. These latest models have opened up new doors for continuing cancer research for not only understanding the colon cancer pathogenesis but also aid in the development of newer chemotherapeutic drugs as they mimic the human disease closely.
Core tip: The murine models of colon cancer represent an important tool for understanding the etiopathogenesis and evaluating management strategies for colorectal cancer, thus representing a resource of immense potential in cancer research. Over the years, the increased understanding of the murine models of human colon cancer have resulted in the development of various models. We evaluated the current literature for various reported orthotopic colon cancer models. Our paper discusses the techniques, results, advantages and limitations of the presently available murine models of colorectal cancer so that a researcher can choose an appropriate colorectal cancer murine model which fits their research goals.
- Citation: Mittal VK, Bhullar JS, Jayant K. Animal models of human colorectal cancer: Current status, uses and limitations. World J Gastroenterol 2015; 21(41): 11854-11861
- URL: https://www.wjgnet.com/1007-9327/full/v21/i41/11854.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i41.11854
National Cancer Institute and SEER (Surveillance, Epidemiology and End Result) Program 2011, reported the incidence of colon cancer as 43.3 and the number of people dying from colon cancer as 15.9 per 100000 men and women per year. Colon cancer remains the third most common cancer among men as well as women and 2nd leading cause of cancer related deaths in United States[1].
Recently, there have been many advances in the understanding of colon cancer epidemiology, pathogenesis, pathology, chemoprevention and therapeutic options, which seem to have emanated from continuing basic and clinical research. Host genetic factors play a critical role in the pathophysiology of most human cancers. The immense biological complexity of colon cancer has kindled the development of more apt research design that could simulate in a natural and spontaneous fashion the pathophysiologic features of cancer biology. Rodent models have many desired attributes and they share a wide variety of characteristics with human that has proven to be important in comprehending many complex molecular facets of colon cancer[2]. They also act as an invaluable tool in the development of newer chemotherapeutic drugs. This has made the laboratory mouse (Mus musculus) as one of the most attractive entity in oncologic research[3,4].
Thus there is a constant endeavor to develop an animal model that closely mimics the malignant disease process of humans. Although many animal models were tried but as per NCI data only two animal models based on breast and colon cancer histology are successfully used in preclinical trials[5,6]. Together with that further studies have displayed that the human xenograft models have shown better results than the murine allograft models in drug development. Till date more than 100000 rodents have been sacrificed for the development of chemotherapeutic drugs. Along with that, this fact also remains that, millions of people all over the world are alive today because of the animal research[7-9].
Colorectal cancer basic research has grown itself on the animal models, which have now become the pillars for understanding the pathogenesis and for developing newer chemotherapeutic drugs. The murine models depict a resource of immense potential, as an intricate disorder like colon cancer can be simultaneously witnessed and manipulated by the researchers. Our knowledge in this field has evolved a lot, and many mouse models have been reported, but each has certain limitations as there is no spontaneous colon cancer and a carcinogen is required for tumor induction in the rodents. Also, many of the mouse models have inter-animal variability in the development of tumors in the intestine. In spite of that animal models have become an important tool in better understanding the effect of genetic alterations on the disease process[10-12].
We reviewed the literature for evaluating the different techniques of developing orthotopic xenograft murine colorectal cancer models, their advantages and limitations. This was done to offer the investigators an appropriate model for research in order to obtain robust and translatable data to aid further understanding of colorectal cancer.
An extensive review of the literature on murine models of colon carcinogenesis was performed by searching the National Library of Medicine Database (PubMed) on 12/28/2014. The MEDLINE search was made using MeSH terms: animal model; chemoprevention; colon-carcinogenesis; min-mice; colon cancer; colorectal cancer; xenograft, heterotopic model, orthotopic model, murine model. We found a total of 5622 literatures by searching above key words. All the 477 relevant articles were analyzed and interpreted in detail and of these 20 manuscripts pertaining to colon cancer orthotopic xenograft model were included in this review.
Based on detailed analysis of all relevant reports on orthotopic colorectal cancer xenograft model, we categorized them into different groups according to the techniques used to create these models. The outcomes using different techniques in various studies are discussed in Table 1.
| Technique | Author | Year | Mice type | Cell-line used | Tumor development (%)/origin | Lymph node metastasis (%) | Distant metastasis (%) |
| Open surgical | Ogata | 1998 | Nude | Human colon carcinoma KM12 SM cells | 100/Mural | 25 | 10 (Lung) 50 (Liver) |
| Hackl | 2012 | Nude | Human colon cancer cells, transfected with hCG and luciferase | 87.5-100/Mural | 50 | 25 (Lung) 50 (Liver) | |
| Priolli | 2012 | Nude | Colorectal adenocarcinoma cell line (CCL-218) | 42.8/Mural | 00 | 00 | |
| Enema model | Takahashi | 2004 | Nude | Human colon cancer cells, LS174T | 95/Mucosal | NS | NS |
| Kishimoto | 2013 | Nude | Mouse rectal cancer cells, expressing green fluorescent protein (GFP) | 100/Mucosal | 90 | 90 (Lung) 09 (Liver) | |
| Microinjection | Donigan | 2009 | Nude | Murine colon cancer (CT26) cells | 65/Mural | NA | 3.3 |
| Zigmond | 2011 | Nude | Murine colon cancer C57BL/6 CRC cells | 95/Mural | NA | NA | |
| Human colon cancer cells SW620, SW480 and LS174T | |||||||
| Transanal low dose electrocoagulation | Bhullar | 2010 | Nude/SCID | Murine cell line CRL-2639, CRL-2638 | 92-100/Mucosal | 50-100 | 41-50 |
| Human cell line HT-29, LS-174T | 58-100/Mucosal | 33-83 | 0-83.33 |
Ogata et al[13] in 1998, used in vivo KM12 SM cell lines and injected them into cecal wall of nude mice through an open surgical technique for making the orthotopic xenograft model. They reported tumor take rate of 100% and metastasis to the regional mesenteric lymph nodes in 25% and to liver in 50%.
Hackl et al[14] in 2012, injected human colon cancer cell lines HT 29 and HCT 116 transfected with human chorionic gonadotropin (b-hCG) and luciferase, orthotopically into the caecal wall of severe combined immunodeficient (SCID) mice. The developing tumor produces b-hCG and luminescent protein luciferin. The levels of these markers correlate with tumor burden, completeness of resection and recurrence. They reported tumour take rates between 87% to 100%, metastases to lymph nodes and liver in 50% and lungs in 25% following intracaecal cell injection.
Priolli et al[15] in 2012, also used the open surgical method for colonic diversion with distal fistula formation. This was followed by injecting of WiDR colorectal (CCL-218) adenocarcinoma cell line into the submucosa of the fistula made in the mice. Tumor growth was reported in 42.8% mice with metastasis in none.
Takahashi et al[16] in 2004, developed a technique by inducing short term colitis in nude mice by an irritant agent, 3% dextran sulfate sodium (DSS) followed by instillation of human colon cancer cells LS174T transanally. They reported a tumor take rate of 95% in rectum after 2 wk but could not observe any significant metastasis.
Kishimoto et al[17] in 2013, used 4% acetic acid solution for two minutes, followed by flushing with 6 ml phosphate buffered saline (PBS) in order to disrupt the epithelial cell layer of the distal rectal mucosa followed by mouse colorectal cancer cell line CT-26 and the human colorectal cancer cell line HCT-116 cells, expressing green fluorescent protein (GFP) were instilled transanally. Authors noted rectal tumor development in 100% of the mice. Spontaneous lymph node metastasis and lung metastasis were found in over 90% of mice.
Donigan et al[18] in 2009, used an optical microscope, to inject murine colon cancer (CT-26) cells into the rectal wall of the nude mice under magnification (10-100 ×) with an overall tumor take rate of 65% and distant metastasis in 3.3%.
Using a similar technique, Zigmond et al[19] in 2011 reported a murine model in which the murine and human colon cancer tumor cells - C57BL/6 CRC tumor cells and SW620, SW480 and LS174T respectively were injected into the wall of distal rectum through a murine colonoscope (Coloview- Karl Storz). They reported tumor take rate of 95% with no metastasis.
Bhullar et al[20] in 2010, transanally instilled human (LS-174T and HT-29) and murine (CRL-2638 and CRL-2639) colon cancer cell lines in SCID and nude mice, after transanal low dose mucosal electrocoagulation of the colon. Overall tumors developed in 87.5% of mice (42/48) i.e., 12 of 12 and 11 of 12 mice with murine tumor lines (CRL-2638 and CRL-2639, respectively) and in 7 of 12 and 12 of 12 mice with human tumor lines (HT-29 and LS-174T, respectively). While overall lymph nodal and distant metastasis was found in 66.66% cases (32/48) i.e., 12 of 12 and 6 of 12 mice with murine tumor lines (CRL-2638 and CRL-2639, respectively) and in 10 of 12 and 4 of 12 mice with human tumor lines (HT-29 and LS-174T, respectively).
After reviewing all the current literature related to orthotopic xenograft murine colorectal cancer models in detail, it is clear that there has been enormous development in the murine models over time. The latest models are more applicative with high tumor take rates, but still they have some limitations.
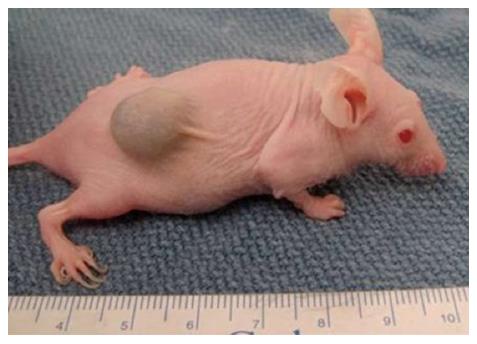
The historical murine cancer models were xenograft heterotopic models which laid the foundation for the development of newer orthotopic models. These xenograft heterotopic models were traditionally made by subcutaneous implantation of human colon cancer cells into either, nude T-cell deficient mice or NOD SCID mice (non-obese diabetic/severe combined immunodeficiency). The formed tumor is located externally in this model, so, its growth can be easily and accurately monitored (Figure 1).
Though making a heterotopic model is easy, but it has multiple pitfalls. It does not mimic the human disease process because of the extra - anatomical location of the tumor and the absence of metastasis from the subcutaneous location made the model inappropriate for study of the spontaneous metastatic process. In the present scenario, the only role of the heterotopic model is its use in making the orthotopic model. Subcutaneously grown tumor is resected after euthanizing the animal and small parts of the tumor are then embedded on the colon to make the orthotopic model[21,22]. The technically more advanced orthotopic xenograft murine models are formed by implanting colorectal cancer cells into colon and they are superior to the traditional subcutaneous xenograft tumor models. The orthotopic model resembles the entire spectrum of colorectal cancer ranging from in situ tumor to metastatic tumor. Commercially available cell lines can be used for making the orthotopic tumors, but these cell lines are altered by years of cultures in vitro, so injecting cells directly from tumors is preferred[23-25].
As discussed earlier, there are various techniques for instilling cancer cells into colon or rectum of murine animal models.
To begin with, investigators have used open surgery methods of implanting tumor cells with many variations to study colorectal tumorigenesis in animal models. Pocard et al[26] in 1996 made subcutaneous xenografts by injection of cancer cells subcutaneously in flanks of the mice. When the tumors acquired a size of > 1 cm3, the mice were euthanized and the tumor sliced into pieces measuring 2 mm × 2 mm × 2 mm. These sections were later implanted under anesthesia through a midline abdominal incision to the serosal surface of cecum and fixed with a stitch and abdomen closed. Identically, in another model, the tumor cells were injected into colonic submucosa from the cecal serosa rather than fixing with a stitch..
Ogata et al[13] in 1998, followed the similar technique for orthotopic inoculation of human colonic cancer cell lines KM12 SM into the cecal wall of nude mice. They found tumors growing in 100% mice and metastasis to the regional mesenteric lymph nodes in 25% and to liver in 50%[27-29].
The open orthotopic models have multiple downsides. The initial immune response following the open and minimally invasive colon resections checks its authenticity. They do not cause mesenteric and retroperitoneal lymphatic metastases. Furthermore, the prior proliferation necessary in these models alters the growth and dissemination potential of the cell lines[30-33].
In 2012, Hackl et al[14] presented only study of its kind which thoroughly compares all three ways of human xenograft models of CRC i.e., subcutaneous, orthotopic and intrasplenic. In this review we have discussed the technical aspects of only orthotopic xenograft done by open method. This new technique to execute xenografts from human colon cancer cell lines, transfected with luciferase and beta-hCG. In the Orthotopic model, open surgical technique was used. Cecum was assessed through midline incision following which trypan blue was injected into cecal wall. The cecum was then being stabilized carefully on a scalpel holder to prevent spillage of tumor cells. Using a 10 mL Hamilton syringe and 30G needle 5 ml cells (5 × 105) were infused into the cecal wall under 4 × magnification. Though this approach has a high tumor take rate of 87.5%-100% but the pitfall is that it is an intricated technique requiring specialized training, expertise hands and suitable instruments. Intrasplenic tumor cell injection was followed by a speedy colonization of the cancer cells to liver and lungs, within a day, which could not be explained by spontaneous metastasis. Mice yield to considerable tumor load within a few weeks and drug efficacy also could not be studied.
Priolli et al[15] in 2012 designed another open surgical method in which colon diversion and distal fistula formed, cancer cells were infused in the submucosa of the fistula. Scintigraphy with 99mTc-MIBI was performed to identify the tumor and monitor its growth. Tumors developed spontaneously in the submucosa and histological examination revealed poorly differentiated tumor cells identically to the cells implanted. Distal fistula allowed easy monitoring of the tumor growth and assessing the effect of cytotoxic therapy. Tumor metastasis was not seen which may be due to insufficient time for tumor spread, insufficient number of cells inoculated or genotypic variance of tumor cells regarding invasiveness and metastatic potential.
Next is the enema model technique which requires induction of colitis followed by instillation of cancer cells transanally. Takahashi and associates in 2004 used a distinct non-surgical technique of orthotopic implantation. They infused 3% dextran sulfate sodium (DSS) to induce colitis followed by which cancer cells were instilled transanally. Clapper et al[34] also analyzed this approach by cyclically administering DSS, which generated colorectal dysplasia and carcinoma with similar pathological characteristics as humans. The frequency and profusion of these lesions differ, depending on the genre of mouse used, dose and schedule of DSS. Also the advancement of these tumors to invasive cancer could be potentiated by delivering DSS simultaneously with a known colon carcinogen {azoxymethane (AOM), 2- amino-3-methylimidazo[4,5-fl] quinoline (IQ), 2-amino-1-methyl-6-phenylimidazo [4,5-b] pyridine (PhIP)} or iron[16].
Kishimoto et al[17] in 2013 also used similar technique for orthotopic implantation of mouse rectal cancer cells, stably expressing green fluorescent protein (GFP). Instilling acetic acid solution disintegrated the epithelial cell layer of the rectal mucosa. CT26-GFP cells in Matrigel were injected in rectal mucosa 4mm from rectal ring. The anus was promptly sealed by tape to restrain cell leakage. The inoculated tumor cells were noninvasively observed by fluorescence microscope. The mice were then euthanized for histological studies and to investigate potential metastasis. The carcinoma developed in 100% of mice, it initiated in rectal mucosa and then invaded submucosa. The tumor metastasized to the lymph nodes and lungs in 90% while dissemination to the liver was seen in only 9% of mice, contrary to the fact that liver is the most common site for metastasis of colorectal carcinoma. The authors elucidated it by the development of tumor in the lower rectum, which primarily drains in systemic venous circulation. As tumors arise only in rectum, which may not be useful in colonic studies, is major limitation of this model.
Another developed modality was microinjection technique which involves instillation of tumor cells directly into the colonic wall by microinjection. Donigan et al[18] in 2009 has devised this non-operative technique by using optical microscope in which the colon cancer cells were injected into the rectal wall of the mouse under magnification (10-100 ×) with an overall uptake rate of 65%. But the hitch was that the injection of the cells into the rectal wall evolved in cancers not emanating from the mucosa. Using the identical notion, Zigmond et al[19] in 2011 reported a murine model in which the tumor cells were injected into the wall of colon through a murine colonoscope (Coloview-Karl Storz). They reported tumor take rate of 95% with no metastasis. But, by the use of coloview the tumor cells can be implantated only in distal rectum[35,36].
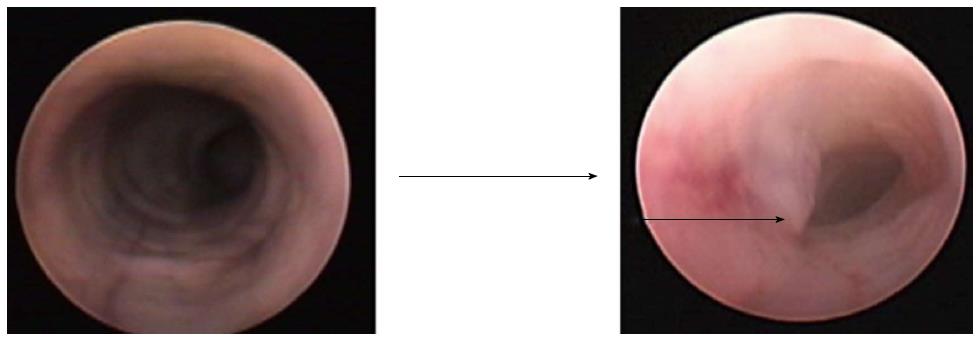
For overcoming problems and deficiencies with the previously described colorectal cancer murine models, Bhullar et al[20] 2010 came up with a very impressive noninvasive technique i.e., transanal low dose electrocoagulation technique. They reported a true orthotopic murine model using tumor cell implantation after low-dose colonic mucosal coagulation through a small transanal electrode resulting in limited mucosal injury. Subsequently, they instilled the tumor cells transanally on the injured mucosa, which then proliferated in the non-ischemic bed. The resultant tumors grew from the mucosal surface and subsequently involved the deeper colonic layers. The colonoscopic (coloview) view of the normal mouse colon shows a smooth circumferential mucosa, while the orthotopically grown tumor from the mucosal surface can be easily detected and followed up (Figure 2).
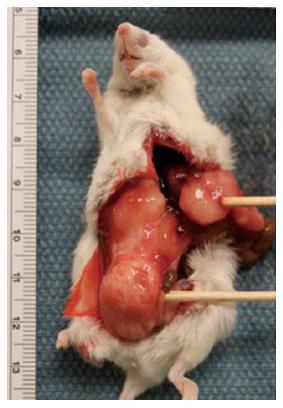
Tumors developed in 87.5% of the mice (42/48) i.e., 12 of 12 and 11 of 12 mice with murine tumor lines (CRL-2638 and CRL 2639, respectively) and in 7 of 12 and 12 of 12 mice with human tumor lines (HT-29 and LS 174T, respectively). The tumors were initially smaller, but over time grew bigger to invade the surrounding structures like the bladder, pelvic bones, etc. (Figure 3). Histologic evaluation revealed that these tumors grew from the mucosal lining (Figure 4). Overall Metastatic disease i.e., lymph nodal in 32/48 i.e., 66.66%, while liver, omentum and peritoneum involvement was seen in 43.75% (21/48) of cases. Orthotopic model using HT-29, LS 174T human cell lines showed tumor development in 58%-100% cases, lymph node and liver metastasis seen in 33-83.33% cases[20].
This noninvasive model has succeeded in dealing with most of the limitations of the antecedent models as: (1) there is no need of laparotomy for tumor implantation at colon or rectum; (2) easy to learn noninvasive technique with high reproducibility; (3) it closely mimics the natural development of disease process in humans; and (4) tumor uptake was 100% and metastasis was 83.33% with human cell line (LS 174T).
This model has come as a boon in the research for human colon cancer. It has enormous potentials and can be used to study tumor development, metastasis, and evaluation of novel chemotherapeutics for the colorectal cancer.
With the escalating worldliness of modelling carcinogenesis in mice, an intricate disorder can be simultaneously witnessed and manipulated by the researchers. The recently developed orthotopic xenograft murine models of colon carcinoma have added a new dimension in the research of colorectal cancer by overcoming limitations of the earlier ones.
The model by our group has overcome most of the shortcomings of the antecedent models and has opened new way for understanding disease process and testing therapeutic drugs that can potentially benefit patients of human colorectal cancer.
At the same time better understanding of the disease process along with the improved technology available to the future researchers, we can expect more advancement in these orthotopic murine models.
The murine models of colon cancer represent an important tool for understanding the etiopathogenesis and evaluating management strategies for colorectal cancer, thus representing a resource of immense potential in cancer medicine. An increasing knowledge in this field has led to development of various murine models, for human colon cancer but they have many limitations.
The present model has enormous potentials and it will open up new dimensions of knowledge in study biology and evaluation of novel chemotherapeutics for the colorectal cancer.
This is easy to learn and highly reproducible noninvasive technique that closely mimics the natural disease process of carcinoma colon in humans with tumor uptake and metastasis of 100% and 83.33% respectively.
This will help in better understanding of disease process and testing therapeutic drugs that can potentially benefit patients of human colorectal cancer.
The authors reported the development of various orthotopic murine model of colorectal cancer and well summarized the advantages and disadvantages of each method with excellent review.
P- Reviewer: Chae SC, Nayak BS S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Colorectal cancer incidence and mortality based on SEER data analyzed by National Cancer Institute. Available from: http://statecancerprofiles.cancer.gov/quickprofiles/index.php?. [Cited in This Article: ] |
| 2. | Taketo MM, Edelmann W. Mouse models of colon cancer. Gastroenterology. 2009;136:780-798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 190] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 3. | Hawk ET, Levin B. Colorectal cancer prevention. J Clin Oncol. 2005;23:378-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 161] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 4. | Voskoglou-Nomikos T, Pater JL, Seymour L. Clinical predictive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models. Clin Cancer Res. 2003;9:4227-4239. [PubMed] [Cited in This Article: ] |
| 5. | Shoemaker RH, Monks A, Alley MC, Scudiero DA, Fine DL, McLemore TL, Abbott BJ, Paull KD, Mayo JG, Boyd MR. Development of human tumor cell line panels for use in disease-oriented drug screening. Prog Clin Biol Res. 1988;276:265-286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107-1112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7084] [Cited by in F6Publishing: 7080] [Article Influence: 208.2] [Reference Citation Analysis (0)] |
| 7. | Johnson JI, Decker S, Zaharevitz D, Rubinstein LV, Venditti JM, Schepartz S, Kalyandrug S, Christian M, Arbuck S, Hollingshead M. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer. 2001;84:1424-1431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 624] [Cited by in F6Publishing: 592] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 8. | Corpet DE, Pierre F. How good are rodent models of carcinogenesis in predicting efficacy in humans? A systematic review and meta-analysis of colon chemoprevention in rats, mice and men. Eur J Cancer. 2005;41:1911-1922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 150] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Bhullar JS, Makarawo T, Subhas G, Alomari A, Silberberg B, Tilak J, Decker M, Mittal VK. A true orthotopic gastric cancer murine model using electrocoagulation. J Am Coll Surg. 2013;217:64-70; discussion 70-1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Reichling T, Goss KH, Carson DJ, Holdcraft RW, Ley-Ebert C, Witte D, Aronow BJ, Groden J. Transcriptional profiles of intestinal tumors in Apc(Min) mice are unique from those of embryonic intestine and identify novel gene targets dysregulated in human colorectal tumors. Cancer Res. 2005;65:166-176. [PubMed] [Cited in This Article: ] |
| 11. | Edelmann L, Edelmann W. Loss of DNA mismatch repair function and cancer predisposition in the mouse: animal models for human hereditary nonpolyposis colorectal cancer. Am J Med Genet C Semin Med Genet. 2004;129C:91-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322-324. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Ogata Y, Hara Y, Akagi Y, Ohkita A, Morodomi T, Shirouzu K. Metastatic model of human colon cancer constructed using orthotopic implantation in nude mice. Kurume Med J. 1998;45:121-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Hackl C, Man S, Francia G, Milsom C, Xu P, Kerbel RS. Metronomic oral topotecan prolongs survival and reduces liver metastasis in improved preclinical orthotopic and adjuvant therapy colon cancer models. Gut. 2013;62:259-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 15. | Priolli DG, Abrantes AM, Neves S, Batista JN, Cardinalli IA, Botelho MF. A novel model of distal colon cancer in athymic mice. Acta Cir Bras. 2012;27:355-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Takahashi T, Morotomi M, Nomoto K. A novel mouse model of rectal cancer established by orthotopic implantation of colon cancer cells. Cancer Sci. 2004;95:514-519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Kishimoto H, Momiyama M, Aki R, Kimura H, Suetsugu A, Bouvet M, Fujiwara T, Hoffman RM. Development of a clinically-precise mouse model of rectal cancer. PLoS One. 2013;8:e79453. [PubMed] [Cited in This Article: ] |
| 18. | Donigan M, Norcross LS, Aversa J, Colon J, Smith J, Madero-Visbal R, Li S, McCollum N, Ferrara A, Gallagher JT. Novel murine model for colon cancer: non-operative trans-anal rectal injection. J Surg Res. 2009;154:299-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Zigmond E, Halpern Z, Elinav E, Brazowski E, Jung S, Varol C. Utilization of murine colonoscopy for orthotopic implantation of colorectal cancer. PLoS One. 2011;6:e28858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Bhullar JS, Subhas G, Silberberg B, Tilak J, Andrus L, Decker M, Mittal VK. A novel nonoperative orthotopic colorectal cancer murine model using electrocoagulation. J Am Coll Surg. 2011;213:54-60; discussion 60-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Flatmark K, Maelandsmo GM, Martinsen M, Rasmussen H, Fodstad Ø. Twelve colorectal cancer cell lines exhibit highly variable growth and metastatic capacities in an orthotopic model in nude mice. Eur J Cancer. 2004;40:1593-1598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Fodstad O. Tumorigenicity and dissemination of human tumors in congenitally immune-deficient mice. J Natl Cancer Inst. 1991;83:1419-1420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Morikawa K, Walker SM, Nakajima M, Pathak S, Jessup JM, Fidler IJ. Influence of organ environment on the growth, selection, and metastasis of human colon carcinoma cells in nude mice. Cancer Res. 1988;48:6863-6871. [PubMed] [Cited in This Article: ] |
| 24. | Fu XY, Besterman JM, Monosov A, Hoffman RM. Models of human metastatic colon cancer in nude mice orthotopically constructed by using histologically intact patient specimens. Proc Natl Acad Sci USA. 1991;88:9345-9349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 287] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 25. | Giavazzi R, Campbell DE, Jessup JM, Cleary K, Fidler IJ. Metastatic behavior of tumor cells isolated from primary and metastatic human colorectal carcinomas implanted into different sites in nude mice. Cancer Res. 1986;46:1928-1933. [PubMed] [Cited in This Article: ] |
| 26. | Pocard M, Tsukui H, Salmon RJ, Dutrillaux B, Poupon MF. Efficiency of orthotopic xenograft models for human colon cancers. In Vivo. 1996;10:463-469. [PubMed] [Cited in This Article: ] |
| 27. | Fidler IJ. Critical factors in the biology of human cancer metastasis: twenty-eighth G.H.A. Clowes memorial award lecture. Cancer Res. 1990;50:6130-6138. [PubMed] [Cited in This Article: ] |
| 28. | Jin H, Liu X, Li VK, Ding Y, Yun S, Liu F, Zhou S, Song Y, Ni M. A simple colostomy implantation model for evaluating colon cancer. Int J Colorectal Dis. 2009;24:41-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Wilmanns C, Fan D, O’Brian CA, Bucana CD, Fidler IJ. Orthotopic and ectopic organ environments differentially influence the sensitivity of murine colon carcinoma cells to doxorubicin and 5-fluorouracil. Int J Cancer. 1992;52:98-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 115] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Bresalier RS, Raper SE, Hujanen ES, Kim YS. A new animal model for human colon cancer metastasis. Int J Cancer. 1987;39:625-630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 86] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Tsutsumi S, Kuwano H, Morinaga N, Shimura T, Asao T. Animal model of para-aortic lymph node metastasis. Cancer Lett. 2001;169:77-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Rashidi B, Gamagami R, Sasson A, Sun FX, Geller J, Moossa AR, Hoffman RM. An orthotopic mouse model of remetastasis of human colon cancer liver metastasis. Clin Cancer Res. 2000;6:2556-2561. [PubMed] [Cited in This Article: ] |
| 33. | Farré L, Casanova I, Guerrero S, Trias M, Capellá G, Mangues R. Heterotopic implantation alters the regulation of apoptosis and the cell cycle and generates a new metastatic site in a human pancreatic tumor xenograft model. FASEB J. 2002;16:975-982. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Clapper ML, Cooper HS, Chang WC. Dextran sulfate sodium-induced colitis-associated neoplasia: a promising model for the development of chemopreventive interventions. Acta Pharmacol Sin. 2007;28:1450-1459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 35. | Céspedes MV, Espina C, García-Cabezas MA, Trias M, Boluda A, Gómez del Pulgar MT, Sancho FJ, Nistal M, Lacal JC, Mangues R. Orthotopic microinjection of human colon cancer cells in nude mice induces tumor foci in all clinically relevant metastatic sites. Am J Pathol. 2007;170:1077-1085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 36. | Kashtan H, Rabau M, Mullen JB, Wong AH, Roder JC, Shpitz B, Stern HS, Gallinger S. Intra-rectal injection of tumour cells: a novel animal model of rectal cancer. Surg Oncol. 1992;1:251-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |