Published online Sep 28, 2015. doi: 10.3748/wjg.v21.i36.10314
Peer-review started: March 18, 2015
Peer-review started: March 18, 2015
First decision: April 13, 2015
First decision: April 13, 2015
Revised: May 21, 2015
Accepted: August 29, 2015
Article in press: August 31, 2015
Article in press: August 31, 2015
Published online: September 28, 2015
Hepatocellular carcinoma (HCC), the predominant form of primary liver cancer, is the fifth most common cancer worldwide and the second leading cause of cancer-related death. Despite the high incidence, treatment options remain limited for advanced HCC, and as a result prognosis continues to be poor. Current therapeutic options, surgery, chemotherapy and radiotherapy, have only modest efficacy. New treatment modalities to prolong survival and to minimize the risk of adverse response are desperately needed for patients with advanced HCC. Tumor immunotherapy is a promising, novel treatment strategy that may lead to improvements in both treatment-associated toxicity and outcome. The strategies have developed in part through genomic studies that have yielded candidate target molecules and in part through basic biology studies that have defined the pathways and cell types regulating immune response. Here, we summarize the various types of HCC immunotherapy and argue that the newfound field of HCC immunotherapy might provide critical advantages in the effort to improve prognosis of patients with advanced HCC. Already several immunotherapies, such as tumor-associated antigen therapy, immune checkpoint inhibitors and cell transfer immunotherapy, have demonstrated safety and feasibility in HCC patients. Unfortunately, immunotherapy currently has low efficacy in advanced stage HCC patients; overcoming this challenge will place immunotherapy at the forefront of HCC treatment, possibly in the near future.
Core tip: Hepatocellular carcinoma (HCC) has a high incidence and poor prognosis worldwide. Tumor immunotherapy is a promising novel therapy that will lead to improvements in both treatment-associated toxicity and outcome. This review summarizes current knowledge concerning the progress of immunotherapy for HCC.
- Citation: Tsuchiya N, Sawada Y, Endo I, Uemura Y, Nakatsura T. Potentiality of immunotherapy against hepatocellular carcinoma. World J Gastroenterol 2015; 21(36): 10314-10326
- URL: https://www.wjgnet.com/1007-9327/full/v21/i36/10314.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i36.10314
Primary liver cancer, which is predominantly diagnosed as hepatocellular carcinoma (HCC), is the fifth most common cancer and the second leading cause of cancer-related deaths worldwide. The annual number of deaths from HCC worldwide is similar to the incidence, with nearly 748300 new cases and 695900 deaths each year[1]. Although patients diagnosed with early stage disease have a relatively good prognosis with a 5-year survival rate of > 70%, the majority of patients are diagnosed with late-stage disease, which leads to a dismal overall 5-year survival rate of < 16%[2].
Protocols for the treatment options currently available tend to adhere to more established traditional methodologies, which includes surgery and adjuvant chemo/radiation therapy. Surgical or local ablative therapy however is generally suitable for only a few HCC patients, as these approaches are limited by tumor size, the number of intra-hepatic metastases, and sufficient hepatic functional reserve. Chemotherapy is moderately tolerated due to the coexistence of liver cirrhosis in most patients with HCC, but more importantly it is not sufficiently effective. Thus, the prognosis remains poor for patients with advanced HCC.
Targeted molecular therapy is one of the more contemporary approaches to cancer treatment and development of these drugs is based on specific molecular attributes of the cancer type in question. Sorafenib, an inhibitor of tyrosine kinases, was developed for the treatment of HCC as well as other cancer types. It has been found to prolong overall survival (OS) in patients with advanced HCC and thus has become the standard drug for first-line systemic treatment[3-5]. However, the response rate for sorafenib is very low according to the Response Evaluation Criteria in Solid Tumors (RECIST), while the incidence of adverse drug reactions is high according to the Common Terminology Criteria for Adverse Events (CTCAE)[3-5]. Sorafenib treatment extends life by only 3 mo as compared to placebo, and no second-line treatment has been established for patients who do not respond to the drug. The results for sorafenib reiterate how desperately new treatment modalities are needed in order to prolong survival while minimizing the risk of adverse reactions in patients with advanced HCC.
Immunotherapy is a step away from small molecule-based cancer therapies and is rapidly gaining broad attention from both basic scientists and clinicians as a viable treatment option for aggressive cancers. Many fundamental studies have demonstrated that tumor cells can be targeted by diverse immune effector mechanisms. Cytokines, peptides vaccines, monoclonal antibodies, and cell-mediated vaccines have been developed as potential treatments or approved cancer therapies that can provoke an immune response against cancers. Moreover, the strategy of adopting immunotherapy with established treatment modalities may increase efficacy and reduce toxicity.
Several characteristics relating to both the treatment and biology of HCC make it amenable to immunotherapy (as detailed below). However, while previous clinical trials have focused on the feasibility and safety of immunotherapy for patients with advanced HCC, non-randomized Phase I or II studies have yet to demonstrate the efficacy of immunotherapy for this disease[6,7]. However, several randomized controlled trials in adjuvant settings have established the ability of immunotherapy to reduce the risk of cancer recurrence[8-10]. Here, we summarize the various types of HCC immunotherapy and argue that the newfound field of HCC immunotherapy might provide critical advantages in the effort to improve the treatment and thus prognosis for HCC.
HCC can be deemed a cancer induced by inflammation. Chronic liver infections caused by hepatitis B and hepatitis C virus (HBV and HCV) are known risk factors for the development of HCC. Other risk factors include obesity, diabetes, alcoholic and nonalcoholic steatohepatitis, and other causes of chronic liver disease. Most patients with chronic hepatitis will develop liver cirrhosis and eventually HCC in a progressive but dynamic process. Liver cirrhosis is a highly genotoxic environment created by a perpetual state of inflammation and fibrogenesis. It is driven by the generation of highly reactive oxygen species, which in turn promote the formation of a type of senescent hepatocyte. Within this environment, some hepatocytes become immortalized and undergo carcinogenesis. Therefore, liver cirrhosis is a crucial disease state promoting the development of HCC.
According to several studies, tumor-specific cellular and humoral immune responses occur in patients with HCC. Spontaneous T cell responses against many tumor antigens, such as alpha-fetoprotein (AFP), glypican-3 (GPC3), NY-ESO-1, SSX-2, MAGE-A10 and p53, have been detected in HCC patients[11-17]. However, these T cell responses have failed to induce tumor regression and/or inhibit disease progression. The failure of the immune system to respond appropriately is likely to be due to the multiple immune-suppressive mechanisms that are regulated by the tumor itself. First, the expression of a number of molecules, such as major histocompatibility complex (MHC) proteins, that normally participate in the immune response is often altered in the context of cancer. Down-regulation of such genes, for example, leads to impairment of tumor-antigen processing and presentation. One study has shown strong MHC class I expression in HCC[18], but the results have not been consistent throughout the literature and the level of MHC class I expression in HCC remains unclear. Furthermore, expression of the co-stimulatory molecules B7-1 and B7-2 is reduced in HCC[19]. Second, impairment of CD4+ T cells has been reported as a mechanism of immune evasion in HCC[20]. Several results from the analysis of mRNA arrays have revealed that MHC class II is one of the most highly expressed genes in HCC tumors, as compared to benign adjacent tissues. These MHC II molecules induce CD4+ T cell anergy in the liver in the absence of interactions with suitable coactivators[21]. Finally, increases in immunosuppressive myeloid and lymphoid cell populations, suppression of natural killer (NK) cells, and up-regulation of immune checkpoint pathways have been demonstrated to impair the effector function of cellular immune responses in patients with HCC[22-25].
Immunosuppressive cell populations, such as T regulatory cells (Tregs) and myeloid-derived suppressor cells (MDSCs), are thought to be key players in cancer evasion from immunosurveillance. The number of Tregs in HCC patients is elevated in both the peripheral blood and the tumor[26], and the accumulation of infiltrated Tregs within the tumor has been correlated with disease progression and poor prognosis[27,28]. Thus, the depletion of Tregs may potentially lead to immune reactivation by facilitating spontaneous AFP-specific T cell responses[29].
MDSCs are a heterogeneous population of early myeloid progenitor cells that have the capacity to suppress the cytotoxic activities of NK cells and the adaptive immune response mediated by CD4+ and CD8+ T cells. MDSCs also promote the expansion of the Treg population. An accumulation of MDSCs has been found within tumors as well as in the peripheral blood, spleen, bone marrow, and liver of HCC patients[30]. It has also been suggested that MDSCs interact with Kupffer cells to induce the expression of programmed death-ligand 1 (PD-L1), which inhibits antigen presentation. The frequency of MDSCs in the peripheral blood has been correlated with a high risk of recurrence in HCC patients who have undergone radiofrequency ablation (RFA) treatment[22]. Recently, a new subset of immune suppressive cells known as regulatory dendritic cells (DCs) has been identified in HCC patients[31]. These regulatory DCs suppress T-cell activity in vitro through the production of interleukin (IL)-10 and indoleamine 2,3-dioxygenase (IDO).
The immune response engaged by a specific antigen and its subsequent intensity is regulated not only by major histocompatibility receptors, but also by co-stimulatory and co-inhibitory molecules that modulate response based on the physiological context. Immune checkpoints function as an extensive inhibitory program that is crucial for maintaining self-tolerance and modulating the duration and extent of physiological immune responses in peripheral tissues, ultimately helping to minimize extra tissue damage. Several immune checkpoint pathways have been shown to be exploited by tumors so as to aid in avoidance of immunosurveillance, particularly involving the T cell responses that are specific for tumor antigens. Many immune checkpoint molecules, such as the cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and PD-L1, have been detected in the tumor microenvironment, and are often overexpressed as well[32-35].
An intriguing finding is the detection of tumor-specific immune responses in patients simply undergoing conventional therapies. For example, RFA has been shown to stimulate activation and enhancement of tumor-specific T cells, but the procedure also increases the frequency of T cells specific for recall antigens[36]. Although this study was not primarily designed to evaluate the effect of RFA on T cell responses, the results indicated that RFA does activate non-specific T cell responses. However, no correlation between T cell response and prevention of HCC relapse was found. Tumor-associated antigen (TAA)-specific T cell responses have also been detected in peripheral blood following RFA[37]. Although patients displayed enhanced immune responses, tumor recurrence was not completely prevented. A second procedure, transarterial chemoembolization (TACE), has also been shown to have an effect on the frequency of tumor-specific T cell response in HCC patients. The expansion of AFP-specific CD4+ T cells in HCC patients after TACE has been described and furthermore was associated with an induction of > 50% tumor necrosis and improved clinical outcome[38].
Tumor-specific immune responses following individual treatment or combined TACE and RFA have been more directly investigated. The results have confirmed that ablative therapies induce TAA-specific T cell responses in individual patients[39,40]. Percutaneous ethanol injections (PEIT) or RFA has also been used to evaluate their impact on the function of dendritic cells in vivo[41]. The results demonstrated that local ablative therapy induces a functional transient activation of myeloid-derived dendritic cells associated with increased levels of TNF-α and IL-1-β. Finally, the frequency of natural killer T (NKT) cells in peripheral blood has also been reported to be increased after RFA treatment[42]. These studies clearly demonstrate that conventional treatment of HCC can unmask both innate and adoptive immune responses and therefore provide a rationale for a treatment strategy that combines immunotherapy with local ablative procedures.
The goal of immunotherapy in the treatment of cancer is to provide a clinical benefit by facilitating the immune response. The induction of long-lasting tumor-specific responses without autoimmunity is the ideal immunotherapeutic scenario. Several clinical trials have been conducted in which immunotherapy has been exploited to enhance anti-tumor responses in patients with advanced HCC, or to reduce the risk of recurrence following curative treatment (Table 1). Although many of these studies were small non-randomized trials, some at least demonstrated the feasibility and safety of the overall strategy. Based on these studies, immunotherapy was found to be safe, even in patients with liver cirrhosis, as severe adverse events were less intense and occurred less often when compared to conventional chemotherapies. However, most of them failed to demonstrate clinical efficacy.
| Author | Country | Year | Indication | Immunotherapy | n | Clinical result | Ref. | |
| Cytokine therapy | Llovet et al | Spain | 2000 | Advanced HCC | RCT: IFN-α2b vs no treatment | 30 and 28 | RR: 2/30 (7%), DCR: NANo significant difference in RR or survival | [91] |
| Ikeda et al | Japan | 2000 | Adjuvant(resection or ethanol injection) | RCT: IFN-βvs no treatment | 10 and 10 | Significantly longer recurrence-free survival after IFN-β therapy (P = 0.0004 | [92] | |
| Sakon et al | Japan | 2002 | Advanced HCC | 5-FU + IFN-α | 11 | RR: 8/11 (73%), DCR: 9/11 (82%)MST: NA | [93] | |
| Kubo et a | Japan | 2001 | Adjuvant (resection) | RCT: IFN-α vs. no treatment | 15 and 15 | Significantly longer recurrence-free survival after IFN-α therapy (P = 0.037) | [94] | |
| Ladhams et al | Australia | 2002 | Advanced HCC | Dendritic cell pulsed with autologous tumor | 2 | Slowing in the rate of tumor growth in one of two patients | [95] | |
| Palmieri et al | Italy | 2002 | Advanced HCC | Low dose IL-2 | 18 | RR: 3/18 (17%), DCR: 16/18 (89%)MST: 24.5 mo | [96] | |
| Reinisch et al | Austria | 2002 | Advanced HCC | GM-CSF + IFN-γ | 15 | RR: 1/15 (7%), DCR: 10/15 (67%)MST: 5.5 mo | [97] | |
| Feun et al | United States | 2003 | Advanced HCC | Doxorubicin + 5-FU + IFN-α2b | 30 | RR: 2/30 (7%), DCR: 3/30 (10%)MST: 3 mo | [99] | |
| Shiratori et al | Japan | 2003 | adjuvant (ethanol injection) | RCT: IFN-αvs no treatment | 49 and 25 | Longer recurrence-free and overall survival after IFN-α therapy (P-value not reported | [100] | |
| Patt et al | United States | 2003 | Advanced HCC | 5-FU + IFN-α2b | 43 | RR: 9/36 (25%), DCR 22/36 (61%)MST: 19.5 mo | [49] | |
| Komorizono et al | Japan | 2003 | Advanced HCC | Cisplatin + 5-FU + IFN-α | 6 | RR: 2/6 (33%), DCR 3/6 (50%)MST: NA | [50] | |
| Sangro et al | Spain | 2004 | Advanced HCC | Intratumoral adenovirus encoding IL-12 genes | 21 (8 HCC) | RR: 1/8 (13%), DCR 7/8 (88%)MST: NA | [102] | |
| Yin et al | China | 2005 | Advanced HCC | Cisplatin + doxorubicin + 5-FU + IFN-2α | 26 | RR: 4/26 (15%), DCR 13/26 (50%)MST: 6 mo | [105] | |
| Vitale et al | Italy | 2007 | Advanced HCC | 5-FU + IFN-α2b | 9 | RR: 3/9 (33%), DCR 4/9 (44%)MST: 11.5 mo | [108] | |
| TAA targeted therapy | Butterfield et al | United States | 2003 | Advanced HCC | AFP peptide vaccination | 6 | RR: 0/6 (0%), DCR 0/6 (0%)MST: 8 mo | [56] |
| Kuang et al | China | 2004 | Adjuvant | RCT: autologous formalin-fixed tumor vaccine vs no treatment | 18 and 21 | Significantly longer recurrence-free survival after vaccination (P = 0.003) | [8] | |
| Greten et al | Germany | 2010 | Advanced HCC | a telomerase peptide vaccine in combination with a low dose cyclophosphamide | 40 | RR: 0/40 (0%), DCR 17/37 (45.9%)MST: 9.8 mo | [114] | |
| Sawada et al | Japan | 2012 | Advanced HCC | GPC3-derived peptide vaccine | 33 | RR: 1/33 (3%), DCR 20/33 (60.6%)MST: 9.0 moOS was significantly longer in patients with high GPC3-specific CTL frequencies | [60] | |
| Zhu et al | United States | 2013 | Advanced HCC | GPC3 monoclonal antibody | 20 | RR: 0/20 (0%), DCR 4/20 (60.6%)MST in GPC3 high was likely to be longer than that in GPC3 low or no expression group [49.4 wk vs 13.0 wk] | [61] | |
| Immune checkpoint inhibitors | Sangro et al | Spain | 2013 | Advanced HCC | anti-CTLA-4 antibody | 21 | RR: 3/21 (17.6%), DCR 13/21 (76.4%)MST: 8.2 mo | [69] |
| Cell transfer immunotherapy | Takayama et al | Japan | 2000 | Adjuvant (resection) | RCT: activated autologous lymphocyte vs no treatment | 76 and 74 | Significantly longer recurrence-free survival after transfer of activated lymphocytes (P = 0.008) | [10] |
| Stift et al | Austria | 2003 | Advanced HCC | Dendritic cell pulsed with autologous tumor | 20 (2 HCC) | RR: NA, DCR: NAMST: 10.5 moPersistent AFP over a period of 6 mo in one of two patients | [98] | |
| Iwashita et al | Japan | 2003 | Advanced HCC | Dendritic cell pulsed with autologous tumor | 10 (8 HCC) | RR: 0/8 (0%), DCR 6/8 (75%)MST: NA | [101] | |
| Shi et al | China | 2004 | Advanced and early HCC | Cytokine induced killer cell | 13 | RR: NA, DCR: NAMST: NA | [78] | |
| Mazzolini et al | Spain | 2005 | Advanced HCC | Dendritic cell transfected with adenovirus encoding IL-12 gene | 17 (8 HCC) | RR: 0/0 (0%), DCR: 2/8 (25%)MST: NA | [103] | |
| Chi | Taiwan | 2005 | Advanced HCC | Local radiation + intratumoral DC injection | 14 | RR: 2/14 (14%), DCR 9/14 (64%)MST: 5.6 mo | [104] | |
| Lee et al | Taiwan | 2005 | Advanced HCC | Dendritic cell pulsed with autologous tumor | 31 | RR: 4/31 (13%), DCR 21/31 (68%)MST: NA | [79] | |
| Kumagi et al | Japan | 2005 | Advanced HCC | Intratumoral dendritic cell injection after ethanol injection | 4 | Feasibility study | [106] | |
| Butterfield et al | United States | 2006 | Advanced HCC | Dendritic cell pulsed with AFP peptide | 10 | RR: 0/10 (0%), DCR 0/10 (0%)MST: 7.5 mo | [55] | |
| Nakamoto et al | Japan | 2007 | Advanced and early HCC | Non-RCT: TACE + dendritic cell vs TACE alone | 10 and 11 | No significant difference in survival | [107] | |
| Weng et al | China | 2008 | Adjuvant (TACE and RFA) | RCT: cytokine induced killer cell vs no treatment | 45 and 40 | Significantly longer recurrence-free survival after immunotherapy (P = 0.01) | [109] | |
| Hui et al | China | 2009 | Adjuvant (resection) | RCT: cytokine induced killer cell 3 courses vs 6 courses vs no treatment | 41, 43 and 43 | Significantly longer recurrence-free survival after immunotherapy (P = 0.001 and 0.004) | [110] | |
| Palmer et al | United Kingdom | 2009 | Advanced HCC | Dendritic cell pulsed with liver tumor cell line lysate (HepG2) | 35 | RR: 1/25 (4%), DCR 7/25 (28%)MST: 5.6 mo | [111] | |
| Olioso et al | Italy | 2009 | Advanced HCC | Cytokine induced killer cell + IFN-α | 12 (1 HCC) | Complete responseSurvival time: 33 mo (alive) | [112] | |
| Hao et al | China | 2010 | Advanced HCC | Non-RCT: TACE + cytokine induced killer cell vs TACE alone | 72 and 74 | Significantly longer survival after combination therapy (P < 0.001) | [113] | |
| Ma et al | China | 2010 | Adjuvant (RFA) | RFA and autologous RetroNectin activated killer cells | 7 | During a 7-mo follow-up, no severe adverse events, recurrences, or deaths | [115] | |
| Nakamoto et al | Japan | 2011 | Adjuvant (TACE) | TACE + OK432-stimulated DCs vs TACE alone | 13 and 22 | Significantly longer recurrence-free survival after immunotherapy (P = 0.046) | [80] | |
| Zhou et al | China | 2011 | HCC with hepatitis B (PMWA) | Immature DCs, CIK, CTL and tumor lysate-pulsed DC | 10 | This Phase I study revealed this therapy was safe and increased the percentage of effector cells | [116] | |
| Qiu et al | China | 2011 | Adjuvant (resection) | RCT: TAA-pulsed DC and CIK vs no treatment | 9 vs 9 | Significantly longer recurrence-free survival after immunotherapy (P = 0.00121) | [117] | |
| Tada et al | Japan | 2012 | Advanced HCC | TACE + multiple TAA-pulsed DC | 5 | This Phase I/II study revealed this therapy was safe and increased the percentage of effector cells | [118] | |
| Cui et al | China | 2014 | Adjuvant (RFA) | RFA + NK cells, γδT cells and CIK cells vs RFA alone | 30 and 32 | Significantly longer recurrence-free survival after immunotherapy | [76] |
Efforts to enhance the immune response in the context of HCC have been undertaken through methods that incorporate immunostimulatory cytokines. For instance, interferon (IFN), which is used in patients with viral hepatitis, has been utilized in patients with HCC[43]. IFN monotherapy has been investigated in an adjuvant setting not only to prevent tumor recurrence but also to inhibit any development of cancer, as in the case of patients with chronic hepatitis B or C virus.
IFN-α induces an antitumor response by enhancing cytotoxicity, tumor antigen presentation and lymphocyte proliferation, and by blocking angiogenesis[44,45]. IFN-α treatment for HCC has demonstrated some clinical efficacy, perhaps by preventing or delaying tumor relapse after curative resection or ablation, with good tolerance[46,47].
Lai et al[48] reported that IFN-α was effective for prolonging survival and inducing tumor regression in patients with advanced HCC. IFN-α has also been used in combination with chemotherapy, such as cisplatin and 5-fluorouracil (5-FU), for advanced HCC[49,50]. Obi et al[51] reported that in HCC patients with portal venous invasion a combination of 5-FU and IFN-γ treatment led to complete response in 16% and partial response in 36%. IFN-γ alone induced apoptosis and inhibited cell growth in HCC[52]. The combination of IFN-γ and granulocyte-macrophage colony-stimulating factor (GM-CSF) was tested and found to be effective in selected advanced HCC patients. However, due to the controversy concerning the effect of IFN on HCC patients and the high rate of toxicity, this regimen cannot be recommended as a standard treatment for all HCC patients.
IL-2, one of the most highly immunostimulatory cytokines, has key functions in the immune system. The effect of IL-2 has been evaluated in various cancers, particularly melanoma and renal cell carcinoma, for its ability to stimulate the proliferation and activity of T cells that would cause tumor regression[53,54]. IL-2 has also been tested for treating HCC patients, but unfortunately, no effect was noted.
A particularly innovative strategy involving the immune system has been to exploit the constellation of unique proteins that are expressed specifically on tumor cells. Such proteins, or so-called TAAs, are mutant or aberrantly expressed antigens that are recognizable by the adaptive immune system. For instance, tumor-specific CD8+ T cells are considered to be critical for cancer control. These T cells recognize 8-11 amino acid peptides that are derived from TAAs and are presented in association with MHC class I complexes. TAAs have been targeted through the isolation of antigen-specific human monoclonal antibodies or the design of humanized or chimeric monoclonal antibodies. Tumor-targeted antibodies such as rituximab (CD20) for lymphoma, trastuzumab (HER2/NEU) for breast cancer, bevacizumab (VEGFA) for metastatic colorectal cancers and lung cancer, and cetuximab (EGFR) for metastatic colorectal cancer and advanced head-and-neck cancer have been shown to be effective and are currently part of standard treatment modalities.
Many TAAs have been targeted for HCC immunotherapy. AFP has been the most thoroughly investigated as a potential HCC tumor-specific antigen. Several AFP-based immunotherapeutic regimens have been reported; however, no dramatic clinical benefits were observed[55,56].
GPC3 is a member of the glypican family of heparan sulfate proteoglycans that are attached to the cell surface via a glycosylphosphatidylinositol (GPI) anchor. GPC3 is not only tumor-specific but also has a role in cell proliferation; therefore, it is an attractive target for HCC. GPC3 is specifically overexpressed in 70%-81% of HCC tumors, and it correlates with poor prognosis[57,58]. GPC3 can be used as a serum biomarker, and immunostaining for GPC3 is recommended for the pathologic diagnosis of early stage HCC by international guidelines[59].
The feasibility of peptide-based vaccine or antibody immunotherapy targeting GPC3 has been investigated in several studies. We have conducted a Phase I clinical trial using GPC3-derived peptide vaccines in patients with advanced HCC[60]. Advanced HCC patients (n = 33) were administered GPC3 vaccination with the following protocol: intradermal injections on days 1, 15 and 29 with dose escalation. GPC3298-306 (EYILSLEEL) was used in HLA-A24-positive patients and GPC3144-152 (FVGEFFTDV) in HLA-A2-positive patients. The GPC3-derived peptide vaccines demonstrated antitumor effects as well as safety. A partial response (PR) was observed in one patient and in 4/19 patients with stable disease (SD); the degree of tumor necrosis or regression exhibited did not meet the criteria for PRs. Two months after the initiation of treatment, the disease control rate (PR + SD) was 60.6%. The serum AFP and/or des-γ-carboxy prothrombin levels associated with HCC decreased temporarily in nine patients. Moreover, we analyzed the GPC3-specific cytotoxic T lymphocyte (CTL) frequency ex vivo with an IFN-γ enzyme-linked immunospot (ELISPOT) assay. Increased numbers of GPC3 peptide-specific CTLs in peripheral blood were found in 30 patients following GPC3 peptide vaccination. The frequency of GPC3 peptide-specific CTLs in the peripheral blood was also correlated with OS in HCC patients who received the peptide vaccination. In fact, the frequency of GPC3 peptide-specific CTLs constituted the only prognostic factor for OS in this trial. The median OS was 12.2 mo (95%CI: 6.5-18.0) in patients with a high frequency of GPC3 peptide-specific CTLs versus 8.5 mo (95%CI: 3.7-13.1) in individuals with a low GPC3-specific CTL frequency (P = 0.033). These observations indicate that GPC3-derived peptide vaccines represent a novel immunotherapeutic strategy for patients with HCC and have the potential to improve OS.
Subsequently, a Phase II study of the GPC3-derived peptide vaccine was conducted as an adjuvant therapy for HCC patients. Patients (n = 40) with primary HCC who had undergone surgery or radiofrequency ablation were enrolled in this open-label, single-arm trial. They received 10 vaccinations over 1 year following curative treatment. The primary endpoints were the 1- and 2-year recurrence rates. The secondary endpoints were immunological responses, as measured by the IFN-γ ELISPOT assay. The correlations between the time of recurrence and immunological responses are currently being analyzed.
We have also conducted a trial to determine whether tumor-infiltrating lymphocytes with an antitumor effect are actually increased in advanced HCC cases. Liver biopsies were performed before and after GPC3 peptide vaccination, as per the protocol. In this trial, GPC3 peptide-specific CTLs were detected in liver biopsy specimens of one patient by flow cytometry with dextramer staining. The results of this study will be reported in the near future.
In another approach, GPC3 antibodies have been developed and are currently under clinical evaluation for the treatment of HCC patients. GC33 is a novel recombinant fully humanized monoclonal antibody that binds to human GPC3. In a Phase I study, patients (n = 20) treated with GC33 showed good tolerance and no dose-limiting toxicity[61]. An SD of more than 26 wk was observed in 4/15 (16.7%) patients, all of who were in the GPC3 high expression group. The median OS in the GPC3 high expression group (49.4 wk, 95%CI: 12.6-81.0 wk) was greater in the GPC3 low or no expression group (13.0 wk, 95%CI: 10.6-54.9 wk; P = 0.142). Thus, antitumor activity associated with the target GPC3 expression was observed in this study.
A Phase I study has also been performed to elucidate the therapeutic benefit of a combination therapy of GC33 and sorafenib. A Phase II study of GC33 in patients with advanced or metastatic HCC has also been completed and the results are currently undergoing analysis.
Other TAAs have also been explored as potential vaccine targets. MAGE-A1 is overexpressed in approximately 65% of HCCs, MAGE-A3 in approximately 70%, and NY-ESO-1 in approximately 45%[62]. However, to our knowledge, there have been no reports of these TAAs in vaccine trials for HCC. On the basis of genomic data sets, a number of TAAs overexpressed in HCC will potentially prove to be highly immunogenic. Furthermore, elucidation of the underlying molecular and cellular mechanisms involved may further advance TAA targeted immunotherapy as a viable treatment option.
The balance between co-stimulatory and co-inhibitory signals determines the cytotoxic T-cell activation and intensity of the immune response[63]. Immune checkpoint receptors are often upregulated in tumor tissue and promote tumor evasion from host immunosurveillance. As receptors, they readily emerged as a novel class of potential targets to be modulated in order to stimulate the immune response. CTLA-4, PD-1, TIM-3, LAG-3, BTLA, VISTA, and OX40 are the most studied immune checkpoint receptors.
CTLA-4 has been the first immune checkpoint receptor to be clinically targeted. It is expressed exclusively on activated T cells, Tregs, and naïve T cells[64-66]. CTLA-4 binds to CD80/CD86 with much higher affinity than CD28, antagonizes the CD28 binding to CD80/CD86, and inhibits T-cell and antigen-presenting cell (APC) activation[17,67]. Ipilimumab blocks the interaction of CTLA-4 with its ligands CD80/CD86 and thereby promotes T-cell activation[68]. The United States Food and Drug Administration (FDA) approved ipilimumab for the treatment of melanoma in 2011 based on a randomized Phase III trial where OS was improved by nearly 4 mo.
Efficacy of a different anti-CTLA-4 antibody, tremelimumab was also examined in a Phase I trial for HCC patients (n = 21) with inoperable cancer and chronic HCV infection[69]. Tremelimumab was well tolerated, and there were no treatment-related deaths. PR and SD rates were 17.6% and 76.4% respectively, and 45% of the patients experienced SD for more than 6 mo. Interestingly, tumor response was greater in patients with stable levels of IFN-γ during the treatment compared to those with lower levels. It was proposed that more active antitumor immunity occurred in these patients in response to the therapy. A Phase I clinical trial combining tremelimumab with RFA or TACE is ongoing.
PD-1 is a CD28 superfamily member that transmits co-inhibitory signals for the TCR receptor and is expressed not only on activated T and B cells, but also Tregs and MDSCs[70]. PD-1 mediates the differentiation and proliferation of Tregs and therefore regulates peripheral tolerance and autoimmunity[71]. Preliminary clinical results for an antibody against PD-1, lambrolizumab, indicate that this protein is a critical target for the enhancement of antitumor immunity and with the potential to produce durable antitumor efficacy. Lambrolizumab induced tumor regression in advanced melanoma patients with a favorable tolerance[72]. Interestingly, lambrolizumab was effective even in patients who failed ipilimumab treatment, indicating a fundamental distinction in the mechanisms underlying PD-1 and CTLA-4 blockade. Finally, the combination of nivolumab, a different PD-1 antibody, with ipilimumab achieved antitumor response with limited toxicity in 40% of patients with advanced hematologic malignancy[73]. A Phase I trial of nivolumab for patients with advanced HCC is currently underway (NCT01658878).
Adoptive cell therapy (ACT) is exploits the cancer patient’s own lymphocytes with anti-tumor activity, and works by expanding the cell population ex vivo and then reinfusing into the patient.
ACT therapy has been demonstrated to be effective in the treatment of a number of tumor types, such as melanoma, renal cell carcinoma and neuroblastoma[62,74,75]. In the first randomized study of ACT therapy in HCC patients, IL-2 and anti-CD3 were used to stimulate autologous peripheral blood mononuclear cells (PBMCs) in an adjuvant setting following curative resection of HCC. The ACT therapy reduced tumor recurrence by 41%[10]. Sequential RFA/ACT has also been shown to significantly reduced the risk of HCC recurrence compared to RFA alone [hazard ratio (HR) = 50.136, 95%CI: 0.049-0.379][76]. While the efficacy of ACT in HCC patients remains an unknown, the feasibility and safety of this therapeutic approach have been evaluated[77,78].
In a second strategy, DC-based immunotherapy has been attempted for HCC patients with various methods of stimulating the cells. In the first Phase I/II clinical trial, AFP peptide-pulsed DCs were used in patients (n = 39), and the disease control rate (PR + SD) was 28%[55]. In a second method, DCs were pulsed with tumor lysate. DCs treated with tumor lysate rather than a specific molecular target demonstrated a 10% PR in HCC patients, confirming the feasibility and safety of DC vaccination in these patients[79]. Finally, DCs were stimulated with OK432, a streptococcus-derived anti-cancer immunotherapeutic agent, and transferred into tumor tissues during/after transcatheter hepatic arterial embolization (TAE)[80]. The immunomodulatory effects and clinical responses were evaluated in HCC patients (n = 13) and compared to historical controls (n = 22) treated with TAE only. The patients treated with OK432 stimulated DCs and TAE had prolonged recurrence-free survival compared to the historical controls (recurrence rates 360 days after treatments were 2/13 and 12/22, respectively; P = 0.046). The results demonstrated that DCs stimulated with OK432 and transferred after TAE reduced tumor recurrence in HCC patients.
The third cell type used in an effort to enhance immunotherapy for HCC has been T cells modified with a chimeric antigen receptor (CAR)[81]. CAR T cells can specifically recognize tumor-associated antigens and eliminate tumor cells in a MHC-independent manner. Second- or third-generation CAR improved the anticancer effect; most of the clinical successes of CAR T cell immunotherapy have been achieved in the setting of CD19-positive B-cell hematologic malignancies where the CR rate was 69%-90%[82-88]. In addition, GPC3-specific CAR T cells have been developed, and their potential for HCC treatment evaluated[89]. The results revealed that T cells expressing third-generation GPC3-targeted CAR have the ability to eliminate HCC xenografts with high GPC3 expression in mice. Finally, NK cell infusions are being tested as therapy for refractory tumors. NK cells have diverse immunological functions that also include recognizing and killing cancer cells. For HCC, the trial of Safety Study of Liver Natural Killer Cell Therapy for Hepatoma Liver Transplantation (MIAMI NK) has only just been completed (NCT01147380).
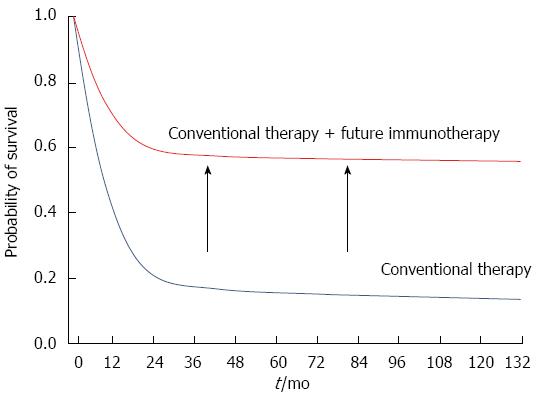
Immunotherapy is one of the most promising and biologically intriguing strategies today for the treatment of aggressive human cancers. The approach is gaining momentum through rigorous clinical and basic research efforts. However, to establish the benefit of tumor immunotherapy in the treatment of HCC and to expand the indications for this approach, several challenges must be addressed. First, it is necessary to better define the patient population that will benefit from immunologic therapies and expand this population to encompass more than a minority of patients. This includes the identification of novel biomarkers to predict who may benefit from immunotherapy. Second, although several immunotherapies demonstrated feasibility and safety, the anti-tumor effect has been inadequate overall. Combination strategies with another immunotherapy or conventional cancer therapy, such as chemotherapy and radiotherapy, may increase the percentage of patients that respond to tumor immunotherapy. Therefore studies are needed to determine which combinations are the most effective. RFA and TACE used routinely in patients with HCC have been shown to induce immune responses and could potentially synergize with tumor immunotherapy. Indeed, several combination strategies have demonstrated augmented antitumor effects and are likely to improve survival rates similar to other cancers (Figure 1). For instance, we recently demonstrated that a PD-1/PD-L1 blockade facilitated the antitumor effects of a peptide vaccine by enhancing the immune response of vaccine-induced CTLs in a murine model[90]. Such studies would lay the foundation for the clinical development of a combination therapy. Third, sufficient evaluation criteria for immunotherapy are needed. Two different response evaluation tools already exist for HCC: RECIST and modified RECIST (mRECIST) (Table 2). Otherwise, the effect of immunotherapy is evaluated by immune-related response criteria (irRC) or irRECIST. The cancer response to immunotherapy is difficult to evaluate because its effect is delayed. These criteria need to complement one another and precisely evaluate the subsequent effect of immunotherapy for HCC. Addressing these problems will greatly facilitate realization of the full potential of these novel immunotherapies for the treatment of this intractable disease.
P- Reviewer: Peltec A, Parvez MK S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23762] [Cited by in F6Publishing: 25182] [Article Influence: 1937.1] [Reference Citation Analysis (3)] |
| 2. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9215] [Cited by in F6Publishing: 9719] [Article Influence: 883.5] [Reference Citation Analysis (3)] |
| 3. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3854] [Cited by in F6Publishing: 4336] [Article Influence: 271.0] [Reference Citation Analysis (0)] |
| 4. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9016] [Cited by in F6Publishing: 9507] [Article Influence: 594.2] [Reference Citation Analysis (1)] |
| 5. | Morimoto M, Numata K, Kondo M, Hidaka H, Takada J, Shibuya A, Kobayashi S, Ohkawa S, Okuse C, Morita S. Higher discontinuation and lower survival rates are likely in elderly Japanese patients with advanced hepatocellular carcinoma receiving sorafenib. Hepatol Res. 2011;41:296-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Breous E, Thimme R. Potential of immunotherapy for hepatocellular carcinoma. J Hepatol. 2011;54:830-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Greten TF, Manns MP, Korangy F. Immunotherapy of hepatocellular carcinoma. J Hepatol. 2006;45:868-878. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Kuang M, Peng BG, Lu MD, Liang LJ, Huang JF, He Q, Hua YP, Totsuka S, Liu SQ, Leong KW. Phase II randomized trial of autologous formalin-fixed tumor vaccine for postsurgical recurrence of hepatocellular carcinoma. Clin Cancer Res. 2004;10:1574-1579. [PubMed] [Cited in This Article: ] |
| 9. | Peng BG, Liang LJ, He Q, Kuang M, Lia JM, Lu MD, Huang JF. Tumor vaccine against recurrence of hepatocellular carcinoma. World J Gastroenterol. 2005;11:700-704. [PubMed] [Cited in This Article: ] |
| 10. | Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356:802-807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 630] [Cited by in F6Publishing: 626] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 11. | Bricard G, Bouzourene H, Martinet O, Rimoldi D, Halkic N, Gillet M, Chaubert P, Macdonald HR, Romero P, Cerottini JC. Naturally acquired MAGE-A10- and SSX-2-specific CD8+ T cell responses in patients with hepatocellular carcinoma. J Immunol. 2005;174:1709-1716. [PubMed] [Cited in This Article: ] |
| 12. | Butterfield LH, Koh A, Meng W, Vollmer CM, Ribas A, Dissette V, Lee E, Glaspy JA, McBride WH, Economou JS. Generation of human T-cell responses to an HLA-A2.1-restricted peptide epitope derived from alpha-fetoprotein. Cancer Res. 1999;59:3134-3142. [PubMed] [Cited in This Article: ] |
| 13. | Cicinnati VR, Zhang X, Yu Z, Ferencik S, Schmitz KJ, Dworacki G, Kaczmarek E, Oldhafer K, Frilling A, Baba HA. Increased frequencies of CD8+ T lymphocytes recognizing wild-type p53-derived epitopes in peripheral blood correlate with presence of epitope loss tumor variants in patients with hepatocellular carcinoma. Int J Cancer. 2006;119:2851-2860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Komori H, Nakatsura T, Senju S, Yoshitake Y, Motomura Y, Ikuta Y, Fukuma D, Yokomine K, Harao M, Beppu T. Identification of HLA-A2- or HLA-A24-restricted CTL epitopes possibly useful for glypican-3-specific immunotherapy of hepatocellular carcinoma. Clin Cancer Res. 2006;12:2689-2697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 139] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 15. | Korangy F, Ormandy LA, Bleck JS, Klempnauer J, Wilkens L, Manns MP, Greten TF. Spontaneous tumor-specific humoral and cellular immune responses to NY-ESO-1 in hepatocellular carcinoma. Clin Cancer Res. 2004;10:4332-4341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 120] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Mizukoshi E, Nakamoto Y, Tsuji H, Yamashita T, Kaneko S. Identification of alpha-fetoprotein-derived peptides recognized by cytotoxic T lymphocytes in HLA-A24+ patients with hepatocellular carcinoma. Int J Cancer. 2006;118:1194-1204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Schneider H, Downey J, Smith A, Zinselmeyer BH, Rush C, Brewer JM, Wei B, Hogg N, Garside P, Rudd CE. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972-1975. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 457] [Cited by in F6Publishing: 463] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 18. | Wadee AA, Paterson A, Coplan KA, Reddy SG. HLA expression in hepatocellular carcinoma cell lines. Clin Exp Immunol. 1994;97:328-333. [PubMed] [Cited in This Article: ] |
| 19. | Fujiwara K, Higashi T, Nouso K, Nakatsukasa H, Kobayashi Y, Uemura M, Nakamura S, Sato S, Hanafusa T, Yumoto Y. Decreased expression of B7 costimulatory molecules and major histocompatibility complex class-I in human hepatocellular carcinoma. J Gastroenterol Hepatol. 2004;19:1121-1127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Alisa A, Ives A, Pathan AA, Navarrete CV, Williams R, Bertoletti A, Behboudi S. Analysis of CD4+ T-Cell responses to a novel alpha-fetoprotein-derived epitope in hepatocellular carcinoma patients. Clin Cancer Res. 2005;11:6686-6694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Crispe IN, Giannandrea M, Klein I, John B, Sampson B, Wuensch S. Cellular and molecular mechanisms of liver tolerance. Immunol Rev. 2006;213:101-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 175] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 22. | Arihara F, Mizukoshi E, Kitahara M, Takata Y, Arai K, Yamashita T, Nakamoto Y, Kaneko S. Increase in CD14+HLA-DR -/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol Immunother. 2013;62:1421-1430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 195] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 23. | Chen Z, Shen S, Peng B, Tao J. Intratumoural GM-CSF microspheres and CTLA-4 blockade enhance the antitumour immunity induced by thermal ablation in a subcutaneous murine hepatoma model. Int J Hyperthermia. 2009;25:374-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, Lehner F, Manns MP, Greten TF, Korangy F. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology. 2009;50:799-807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 447] [Cited by in F6Publishing: 468] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 25. | Ilkovitch D, Lopez DM. The liver is a site for tumor-induced myeloid-derived suppressor cell accumulation and immunosuppression. Cancer Res. 2009;69:5514-5521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 26. | Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65:2457-2464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 452] [Cited by in F6Publishing: 462] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 27. | Chen KJ, Lin SZ, Zhou L, Xie HY, Zhou WH, Taki-Eldin A, Zheng SS. Selective recruitment of regulatory T cell through CCR6-CCL20 in hepatocellular carcinoma fosters tumor progression and predicts poor prognosis. PLoS One. 2011;6:e24671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 176] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 28. | Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586-2593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 787] [Cited by in F6Publishing: 845] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 29. | Greten TF, Ormandy LA, Fikuart A, Höchst B, Henschen S, Hörning M, Manns MP, Korangy F. Low-dose cyclophosphamide treatment impairs regulatory T cells and unmasks AFP-specific CD4+ T-cell responses in patients with advanced HCC. J Immunother. 2010;33:211-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 30. | Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Krüger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 601] [Cited by in F6Publishing: 603] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 31. | Han Y, Chen Z, Yang Y, Jiang Z, Gu Y, Liu Y, Lin C, Pan Z, Yu Y, Jiang M. Human CD14+ CTLA-4+ regulatory dendritic cells suppress T-cell response by cytotoxic T-lymphocyte antigen-4-dependent IL-10 and indoleamine-2,3-dioxygenase production in hepatocellular carcinoma. Hepatology. 2014;59:567-579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 32. | Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971-979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 563] [Cited by in F6Publishing: 625] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 33. | Kalathil S, Lugade AA, Miller A, Iyer R, Thanavala Y. Higher frequencies of GARP(+)CTLA-4(+)Foxp3(+) T regulatory cells and myeloid-derived suppressor cells in hepatocellular carcinoma patients are associated with impaired T-cell functionality. Cancer Res. 2013;73:2435-2444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 207] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 34. | Shi F, Shi M, Zeng Z, Qi RZ, Liu ZW, Zhang JY, Yang YP, Tien P, Wang FS. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer. 2011;128:887-896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 340] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 35. | Wang BJ, Bao JJ, Wang JZ, Wang Y, Jiang M, Xing MY, Zhang WG, Qi JY, Roggendorf M, Lu MJ. Immunostaining of PD-1/PD-Ls in liver tissues of patients with hepatitis and hepatocellular carcinoma. World J Gastroenterol. 2011;17:3322-3329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 104] [Cited by in F6Publishing: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 36. | Zerbini A, Pilli M, Penna A, Pelosi G, Schianchi C, Molinari A, Schivazappa S, Zibera C, Fagnoni FF, Ferrari C. Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses. Cancer Res. 2006;66:1139-1146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 183] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 37. | Mizukoshi E, Yamashita T, Arai K, Sunagozaka H, Ueda T, Arihara F, Kagaya T, Yamashita T, Fushimi K, Kaneko S. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology. 2013;57:1448-1457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 180] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 38. | Ayaru L, Pereira SP, Alisa A, Pathan AA, Williams R, Davidson B, Burroughs AK, Meyer T, Behboudi S. Unmasking of alpha-fetoprotein-specific CD4(+) T cell responses in hepatocellular carcinoma patients undergoing embolization. J Immunol. 2007;178:1914-1922. [PubMed] [Cited in This Article: ] |
| 39. | Hiroishi K, Eguchi J, Baba T, Shimazaki T, Ishii S, Hiraide A, Sakaki M, Doi H, Uozumi S, Omori R. Strong CD8(+) T-cell responses against tumor-associated antigens prolong the recurrence-free interval after tumor treatment in patients with hepatocellular carcinoma. J Gastroenterol. 2010;45:451-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 40. | Nobuoka D, Motomura Y, Shirakawa H, Yoshikawa T, Kuronuma T, Takahashi M, Nakachi K, Ishii H, Furuse J, Gotohda N. Radiofrequency ablation for hepatocellular carcinoma induces glypican-3 peptide-specific cytotoxic T lymphocytes. Int J Oncol. 2012;40:63-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Ali MY, Grimm CF, Ritter M, Mohr L, Allgaier HP, Weth R, Bocher WO, Endrulat K, Blum HE, Geissler M. Activation of dendritic cells by local ablation of hepatocellular carcinoma. J Hepatol. 2005;43:817-822. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 42. | Okumura A, Ishikawa T, Maeno T, Sato K, Ayada M, Hotta N, Yamauchi T, Fukuzawa Y, Kakumu S. Changes in natural killer T cells subsets during therapy in type C hepatitis and hepatocellular carcinoma. Hepatol Res. 2005;32:213-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Rougier P, Mitry E, Barbare JC, Taieb J. Hepatocellular carcinoma (HCC): an update. Semin Oncol. 2007;34:S12-S20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Belardelli F, Ferrantini M, Proietti E, Kirkwood JM. Interferon-alpha in tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:119-134. [PubMed] [Cited in This Article: ] |
| 45. | Singh RK, Gutman M, Bucana CD, Sanchez R, Llansa N, Fidler IJ. Interferons alpha and beta down-regulate the expression of basic fibroblast growth factor in human carcinomas. Proc Natl Acad Sci USA. 1995;92:4562-4566. [PubMed] [Cited in This Article: ] |
| 46. | Sun HC, Tang ZY, Wang L, Qin LX, Ma ZC, Ye QH, Zhang BH, Qian YB, Wu ZQ, Fan J. Postoperative interferon alpha treatment postponed recurrence and improved overall survival in patients after curative resection of HBV-related hepatocellular carcinoma: a randomized clinical trial. J Cancer Res Clin Oncol. 2006;132:458-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 172] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 47. | Lin SM, Lin CJ, Hsu CW, Tai DI, Sheen IS, Lin DY, Liaw YF. Prospective randomized controlled study of interferon-alpha in preventing hepatocellular carcinoma recurrence after medical ablation therapy for primary tumors. Cancer. 2004;100:376-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 48. | Lai CL, Lau JY, Wu PC, Ngan H, Chung HT, Mitchell SJ, Corbett TJ, Chow AW, Lin HJ. Recombinant interferon-alpha in inoperable hepatocellular carcinoma: a randomized controlled trial. Hepatology. 1993;17:389-394. [PubMed] [Cited in This Article: ] |
| 49. | Patt YZ, Hassan MM, Lozano RD, Brown TD, Vauthey JN, Curley SA, Ellis LM. Phase II trial of systemic continuous fluorouracil and subcutaneous recombinant interferon Alfa-2b for treatment of hepatocellular carcinoma. J Clin Oncol. 2003;21:421-427. [PubMed] [Cited in This Article: ] |
| 50. | Komorizono Y, Kohara K, Oketani M, Maeda M, Shibathou T, Shigenobu S, Hiramine Y, Yamasaki N, Arima T, Kazuaki I. Systemic combined chemotherapy with low dose of 5-fluorouracil, cisplatin, and interferon-alpha for advanced hepatocellular carcinoma: a pilot study. Dig Dis Sci. 2003;48:877-881. [PubMed] [Cited in This Article: ] |
| 51. | Obi S, Yoshida H, Toune R, Unuma T, Kanda M, Sato S, Tateishi R, Teratani T, Shiina S, Omata M. Combination therapy of intraarterial 5-fluorouracil and systemic interferon-alpha for advanced hepatocellular carcinoma with portal venous invasion. Cancer. 2006;106:1990-1997. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 223] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 52. | Vadrot N, Legrand A, Nello E, Bringuier AF, Guillot R, Feldmann G. Inducible nitric oxide synthase (iNOS) activity could be responsible for resistance or sensitivity to IFN-gamma-induced apoptosis in several human hepatoma cell lines. J Interferon Cytokine Res. 2006;26:901-913. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Rosenberg SA, Yang JC, White DE, Steinberg SM. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann Surg. 1998;228:307-319. [PubMed] [Cited in This Article: ] |
| 54. | Cheng Y, Zhang G, Li G. Targeting MAPK pathway in melanoma therapy. Cancer Metastasis Rev. 2013;32:567-584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 55. | Butterfield LH, Ribas A, Dissette VB, Lee Y, Yang JQ, De la Rocha P, Duran SD, Hernandez J, Seja E, Potter DM. A phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four alpha-fetoprotein peptides. Clin Cancer Res. 2006;12:2817-2825. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 56. | Butterfield LH, Ribas A, Meng WS, Dissette VB, Amarnani S, Vu HT, Seja E, Todd K, Glaspy JA, McBride WH. T-cell responses to HLA-A*0201 immunodominant peptides derived from alpha-fetoprotein in patients with hepatocellular cancer. Clin Cancer Res. 2003;9:5902-5908. [PubMed] [Cited in This Article: ] |
| 57. | Nakatsura T, Yoshitake Y, Senju S, Monji M, Komori H, Motomura Y, Hosaka S, Beppu T, Ishiko T, Kamohara H. Glypican-3, overexpressed specifically in human hepatocellular carcinoma, is a novel tumor marker. Biochem Biophys Res Commun. 2003;306:16-25. [PubMed] [Cited in This Article: ] |
| 58. | Shirakawa H, Suzuki H, Shimomura M, Kojima M, Gotohda N, Takahashi S, Nakagohri T, Konishi M, Kobayashi N, Kinoshita T. Glypican-3 expression is correlated with poor prognosis in hepatocellular carcinoma. Cancer Sci. 2009;100:1403-1407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 192] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 59. | International Consensus Group for Hepatocellular NeoplasiaThe International Consensus Group for Hepatocellular Neoplasia. Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology. 2009;49:658-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 610] [Cited by in F6Publishing: 554] [Article Influence: 36.9] [Reference Citation Analysis (1)] |
| 60. | Sawada Y, Yoshikawa T, Nobuoka D, Shirakawa H, Kuronuma T, Motomura Y, Mizuno S, Ishii H, Nakachi K, Konishi M. Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: immunologic evidence and potential for improving overall survival. Clin Cancer Res. 2012;18:3686-3696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 208] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 61. | Zhu AX, Gold PJ, El-Khoueiry AB, Abrams TA, Morikawa H, Ohishi N, Ohtomo T, Philip PA. First-in-man phase I study of GC33, a novel recombinant humanized antibody against glypican-3, in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2013;19:920-928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 62. | Rosenberg SA. Cell transfer immunotherapy for metastatic solid cancer--what clinicians need to know. Nat Rev Clin Oncol. 2011;8:577-585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 243] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 63. | Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1770] [Cited by in F6Publishing: 1999] [Article Influence: 181.7] [Reference Citation Analysis (0)] |
| 64. | Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771-775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 853] [Cited by in F6Publishing: 885] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 65. | Manzotti CN, Liu MK, Burke F, Dussably L, Zheng Y, Sansom DM. Integration of CD28 and CTLA-4 function results in differential responses of T cells to CD80 and CD86. Eur J Immunol. 2006;36:1413-1422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 66. | Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 864] [Cited by in F6Publishing: 882] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 67. | Collins AV, Brodie DW, Gilbert RJ, Iaboni A, Manso-Sancho R, Walse B, Stuart DI, van der Merwe PA, Davis SJ. The interaction properties of costimulatory molecules revisited. Immunity. 2002;17:201-210. [PubMed] [Cited in This Article: ] |
| 68. | Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer. 2007;7:95-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 453] [Cited by in F6Publishing: 480] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 69. | Sangro B, Gomez-Martin C, de la Mata M, Iñarrairaegui M, Garralda E, Barrera P, Riezu-Boj JI, Larrea E, Alfaro C, Sarobe P. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 634] [Cited by in F6Publishing: 686] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 70. | Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015-3029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1346] [Cited by in F6Publishing: 1511] [Article Influence: 100.7] [Reference Citation Analysis (0)] |
| 71. | Nikolova M, Lelievre JD, Carriere M, Bensussan A, Lévy Y. Regulatory T cells differentially modulate the maturation and apoptosis of human CD8+ T-cell subsets. Blood. 2009;113:4556-4565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 72. | Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3192] [Cited by in F6Publishing: 3187] [Article Influence: 289.7] [Reference Citation Analysis (0)] |
| 73. | Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, Koren-Michowitz M, Shimoni A, Nagler A. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14:3044-3051. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 533] [Cited by in F6Publishing: 568] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 74. | Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346-2357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1220] [Cited by in F6Publishing: 1194] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 75. | Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, Huls MH, Liu E, Gee AP, Mei Z. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264-1270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 864] [Cited by in F6Publishing: 891] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 76. | Cui J, Wang N, Zhao H, Jin H, Wang G, Niu C, Terunuma H, He H, Li W. Combination of radiofrequency ablation and sequential cellular immunotherapy improves progression-free survival for patients with hepatocellular carcinoma. Int J Cancer. 2014;134:342-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 77. | Kawata A, Une Y, Hosokawa M, Wakizaka Y, Namieno T, Uchino J, Kobayashi H. Adjuvant chemoimmunotherapy for hepatocellular carcinoma patients. Adriamycin, interleukin-2, and lymphokine-activated killer cells versus adriamycin alone. Am J Clin Oncol. 1995;18:257-262. [PubMed] [Cited in This Article: ] |
| 78. | Shi M, Zhang B, Tang ZR, Lei ZY, Wang HF, Feng YY, Fan ZP, Xu DP, Wang FS. Autologous cytokine-induced killer cell therapy in clinical trial phase I is safe in patients with primary hepatocellular carcinoma. World J Gastroenterol. 2004;10:1146-1151. [PubMed] [Cited in This Article: ] |
| 79. | Lee WC, Wang HC, Hung CF, Huang PF, Lia CR, Chen MF. Vaccination of advanced hepatocellular carcinoma patients with tumor lysate-pulsed dendritic cells: a clinical trial. J Immunother. 2011;28:496-504. [PubMed] [Cited in This Article: ] |
| 80. | Nakamoto Y, Mizukoshi E, Kitahara M, Arihara F, Sakai Y, Kakinoki K, Fujita Y, Marukawa Y, Arai K, Yamashita T. Prolonged recurrence-free survival following OK432-stimulated dendritic cell transfer into hepatocellular carcinoma during transarterial embolization. Clin Exp Immunol. 2011;163:165-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 81. | Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432-1433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1306] [Cited by in F6Publishing: 1404] [Article Influence: 140.4] [Reference Citation Analysis (0)] |
| 82. | Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1402] [Cited by in F6Publishing: 1536] [Article Influence: 139.6] [Reference Citation Analysis (0)] |
| 83. | Brentjens RJ, Rivière I, Park JH, Davila ML, Wang X, Stefanski J, Taylor C, Yeh R, Bartido S, Borquez-Ojeda O. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817-4828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 923] [Cited by in F6Publishing: 972] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 84. | Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska M. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1670] [Cited by in F6Publishing: 1839] [Article Influence: 183.9] [Reference Citation Analysis (0)] |
| 85. | Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509-1518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2465] [Cited by in F6Publishing: 2566] [Article Influence: 233.3] [Reference Citation Analysis (0)] |
| 86. | Kochenderfer JN, Dudley ME, Carpenter RO, Kassim SH, Rose JJ, Telford WG, Hakim FT, Halverson DC, Fowler DH, Hardy NM. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013;122:4129-4139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 435] [Cited by in F6Publishing: 463] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 87. | Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes MS, Sherry RM, Yang JC, Kammula US, Devillier L, Carpenter R, Nathan DA, Morgan RA, Laurencot C, Rosenberg SA. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709-2720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1061] [Cited by in F6Publishing: 1124] [Article Influence: 86.5] [Reference Citation Analysis (0)] |
| 88. | Till BG, Jensen MC, Wang J, Qian X, Gopal AK, Maloney DG, Lindgren CG, Lin Y, Pagel JM, Budde LE. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood. 2012;119:3940-3950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 372] [Cited by in F6Publishing: 391] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 89. | Gao H, Li K, Tu H, Pan X, Jiang H, Shi B, Kong J, Wang H, Yang S, Gu J. Development of T cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clin Cancer Res. 2014;20:6418-6428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 195] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 90. | Sawada Y, Yoshikawa T, Shimomura M, Iwama T, Endo I, Nakatsura T. Programmed death-1 blockade enhances the antitumor effects of peptide vaccine-induced peptide-specific cytotoxic T lymphocytes. Int J Oncol. 2015;46:28-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 91. | Llovet JM, Sala M, Castells L, Suarez Y, Vilana R, Bianchi L, Ayuso C, Vargas V, Rodés J, Bruix J. Randomized controlled trial of interferon treatment for advanced hepatocellular carcinoma. Hepatology. 2000;31:54-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 202] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 92. | Ikeda K, Arase Y, Saitoh S, Kobayashi M, Suzuki Y, Suzuki F, Tsubota A, Chayama K, Murashima N, Kumada H. Interferon beta prevents recurrence of hepatocellular carcinoma after complete resection or ablation of the primary tumor-A prospective randomized study of hepatitis C virus-related liver cancer. Hepatology. 2000;32:228-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 288] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 93. | Sakon M, Nagano H, Dono K, Nakamori S, Umeshita K, Yamada A, Kawata S, Imai Y, Iijima S, Monden M. Combined intraarterial 5-fluorouracil and subcutaneous interferon-alpha therapy for advanced hepatocellular carcinoma with tumor thrombi in the major portal branches. Cancer. 2002;94:435-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 161] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 94. | Kubo S, Nishiguchi S, Hirohashi K, Tanaka H, Shuto T, Yamazaki O, Shiomi S, Tamori A, Oka H, Igawa S. Effects of long-term postoperative interferon-alpha therapy on intrahepatic recurrence after resection of hepatitis C virus-related hepatocellular carcinoma. A randomized, controlled trial. Ann Intern Med. 2001;134:963-967. [PubMed] [Cited in This Article: ] |
| 95. | Ladhams A, Schmidt C, Sing G, Butterworth L, Fielding G, Tesar P, Strong R, Leggett B, Powell L, Maddern G. Treatment of non-resectable hepatocellular carcinoma with autologous tumor-pulsed dendritic cells. J Gastroenterol Hepatol. 2002;17:889-896. [PubMed] [Cited in This Article: ] |
| 96. | Palmieri G, Montella L, Milo M, Fiore R, Biondi E, Bianco AR, Martignetti A. Ultra-low-dose interleukin-2 in unresectable hepatocellular carcinoma. Am J Clin Oncol. 2002;25:224-226. [PubMed] [Cited in This Article: ] |
| 97. | Reinisch W, Holub M, Katz A, Herneth A, Lichtenberger C, Schoniger-Hekele M, Waldhoer T, Oberhuber G, Ferenci P, Gangl A. Prospective pilot study of recombinant granulocyte-macrophage colony-stimulating factor and interferon-gamma in patients with inoperable hepatocellular carcinoma. J Immunother. 2003;25:489-499. [PubMed] [Cited in This Article: ] |
| 98. | Stift A, Friedl J, Dubsky P, Bachleitner-Hofmann T, Schueller G, Zontsich T, Benkoe T, Radelbauer K, Brostjan C, Jakesz R. Dendritic cell-based vaccination in solid cancer. J Clin Oncol. 2003;21:135-142. [PubMed] [Cited in This Article: ] |
| 99. | Feun LG, O’Brien C, Molina E, Rodriguez M, Jeffers L, Schiff ER, Marini A, Savaraj N, Ardalan B. Recombinant leukocyte interferon, doxorubicin, and 5FUDR in patients with hepatocellular carcinoma-A phase II trial. J Cancer Res Clin Oncol. 2003;129:17-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 100. | Shiratori Y, Shiina S, Teratani T, Imamura M, Obi S, Sato S, Koike Y, Yoshida H, Omata M. Interferon therapy after tumor ablation improves prognosis in patients with hepatocellular carcinoma associated with hepatitis C virus. Ann Intern Med. 2003;138:299-306. [PubMed] [Cited in This Article: ] |
| 101. | Iwashita Y, Tahara K, Goto S, Sasaki A, Kai S, Seike M, Chen CL, Kawano K, Kitano S. A phase I study of autologous dendritic cell-based immunotherapy for patients with unresectable primary liver cancer. Cancer Immunol Immunother. 2003;52:155-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 102. | Sangro B, Mazzolini G, Ruiz J, Herraiz M, Quiroga J, Herrero I, Benito A, Larrache J, Pueyo J, Subtil JC. Phase I trial of intratumoral injection of an adenovirus encoding interleukin-12 for advanced digestive tumors. J Clin Oncol. 2004;22:1389-1397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 230] [Cited by in F6Publishing: 222] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 103. | Mazzolini G, Alfaro C, Sangro B, Feijoó E, Ruiz J, Benito A, Tirapu I, Arina A, Sola J, Herraiz M. Intratumoral injection of dendritic cells engineered to secrete interleukin-12 by recombinant adenovirus in patients with metastatic gastrointestinal carcinomas. J Clin Oncol. 2005;23:999-1010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 141] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 104. | Chi KH, Liu SJ, Li CP, Kuo HP, Wang YS, Chao Y, Hsieh SL. Combination of conformal radiotherapy and intratumoral injection of adoptive dendritic cell immunotherapy in refractory hepatoma. J Immunother. 2005;28:129-135. [PubMed] [Cited in This Article: ] |
| 105. | Yin XY, Lü MD, Liang LJ, Lai JM, Li DM, Kuang M. Systemic chemo-immunotherapy for advanced-stage hepatocellular carcinoma. World J Gastroenterol. 2005;11:2526-2529. [PubMed] [Cited in This Article: ] |
| 106. | Kumagi T, Akbar SM, Horiike N, Kurose K, Hirooka M, Hiraoka A, Hiasa Y, Michitaka K, Onji M. Administration of dendritic cells in cancer nodules in hepatocellular carcinoma. Oncol Rep. 2005;14:969-973. [PubMed] [Cited in This Article: ] |
| 107. | Nakamoto Y, Mizukoshi E, Tsuji H, Sakai Y, Kitahara M, Arai K, Yamashita T, Yokoyama K, Mukaida N, Matsushima K. Combined therapy of transcatheter hepatic arterial embolization with intratumoral dendritic cell infusion for hepatocellular carcinoma: clinical safety. Clin Exp Immunol. 2007;147:296-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 38] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 108. | Vitale FV, Romeo P, Vasta F, Panebianco V, Calì S, Rotondo S, Ferraù F, La Greca M. Hepatic intra-arterial interferon alpha 2b-based immunotherapy combined with 5-fluorouracil (5-FU)-based systemic chemotherapy for patients with hepatocellular carcinoma (HCC) not responsive and/or not eligible for conventional treatments: a pilot study. Anticancer Res. 2008;27:4077-4081. [PubMed] [Cited in This Article: ] |
| 109. | Weng DS, Zhou J, Zhou QM, Zhao M, Wang QJ, Huang LX, Li YQ, Chen SP, Wu PH, Xia JC. Minimally invasive treatment combined with cytokine-induced killer cells therapy lower the short-term recurrence rates of hepatocellular carcinomas. J Immunother. 2008;31:63-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 110. | Hui D, Qiang L, Jian W, Ti Z, Da-Lu K. A randomized, controlled trial of postoperative adjuvant cytokine-induced killer cells immunotherapy after radical resection of hepatocellular carcinoma. Dig Liver Dis. 2009;41:36-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 111. | Palmer DH, Midgley RS, Mirza N, Torr EE, Ahmed F, Steele JC, Steven NM, Kerr DJ, Young LS, Adams DH. A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology. 2009;49:124-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 201] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 112. | Olioso P, Giancola R, Di Riti M, Contento A, Accorsi P, Iacone A. Immunotherapy with cytokine induced killer cells in solid and hematopoietic tumours: a pilot clinical trial. Hematol Oncol. 2009;27:130-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 113. | Hao MZ, Lin HL, Chen Q, Ye YB, Chen QZ, Chen MS. Efficacy of transcatheter arterial chemoembolization combined with cytokine-induced killer cell therapy on hepatocellular carcinoma: a comparative study. Chin J Cancer. 2010;29:172-177. [PubMed] [Cited in This Article: ] |
| 114. | Greten TF, Forner A, Korangy F, N’Kontchou G, Barget N, Ayuso C, Ormandy LA, Manns MP, Beaugrand M, Bruix J. A phase II open label trial evaluating safety and efficacy of a telomerase peptide vaccination in patients with advanced hepatocellular carcinoma. BMC Cancer. 2010;10:209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 115. | Ma H, Zhang Y, Wang Q, Li Y, He J, Wang H, Sun J, Pan K, Chen M, Xia J. Therapeutic safety and effects of adjuvant autologous RetroNectin activated killer cell immunotherapy for patients with primary hepatocellular carcinoma after radiofrequency ablation. Cancer Biol Ther. 2010;9:903-907. [PubMed] [Cited in This Article: ] |
| 116. | Zhou P, Liang P, Dong B, Yu X, Han Z, Xu Y. Phase I clinical study of combination therapy with microwave ablation and cellular immunotherapy in hepatocellular carcinoma. Cancer Biol Ther. 2011;11:450-456. [PubMed] [Cited in This Article: ] |
| 117. | Qiu Y, Xu MB, Yun MM, Wang YZ, Zhang RM, Meng XK, Ou-Yang XH, Yun S. Hepatocellular carcinoma-specific immunotherapy with synthesized α1,3- galactosyl epitope-pulsed dendritic cells and cytokine-induced killer cells. World J Gastroenterol. 2011;17:5260-5266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 32] [Cited by in F6Publishing: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 118. | Tada F, Abe M, Hirooka M, Ikeda Y, Hiasa Y, Lee Y, Jung NC, Lee WB, Lee HS, Bae YS. Phase I/II study of immunotherapy using tumor antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Int J Oncol. 2012;41:1601-1609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 119. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. [PubMed] [Cited in This Article: ] |
| 120. | Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412-7420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2311] [Cited by in F6Publishing: 2361] [Article Influence: 157.4] [Reference Citation Analysis (0)] |
| 121. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2583] [Cited by in F6Publishing: 2903] [Article Influence: 207.4] [Reference Citation Analysis (36)] |