Published online Sep 28, 2015. doi: 10.3748/wjg.v21.i36.10461
Peer-review started: March 11, 2015
First decision: March 26, 2015
Revised: May 15, 2015
Accepted: June 26, 2015
Article in press: June 26, 2015
Published online: September 28, 2015
This is the first report describing a case where prolonged, severe malabsorption from brown bowel syndrome progressed to multifocally spread small bowel adenocarcinoma. This case involves a female patient who was initially diagnosed with chronic jejunitis associated with primary diffuse lymphangiectasia at the age of 26 years. The course of the disease was clinically, endoscopically, and histologically followed for 21 years until her death at the age 47 due to multifocal, metastasizing adenocarcinoma of the small bowel. Multiple lipofuscin deposits (so-called brown bowel syndrome) and severe jejunitis were observed microscopically, and sections of the small bowel showed dense lymphoplasmacytic infiltration of the lamina propria as well as blocked lymphatic vessels. After several decades, multifocal nests of adenocarcinoma cells and extensive, flat, neoplastic mucosal proliferations were found only in the small bowel, along with a loss of the mismatch repair protein MLH1 as a long-term consequence of chronic jejunitis with malabsorption. No evidence was found for hereditary nonpolyposis colon carcinoma syndrome. This article demonstrates for the first time multifocal carcinogenesis in the small bowel in a malabsorption syndrome in an enteritis-dysplasia-carcinoma sequence.
Core tip: Severe malabsorption associated with brown bowel syndrome can progress to multifocally spread small bowel adenocarcinoma. This report describes the clinical course of a woman suffering from a long-lasting malabsorption syndrome who developed small bowel adenocarcinoma in an enteritis-dysplasia-carcinoma sequence. After several decades of chronic jejunitis with malabsorption, multifocal nests of adenocarcinoma cells were found only in the small bowel without evidence of hereditary nonpolyposis colon carcinoma syndrome.
- Citation: Raithel M, Rau TT, Hagel AF, Albrecht H, Rossi T, Kirchner T, Hahn EG. Jejunitis and brown bowel syndrome with multifocal carcinogenesis of the small bowel. World J Gastroenterol 2015; 21(36): 10461-10467
- URL: https://www.wjgnet.com/1007-9327/full/v21/i36/10461.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i36.10461
Brown bowel syndrome is caused by a lipofuscinosis, primarily in the small bowel, which is characterized macroscopically by an orange-brown appearance. The brown coloring occurs from increased lipofuscin aggregates, particularly in the apical enterocytes, which are thought to result from vitamin E deficiency caused by longtime chronic malabsorption[1-3]. This report describes the case of a woman with brown bowel syndrome that progressed over several decades into multifocally spread small bowel adenocarcinoma. To the best of our knowledge, this is the first presentation of a small bowel adenocarcinoma caused by long-term malabsorption and brown bowel syndrome.
The patient initially complained of watery diarrhea, recurrent attacks of vomiting bile, stomach cramps, recurrent edemas, and tympanites at the age of 26 years. Although both of her sisters were healthy, her father suffered from Menière’s disease and her mother had hypertension and hypercholesterolemia. Otherwise, her family history was unremarkable and contained no reports of malignancies, including colorectal cancer. The patient was allergic to pork and to several medications, but was otherwise healthy. A clinical examination at this time showed that she was considerably underweight (body mass index, 17.7 kg/m2) and short (158 cm). She had increased kyphosis of the thoracic spine associated with funnel chest, a clubfoot position with pronounced edema in the ankle and lower leg, ascites, and periodontitis with heavy gum bleeding. Laboratory results indicated low-grade, normocytic anemia (hemoglobin, 11.8 g/dL; normal range: 12-16 g/dL), pronounced hypoproteinemia (4.5 g/dL; normal range: 6.6-8.3 g/dL), decreased erythrocyte sedimentation rate (1/3 mm/h), and hypogammaglobulinemia (IgG 509 mg/dL; normal range: 700-1600 mg/dL). The Gordon test (iv administration of 40 μCi 51Cr-labelled albumin) showed abnormal (> 9%) excretion of the test substance in stool samples collected over 96 h (normal: < 1.5%), and confirmed a high-grade enteral loss of protein. These findings remained unchanged throughout the course of her disease, namely prominent intestinal protein loss (persistent hypoproteinemia, hypogammaglobulinemia) and vitamin (folate, vitamin B12, and D), electrolyte and zinc deficiencies, but without remarkable signs of systemic inflammatory reactivity (C-reactive protein < 10 mg/dL), and frequently with increased stool weights up to 780 g.
Transabdominal ultrasonography revealed normal parenchymatous organs, but with accentuated sonomorphology of the small bowel observed as increased wall thickness (4-6 mm), pronounced submucosa, and strict layer widening, which later progressed to a more hypoechoic small bowel appearance with layer loosening. Small bowel double-contrast imaging initially revealed hyperperistalsis and lymphonodular hyperplasia without any stenosis. Evaluations 12 years later revealed moderately dilated intestinal jejunal segments with flattened mucosa, diffuse edema, increased distance between loops, with some tubular, smooth narrowed loops.
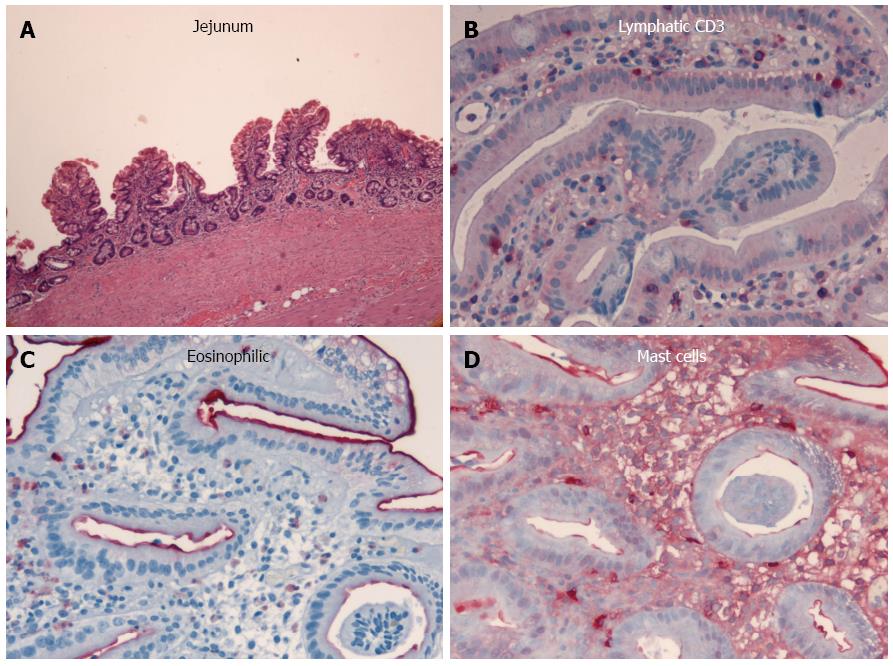
The colonoscopic findings were normal at the initial presentation and throughout the course of disease. However, diffuse lymphangiectasia in the small bowel and chronic jejunitis were detected initially with esophagogastroduodenoscopy, and later with push enteroscopy. Differential diagnoses of malignant lymphoma, celiac disease, mesenteric lymph node metastases, Crohn’s disease, rare intestinal manifestations of sarcoidosis or tuberculosis, Whipple’s disease, retroperitoneal fibrosis, and cardiovascular disease (right ventricular insufficiency, vena cava or portal vein thrombosis) were excluded as possible causes. Endoscopic and histologic examinations subsequently confirmed a diagnosis of chronic malabsorption syndrome associated with exudative enteropathy as a result of prolonged intestinal primary lymphangectasia and chronic jejunitis of undefined etiology (Figure 1).
Over the 21-year course of her disease, the patient had > 30 hospital admissions at two university centers and two local hospitals. Her food tolerance declined and she failed to gain any weight, remaining at a body mass index of 16-18 kg/m2 and requiring parenteral nourishment. Multiple drug treatment attempts (including loperamide, cholestyramine, and pancreatic enzymes) failed, and despite continued nutrition by percutaneous endoscopic gastrostomy (to avoid complications such as venous thrombosis), the diarrhea and intestinal protein loss persisted.
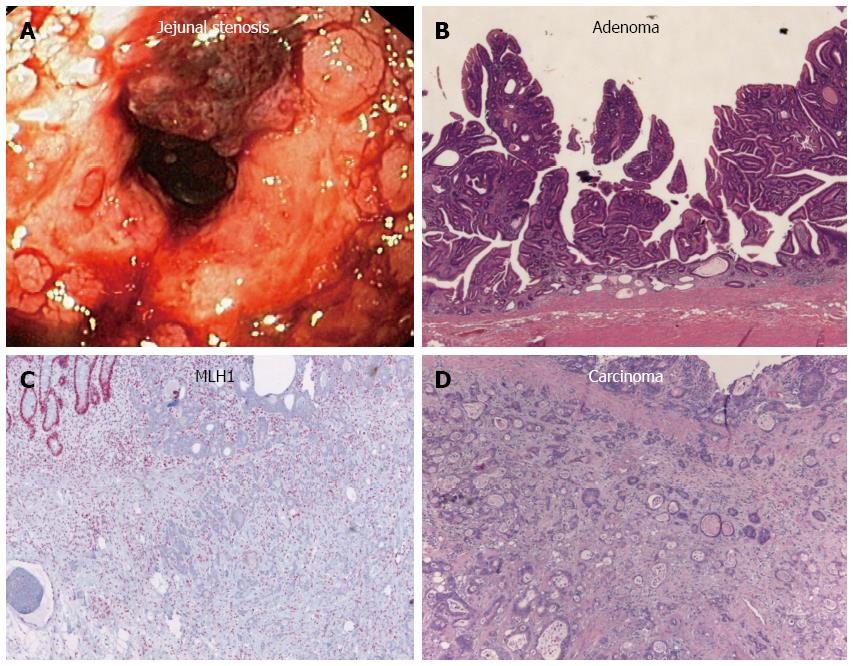
Twenty years after the initial diagnosis, the patient was again admitted to the hospital because of increasing abdominal colic, nausea, vomiting, and melena. An ultrasound revealed fluid-filled small bowel loops indicative of subileus, but without ascites. A short stenosis (about 25 mm long) was visualized in the middle part of the jejunum upon double-contrast small bowel (Sellink) imaging. Abdominal magnetic resonance imaging showed an abrupt transition from substantially dilated small bowel loops to non-dilated loops in the middle-right abdomen, but did not indicate a solid tumor mass. Thus, push enteroscopy and high-pressure balloon dilatation of the stenosis were performed (Figure 2A). Histology of the biopsy samples taken during enteroscopy showed the same features of chronic jejunitis associated with lymphangectasia that were observed 20 years before, but no indication of malignant disease. Afterwards, a hemoglobin-effective (10.5 g/dL) hematemesis and hematochezia developed, which required emergency push enteroscopy. A Forrest IIb lesion in the area of the dilated stenosis was observed and treated with injections of suprarenin (1:100.000) and fibrin glue. However, the patient aspirated intestinal fluid and blood during the endoscopic procedure, developed asystole, and required cardiopulmonary resuscitation. She was then transferred to the intensive care unit for further treatment.
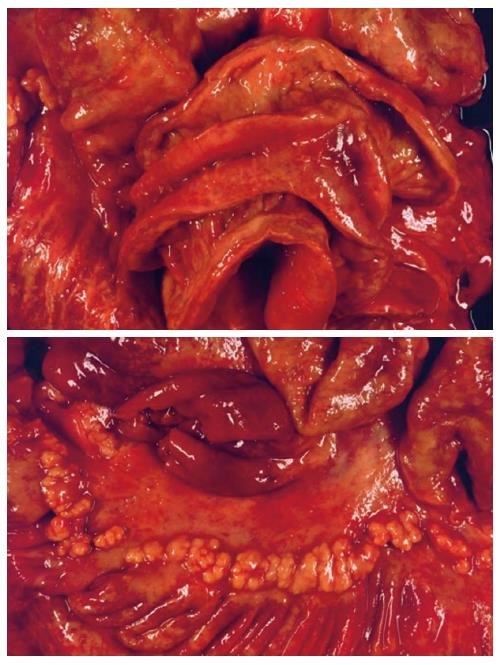
The patient subsequently underwent segment resection of the jejunum. Histopathologic examination revealed a multicentric, poorly differentiated adenocarcinoma (Figure 2B-D). The tumor had infiltrated the blocked lymphatic vessel system (lymphangiosis carcinomatosa), and peritoneal carcinomatosis was detected at an uncurable stage (pT4 [m] pN0 [0/1] L1 V0 R1). Other portions of the resected tissue exhibited foci of extensive, flat, neoplastic mucosal proliferation transitioning to adenocarcinoma, with signs of an enteritis-dysplasia-adenocarcinoma sequence, which is unusual in the small bowel. In addition, multiple lipofuscin deposits were discovered in the small bowel tissue, indicating brown bowel syndrome.
Eight months following the surgical resection, the patient’s general condition markedly deteriorated with colicky stomach pain, dyspnea, and additional weight loss (5 kg in 3 wk). In addition to ascites, bilateral pleural effusion, and pressure pain throughout the abdomen, bilateral pneumonic infiltrates were detected upon radiologic examination, and laboratory testing showed hypokalemia, hypocalcemia, hypoalbuminemia, and hypoproteinemia. Antibiotics and human albumin substitution therapy were administered and pleural and ascites puncture and drainage procedures were initiated. As a result, her general condition stabilized. However, she developed clinical symptoms of small bowel obstruction, which were confirmed radiologically and sonographically as mechanical ileus. An exploratory laparotomy was then performed, though further treatment was prohibited by the pronounced peritoneal carcinomatosis and obstructed ileus. The patient died 3 d later.
A postmortem examination demonstrated that, in addition to the known peritoneal carcinomatosis of the multifocal small bowel carcinomas and brown bowel syndrome (Figure 3), pleural carcinomatosis, lymphangiosis carcinomatosa, and pulmonary artery emboli had extended to the lungs. Further scientific evaluation of this disease was undertaken, including microsatellite instability diagnosis. Immunohistochemistry for MLH1 showed a considerable deficiency of the mismatch repair protein in the small bowel tissue (Figure 2D). In contrast, tumor specimens were immunopositive for DNA repair proteins MSH2 and MSH6. Unfortunately, degradation of the DNA in the tumor specimen prohibited any further DNA analyses. However, the loss of the MLH1 repair protein was likely a consequence of the long-term chronic jejunitis and malabsorption, as there was no clinical evidence for hereditary nonpolyposis colon carcinoma syndrome.
The adenoma-dysplasia-carcinoma sequence constitutes an important underlying cause of malignant tumor development in the lower gastrointestinal tract[4,5]. Moreover, diseases associated with chronic inflammation are risk factors for pancreatic carcinoma (chronic pancreatitis), cholangiocellular carcinoma, and colitis-associated colorectal carcinoma[4,5]. In the small bowel, celiac disease or polyposis syndromes (e.g., familial adenomatous polyposis and Peutz-Jeghers syndrome) are risk factors for developing malignancy or primary extranodal lymphoma[5,6]. However, progression from enteritis to dysplasia and/or flat neoplasia and then to small bowel carcinoma as a consequence of a chronic inflammation has only been postulated in a few conditions, such as celiac and Crohn’s diseases, and has not yet been systematically investigated or observed in individual patients[4,5,7]. Indeed, small bowel adenocarcinomas rarely develop beyond the ligament of Treitz, and tumor incidence further decreases distal to this point[5,7,8]. The patient described in the present case did not have celiac disease (there was no response to a gluten-free diet and tests for celiac-specific antibodies [anti-TG2 and anti-endomysium IgA] were negative), Crohn’s disease, or any of the polyposis syndromes. Rather, her chronic jejunitis and long-standing malabsorption syndrome were associated with primary diffuse lymphangiectasia. The clinical characterization of symptoms remained consistent throughout the > 20-year course of her disease, including the loss of intestinal proteins, electrolytes, nutrients and vitamins, subsequent vitamin E deficiency, and ultimately brown bowel syndrome. We postulate that the transformation to malignancy was caused by molecular and genetic changes in the small bowel that occurred during the longstanding period of malabsorption, similar to what has been described for colorectal carcinogenesis[9]. To our knowledge, this is the first report of multifocal carcinogenesis within the small bowel associated with severe malabsorption and brown bowel syndrome.
Histopathologic examinations from this patient over more than 20 years consistently indicated local, persistent inflammation, which induced neoplastic mucosal changes, and finally led to multifocal nests of adenocarcinoma cells. Thus, an enteritis-dysplasia-carcinoma sequence played a carcinogenic role in conjunction with a loss of the mismatch repair protein MLH1. Similar mechanisms are known to be involved in the carcinogenesis of colon carcinomas associated with chronically active ulcerative colitis[4,9]. Additionally, the loss of mismatch repair mechanisms involving MLH1 or MSH2 have also been described in patients with celiac disease[10,11]. Thus, it is likely that the chronic, long-standing enteritis with continuous intestinal repair in the patient presented here resulted in inactivation of mismatch repair mechanisms. Loss of MLH1 in hereditary nonpolyposis colon carcinoma syndrome has been reported to contribute to additional DNA replication errors and/or apoptosis failure[12,13], which can cause multifocal spread of adenocarcinoma. However, the family history of the patient in the present case was negative for malignancy and no inflammatory or neoplastic lesions of the colon were found.
The severity of the malabsorptive state in this patient was reflected in the appearance of the brown bowel syndrome. The syndrome is thought to be the result of chronic malabsorption and vitamin E deficiency caused by intestinal lymphangiectasia associated with chronic jejunitis[14-16] or small bowel inflammation associated with intestinal malabsorption syndromes, such as celiac disease[3]. The characteristic lipofuscin deposits are typically found in the smooth muscle cells of the tunica muscularis, but can also occur in the lamina muscularis mucosae[1,2,16]. It is possible that the vitamin E deficiency or lipofuscinosis alters neuronal function in the small bowel and thus affects motility[1-3,17-19]. As conventional histologic staining methods often fail to reveal the lipofuscin deposits, specialized examinations, such as UV or electron microscopy, are required when this disease is suspected[1,14,16]. The deposits are thought to be products of mitochondria degeneration, and are rarely detected in other organs, e.g., thyroid or intestinal lymph nodes, in brown bowel syndrome[1,2,15,16].
The combined presence of brown bowel syndrome and small bowel adenocarcinoma has only been described previously in two reports[20,21], though the connection between the two has not been clearly established. However, the role of vitamin E deficiency in small bowel carcinogenesis cannot be excluded and requires further study[21,22]. The appearance of brown bowel syndrome may also indicate clinical deficiencies in other nutrients involved in the regulation of intestinal epithelial proliferation, such as folate and vitamin D. Despite the continuous supplementation with essential nutrients that our patient received, reduced levels of folate occurred intermittently, which could have contributed to the inactivation of mismatch repair mechanisms, as has been reported in ulcerative colitis[12].
In conclusion, this article provides the clinical observations from a greater than 20-year disease course of primary diffuse lymphangiectasia with persistent chronic jejunitis. Moreover, this report demonstrates for the first time multifocal carcinogenesis within the small bowel in association with loss of a DNA mismatch repair mechanism in a malabsorption syndrome. The evidence suggests that local enteritis activity caused the dysplasia and neoplastic transformation and resulted in multifocal nests of small bowel adenocarcinomas, resembling the transformation process in chronic colitis[4,5]. The accompanying brown bowel syndrome and severe malabsorption may have further influenced the malignant transformation.
The patient initially complained of watery diarrhea, recurrent attacks of vomiting bile, stomach cramps, recurrent edemas, and tympanites.
Severe malabsorption with a brown bowel syndrome progressed to multifocally spread small bowel adenocarcinoma.
Incidental small bowel adenocarcinoma.
Low-grade, normocytic anemia (hemoglobin, 11.8 g/dL); pronounced hypoproteinemia (4.5 g/dL); decreased erythrocyte sedimentation rate (1/3 mm/h); hypogammaglobulinemia (IgG 509 mg/dL); > 9% excretion in the Gordon test; C-reactive protein < 10 mg/dL; and increased stool weights, often up to 780 g.
Small bowel obstruction that was confirmed as mechanical ileus by radiography, sonography, and endoscopy.
Repeated histopathologic examinations consistently demonstrated local, chronic (20 years), persistent inflammation resulting in multifocal nests of adenocarcinoma cells.
The patient received supplementary nutritional treatment and was later fed by percutaneous endoscopic gastrostomy. Multiple drug treatment attempts (including loperamide, cholestyramine, and pancreatic enzymes) failed to reduce malabsorption.
This is the first known report of a small bowel adenocarcinoma caused by long-term malabsorption and brown bowel syndrome.
Brown bowel syndrome is caused by long-term chronic malabsorption, which finally leads to formation of lipofuscin aggregates/lipofuscinosis of the small bowel. Small bowel adenocarcinoma is a rare malignant tumor of the small intestine.
This article provides observations from a greater than 20-year disease course of primary diffuse lymphangiectasia with persistent chronic jejunitis, and demonstrates for the first time multifocal carcinogenesis within the small bowel in association with loss of a DNA mismatch repair mechanism in a malabsorption syndrome.
The authors conclude that long-term malabsorption associated with brown bowel syndrome may cause dysplasia and neoplastic transformation, ultimately leading to the formation of multifocal nests of small bowel adenocarcinoma, resembling the transformation process in chronic colitis. The article highlights the clinical characteristics of this tumor and provides information about its development over course of the disease.
P- Reviewer: Li YY, Riccioni ME, Soares RLS S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Gallager RL. Intestinal ceroid deposition - “brown bowel syndrome”. A light and electron microscopic study. Virchows Arch A Pathol Anat Histol. 1980;389:143-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Foster CS. The brown bowel syndrome: a possible smooth muscle mitochondrial myopathy? Histopathology. 1979;3:1-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Lee SP. Vitamin E treatment for brown bowel syndrome. Mayo Clin Proc. 1979;54:752. [PubMed] [Cited in This Article: ] |
| 4. | Hermanek P. [Adenoma/dysplasia-carcinoma sequence in the small intestine]. Z Gastroenterol. 1987;25:166-167. [PubMed] [Cited in This Article: ] |
| 5. | Solem CA, Harmsen WS, Zinsmeister AR, Loftus EV. Small intestinal adenocarcinoma in Crohn’s disease: a case-control study. Inflamm Bowel Dis. 2004;10:32-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Giardiello FM, Brensinger JD, Tersmette AC, Goodman SN, Petersen GM, Booker SV, Cruz-Correa M, Offerhaus JA. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000;119:1447-1453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 961] [Cited by in F6Publishing: 815] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 7. | Dabaja BS, Suki D, Pro B, Bonnen M, Ajani J. Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer. 2004;101:518-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 357] [Cited by in F6Publishing: 316] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 8. | Abrahams NA, Halverson A, Fazio VW, Rybicki LA, Goldblum JR. Adenocarcinoma of the small bowel: a study of 37 cases with emphasis on histologic prognostic factors. Dis Colon Rectum. 2002;45:1496-1502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 21] [Reference Citation Analysis (0)] |
| 9. | Arber N, Neugut AI, Weinstein IB, Holt P. Molecular genetics of small bowel cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:745-748. [PubMed] [Cited in This Article: ] |
| 10. | Potter DD, Murray JA, Donohue JH, Burgart LJ, Nagorney DM, van Heerden JA, Plevak MF, Zinsmeister AR, Thibodeau SN. The role of defective mismatch repair in small bowel adenocarcinoma in celiac disease. Cancer Res. 2004;64:7073-7077. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Bergmann F, Singh S, Michel S, Kahlert C, Schirmacher P, Helmke B, Von Knebel Doeberitz M, Kloor M, Bläker H. Small bowel adenocarcinomas in celiac disease follow the CIM-MSI pathway. Oncol Rep. 2010;24:1535-1539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Schulmann K, Brasch FE, Kunstmann E, Engel C, Pagenstecher C, Vogelsang H, Krüger S, Vogel T, Knaebel HP, Rüschoff J. HNPCC-associated small bowel cancer: clinical and molecular characteristics. Gastroenterology. 2005;128:590-599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 145] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 13. | Lynch HT, Lynch PM, Lanspa SJ, Snyder CL, Lynch JF, Boland CR. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet. 2009;76:1-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 543] [Cited by in F6Publishing: 550] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 14. | Toffler AH, Hukill PB, Spiro HM. Brown bowel syndrome. Ann Intern Med. 1963;58:872-877. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 34] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Horn T, Svendsen LB, Nielsen R. Brown-bowel syndrome. Review of the literature and presentation of cases. Scand J Gastroenterol. 1990;25:66-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Dudorkinová D, Povýsil C. [The brown bowel syndrome]. Cesk Patol. 1994;30:23-26. [PubMed] [Cited in This Article: ] |
| 17. | Hozyasz KK, Chelchowska M, Laskowska-Klita T. [Vitamin E levels in patients with celiac disease]. Med Wieku Rozwoj. 2003;7:593-604. [PubMed] [Cited in This Article: ] |
| 18. | Ruchti C, Eisele S, Kaufmann M. Fatal intestinal pseudo-obstruction in brown bowel syndrome. Arch Pathol Lab Med. 1990;114:76-80. [PubMed] [Cited in This Article: ] |
| 19. | Hitzman JL, Weiland LH, Oftedahl GL, Lie JT. Ceroidosis in the “brown bowel syndrome”. Mayo Clin Proc. 1979;54:251-257. [PubMed] [Cited in This Article: ] |
| 20. | Shiller M, Cohen I, Munichor M, Loberant N, Bickel A, Reshef R. The “brown bowel syndrome” associated with jejunal carcinoma. Am J Gastroenterol. 1993;88:1788-1789. [PubMed] [Cited in This Article: ] |
| 21. | Reynaert H, Devis G. The brown bowel syndrome and gastrointestinal adenocarcinoma. Am J Gastroenterol. 1994;89:812-813. [PubMed] [Cited in This Article: ] |
| 22. | Reynaert H, Debeuckelaere S, De Waele B, Meysman M, Goossens A, Devis G. The brown bowel syndrome and gastrointestinal adenocarcinoma. Two complications of vitamin E deficiency in celiac sprue and chronic pancreatitis? J Clin Gastroenterol. 1993;16:48-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |