Published online Jul 7, 2015. doi: 10.3748/wjg.v21.i25.7869
Peer-review started: August 21, 2014
First decision: November 14, 2014
Revised: March 4, 2015
Accepted: May 11, 2015
Article in press: May 11, 2015
Published online: July 7, 2015
AIM: To evaluate the clinical outcomes of 240-wk treatment with entecavir (0.5 mg) in Chinese nucleoside-naive patients with cirrhosis.
METHODS: A total of 204 nucleoside-naive patients with compensated (n = 96) or decompensated (n = 108) hepatitis B virus (HBV)-induced cirrhosis at the Department of Gastroenterology of the China-Japan Union Hospital (Jilin University, Changchun, China) who were treated with entecavir (0.5 mg) for 240 wk were enrolled in this study. Liver biopsy samples obtained from 38 patients prior to treatment (baseline) and at week 240 were evaluated by different independent histopathologists. Efficacy assessments included the proportions of patients who achieved an HBV DNA level < 500 copies/mL, the association of interleukin-28B genetic variation with antivirus therapy, clinical outcomes, and histologic improvement. Changes in liver disease severity were analyzed, and liver histologic evaluation was performed in 38 patients with paired biopsies. Student t tests were used to compare the means of continuous variables between the groups, and the proportions of patients who achieved the endpoints were compared using the χ2 test.
RESULTS: At week 240, 87.5% of the patients with compensated cirrhosis and 92.6% of the patients with decompensated cirrhosis achieved a HBV DNA level < 500 copies/mL. Three patients had genotypic entecavir resistance within the 240-wk period. No significant association was observed between virologic response and interleukin-28 genotype (CT, 88.2% vs CC, 90.6%). The proportion of patients with Child-Pugh class A disease was significantly increased at week 240 (68%) from the baseline (47%; P < 0.01). The proportion of patients with Child-Pugh class B disease was significantly decreased at week 240 (25%) from the baseline (39%; P = 0.02). In the patients with paired liver biopsies, the mean reduction in the Knodell necroinflammatory score from the baseline was 3.58 ± 1.03 points (7.11 ± 1.80 vs 3.53 ± 1.35, P < 0.01). The mean reduction in Ishak fibrosis score from the baseline was 1.26 ± 0.64 points (5.58 ± 0.50 vs 4.32 ± 0.81, P < 0.01).
CONCLUSION: Entecavir is an effective treatment option for patients with HBV-related compensated or decompensated cirrhosis that can result in sustained virologic suppression and histologic improvement.
Core tip: Entecavir is a potent antiviral agent that is effective and safe for the treatment of chronic hepatitis B. However, data on its clinical benefits in patients with cirrhosis, especially in long-term treatment, are limited. The aims of this prospective study were to evaluate the antiviral efficacy and clinical outcomes of entecavir treatment for 240 wk in nucleoside-naive Chinese patients with chronic hepatitis B, and compensated or decompensated cirrhosis.
- Citation: Xu Y, Zhang YG, Wang X, Qi WQ, Qin SY, Liu ZH, Jiao J, Wang JB. Long-term antiviral efficacy of entecavir and liver histology improvement in Chinese patients with hepatitis B virus-related cirrhosis. World J Gastroenterol 2015; 21(25): 7869-7876
- URL: https://www.wjgnet.com/1007-9327/full/v21/i25/7869.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i25.7869
Chronic hepatitis B (CHB) remains a serious global public health problem, with an estimated 350-400 million people affected worldwide[1]. Such patients are at increased risk of developing cirrhosis, hepatic decompensation, and hepatocellular carcinoma (HCC)[2]. In the absence of treatment, 15%-20% of patients develop cirrhosis within five years[3,4]. Patients who subsequently progress to decompensated cirrhosis have a poor prognosis, with a five-year survival rate of only 14%-28% compared with 84% for patients with compensated cirrhosis[5,6]. Elevated serum hepatitis B virus (HBV) DNA levels are an independent risk factor of progression to cirrhosis, hepatic decompensation, HCC, and death[7,8]. Conversely, sustained reductions in viral load associated with antiviral therapy are strongly correlated with decreased risk of disease progression and improvements in liver histology and clinical signs or symptoms[9,10].
Multiple clinical studies have demonstrated that nucleos(t)ide analogs are effective in suppressing viral replication and reducing disease progression in patients with HBV-related cirrhosis[11-13]. In a randomized clinical trial, long-term lamivudine treatment (median duration, 32.4 mo) significantly reduced overall disease progression (increase in Child-Pugh score, hepatic decompensation, or HCC) compared with placebo (7.8% vs 17.7%, P = 0.001) in patients with hepatitis B e antigen-positive CHB and advanced fibrosis/compensated cirrhosis[12]. In contrast, data on clinical outcomes with nucleos(t)ide analogs in patients with decompensated cirrhosis are limited.
Entecavir is a potent antiviral agent that has been shown to be effective and safe for the treatment of CHB[14-17]. A subanalysis of phase III clinical data found that 57%-59% of patients with CHB and advanced liver fibrosis/cirrhosis experienced improvements in terms of Ishak fibrosis score at 48 wk of entecavir therapy[18]. More recently, the Shim et al[19] research group observed the clinical efficacy of one-year entecavir therapy in 55 patients with decompensated cirrhosis and found that 66% of the patients had improved Child-Turcotte-Pugh scores, which comprises individual scores for five parameters, namely total bilirubin level, serum albumin level, prothrombin time, ascites level, and hepatic encephalopathy. Patients with scores of 5 or 6, 7-9, or 10-15 were classified as having Child-Pugh class A, B, or C liver disease, respectively. Of the patients, 49% had increased Child-Turcotte-Pugh scores by ≥ 2. Clinical trial data from patients with advanced fibrosis/cirrhosis found that after approximately six years of cumulative entecavir therapy, all ten patients showed improvement in liver histology and Ishak fibrosis score. In particular, four patients had Ishak fibrosis scores ≤ 4 after the entecavir therapy[20].
Although the efficacy and safety of entecavir in nucleoside-naive patients without cirrhosis have been demonstrated in multiple studies, limited data are available on the clinical benefits in patients with cirrhosis. The aims of this prospective study were to evaluate the antiviral efficacy and clinical outcomes of entecavir treatment for 240 wk in nucleoside-naive Chinese patients with CHB and compensated or decompensated cirrhosis.
This prospective study evaluated the efficacy of entecavir (Bristol-Myers Squibb, Wallingford, CT, United States) at 0.5 mg once daily for 240 wk in patients with cirrhosis. Nucleoside-naive patients (n = 204) with HBV-related cirrhosis who attended the Department of Gastroenterology at the China-Japan Union Hospital (Jilin University, Changchun, China) were recruited and enrolled in the study beginning in June 2006. Diagnoses of compensated and decompensated cirrhosis were based on liver biopsy and/or clinical, radiologic, and laboratory criteria according to disease management guidelines[21]. Liver disease severity was graded according to Child-Pugh score. Patients with scores of 5 or 6, 7-9, or 10-15 were classified as Child-Pugh class A, B, or C, respectively. This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Jilin University. Written informed consent was obtained from all participants.
Eligible patients were adults aged ≥ 16 years with CHB infection (defined as hepatitis B surface antigen positive for ≥ 6 mo with persistent detectable hepatitis B surface antigen and/or serum HBV DNA) and compensated or decompensated cirrhosis. All of the patients were nucleoside-naive prior to entecavir treatment and had serum HBV DNA levels ≥ 500 copies/mL as measured using a PCR assay (Da An Gene Co. Ltd, Guangzhou, China; lower limit of detection, 500 copies/mL). The exclusion criteria included patients with coinfection with HIV or hepatitis A, C, D, or E viruses, and active alcohol abuse or dependence. Women who were pregnant or breastfeeding were also excluded.
HBV DNA, interleukin (IL)-28B genotype, and serum biochemical profiles were analyzed at baseline, weeks 4 and 12 of treatment, and every 12 wk thereafter up to 240 wk of treatment. Efficacy was defined when patients achieved a HBV DNA level < 500 copies/mL (via PCR) at week 240. The clinical endpoint assessed was disease progression in the total study population. Disease progression was defined as an increase in Child-Pugh score of ≥ 2, hepatic decompensation, HCC, spontaneous bacterial peritonitis, bleeding gastroesophageal varices, or death related to liver disease.
Liver biopsy samples obtained from 38 patients prior to treatment (baseline) and at week 240 were evaluated by different independent histopathologists. The proportions of patients with improvements in Knodell histologic activity index (HAI), fibrosis, and necroinflammatory scores from the baseline were assessed at week 240. Histologic improvement was defined as a decrease in Knodell necroinflammatory score of ≥ 2 points from the baseline and no worsening of fibrosis score, or a decrease in Ishak fibrosis score of ≥ 1 point from the baseline. Histologic worsening was defined as an increase in Knodell necroinflammatory score of ≥ 2 points or an increase in Ishak fibrosis score of ≥ 1 point from the baseline.
Patients with a virologic breakthrough (> 1-log10 increase in HBV DNA level higher than the nadir) were monitored for resistance mutations. Nucleotide sequence analysis of the HBV polymerase gene to detect genotypic entecavir resistance was performed for on-treatment samples by an independent laboratory (TaKaRa Biotechnology Co., Ltd., Dalian, China).
The incidence of adverse events, treatment discontinuation, deaths, and on-treatment alanine aminotransferase flares (defined as a serum level > 2 × baseline and > 10 × upper limit of normal) were documented. Renal impairment (defined as an elevation in serum creatinine to > 3 × upper limit of normal vs baseline) was also monitored. In all the patients with increased lactate serum concentrations, arterial blood gas analysis was performed immediately.
Statistical analysis was performed using SPSS v13.0 (SPSS Inc., Chicago, IL, United States). Continuous variables are expressed as mean ± SD and categorical data are expressed as proportions or percentages. The Student’s t test was used to compare the means of the continuous variables between the groups. The proportions of patients who achieved the end points were compared using the χ2 test. All of the tests were two-sided, and P < 0.05 was considered as statistically significant.
A total of 204 patients with HBV-related cirrhosis who were treated with entecavir for 240 wk at the China-Japan Union Hospital (Jilin University, Changchun, China) beginning in June 2006 were enrolled in this study. Of these patients, 96 had compensated cirrhosis (Child-Pugh class A) and 108 had decompensated cirrhosis (Child-Pugh classes B and C). Thirty-eight patients had paired liver biopsies at baseline and week 240 of treatment. The patients were predominantly male (67%-78%), and patients in the compensated cirrhosis group were significantly younger (P < 0.05) (Table 1). The compensated cirrhosis group also had higher alanine aminotransferase levels and fewer patients with HBV genotype C than the decompensated cirrhosis group (both P < 0.05).
| Characteristic | Compensated cirrhosis group(n = 96) | Decompensated cirrhosis group(n = 108) | P value |
| Age (yr) | 33.4 ± 10.6 | 42.4 ± 14.5 | < 0.05 |
| Male | 64 (67) | 84 (78) | 0.085 |
| HBeAg-positive | 72 (75) | 46 (43) | < 0.05 |
| HBV DNA (log10 copies/mL) | 6.5 ± 1.3 | 5.6 ± 1.5 | 0.077 |
| ALT (IU/L) | 131.4 ± 125.7 | 72.5 ± 63.1 | < 0.05 |
| HBV genotype | |||
| B | 40 (42) | 16 (15) | < 0.05 |
| C | 48 (50) | 78 (72) | < 0.05 |
| Other (A and D) | 8 (8) | 14 (13) | 0.367 |
At 240 wk of treatment, 87.5% of the patients with compensated cirrhosis and 92.6% of the patients with decompensated cirrhosis had achieved serum HBV DNA levels < 500 copies/mL. No significant differences in virologic response were observed between the two groups at week 240.
The genotype distributions of rs12979860 C/T in all the patients were analyzed. For the genotypes, the proportion of the CT genotype in the patients was 16.7% and that of the CC genotype was 83.3%. No significant association was observed between virologic response and IL-28 genotype (CT, 88.2% vs CC, 90.6%).
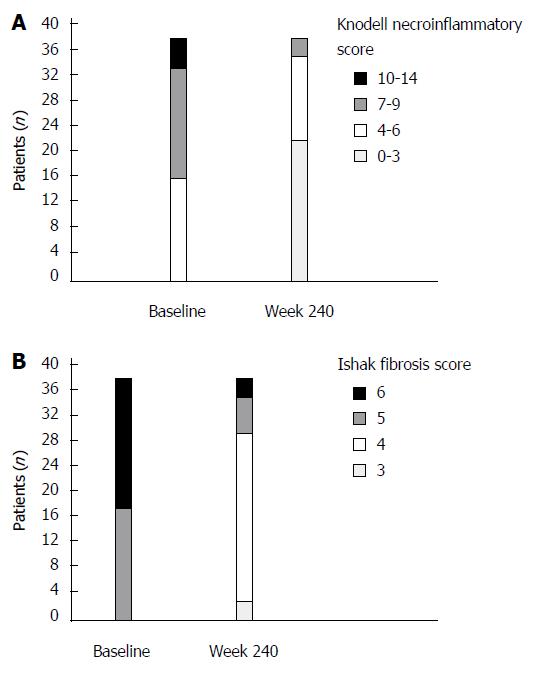
Histologic evaluation of liver biopsy samples from 38 patients with HBV-related cirrhosis indicated that 20 (52.6%) patients had a Knodell HAI score of 0-3 points at week 240 of entecavir treatment. The mean reduction in the Knodell necroinflammatory score from the baseline was 3.58 ± 1.03 points (7.11 ± 1.80 vs 3.53 ± 1.35, P < 0.01; Figure 1A).
With respect to fibrosis, 89.5% of the patients achieved improvement (≥ 1 point decrease from the baseline) in terms of Ishak fibrosis score. The mean reduction in Ishak fibrosis score from the baseline was 1.26 ± 0.64 points (5.58 ± 0.50 vs 4.32 ± 0.81, P < 0.01; Figure 1B).
A total of 89.5% of the patients achieved improvement (decrease in Knodell necroinflammatory score of ≥ 2 points from the baseline and no worsening of fibrosis score, or a decrease in Ishak fibrosis score of ≥ 1 point from the baseline).
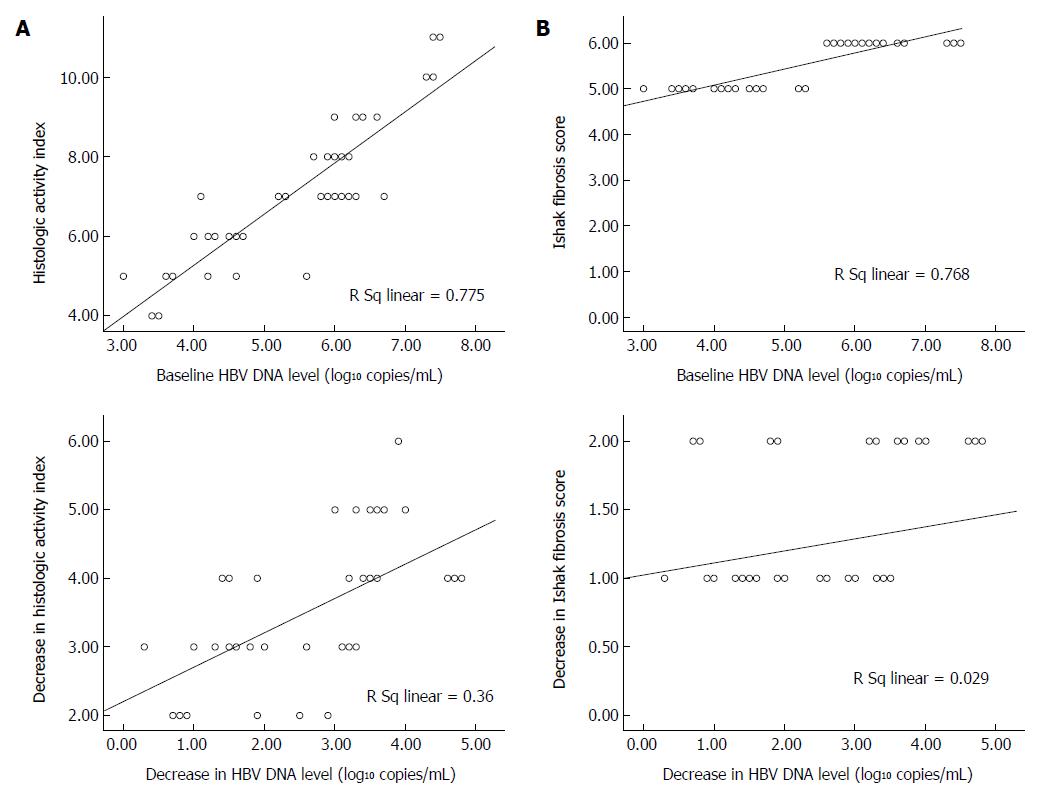
The relationships between HBV DNA level and the Knodell HAI and Ishak fibrosis scores at baseline and after entecavir treatment were analyzed by performing linear regression using data from the histology subgroup of patients (n = 38). As shown in Figure 2A, viral load at baseline was significantly correlated with Knodell HAI and Ishak fibrosis scores in the untreated patients (r = 0.880 and r = 0.876, respectively; P = 0.01). Similarly, decreases in HBV DNA level from the baseline were strongly correlated with decreases in Knodell HAI (r = 0.60; P = 0.01), but not Ishak fibrosis score (r = 0.17) at week 240 of entecavir treatment (Figure 2B).
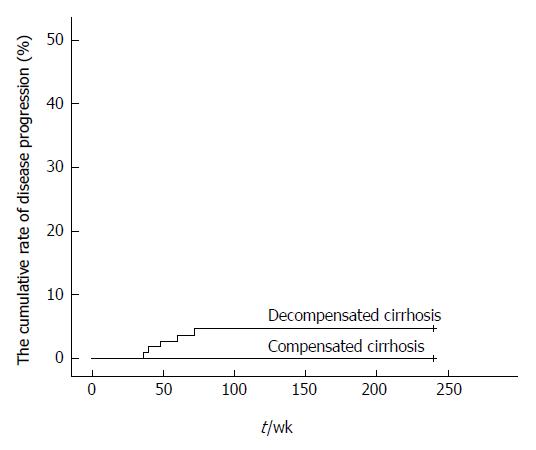
The proportion of patients with disease progression in the decompensated cirrhosis group was 4.6% within the 240 wk. Three patients were found to have HCC at weeks 40, 60, and 72, and two patients had bleeding gastroesophageal varices at weeks 36 and 48. None of the patients had worsened compensated cirrhosis (Figure 3).
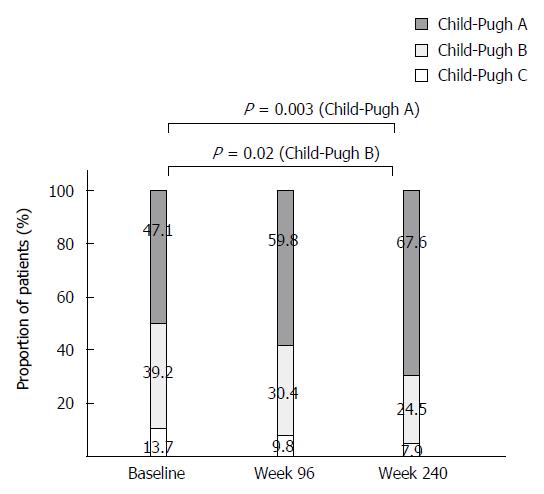
Liver disease severity (Child-Pugh class) at baseline, week 96, and week 240 in the total study population is shown in Figure 4. The proportion of patients with Child-Pugh class A disease significantly increased from the baseline at week 240 (47% vs 68%, P < 0.01). The proportion of patients with Child-Pugh class B disease significantly decreased from the baseline at week 240 (39% vs 25%, P = 0.02), with corresponding decreases occurring in the proportions of patients with Child-Pugh class C disease.
Three patients (1.5%) experienced a virologic breakthrough during 240 wk of entecavir treatment. They had the same mutations, and resistance mutation occurred in rtM204I/V, rtL180M, and rtT184, respectively.
None of the patients discontinued treatment with entecavir, experienced renal function impairment, or developed lactic acidosis throughout the 240-wk period of treatment with entecavir.
Data on the efficacy and safety of entecavir in patients with CHB-related cirrhosis are limited. This prospective study demonstrates that entecavir is an effective treatment option for Chinese nucleoside-naive patients with CHB and compensated or decompensated cirrhosis. Most of the patients achieved virologic suppression (HBV DNA level < 500 copies/mL) by week 240 of therapy. Furthermore, histologic improvement was observed in most (89.5%) of the patients with paired biopsies at baseline and week 240. The most important finding is that the entecavir treatment was associated with significant improvements in hepatic functional reserve in the patients with decompensated cirrhosis.
Sustained suppression of HBV replication is recommended as a primary aim of therapy for CHB[22-24]. In this study, 88% and 93% of patients with compensated and decompensated cirrhosis, respectively, achieved an HBV DNA level < 500 copies/mL by week 240. These results confirm previous findings that demonstrated the efficacy of entecavir in nucleoside-naive patients and extended these findings to patients with CHB and compensated or decompensated cirrhosis.
The long-term goal of treatment for CHB is to arrest or reverse liver disease progression[22,23]. After week 240 of entecavir therapy, all of the 38 patients showed improvement in liver histology and Ishak fibrosis score. The mean change in Knodell necroinflammatory and Ishak fibrosis scores from the baseline were -1.3 and -3.5, respectively. These findings are consistent with previous studies that demonstrated histologic improvement with nucleoside analog treatment in patients with bridging fibrosis/cirrhosis[12,18,20,25,26]. Clinical trial data from ten patients with advanced fibrosis/cirrhosis (Ishak fibrosis scores, 4-6) found that after approximately six years of cumulative entecavir therapy (range: 267-297 wk), all of the patients showed improvement in liver histology and Ishak fibrosis score; mean changes in Ishak fibrosis and Knodell necroinflammatory scores from the baseline were 2.2 and 7.6, respectively. A reduction in Ishak fibrosis score to 4 or lower was observed for all four patients who had cirrhosis at baseline[20].
In a previous study of one-year entecavir treatment in Korean patients with decompensated cirrhosis, genotypic resistance to entecavir was not evaluated[7]. In contrast, comprehensive resistance monitoring of all the patients in the present study found a virologic breakthrough in three patients. These results are consistent with the low cumulative probability of genotypic entecavir resistance (0.5% at two years to 1.2% over six years) observed in clinical trials with nucleoside-naive patients without cirrhosis[27,28]. Considering that current CHB guidelines recommend long-term treatment for patients with cirrhosis[22-24], the low rate of genotypic entecavir resistance in this study provides further evidence to support the use of entecavir in patients with CHB and either decompensated or compensated cirrhosis.
Recently, genome-wide association studies have shown that several single-nucleotide polymorphisms in the IL-28B gene (IL28B) on chromosome 19q13, which encodes type III interferon (IFN; also named IFN-λ3), are strongly associated with not only spontaneous and treatment-induced clearance of hepatitis V virus (HCV) infection, but also the course of HCV-related disease[29]. Moreover, our recent study also showed that IL28B polymorphism rs12979860 is associated with response to treatment in Chinese hepatitis C patients[30]. Considering that HBV and HCV are both hepatotropic viruses that can establish chronic infections that persist for the lifetime of the host and are sensitive to the antiviral activity of IFN-λ in cell culture models of virus replication, it might be possible that genetic variants of IL28B play a similar functional role during chronic HBV infection[31]. Several previous studies in different ethnic groups have suggested that IL28B genetic variation is associated with HBV-related disease and IFN-based treatment outcomes[32-35]. However, the present study shows that virologic response and IL-28 genotype are not significantly associated.
In conclusion, this study demonstrates that entecavir is safe and provides potent virologic suppression and improvement in overall liver disease severity in nucleoside-naive patients with HBV-related decompensated or compensated cirrhosis. Sustained virologic suppression, biochemical response, and improvements in liver histology were achieved by most of the patients throughout the 240 wk of treatment. These findings, together with a high genetic barrier to resistance, provide evidence that support the use of entecavir as a first-line treatment for patients with CHB and advanced liver disease.
Entecavir is a potent antiviral agent that has been shown to be effective and safe for the treatment of chronic hepatitis B (CHB). However, data on its clinical benefits in patients with cirrhosis, especially in long-term treatment, are limited.
Prospectively evaluate the antiviral efficacy and clinical outcomes of entecavir treatment for 240 wk in nucleoside-naive Chinese patients with CHB, and compensated or decompensated cirrhosis.
This study demonstrates that entecavir is an effective treatment option for Chinese nucleoside-naive patients with CHB, and compensated or decompensated cirrhosis, can result in sustained virological suppression and histological improvement.
The paper is informative and very interesting, which evaluate the efficacy of entecavir in the treatment of hepatitis B virus-cirrhosis. Although it does not offer any very novel insights, it does provide worthwhile “real world” data in this group of patients.
P- Reviewer: Douglas MW, Li Q, Picardi A S- Editor: Yu J L- Editor: AmEditor E- Editor: Liu XM
| 1. | Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359:1486-1500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 689] [Cited by in F6Publishing: 663] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 2. | Fattovich G. Natural history of hepatitis B. J Hepatol. 2003;39 Suppl 1:S50-S58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 3. | Fattovich G, Brollo L, Giustina G, Noventa F, Pontisso P, Alberti A, Realdi G, Ruol A. Natural history and prognostic factors for chronic hepatitis type B. Gut. 1991;32:294-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 300] [Cited by in F6Publishing: 281] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 4. | Liaw YF, Tai DI, Chu CM, Chen TJ. The development of cirrhosis in patients with chronic type B hepatitis: a prospective study. Hepatology. 1998;8:493-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 440] [Cited by in F6Publishing: 428] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 5. | Fattovich G, Giustina G, Schalm SW, Hadziyannis S, Sanchez-Tapias J, Almasio P, Christensen E, Krogsgaard K, Degos F, Carneiro de Moura M. Occurrence of hepatocellular carcinoma and decompensation in western European patients with cirrhosis type B. The EUROHEP Study Group on Hepatitis B Virus and Cirrhosis. Hepatology. 1995;21:77-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 70] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Realdi G, Fattovich G, Hadziyannis S, Schalm SW, Almasio P, Sanchez-Tapias J, Christensen E, Giustina G, Noventa F. Survival and prognostic factors in 366 patients with compensated cirrhosis type B: a multicenter study. The Investigators of the European Concerted Action on Viral Hepatitis (EUROHEP). J Hepatol. 1994;21:656-666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 213] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2309] [Cited by in F6Publishing: 2210] [Article Influence: 122.8] [Reference Citation Analysis (0)] |
| 8. | Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678-686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1164] [Cited by in F6Publishing: 1101] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 9. | Mommeja-Marin H, Mondou E, Blum MR, Rousseau F. Serum HBV DNA as a marker of efficacy during therapy for chronic HBV infection: analysis and review of the literature. Hepatology. 2003;37:1309-1319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 221] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 10. | Davies L, Knutson KC. Warning signals for malnutrition in the elderly. J Am Diet Assoc. 1991;91:1413-1417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 238] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 11. | Gigi E, Sykia A, Sinakos E, Koumerkeridis G, Raptopoulou-Gigi M, Bellou A, Mougiou D, Orfanou E, Vrettou E. Virologic response and resistance to lamivudine in patients with chronic hepatitis B: a ten-year retrospective analysis. Hippokratia. 2012;16:342-346. [PubMed] [Cited in This Article: ] |
| 12. | Chung GE, Lee JH, Kim YJ. Does antiviral therapy reduce complications of cirrhosis? World J Gastroenterol. 2014;20:7306-7311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 12] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Nishida T, Kobashi H, Fujioka S, Fujio K, Takaguchi K, Ikeda H, Kawaguchi M, Ando M, Araki Y, Higashi T. A prospective and comparative cohort study on efficacy and drug resistance during long-term lamivudine treatment for various stages of chronic hepatitis B and cirrhosis. J Gastroenterol Hepatol. 2008;23:794-803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, DeHertogh D, Wilber R, Zink RC, Cross A. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354:1011-1020. [PubMed] [Cited in This Article: ] |
| 15. | Yao G, Chen C, Lu W, Ren H, Tan D, Wang Y, Xu D, Jiang Z, Liu J, Xu D. Efficacy and safety of entecavir compared to lamivudine in nucleoside-naïve patients with chronic hepatitis B: a randomized double-blind trial in China. Hepatol Int. 2007;1:365-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Yao G, Chen C, Lu W, Ren H, Tan D, Wang Y, Xu D, Liu J, Xu D, Llamoso C. Virologic, serologic, and biochemical outcomes through 2 years of treatment with entecavir and lamivudine in nucleoside-naïve Chinese patients with chronic hepatitis B: a randomized, multicenter study. Hepatol Int. 2008;2:486-493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Wang J. Clinical utility of entecavir for chronic hepatitis B in Chinese patients. Drug Des Devel Ther. 2013;8:13-24. [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Schiff E, Simsek H, Lee WM, Chao YC, Sette H, Janssen HL, Han SH, Goodman Z, Yang J, Brett-Smith H. Efficacy and safety of entecavir in patients with chronic hepatitis B and advanced hepatic fibrosis or cirrhosis. Am J Gastroenterol. 2008;103:2776-2783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | Shim JH, Lee HC, Kim KM, Lim YS, Chung YH, Lee YS, Suh DJ. Efficacy of entecavir in treatment-naïve patients with hepatitis B virus-related decompensated cirrhosis. J Hepatol. 2010;52:176-182. [PubMed] [Cited in This Article: ] |
| 20. | Schiff ER, Lee SS, Chao YC, Kew Yoon S, Bessone F, Wu SS, Kryczka W, Lurie Y, Gadano A, Kitis G. Long-term treatment with entecavir induces reversal of advanced fibrosis or cirrhosis in patients with chronic hepatitis B. Clin Gastroenterol Hepatol. 2011;9:274-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 21. | Chinese Society for Infectious Diseases, Chinese Society for Hepatology, Chinese Medical Association. Guideline on prevention and treatment of chronic hepatitis B in China (2005). Zhonghuayixue Zazhi. 2007;120:2159-2173. [Cited in This Article: ] |
| 22. | European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1152] [Cited by in F6Publishing: 1122] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 23. | Liaw YF, Leung N, Kao JH, Piratvisuth T, Gane E, Han KH, Guan R, Lau GK, Locarnini S. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2:263-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 666] [Cited by in F6Publishing: 724] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 24. | Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2125] [Cited by in F6Publishing: 2114] [Article Influence: 140.9] [Reference Citation Analysis (0)] |
| 25. | Park H, Park JY, Kim SU, Kim do Y, Han KH, Chon CY, Ahn SH. Efficacy of switching to telbivudine plus adefovir in suboptimal responders to lamivudine plus adefovir. World J Gastroenterol. 2013;19:7671-7679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Kim JK, Ma DW, Lee KS, Paik YH. Assessment of hepatic fibrosis regression by transient elastography in patients with chronic hepatitis B treated with oral antiviral agents. J Korean Med Sci. 2014;29:570-575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Ridruejo E. Treatment of chronic hepatitis B in clinical practice with entecavir or tenofovir. World J Gastroenterol. 2014;20:7169-7180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 15] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Ke W, Liu L, Zhang C, Ye X, Gao Y, Zhou S, Yang Y. Comparison of efficacy and safety of tenofovir and entecavir in chronic hepatitis B virus infection: a systematic review and meta-analysis. PLoS One. 2014;9:e98865. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 29. | Donnelly RP, Dickensheets H, O’Brien TR. Interferon-lambda and therapy for chronic hepatitis C virus infection. Trends Immunol. 2011;32:443-450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Yu ML, Huang CF, Huang JF, Chang NC, Yang JF, Lin ZY, Chen SC, Hsieh MY, Wang LY, Chang WY. Role of interleukin-28B polymorphisms in the treatment of hepatitis C virus genotype 2 infection in Asian patients. Hepatology. 2011;53:7-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 31. | Pagliaccetti NE, Robek MD. Interferon-lambda in the immune response to hepatitis B virus and hepatitis C virus. J Interferon Cytokine Res. 2010;30:585-590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Li W, Jiang Y, Jin Q, Shi X, Jin J, Gao Y, Pan Y, Zhang H, Jiang J, Niu J. Expression and gene polymorphisms of interleukin 28B and hepatitis B virus infection in a Chinese Han population. Liver Int. 2011;31:1118-1126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Fabris C, Falleti E, Cussigh A, Bitetto D, Fontanini E, Bignulin S, Cmet S, Fornasiere E, Fumolo E, Fangazio S. IL-28B rs12979860 C/T allele distribution in patients with liver cirrhosis: role in the course of chronic viral hepatitis and the development of HCC. J Hepatol. 2011;54:716-722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 34. | Martin MP, Qi Y, Goedert JJ, Hussain SK, Kirk GD, Hoots WK, Buchbinder S, Carrington M, Thio CL. IL28B polymorphism does not determine outcomes of hepatitis B virus or HIV infection. J Infect Dis. 2010;202:1749-1753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 35. | Sonneveld MJ, Wong VW, Woltman AM, Wong GL, Cakaloglu Y, Zeuzem S, Buster EH, Uitterlinden AG, Hansen BE, Chan HL. Polymorphisms near IL28B and serologic response to peginterferon in HBeAg-positive patients with chronic hepatitis B. Gastroenterology. 2012;142:513-520.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |