Published online Sep 21, 2015. doi: 10.3748/wjg.v21.i35.10091
Peer-review started: February 22, 2015
First decision: March 26, 2015
Revised: May 25, 2015
Accepted: July 3, 2015
Article in press: July 3, 2015
Published online: September 21, 2015
AIM: To investigate the effects of broccoli sprout extract (BSEx) on liver gene expression and acute liver injury in the rat.
METHODS: First, the effects of BSEx on liver gene expression were examined. Male rats were divided into two groups. The Control group was fed the AIN-76 diet, and the BSEx group was fed the AIN-76 diet containing BSEx. After a 10-d feeding period, rats were sacrificed and their livers were used for DNA microarray and real-time reverse transcription-polymerase chain reaction (RT-PCR) analyses. Next, the effects of BSEx on acute liver injury were examined. In experiments using acute liver injury models, 1000 mg/kg acetaminophen (APAP) or 350 mg/kg D-galactosamine (D-GalN) was used to induce injury. These male rats were divided into four groups: Control, BSEx, Inducer (APAP or D-GalN), and Inducer+BSEx. The feeding regimens were identical for the two analyses. Twenty-four hours following APAP administration via p.o. or D-GalN administration via i.p., rats were sacrificed to determine serum aspartate transaminase (AST) and alanine transaminase (ALT) levels, hepatic glutathione (GSH) and thiobarbituric acid-reactive substances accumulation and glutathione-S-transferase (GST) activity.
RESULTS: Microarray and real-time RT-PCR analyses revealed that BSEx upregulated the expression of genes related to detoxification and glutathione synthesis in normal rat liver. The levels of AST (70.91 ± 15.74 IU/mL vs 5614.41 ± 1997.83 IU/mL, P < 0.05) and ALT (11.78 ± 2.08 IU/mL vs 1297.71 ± 447.33 IU/mL, P < 0.05) were significantly suppressed in the APAP + BSEx group compared with the APAP group. The level of GSH (2.61 ± 0.75 nmol/g tissue vs 1.66 ± 0.59 nmol/g tissue, P < 0.05) and liver GST activity (93.19 ± 16.55 U/g tissue vs 51.90 ± 16.85 U/g tissue, P < 0.05) were significantly increased in the APAP + BSEx group compared with the APAP group. AST (4820.05 ± 3094.93 IU/mL vs 12465.63 ± 3223.97 IU/mL, P < 0.05) and ALT (1808.95 ± 1014.04 IU/mL vs 3936.46 ± 777.52 IU/mL, P < 0.05) levels were significantly suppressed in the D-GalN + BSEx group compared with the D-GalN group, but the levels of AST and ALT in the D-GalN + BSEx group were higher than those in the APAP + BSEx group. The level of GST activity was significantly increased in the D-GalN + BSEx group compared with the D-GalN group (98.04 ± 15.75 U/g tissue vs 53.15 ± 8.14 U/g tissue, P < 0.05).
CONCLUSION: We demonstrated that BSEx protected the liver from various types of xenobiotic substances through induction of detoxification enzymes and glutathione synthesis.
Core tip: The aim of this study was to investigate the effects of broccoli sprout extract (BSEx) on gene expression and acute liver injury in rat liver. Gene expression analyses revealed that BSEx upregulated the expression of genes related to detoxification and glutathione synthesis. Experiments using acute liver injury models revealed that BSEx suppressed acetaminophen- and D-galactosamine-induced liver injury and increased liver glutathione concentration and glutathione-S-transferase activity. These findings suggest that consuming BSEx daily protected the liver from various types of xenobiotic substances through induction of detoxification enzymes and glutathione synthesis.
- Citation: Yoshida K, Ushida Y, Ishijima T, Suganuma H, Inakuma T, Yajima N, Abe K, Nakai Y. Broccoli sprout extract induces detoxification-related gene expression and attenuates acute liver injury. World J Gastroenterol 2015; 21(35): 10091-10103
- URL: https://www.wjgnet.com/1007-9327/full/v21/i35/10091.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i35.10091
Many drug-metabolizing enzymes are expressed in the liver, including phase I enzymes, such as cytochrome P450s (CYPs), and phase II enzymes, such as glutathione S-transferases (GSTs), UDP-glucuronosyltransferases (UGTs), and sulfotransferases. The phase I enzymes, composed mainly of the CYP supergene family, are involved in the oxidation and hydroxylation of xenobiotics. Consequently, reactive molecules, which may be more toxic than the parent molecules, are produced. Phase II enzymes convert activated, hydrophobic xenobiotics into hydrophilic forms via conjugation reactions with glutathione, glucuronide, sulfate, and other molecules[1].
Sulforaphane (4-methylsulfinylbutyl isothiocyanate) has been identified as the most potent naturally occurring inducer of phase II enzymes[2-4]. Dietary sulforaphane is known to protect against liver injuries caused by carbon tetrachloride, intestinal ischemia reperfusion, and cisplatin[5-7]. In these reports, the ability of orally ingested sulforaphane to induce phase II detoxification enzymes was suggested as the basis for protection from these injuries.
Sulforaphane is a metabolite of glucoraphanin, a thioglycoside compound released upon chewing or macerating cruciferous plants[8]. Glucoraphanin is hydrolyzed to sulforaphane by myrosinase, an enzyme released when plant cells are damaged[9]. Cruciferous vegetables are a main source of glucoraphanin, and several-day-old broccoli sprouts have 15-fold more glucoraphanin than mature plants[2]. Broccoli sprouts have been reported to induce phase II enzymes in vitro and in vivo[2,10]. Moreover, interventional studies have shown that dietary broccoli sprouts induce phase II enzymes[11] and can modulate the excretion patterns of aflatoxin[12]. These reports show that detoxification enzymes are induced by intake of broccoli sprouts in vivo; however, few reports have evaluated the effects of continuous ingestion of broccoli sprouts on liver function.
DNA microarray technology has allowed us to comprehensively analyze the expression of a large number of genes in target cells or tissues[13]. This technology has been used to study how administration of sulforaphane modulates gene expression in animal liver[14,15]. To our knowledge, there have been no reports showing a detailed analysis of daily administration of dietary broccoli sprouts on liver gene expression.
In this study, we used DNA microarray and real-time reverse transcription-polymerase chain reaction (RT-PCR) analyses to investigate the effects of broccoli sprout extract (BSEx) on gene expression in rat liver. Moreover, we investigated the effects of BSEx on the intoxication produced by acetaminophen (APAP) and D-galactosamine (D-GalN), which are model compounds for drug-induced liver injury and virus-induced liver injury, respectively[16].
It was reported that glucoraphanin content in broccoli sprout at the early stage of germination (within 24 h) was almost same as the glucoraphanin content at 72 h of germination[17]. Therefore, BSEx was industrially processed using 1-d-old broccoli sprouts to conduct experiments in a short period. Briefly, the broccoli sprouts were extracted with water at 95 °C for 1 h to remove erucic acid, which has been reported to be a potential risk factor for heart disease[18]. The extract was concentrated to concentrate the glucoraphanin content up to approximately 10 times (54.45 mg/g) using a centrifugal thin film vacuum evaporator. Finally, the concentrated extract was sterilized by filtration through MF-Millipore filters (EMD Millipore, Billerica, MA, United States), packed in plastic bags, and stored at -20 °C until use.
The BSEx diet was prepared based on the composition of the AIN-76 diet, not of the AIN-93 diet. This is because tert-butylhydroquinone, which is a constituent of AIN-93, is known as a representative inducer of drug-metabolizing enzymes. We used AIN-76, which does not contain tert-butylhydroquinone, to more precisely evaluate the effects of BSEx on the liver detoxification system. The amount of cornstarch contained in the AIN-76 diet was replaced with BSEx. The concentration of glucoraphanin in the BSEx diet was adjusted to 340 mg/100 g diet, a concentration based on pilot animal experiments using acute liver injury models (unpublished results). The compositions of the control and BSEx diets are described in Table 1.
| Ingredients | Control diet (AIN-76) | BSEx diet |
| Casein | 25.00% | 25.00% |
| Cornstarch | 40.10% | 33.86% |
| Sucrose | 20.00% | 20.00% |
| Corn oil | 5.00% | 5.00% |
| Mineral mixture | 3.50% | 3.50% |
| Vitamin mixture | 1.00% | 1.00% |
| Choline bitartrate | 0.40% | 0.40% |
| Cellulose | 5.00% | 5.00% |
| BSEx | ||
| Glucoraphanin | - | 0.34% |
| Others | - | 5.90% |
Specific pathogen-free, 7-wk-old male Wistar rats were purchased from Japan SLC (Hamamatsu, Japan). They were housed at 20 °C-24 °C and 45%-65% humidity in an animal laboratory with a 12-h light/12-h dark cycle timed from 7:00 AM. They were fed a normal commercial diet (CE-2; CLEA Japan, Tokyo, Japan) and sterile water during the 5-d acclimatization period before the experiment. The Animal Care and Use Committee of the Institute of Kagome Company Limited approved all protocols, which were in accordance with the guidelines established by the Japanese Society of Nutrition and Food Science (Law and Notification 6 of the Japanese Government).
Animals were divided into two groups with similar average body weights. Each group was composed of six rats; the Control group was fed the AIN-76 diet, and the BSEx group was fed the BSEx diet. After a 10-d feeding period, rats were sacrificed and their livers were immediately frozen in liquid nitrogen and stored at -80 °C until DNA microarray and real time RT-PCR processing.
Four rats from each group, whose final body weights and relative liver weights approximated the mean values for the six rats in each group, were selected for further DNA microarray analysis. Approximately 50 mg of liver was homogenized in TRIzol reagent (Invitrogen, Carlsbad, CA, United States). Total RNA was isolated from each liver according to the manufacturer’s instructions and purified using the RNeasy Plus Mini Kit (Qiagen, Hilden, Germany). The quality and quantity were evaluated by agarose gel electrophoresis and spectrophotometry, respectively. Total RNA from individual samples was analyzed by DNA microarray as previously described[19]. Briefly, 100 ng of purified total RNA was used to synthesize complementary DNA, and then biotinylated amplified RNA (aRNA) was transcribed using the GeneChip 3’ IVT Express Kit (Affymetrix, Santa Clara, CA, United States). aRNA was fragmented and then hybridized to an Affymetrix GeneChip rat genome 230 2.0 array. The array was hybridized at 45 °C for 16 h, and then washed and stained with phycoerythrin. Fluorescence signals were scanned with the Affymetrix GeneChip System, and Affymetrix GeneChip Command Console software was used to reduce the images to the intensity values for each probe (CEL files).
Six rats from each group were used for real-time RT-PCR analysis. Approximately 50 mg of liver was homogenized in TRIzol reagent and total RNA, which is treated with DNase, was isolated from each liver. The quantity was measured by spectrophotometry and cDNA was prepared from 2 μg of the total RNA. Real-time RT-PCR was performed with SYBR Premix Ex Taq II (Takara Bio, Otsu, Japan) and the 7900HT Fast Real-Time PCR System (Applied Biosystems, Tokyo, Japan). After denaturing at 95 °C for 30 s, PCR was performed with 40 cycles of denaturing at 95 °C for 5 s, annealing at 60 °C for 30 s, and dissociation at 95 °C for 15 s, followed by 60 °C for 60 s and 95 °C for 15 s. The expression of each gene was normalized to that of β-actin mRNA. The sequence of each primer is shown in Table 2. The concentration of each primer used in real-time PCR was 0.4 μmol/L.
| Gene symbol | Sequence (5'→3') | |
| Rpl10a | Forward | GAAGAAGGTGCTGTGTTTGGC |
| Reverse | TCGGTCATCTTCACGTGGC | |
| Arbp | Forward | GGCGACCTGGAAGTCCAACTA |
| Reverse | CATTGTCTGCTCCCACAATGAA | |
| Rpl15 | Forward | GCTTTAGTAGCAGCTGGTGTGTGA |
| Reverse | ACCCAAGACGAATTGATTGGAA | |
| Rps16 | Forward | TCCAAGGGTCCGCTGCAGTC |
| Reverse | CGTTCACCTTGATGAGCCCATT | |
| Rpl3 | Forward | TGTATTGGAGCTTGGCATCCTG |
| Reverse | ACCATCCTTGATGAGGTAGCCTTG | |
| Rps18 | Forward | AAGTTTCAGCACATCCAGCGAGTA |
| Reverse | TTGGTGAGGTCAATGTCTGCTTTC | |
| Rps3 | Forward | GATCATGTGAGCATTGTGGAACCTA |
| Reverse | CTCCAGATGCAGCTGCCAAG | |
| Cyp1a2 | Forward | TCAACCTCGTGAAGAGCAGCA |
| Reverse | GTCCTGGATACTGTTCTTGTTGAAGTC | |
| Gstm1 | Forward | TTCGTGCAGACATTGTGGAGA |
| Reverse | CTTGCCCAGGAACTCAGAGTAGA | |
| Gclc | Forward | GTGGACACCCGATGCAGTATTC |
| Reverse | CATCCACCTGGCAACAGTCATTAG | |
| Gsta3 | Forward | AATAGGCTGAGCAGGGCTGATG |
| Reverse | GGTTGCTGACTCTGGTTCTCAGG | |
| Gstt3 | Forward | ATGCCTTTGCCCAGGTGAAC |
| Reverse | GTGTGCTGCCAAGCCAGGTA | |
| Gstp1 | Forward | GAGGACCTTCGATGCAAATATGGTA |
| Reverse | CTGGGACAGCAGGGTCTCAA | |
We used two types of acute liver injury models, APAP- and D-GalN-induced liver injury[16]. APAP and D-GalN were purchased from Sigma-Aldrich (St. Louis, MO, United States). For the experiment using the APAP-induced liver injury model, 34 male Wistar rats aged 6 wk were purchased from Japan SLC. Acclimatization was performed as described in DNA microarray analysis. After the acclimatization period, rats were divided into Control (n = 6), BSEx (n = 8), APAP (n = 10), and APAP + BSEx (n = 10) groups. Control and APAP groups were fed the AIN-76 diet, and the BSEx and APAP + BSEx groups were fed the BSEx diet for 10 d. Liver injury was induced in the APAP and APAP + BSEx groups by oral administration of 1000 mg/kg APAP dissolved in 1% methylcellulose. The Control and BSEx groups were orally administered 1% methylcellulose.
For the experiment using the D-GalN-induced liver injury model, 36 male Wistar rats aged 7 wk were purchased from Japan SLC. Acclimatization was performed as described in DNA microarray analysis. After the acclimatization period, rats were divided into Control (n = 6), BSEx (n = 6), D-GalN (n = 12) and D-GalN + BSEx (n = 12) groups. The method of feeding was as described above. Liver injury was induced in the D-GalN and D-GalN + BSEx groups by intraperitoneal administration of 350 mg/kg D-GalN dissolved in saline. The Control and BSEx groups were intraperitoneally administered saline.
Twenty-four hours following administration of the inducers and vehicles, rats were anaesthetized and blood and livers were collected. Serum samples were separated by centrifugation at 2000 g for 10 min and tested for aspartate transaminase (AST) and alanine transaminase (ALT). Livers were washed with saline and immediately stored at -80 °C for further analyses.
Plasma AST and ALT levels were determined using a commercially available analytical kit (Wako Pure Chemical Industries, Osaka, Japan). Approximately 100 mg of liver was homogenized with 0.5-1.0 mL of 5% 5-sulfosalicylic acid, and hepatic glutathione (GSH) concentration was determined by a commercial kit (Dojindo Molecular Technologies, Kumamoto, Japan). Hepatic GST activity was measured according to the method of Habig et al[20]. Approximately 0.5 g of liver was homogenized with 10 volumes of 0.1 mol/L potassium phosphate buffer (pH 7.4), and the supernatant was collected. Five-hundred microliters of 0.2 mol/L potassium phosphate, 100 μL of 10 mmol/L GSH, and 100 μL of 10 mmol/L 1-chloro-2,4-dinitrobenzene (CDNB) were added to 100 μL of supernatant. GST activity was determined by monitoring the absorbance at 340 nm for 3 min. Hepatic thiobarbituric acid-reactive substances (TBARS) were measured according to the method of Kikugawa et al[21]. Approximately 0.5 g of liver was homogenized with 9 volumes of 10 mmol/L Tris-HCl (pH 7.4). The liver homogenate (200 μL) was mixed with 650 μL of extraction reagent (0.2 mL of 5.2% sodium dodecyl sulfate (SDS), 50 μL of 0.8% butylated hydroxytoluene in glacial acetic acid, 1.5 mL of 0.8% TBA, 1.7 mL of water) and 150 μL of 20% acetate buffer to a final volume of 1.0 mL. The mixture was heated at 100 °C for 60 min, cooled to room temperature, and extracted with 1.0 mL of a mixture of 1-butanol/pyridine (15:1, v/v). TBARS concentration was determined by measuring the absorbance at 532 nm of the extract.
CEL files were quantified with the Factor Analysis for Robust Microarray Summarization (FARMS) algorithm[22] using the statistical language R[23] and Bioconductor[24]. To detect the differentially expressed genes between the Control and BSEx groups, the rank products (RP) method was used[25]. RP offers several advantages over linear modeling, including a biologically intuitive fold-change criterion; this model contains fewer assumptions and increased performance with noisy data and/or low numbers of replicates[26]. A recent study revealed that the combination of the RP method and the FARMS with quantile normalization (qFARMS) preprocessing algorithm is one of the best combinations for accurately detecting differentially expressed genes[27], and these were applied to our microarray data. Functional classification of the differentially expressed genes according to Biological Process in Gene Ontology (GO) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway were performed using the Database for Annotation, Visualization, and Integrated Discovery (DAVID)[28], a web-accessible program, in accordance with the manuals available from the website (http://david.abcc.ncifcrf.gov/home.jsp). Enrichment analyses were performed based on EASE score, a modified Fisher’s exact P value[29] with the Benjamini and Hochberg false discovery rate corrections[30].
Ingenuity Pathway Analysis (IPA)[31] was used to search for canonical pathways. IPA is software licensed by Ingenuity Systems (Redwood City, CA, United States) and is a commercial tool based on an appropriate database to facilitate the identification of biological themes in microarray gene expression data. IPA uses a right-tailed Fisher’s exact test to calculate a P value determining the probability that each biological function, canonical pathway, or transcriptional network assigned to the dataset is due to chance alone. A data set containing only the IDs of the significantly upregulated genes was uploaded as a tab-delimited text file into the Ingenuity software.
Statistical analysis to compare gene expression by real-time RT-PCR analysis was performed by Student’s t-test. Statistical analysis to compare body and liver weights, and blood and liver parameters was performed by the Tukey-Kramer method using SPSS 15.0 for Windows (SPSS Japan, Tokyo, Japan). Differences were considered significant at P < 0.05.
BSEx had no effect on the amount of dietary intake in rats (data not shown). In DNA microarray analysis, genes showing a false discovery rate (FDR) of less than 0.05 between the Control and BSEx groups were defined as differentially expressed genes. The RP method combined with a qFARMS preprocessing algorithm revealed 403 upregulated and 515 downregulated probe sets in the group fed the BSEx diet. Of those, overlapping probe sets and probe sets lacking defined gene titles were removed, resulting in 356 upregulated genes and 426 downregulated genes that were identified.
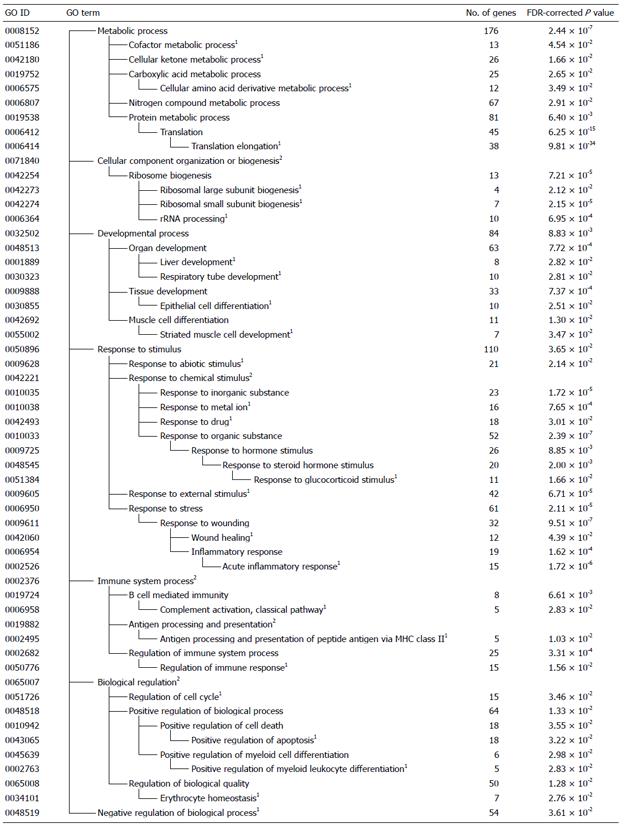
Using DAVID, the differentially expressed genes induced by intake of the BSEx diet were classified into functional categories according to GO. The significantly enriched categories of genes that were up- or downregulated by the intake of BSEx diet are summarized in Figures 1 and 2, respectively. The GO classes upregulated by BSEx were ranked according to the P value of each GO class as follows: “translation elongation”, “response to organic substance”, “response to wounding”, “response to inorganic substance”, “response to external stimulus”, and “ribosome biogenesis” (Figure 1). Similarly, the GO classes downregulated by BSEx were ranked as follows: “carboxylic acid metabolic process”, “lipid metabolic process”, “alcohol metabolic process”, “response to organic substance”, “coenzyme metabolic process”, and “glucose metabolic process” (Figure 2).
The KEGG analysis identified functional classes of the differentially expressed genes. The up- and downregulated genes fell into significantly enriched KEGG pathways [three for the upregulated genes and eighteen for the downregulated genes (Table 3)]. The KEGG classes upregulated by BSEx were “Ribosome”, “Metabolism of xenobiotics by cytochrome P450” and “Drug metabolism”. The KEGG class “Ribosome” included about 40 kinds of genes encoding ribosomal proteins. The KEGG classes “Metabolism of xenobiotics by cytochrome P450” and “Drug metabolism” included genes encoding phase I and II detoxification enzymes, such as Cyps, Gsts, and Ugts. The KEGG classes downregulated by BSEx contained pathways related to lipid and carbohydrate metabolism.
| KEGG ID | Pathway | FDR-corrected P value | |
| Up-regulated | rno03010 | Ribosome | 3.97 × 10-43 |
| rno00980 | Metabolism of xenobiotics by cytochrome P450 | 0.001186149 | |
| rno00982 | Drug metabolism | 0.004009591 | |
| Down-regulated | rno00071 | Fatty acid metabolism | 2.42 × 10-17 |
| rno03320 | PPAR signaling pathway | 2.42 × 10-12 | |
| rno00280 | Valine, leucine and isoleucine degradation | 5.75 × 10-8 | |
| rno00620 | Pyruvate metabolism | 8.79 × 10-7 | |
| rno01040 | Biosynthesis of unsaturated fatty acids | 8.34 × 10-7 | |
| rno00650 | Butanoate metabolism | 1.21 × 10-6 | |
| rno00830 | Retinol metabolism | 5.97 × 10-6 | |
| rno00982 | Drug metabolism | 7.15 × 10-6 | |
| rno00640 | Propanoate metabolism | 1.06 × 10-5 | |
| rno00020 | Citrate cycle (TCA cycle) | 4.94 × 10-5 | |
| rno00120 | Primary bile acid biosynthesis | 5.63 × 10-5 | |
| rno00140 | Steroid hormone biosynthesis | 7.09 × 10-4 | |
| rno00561 | Glycerolipid metabolism | 7.78 × 10-4 | |
| rno00010 | Glycolysis/gluconeogenesis | 0.003660561 | |
| rno00380 | Tryptophan metabolism | 0.003952492 | |
| rno00983 | Drug metabolism | 0.004286518 | |
| rno00500 | Starch and sucrose metabolism | 0.009275005 | |
| rno00980 | Metabolism of xenobiotics by cytochrome P450 | 0.024561245 |
The 356 upregulated genes were imported into the IPA software to identify biological networks and pathways. Fifteen highly significant canonical pathways with a score of P < 0.05 were identified from the 356 genes upregulated by intake of the BSEx diet. As shown in Table 4, this analysis validated EIF2 signaling, regulation of eIF4 and p70S6K signaling, and mTOR signaling as major pathways in the first network. These included genes that encoded ribosomal proteins. As shown in Table 4, at least 6 of the 15 significant canonical pathways were related to xenobiotic metabolism, including NRF2-mediated oxidative stress response, metabolism of xenobiotics by cytochrome P450, glutathione metabolism, aryl hydrocarbon receptor signaling, xenobiotic metabolism signaling and pentose and glucuronate interconversions. These included genes that are involved in phase I and II detoxification and glutathione metabolism such as Cyp1a2, Gsts, Usts, Akrs and Gclc.
| Ingenuity canonical pathways | -log(B-H P value) | Ratio | Molecules |
| EIF2 signaling | 22.5 | 0.1830 | RPL18, RPL14, RPLP0, RPL7, Rpl7a, RPL41, RPS4X, RPS12, RPL22L1, RPL31, RPS26, RPS13, RPL15, RPS16, RPS7, RPS19, RPS6, RPS15A, RPL11, RPS2, RPS5, RPL26, RPS18, RPL34, RPSA, RPL13, Gm11425, RPS9, RPS3, RPS23, RPL23A, RPL27A, RPS8, RPL18A, RPS11, RPL3, RPLP2 |
| Regulation of eIF4 and p70S6K Signaling | 7.57 | 0.1150 | RPS18, RPS12, RPS4X, RPS26, RPS13, RPSA, RPS3, RPS16, RPS9, RPS7, RPS23, PPP2CA, RPS19, RPS6, RPS8, ITGB1, RPS15A, RPS11, RPS2, RPS5 |
| mTOR Signaling | 6.49 | 0.1000 | RND3, RPS18, RPS12, RPS4X, HMOX1, RPS26, RPS13, RPSA, RPS3, RPS16, RPS9, RPS7, RPS23, PPP2CA, RPS19, RPS6, RPS8, RPS15A, RPS11, RPS2, RPS5 |
| Nrf2-mediated oxidative stress response | 3.85 | 0.0890 | GPX2, GSTM5, ACTG1, AKR1A1, GCLC, HSPB8, GSTP1, GSTA1, HMOX1, GSTM1, FTL, JUN, EPHX1, NFE2L2, AKR7A3, ACTB, NQO1 |
| Metabolism of xenobiotics by cytochrome P450 | 3.84 | 0.0612 | GSTM5, GSTM1, Gstt3, CYP2B6, AKR1A1, UGT2B15, Cyp2c40, CYP1A2, GSTP1, GSTA1, AKR1C3, UGT2B4 |
| Glutathione metabolism | 2.88 | 0.0899 | GPX2, GSTM5, GSTM1, Gstt3, GCLC, PRDX6, GSTP1, GSTA1 |
| Arachidonic acid metabolism | 2.09 | 0.0485 | GPX2, LTC4S, CYP2B6, CYP4A22, PRDX6, PLA2G2A, Cyp2c40, PLA2G16, CYP1A2, AKR1C3 |
| Acute phase response signaling | 2.00 | 0.0734 | APOA1, A2M, C4BPA, SERPINA3, RBP7, C4B, HMOX1, ORM1/ORM2, C4BPB, FTL, JUN, HP, HPX |
| B cell development | 1.78 | 0.1210 | HLA-DMA, HLA-DRB1, HLA-DRA, HLA-DMB |
| Aryl hydrocarbon receptor signaling | 1.72 | 0.0692 | HSPB1, GSTM5, GSTM1, MYC, RARB, JUN, NFE2L2, CYP1A2, GSTP1, NQO1, GSTA1 |
| ERK/MAPK Signaling | 1.56 | 0.0588 | HSPB1, DUSP1, MYC, YWHAZ, PPP2CA, PRKAR2A, YWHAG, DUSP6, PLA2G2A, ITGB1, PPP1R3C, ETS1 |
| Xenobiotic metabolism signaling | 1.56 | 0.0508 | GSTM5, CYP2B6, GCLC, UGT2B15, CYP1A2, GSTP1, GSTA1, HMOX1, GSTM1, FTL, PPP2CA, NFE2L2, FMO5, NQO1, UGT2B4 |
| Eicosanoid signaling | 1.56 | 0.0759 | LTC4S, PTGER3, PRDX6, PLA2G2A, PLA2G16, AKR1C3 |
| Complement system | 1.56 | 0.1430 | C4BPB, C6, C4BPA, C4B, C1QB |
| Pentose and glucuronate interconversions | 1.56 | 0.0352 | AKR1A1, UGT2B15, AKR7A3, AKR1C3, UGT2B4 |
DNA microarray analysis and IPA revealed that the expression of genes related to protein synthesis, xenobiotic metabolism, and glutathione metabolism were upregulated by BSEx. Therefore, we analyzed expression of these genes by real-time RT-PCR analysis. One sample in the BSEx group showed abnormal expression of β-actin and was eliminated from the analysis. Table 5 shows FDR-corrected P values and the real-time RT-PCR results. Among 7 genes related to ribosomal proteins showing FDR < 0.01 in the microarray analysis, only the expression of Rps3 was significantly (P < 0.05) increased in the BSEx group and the expression of other genes was not increased. On the other hand, among 6 genes related to xenobiotic and glutathione metabolism, the expression of Cyp1a2, Gclc and Gsta3 were significantly (P < 0.01, 0.05 and 0.05, respectively) increased in the BSEx group.
| Gene symbol | FDR-corrected P value | Control | BSEx | ||
| Mean | SD | Mean | SD | ||
| Ribosome | |||||
| Rpl10a | 0.0018 | 1.00 | 0.06 | 0.82b | 0.05 |
| Arbp | 0.0056 | 1.00 | 0.13 | 0.86 | 0.24 |
| Rpl15 | 0.0058 | 1.00 | 0.14 | 0.80 | 0.23 |
| Rps16 | 0.0058 | 1.00 | 0.11 | 0.75a | 0.18 |
| Rpl3 | 0.0068 | 1.00 | 0.12 | 1.07 | 0.20 |
| Rps18 | 0.0086 | 1.00 | 0.06 | 1.00 | 0.17 |
| Rps3 | 0.0096 | 1.00 | 0.05 | 1.34a | 0.27 |
| Xenobiotic/glutathione metabolism | |||||
| Cyp1a2 | < 0.0001 | 1.00 | 0.32 | 2.43b | 0.66 |
| Gstm1 | < 0.0001 | 1.00 | 0.13 | 1.23 | 0.30 |
| Gclc | 0.0014 | 1.00 | 0.06 | 1.32a | 0.23 |
| Gsta3 | 0.0018 | 1.00 | 0.13 | 1.58a | 0.43 |
| Gstt3 | 0.0018 | 1.00 | 0.19 | 1.03 | 0.05 |
| Gstp1 | 0.0287 | 1.00 | 0.14 | 0.94 | 0.30 |
BSEx had no effect on the amount of dietary intake in rats (data not shown). The levels of body weight gain after APAP administration, liver weight, liver/body weight, and GSH were significantly decreased in the APAP group compared with the Control group. Decreases in these parameters in the APAP group were significantly reduced in the APAP + BSEx group. The levels of AST, ALT, and TBARS were significantly increased in the APAP group compared with the Control group. Increases in these parameters in the APAP group were significantly suppressed in the APAP + BSEx group (Table 6).
| Marker | Control | BSEx | APAP | APAP + BSEx | ||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Body weight (g) | 188.45 | 10.38 | 190.90 | 11.36 | 172.39 | 9.91 | 183.63 | 8.87 |
| Body weight gain after i.p (g) | 3.88 | 1.77 | 3.59 | 1.53 | -11.21a | 3.30 | -1.05b | 2.84 |
| Liver weight (g) | 8.46 | 0.44 | 9.13 | 0.69 | 6.90a | 0.58 | 8.11d | 0.85 |
| Liver/body | 0.045 | 0.001 | 0.048 | 0.002 | 0.04a | 0.002 | 0.044d | 0.003 |
| AST (IU/L) | 71.41 | 6.99 | 59.98 | 8.46 | 5614.41a | 1997.83 | 70.91 | 15.74 |
| ALT (IU/L) | 12.51 | 0.57 | 9.81 | 1.84 | 1297.71a | 447.33 | 11.78 | 2.08 |
| GST activity (U/g tissue) | 61.78 | 4.95 | 77.89 | 4.62 | 51.90c | 16.85 | 93.19b | 16.55 |
| TBARS (μmol/g tissue) | 462.68 | 36.57 | 420.95 | 23.66 | 751.12a | 220.87 | 496.63 | 37.21 |
| GSH (nmol/g tissue) | 2.73 | 0.26 | 2.99 | 0.29 | 1.66a | 0.59 | 2.61 | 0.75 |
The levels of body weight gain after D-GalN administration, liver weight, liver/body weight, and GST activity were significantly decreased in the D-GalN group compared with the Control group. Decreases in these parameters in the D-GalN group were significantly reduced in the D-GalN + BSEx group. The levels of AST, ALT, and TBARS were significantly increased in the D-GalN group compared with the Control group. Increases in AST and ALT in the D-GalN group were significantly suppressed in the D-GalN + BSEx group, but the levels of AST and ALT were much higher than that in the APAP+BSEx. Increases in TBARS was not significantly suppressed (Table 7).
| Marker | Control | BSEx | D-GalN | D-GalN + BSEx | ||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Body weight (g) | 237.31 | 10.49 | 235.13 | 16.38 | 224.30 | 11.14 | 225.39 | 10.76 |
| Body weight gain after i.p (g) | 1.85 | 1.32 | 3.50 | 0.97 | -7.74a | 2.42 | -4.10b | 3.67 |
| Liver weight (g) | 10.38 | 0.32 | 11.16 | 1.25 | 7.40a | 0.60 | 8.32b | 1.33 |
| Liver/body | 0.044 | 0.001 | 0.048 | 0.002 | 0.032a | 0.001 | 0.036b | 0.005 |
| AST (IU/L) | 55.73 | 8.66 | 66.30 | 24.08 | 12465.63a | 3223.97 | 4820.05b | 3094.93 |
| ALT (IU/L) | 11.12 | 1.01 | 12.47 | 3.48 | 3936.46a | 777.52 | 1808.95b | 1014.04 |
| GST activity (U/g tissue) | 87.92 | 10.42 | 109.43 | 23.19 | 53.15a | 8.14 | 98.04 | 15.75 |
| TBARS (μmol/g tissue) | 418.96 | 33.06 | 442.34 | 41.10 | 543.81c | 45.35 | 495.42d | 54.46 |
DNA microarray technology has been used for comprehensive analysis of gene expression changes induced by food or food components in target cells or tissues. We examined the effects of continuous ingestion of BSEx on comprehensive gene expression in normal rats and found that the expression of genes involved in phase I and II detoxification and glutathione synthesis were upregulated. Also, BSEx protected liver from APAP- and D-GalN-induced injury.
In the previous study, mice consuming the broccoli sprout extract-containing diet gained significantly less weight than mice consuming the normal diets and the reason is thought that the bitter flavor imparted by the broccoli sprout extract may have resulted in decreased consumption compared to the normal diet[32]. However, we show no decrease (Tables 6 and 7) of weight gain in the animals fed with BSEx diet, with no difference in food consumption (data not shown). We think that the following two factors are related with the controversy. The first one is the extraction temperature. In the previous study, broccoli sprout extracts were prepared by 60 °C mildly heating or 5-min steaming of broccoli sprouts[32]. Under the condition of 60 °C mildly heating, myrosinase has its enzyme activity and converts glucosinolates to isothiocyanates. The second one is the germination stage of broccoli sprouts. We used 1-d-old broccoli sprout for extraction of BSEx, but, in the previous study, 6-d-old broccoli sprouts were used[32]. It was reported that the content of phenolic compounds in broccoli sprouts increased dependent on its germination stage, so 6-d-old sprouts were thought to contain more phenolic compounds than 1-d broccoli sprouts[33]. Because of the bitter taste of isothiocyanates and/or phenolic compounds, rats might avoid eating broccoli extract containing diet in the previous study.
In this study, we prepared the BSEx diet containing 340 mg glucoraphanin/100 g diet. If calculated by the daily food intake (approximately 15 g) by a rat, and the body weight of a rat (approximately 200 g), we assumed that rats consumed about 200-300 mg/kg of glucoraphanin every day. Previous studies reported that 30-60 mg/kg of glucoraphanin administration was safe and effectively enhanced NQO1 (phase II enzyme) in various tissues and 120-240 mg/kg of glucoraphanin administration caused oxidative stress in rat liver[34,35]. However, in this study, rats didn’t show any of AST, ALT, TBARS increase and GSH decrease after 10 days of BSEx diet administration (Tables 6 and 7). These results suggested that BSEx used in this study had higher level of safety than used in the previous study. On the other hand, a pilot study (data not shown) carried out before this study showed that APAP-induced liver injury was strongly suppressed by the administration of BSEx diet containing 170 mg glucoraphanin/100 g diet (about 100-150 mg/kg of glucoraphanin every day). Moreover, APAP-induced liver injury was weakly suppressed by the administration of BSEx diet containing 34 mg glucoraphanin/100 g diet (about 20-30 mg/kg of glucoraphanin every day). These results suggest that acute liver injuries were suppressed by the low BSEx administration than in this study. We conducted experiment using high glucoraphanin contained diet to clarify the effect of BSEx on the liver injuries, but we think that the investigation of the dose-response effect of BSEx will be needed in future studies.
Sulforaphane is a well-known inducer of phase II drug-metabolizing enzymes[2-4], but the contribution of sulforaphane to the regulation of phase I enzymes is negligible. Nuclear factor erythroid 2-related factor 2 (Nrf2) and aryl hydrocarbon receptor (AHR) are well-known transcription factors related to the upregulation of detoxification genes[36]. Our IPA results also suggest that they contribute to the BSEx-induced upregulation of detoxification genes (Table 4). It is known that sulforaphane upregulates detoxification genes through activation of Nrf2[4,37], but little is known about the activation of AHR by sulforaphane. Compounds other than sulforaphane contained in BSEx possibly activated AHR. BSEx ingestion coordinately induced Nrf2 activation by sulforaphane and AHR activation by other unknown compounds, and consequently detoxification genes were upregulated.
We did not clarify the complete glucosinolate profile of BSEx in this study, but can estimate this parameter based on previous reports[2,10,38,39]. Previous studies have shown that glucoraphanin accounts for about 70% of glucosinolates in broccoli sprouts and is thought to be the major glucosinolate in BSEx. Glucoraphanin is converted to sulforaphane in vivo; hence, it is likely that glucoraphanin in BSEx contributed to the Nrf2 activation. Therefore, glucoraphanin, glucoiberin, glucoerucin, 4-methylthiobutylblucosinolate, and some indole glucosinolates may be present in BSEx, although their rates are thought to be lower than that of glucoraphanin[2,10,38,39]. The converted forms of indole glucosinolates are known to activate AHR[40,41]; thus, they may contribute to AHR activation by BSEx.
Microarray analysis revealed remarkable downregulation of genes related to lipid metabolism by ingestion of BSEx (Figures 1 and 2). Proteomic analysis of Nrf2-deficient transgenic mice has shown that many proteins involved in lipid metabolism are upregulated in the absence of Nrf2, suggesting a negative regulation of their expression by Nrf2[42]. In light of this report, Nrf2 activation by BSEx may contribute to the downregulation of genes involved in lipid metabolism, although its significance in liver protection remains unclear.
Because gene expression analysis showed that BSEx caused significant changes in the expression of genes related to liver protection from toxicity, we examined the effects of BSEx on liver injury induced by a toxicant. APAP causes liver injury through loss of GSH with an increased formation of reactive oxygen and nitrogen species in hepatocytes[43]. Moreover, liver GST activity was reported to decrease in APAP-induced acute liver injury[44]. In the present study, APAP-induced acute liver injury was followed by an increase in TBARS and decreases in liver GSH concentration and GST activity (Table 6). BSEx protected the liver from APAP-induced injury and improved the liver TBARS, GST activity, and GSH concentration (Table 6). Microarray and real-time RT-PCR analysis showed that Gclc, one of the rate-limiting enzymes of glutathione synthesis, was upregulated by BSEx (Table 5). It has been suggested that upregulation of Gclc contributes to the improvement of liver TBARS and GSH concentrations. N-acetyl-p-benzoquinoneimine, an intermediate of APAP that causes acute liver injury, is conjugated with the reduced form of GSH, a reaction that is mediated by GST[45]. Microarray and real-time RT-PCR analyses showed upregulation of Gsts by intake of BSEx (Table 5). These results suggest that upregulation of Gsts demonstrated by gene expression analysis influenced liver GST activity and resulted in protection from APAP-induced liver injury by BSEx.
Liver GST activity decreased and TBARS increased in D-GalN-induced acute liver injury[46,47]. In the present study, D-GalN-induced acute liver injury was followed by an increase in TBARS and a decrease in liver GST activity (Table 7). BSEx suppressed D-GalN-induced liver injury and improved GST activity and TBARS (Table 7), but the effect of BSEx on D-GalN-induced liver injury was weaker than that on APAP-induced liver injury. These results may indicate that suppression of D-GalN-induced liver injury by BSEx is caused partly by induction of GST activity, but some mechanisms other than improvement of GST activity and oxidative stress may contribute to suppression of D-GalN-induced liver injury. D-GalN caused a marked decrease in UTP with an accompanying inhibition of RNA and protein synthesis in rats[48,49]. Because microarray analysis showed upregulation of ribosomal proteins by intake of BSEx (Tables 3 and 4), D-GalN-induced liver injury might be suppressed through induction of protein synthesis, although real-time RT-PCR analysis showed that the expression of genes related to ribosomal proteins was not increased by BSEx, except for the expression of Rps3.
In summary, we showed that BSEx upregulated the expression of genes related to detoxification and glutathione synthesis in normal rat liver using DNA microarray and real-time PCR analyses. Moreover, BSEx suppressed APAP- and D-GalN-induced liver injury. Our data suggest that protection from these liver injuries resulted from induction of GSH synthesis and GST activity, although some other mechanisms may contribute to suppression of D-GalN-induced liver injury. We conclude that BSEx enhanced defensive functions and protected against the toxicities of various types of xenobiotic substances through induction of detoxification enzymes and glutathione synthesis in the liver.
We thank T. Matsumoto, K. Aizawa (Kagome Co. Ltd.), and K. Sugiyama (Shizuoka University) for technical advice.
Broccoli sprout is a unique plant which is abundant in sulforaphane, known as the most potent naturally occurring inducer of phase II drug-metabolizing enzymes. However, few reports have evaluated the effects of continuous ingestion of broccoli sprouts on liver function. Thus, it was very important to clarify the effects of broccoli sprout intake on gene expression in liver.
There have been no reports showing a detailed analysis of daily administration of dietary broccoli sprouts on liver gene expression. In this study, the authors used DNA microarray to investigate the effects of broccoli sprout extract (BSEx) on gene expression in rat liver. That technology allowed us to comprehensively analyze the expression of a large number of genes in liver.
The authors showed that BSEx upregulated the expression of genes related to detoxification and glutathione synthesis in normal rat liver using DNA microarray and real-time polymerase chain reaction analyses. Moreover, BSEx suppressed APAP- and D-galactosamine (D-GalN)-induced liver injury. They conclude that BSEx enhanced defensive functions and protected against the toxicities of various types of xenobiotic substances through induction of detoxification enzymes and glutathione synthesis in the liver.
Broccoli sprout is a commercially available plant and it is fit for food. The results of this study are applicable for development of functional foods using BSEx, although human trial will be needed in the future.
It is an interesting study investigating the effect of BSEx on gene expression in rat liver, and on the intoxication produced by acetaminophen and D-GalN. The results from these studies may provide better insights into the hepatoprotective effects of broccoli sprouts.
P- Reviewer: Pajares MA, Vynios D S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Liska DJ. The detoxification enzyme systems. Altern Med Rev. 1998;3:187-198. [PubMed] [Cited in This Article: ] |
| 2. | Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci USA. 1997;94:10367-10372. [PubMed] [Cited in This Article: ] |
| 3. | Gerhäuser C, You M, Liu J, Moriarty RM, Hawthorne M, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive potential of sulforamate, a novel analogue of sulforaphane that induces phase 2 drug-metabolizing enzymes. Cancer Res. 1997;57:272-278. [PubMed] [Cited in This Article: ] |
| 4. | Fahey JW, Talalay P. Antioxidant functions of sulforaphane: a potent inducer of Phase II detoxication enzymes. Food Chem Toxicol. 1999;37:973-979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 351] [Cited by in F6Publishing: 333] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 5. | Baek SH, Park M, Suh JH, Choi HS. Protective effects of an extract of young radish (Raphanus sativus L) cultivated with sulfur (sulfur-radish extract) and of sulforaphane on carbon tetrachloride-induced hepatotoxicity. Biosci Biotechnol Biochem. 2008;72:1176-1182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Zhao HD, Zhang F, Shen G, Li YB, Li YH, Jing HR, Ma LF, Yao JH, Tian XF. Sulforaphane protects liver injury induced by intestinal ischemia reperfusion through Nrf2-ARE pathway. World J Gastroenterol. 2010;16:3002-3010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 79] [Cited by in F6Publishing: 84] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 7. | Gaona-Gaona L, Molina-Jijón E, Tapia E, Zazueta C, Hernández-Pando R, Calderón-Oliver M, Zarco-Márquez G, Pinzón E, Pedraza-Chaverri J. Protective effect of sulforaphane pretreatment against cisplatin-induced liver and mitochondrial oxidant damage in rats. Toxicology. 2011;286:20-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Zhang Y, Talalay P. Mechanism of differential potencies of isothiocyanates as inducers of anticarcinogenic Phase 2 enzymes. Cancer Res. 1998;58:4632-4639. [PubMed] [Cited in This Article: ] |
| 9. | Bones AM, Rossiter JT. The myrosinase-glucosinolate system, its organisation and biochemistry. Physiol Plant. 1996;97:194-208. [DOI] [Cited in This Article: ] |
| 10. | Zhang Y, Munday R, Jobson HE, Munday CM, Lister C, Wilson P, Fahey JW, Mhawech-Fauceglia P. Induction of GST and NQO1 in cultured bladder cells and in the urinary bladders of rats by an extract of broccoli (Brassica oleracea italica) sprouts. J Agric Food Chem. 2006;54:9370-9376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Riedl MA, Saxon A, Diaz-Sanchez D. Oral sulforaphane increases Phase II antioxidant enzymes in the human upper airway. Clin Immunol. 2009;130:244-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 164] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 12. | Kensler TW, Chen JG, Egner PA, Fahey JW, Jacobson LP, Stephenson KK, Ye L, Coady JL, Wang JB, Wu Y. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, Qidong, People’s Republic of China. Cancer Epidemiol Biomarkers Prev. 2005;14:2605-2613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 230] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 13. | Barrett JC, Kawasaki ES. Microarrays: the use of oligonucleotides and cDNA for the analysis of gene expression. Drug Discov Today. 2003;8:134-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Hu R, Hebbar V, Kim BR, Chen C, Winnik B, Buckley B, Soteropoulos P, Tolias P, Hart RP, Kong AN. In vivo pharmacokinetics and regulation of gene expression profiles by isothiocyanate sulforaphane in the rat. J Pharmacol Exp Ther. 2004;310:263-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 182] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 15. | Hu R, Xu C, Shen G, Jain MR, Khor TO, Gopalkrishnan A, Lin W, Reddy B, Chan JY, Kong AN. Gene expression profiles induced by cancer chemopreventive isothiocyanate sulforaphane in the liver of C57BL/6J mice and C57BL/6J/Nrf2 (-/-) mice. Cancer Lett. 2006;243:170-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 197] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 16. | Tuñón MJ, Alvarez M, Culebras JM, González-Gallego J. An overview of animal models for investigating the pathogenesis and therapeutic strategies in acute hepatic failure. World J Gastroenterol. 2009;15:3086-3098. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 148] [Cited by in F6Publishing: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 17. | Gu Y, Guo Q, Zhang L, Chen Z, Han Y, Gu Z. Physiological and biochemical metabolism of germinating broccoli seeds and sprouts. J Agric Food Chem. 2012;60:209-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Borg K. Physiopathological effects of rapeseed oil: a review. Acta Med Scand Suppl. 1975;585:5-13. [PubMed] [Cited in This Article: ] |
| 19. | Fukui Y, Sasaki E, Fuke N, Nakai Y, Ishijima T, Abe K, Yajima N. Effect of Lactobacillus brevis KB290 on the cell-mediated cytotoxic activity of mouse splenocytes: a DNA microarray analysis. Br J Nutr. 2013;110:1617-1629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130-7139. [PubMed] [Cited in This Article: ] |
| 21. | Kikugawa K, Yasuhara Y, Ando K, Koyama K, Hiramoto K, Suzuki M. Protective effect of supplementation of fish oil with high n-3 polyunsaturated fatty acids against oxidative stress-induced DNA damage of rat liver in vivo. J Agric Food Chem. 2003;51:6073-6079. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Hochreiter S, Clevert DA, Obermayer K. A new summarization method for Affymetrix probe level data. Bioinformatics. 2006;22:943-949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 196] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 23. | R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing 2006. Available from: http://www.gbif.org/resource/81287. [Cited in This Article: ] |
| 24. | Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9318] [Cited by in F6Publishing: 9280] [Article Influence: 464.0] [Reference Citation Analysis (0)] |
| 25. | Breitling R, Armengaud P, Amtmann A, Herzyk P. Rank products: a simple, yet powerful, new method to detect differentially regulated genes in replicated microarray experiments. FEBS Lett. 2004;573:83-92. [PubMed] [Cited in This Article: ] |
| 26. | Breitling R, Herzyk P. Rank-based methods as a non-parametric alternative of the T-statistic for the analysis of biological microarray data. J Bioinform Comput Biol. 2005;3:1171-1189. [PubMed] [Cited in This Article: ] |
| 27. | Kadota K, Nakai Y, Shimizu K. Ranking differentially expressed genes from Affymetrix gene expression data: methods with reproducibility, sensitivity, and specificity. Algorithms Mol Biol. 2009;4:7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24735] [Cited by in F6Publishing: 26229] [Article Influence: 1748.6] [Reference Citation Analysis (0)] |
| 29. | Hosack DA, Dennis G, Sherman BT, Lane HC, Lempicki RA. Identifying biological themes within lists of genes with EASE. Genome Biol. 2003;4:R70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1338] [Cited by in F6Publishing: 1465] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 30. | Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289-300. [DOI] [Cited in This Article: ] |
| 31. | Thomas S, Bonchev D. A survey of current software for network analysis in molecular biology. Hum Genomics. 2010;4:353-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 32. | Bricker GV, Riedl KM, Ralston RA, Tober KL, Oberyszyn TM, Schwartz SJ. Isothiocyanate metabolism, distribution, and interconversion in mice following consumption of thermally processed broccoli sprouts or purified sulforaphane. Mol Nutr Food Res. 2014;58:1991-2000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 33. | Waje CK, Jun SY, Lee YK, Moon KD, Choi YH, Kwon JH. Seed viability and functional properties of broccoli sprouts during germination and postharvest storage as affected by irradiation of seeds. J Food Sci. 2009;74:C370-C374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Perocco P, Bronzetti G, Canistro D, Valgimigli L, Sapone A, Affatato A, Pedulli GF, Pozzetti L, Broccoli M, Iori R. Glucoraphanin, the bioprecursor of the widely extolled chemopreventive agent sulforaphane found in broccoli, induces phase-I xenobiotic metabolizing enzymes and increases free radical generation in rat liver. Mutat Res. 2006;595:125-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Lai RH, Keck AS, Wallig MA, West LG, Jeffery EH. Evaluation of the safety and bioactivity of purified and semi-purified glucoraphanin. Food Chem Toxicol. 2008;46:195-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Nakata K, Tanaka Y, Nakano T, Adachi T, Tanaka H, Kaminuma T, Ishikawa T. Nuclear receptor-mediated transcriptional regulation in Phase I, II, and III xenobiotic metabolizing systems. Drug Metab Pharmacokinet. 2006;21:437-457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 146] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 37. | McMahon M, Itoh K, Yamamoto M, Chanas SA, Henderson CJ, McLellan LI, Wolf CR, Cavin C, Hayes JD. The Cap’n’Collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res. 2001;61:3299-3307. [PubMed] [Cited in This Article: ] |
| 38. | Mewis I, Schreiner M, Nguyen CN, Krumbein A, Ulrichs C, Lohse M, Zrenner R. UV-B irradiation changes specifically the secondary metabolite profile in broccoli sprouts: induced signaling overlaps with defense response to biotic stressors. Plant Cell Physiol. 2012;53:1546-1560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 39. | Pereira FM, Rosa E, Fahey JW, Stephenson KK, Carvalho R, Aires A. Influence of temperature and ontogeny on the levels of glucosinolates in broccoli (Brassica oleracea Var. italica) sprouts and their effect on the induction of mammalian phase 2 enzymes. J Agric Food Chem. 2002;50:6239-6244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 82] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 40. | Jellinck PH, Forkert PG, Riddick DS, Okey AB, Michnovicz JJ, Bradlow HL. Ah receptor binding properties of indole carbinols and induction of hepatic estradiol hydroxylation. Biochem Pharmacol. 1993;45:1129-1136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 144] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 41. | Renwick AB, Mistry H, Barton PT, Mallet F, Price RJ, Beamand JA, Lake BG. Effect of some indole derivatives on xenobiotic metabolism and xenobiotic-induced toxicity in cultured rat liver slices. Food Chem Toxicol. 1999;37:609-618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Kitteringham NR, Abdullah A, Walsh J, Randle L, Jenkins RE, Sison R, Goldring CE, Powell H, Sanderson C, Williams S. Proteomic analysis of Nrf2 deficient transgenic mice reveals cellular defence and lipid metabolism as primary Nrf2-dependent pathways in the liver. J Proteomics. 2010;73:1612-1631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 43. | Hinson JA, Roberts DW, James LP. Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol. 2010;369-405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 543] [Cited by in F6Publishing: 576] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 44. | Acharya M, Lau-Cam CA. Comparison of the protective actions of N-acetylcysteine, hypotaurine and taurine against acetaminophen-induced hepatotoxicity in the rat. J Biomed Sci. 2010;17 Suppl 1:S35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 45. | Henderson CJ, Wolf CR, Kitteringham N, Powell H, Otto D, Park BK. Increased resistance to acetaminophen hepatotoxicity in mice lacking glutathione S-transferase Pi. Proc Natl Acad Sci USA. 2000;97:12741-12745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 170] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 46. | Yoo YM, Nam JH, Kim MY, Choi J, Park HJ. Pectolinarin and Pectolinarigenin of Cirsium setidens Prevent the Hepatic Injury in Rats Caused by D-Galactosamine via an Antioxidant Mechanism. Biol Pharm Bull. 2008;31:760-764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 47. | Zhou Y, Park CM, Cho CW, Song YS. Protective effect of pinitol against D-galactosamine-induced hepatotoxicity in rats fed on a high-fat diet. Biosci Biotechnol Biochem. 2008;72:1657-1666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Farber JL, Gill G, Konishi Y. Prevention of galactosamine-induced liver cell necrosis by uridine. Am J Pathol. 1973;72:53-62. [PubMed] [Cited in This Article: ] |
| 49. | Funatsu K, Ishii H, Shigeta Y, Morita A, Tsuchiya M. D-galactosamine induced hepatic cirrhosis: its ultrastructural and biochemical studies in rat. Acta Hepatogastroenterol (Stuttg). 1978;25:97-104. [PubMed] [Cited in This Article: ] |