Published online Sep 21, 2015. doi: 10.3748/wjg.v21.i35.10192
Peer-review started: March 2, 2015
First decision: April 23, 2015
Revised: May 18, 2015
Accepted: July 15, 2015
Article in press: July 15, 2015
Published online: September 21, 2015
AIM: To assess the relationship between non-alcoholic fatty liver disease (NAFLD) with metabolic risk factors and brachial ankle pulse wave velocity (baPWV).
METHODS: A total of 8603 subjects (6662 males and 1941 females) were enrolled during an annual health check-up. Fatty liver was examined using a Philips HD 11 XE multi-function color Doppler diagnostic instrument, and baPWV was determined using a novel arteriosclerosis detection device. Blood pressure (BP), fasting plasma glucose (FPG), waist circumference (WC), plasma triglycerides (TG), high-density lipoprotein (HDL), total cholesterol (TC), low-density lipoprotein (LDL) and uric acid (UA) were measured using standard methods. The relationship between fatty liver with metabolic risk factors and baPWV was analyzed using regression analysis and the χ2 test.
RESULTS: The values and abnormal rates of baPWV were significantly different between NAFLD patients and non-NAFLD subjects (P < 0.001). In addition, the values of baPWV were different by gender between NAFLD patients and non-NAFLD subjects. The OR values in females, males, and the entire population were 3.33, 1.67, and 2.13, respectively (P < 0.001). The incidence of high baPWV increased with increasing degree of NAFLD (levels 0, 1, 2, and 3) (P < 0.001), which was 45.9%, 54.5%, 60.2%, and 71.4% in males and 27.0%, 49.1%, 55.60%, and 60.0% in females (P < 0.001), respectively. Logistic regression analysis showed that the OR value for baPWV in the non-metabolic syndrome group and the metabolic syndrome group was 1.28 vs 1.14 (males) and 2.55 vs 0.98 (females). The OR values for baPWV in the non-high-BP and high-BP, non-high-WC and high-WC, non-high-FPG and high-FPG, non-high-TG and high-TG, non-high-HDL and high-HDL, non-high-TC and high-TC, non-high-LDL and high-LDL, non-high-UA and high-UA groups were 3.38 vs 1.19, 3.50 vs 1.44, 2.80 vs 2.30, 3.29 vs 1.88, 3.03 vs 3.28, 3.35 vs 2.70, 3.93 vs 1.66, and 3.20 vs 2.34, respectively, in females (P < 0.001), and were 1.37 vs 1.34, 1.56 vs 1.26, 1.51 vs 1.28, 1.49 vs 1.52, 1.71 vs 1.61, 1.59 vs 1.74, 1.76 vs 1.47, and 1.73 vs 1.54, respectively, in males (P < 0.01). The OR value for baPWV was still higher than 1.2 (1.21 in males and 1.40 in females) after adjustment for the metabolic component (0, 1, 2, 3, 4, 5, 6 and above) (P < 0.01).
CONCLUSION: NAFLD is closely correlated with baPWV, particularly in females. NAFLD has a large impact on baPWV, no matter whether the metabolic index is increased or not. NAFLD may be a useful indicator for assessing early arteriosclerosis.
Core tip: Non-alcoholic fatty liver disease (NAFLD) is emerging as an independent risk factor for the occurrence and progression of ischemic cardiovascular disease. However, the association between NAFLD and arterial stiffness is not fully elucidated. This study showed that NAFLD is closely related to brachial ankle pulse wave velocity. NAFLD has a noticeable impact when considering gender in the metabolic risk factor group, especially in females. NAFLD may be a useful indicator for assessing early arteriosclerosis.
- Citation: Zhu WH, Fang LZ, Lu CR, Dai HL, Chen JH, Qiao QH, Chen LY. Correlation between non-alcoholic fatty liver with metabolic risk factors and brachial-ankle pulse wave velocity. World J Gastroenterol 2015; 21(35): 10192-10199
- URL: https://www.wjgnet.com/1007-9327/full/v21/i35/10192.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i35.10192
Non-alcoholic fatty liver disease (NAFLD) is a clinicopathologic syndrome with pathological changes in liver tissue similar to alcoholic fatty liver disease, but without a history of excessive alcohol consumption. It is a manifestation of the metabolic syndrome in the liver[1]. Many studies have shown that NAFLD can co-exist with metabolic risk factors; it is closely related to atherosclerosis and can cause early changes in the function of arterial wall. NAFLD is accompanied by the release of a variety of proinflammatory molecules also implicated in cardiovascular disease (CVD) and metabolic syndrome (MetS), and it is an early stage in the process of systemic endothelial damage[2]. In a long-term follow-up study, Rafiq et al[3] concluded that NAFLD was not only a sign of atherosclerosis, but may also be involved in the development of early atherosclerosis; the prognosis of NAFLD is mainly associated with the occurrence of acute coronary events and stroke events. NAFLD is associated with coronary plaque, independent of traditional cardiovascular diseases[4]. Brachial-ankle pulse wave velocity (baPWV) is currently well recognized as an ideal indicator for assessing early atherosclerosis[5]. Metabolic risk factors are defined as a cluster of multiple cardiovascular risk factors[6]. The current study was designed to analyze the relationship between NAFLD and baPWV and explore the potential impact of NAFLD and metabolic risk factors on early changes in the function of arterial wall, in order to provide evidence for the early assessment of cardiovascular diseases.
A total of 9613 subjects (7180 males and 2433 females) who received health check-ups in our hospital from January 2013 to June 2014 were enrolled in this study. Metabolic risk factors, NAFLD, and baPWV were determined in these subjects. The inclusion criteria were as follows: (1) healthy residents in Zhejiang Province, China; and (2) subjects aged 25-75 years. The exclusion criteria were: (1) subjects with cardiovascular disease, other types of viral hepatitis and liver disease, abnormal liver function, drug-induced hepatitis, decompensated cirrhosis, autoimmune liver disease, hyperthyroidism, or cancer; or (2) subjects with a history of heavy drinking (equivalent of 40 g ethanol intake per week). Excluding 1010 subjects who were lost to follow-up, a total of 8603 subjects (6662 males and 1941 females) were eventually included with informed consent. This study was approved by the Medical Ethics Committee in Sir Run Run Shaw Hospital. All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
NAFLD was diagnosed by examining fatty livers using a Philips HD 11 XE multi-function color Doppler diagnostic instrument (Philips Ultrasound, United States). Subjects were then divided into the non-fatty liver group (group 0) and the fatty liver groups (including the mild, moderate, and severe fatty liver groups which were labeled as groups 1, 2, and 3).
The baPWV was determined using a novel arteriosclerosis detection device VP-1000 (BP-203RPE III) (Omron Corporation, Japan). The subjects were asked to rest quietly before taking three deep breaths in the spinal position, then remain quiet for another 5 min, and the pulse wave at the brachial-ankle artery was measured, and the baPWV value and its abnormal change rate were determined[7]. Here, the “abnormal change rate” refers to the percentage of the changed baPWV values relative to the reference values in subjects of the same gender and age. The means at the left and right sides were obtained, and then the median and values higher than the median were determined in the increased group.
The metabolic risk factors (closely related to fatty liver) were selected according to the guidelines for metabolic syndrome, hyperlipidemia and hyperuricemia prevention. The metabolic risk factors were determined, including: blood pressure (BP), waist circumference (WC), fasting plasma glucose (FPG), triglycerides (TG), total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL) and uric acid (UA). BP was measured using the standard method recommended in the 2010 Chinese Guidelines for the Management of Hypertension. WC was measured using the World Health Organization (WHO) standard method (the subjects were asked to stand and breathe normally, and the WC at the midpoint between the lowest rib and the iliac crest was measured). TG and TC were measured using the oxidase method, and HDL and LDL were measured using direct homogeneous methods. Uric acid (UA) was measured using enzymatic colorimetric method.
Criteria for assessing NAFLD: Ultrasonography was performed to assess NAFLD in accordance with the Diagnostic Criteria of Non-alcoholic Fatty Liver Disease by the Chinese Society of Hepatology in 2010[8]. Fatty liver was defined as “diffuse” if it met two of the following three criteria: (1) there was diffuse enhancement of near-field echo in the hepatic region, with the echo stronger than that in the kidneys; (2) the intrahepatic ductal structures were not clearly shown; and (3) the far-field echo in the hepatic region gradually became attenuated. According to the NALFD grading proposed by the Chinese Society of Hepatology and those described in a national document[9], we categorized the degree of echo attenuation in the posterior field, the intensities of hepatic dotted echoes, and the clarity of intrahepatic portal vein into I (low), II (intermediate), and III (high). The posterior-field echo attenuation in fatty liver patients was further graded: degree I, attenuated by < 1/3; degree II, attenuated by 1/3-2/3; and degree III, attenuated by > 2/3.
Diagnostic criteria for the metabolic syndrome and other metabolic risk factors: The diagnostic criteria for MS[10] established by the International Diabetes Federation in 2005 were adopted. More specifically, the subjects were determined to have MS if they had central obesity (waist circumference ≥ 90 cm in males and ≥ 80 cm in females) accompanied by two or more of the following features: (1) triglyceride (TG) level > 1.7 mmol/L; (2) systolic blood pressure (SBP) ≥ 130 mmHg; or diastolic blood pressure (DBP) ≥ 85 mmHg; or had been diagnosed with hypertension; (3) fasting plasma glucose (FPG) ≥ 5.6 mmol/L, or had been diagnosed with type 2 diabetes; (4) high-density lipoprotein cholesterol (HDL-C) < 1.04 mmol/L in males and < 1.30 mmol/L in females. According to the diagnostic criteria proposed in the Guidelines on Adult Lipid Control in China (2007 Edition)[11], hypercholesteremia was defined as ≥ 5.2 mmol/L and high LDL-C was defined as ≥ 3.1 mmol/L; and (5) According to the diagnostic criteria of the American Rheumatism Association in 1997, hyperuricemia is defined as a serum uric acid level ≥ 420 mmol/L in males or ≥ 357 mmol/L in females.
All measurement data were entered into the database and then analyzed using SPSS 19.0 software. Data are presented as mean ± SD, categorical variables of medians, and percentages (%). t test was used for analysis of metabolic index and baPWV in non-alcoholic fatty liver and alcoholic fatty liver. The potential correlation between NAFLD and baPWV was analyzed using the logistic regression method. The potential impact of NAFLD on baPWV in subjects with high metabolic risk factors and in those with normal metabolic risk factors was also analyzed using the logistic regression method and multiple linear regression analysis. The increased percentage in baPWV in patients with different degrees of fatty liver was analyzed using the χ2 test. A P value of less than 0.05 was considered statistically significant.
The baseline SBP, DBP, WC, FPG, body mass index, TG, HDL, TC, LDL, baPWV value, and rate of change in baPWV differed significantly between the fatty liver group and the non-fatty liver group (Table 1).
| Variables | NAFLD groups | non-NAFLD group | P value |
| (n = 4025) | (n = 4578) | ||
| Age (yr) | 46.92 ± 9.12 | 45.67 ± 9.95 | < 0.001 |
| BMI (kg/m2) | 26.42 ± 2.83 | 22.93 ± 2.63 | < 0.001 |
| SBP (mmHg) | 127.68 ± 15.49 | 119.46 ± 16.17 | < 0.001 |
| DBP (mmHg) | 79.65 ± 11.38 | 72.78 ± 11.04 | < 0.001 |
| WC (cm) | 90.70 ± 7.88 | 80.08 ± 8.23 | < 0.001 |
| FBG (mmol/L) | 5.46 ± 1.36 | 4.97 ± 0.87 | < 0.001 |
| TG (mmol/L) | 2.18 ± 1.64 | 1.25 ± 0.81 | < 0.001 |
| HDL-C (mmol/L) | 1.12 ± 0.28 | 1.29 ± 0.29 | < 0.001 |
| TC (mmol/L) | 5.02 ± 0.98 | 4.68 ± 0.89 | < 0.001 |
| LDL (mmol/L) | 2.82 ± 0.78 | 2.65 ± 0.73 | < 0.001 |
| UA (mmol/L) | 386.60 ± 88.75 | 319.00 ± 87.17 | < 0.001 |
| Mean baPWV value (cm/s) | 1384.09 ± 228.86 | 1297.38 ± 237.58 | < 0.001 |
High baPWV was defined as a value equal to or higher than the median [median baPWV value: 1321 cm/s (males) and 1219 cm/s (females), and 1302 cm/s (entire population)]. With NAFLD as the independent variable and high baPWV as the dependent variable, logistic regression and multiple linear regression analysis of the relationship between NAFLD and baPWV showed that NAFLD had an impact on high baPWV in males (OR = 1.667), females (OR = 3.328), and the entire population (OR = 2.134) (Table 2).
| High baPWV | Non-high baPWV | OR | 95%CI | P value | |
| Males with NAFLD | |||||
| No | 1436 (46.4) | 1219 (34.2) | - | - | - |
| Yes | 1659 (53.6) | 2348 (65.8) | 1.667 | 1.510-1.841 | < 0.001 |
| Females with NAFLD | |||||
| No | 1004 (79.9) | 372 (54.4) | - | - | |
| Yes | 253 (20.1) | 312 (45.6) | 3.328 | 2.714-4.082 | < 0.001 |
| Entire population | |||||
| No | 2440 (56.1) | 1591 (37.4) | - | - | - |
| Yes | 1912 (43.9 ) | 2660 (62.6) | 2.134 | 1.957-2.326 | < 0.001 |
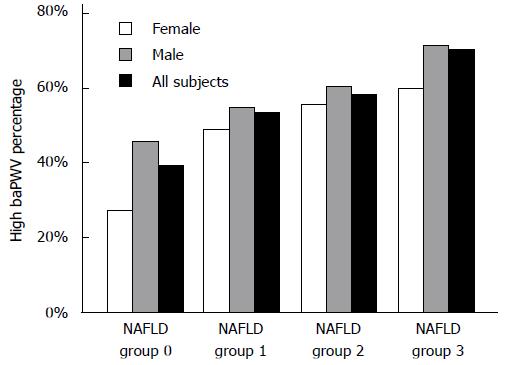
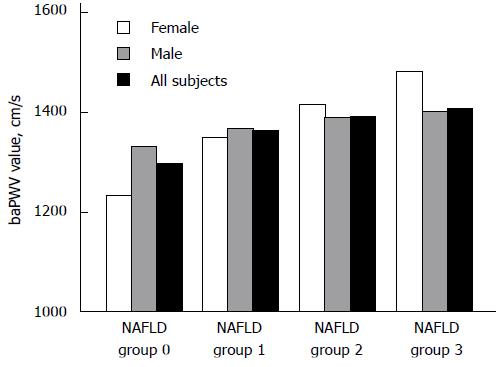
We analyzed the proportions of high baPWV in four NAFLD groups (groups 0, 1, 2, and 3) and found that the incidence of high baPWV gradually increased in the entire population (39.5%, 53.6%, 58.1%, and 70.5%, respectively; χ2 = 320.3, P < 0.001), in males (45.9%, 54.5%, 60.2%, and 71.4%, respectively; χ2 = 118.0, P < 0.001), and in females (27.0%, 49.1%, 55.60%, and 60.0%, respectively; χ2 = 145.8, P < 0.001) with an increase in the degree of NAFLD (Figure 1). The value of baPWV increased gradually in females (from group 1 to group 4, P < 0.001), and males (from group 1 to group 3, P < 0.01; Figure 2).
The metabolic risk factors were stratified into two groups according to the diagnostic criteria of MS and metabolic factors. With the presence of fatty liver as the independent variable and high baPWV as the dependent variable (high baPWV was defined as a value equal to or higher than the median), logistic regression analysis was performed to determine the relationship between fatty liver and high baPWV. The results showed that, in all groups of non-high metabolic risk factors and high metabolic risk factors, NAFLD has an effect on baPWV, particularly in females - except for females with MS (P < 0.01) (Table 3).
| Variable | NAFLD | Males (n = 6662) | Females (n = 1941) | ||||
| Subjects with high baPWV | OR (95%CI) | P value | Subjects with high baPWV | OR (95%CI) | P value | ||
| MS | |||||||
| No | No | 1033 (39.5) | Ref | 296 (23.3) | Ref | - | |
| Yes | 1089 (58.2) | 1.280 (1.139-1.439) | < 0.001 | 139 (43.7) | 2.550 (1.972-3.297) | < 0.001 | |
| Yes | No | 185 (66.5) | Ref | - | 76 (70.4) | Ref | - |
| Yes | 1259 (69.4) | 1.142 (0.873-1.494) | 0.331 | 173 (70.0) | 0.984 (0.600-1.614) | 0.950 | |
| High BP | |||||||
| No | No | 698 (35.9) | Ref | - | 185 (16.3) | Ref | - |
| Yes | 990 (43.5) | 1.374 (1.214-1.556) | <0.001 | 139 (39.7) | 3.383 (2.593-4.413) | < 0.001 | |
| Yes | No | 520 (73.6) | Ref | - | 187 (77.6) | Ref | - |
| Yes | 1352 (78.8) | 1.339 (1.093-1.641) | 0.005 | 173 (80.5) | 1.189 (0.756-1.871) | 0.453 | |
| High WC | |||||||
| No | No | 947 (43.7) | Ref | - | 218 (20.5) | Ref | - |
| Yes | 886 (54.8) | 1.559 (1.369-1.775) | < 0.001 | 75 (47.5) | 3.503 (2.478-4.950) | < 0.001 | |
| Yes | No | 271 (55.5) | Ref | - | 154 (48.2) | ||
| Yes | 1462 (61.2) | 1.262 (1.036-1.536) | 0.021 | 237 (58.2) | 1.439 (1.070-1.936) | 0.016 | |
| High FPG | |||||||
| No | No | 1019 (43.2) | Ref | - | 326 (25.1) | Ref | - |
| Yes | 1573 (53.4) | 1.508 (1.352-1.681) | < 0.001 | 210 (48.4) | 2.801 (2.234-3.512) | < 0.001 | |
| Yes | No | 199 (67.9) | Ref | - | 46 (60.5) | Ref | _ |
| Yes | 775 (73.0) | 1.280 (0.967-1.694) | 0.084 | 102 (77.9) | 2.294 (1.237-4.255) | 0.008 | |
| High TG | |||||||
| No | No | 919 (44.2) | Ref | - | 315 (25.2) | Ref | - |
| Yes | 959 (54.1) | 1.486 (1.308-1.688) | < 0.001 | 199 (52.6) | 3.296 (2.596-4.186) | < 0.001 | |
| Yes | No | 299 (52.0) | Ref | - | 57 (49.9) | Ref | - |
| Yes | 1389 (62.2) | 1.517 (1.262-1.825) | < 0.001 | 113 (60.4) | 1.875 (1.188-2.960) | 0.007 | |
| Low HDL | |||||||
| No | No | 933 (45.5) | Ref | - | 210 (25.4) | Ref | - |
| Yes | 1368 (59.2) | 1.709 (1.515-1.927) | < 0.001 | 108 (50.7) | 3.027 (2.218-4.131) | < 0.001 | |
| Yes | No | 285 (45.9) | Ref | - | 162 (29.6) | Ref | - |
| Yes | 980 (57.8) | 1.614 (1.341-1.941) | 204 (58.0) | 3.284 (2.482-4.346) | < 0.001 | ||
| High TC | |||||||
| No | No | 868 (45.1) | Ref | - | 245 (22.6) | Ref | - |
| Yes | 1373 (56.6) | 1.588 (1.408-1.791) | < 0.001 | 190 (49.5) | 3.350 (2.621-4.282) | < 0.001 | |
| Yes | No | 350 (48.1) | Ref | - | 127 (43.3) | Ref | _ |
| Yes | 975 (61.7) | 1.736 (1.454-2.073) | < 0.001 | 122 (67.4) | 2.703 (1.835-3.981) | < 0.001 | |
| High LDL | |||||||
| No | No | 867 (44.9) | Ref | - | 258 (22.7) | Ref | - |
| Yes | 1544 (58.9) | 1.758 (1.561-1.979) | < 0.001 | 224 (53.6) | 3.925 (3.096-4.975) | < 0.001 | |
| Yes | No | 351 (48.5) | Ref | - | 114 (47.3) | Ref | - |
| Yes | 804 (58.0) | 1.466 (1.223-1.756) | < 0.001 | 88 (59.9) | 1.662 (1.097-2.518) | 0.017 | |
| High UA | |||||||
| No | No | 797 (45.6) | Ref | - | 384 (26.4) | Ref | - |
| Yes | 1544 (58.9) | 1.725 (1.523-1.924) | < 0.001 | 260 (53.3) | 3.202 (2.580-3.973) | < 0.001 | |
| Yes | No | 239 (45.7) | Ref | - | 24 (47.0) | Ref | - |
| Yes | 857 (59.7) | 1.543 (1.260-1.890) | < 0.001 | 52 (67.5) | 2.340 (1.130-4.843) | 0.022 | |
The metabolism risk factors were divided into 7 groups (0, 1, 2, 3, 4, 5, 6 and above groups) according to metabolic component number. Logistic regression analysis was also performed to determine the relationship between fatty liver and baPWV after adjusting for metabolic components (the number of metabolism risk factors to be assumed dummy variable). OR value for baPWV was still higher than 1.2 (in males and females) (P < 0.05) (Table 4).
| Group | Males (n = 6662) | Females (n = 1941) | ||||
| n (%) | OR (95%CI) | P value | n (%) | OR (95%CI) | P value | |
| NAFLD0 | 2653 (39.8) | Ref | - | 1376 (70.9) | Ref | - |
| NAFLD1 | 4009 (60.2) | 1.21 (1.09-1.33) | < 0.001 | 565 (29.1) | 1.40 (1.08-1.82) | < 0.001 |
| MC0 | 787 (11.8) | Ref | - | 451 (23.2) | Ref | - |
| MC1 | 955 (14.3) | 1.59 (1.29-1.97) | < 0.001 | 511 (26.3) | 3.38 (2.19-5.20) | < 0.001 |
| MC2 | 1288 (19.3) | 2.03 (1.66-2.48) | < 0.001 | 368 (19.0) | 5.04 (3.23-7.87) | < 0.001 |
| MC3 | 1282 (19.2) | 2.66 (2.16-3.27) | < 0.001 | 272 (14.0) | 7.40 (4.67-11.80) | < 0.001 |
| MC4 | 1117 (16.8) | 3.22 (2.60-3.99) | < 0.001 | 187 (9.60) | 9.84 (5.83-16.60) | < 0.001 |
| MC5 | 763 (11.5) | 4.35 (3.42-5.52) | < 0.001 | 106 (5.50) | 11.87 (6.40-22.03) | < 0.001 |
| MC6 and above | 470 (7.10) | 5.59 (4.23-7.38) | < 0.001 | 46 (2.40) | 20.26 (7.45-55.12) | < 0.001 |
NAFLD is associated with atherosclerosis and coronary heart disease. Research has shown that liver histopathology is closely correlated with early atherosclerosis in NAFLD patients[12]. Although it has been proposed that carotid-femoral pulse wave velocity may be the gold standard during the non-invasive examination of arteriosclerosis, arterial pulse wave velocity remains a well-recognized parameter for assessing vascular elasticity in the early phases of atherosclerosis; it is also a method for assessing arterial stiffness and reflecting the risk of cardiovascular lesions. Together with advances in the non-invasive detection of arteriosclerosis, monitoring of arterial pulse wave velocity has been widely used to screen individuals with early atherosclerotic vascular disease and for assessing cardiovascular risks[13].
In the current study, we determined the relationship between baPWV and NAFLD, and found that baPWV was significantly increased in the NAFLD group compared with the control group, suggesting a close relationship between baPWV and NAFLD. Logistic regression analysis showed that NAFLD affected baPWV in both males and females in the entire population. In addition, the incidence of high baPWV gradually increased with exacerbation of NAFLD, further indicating the clear relationship between NAFLD and baPWV. Many studies have shown that NAFLD is a marker of subclinical CVD, and the pathogenesis of NAFLD may involve early atherosclerotic cardiovascular disease[14], while the role of NAFLD in promoting the development of atherosclerosis remains unclear. The following mechanisms may be involved: (1) patients with NAFLD also have other metabolic risk factors, and the number and degree of these factors are associated with early atherosclerosis; (2) NAFLD promotes systemic inflammation and oxidative stress, intensifies insulin resistance, and triggers the occurrence of atherosclerosis and coronary heart disease. Notably, NAFLD can cause changes in vascular structure and function. By affecting vascular endothelial function and the hypercoagulation/hypofibrinolysis status, NAFLD can cause endothelium-dependent flow-mediated vasodilation as well as oxidative stress-lipid peroxidation, thus leading to arteriosclerosis[15]; and (3) NAFLD may lead to an increase in free fatty acids, mainly from visceral adipose tissues with increased flow and may cause a significant increase in the prevalence of cardiocerebrovascular diseases. Therefore, NAFLD may be a marker of subclinical cardiovascular disease at the early stages of atherosclerosis[16].
NAFLD has been documented to be a sign of MS in the liver, and most NAFLD patients have cardiovascular disease or cancer. Arteriosclerosis is the pathogenic basis for most cardiocerebrovascular diseases[17]. The change in vascular elasticity is often earlier than the change in vascular morphology. Among the currently available non-invasive methods for detecting arterial stiffness, baPWV can adequately reflect the degree of change in systemic arterial elasticity. Thus, by detecting vascular indicators such as baPWV, assessments and interventions in populations at different levels of risk can be carried out to lower the incidence of early atherosclerosis and its associated cardiocerebrovascular diseases, thus decreasing the NAFLD mortality[18].
Several recent studies reported that NAFLD is probably an independent risk factor for the occurrence of cardiovascular disease[19]. In the current study, we found that no matter whether the metabolic index increased, NAFLD affected baPWV. After adjustment for metabolic components (the number of metabolism risk factors assumed as dummy variable), NAFLD still affected baPWV in males and females. These findings suggest that NAFLD not only affects baPWV independently, but also has a close relationship with arteriosclerosis during an increase in the non-cardiovascular metabolic risk factors. In addition, our study further suggested that NAFLD does not affect baPWV by relying solely on increased metabolic risk factors. NAFLD may be a marker of increased baPWV in early atherosclerosis. A possible mechanism for this may involve hepatocytes, which are rich in mitochondria, the major sites for synthesizing ATP and reactive oxygen species (ROS) in cells. In patients with fatty liver, mitochondria have significantly increased respiratory activities and elevated ROS production, causing oxidative stress and lipid peroxidation. The presence of massive numbers of ROS and lipid peroxides can result in an imbalance between oxidation and antioxidation in the body, leading to angiogenesis and endothelial dysfunction[20], and affect the formation of arterial plaques[21]. Another possible mechanism is that NAFLD is related to oxidative stress and procoagulation and can increase the circulating levels of oxidized low-density lipoprotein, nitrotyrosine, and plasminogen activator inhibitor-1[22], thus leading to early atherosclerosis. The increase in adipose tissue and chronic inflammation cause an imbalance in adipokine secretion, in particular a reduction of adiponectin improving vascular damage[23].
We also found that the correlation between NAFLD and baPWV was closer in females than in males, particularly in individuals with normal metabolic risk factors. This may be explained by the decreased estrogen level in some post-menopausal women. Estrogen produced by the ovaries can inhibit the accumulation of visceral fats and increase the formation of subcutaneous fats[24]. Furthermore, the distribution profiles of dietary fatty acids may also differ significantly between males and females. The fatty acids in females are more likely to be transformed into ketone bodies and are involved in energy metabolism, and thus cause fat deposition in the liver[25] .
Our research had some limitations: (1) it was a cross-sectional analysis with subjects enrolled following a health check-up. Although the baPWV was adjusted for age and gender to reflect a natural population, sampling bias may still exist; and (2) while the baPWV used in our study is a good parameter for measuring arterial stiffness, it is not the gold standard for arteriosclerosis.
In summary, NAFLD is independently correlated with baPWV, and NAFLD is closely associated with an increased risk of cardiovascular diseases. Measurement of baPWV in NAFLD populations can facilitate the early prediction and assessment of vascular lesions. In patients with NAFLD, clinicians should not only treat the liver disease, but also comprehensively assess the risk of ischemic cardiovascular diseases, which should include the pathogenic effects of different risk factors on the individuals and potential early atherosclerotic disease caused by these risk factors. Such a comprehensive assessment of the various risk factors in NAFLD patients may help to identify the absolute risk of ischemic cardiovascular diseases and thus provide different interventions for populations at different levels of risk to lower the morbidity and mortality associated with NAFLD[26]. Early monitoring of NAFLD may benefit preventive strategies to help decrease the risk of developing arteriosclerosis. In NAFLD patients with a low risk of cardiovascular disease, assessment and management of early arteriosclerosis is still required. In addition, appropriate interventions should be undertaken to lower cardiovascular risks and reduce underlying cardiovascular diseases. However, further prospective studies are needed to clarify the mechanisms governing the change of baPWV in the early stages of arteriosclerosis in NAFLD patients and the subsequent progression of cardiovascular diseases.
We thank Prof. YunXian Yu for instruction on the statistical analysis of the manuscript.
Non-alcoholic fatty liver disease (NAFLD) is well known as an important risk factor for cardiovascular disease. Brachial ankle pulse wave velocity is closely related to atherosclerosis and cardiovascular disease. However, correlation between non-alcoholic fatty liver disease and arterial stiffness is not completely elucidated.
The authors determined the relationship between brachial ankle pulse wave velocity (baPWV) and NAFLD, and found that baPWV was significantly increased in the NAFLD group compared with the control group, suggesting a close relationship between baPWV and NAFLD. Logistic regression analysis showed that NAFLD affected baPWV in both males and females in the entire population. In addition, the incidence of high baPWV gradually increased with exacerbation of NAFLD, further indicating the clear relationship between NAFLD and baPWV.
This study found that no matter whether the metabolic index is increased or not, NAFLD is closely related to baPWV, and NAFLD may be a useful indicator for assessing early arteriosclerosis.
NAFLD can be used to predict early atherosclerosis. This will provide the basis for the prevention and early treatment of atherosclerosis and cardiovascular disease.
NAFLD is a clinicopathologic syndrome with pathological changes in liver tissue similar to alcoholic fatty liver disease.
It is important to study the correlation between non-alcoholic fatty liver disease and brachial ankle pulse wave velocity.
P- Reviewer: Chen LZ S- Editor: Yu J L- Editor: Logan S E- Editor: Ma S
| 1. | Me DA, Zhang JJ, Li B, Chen XQ, Lu XH. A study on the relationship between non-alcoholic fatty liver and atherosclerosis. Zhonghua Jiankangguanlixue Zazhi. 2012;6:434-435. [DOI] [Cited in This Article: ] |
| 2. | Gutiérrez-Grobe Y, Gavilanes-Espinar JG, Masso-Rojas FA, Sánchez-Valle V, Páez-Arenas A, Ponciano-Rodríguez G, Chávez-Tapia NC, Uribe M, Méndez-Sánchez N. Metabolic syndrome and nonalcoholic fatty liver disease. The role of endothelial progenitor cells. Ann Hepatol. 2013;12:908-914. [PubMed] [Cited in This Article: ] |
| 3. | Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, Younossi ZM. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7:234-238. [PubMed] [Cited in This Article: ] |
| 4. | VanWagner LB, Ning H, Lewis CE, Shay CM, Wilkins J, Carr JJ, Terry JG, Lloyd-Jones DM, Jacobs DR, Carnethon MR. Associations between nonalcoholic fatty liver disease and subclinical atherosclerosis in middle-aged adults: the Coronary Artery Risk Development in Young Adults Study. Atherosclerosis. 2014;235:599-605. [PubMed] [Cited in This Article: ] |
| 5. | Branch of Cardiologists, Chinese Medical Doctor Association, Editorial Board of Chinese Journal of Internal Medicine. Chinese Expert Concensus on the Primary Prevention of Cardiovascular Diseases. Zhonghua Neike Zazhi. 2010;45:174-185. [DOI] [Cited in This Article: ] |
| 6. | Chen LY, Qiao QH, Zhang SC, Chen YH, Chao GQ, Fang LZ. Metabolic syndrome and gallstone disease. World J Gastroenterol. 2012;18:4215-4220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 110] [Cited by in F6Publishing: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 7. | Zhu WH, Zhu YM, Chen JH, Zhou YH. [Brachial ankle pulse wave velocity in the patients of metabolic syndrome]. Zhonghua Yi Xue Zazhi. 2013;93:566-568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 8. | Jian-gao F; Chinese Liver Disease Association. Guidelines for management of nonalcoholic fatty liver disease: an updated and revised edition. Zhonghua Ganzangbing Zazhi. 2010;18:163-166. [PubMed] [Cited in This Article: ] |
| 9. | Xu HM, Zeng QY. An easy way to estimate the degree of homogeneous fatty liver with ultrasonography. Zhongguo Jieru Yu Yingxiangxue Zazhi. 2008;5:148-151. [Cited in This Article: ] |
| 10. | Pan CY. Advances in the understanding and management of metabolic syndrome: a comment on the International Diabetes Federation Consensus Worldwide Definition Of The Metabolic Syndrome. Zhonghua Linchuang Neifenmi Zazhi. 2005;21:298-300. [DOI] [Cited in This Article: ] |
| 11. | Joint Commission for Establishing the Guidelines on the Management of Abnormal Blood lipids among Chinese Adults. Guidelines on the Management of Abnormal Blood lipids among Chinese Adults. Zhonghua Xinxueguanbingxue Zazhi. 2007;35:390-419. [DOI] [Cited in This Article: ] |
| 12. | Zhou Q, Fan JG. Research advances in the relationship between non-alcoholic fatty liver disease and coronary heart disease. Zhonghua Dongmaizhouyangyinghua Zazhi. 2008;16:669-671. [DOI] [Cited in This Article: ] |
| 13. | Lin GY, Lin JX. Research advances in pulse wave velocity and its clinical relevances. Zonghua Xinxueguanbing Zazhi. 2011;9:539-542. [DOI] [Cited in This Article: ] |
| 14. | Kim BJ, Kim NH, Kim BS, Kang JH. The association between nonalcoholic fatty liver disease, metabolic syndrome and arterial stiffness in nondiabetic, nonhypertensive individuals. Cardiology. 2012;123:54-61. [PubMed] [Cited in This Article: ] |
| 15. | Zhong JM, Huang ZY, Hu SH. Relationship between non-alcoholic fatty liver disease and peripheral arteriosclerosis. Zhonghua Dongmaizhouyangyinghua Zazhi. 2011;19:66-68. [Cited in This Article: ] |
| 16. | Cartier A, Lemieux I, Alméras N, Tremblay A, Bergeron J, Després JP. Visceral obesity and plasma glucose-insulin homeostasis: contributions of interleukin-6 and tumor necrosis factor-alpha in men. J Clin Endocrinol Metab. 2008;93:1931-1938. [PubMed] [Cited in This Article: ] |
| 17. | Cao Y, Li L. [Relationship of non-alcoholic steatohepatitis with arterial endothelial function and atherosclerosis]. Zhonghua Gan Zang Bing Zazhi. 2014;22:205-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 18. | Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341-1350. [PubMed] [Cited in This Article: ] |
| 19. | Fargion S, Porzio M, Fracanzani AL. Nonalcoholic fatty liver disease and vascular disease: state-of-the-art. World J Gastroenterol. 2014;20:13306-13324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 144] [Cited by in F6Publishing: 154] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 20. | Elsheikh E, Younoszai Z, Otgonsuren M, Hunt S, Raybuck B, Younossi ZM. Markers of endothelial dysfunction in patients with non-alcoholic fatty liver disease and coronary artery disease. J Gastroenterol Hepatol. 2014;29:1528-1534. [PubMed] [Cited in This Article: ] |
| 21. | De Paola L, Silvado C, Souza L, Crippa A, Twardowschy CA, Germiniani F, Mäder MJ, Palmini A. Right and left mesial temporal lobe seizures in one patient: bona fide semiological, interictal, ictal, and MRI evidence. Epilepsy Behav. 2009;14:418-420. [PubMed] [Cited in This Article: ] |
| 22. | Sookoian S, Castaño GO, Burgueño AL, Rosselli MS, Gianotti TF, Mallardi P, Martino JS, Pirola CJ. Circulating levels and hepatic expression of molecular mediators of atherosclerosis in nonalcoholic fatty liver disease. Atherosclerosis. 2010;209:585-591. [PubMed] [Cited in This Article: ] |
| 23. | Marra F, Gastaldelli A, Svegliati Baroni G, Tell G, Tiribelli C. Molecular basis and mechanisms of progression of non-alcoholic steatohepatitis. Trends Mol Med. 2008;14:72-81. [PubMed] [Cited in This Article: ] |
| 24. | Hao ZH, Wang JM, Li Y. Prevalence of fatty liver in populations with different occupations and its risk factors. Zhonghua Quanke Yixue. 2012;15:2444-2447. [DOI] [Cited in This Article: ] |
| 25. | Wang YD, Zhang YZ, Zhao CY, Cao W. [Role of sex hormones in nonalcoholic fatty liver disease pathogenesis]. Zhonghua Gan Zang Bing Zazhi. 2012;20:398-400. [PubMed] [Cited in This Article: ] |
| 26. | Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2292-2333. [PubMed] [Cited in This Article: ] |