Published online Jul 7, 2015. doi: 10.3748/wjg.v21.i25.7683
Peer-review started: February 6, 2015
First decision: March 26, 2015
Revised: April 7, 2015
Accepted: June 10, 2015
Article in press: June 10, 2015
Published online: July 7, 2015
Primary biliary cirrhosis (PBC) is a chronic progressive cholestatic granulomatous, and destructive inflammatory lesion of small intralobular and septal bile ducts, which is likely to be caused by an autoimmune mechanism with a the presence of serum antimitochondrial antibodies and a potential tendency to progress to cirrhosis. Despite the fact that the etiology of this disease has been unknown so far, there has been a considerable body of scientific evidence that can reveal the clinical and laboratory signs of PBC and the individual components of its pathogenesis and elaborate diagnostic criteria for the disease and its symptomatic therapy. Deficiencies in autoimmune tolerance are critical factors for the initiation and perpetuation of the disease. The purpose of this review is to summarize the data available in the literature and the author’s findings on clinical and laboratory criteria for the diagnosis of PBC. This review describes the major clinical manifestations of the disease and the mechanisms of its development. It presents the immunological, biochemical, and morphological signs of PBC and their significance for its diagnosis. A great deal of novel scientific evidence for the problem of PBC has been accumulated. However, the inadequate efficiency of therapy for the disease lends impetus to the quest for its etiological factors and to further investigations of its pathogenetic mechanisms and, on this basis, to searches for new methods for its early diagnosis.
Core tip: Primary biliary cirrhosis is a chronic autoimmune cholestatic liver disease. This review summarizes current literature data and our own experiences on clinical and laboratory criteria for the diagnosis of primary biliary cirrhosis. Thanks to advances in biochemistry, molecular biology and genetics, it became possible to present these data with regard to the pathophysiological mechanisms of their development.
- Citation: Reshetnyak VI. Primary biliary cirrhosis: Clinical and laboratory criteria for its diagnosis. World J Gastroenterol 2015; 21(25): 7683-7708
- URL: https://www.wjgnet.com/1007-9327/full/v21/i25/7683.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i25.7683
Primary biliary cirrhosis (PBC) is a chronic autoimmune cholestatic liver disease characterized by a striking female predominance, high-titer serum antimitochondrial autoantibodies (AMAs), disease-specific antinuclear autoantibodies (ANAs), and an autoimmune-mediated progressive granulomatous destruction of small and medium-sized intralobular and septal intrahepatic bile ducts, leading to cirrhosis and ultimately liver transplantation or death[1-5]. Deficiencies in autoimmune tolerance are critical factors for the initiation and perpetuation of the disease[6]. Immunologically, PBC is distinguished by immune-mediated destruction of the intrahepatic bile ducts and the presence of high-titer antimitochondrial autoantibodies[3] directed against a highly specific epitope within the lipoic acid binding domain of the pyruvate dehydrogenase E2 subunit (PDC-E2)[7]. The natural history of the disease is 10 to 20 years[6].
According to the presence or absence of cirrhosis, the mean survival was 9.17 years (95%CI: 6.79-11.56) and 10.7 years (95%CI: 9.27-12.14), respectively (P = 0.03)[8]. Mortality from PBC is 2.2% of all deaths due to liver cirrhosis[9].
In 1851, Addison et al[10] were the first to observe an association of skin changes with liver disease in women. Elevated serum cholesterol levels in these patients and the presence of cutaneous xanthelasmas served as a basis for employing the term “xanthomatous biliary cirrhosis” to denote this disease[11,12]. Almost 100 years ago, its clinical picture was described in detail and the term “primary biliary cirrhosis” was offered[11,12]. In 1965, a group of morphologists under the supervision of H. Popper proposed the term “chronic non-purulent destructive cholangitis”[13].
PBC is encountered in all parts of the world among people of all races and nationalities[14,15]. No differences were observed in the geographical distributions of the disease[15]. According to different authors, the prevalence of PBC is 4-14 cases per 100000 population[15-17]. PBC is mostly found in patients in Northern Europe, the United Kingdom, and the northern United States. The prevalence of familial PBC was later reported to be 6.4% in United Kingdom[18], and between 3.8% and 9.0% in a number of studies from North America, Europe, and Japan[5]. In Asia, Japan is the only country with a known prevalence of PBC, at 27-54 per million[19,20]. The annual incidence rates range between 0.7 and 49 cases per million persons, while the global prevalence rates range between 6.7 and 402 cases per million persons[3,5,6,20-24]. A study conducted by Gershwin et al[25] indicated that having a first-degree relative with PBC was significantly associated with increased risk of PBC, with an odds ratio of 10.7. This is supported by the high concordance rate of PBC among first-degree relatives and homozygous twins (approximately 60%)[26,27]. A pairwise concordance rate of 0.63 for PBC in monozygotic twin pairs has been published, which is one of the highest reported in autoimmunity[28]. Specifically, the high concordance for PBC in monozygotic twins, family clustering, and female predominance suggest that genetic factors may play an important role in the development of PBC[29-31].
PBC is a typical female disease that occurs from 40-60 years of age[32,33]. Much less is logged PBC in persons under the age of 25 years[34,35]. There is a high female:male incidence ratio (8:1), with suggestions of a significant role for X chromosome defects in PBC, based on the observation that women with PBC have a significantly enhanced monosomy X frequency in peripheral white blood cells compared to age-matched healthy women[36] and that the X chromosome loss is preferential[37]. Smoking behavior, age at menarche, age at first pregnancy, gravidity, and number of children were not significantly different between PBC cases and controls[15].
The etiology of PBC is still unknown[1,38]. Currently, it is believed that PBC is likely to be triggered by a combination of environmental factors, including infection in a genetically susceptible individual[2,7]. Many authors regard the disease as impaired immunoregulation with a loss of tolerance of histocompatibility antigen-enriched tissues. PBC is characterized by T-cell-mediated destruction of small bile duct epithelial cells[39]. This leads to ductulopenia and persistent cholestasis, by developing end-stage hepatic-cell failure. Recent data point towards apoptosis as a leading mechanism for ductopenia[40]. The loss of bile ducts leads to decreased bile secretion and the retention of toxic substances within the liver. This results in further hepatic damage, fibrosis, cirrhosis, and eventually liver failure[35]. A vital question in the pathogenesis of PBC is why biliary epithelial cells (BECs) in particular are the primary target of disease despite the ubiquitous presence of the pyruvate dehydrogenase complex (PDC) autoantigen in all tissue cells[39]. How and why the bile ducts are involved in this process remains unknown. Viruses[41,42], bacteria, xenobiotics[43], and human immunoregulatory defect may be possible PBC triggers that initiate the immunopathological cascade. Infection, either viral or bacterial, can either directly induce BEC apoptosis or more probably trigger an immune attack on epithelial cells as a result of molecular mimicry[28]. Initiating mimotopes of the vulnerable epitope of the E2 subunit of the pyruvate dehydrogenase comple (PDC-E2) autoantigen can be derived from microbes that utilize the PDC enzyme or, alternatively, environmental xenobiotics/chemical compounds that modify the structure of native proteins to make them immunogenic. A further alternative as a source of antigen is PDC-E2 derived from apoptotic cells. In the effector phase the biliary ductular cell, by reason of its proclivity to express the antigen PDC-E2 in the course of apoptosis, undergoes a multilineage immune attack comprised of CD4+ and CD8+ T cells and antibody[39]. The liver can encounter a number of pathogenic microorganisms and their by-products from the gut by acting as a traffic hub. Kouroumalis et al[28] propose a pathogenetic model for PBC, which plays an important role in primary dysfunction of endothelial cells overproducing endothelin-2.
The pathogenesis of PBC involves environmental factors, genetic predisposition and loss of immune tolerance[6]. In recent years, it has become univocally accepted that an inappropriately activated immune response is one of the most important factors in PBC. A number of models for the disease have been proposed to systematize the ideas on its development mechanisms[1,28,39]. However, the existing concepts of PBC cannot fully explain the specificity of biliary epithelial injury, the expression of mitochondrial PDC-E2 of bile duct epithelial cells as autoantigens, and the higher prevalence of this disease in women than men (9:1).
A few classifications of PBC have been proposed according to its clinical course (Table 1).
The development of PBC is preceded by a long asymptomatic period. The wide use of computer-aided screening biochemical and immunological studies has significantly increased the detection of asymptomatic patients. In this period, there are generally no physical signs of PBC, at the same time anti-mitochondrial autoantibodies are detectable in the serum of virtually all patients (95%)[1]. The fact that AMAs are detectable many years before PBC manifests itself is indicative of their primary immunopathological role rather than a secondary phenomenon that occurs in the presence of cholestasis. The production of AMAs is not an epiphenomenon, and an understanding of the mechanism of AMAs induction will shed light on the etiology of PBC. The clinical manifestations of PBC may mask those of other diseases. The blood of PBC patients shows a moderate increase in the activity of gamma-glutamyltransferase (γ-GT), alkaline phosphatase (ALP), 5’-nucleotidase (5’-NT), and leucine aminopeptidase (LAP). The level of serum cholesterol and aminotransferases in this period are within normal limits. Morphological examination of liver biopsy specimens allows a diagnosis of one of the stages of PBC. The signs of hepatocellular insufficiency or those of portal hypertension and their complications (esophageal varices, ascites, hepatic encephalopathy and others) occur only in the advanced stages of the disease[46].
Il’ichenko et al[47] identify the following course options PBC: (1) Asymptomatic PBC (10.9%); (2) AMA-positive (classical) PBC (85.4%). It is characterized by the typical clinical manifestations of the disease, such as skin itching and jaundice, by the biochemical signs of cholestasis and serum AMAs at diagnostic titers of higher than 1:40; (3) АМА-negative PBC (14.6%). It is characterized by decreased biochemical and immunological activities and the lower frequency of extrahepatic manifestations, which has no impact on the prognosis of liver cirrhosis and the time of its progression; and (4) PBC-autoimmune hepatitis (AIH) overlap-syndrome (PBC/AIH) (9.4%); Patients are observed to have simultaneously signs of PBC and AIH (those of cholestasis and cytolysis and the presence of autoantibodies (antimitochondrial, antinuclear, anti-smooth muscle autoantibodies); their liver biopsy specimens exhibit the morphological signs of non-purulent destructive cholangitis, as well as bridging necroses and plasma cells.
Weakness, fatigue, daytime somnolence; Pruritus; Weight loss; Xanthelasma; Skin hyperpigmentation; Jaundice; Hepatomegaly and less - splenomegaly; Malabsorption syndrome; Osteodystrophy, osteoporosis; Cholelithiasis; Extrahepatic manifestations of autoimmune nature.
Primary biliary cirrhosis is latent and oligosymptomatic disease for many years[48,49]. The disease begins quietly and is long manifested only by weakness, malaise, fatigue, daytime somnolence and/or low working efficiency.
Fatigue and pruritus are the most common symptoms of PBC, but the majority of patients are asymptomatic at first presentation[50]. Fatigue is considered to be a specific manifestation of PBC[51,52]. The pathogenesis of fatigue in PBC is unclear, but preliminary studies suggest it has central mechanisms and may have peripheral manifestations[53]. Comorbidities and depression might have played a role in its pathogenesis[52]. The asthenic sign of PBC is more pronounced than that of other chronic liver diseases.
The leading and early pathognomonic symptom of PBC is the appearance of skin itching that may be the only manifestation of the disease within a few months and even few years. The skin shows multiple scratched traces that further display hyperpigmentation portions. The itching is characterized by extension (local or systemic), degrees (moderate or severe), and duration (persistent or transient). Itching may be excruciating, may seriously impair quality of life and even induce suicidal ideation in the most severe cases[54]. Itching is more marked at night and frequently enhanced in contact with fabrics and also in warmth.
Itching is not relieved with symptomatic (antihistamine, sedative) agents; it often causes excruciating insomnia, emotional changes, and depression. Poor sleep at night leads to daytime somnolence, chronic fatigue and reduced ability to work. Durazzo et al[33] indicate that female sex hormones may cause constitutional symptoms (malaise, anorexia, weight loss, and fatigue). The patients frequently seek the advice of a dermatologist, an allergist, or a neurologist.
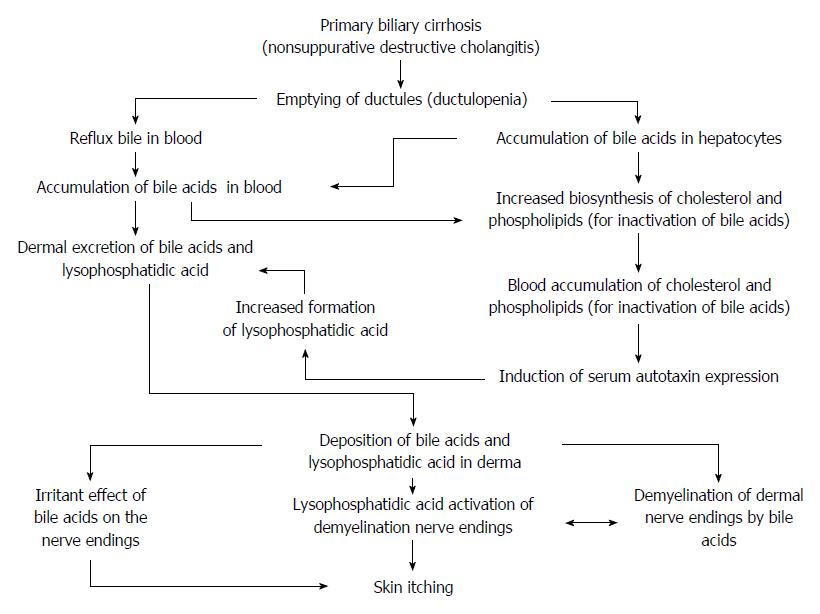
The molecular mechanism of itch signal transduction in cholestasis is largely unclear. It may be caused or potentiated by compounds that accumulate in the circulating blood during cholestasis[54]. Increased concentrations of bile salts[1], histamine, female steroid hormones and their metabolites[33], endogenous opioids, and lysophosphatidic acid (LPA)[55-57] have been controversially discussed as potential pruritogens in cholestasis[58-60]. Fatigue, pruritus, and Sjögren’s syndrome are more common in women than men, but other signs and symptoms do not differ in the two sexes[61]. Females experience pruritus as a single symptom more often than males. It has been suggested that female sex hormones may be linked with pruritus[33].
It has been suggested that opiates and their receptors are involved in the development of itchy skin in cholestatic liver diseases. Excessive amount of endogenous lipophilic bile acids are likely to promote the release of so far unknown substances that stimulate opioid receptors. There are data on the use of opiate agonists to alleviate itching[62].
It has been assumed that retention of bile salts, with their deposition in the skin, is related in some way to the development of the pruritus. This assumption is confirmed by two facts: (1) Purified bile acids have produced itch when injected into the skin[63]; and (2) The bile salt binding agent cholestyramine effectively relieves the itch of cholestasis, and concomitantly lowers serum bile salt concentrations. The poor correlation between serum bile salt concentration and pruritus may thus be due to variation in bile salt composition[63]. The various bile salts might differ in their ability to provoke pruritus. Unconjugated bile acids were found to be more pruritogenic than conjugates, and the dihydroxy bile salts (particularly unconjugated chenodeoxycholate) are more effective pruritogens than the trihydroxy (cholic acid) salts[63]. 50%-85% of unconjugated bile acids and less than 20% of sulfate esters are detected in the skin of PBC patients[64]. Large differences in the retention of either sulfated or nonsulfated fractions could correlate with pruritus. The intensity of itching may depend on the ratio of sulfated (conjugated, glucuronized) and nonsulfated (unconjugated, nonglucuronized) bile cells accumulating in the skin of PBC patients.
Most recently, novel itch-specific neuronal pathways, itch mediators and their relevant receptors have been identified[55]. Screening plasma samples of a large group of patients with various cholestatic conditions revealed LPA as the active itchy compound[57]. LPA is a very potent signaling lipid that can activate cells through various LPA receptors. Subsequently, authors could demonstrate that cholestatic patients with pruritus have highly elevated levels of serum autotaxin (ATX), the enzyme that converts lysophosphatidylcholine into LPA[54,57].
Kremer et al[59] highlight that increased serum ATX levels are specific for pruritus of cholestasis, but not pruritus of uremia, Hodgkin’s disease, or atopic dermatitis. Oude Elferink et al[57] hypothesize that during cholestasis, expression of ATX is induced and gives rise to increased local formation of LPA near demyelinated nerve endings of itch fibers. LPA then activates these neurons through one of the LPA receptors, which in turn potentiates action potentials along itch fibers[54,57].
Serum autotaxin activity correlates with pruritus intensity, but its causal relationship, expression pattern and exact mode of action during cholestasis remain to be established.
Analysis of available scientific data may suggest the following hypothetical scheme of the mechanism responsible for developing skin itching in patients with PBC (Figure 1).
In a small number (8.6%) of patients, itching may be absent in the whole period of the disease practically until terminal complications develop[47].
Varying skin changes are observed. Skin elasticity loss and dryness, scratching traces, hyperpigmentation, xanthomas, and xanthelasmas engage attention when examining patients with PBC.
According to Sherlock et al[65], skin hyperpigmentation is due to excessive melanin biosynthesis in the melanocytes. Its initial reaction is catalyzed by the copper-containing enzyme tyrosinase. According to one of the hypotheses, elevated serum copper levels in patients with PBC may lead to the enhanced activity of a tyrosinase reaction and the increased biosynthesis of melanins whose skin deposition does induce hyperpigmentation[66]. Copper accumulation imparts a bronzish color to the skin.
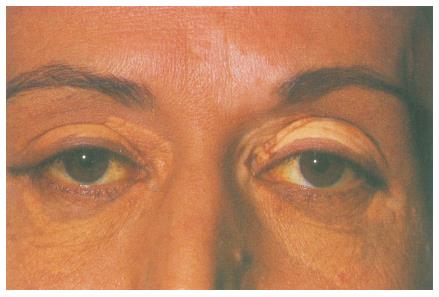
Xanthelasmas vary in shape, may be solitary or multiple, flat, pale yellow color slightly raised above the skin. In PBC, there are xanthelasmas on the upper or lower eyelids (Figure 2), as well as in the palmar creases, under the breasts, in the areas of the joints, tendons, buttocks, etc[67-69].
According to the data obtained by Ahrens et al[70], xanthelasmas is formed in elevated blood concentration of cholesterol (more than 400 mg/dL) persisting for at least three months. Xanthelasmas may disappear after normalization of cholesterol levels and in end-stage disease due to the progression of hepatocellular damage[65].
Jaundice is an important clinical symptom of PBC, but may be absent long (for 2 years or more). Jaundice develops in the end-stage disease[61]. In its eаrly stages, jaundice is generally undulating. Later on, occur its steady progression with the simultaneously increased blood levels of conjugated bilirubin. Patients with PBC show individual variations in bilirubin levels. Jaundice and skin itching concurrently occur in about one fourth of patients[32]. When jaundice is an initial manifestation of PBC, the patients exhibit a more rapid development of its end-stage, lower survival rates, and an earlier outcome than do those with its anicteric type[47,71].
In the early stages of PBC, a moderate enlargement of the liver is detectable in 70%-80% of cases. The liver slowly increases in size during the whole period of the disease. The enlarged liver is associated with its compensatory regeneration in response to the decreased hepatocytes’ functional ability caused by the excess accumulation and toxic action of bile acids. Hepatomegaly suggests a lesion of the liver on the one hand and the preserved regenerative ability of the organ on the other. In most cases, the liver is of moderate consistency, its surface is smooth and, in end-stage PBC, finely tuberous, painless on palpation.
The enlarged spleen is untypical of PBC, observed in its end stages in 20% of cases, and indicative of portal hypertension.
In PBC, reduced secretion of bile acids gives rise to steatorrhea (fecal fat excretion of more than 7 g/d). Intestinal bile salts deficiency impairs fat absorption in PBC patients[72] and result in bacterial overgrowth. DiBaise et al[73] suggest that bacterial overgrowth plays a significant role in the development of steatorrhea in patients with PBC and that an assessment for bacterial overgrowth should be performed on persons with steatorrhea in PBC. The severity of steatorrhea is associated with reduced bile acid outputs and concentrations (r = 0.82; P < 0.0001), degree of cholestasis (serum bilirubin; r = 0.88; P < 0.001) and advanced histologic stages (P < 0.005)[74]. All patients with a total serum bilirubin level of more than 4.5 mg/dL had severe steatorrhea (fecal fat excretion was above 25 g/d).
The results obtained Ros et al[72] indicate that overt pancreatic failure is uncommon in PBC and that fat maldigestion and steatorrhea, regardless of what degree, are due mainly to low intestinal bile salt levels secondary to bile secretory failure. The stool in the patients with PBC usually shows admixtures of incompletely digested fats. Despite steatorrhea, the patients with PBC have constipation. The latter seems to be related to the inadequate effect of a small amount of intestinal bile acids on the small and large intestinal motility.
The activity of pancreatic ALP in the serum of patients with PBC does not correlate with the severity of steatorrhea and that of amylase is within the normal range.
Skin itching accompanied by sleep disorder, as well as malabsorption of fats and fat-soluble vitamins lead to a slowly progressive weight loss in patients with PBC.
The clinical picture may be determined by complications and comorbidities.
Metabolic bone disease has been recognized as an important complication of chronic liver disease particularly in PBC and after liver transplantation[75]. It includes osteoporosis and more rarely osteomalacia, which is more frequent in severe malabsorption and advanced liver disease.
The molecular mechanisms of osteoporosis in patients with PBC are associated with the impaired enterohepatic circulation of bile acids.
Decreased small intestinal bile acid concentrations in PBC can lead to impaired absorption of fats and fat-soluble vitamins, resulting in deficiencies in vitamins A, D, E, and K[76].
Malabsorption of calcium ions and fat-soluble vitamin D in the small intestine gives rise to osteodystrophy[77,78]. The latter may manifest as bone pain in early stages of PBC; in its severe form, osteoporosis develops. Osteoporosis is a systemic skeletal disease characterized by low bone mass and bone tissue microarchitectural deterioration, resulting in increased bone fragility and fracture risk[79-81].
Osteopenia is a recognized complication of cholestatic liver diseases, usually ascribed to metabolic bone diseases such as osteomalacia or osteoporosis, with a prevalence of 10% to 56%, depending on the nature of liver disease[82]. PBC is the condition causing osteopenia more frequently than other cholestatic liver disease[78]. Regardless of the etiology of osteoporosis in PBC patients, they have an increased risk of spontaneous or low-trauma fracturing leading to significant patient morbidity, deterioration of quality of life, and even patient mortality[75]. Osteopenia predisposes to atraumatic fractures, particularly in PBC patients undergoing orthotopic liver transplantation and treated with high corticosteroid doses. There are pathological fractures of vertebrae and ribs commonly and those of the pelvis and long bones more rаrely.
Kehayoglou et al[83] have reported that the mean absorption of calcium ions is significantly less in patients with PBC than in controls. Impaired calcium absorption correlated well with increased fecal fat excretion and less well with the intensity of jaundice. The degree of osteoporosis depends on physical activity, nutritional, hormonal, and genetic factors.
The pathogenesis of bone disease in both adults and children with chronic cholestasis is not completely understood. Various potential inciting factors that either directly or indirectly alter bone mass are insulin-like growth factor 1 (IGF-I) deficiency, hyperbilirubinemia, hypogonadism, alcohol, subnormal 25-hydroxyvitamin D3 levels, vitamin D receptor genotypes, vitamin K, osteoprotegerin and receptor activator of nuclear factor Kb ligand interactions and concurrent use of drugs like cholestyramine, furosemide, glucocorticoids and immunosuppressive agents[78].
Hypogonadism is an established risk factor for osteoporosis in chronic liver disease.
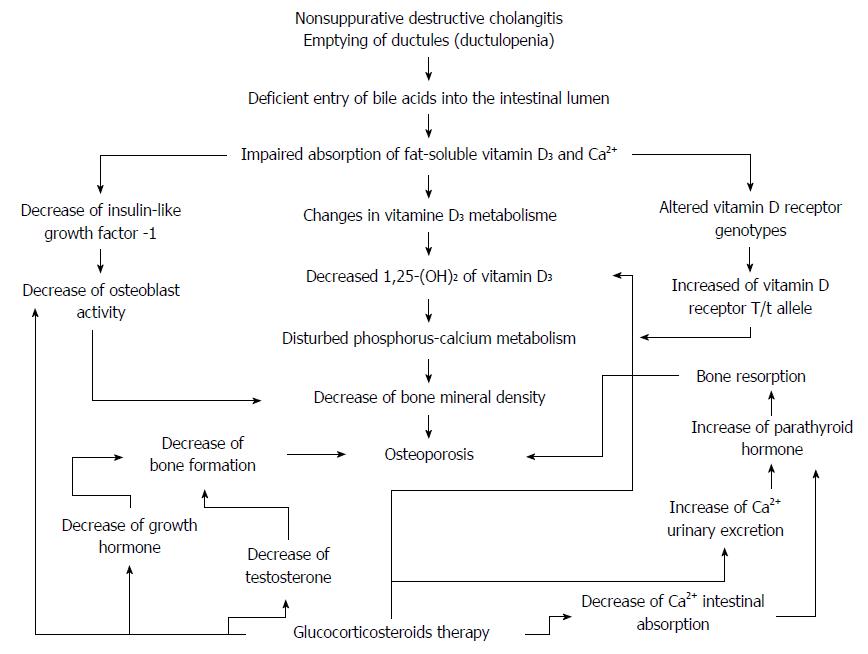
The pathogenesis of osteoporosis in patients with PBC is complex and is likely to be multifactorial[75,84] and involves impairments of vitamin D3 absorption and metabolism[85], decreased intestinal calcium ion absorption[86,87], genetic predisposition[88], and impact of corticosteroid therapy[89] (Figure 3).
Deficient entry of bile acids into the intestinal lumen substantially diminishes the absorption of fats and fat-soluble vitamins (А, D, and K)[90]. Vitamin D3 deficiency and metabolic bone disease are common complications of PBC. In patients with severe cholestasis, malabsorption of dietary vitamin D is an important contributing factor to vitamin D3 deficiency[91]. Along with insufficient vitamin D absorption, there is a lower formation of 1,25-dihydroxyvitamin D3 on cytochrome Р450 (competitive inhibition of monooxygenases due to the enhanced biosynthesis of bile acids)[1]. This all brings about inadequate calcium ion absorption in the small bowel and impaired phosphorus-calcium metabolism.
Lower serum IGF-I levels were seen in patients with chronic liver diseases[92]. IGF-I is synthesized by the liver. It is a bone collagen and osteoblast stimulator. IGF-I together with other genetic and environmental factors may be involved in the complex regulation of bone mineral density (BMD) in PBC.
Genetic factors have been implicated in the pathogenesis of osteoporosis, which is a common disorder in PBC. IGF-I gene microsatellite repeat polymorphism was found to be associated with osteoporosis and lower BMD in PBC[88].
Reduced tissue sensitivity to circulating vitamin D due to altered vitamin D receptor genotypes may play a role in the development of hepatic osteodystrophy. Vitamin D receptor allelic polymorphisms, designated B/b, A/a, and T/t alleles, correlate with BMD. The risk of developing a vertebral fracture increased 2- to 3-fold with the presence of a T/t allele[93].
Prolonged steroid therapy of PBC patients may results in clinically significant bone loss with an increase in fracture risk by greater than 2-fold[94]. Glucocorticosteroids (GCSs) diminish intestinal calcium absorption, by reducing the production of 1,25(OH)2 vitamin D3, increasing urinary calcium excretion, and depressing canalicular reabsorption. As a result, there is a compensatory increase in parathyroid hormone production and bone resorption. In addition, GCSs directly increase parathyroid hormone release and enhance its sensitivity. Furthermore, GCSs inhibit bone formation indirectly, by suppressing the synthesis of testosterone in gonads and by decreasing the production of growth hormone, IGF, and accordingly type 1 collagen, as well as directly by suppressing the function of osteoblasts[95,96]. Steroids exert a direct effect on bone cells by increasing osteoclastic activity by increasing IL1 and IL6 and decreasing differentiation, recruitment and life span of osteoblasts[78].
García-Suárez et al[97] suggest that serum leptin is associated with BMD. Szalay et al[98] found a lower serum leptin level and a higher soluble leptin receptor in patients with PBC, which could not be explained by the difference in body mass index. As leptin is associated with BMD, it may be hypothesized that leptin is involved in the complex regulation of bone metabolism in PBC. There is a clear increase in serum leptin levels according to its histological stage[97].
The best course of management for PBC patients is to review the individual risk factors for osteoporosis, to obtain a bone mass measurement, and to prescribe age- and disease-specific therapies[75,78]. The development of bone densitometry has allowed assessment of bone mass and then contributed in estimating the fracture risk[78]. The risk of fracture shows a correlation with BMD but no fracture threshold is determined[75].
BMD measurement is the best way to assess the presence and severity of osteopenia in PBC patients, while laboratory tests give important information about the metabolic status of the bone. Newer diagnostic modalities have improved the detection of hepatic osteodystrophy and vitamin D repletion, calcium supplementation and bisphosphonates seem promising[78].
There are different immune extrahepatic diseases (Table 2) in PBC, which make its diagnosis difficult. PBC is associated with a large variety of other diseases, like arthropathy, CREST syndrome, autoimmune thyroiditis, and so on, which in addition will or will not produce symptoms[61].
According to Gu et al[101], the most common comorbidities were Sjögren’s syndrome (9.14%), rheumatoid arthritis (3.95%) and type 2 diabetes (2.54%). Concomitant autoimmune diseases, such as Sicca syndrome, scleroderma and Raynaud’s phenomenon, have been shown to be less prevalent in men. These findings suggest that females are more likely to suffer concomitant autoimmune disease than males[33].
The coexistence of PBC and AIH in PBC patients has been described as overlap syndrome[102-109]. The prevalence of typical PBC possessing features of AIH has been reported to range from 5% to 19%[109-111]. PBC/AIH overlap-syndrome has the histological features of AIH and PBC, with AMAs, ANAs, or smooth muscle antibodies (SMAs)[61,112]. Recent studies have shown that PBC patients with features of PBC-AIH overlap may have a rapid progression toward cirrhosis and liver failure and greater risk of varices, ascites, portal hypertension, transplantation[113,114]. Steroids or immunosuppressive therapies may be effective in these patients[17,104,107-109,115-117]. The combination therapy with UDCA and corticosteroids was more effective for PBC-AIH[118].
Hepatocellular carcinoma (HCC) in PBC is rare[119,120]. However, PBC in the advanced stage, corresponding to PBC stage IV, was shown in the past to be associated with an increased incidence of HCC[121]. Hepatobiliary malignancies had a relative risk of 46 (P < 0.0001) for women and 55 (P < 0.0001) for men[120]. Whereas it is a relatively rare complication of cirrhotic PBC in women, HCC is a relatively common cause of death in male PBC patients with cirrhosis[33,61,119,122]. As far as the development of HCC is concerned, instead, PBC patients should undergo the usual surveillance reserved to other categories of cirrhotic patients, according to published guidelines for the management of HCC[121]. Such surveillance should start only when PBC patients have reached stage IV disease.
The risk of extrahepatic malignancies is higher than that of hepatocellular carcinoma, but it is not influenced by the histologic stage of the liver disease[123-125]. For stomach and pancreas cancers, the results of one study that only examined male patients with PBC indicated that PBC patients had increased risk of stomach cancer and pancreatic cancer, whereas the results of other studies of mixed-sex patients showed no significant association[124-126]. Therefore, despite inconsistent results, the meta-analysis could not be conducted for assessing the association. PBC was not significantly associated with increased risk of other cancers[126].
PBC patients might benefit from more aggressive surveillance for hepatobiliary malignancies during their lifetime[120]. The risk of HCC development may be an additional reason to consider earlier transplantation in these patients.
In recent years, it has become univocally accepted that an inappropriately activated immune response is one of the most important factors in PBC[6]. Substantial amounts of data to date have illustrated that autoimmunity plays a critical role in the pathogenesis of PBC[6]. There is concrete evidence that apoptosis is possibly the most important mechanism of biliary epithelial cells loss[28]. Enhanced BEC apoptosis is a critical step in ductular destruction in PBC[127,128]. BECs apoptosis in PBC is assumed to cause tissue-specific autoimmune reactions, as evidenced by the detection of АМАs[2,3]. High titers of antibodies against mitochondrial elements are characteristic of the disease[28].
Walker et al[129] were the first to find AMAs in the sera of patients with PBC. АМАs are detectable in different diseases; but anti-M2 AMAs are predominantly PBC-specific[130]. The highly PBC-specific autoantibody, AMA-M2, recognizes components of the oxo-acid dehydrogenase complex, which are ubiquitously expressed on the inner mitochondrial membrane, including the E2 subset of the pyruvate dehydrogenase complex (PDC-E2)[6]. The serological hallmark of PBC is the presence of high-titer (1:40 and more) AMAs directed against the E2 subunit of 2-oxo-acid dehydrogenase enzymes, chiefly PDC-E2 enzyme complexes located on the inner mitochondrial membrane[39,131,132]. These reflect the presence of autoreactive T and B cells to the culprit antigens.
The presence of serum AMAs and autoreactive B cells strongly endorses the concept of an autoimmune pathogenesis of PBC[133-135]. A. Lleo and colleagues[40,136] demonstrate that PDC-E2 with antigenic reactivity is only detectable in apoptotic blebs of human intra-hepatic BECs. Interestingly, in vitro caspase cleavage of PDC-E2 has been shown to generate immunologically active protein fragments[137]. The antigens are released from apoptotic blebs of the BECs, or come from molecular mimicry of infectious agents, or from alteration by xenobiotics[138].
AMAs can be detected even before clinical and morphological symptoms or biochemical abnormalities. Although most patients with PBC have AMAs against PDC-E2, there is no direct correlation between the titer of AMAs and disease severity, as well as histological progression of BECs damage[39,61]. AMAs titer measurement is, first of all, of diagnostic value.
The activity of antibodies to antigens of varying specificities (exogenous and autologous antigens) is associated with different classes of immunoglobulins - A, D, E, G, and M. In addition to high titers of circulating AMAs, PBC patients have high levels of serum IgM that are not related to titers of AMAs. IgM levels in PBC patients average 6.27 ± 0.66 g/L (normal human plasma IgM concentrations are 1-2 g/L)[139]. The mechanism of IgM elevation is still unclear in PBC, but abnormal Ig class switching may be involved[6]. The appearance of IgM antibodies is the earliest immune response to antigen.
Specific IgA-type AMAs that have specificity for PDC-E2 can be detected in almost all body fluids of patients with PBC, including saliva, urine and bile[140,141]. The mechanism responsible is likely that a greater concentration of IgA in the bile ducts can make cells more susceptible to apoptosis through constant transcytosis, resulting in subsequent bile duct damage[142]. The presence of IgA-anti-PDC-E2 in sera or saliva might be associated with the progression of PBC[143].
In addition to AMAs, PBC sera can exhibit other disease-specific autoantibodies, particularly ANAs and SMAs[144].
Disease-specific ANAs are present in about one third of patients with PBC[144]. ANAs belong mainly to the IgG class. PBC-specific ANAs reactants include nuclear pore glycoproteins of the inner nuclear membrane, Gp210[145,146] and p62[146,147]. This subtype of PBC-specific ANAs has been shown to correlate with disease severity and progression[144,148,149]. High blood ANA concentrations in patients with PBC have the least favorable prognosis. Rigopoulou et al[150] have found that AMA-IgG3 is associated with a more severe disease.
The study conducted by Granito et al[151] has identified Sp140 protein as a new, highly specific autoantigen in early-stage PBC. Anti-Sp140 antibodies were present in 15% of PBC patients with a higher frequency in AMA-negative cases (53% vs 9%, P < 0.0001). Anti-Sp140 positivity was not associated with a specific clinical feature of PBC. Anti-Sp140 antibodies were found together with anti-Sp100 antibodies in 90% of cases and with anti-promyelocytic leukemia protein antibodies in 60% of cases[151].
The detection of antinuclear antibodies is of diagnostic and prognostic value. That of antibodies against nuclear envelop antigens in the presence of clinical signs of the disease, but in the absence of AMAs can verify the diagnosis of PBC and suggest its unfavorable course.
SMAs against contractile proteins are found less frequently than АNAs. The SMAs are detected simultaneously with the latter ones in about one third of patients. Smooth muscle antibodies are not organ- or species-specific. F-actin serves as an autoantigen for these antibodies. SMAs in PBC belong mainly to the IgM class.
AMA-negative PBC patients account for about 10% to 15%[47]. When AMAs are not detected, then ANAs (autoantibodies against Gp210 and others) can be detected in 50% of AMAs-negative patients. Routine biochemical tests are not different from AMA-positive patients, but usually higher ANAs, SMAs, and IgG concentrations are detected. Serum IgM levels were lower in AMA-negative patients when compared with AMA-positive PBC patients[152,153]. Histologically, it is PBC[61].
The proportion of AMA-negative patients has been minimized due to the development of sensitive detection technology of autoantibodies[154,155]. AMAs are highly specific for PBC and can be detected in nearly 100% of patients when sensitive diagnostic methodologies based on recombinant antigens are used[154].
The production of AMA-IgM from peripheral blood mononuclear cells from PBC patients is reduced after exposure to UDCA[156].
The etiology of PBC remains enigmatic, recent evidence has strengthened the importance of genetic factors in determining the susceptibility to the disease[5]. Hirschfield et al[157-159] have recently reported that PBC is associated with the mutation of genes, such as HLA, IL12A, IL12RB2, and IRF5-TNPO3. Mells et al[160] have a correlation of PBC with 12 new candidate genes, including STAT4, DENND1B, CD80, IL7R, CXCR5, TNFRSF1A, CLEC16A, and NFKB1. Active genetic research is expected to provide significant achievements for prevention and early diagnosis of PBC.
Class II human leukocyte antigen (HLA) genes fully return to attract interest thanks to recent genome-wide association studies (GWAS), which clearly demonstrates that the major components of the genetic architecture of PBC are within the HLA region. PBC is exceptional among autoimmune diseases in having controversially variable associations with alleles of the major histocompatibility complex (MHC, HLA); only a weak and regional association with HLA DRB1*08 has been widely confirmed[161], although there is growing evidence on a protective association with HLA DRB1*11 and *13[162,163].
Investigations by Qin et al[164] suggest that distinct HLA class II genetic variants conferred both a predisposition and a resistance to PBC. HLA-DQB1 (*02, *04, *0401, *0402 and *0601) and HLA-DRB1 (*01, *03, *0405, *07, *08, *0801, and *0803) were identified as risk factors for PBC, whereas HLA-DQB1 (*0301, *06, *0602 and *0604), and HLA-DRB1 (*11, *1101, *13 and *1501) were potent protective factors. Also, DR8 was identified to be a predisposing factor. A total of 13 studies contained data on serological HLA-DR from 5400 subjects (788 cases of PBC and 4612 controls). A high degree of heterogeneity was found to exist between DR8 and PBC risk (I2 = 54.8%, P = 0.011)[164]. These results expand the repertoire of HLA-Class II genes with potential roles in PBC pathogenesis, however follow-up biological studies are needed to confirm these associations[164].
PDC-E2 specific T-cells are present in the liver of PBC patients[165,166], mostly during the earliest disease stages[167,168] that are essential in the pathogenesis and diagnosis of this disease[168-170]. Autoreactive CD4+ and CD8+ T cells are demonstrably involved in the pathogenesis of PBC and, histologically, infiltration of presumably autoreactive T cells in the liver and periductular spaces is one of the major features of PBC[171,172]. CD4+ and CD8+ T lymphocytes reactive with subsets recognize epitopes of PDC-E2 have been identified in the peripheral blood and liver biopsy samples of PBC patients[28,165,167,168]. Several experiments using murine models have indicated a central role of CD4+ T cells in the pathogenesis of PBC[173]. CD4+CD25high regulatory T (Treg) cells play a critical role in self-tolerance and the prevention of autoimmune disease. Patients with PBC display a relative reduction of circulating CD4+CD25high Tregs compared to controls[174,175]. This may be associated with decreased estrogen levels in patients with PBC. Previous studies have examined gender differences in the immune system, and suggest that estrogen and androgen may modulate the immune system. Women have significantly higher CD4+ T lymphocyte counts and a higher CD4+/CD8+ ratio than men[33,176].
The frequency of circulating Tregs can increase after 1 year of treatment with UDCA[177].
Compared to CD4+ T cells, CD8+ T cells play a more significant role in mediating the destruction of the bile duct[6]. BECs apoptosis is considered to result from the attack of effector cells like CD8 T cells[169]. CD8+ T cells may mediate bile ductular injury in the presence of Treg function loss[178]. There is concrete evidence that apoptosis is possibly the most important mechanism of BECs loss. Apoptosis is considered to result from the attack of effector cells like CD8+ T cells[169]. Markers of ongoing apoptosis have been reported within affected portal tracts[179,180]. Interestingly, in vitro caspase cleavage of PDC-E2 has been shown to generate immunologically active protein fragments[137].
It is thought that activated CD4+ T cells can recognize peptide PDC-E2163-176 while activated CD8+ T cells can recognize peptide PDC-E2159-167 and PDC-E2165-174 in PBC[6,181,182]. HLA-A2-restricted CD8+ T cell lines reactive with PDC-E2 residues 159-167 have been characterized[181,182]. Interestingly, CD8+ T cells from livers of PBC patients demonstrate cytotoxicity against PDC-E2 159-167 pulsed autologous cells[169].
PBC is characterized by changes in many blood biochemical parameters. Patients’ sera show the enhanced activity of alkaline phosphatase (ALP), γ-glutamyltransferase (gamma glutamyltranspeptidase, γ-GT), 5’-nucleotidase (5’-NT), and leucineaminopeptidase (LAP), the higher levels of bile acids, cholesterol, phospholipids, copper, γ-globulins, and bilirubin, and the lower level of total protein mainly at the expense of albumin fractions.
In PBC, there is a decline in the levels of bile acids, cholesterol, and lecithin in the hepatic bile portion and their simultaneous rises in hepatocytes and blood (Table 3). This suggests that enterohepatic bile acid circulation is impaired in PBC.
| Parameters | Parameter values inthe groups | mean1/mean2 | ||
| Control(mean1± SE) | Patients with PBC(mean2± SE) | |||
| Bile acids (g/L) | Bile | 3.9 ± 0.8 | 0.65 ± 0.02 | 6.0 |
| Blood | 0.012 ± 0.008 | 0.054 ± 0.008 | -0.2 | |
| Cholesterol (μmol/L) | Bile | 0.91 ± 0.06 | 0.38 ± 0.08 | 2.4 |
| Blood | 4.2 ± 0.6 | 11.8 ± 1.6 | -0.4 | |
| Lecithin1 | Bile | 2.1 ± 0.3 | 0.5 ± 0.1 | 4.6 |
| Orthophosphate1 | Bile | 1.2 ± 0.3 | 0.30 ± 0.07 | 4.0 |
Intrahepatic cholestasis in PBC is a multifactorial process that leads to biochemical disorders and damages to subcellular structures, with changes in the metabolism of bile acids and their transmembrane transport that is done by carrier proteins in the sinusoidal and canalicular membranes[183,184].
Serum bile acid concentrations increase in patients with PBC (Table 3) just in its asymptomatic stage, which is associated with the occurrence of skin itching, the first clinical sign of the disease[66]. All fractions of conjugated bile acids appear in appreciable amounts in the blood of patients with PBC. Unconjugated bile acids are rarely detectable in the serum of PBC patients.
Autoimmune pathological processes in PBC gradually lead to ductulopenia and impair the mechanism responsible for the metabolism of bile acids. The increased number of desolated bile ductules result in impaired bile excretion and insufficient entry of bile acids into the duodenum. As a response, the hepatocyte increases the synthesis of bile acids. A reduction in intestinal bile acid levels via a feedback mechanism induces a compensatory hepatocyte increase in the biosynthesis of bile acids and cholesterol. Cholesterol is the major substrate for bile acid biosynthesis. But ductulopenia does not diminish. The hepatocytic concentration of bile acids gradually elevates and their entry into the intestinal lumen remains insufficient, giving rise to a closed vicious circle that results in the accumulation of bile acids in the liver cells. Due to the increased hepatocytic level of bile acids, their reabsorption from the portal venous bed decreases, leading to the entry and progressive accumulation of bile acids in systemic circulation.
Bile acids, unconjugated ones in particular, are potent detergents that are able to impair cell membranes and have irritant effects on nerve endings. In vitro experiments have shown that several bile acids cause hepatocyte injury with a concomitant generation of hydroperoxide by mitochondria[185,186] and also induce hepatocyte apoptosis in a time- and concentration-dependent manner via reactive oxygen species (ROS) generation by mitochondria[187].
The blood of patients with PBC shows a higher ratio of trihydroxy-/dihydroxycholanic acids and a lower glycine/taurine coefficient[188]. According to Greim et al[189,190], cholic (trihydroxycholic) bile acid has lower detergent properties than dihydroxy- (deoxycholic and chenodeoxycolic) bile acids.
The conjugation, sulfation, and glucuronidation of bile acids are directed towards decreasing their detergent and irritant effects on somatic cells and nerve endings. These also lead to their enhanced release from the systemic circulation through the skin, kidneys, and bowel. García-Marín et al[191] have indicated that taurine-conjugated bile acids stimulate the production of micelles with cholesterol and phosphatidylcholine (lecithin).
Thus, the higher ratio of trihydroxy-/dihydroxycholanic bile acids and the appearance of glycine- or taurine-conjugated, sulfated, and glucuronidated bile acids in the systemic circulation in PBC may be considered as the body’s compensatory and detoxifying response to cholestasis and considerably increased cholanic acids in circulating blood.
Atypical, non-physiological bile acids appear in the blood and urine of patients with PBC[192]. The latter have more potent detergent effects on cell membranes and a stronger irritant effect on nerve receptors than primary and secondary bile acids. The atypical, non-physiological bile acids can be released from the systemic circulation through the skin, taking part in the mechanisms of skin itching[66]. The intensity of the latter may depend on the amount of atypical bile acids in the skin of patients with PBC.
Biochemical studies have revealed that hepatic bile portions from PBC patients contain lower levels of not only bile acids, but also phosphatidylcholine (lecithin) and cholesterol (Table 3). The hepatic bile portions from the patients with PBC and those in the comparison group show a ratio of lipid components (bile acids, lecithin, and cholesterol) of 6:4.6:2.4 (Table 3), which is indicative of high bile lithogenicity in patients with PBC. The appearance of gallbladder concrements complicates PBC in 35%-40% of cases. As a rule, is formed pigment gallstones.
The altered concentration of phosphatidylcholine in the hepatic bile portions can be induced by a change in the relative amounts of bile acids[193]. Hepatocytic lecithin secretion is acid-dependent (dependent on the secretion of bile acids)[194]. The regulatory effects of bile acids on the liver and biliary tract are largely dependent on the hydrophobic-hydrophilic balance of the recirculating bile acid pool[195].
The hepatic biopsy specimens from patients with PBC show a 1.5-fold (P = 0.044) increase in the total amount of phospholipids[196]. Moreover, the same membranes contain the lower levels of lysophosphatidylcholine, sphingomyelin, phosphatidylserine, phosphatidylinositol, and phosphatidylethanolamine[1,196]. There is a 2-fold decrease in the ratio of cholesterol to phospholipids in the hepatocyte membranes of PBC patients.
The blood of patients with early-stage PBC contains higher levels of phospholipids, cholesterol[197,198], and bile acids[199-201] (Table 3). The change in the content of cholesterol and phospholipids in the blood of patients with PBC is associated with their increased formation in the liver and regurgitation into blood flow. Cholesterol and phospholipids are able to bind bile acids and to inactivate their solubilizing effect. Hyperlipidemia in PBC is a compensatory reaction of the organism in response to cholestasis. The high levels of cholesterol and phosphatidylcholine in the blood of PBC patients are apparently associated with the neutralization of the detergent effect of excess bile acids.
In PBC, serum cholesterol levels markedly increase with worsening of cholestasis, and decrease in the late disease stages, despite a severe reduction in biliary secretion[197]. At the same time, there were no significant differences in the levels of major lipoprotein classes in healthy individuals and patients with PBC[66] (Table 4).
| Lipoproteins | Parameter values in the groups | ||
| Control (n = 21) | PBC (n = 18) | ||
| β-lipoproteins | mean ± SD | 50.1 ± 6.0 | 56.4 ± 16.6 |
| Range | 39.2-59.1 | 34.8-86.4 | |
| α-lipoproteins | mean ± SD | 28.4 ± 6.5 | 23.9 ± 12.2 |
| Range | 15.5-42.7 | 6.4-46.8 | |
| Pre-β-lipoproteins | mean ± SD | 20.5 ± 7.5 | 18.2 ± 8.5 |
| Range | 10.5-34.7 | 6.3-43.5 | |
Marked hypercholesterolemia that is typical for longstanding cholestasis is unassociated with an excess risk of cardiovascular disease[197] and the risk of developing atherosclerosis in PBC[202].
PBC can serve as a model disease, showing that only hypercholesterolemia is insufficient to develop atherosclerosis and cardiovascular diseases.
Blood triglyceride levels in PBC are virtually unchanged.
Elevated blood levels of many biliary constituents are observed in patients with PBC. However, the higher bilirubin concentrations are untypical of early-stage PBC[203]. The blood total and conjugated bilirubin levels are increased in patients with PBC in the stage of its obvious clinical symptoms and rarely reach high values. Hyperbilirubinemia results from intrahepatic cholestasis when, in the absence of extrahepatic obstruction, conjugated bilirubin comprises a high fraction of the total bilirubin[204]. Significant individual variations in bilirubin levels are noted in patients with PBC. However, the blood bilirubin concentrations generally correspond to the stage of the disease and the activity of the pathological process. In PBC, the levels of bilirubin are elevated mainly by its conjugated fraction. This suggests that the normal hepatocytic activity of glycosylating enzymes is retained to produce conjugated bilirubin.
The development of hyperbilirubinemia in PBC is associated with impaired bilirubin excretion. The elevation of conjugated bilirubin may result from obstructed bile flow, altered bile ductular integrity, or reduced production of bile due to defective activity of bile efflux transporters[204]. The most probable pathway for the leak of bile back into the systemic circulation is via the intercellular tight junctions[204]. Damage to small and medium-sized bile ducts in PBC and the resultant increased pressure in the biliary system produce reflux of conjugated bilirubin into the plasma. Bile reflux can occur from the bile capillary lumen or hepatocyte into the Disse space and then into blood. Bilirubin accumulations are noted in liver biopsies[205].
In the late stages of the disease, hyperbilirubinemia may be caused by not only bile reflux, but also by increased hemolysis. Enhanced hemolysis is favored by excess serum bile acids in patients with PBC. As potent detergents, bile acids can solubilize the cytoplasm membranes of not only erythrocytes, but also other blood cells, causing them to hemolyze. This favors the development of a number of hematological complications in the end stage of the disease. The magnitude of hyperbilirubinemia at this time is characterized by not only the conjugated fraction of bilirubin, but also by its unconjugated one. Unconjugated and conjugated hyperbilirubinemia is usually the result of the so-called hepatocellular injury, such as cirrhosis.
Conjugated bilirubin may be also detected in the urine of PBC patients. Moreover, urobilinogen is excreted in the urine in proportion with the amount of bile entering the duodenum[32].
Progressively increased serum bilirubin concentrations in PBC are the best indicator for prognostic purposes[61,206]. A study conducted in 1979 claimed that when total bilirubin, a prognostic factor of PBC, is 10 mg/dL or greater, the average survival period is 1.4 years[207]. Pretreatment levels of serum bilirubin, bilirubin levels during follow-up, and the occurrence of normal levels of serum bilirubin were significantly associated with prognosis[208].
A number of studies have reported that the Mayo risk score is an effective means of predicting prognoses in PBC patients[209,210]. The Mayo Clinic published a study that excluded the invasive technique of biopsy, and predicted prognosis based only on clinical and biochemical factors, including age, serum bilirubin level, serum albumin level, prothrombin time, and existence of peripheral edema[211].
Total bilirubin, the Mayo risk score, and the revised International AIH Group score are significantly important as prognostic factors of PBC[206].
Serum ALP and γ-GT levels were markedly elevated in PBC patients[101]. The activity of these enzymes increases in patients with PBC already in the asymptomatic stage of the disease[212,213]. ALP and γ-GT increase to 10 or more times the upper limit of normal in PBC patients[61]. In PBC, there is an increased activity mainly of the hepatic fraction of ALP. The amount of ALP does not correlate with disease progression or stage of the disease[61]. Laboratory studies show a rise in the serum ALP level following the onset of pruritus[203]. The biochemical levels of ALP and γ-GT were reported to be slightly higher in symptomatic males compared to asymptomatic males, but both were higher than in females[214].
The enhanced activity of ALP and 5’-NT is most likely associated with the increased biosynthesis of phospholipids in PBC patients. The biosynthesis of phospholipids occurs with using of orthophosphate which formed by hydrolysis of organic phosphorus compounds under the action of phosphatases: ALP and 5’-NT. ALP activates the hydrolysis of glycerophosphate, glucose-1-phosphate and glucose-6-phosphate. 5’-NT does that of adenosine monophosphate, guanosine monophosphate, cimetidine monophosphate and uridine monophosphate. It is theorized that ALP and 5’-NT give rise to orthophosphate, where required[215]. Based on this theory, the enhancement in the activity of ALP and 5’-nucleotidase in PBC indicates the higher needs of hepatocytes in the orthophosphate
The decreased quantity of orthophosphate in the hepatic bile portion (Table 3) from PBC patients indicates a reduction in its secretion in bile and simultaneously its reduction in liver cells since the secretion of orthophosphate from hepatocytes to bile capillary are passively effected by the concentration gradient. The lower orthophosphate concentrations (despite the enhanced activity of ALP and 5’-NT) indicate the intensive utilization of a phosphorus group in the metabolic processes which take place in hepatocytes in PBC. One of the possible ways the increased using of orthophosphate in patients with PBC is increase the biosynthesis of phospholipids.
The enhanced activity of ALP and 5’-NT is presumably due to their increased biosynthesis in the hepatocytes[216-218]. The mechanism by which cholestasis results in an elevated serum ALP level is thought to involve the induction of ALP synthesis as a result of the enhanced translation of ALP mRNA[219]. To enhance the synthesis of these enzymes, it is necessary to increase the delivery of amino acids to the cells. This can be achieved by increasing the activity of γ-GT in PBC patients. Loginov[212] has noted that the change in blood γ-GT activity in patients with PBC outstrips the increase in the activities of ALP and 5’-NT. Some PBC patients are observed to have an early increase in the activity of γ-GT (> 3 upper limits of normal) before enhancing the activity of ALP. When the effect of alcohol or medications is ruled out, this test is highly sensitive in identifying cholestasis in PBC patients.
Elevated serum ALP and γ-GT levels, together with a positive AMA/AMA-M2, can help diagnose PBC[219].
During the formation of the PBC, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities remain normal or only modestly is increased[46,203,219]. The normal (or slightly enhanced) activity of aminotransferases for several years suggests the preserved integrity and normal permeability of the cytoplasmic membrane of hepatocytes in most patients with PBC. The biochemical level of ALT is reported to be slightly higher in symptomatic males than asymptomatic males, but both were higher than in females[33,214]. From biological and physiological points of view, the differences between men and women may be explained by differences in the presence of risk factors, protective/aggravating effects of sexual hormones, variances linked to genetics and various corporal structures[33,220].
During the last decade many studies have been designed to identify non-invasive markers capable of providing accurate information about liver fibrogenesis activity and liver fibrosis stage in patients with chronic, potentially progressive hepatic diseases[35,221].
Sebastiani et al[222] analyze the value of AST/ALT ratio as an indicator of cirrhosis in patients with PBC. This study includes 160 patients with PBC and laboratory and liver histology data are available for 121 patients. The authors analyze the clinical and laboratory data and follow-up outcomes: liver-related death, liver transplantation and survival. The AST/ALT ratio was also used for assessment in alcohol-induced liver cirrhosis prediction of oesophageal varices and ascites presence. It is suggested that the AST/ALT ratio increases in patients who develop liver cirrhosis, regardless of its cause. The reason for the increased AST/ALT ratio is unknown. It is suggested that the sinusoidal clearance of AST decreases in cirrhotic patients[223-225]. The higher AST/ALT ratio in PBC patients may be due to decreased hepatocyte adhesion[1]. A quantitative method used to evaluate adhesive interactions of hepatocytes has revealed decreased intercellular interactions, particularly at the site of tight junction, probably due to cholestasis and its related elevated pressure in the bile capillaries[226]. The divergence of hepatocytes can be accompanied by the increased permeability of the liver cell cytoplasmic membrane for AST.
Nyblom et al[223] reported the use of this ratio for discrimination between cirrhotic and non-cirrhotic patients with a sensitivity of 82% and a specificity of 79%, for a cut-off value 1.1. The explanation for such high sensitivity is that a large number of cirrhotic patients were included, while sampling variability in the liver biopsies contributed to low specificity. The study concluded that the AST/ALT ratio is of clinical value as a predictor of cirrhosis in patients with PBC, but not as a prognostic factor.
Alempijevic et al[35] observed lower sensitivity (47.5%) and specificity (75%) for the AST/ALT ratio for staging the disease that could be explained by different study design. The authors found a statistically significant correlation between PBC stage and AST, ALT to platelet ratio, ALT/platelet count, AST/ALT, ALT/AST and ALT/cholesterol ratios, with the values of Spearman’s rho of 0.338, 0.476, 0.404, 0.356, 0.351 and 0.325, respectively. The best sensitivity and specificity were shown for AST/ALT, with an area under ROC of 0.660. The authors suggest that the potential predictive value of aminotransferase and platelet count ratios in predicting the stage of PBC may be used to evaluate PBC evolution, despite their limited sensitivity and specificity, especially when considering their availability and cost effectiveness[35].
LAP is highly active in the liver where it is present mainly in the biliary epithelium. It is an enzyme involved in protein metabolism. The enhanced serum activity of LAP, like 5’-NT and, to a lesser extent, γ-GT, is specific to all forms of intra- and extrahepatic cholestasis. In PBC, there is increased LAP activity, which may indicate bile duct epithelial damage[71].
The liver is known to play an important role in the metabolism of copper, giving rise to hepatocytic protein-copper complexes and its biliary excretion[227]. In health, about 80% of copper is excreted from the body into bile and feces.
Liver copper levels are significantly increased in PBC compared with other liver chronic diseases[228,229]. In PBC, liver cell copper retention occurs as a complication of impaired biliary copper excretion[230]. Elevated blood copper concentrations are due to hepatocyte adhesion impairment and reflux of biliary components into blood. The copper levels may exceed 25 mg/100 g of dry liver tissue (normal range up to 6 mg/100 g) in PBC[229]. Despite the retention of copper in hepatocytes, liver cell function is well preserved[230] and there are no clinical signs of toxic effects of copper on liver cells.
In PBC patients with increased liver copper concentrations, the latter do not correlate with the biochemical parameters of liver damage[230], but generally do with the stage of the disease[231]. The characteristic organelle (nuclear vacuolation and steatosis) changes associated with copper toxicity in Wilson’s disease are not observed in PBC[230]. A Kayser-Fleischer ring is not detected in patients with PBC. Copper-associated proteins are found in the disease[227]. Detection of protein-binding copper in PBC is suggestive of the normal hepatocytic metabolism of copper and its reflux with biliary components into blood. This can account for that there is no Kayser-Fleischer ring or toxic effect of copper on the body.
The liver is the only site for the synthesis of albumin, fibrinogen, prothrombin, and some other blood coagulation factors. Furthermore, the liver plays a leading role in producing α-globulins, a major portion of β-globulins, heparin, and enzymes. In patients with early-stage PBC, the blood levels of albumins and globulins are within the normal range. Liver cell synthetic function is well preserved in PBC patients. All the patients have normal prothrombin times and subnormal serum albumin concentrations[230]. With progression of PBC observed increased levels of γ-globulins, IgM in particular. At the same time, there is simultaneously a relative decline in blood albumin. A test for an alkaline albumin fraction is a highly sensitive method in hepatocellular failure. In health, the alkaline fraction accounts for about 3% of a total of albumin. The half-life of the alkaline fraction is longer than that of other albumin fractions; therefore, when its hepatic synthesis is impaired, the percentage of the alkaline fraction is increased (as high as 50% in end-stage cirrhosis). Unfortunately, this test is not practically used in the diagnosis of PBC.
In end-stage disease, impairments in fat-soluble vitamin absorption and hepatic protein synthetic function can result in vitamin K deficiency, coagulopathies, and decreased vitamin A levels, which can promote visual disorder.
The most marked biochemical changes suggesting hepatocytic protein synthetic dysfunction are usually found in more advanced stage (3-4) PBC.
Thus, cholestasis in PBC is accompanied by the enhanced activity of ALP, LAP, γ-GT, 5’-NT; hypercholesterolemia, elevated levels of bile acids, phospholipids, and β-lipoproteins, and hyperbilirubinemia.
Imaging procedures are not helpful for the diagnosis of PBC, except for liver histology. Histologically, PBC is characterized by portal inflammation and immune-mediated destruction of the intrahepatic bile ducts. These changes occur at different rates and with varying degrees of severity in different patients. The loss of bile ducts leads to decreased bile secretion and the retention of toxic substances within the liver, resulting in further hepatic damage, fibrosis, cirrhosis, and eventually liver failure[3,232].
Histological findings after liver biopsy included focal and piecemeal necrosis, portal, periportal, and lobular inflammation, fibrosis and overall inflammation and liver cell damage (“histological activity”)[230]. The histological manifestations are damaged BECs and infiltration of T cells, B cells, macrophages, eosinophils and natural killer cells in the portal area[3,6]. In the field nucleopore frequently detected complexes of autoantibodies with Gp210, p62 and sp100, which to form a ring around the nucleus[146,227]. Liver histology in PBC patients is of interest for the assessment of the diagnosis and for staging of the disease[61].
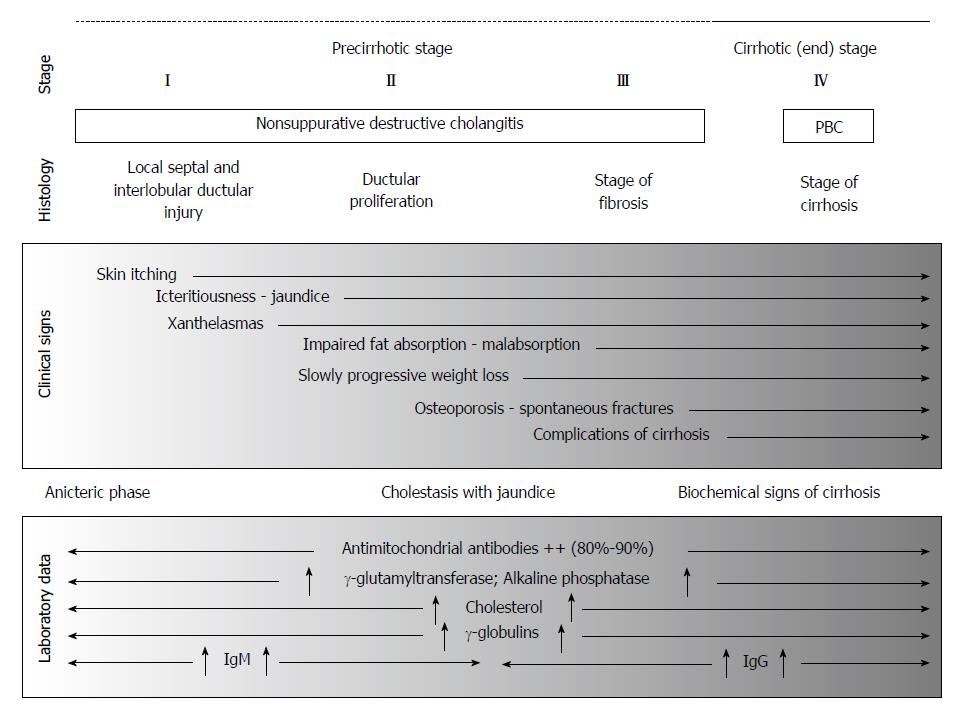
According to its morphological characteristics, PBC is classified in four stages ranging from florid bile duct lesions, ductular proliferation, and fibrosis to liver cirrhosis (Table 5). Stage I-IV disease classification was used by an experienced pathologist who assessed liver biopsies[236].
Stage I is defined by the localization of inflammation to the portal triads. In stage II, the number of normal bile ducts is reduced, and inflammation extends beyond the portal triads into the surrounding parenchyma. Fibrous septa link adjacent portal triads in stage III, while stage IV represents end-stage liver disease characterized by obvious cirrhosis with regenerative nodules. The morphogenesis of PBC displays a gradual transition from early to later stages. Liver biopsy specimens from PBC patients can frequently exhibit the histological features of different stages of the disease[227,237]. According to Klöppel et al[238], despite this fact, in most cases liver puncture biopsy enables one to reveal the signs characterizing this or that stage of PBC. The histological findings after liver biopsy in PBC patients point to the predominant histological pattern of one of the disease stages.
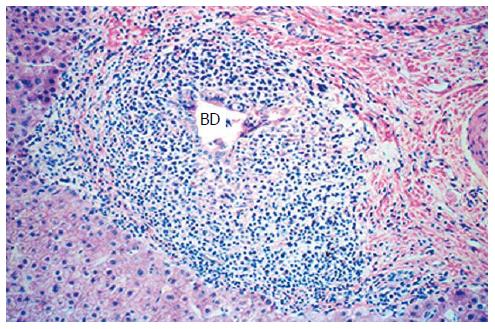
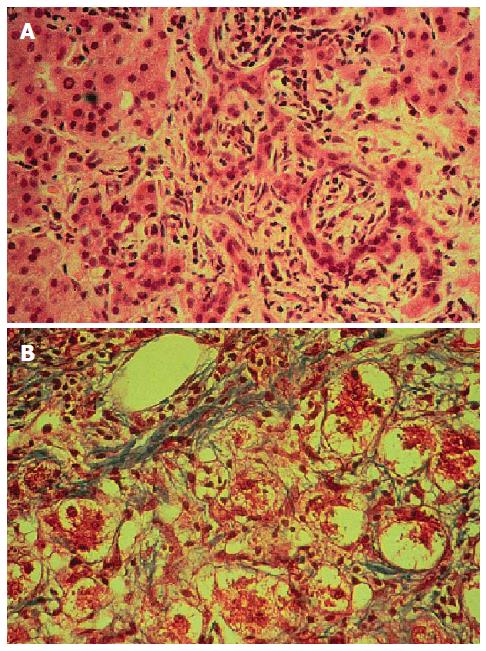
In stages I-II PBC, biopsy specimens show different phases of bile duct injury (Figure 4). According to G. Roschlau, these changes may precede the clinical manifestations of PBC[239]. Early injuries develop in the interlobular ducts 45-75 μm in diameter. Dystrophy of ductal epithelial cells should be considered the earliest sign. Their cytoplasm becomes granular or homogenic eosinophilic, turgid, vacuolated; the nuclei get pycnotic. There is further necrosis of a small canalicular segment, but its outlines are still retained and finally the wall is destroyed, giving rise to a pattern of destructive cholangitis[227].
Lymphoid/plasma cell infiltration is observed in the periportal fields around the epithelium lining the bile ducts. Moreover, the epithelial cells appear compressed, in the basal part in particular. Examination of inflammatory infiltrates reveals the elevated CD4 lymphocyte levels exceeding the CD8 lymphocyte counts (4:1).
There are also large lymph follicles, overexuberant portal tract infiltration, sometimes with an impurity of xanthoma cells, as well as some histiocytic and epithelioid cell granulomas.
Damage to the septal or interlobular bile ducts is a pathognomonic sign of PBC, but such changes are rarely found in the puncture biopsy specimens[240]. The hepatocytes in this stage have a usual structure; the stellate reticuloendothelial cells are hyperplastic.
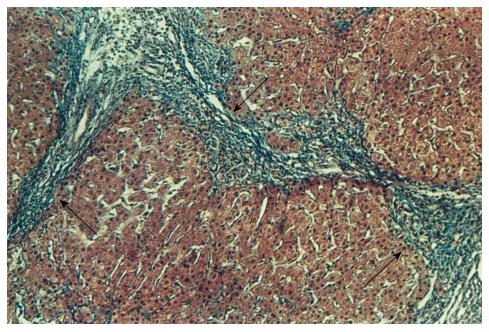
Proliferation of ductuli that penetrate the terminal plate, periductal fibrosis, and sclerotic processes to form blind septa are dominant in stages II-III PBC (Figure 5A and B).
Fibrosis of the portal fields may lead to portal hypertension just as long before liver cirrhosis develops. The epithelium of some proliferating canaliculi is dystrophic. This may be regarded as the presence of signs of an exacerbation or progredient course of disease. Bile ducts not in all portal tracts are detected. Their sites show scars or small groups of epithelial cells. Bile thrombi are seen rarely. The parenchymal structure is usually preserved. Lobular necroses are detectable at the site of destroyed hepatocytes in the liver lobule.
Stage IV PBC is characterized by a pattern of significant micronodular cirrhosis. At the same time, there may also be signs typical of earlier-stage PBC, as well as granulomas. Inflammatory infiltration is found predominantly around the latter of the remaining bile ducts and resolves after their destruction. The developed cirrhosis in PBC patients is often difficult and sometimes impossible to differentiate from liver cirrhosis of another etiology.
Thus, only the last stage of PBC complies with the conventional criteria for cirrhosis. In the others, there is no diffuse fibrosis or nodular transformation, the necessary signs of cirrhosis, which are apparent from its definition. Much significance is attached to piecemeal and bridging parenchymal necrosis in the development of the final stage of liver cirrhosis (Figure 6)[241].
In cholestasis, extralysosomal copper is often present in the hepatocellular cytoplasm[231]. Orsein-positive granules are found in biopsy specimens in cholestatic liver diseases[242]. In PBC, these granules are found in the cytoplasm of periportal hepatocytes. As shown by the test with rubean-hydrogen acid, copper is found in combination with these granules. The detection of orsein-positive granules in combination with copper in the cytoplasm of periportal hepatocytes is an additional informative sign in favor of PBC[242].
For diagnosis, staining for copper and for copper-associated protein may assist in the differentiation of PBC from chronic active hepatitis[231]. Symptomatic male patients with PBC had more stainable copper deposits in the histological samples than asymptomatic males.
Hepatic damage may produce different consequences in men and women in ongoing primitive diseases and during acquired conditions[33,220]. The only histological difference identified is that symptomatic female patients had more piecemeal necrosis of the liver and that symptomatic males had more stainable copper storage than asymptomatic males[33]. Additionally, symptomatic females were reported to have more pseudoxanthomatous transformation than asymptomatic females[33,243].
In PBC patients, liver biopsy is used as the gold standard for assessing liver fibrosis. There are four stages of fibrosis in PBC[244]: (1) no fibrosis (stage I); (2) periportal fibrosis (stage II); (3) bridging fibrosis (stage III); and (4) cirrhosis (stage IV).
Many fibrosis experts would therefore consider serum fibrosis tests with an ROC area of 0.85-0.90 to be as good as liver biopsy for staging fibrosis[35,245].
Biochemical markers and their ratios do correlate with different sensitivity to and specificity of PBC disease stage. The use of biochemical markers and their ratios in clinical evaluation of PBC patients may reduce, but not eliminate, the need for liver biopsy[35].
New technology has been developed based on the fact that liver stiffness increases as liver fibrosis progresses[246]. Transient elastography is a new modality developed for non-invasive evaluation of liver stiffness. Liver stiffness correlates well with the histological stage of fibrosis. Changes in liver fibrosis stage may thus be estimated non-invasively using transient elastography[247]. Nevertheless, further studies are needed to confirm the value of this method in different chronic liver diseases.
Diagnosis of PBC is based on clinical, laboratory and morphological criteria (Figure 7). In the early stages of PBC, its diagnosis presents no great problems, particularly if at examination a middle-aged woman is detected to have skin itching, more than 6-mo increases in the activity of ALP and/or γ-GT, an AMAs titer of over 1:40, and morphological changes corresponding to nonsuppurative destructive cholangitis[66,248]. Currently, the diagnosis of PBC is often made when the patient is still asymptomatic, with abnormal liver biochemistry and/or AMAs[248,249]. The symptomatic patients may have fatigue, generalized pruritus, osteoporosis, fat-soluble vitamin deficiencies and portal hypertension[245,250].
Owing to easy-to-use biochemical tests, such as quantification of ALP, γ-GT, bile acids, AST, ALT, and total bilirubin, and other tests for AMAs, ANAs, SMAs, IgM, and IgG, PBC is often diagnosed in its early stages[206]. The early criteria for PBC are (1) skin itching; (2) a positive test for AMAs; (3) ALP levels at least two times higher than the upper limit of normal (ULN) and/or γ-GT levels at least five times higher than the ULN; and (4) a liver biopsy specimen showing florid bile duct lesions[61,118].
AMAs detection with evidence of normal ALP activity suggests a lifetime risk for PBC. There is a concurrent increase in ALP and γ-GT activities. The enhanced activity of γ-GT only is not highly specific for PBC and may be caused by alcohol or drugs. That of ALP only may also be due to bone changes, pregnancy, or familial cholestasis.
Chronic cholestasis is a disruption in bile synthesis and outflow for more than 6 mo. Biochemical tests cannot differentiate intra- and extrahepatic cholestasis. Abdominal ultrasonography (AUS), magnetic resonance cholangiopancreatography (MRCP), endoscopic ultrasound (EUS), endoscopic retrograde cholangiopancreatography (ERCP) with sphincteropapillotomy used to improve bile outflow are performed to evaluate intra- and extrahepatic bile ducts. ERCP is particularly important in the differential diagnosis of primary sclerosing cholangitis (PSC). Laparoscopy allows the presence of stage IV PBC to be specified.
MRCP and EUS are a great advantage in interpreting the biliary system. ERCP may cause pancreatitis (3%-5%), bleeding (2%), and cholangitis (1%), at sphincteropapillotomy in particular. ERCP-related mortality is 0.4%[251].
Liver biopsy is indicated when serum AMAs are absent in the typical pattern of the disease, when its stage and activity should be determined, or when the overlap syndrome is suggested[252]. The PBC-associated morphological changes are characterized by a peculiar mosaicism of lesions and a stereotypy of liver tissue reactions. This is due to the fact that sequential progression from one to another stage may be manifested in other segments of the organ.
PBC should be differentially diagnosed with extrahepatic cholestasis, sarcoidosis, drug-induced hepatitis, PSC, and AIH. Moreover, the diagnosis of PBC in the absence of AMAs may pose particular problems[47,152].
In asymptomatic PBC, it may be difficult to make a differential diagnosis of Paget’s disease characterized by enhanced serum ALP activity. Determination of the activities of γ-GT and ALP isoforms permits a reliable diagnosis. Enhanced γ-GT activity is typical of PBC rather than Paget’s disease. The determination of hepatic and osseous ALP isoforms reveals an increase in the former in PBC and in the latter in Paget’s disease.
AUS, EUS, MRCP, and ERCP data are of decisive importance in differentially diagnosing PBC and extrahepatic cholestasis-accompanied diseases.
The detection of tissue granulomas may suggest cholestatic sarcoidosis. In sarcoidosis, serum AMAs are absent and the Kveim-Siltzbach test is positive (75%). Great importance is attached to biochemical functional tests and morphological examination of liver biopsy specimens. Thus, well-formed granulomas with minimal bile duct injury are found in sarcoidosis. On the contrary, there may be biliary epithelial changes, mild hepatocyte necrosis, and lymphoid cell infiltration just in the early stages of PBC.
Drug-induced hepatitis may also have a clinical picture similar to that of PBC. In these cases, hepatic damage is associated with the use of drugs and characterized by an acuter onset and rapid development of jaundice (about 4-6 wk).
The etiology of liver diseases concurrent with cholestasis caused by exogenous factors is established by carefully collecting the history data of a patient and those around him; that in viral hepatitis is determined using the serological markers of hepatitis B, C, D and other viruses.
Patient age, the presence or absence of AMAs and other autoantibodies (most commonly, perinuclear antineutrophilic cytoplasmic antibodies), and ERCP or MRCP evidence are of importance for the differential diagnosis of PBC and PSC. The latter affects mainly young or middle-aged men. PSC is characterized by either none or low (< 1:40) AMA titers.
After ERCP and MRCP, PSC patients are noted to have an impaired typical structure of bile ducts as unevenness in the lumen of the common bile duct, deformations of the extra- and intrahepatic bile ducts, and the appearance of well-defined irregularity segments, by alternating stenosis and saccular enlargements.
The differential diagnosis of AIH frequently presents problems in advanced-stage PBC. There are simplified criteria for the diagnosis of AIH, such as serum ANAs, SMAs (1:80 or more), antibodies to soluble liver antigen, or autoantibodies to liver/kidney microsomal antigen in a titer of ≥ 1:40; elevated IgG levels above 1.1 of the upper limit of normal; liver tissue morphological changes corresponding to chronic hepatitis, as well as no markers for hepatitis viruses[252].
Furthermore, PBC is characterized by higher IgM levels than IgG levels, which also enables PBC and AIH to be differentiated[253,254].
When ANAs and SMAs at the diagnostic titer are detected in a PBC patient, the overlap syndrome should be diagnosed[255]. Histological examination shows the signs of the two diseases, such as intrahepatic bile duct injury, lymphocytic plasma cell infiltrates, bridging hepatocyte necrosis, etc.
Incidence and prevalence rates of PBC are increasing over time[15]. During the last several years, advanced biochemical assays, further delineation of specific liver histological findings, more effective serum autoantibody detection methods and improved diagnostic abilities have led to higher prevalence estimates worldwide[2,6,22,29,256]. PBC is diagnosed more frequently than it was a decade ago because of its greater recognition by physicians, the widespread use of automated blood testing and the AMA test, which is relatively specific for the diseases[35,245].
Due to advances in testing methodology, many cases of PBC are diagnosed early in the asymptomatic phase, and early diagnosis of PBC provides a much better prognosis. Early diagnosis and treatment of PBC of UDCA is effective in delaying the development of hepatic fibrosis and esophageal varix[3,206,257-259].
Further improvements in immunological assays for detecting autoantibodies (to bile duct epithelial proteins) will be able to optimize a diagnostic search in cholestatic liver damages in the absence of AMAs, in very advanced-stage PBC, and bile duct injuries of unknown origin.
Clinicians should be practically alert to the need for early diagnosis, during the long latent period of the disease, in the susceptible middle-aged female population to ensure that such subjects do gain benefit from disease-retarding therapy, at whatever stage their disease may be[260]. This is already happening to some degree, if we compare what PBC was like 20 years ago[261] to what it is today[262].
P- Reviewer: Hu ZY, Kikuchi K, Temel T S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261-1273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 383] [Cited by in F6Publishing: 385] [Article Influence: 13.8] [Reference Citation Analysis (1)] |
| 2. | Reshetnyak VI. Concept on the pathogenesis and treatment of primary biliary cirrhosis. World J Gastroenterol. 2006;12:7250-7262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 3. | Hu CJ, Zhang FC, Li YZ, Zhang X. Primary biliary cirrhosis: what do autoantibodies tell us? World J Gastroenterol. 2010;16:3616-3629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 39] [Cited by in F6Publishing: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Invernizzi P, Selmi C, Gershwin ME. Update on primary biliary cirrhosis. Dig Liver Dis. 2010;42:401-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Invernizzi P. Human leukocyte antigen in primary biliary cirrhosis: an old story now reviving. Hepatology. 2011;54:714-723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Shi TY, Zhang FC. Role of autoimmunity in primary biliary cirrhosis. World J Gastroenterol. 2012;18:7141-7148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Leung PS, Wang J, Naiyanetr P, Kenny TP, Lam KS, Kurth MJ, Gershwin ME. Environment and primary biliary cirrhosis: electrophilic drugs and the induction of AMA. J Autoimmun. 2013;41:79-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Sánchez A, Hernández N, Chiodi D, Berrueta J, Robaina G, Pollio C, Mescia G. Primary biliary cirrhosis: clinical and epidemiological features in an Uruguayan population. Acta Gastroenterol Latinoam. 2013;43:288-293. [PubMed] [Cited in This Article: ] |
| 9. | Hamlyn AN, Sherlock S. The epidemiology of primary biliary cirrhosis: a survey of mortality in England and Wales. Gut. 1974;15:473-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Addison T, Gull W. On a certain affection of the skin, vitiligoidea plana and vitiligoidea tuberosa, with remarks. Guy’s Hosp Rep. 1851;7:265-276. [Cited in This Article: ] |
| 11. | Dauphinee JA, Sinclair JC. Primary biliary cirrhosis. Can Med Assoc J. 1949;61:1-6. [PubMed] [Cited in This Article: ] |
| 12. | Ahrens EH, Payne MA, Kunkel HG, Eisenmenger WJ, Blondheim SH. Primary biliary cirrhosis. Medicine (Baltimore). 1950;29:299-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 325] [Cited by in F6Publishing: 257] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Rubin E, Schaffner F, Popper H. Primary biliary cirrhosis. chronic non-suppurative destructive cholangitis. Am J Pathol. 1965;46:387-407. [PubMed] [Cited in This Article: ] |
| 14. | Koulentaki M, Mantaka A, Sifaki-Pistolla D, Thalassinos E, Tzanakis N, Kouroumalis E. Geoepidemiology and space-time analysis of Primary biliary cirrhosis in Crete, Greece. Liver Int. 2014;34:e200-e207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Boonstra K, Kunst AE, Stadhouders PH, Tuynman HA, Poen AC, van Nieuwkerk KM, Witteman EM, Hamann D, Witteman BJ, Beuers U. Rising incidence and prevalence of primary biliary cirrhosis: a large population-based study. Liver Int. 2014;34:e31-e38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 16. | Newton JL, Hudson M, Tachtatzis P, Sutcliffe K, Pairman J, Burt JA, Jones DE. Population prevalence and symptom associations of autonomic dysfunction in primary biliary cirrhosis. Hepatology. 2007;45:1496-1505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Poupon R. Primary biliary cirrhosis: a 2010 update. J Hepatol. 2010;52:745-758. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 215] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 18. | Jones DE, Watt FE, Metcalf JV, Bassendine MF, James OF. Familial primary biliary cirrhosis reassessed: a geographically-based population study. J Hepatol. 1999;30:402-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 165] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Inoue K, Hirohara J, Nakano T, Seki T, Sasaki H, Higuchi K, Ohta Y, Onji M, Muto Y, Moriwaki H. Prediction of prognosis of primary biliary cirrhosis in Japan. Liver. 1995;15:70-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 61] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Kim KA, Jeong SH, Lee JI, Yeon JE, Lee HJ, Kwon SY, Chang UI, Min HJ. [Clinical features and prognosis of primary biliary cirrhosis in Korea]. Korean J Hepatol. 2010;16:139-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Metcalf J, James O. The geoepidemiology of primary biliary cirrhosis. Semin Liver Dis. 1997;17:13-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 73] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Prince MI, James OF. The epidemiology of primary biliary cirrhosis. Clin Liver Dis. 2003;7:795-819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Sood S, Gow PJ, Christie JM, Angus PW. Epidemiology of primary biliary cirrhosis in Victoria, Australia: high prevalence in migrant populations. Gastroenterology. 2004;127:470-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Lazaridis KN, Talwalkar JA. Clinical epidemiology of primary biliary cirrhosis: incidence, prevalence, and impact of therapy. J Clin Gastroenterol. 2007;41:494-500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Gershwin ME, Selmi C, Worman HJ, Gold EB, Watnik M, Utts J, Lindor KD, Kaplan MM, Vierling JM. Risk factors and comorbidities in primary biliary cirrhosis: a controlled interview-based study of 1032 patients. Hepatology. 2005;42:1194-1202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 435] [Cited by in F6Publishing: 409] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 26. | Selmi C, Mayo MJ, Bach N, Ishibashi H, Invernizzi P, Gish RG, Gordon SC, Wright HI, Zweiban B, Podda M. Primary biliary cirrhosis in monozygotic and dizygotic twins: genetics, epigenetics, and environment. Gastroenterology. 2004;127:485-492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 365] [Cited by in F6Publishing: 378] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 27. | Lazaridis KN, Juran BD, Boe GM, Slusser JP, de Andrade M, Homburger HA, Ghosh K, Dickson ER, Lindor KD, Petersen GM. Increased prevalence of antimitochondrial antibodies in first-degree relatives of patients with primary biliary cirrhosis. Hepatology. 2007;46:785-792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Kouroumalis E, Notas G. Pathogenesis of primary biliary cirrhosis: a unifying model. World J Gastroenterol. 2006;12:2320-2327. [PubMed] [Cited in This Article: ] |
| 29. | Selmi C, Invernizzi P, Zuin M, Podda M, Gershwin ME. Genetics and geoepidemiology of primary biliary cirrhosis: following the footprints to disease etiology. Semin Liver Dis. 2005;25:265-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Smyk D, Cholongitas E, Kriese S, Rigopoulou EI, Bogdanos DP. Primary biliary cirrhosis: family stories. Autoimmune Dis. 2011;2011:189585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Mitchell MM, Lleo A, Zammataro L, Mayo MJ, Invernizzi P, Bach N, Shimoda S, Gordon S, Podda M, Gershwin ME. Epigenetic investigation of variably X chromosome inactivated genes in monozygotic female twins discordant for primary biliary cirrhosis. Epigenetics. 2011;6:95-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Sherlock S, Dooley J. Diseases of the liver and biliary cirrhosis. Oxford: Blackwell Sci Pub 1993; 236-253. [Cited in This Article: ] |
| 33. | Durazzo M, Belci P, Collo A, Prandi V, Pistone E, Martorana M, Gambino R, Bo S. Gender specific medicine in liver diseases: a point of view. World J Gastroenterol. 2014;20:2127-2135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 34] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Prince M, Chetwynd A, Newman W, Metcalf JV, James OF. Survival and symptom progression in a geographically based cohort of patients with primary biliary cirrhosis: follow-up for up to 28 years. Gastroenterology. 2002;123:1044-1051. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 219] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 35. | Alempijevic T, Krstic M, Jesic R, Jovanovic I, Sokic Milutinovic A, Kovacevic N, Krstic S, Popovic D. Biochemical markers for non-invasive assessment of disease stage in patients with primary biliary cirrhosis. World J Gastroenterol. 2009;15:591-594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Invernizzi P, Miozzo M, Battezzati PM, Bianchi I, Grati FR, Simoni G, Selmi C, Watnik M, Gershwin ME, Podda M. Frequency of monosomy X in women with primary biliary cirrhosis. Lancet. 2004;363:533-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 218] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 37. | Miozzo M, Selmi C, Gentilin B, Grati FR, Sirchia S, Oertelt S, Zuin M, Gershwin ME, Podda M, Invernizzi P. Preferential X chromosome loss but random inactivation characterize primary biliary cirrhosis. Hepatology. 2007;46:456-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 38. | Leung PS, Coppel RL, Gershwin ME. Etiology of primary biliary cirrhosis: the search for the culprit. Semin Liver Dis. 2005;25:327-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Lleo A, Invernizzi P, Mackay IR, Prince H, Zhong RQ, Gershwin ME. Etiopathogenesis of primary biliary cirrhosis. World J Gastroenterol. 2008;14:3328-3337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 69] [Cited by in F6Publishing: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Lleo A, Bowlus CL, Yang GX, Invernizzi P, Podda M, Van de Water J, Ansari AA, Coppel RL, Worman HJ, Gores GJ. Biliary apotopes and anti-mitochondrial antibodies activate innate immune responses in primary biliary cirrhosis. Hepatology. 2010;52:987-998. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 41. | Sutton I, Neuberger J. Primary biliary cirrhosis: seeking the silent partner of autoimmunity. Gut. 2002;50:743-746. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 42. | Gershwin ME, Selmi C. Apocalypsal versus apocryphal: the role of retroviruses in primary biliary cirrhosis. Am J Gastroenterol. 2004;99:2356-2358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 43. | Amano K, Leung PS, Rieger R, Quan C, Wang X, Marik J, Suen YF, Kurth MJ, Nantz MH, Ansari AA. Chemical xenobiotics and mitochondrial autoantigens in primary biliary cirrhosis: identification of antibodies against a common environmental, cosmetic, and food additive, 2-octynoic acid. J Immunol. 2005;174:5874-5883. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 44. | Sasaki H, Inoue K, Higuchi K, Yasuyama T, Koyata H, Kuroki T, Yamamoto S, Ichida F. Primary biliary cirrhosis in Japan: national survey by the Subcommittee on Autoimmune hepatitis. Gastroenterol Jpn. 1985;20:476-485. [PubMed] [Cited in This Article: ] |
| 45. | Poupon R. [Primary biliary cirrhosis]. Rev Prat. 1991;41:1161-1165. [PubMed] [Cited in This Article: ] |
| 46. | Poupon R. [Primary biliary cirrhosis]. Schweiz Med Wochenschr. 1991;121:727-732. [PubMed] [Cited in This Article: ] |
| 47. | Il’ichenko LIu, Golovanova EV, Tsaregorodtseva TM, Serova TI, Gudkova RB. Current understanding of primary biliary cirrhosis. Ter Arkh. 2005;77:50-54. [PubMed] [Cited in This Article: ] |
| 48. | Springer J, Cauch-Dudek K, O’Rourke K, Wanless IR, Heathcote EJ. Asymptomatic primary biliary cirrhosis: a study of its natural history and prognosis. Am J Gastroenterol. 1999;94:47-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 49. | Prince MI, Chetwynd A, Craig WL, Metcalf JV, James OF. Asymptomatic primary biliary cirrhosis: clinical features, prognosis, and symptom progression in a large population based cohort. Gut. 2004;53:865-870. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 193] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 50. | Abbas G, Jorgensen RA, Lindor KD. Fatigue in primary biliary cirrhosis. Nat Rev Gastroenterol Hepatol. 2010;7:313-319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 51. | Newton JL, Gibson GJ, Tomlinson M, Wilton K, Jones D. Fatigue in primary biliary cirrhosis is associated with excessive daytime somnolence. Hepatology. 2006;44:91-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 134] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 52. | Biagini MR, Tozzi A, Milani S, Grippo A, Amantini A, Capanni M, Galli A, Surrenti C. Fatigue in primary biliary cirrhosis: a possible role of comorbidities. Eur J Gastroenterol Hepatol. 2008;20:122-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 53. | Newton JL. Fatigue in primary biliary cirrhosis. Clin Liver Dis. 2008;12:367-383; ix. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 54. | Oude Elferink RP, Kremer AE, Beuers U. Mediators of pruritus during cholestasis. Curr Opin Gastroenterol. 2011;27:289-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 55. | Kremer AE, Beuers U, Oude-Elferink RP, Pusl T. Pathogenesis and treatment of pruritus in cholestasis. Drugs. 2008;68:2163-2182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 56. | Kremer AE, Oude Elferink RP, Beuers U. Cholestatic pruritus: new insights into pathophysiology and current treatment. Hautarzt. 2012;63:532-538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 57. | Oude Elferink RP, Kremer AE, Martens JJ, Beuers UH. The molecular mechanism of cholestatic pruritus. Dig Dis. 2011;29:66-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 58. | Kremer AE, Oude Elferink RP, Beuers U. Pathophysiology and current management of pruritus in liver disease. Clin Res Hepatol Gastroenterol. 2011;35:89-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 59. | Kremer AE, van Dijk R, Leckie P, Schaap FG, Kuiper EM, Mettang T, Reiners KS, Raap U, van Buuren HR, van Erpecum KJ. Serum autotaxin is increased in pruritus of cholestasis, but not of other origin, and responds to therapeutic interventions. Hepatology. 2012;56:1391-1400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 201] [Article Influence: 16.8] [Reference Citation Analysis (1)] |
| 60. | Bolier R, Oude Elferink RP, Beuers U. Advances in pathogenesis and treatment of pruritus. Clin Liver Dis. 2013;17:319-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 61. | Leuschner U. Primary biliary cirrhosis--presentation and diagnosis. Clin Liver Dis. 2003;7:741-758. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 61] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 62. | Jones EA, Bergasa NV. The pruritus of cholestasis: from bile acids to opiate agonists. Hepatology. 1990;11:884-887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 146] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 63. | Kirby J, Heaton KW, Burton JL. Pruritic effect of bile salts. Br Med J. 1974;4:693-695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 75] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 64. | Ghent CN, Bloomer JR. Itch in liver disease: facts and speculations. Yale J Biol Med. 1979;52:77-82. [PubMed] [Cited in This Article: ] |
| 65. | Sherlock S, Dooley J. Diseases of the liver and biliary cirrhosis. Oxford: Blackwell Sci Pub 1993; 44-58. [Cited in This Article: ] |
| 66. | Reshetnyak VI. Mechanisms of bile formation and primary biliary cirrhosis. Moscow: Publishing house “Red square” 2003; 144. [Cited in This Article: ] |
| 67. | Hsu JC, Su TC, Chen MF, Liau CS, Lee YT. Xanthoma striatum palmare in a patient with primary biliary cirrhosis and hypercholesterolemia. J Gastroenterol Hepatol. 2005;20:1799-1800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 68. | Macías-Rodríguez RU, Torre-Delgadillo A. Xanthelasmas and xanthomatas striatum palmare in primary biliary cirrhosis. Ann Hepatol. 2006;5:49. [PubMed] [Cited in This Article: ] |
| 69. | Smith AD, Gentile JW, Desai DM, Tuttle-Newhall JE. Palmar lipid deposits and profound hypercholesterolemia that resolved after orthotopic liver transplantation for primary biliary cirrhosis. Am J Gastroenterol. 2007;102:210-211; author reply 211. [PubMed] [Cited in This Article: ] |
| 70. | Ahrens EH, Kunkel HG. The relationship between serum lipids and skin xanthomata in 18 patients with primary biliary cirrhosis. J Clin Invest. 1949;28:1565-1574. [PubMed] [Cited in This Article: ] |
| 71. | Ilchenko LY, Reshetnyak VI. Primary biliary cirrhosis: Clinical and laboratory criteria of diagnostics and up-to-date treatment. Russian J Gastroenterol, Hepatology, Coloproctology. 2011;5:41-51. [Cited in This Article: ] |
| 72. | Ros E, García-Pugés A, Reixach M, Cusó E, Rodés J. Fat digestion and exocrine pancreatic function in primary biliary cirrhosis. Gastroenterology. 1984;87:180-187. [PubMed] [Cited in This Article: ] |
| 73. | DiBaise JK, Paustian FF. Steatorrhea and weight loss in a 72-year-old man: primary biliary cirrhosis? Celiac disease? Bacterial overgrowth? What else? Am J Gastroenterol. 1998;93:2226-2230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 74. | Lanspa SJ, Chan AT, Bell JS, Go VL, Dickson ER, DiMagno EP. Pathogenesis of steatorrhea in primary biliary cirrhosis. Hepatology. 1985;5:837-842. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 48] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 75. | Wariaghli G, Allali F, El Maghraoui A, Hajjaj-Hassouni N. Osteoporosis in patients with primary biliary cirrhosis. Eur J Gastroenterol Hepatol. 2010;22:1397-1401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 76. | Kowdley KV. Lipids and lipid-activated vitamins in chronic cholestatic diseases. Clin Liver Dis. 1998;2:373-389, x. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 77. | Rosen H. Primary biliary cirrhosis and bone disease. Hepatology. 1995;21:253-255. [PubMed] [DOI] [Cited in This Article: ] |
| 78. | Goel V, Kar P. Hepatic osteodystrophy. Trop Gastroenterol. 2010;31:82-86. [PubMed] [Cited in This Article: ] |
| 79. | Consensus development conference: prophylaxis and treatment of osteoporosis. Osteoporos Int. 1991;1:114-117. [PubMed] [Cited in This Article: ] |
| 80. | Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94:646-650. [PubMed] [Cited in This Article: ] |
| 81. | NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy, March 7-29, 2000: highlights of the conference. South Med J. 2001;94:569-573. [PubMed] [Cited in This Article: ] |
| 82. | Isaia G, Di Stefano M, Roggia C, Ardissone P, Rosina F. Bone disorders in cholestatic liver diseases. Forum (Genova). 1998;8:28-38. [PubMed] [Cited in This Article: ] |
| 83. | Kehayoglou K, Hadziyannis S, Kostamis P, Malamos B. The effect of medium-chain triglyceride on 47 calcium absorption in patients with primary biliary cirrhosis. Gut. 1973;14:653-656. [PubMed] [Cited in This Article: ] |
| 84. | Farias AQ, Gonçalves LL, Cançado EL, Seguro AC, Campos SB, Abrantes-Lemos CP, Carrilho FJ. Bone disease in primary biliary cirrhosis: lack of association with distal renal tubular acidosis. J Gastroenterol Hepatol. 2005;20:147-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 85. | Levy C, Lindor KD. Management of osteoporosis, fat-soluble vitamin deficiencies, and hyperlipidemia in primary biliary cirrhosis. Clin Liver Dis. 2003;7:901-910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 86. | Guañabens N, Parés A, Mariñoso L, Brancós MA, Piera C, Serrano S, Rivera F, Rodés J. Factors influencing the development of metabolic bone disease in primary biliary cirrhosis. Am J Gastroenterol. 1990;85:1356-1362. [PubMed] [Cited in This Article: ] |
| 87. | Verma A, Maxwell JD, Ang L, Davis T, Hodges S, Northfield TC, Zaidi M, Pazianas M. Ursodeoxycholic acid enhances fractional calcium absorption in primary biliary cirrhosis. Osteoporos Int. 2002;13:677-682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 88. | Lakatos PL, Bajnok E, Tornai I, Folhoffer A, Horváth A, Lakatos P, Szalay F. Decreased bone mineral density and gene polymorphism in primary biliary cirrhosis. Orv Hetil. 2004;145:331-336. [PubMed] [Cited in This Article: ] |
| 89. | Pereira SP, O’Donohue J, Moniz C, Phillips MG, Abraha H, Buxton-Thomas M, Williams R. Transdermal hormone replacement therapy improves vertebral bone density in primary biliary cirrhosis: results of a 1-year controlled trial. Aliment Pharmacol Ther. 2004;19:563-570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 90. | Phillips JR, Angulo P, Petterson T, Lindor KD. Fat-soluble vitamin levels in patients with primary biliary cirrhosis. Am J Gastroenterol. 2001;96:2745-2750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 91. | Sitrin MD, Bengoa JM. Intestinal absorption of cholecalciferol and 25-hydroxycholecalciferol in chronic cholestatic liver disease. Am J Clin Nutr. 1987;46:1011-1015. [PubMed] [Cited in This Article: ] |
| 92. | Eriksen EF, Kassem M, Langdahl B. Growth hormone, insulin-like growth factors and bone remodelling. Eur J Clin Invest. 1996;26:525-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 93. | Springer JE, Cole DE, Rubin LA, Cauch-Dudek K, Harewood L, Evrovski J, Peltekova VD, Heathcote EJ. Vitamin D-receptor genotypes as independent genetic predictors of decreased bone mineral density in primary biliary cirrhosis. Gastroenterology. 2000;118:145-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 117] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 94. | Adler RA, Rosen CJ. Glucocorticoids and osteoporosis. Endocrinol Metab Clin North Am. 1994;23:641-654. [PubMed] [Cited in This Article: ] |
| 95. | Chavassieux P, Pastoureau P, Chapuy MC, Delmas PD, Meunier PJ. Glucocorticoid-induced inhibition of osteoblastic bone formation in ewes: a biochemical and histomorphometric study. Osteoporos Int. 1993;3:97-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 88] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 96. | Libanati CR, Baylink DJ. Prevention and treatment of glucocorticoid-induced osteoporosis. A pathogenetic perspective. Chest. 1992;102:1426-1435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 97. | García-Suárez C, Crespo J, Fernández-Gil PL, Amado JA, García-Unzueta MT, Pons Romero F. [Plasma leptin levels in patients with primary biliary cirrhosis and their relationship with degree of fibrosis]. Gastroenterol Hepatol. 2004;27:47-50. [PubMed] [Cited in This Article: ] |
| 98. | Szalay F, Folhoffer A, Horváth A, Csak T, Speer G, Nagy Z, Lakatos P, Horváth C, Habior A, Tornai I. Serum leptin, soluble leptin receptor, free leptin index and bone mineral density in patients with primary biliary cirrhosis. Eur J Gastroenterol Hepatol. 2005;17:923-928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 99. | Selmi C, Meroni PL, Gershwin ME. Primary biliary cirrhosis and Sjögren’s syndrome: autoimmune epithelitis. J Autoimmun. 2012;39:34-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 100. | Kishor S, Turner ML, Borg BB, Kleiner DE, Cowen EW. Cutaneous sarcoidosis and primary biliary cirrhosis: A chance association or related diseases? J Am Acad Dermatol. 2008;58:326-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 101. | Gu EL, Yao GB. [The clinical characteristics of primary biliary cirrhosis in China: a systematic review]. Zhonghua Gan Zang Bing Za Zhi. 2009;17:861-866. [PubMed] [Cited in This Article: ] |
| 102. | Czaja AJ. The variant forms of autoimmune hepatitis. Ann Intern Med. 1996;125:588-598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 154] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 103. | Poupon R. Autoimmune overlapping syndromes. Clin Liver Dis. 2003;7:865-878. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 104. | Boberg KM, Chapman RW, Hirschfield GM, Lohse AW, Manns MP, Schrumpf E. Overlap syndromes: the International Autoimmune Hepatitis Group (IAIHG) position statement on a controversial issue. J Hepatol. 2011;54:374-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 350] [Cited by in F6Publishing: 315] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 105. | Coss Adame E, Granados J, Uribe M, Torre A. Does HLA-DR7 differentiate the overlap syndrome of auto-immune hepatitis-primary biliary cirrhosis (AIH-PBC) from those with auto-immune hepatitis type 1? Ann Hepatol. 2011;10:28-32. [PubMed] [Cited in This Article: ] |
| 106. | Wang Q, Selmi C, Zhou X, Qiu D, Li Z, Miao Q, Chen X, Wang J, Krawitt EL, Gershwin ME. Epigenetic considerations and the clinical reevaluation of the overlap syndrome between primary biliary cirrhosis and autoimmune hepatitis. J Autoimmun. 2013;41:140-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 107. | Tanaka A, Harada K, Ebinuma H, Komori A, Yokokawa J, Yoshizawa K, Abe M, Miyake Y, Kikuchi K, Ohira H. Primary biliary cirrhosis - Autoimmune hepatitis overlap syndrome: A rationale for corticosteroids use based on a nation-wide retrospective study in Japan. Hepatol Res. 2011;41:877-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 108. | Rust C, Beuers U. Overlap syndromes among autoimmune liver diseases. World J Gastroenterol. 2008;14:3368-3373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 89] [Cited by in F6Publishing: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 109. | Chazouillères O, Wendum D, Serfaty L, Montembault S, Rosmorduc O, Poupon R. Primary biliary cirrhosis-autoimmune hepatitis overlap syndrome: clinical features and response to therapy. Hepatology. 1998;28:296-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 506] [Cited by in F6Publishing: 443] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 110. | Talwalkar JA, Keach JC, Angulo P, Lindor KD. Overlap of autoimmune hepatitis and primary biliary cirrhosis: an evaluation of a modified scoring system. Am J Gastroenterol. 2002;97:1191-1197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 111. | Poupon R, Chazouilleres O, Corpechot C, Chrétien Y. Development of autoimmune hepatitis in patients with typical primary biliary cirrhosis. Hepatology. 2006;44:85-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 112. | Kobayashi M, Kakuda Y, Harada K, Sato Y, Sasaki M, Ikeda H, Terada M, Mukai M, Kaneko S, Nakanuma Y. Clinicopathological study of primary biliary cirrhosis with interface hepatitis compared to autoimmune hepatitis. World J Gastroenterol. 2014;20:3597-3608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 18] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 113. | Silveira MG, Talwalkar JA, Angulo P, Lindor KD. Overlap of autoimmune hepatitis and primary biliary cirrhosis: long-term outcomes. Am J Gastroenterol. 2007;102:1244-1250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 114. | Neuhauser M, Bjornsson E, Treeprasertsuk S, Enders F, Silveira M, Talwalkar J, Lindor K. Autoimmune hepatitis-PBC overlap syndrome: a simplified scoring system may assist in the diagnosis. Am J Gastroenterol. 2010;105:345-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 115. | Portman BC, Nakanuma Y. Diseases of bile ducts. MacSween’s pathology of the liver. 5th ed. London: Churchill Livingstone 2006; 517-581. [Cited in This Article: ] |
| 116. | Nakanuma Y, Saito K, Unoura M. Semiquantitative assessment of cholestasis and lymphocytic piecemeal necrosis in primary biliary cirrhosis: a histologic and immunohistochemical study. J Clin Gastroenterol. 1990;12:357-362. [PubMed] [Cited in This Article: ] |
| 117. | Gossard AA, Lindor KD. Development of autoimmune hepatitis in primary biliary cirrhosis. Liver Int. 2007;27:1086-1090. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 118. | Zhang Y, Lu J, Dai W, Wang F, Shen M, Yang J, Zhu R, Zhang H, Chen K, Cheng P. Combination therapy of ursodeoxycholic Acid and corticosteroids for primary biliary cirrhosis with features of autoimmune hepatitis: a meta-analysis. Gastroenterol Res Pract. 2013;2013:490731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 119. | Jones DE, Metcalf JV, Collier JD, Bassendine MF, James OF. Hepatocellular carcinoma in primary biliary cirrhosis and its impact on outcomes. Hepatology. 1997;26:1138-1142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 120. | Nijhawan PK, Therneau TM, Dickson ER, Boynton J, Lindor KD. Incidence of cancer in primary biliary cirrhosis: the Mayo experience. Hepatology. 1999;29:1396-1398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 72] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 121. | Piscaglia F, Sagrini E. Malignancies in primary biliary cirrhosis. Eur J Gastroenterol Hepatol. 2008;20:1-4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 122. | Lucey MR, Neuberger JM, Williams R. Primary biliary cirrhosis in men. Gut. 1986;27:1373-1376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 57] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 123. | Deutsch M, Papatheodoridis GV, Tzakou A, Hadziyannis SJ. Risk of hepatocellular carcinoma and extrahepatic malignancies in primary biliary cirrhosis. Eur J Gastroenterol Hepatol. 2008;20:5-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 124. | Floreani A, Farinati F. Risk factors associated with hepatocellular carcinoma in primary biliary cirrhosis. Hepatology. 2013;58:1520-1521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 125. | Yang Z, Liang Y, Zhong R. Reply to Risk factors associated with hepatocellular carcinoma in primary biliary cirrhosis. Hepatology. 2013;58:1521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 126. | Liang Y, Yang Z, Zhong R. Primary biliary cirrhosis and cancer risk: a systematic review and meta-analysis. Hepatology. 2012;56:1409-1417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 127. | Peng Y, Martin DA, Kenkel J, Zhang K, Ogden CA, Elkon KB. Innate and adaptive immune response to apoptotic cells. J Autoimmun. 2007;29:303-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 134] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 128. | Salunga TL, Cui ZG, Shimoda S, Zheng HC, Nomoto K, Kondo T, Takano Y, Selmi C, Alpini G, Gershwin ME. Oxidative stress-induced apoptosis of bile duct cells in primary biliary cirrhosis. J Autoimmun. 2007;29:78-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 129. | Walker JG, Doniach D, Roitt IM, Sherlock S. Serological tests in diagnosis of primary biliary cirrhosis. Lancet. 1965;1:827-831. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 341] [Cited by in F6Publishing: 379] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 130. | Jiang XH, Zhong RQ, Yu SQ, Hu Y, Li WW, Kong XT. Construction and expression of a humanized M2 autoantigen trimer and its application in the diagnosis of primary biliary cirrhosis. World J Gastroenterol. 2003;9:1352-1355. [PubMed] [Cited in This Article: ] |
| 131. | Ninomiya M, Kondo Y, Funayama R, Nagashima T, Kogure T, Kakazu E, Kimura O, Ueno Y, Nakayama K, Shimosegawa T. Distinct microRNAs expression profile in primary biliary cirrhosis and evaluation of miR 505-3p and miR197-3p as novel biomarkers. PLoS One. 2013;8:e66086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 132. | Braun S, Berg C, Buck S, Gregor M, Klein R. Catalytic domain of PDC-E2 contains epitopes recognized by antimitochondrial antibodies in primary biliary cirrhosis. World J Gastroenterol. 2010;16:973-981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 133. | Pasquali JL, Soulas-Sprauel P, Korganow AS, Martin T. Auto-reactive B cells in transgenic mice. J Autoimmun. 2007;29:250-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 134. | Rowley B, Tang L, Shinton S, Hayakawa K, Hardy RR. Autoreactive B-1 B cells: constraints on natural autoantibody B cell antigen receptors. J Autoimmun. 2007;29:236-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 135. | Invernizzi P, Lleo A, Podda M. Interpreting serological tests in diagnosing autoimmune liver diseases. Semin Liver Dis. 2007;27:161-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 136. | Lleo A, Selmi C, Invernizzi P, Podda M, Coppel RL, Mackay IR, Gores GJ, Ansari AA, Van de Water J, Gershwin ME. Apotopes and the biliary specificity of primary biliary cirrhosis. Hepatology. 2009;49:871-879. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 137. | Matsumura S, Van De Water J, Kita H, Coppel RL, Tsuji T, Yamamoto K, Ansari AA, Gershwin ME. Contribution to antimitochondrial antibody production: cleavage of pyruvate dehydrogenase complex-E2 by apoptosis-related proteases. Hepatology. 2002;35:14-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 138. | Selmi C, De Santis M, Cavaciocchi F, Gershwin ME. Infectious agents and xenobiotics in the etiology of primary biliary cirrhosis. Dis Markers. 2010;29:287-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 17] [Reference Citation Analysis (0)] |
| 139. | Loginov AS, Tsaregorodtseva TM, Zotina MM. The immune system and digestive diseases. Moscow: Meditsina Publishers 1986; 42-65. [Cited in This Article: ] |
| 140. | Tanaka A, Nalbandian G, Leung PS, Benson GD, Munoz S, Findor JA, Branch AD, Coppel RL, Ansari AA, Gershwin ME. Mucosal immunity and primary biliary cirrhosis: presence of antimitochondrial antibodies in urine. Hepatology. 2000;32:910-915. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 141. | Reynoso-Paz S, Leung PS, Van De Water J, Tanaka A, Munoz S, Bass N, Lindor K, Donald PJ, Coppel RL, Ansari AA. Evidence for a locally driven mucosal response and the presence of mitochondrial antigens in saliva in primary biliary cirrhosis. Hepatology. 2000;31:24-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 142. | Matsumura S, Van De Water J, Leung P, Odin JA, Yamamoto K, Gores GJ, Mostov K, Ansari AA, Coppel RL, Shiratori Y. Caspase induction by IgA antimitochondrial antibody: IgA-mediated biliary injury in primary biliary cirrhosis. Hepatology. 2004;39:1415-1422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 143. | Tanaka A, Nezu S, Uegaki S, Mikami M, Okuyama S, Kawamura N, Aiso M, Gershwin ME, Takahashi S, Selmi C. The clinical significance of IgA antimitochondrial antibodies in sera and saliva in primary biliary cirrhosis. Ann N Y Acad Sci. 2007;1107:259-270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 144. | Invernizzi P, Selmi C, Ranftler C, Podda M, Wesierska-Gadek J. Antinuclear antibodies in primary biliary cirrhosis. Semin Liver Dis. 2005;25:298-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 145. | Courvalin JC, Lassoued K, Bartnik E, Blobel G, Wozniak RW. The 210-kD nuclear envelope polypeptide recognized by human autoantibodies in primary biliary cirrhosis is the major glycoprotein of the nuclear pore. J Clin Invest. 1990;86:279-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 94] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 146. | Worman HJ, Courvalin JC. Antinuclear antibodies specific for primary biliary cirrhosis. Autoimmun Rev. 2003;2:211-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 147. | Wesierska-Gadek J, Hohenuer H, Hitchman E, Penner E. Autoantibodies against nucleoporin p62 constitute a novel marker of primary biliary cirrhosis. Gastroenterology. 1996;110:840-847. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 72] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 148. | Wesierska-Gadek J, Penner E, Battezzati PM, Selmi C, Zuin M, Hitchman E, Worman HJ, Gershwin ME, Podda M, Invernizzi P. Correlation of initial autoantibody profile and clinical outcome in primary biliary cirrhosis. Hepatology. 2006;43:1135-1144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 149. | Nakamura M, Kondo H, Mori T, Komori A, Matsuyama M, Ito M, Takii Y, Koyabu M, Yokoyama T, Migita K. Anti-gp210 and anti-centromere antibodies are different risk factors for the progression of primary biliary cirrhosis. Hepatology. 2007;45:118-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 247] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 150. | Rigopoulou EI, Davies ET, Bogdanos DP, Liaskos C, Mytilinaiou M, Koukoulis GK, Dalekos GN, Vergani D. Antimitochondrial antibodies of immunoglobulin G3 subclass are associated with a more severe disease course in primary biliary cirrhosis. Liver Int. 2007;27:1226-1231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 151. | Granito A, Yang WH, Muratori L, Lim MJ, Nakajima A, Ferri S, Pappas G, Quarneti C, Bianchi FB, Bloch DB. PML nuclear body component Sp140 is a novel autoantigen in primary biliary cirrhosis. Am J Gastroenterol. 2010;105:125-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 152. | Zhang FK, Jia JD, Wang BE. Clinical evaluation of serum antimitochondrial antibody-negative primary biliary cirrhosis. Hepatobiliary Pancreat Dis Int. 2004;3:288-291. [PubMed] [Cited in This Article: ] |
| 153. | Hu C, Deng C, Song G, Zhang W, Zhang S, Li X, Li P, Zhang F, Li Y. Prevalence of autoimmune liver disease related autoantibodies in Chinese patients with primary biliary cirrhosis. Dig Dis Sci. 2011;56:3357-3363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 154. | Oertelt S, Rieger R, Selmi C, Invernizzi P, Ansari AA, Coppel RL, Podda M, Leung PS, Gershwin ME. A sensitive bead assay for antimitochondrial antibodies: Chipping away at AMA-negative primary biliary cirrhosis. Hepatology. 2007;45:659-665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 155. | Bizzaro N, Covini G, Rosina F, Muratori P, Tonutti E, Villalta D, Pesente F, Alessio MG, Tampoia M, Antico A. Overcoming a “probable” diagnosis in antimitochondrial antibody negative primary biliary cirrhosis: study of 100 sera and review of the literature. Clin Rev Allergy Immunol. 2012;42:288-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 156. | Kikuchi K, Hsu W, Hosoya N, Moritoki Y, Kajiyama Y, Kawai T, Takai A, Hayami E, Selmi C, Gershwin ME. Ursodeoxycholic acid reduces CpG-induced IgM production in patients with primary biliary cirrhosis. Hepatol Res. 2009;39:448-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 157. | Hirschfield GM, Liu X, Xu C, Lu Y, Xie G, Lu Y, Gu X, Walker EJ, Jing K, Juran BD. Primary biliary cirrhosis associated with HLA, IL12A, and IL12RB2 variants. N Engl J Med. 2009;360:2544-2555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 475] [Cited by in F6Publishing: 444] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 158. | Hirschfield GM, Liu X, Han Y, Gorlov IP, Lu Y, Xu C, Lu Y, Chen W, Juran BD, Coltescu C. Variants at IRF5-TNPO3, 17q12-21 and MMEL1 are associated with primary biliary cirrhosis. Nat Genet. 2010;42:655-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 177] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 159. | Hirschfield GM, Xie G, Lu E, Sun Y, Juran BD, Chellappa V, Coltescu C, Mason AL, Milkiewicz P, Myers RP. Association of primary biliary cirrhosis with variants in the CLEC16A, SOCS1, SPIB and SIAE immunomodulatory genes. Genes Immun. 2012;13:328-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 160. | Mells GF, Floyd JA, Morley KI, Cordell HJ, Franklin CS, Shin SY, Heneghan MA, Neuberger JM, Donaldson PT, Day DB. Genome-wide association study identifies 12 new susceptibility loci for primary biliary cirrhosis. Nat Genet. 2011;43:329-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 368] [Cited by in F6Publishing: 370] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 161. | Invernizzi P, Selmi C, Mackay IR, Podda M, Gershwin ME. From bases to basis: linking genetics to causation in primary biliary cirrhosis. Clin Gastroenterol Hepatol. 2005;3:401-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 162. | Invernizzi P, Battezzati PM, Crosignani A, Perego F, Poli F, Morabito A, De Arias AE, Scalamogna M, Zuin M, Podda M. Peculiar HLA polymorphisms in Italian patients with primary biliary cirrhosis. J Hepatol. 2003;38:401-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 163. | Donaldson PT, Baragiotta A, Heneghan MA, Floreani A, Venturi C, Underhill JA, Jones DE, James OF, Bassendine MF. HLA class II alleles, genotypes, haplotypes, and amino acids in primary biliary cirrhosis: a large-scale study. Hepatology. 2006;44:667-674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 164. | Qin B, Wang J, Chen J, Liang Y, Yang Z, Zhong R. Association of human leukocyte antigen class II with susceptibility to primary biliary cirrhosis: a systematic review and meta-analysis. PLoS One. 2013;8:e79580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 165. | Van de Water J, Ansari A, Prindiville T, Coppel RL, Ricalton N, Kotzin BL, Liu S, Roche TE, Krams SM, Munoz S. Heterogeneity of autoreactive T cell clones specific for the E2 component of the pyruvate dehydrogenase complex in primary biliary cirrhosis. J Exp Med. 1995;181:723-733. [PubMed] [Cited in This Article: ] |
| 166. | Shimoda S, Nakamura M, Ishibashi H, Hayashida K, Niho Y. HLA DRB4 0101-restricted immunodominant T cell autoepitope of pyruvate dehydrogenase complex in primary biliary cirrhosis: evidence of molecular mimicry in human autoimmune diseases. J Exp Med. 1995;181:1835-1845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 232] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 167. | Jones DE, Palmer JM, James OF, Yeaman SJ, Bassendine MF, Diamond AG. T-cell responses to the components of pyruvate dehydrogenase complex in primary biliary cirrhosis. Hepatology. 1995;21:995-1002. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 168. | Shimoda S, Van de Water J, Ansari A, Nakamura M, Ishibashi H, Coppel RL, Lake J, Keeffe EB, Roche TE, Gershwin ME. Identification and precursor frequency analysis of a common T cell epitope motif in mitochondrial autoantigens in primary biliary cirrhosis. J Clin Invest. 1998;102:1831-1840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 195] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 169. | Kita H, Matsumura S, He XS, Ansari AA, Lian ZX, Van de Water J, Coppel RL, Kaplan MM, Gershwin ME. Quantitative and functional analysis of PDC-E2-specific autoreactive cytotoxic T lymphocytes in primary biliary cirrhosis. J Clin Invest. 2002;109:1231-1240. [PubMed] [Cited in This Article: ] |
| 170. | Kita H, Naidenko OV, Kronenberg M, Ansari AA, Rogers P, He XS, Koning F, Mikayama T, Van De Water J, Coppel RL. Quantitation and phenotypic analysis of natural killer T cells in primary biliary cirrhosis using a human CD1d tetramer. Gastroenterology. 2002;123:1031-1043. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 186] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 171. | Wakabayashi K, Lian ZX, Moritoki Y, Lan RY, Tsuneyama K, Chuang YH, Yang GX, Ridgway W, Ueno Y, Ansari AA. IL-2 receptor alpha(-/-) mice and the development of primary biliary cirrhosis. Hepatology. 2006;44:1240-1249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 193] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 172. | Aoki CA, Roifman CM, Lian ZX, Bowlus CL, Norman GL, Shoenfeld Y, Mackay IR, Gershwin ME. IL-2 receptor alpha deficiency and features of primary biliary cirrhosis. J Autoimmun. 2006;27:50-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 136] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 173. | Irie J, Wu Y, Wicker LS, Rainbow D, Nalesnik MA, Hirsch R, Peterson LB, Leung PS, Cheng C, Mackay IR. NOD.c3c4 congenic mice develop autoimmune biliary disease that serologically and pathogenetically models human primary biliary cirrhosis. J Exp Med. 2006;203:1209-1219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 174. | Lan RY, Cheng C, Lian ZX, Tsuneyama K, Yang GX, Moritoki Y, Chuang YH, Nakamura T, Saito S, Shimoda S. Liver-targeted and peripheral blood alterations of regulatory T cells in primary biliary cirrhosis. Hepatology. 2006;43:729-737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 220] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 175. | Wang D, Zhang H, Liang J, Gu Z, Zhou Q, Fan X, Hou Y, Sun L. CD4+ CD25+ but not CD4+ Foxp3+ T cells as a regulatory subset in primary biliary cirrhosis. Cell Mol Immunol. 2010;7:485-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 176. | Amadori A, Zamarchi R, De Silvestro G, Forza G, Cavatton G, Danieli GA, Clementi M, Chieco-Bianchi L. Genetic control of the CD4/CD8 T-cell ratio in humans. Nat Med. 1995;1:1279-1283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 304] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 177. | Liu B, Shi XH, Zhang FC, Zhang W, Gao LX. Antimitochondrial antibody-negative primary biliary cirrhosis: a subset of primary biliary cirrhosis. Liver Int. 2008;28:233-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 178. | Hsu W, Zhang W, Tsuneyama K, Moritoki Y, Ridgway WM, Ansari AA, Coppel RL, Lian ZX, Mackay I, Gershwin ME. Differential mechanisms in the pathogenesis of autoimmune cholangitis versus inflammatory bowel disease in interleukin-2Ralpha(-/-) mice. Hepatology. 2009;49:133-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 179. | Graham AM, Dollinger MM, Howie SE, Harrison DJ. Bile duct cells in primary biliary cirrhosis are ‘primed’ for apoptosis. Eur J Gastroenterol Hepatol. 1998;10:553-557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 180. | Tinmouth J, Lee M, Wanless IR, Tsui FW, Inman R, Heathcote EJ. Apoptosis of biliary epithelial cells in primary biliary cirrhosis and primary sclerosing cholangitis. Liver. 2002;22:228-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 181. | Kita H, Lian ZX, Van de Water J, He XS, Matsumura S, Kaplan M, Luketic V, Coppel RL, Ansari AA, Gershwin ME. Identification of HLA-A2-restricted CD8(+) cytotoxic T cell responses in primary biliary cirrhosis: T cell activation is augmented by immune complexes cross-presented by dendritic cells. J Exp Med. 2002;195:113-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 230] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 182. | Matsumura S, Kita H, He XS, Ansari AA, Lian ZX, Van De Water J, Yamamoto K, Tsuji T, Coppel RL, Kaplan M. Comprehensive mapping of HLA-A0201-restricted CD8 T-cell epitopes on PDC-E2 in primary biliary cirrhosis. Hepatology. 2002;36:1125-1134. [PubMed] [Cited in This Article: ] |
| 183. | Reshetnyak VI. Physiological and molecular biochemical mechanisms of bile formation. World J Gastroenterol. 2013;19:7341-7360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 69] [Cited by in F6Publishing: 58] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 184. | Reshetnyak VI. Physiological and molecular biochemical mechanisms of bile formation. World Clinical Gastroenterology (WCG). 1st ed. Pleasanton (CA): Baishideng Publishing Group 2014; 37-56. [Cited in This Article: ] |
| 185. | Sokol RJ, Winklhofer-Roob BM, Devereaux MW, McKim JM. Generation of hydroperoxides in isolated rat hepatocytes and hepatic mitochondria exposed to hydrophobic bile acids. Gastroenterology. 1995;109:1249-1256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 214] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 186. | Sokol RJ, Straka MS, Dahl R, Devereaux MW, Yerushalmi B, Gumpricht E, Elkins N, Everson G. Role of oxidant stress in the permeability transition induced in rat hepatic mitochondria by hydrophobic bile acids. Pediatr Res. 2001;49:519-531. [PubMed] [Cited in This Article: ] |
| 187. | Sokol RJ, Dahl R, Devereaux MW, Yerushalmi B, Kobak GE, Gumpricht E. Human hepatic mitochondria generate reactive oxygen species and undergo the permeability transition in response to hydrophobic bile acids. J Pediatr Gastroenterol Nutr. 2005;41:235-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 188. | Molostova LV. Secretion and metabolism of bile acids in different forms of their impaired hepatoenteric circulation and in chronic liver diseases (Experimental and clinical studies). Dissertation for the academic degree of doctor of biological sciences. Moscow: biological sciences 1982; 284. [Cited in This Article: ] |
| 189. | Greim H, Trülzsch D, Roboz J, Dressler K, Czygan P, Hutterer F, Schaffner F, Popper H. Mechanism of cholestasis. 5. Bile acids in normal rat livers and in those after bile duct ligation. Gastroenterology. 1972;63:837-845. [PubMed] [Cited in This Article: ] |
| 190. | Greim H, Trülzsch D, Czygan P, Rudick J, Hutterer F, Schaffner F, Popper H. Mechanism of cholestasis. 6. Bile acids in human livers with or without biliary obstruction. Gastroenterology. 1972;63:846-850. [PubMed] [Cited in This Article: ] |
| 191. | García-Marín JJ, González J, Esteller A. Influence of dehydrocholate on bilirubin transport into bile in the rat. Digestion. 1986;33:80-88. [PubMed] [Cited in This Article: ] |
| 192. | Alme B, Bremmelgaard A, Sjovall J, Thomassen G. Complexity of bile acid mixture in human urine. Stutgart, New York: Advences in bile acid research 1974; 145-148. [Cited in This Article: ] |
| 193. | Roda E, Cipolla A, Salzetta A, Marchetto S, Pezzoli A, Accogli E, Novelli V, Polimeni C, Cerrè C, Mazzella G. Influence of ursodeoxycholic acid on biliary lipids. Scand J Gastroenterol Suppl. 1994;204:16-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 194. | Alvaro D, Angelico M, Cantafora A, Di Biase A, De Santis A, Bracci F, Minervini G, Ginanni Corradini S, Attili AF, Capocaccia L. Biliary secretion of phosphatidylcholine and its molecular species in cholecystectomized T-tube patients: effects of bile acid hydrophilicity. Biochem Med Metab Biol. 1986;36:125-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 195. | Carulli N, Bertolotti M, Carubbi F, Concari M, Martella P, Carulli L, Loria P. Review article: effect of bile salt pool composition on hepatic and biliary functions. Aliment Pharmacol Ther. 2000;14 Suppl 2:14-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 196. | Reshetnyak VI, Ozerova IN, Perova NV, Ilchenko LIu, Tkachev VD, Golovanova EV, Vinnickaya EV. Investigation of the lipid composition of liver cells and serum in patients with primary biliary cirrhosis (PBC). Ross Gastroenterol Zh. 2001;143-144. [Cited in This Article: ] |
| 197. | Longo M, Crosignani A, Battezzati PM, Squarcia Giussani C, Invernizzi P, Zuin M, Podda M. Hyperlipidaemic state and cardiovascular risk in primary biliary cirrhosis. Gut. 2002;51:265-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 141] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 198. | Kanda T, Yokosuka O, Kojima H, Imazeki F, Nagao K, Tatsuno I, Saito Y, Saisho H. Severe hypercholesterolemia associated with primary biliary cirrhosis in a 44-year-old Japanese woman. World J Gastroenterol. 2004;10:2607-2608. [PubMed] [Cited in This Article: ] |
| 199. | Stiehl A. Disturbances of bile acid metabolism in cholestasis. Clin Gastroenterol. 1977;6:45-67. [PubMed] [Cited in This Article: ] |
| 200. | Markova MN. Cholestatic hyperlipidemia in patients with primary biliary cirrhosis. Collected Articles. Vilnius: “Gastroenterology-78” 1978; 150-152. [Cited in This Article: ] |
| 201. | Molostova LV. Hyperlipidemia in chronic intrahepatic cholestasis. Collected Articles. Moscow: “Chronic Hepatitis ” 1988; 51-59. [Cited in This Article: ] |
| 202. | Allocca M, Crosignani A, Gritti A, Ghilardi G, Gobatti D, Caruso D, Zuin M, Podda M, Battezzati PM. Hypercholesterolaemia is not associated with early atherosclerotic lesions in primary biliary cirrhosis. Gut. 2006;55:1795-1800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 203. | Nguyen KD, Sundaram V, Ayoub WS. Atypical causes of cholestasis. World J Gastroenterol. 2014;20:9418-9426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 51] [Cited by in F6Publishing: 47] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 204. | Levitt DG, Levitt MD. Quantitative assessment of the multiple processes responsible for bilirubin homeostasis in health and disease. Clin Exp Gastroenterol. 2014;7:307-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 205. | Bénichou C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol. 1990;11:272-276. [PubMed] [Cited in This Article: ] |
| 206. | Jung HE, Jang JY, Jeong SW, Kim JN, Jang HY, Cho YJ, Woo SA, Lee SH, Kim SG, Cha SW. Prognostic indicators in primary biliary cirrhosis: significance of revised IAHG (International Autoimmune Hepatitis Group) score. Clin Mol Hepatol. 2012;18:375-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 207. | Shapiro JM, Smith H, Schaffner F. Serum bilirubin: a prognostic factor in primary biliary cirrhosis. Gut. 1979;20:137-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 241] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 208. | van Hoogstraten HJ, Hansen BE, van Buuren HR, ten Kate FJ, van Berge-Henegouwen GP, Schalm SW. Prognostic factors and long-term effects of ursodeoxycholic acid on liver biochemical parameters in patients with primary biliary cirrhosis. Dutch Multi-Centre PBC Study Group. J Hepatol. 1999;31:256-262. [PubMed] [Cited in This Article: ] |
| 209. | Angulo P, Lindor KD, Therneau TM, Jorgensen RA, Malinchoc M, Kamath PS, Dickson ER. Utilization of the Mayo risk score in patients with primary biliary cirrhosis receiving ursodeoxycholic acid. Liver. 1999;19:115-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 136] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 210. | Dickson ER, Grambsch PM, Fleming TR, Fisher LD, Langworthy A. Prognosis in primary biliary cirrhosis: model for decision making. Hepatology. 1989;10:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 588] [Cited by in F6Publishing: 516] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 211. | Parés A, Rodés J. Natural history of primary biliary cirrhosis. Clin Liver Dis. 2003;7:779-794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 212. | Loginov AS. Intrahepatic cholestasis: A cardinal problem of modern hepatology. Collected Articles. Moscow: “Topical aspects of gastroenterology” 1977; 3-12. [Cited in This Article: ] |
| 213. | Zhang F, Jia J, Wang B, Qian L, Yin S, Wang Y, Cui Y, You H, Ma H, Wang H. [Clinical characteristics of primary biliary cirrhosis: a report of 45 cases]. Zhonghua Nei Ke Za Zhi. 2002;41:163-167. [PubMed] [Cited in This Article: ] |
| 214. | Muratori P, Granito A, Pappas G, Muratori L, Quarneti C, De Molo C, Cipriano V, Vukotic R, Andreone P, Lenzi M. Clinical and serological profile of primary biliary cirrhosis in men. QJM. 2007;100:534-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 215. | Metzler DE. Biochemistry. V.2. Moscow: Mir Publishers 1980; 119. [Cited in This Article: ] |
| 216. | Hatoff DE, Toyota N, Wong C, Miller AL, Takeya M, Miyai K. Rat liver alkaline phosphatases. Evidence hepatocyte and portal triad enzymes differ. Dig Dis Sci. 1985;30:564-572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 217. | Kaplan MM, Righetti A. Induction of rat liver alkaline phosphatase: the mechanism of the serum elevation in bile duct obstruction. J Clin Invest. 1970;49:508-516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 177] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 218. | Kryszewski AJ, Neale G, Whitfield JB, Moss DW. Enzyme changes in experimental biliary obstruction. Clin Chim Acta. 1973;47:175-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 54] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 219. | Zhang F, Jia J, Cui R, Wang B, Wang H. Clinical features of forty patients with primary biliary cirrhosis. Chin Med J (Engl). 2002;115:904-908. [PubMed] [Cited in This Article: ] |
| 220. | Floreani A, Cazzagon N, Boemo DG, Baldovin T, Baldo V, Egoue J, Antoniazzi S, Minola E. Female patients in fertile age with chronic hepatitis C, easy genotype, and persistently normal transaminases have a 100% chance to reach a sustained virological response. Eur J Gastroenterol Hepatol. 2011;23:997-1003. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 221. | Williams AL, Hoofnagle JH. Ratio of serum aspartate to alanine aminotransferase in chronic hepatitis. Relationship to cirrhosis. Gastroenterology. 1988;95:734-739. [PubMed] [Cited in This Article: ] |
| 222. | Sebastiani G, Alberti A. Non invasive fibrosis biomarkers reduce but not substitute the need for liver biopsy. World J Gastroenterol. 2006;12:3682-3694. [PubMed] [Cited in This Article: ] |
| 223. | Nyblom H, Berggren U, Balldin J, Olsson R. High AST/ALT ratio may indicate advanced alcoholic liver disease rather than heavy drinking. Alcohol Alcohol. 2004;39:336-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 258] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 224. | Nyblom H, Björnsson E, Simrén M, Aldenborg F, Almer S, Olsson R. The AST/ALT ratio as an indicator of cirrhosis in patients with PBC. Liver Int. 2006;26:840-845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 225. | Park GJ, Lin BP, Ngu MC, Jones DB, Katelaris PH. Aspartate aminotransferase: alanine aminotransferase ratio in chronic hepatitis C infection: is it a useful predictor of cirrhosis? J Gastroenterol Hepatol. 2000;15:386-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 115] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 226. | Loginov AS, Iamskova VP, Tumanova NB, Tkachev VD, Reshetniak VI. [Study of hepatocyte adhesion in chronic diffuse diseases of the liver]. Biull Eksp Biol Med. 1989;108:160-162. [PubMed] [Cited in This Article: ] |
| 227. | Loginov AS, Aruin LI. Clinical morphology of the liver. Moscow: Meditsina Publishers 1985; 181. [Cited in This Article: ] |
| 228. | Smallwood RA, Williams HA, Rosenoer VM, Sherlock S. Liver-copper levels in liver disease: studies using neutron activation analysis. Lancet. 1968;2:1310-1313. [PubMed] [Cited in This Article: ] |
| 229. | Schwabe U, Friedrich K. Significance of the iron and copper content of the liver for the differential diagnosis of chronic liver diseases. Z Gastroenterol. 1990;28:353-357. [PubMed] [Cited in This Article: ] |
| 230. | Epstein O, Arborgh B, Sagiv M, Wroblewski R, Scheuer PJ, Sherlock S. Is copper hepatotoxic in primary biliary cirrhosis? J Clin Pathol. 1981;34:1071-1075. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 231. | Goldfischer S, Popper H, Sternlieb I. The significance of variations in the distribution of copper in liver disease. Am J Pathol. 1980;99:715-730. [PubMed] [Cited in This Article: ] |
| 232. | Myszor M, James OF. The epidemiology of primary biliary cirrhosis in north-east England: an increasingly common disease? Q J Med. 1990;75:377-385. [PubMed] [Cited in This Article: ] |
| 233. | Popper H, Schaffner F. Nonsuppurative destructive chronic cholangitis and chronic hepatitis. Prog Liver Dis. 1970;3:336-354. [PubMed] [Cited in This Article: ] |
| 234. | Scheuer P. Liver biopsy interpretation. London: Bailliere Tindall 1974; 172. [Cited in This Article: ] |
| 235. | Ludwig J, Dickson ER, McDonald GS. Staging of chronic nonsuppurative destructive cholangitis (syndrome of primary biliary cirrhosis). Virchows Arch A Pathol Anat Histol. 1978;379:103-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 586] [Cited by in F6Publishing: 518] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 236. | Afdhal NH, Nunes D. Evaluation of liver fibrosis: a concise review. Am J Gastroenterol. 2004;99:1160-1174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 424] [Cited by in F6Publishing: 410] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 237. | Leuschner U. Autoimmunkrankheiten der Leber und Overlapsyndrome. 2 Auflage. Bremen: UNIMED 2005; 48-64. [Cited in This Article: ] |
| 238. | Klöppel G, Kirchhof M, Berg PA. Natural course of primary biliary cirrhosis. I. A morphological, clinical and serological analysis of 103 cases. Liver. 1982;2:141-151. [PubMed] [Cited in This Article: ] |
| 239. | Roschlau G. Morphology and differential diagnosis of primary biliary cirrhosis and its pre-stages. Dtsch Z Verdau Stoffwechselkr. 1976;36:113-115. [PubMed] [Cited in This Article: ] |
| 240. | Sherlock S. Primary billiary cirrhosis (chronic intrahepatic obstructive jaundice). Gastroenterology. 1959;37:574-586. [PubMed] [Cited in This Article: ] |
| 241. | Portmann BC, Nakanuma Y. Diseases affecting primarily the intrahepatic bile ducts. MacSween’s pathology of the liver. Philadelphia: Churchill Livingston (Elsevier) 2007; 535-548. [Cited in This Article: ] |
| 242. | Loginov AS, Aruin LI, Shepeleva SD. Protein-copper complex in primary biliary cirrhosis and other chronic diffuse diseases of the liver. Arkh Patol. 1980;42:50-55. [PubMed] [Cited in This Article: ] |
| 243. | Rubel LR, Rabin L, Seeff LB, Licht H, Cuccherini BA. Does primary biliary cirrhosis in men differ from primary biliary cirrhosis in women? Hepatology. 1984;4:671-677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 244. | Hubscher SG. Histological classification of autoimmune cholestatic liver diseases. Kluwer, Dordrecht, Boston, London: Immunology and liver 2000; 223-243. [Cited in This Article: ] |
| 245. | Kaplan MM. Primary biliary cirrhosis: past, present, and future. Gastroenterology. 2002;123:1392-1394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 246. | Yeh WC, Li PC, Jeng YM, Hsu HC, Kuo PL, Li ML, Yang PM, Lee PH. Elastic modulus measurements of human liver and correlation with pathology. Ultrasound Med Biol. 2002;28:467-474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 329] [Cited by in F6Publishing: 285] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 247. | Takeda T, Yasuda T, Nakayama Y, Nakaya M, Kimura M, Yamashita M, Sawada A, Abo K, Takeda S, Sakaguchi H. Usefulness of noninvasive transient elastography for assessment of liver fibrosis stage in chronic hepatitis C. World J Gastroenterol. 2006;12:7768-7773. [PubMed] [Cited in This Article: ] |
| 248. | Heathcote EJ. Management of primary biliary cirrhosis. The American Association for the Study of Liver Diseases practice guidelines. Hepatology. 2000;31:1005-1013. [PubMed] [Cited in This Article: ] |
| 249. | Biagini MR, Tozzi A, Marcucci R, Paniccia R, Fedi S, Milani S, Galli A, Ceni E, Capanni M, Manta R. Hyperhomocysteinemia and hypercoagulability in primary biliary cirrhosis. World J Gastroenterol. 2006;12:1607-1612. [PubMed] [Cited in This Article: ] |
| 250. | Talwalkar JA, Lindor KD. Primary biliary cirrhosis. Lancet. 2003;362:53-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 251] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 251. | Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, Moore JP, Fennerty MB, Ryan ME, Shaw MJ. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909-918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1716] [Cited by in F6Publishing: 1607] [Article Influence: 57.4] [Reference Citation Analysis (2)] |
| 252. | Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, Bittencourt PL, Porta G, Boberg KM, Hofer H. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1205] [Cited by in F6Publishing: 1132] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 253. | Daniels JA, Torbenson M, Anders RA, Boitnott JK. Immunostaining of plasma cells in primary biliary cirrhosis. Am J Clin Pathol. 2009;131:243-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 254. | Moreira RK, Revetta F, Koehler E, Washington MK. Diagnostic utility of IgG and IgM immunohistochemistry in autoimmune liver disease. World J Gastroenterol. 2010;16:453-457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 33] [Cited by in F6Publishing: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 255. | Heurgué A, Vitry F, Diebold MD, Yaziji N, Bernard-Chabert B, Pennaforte JL, Picot R, Louvet H, Frémond L, Geoffroy P. Overlap syndrome of primary biliary cirrhosis and autoimmune hepatitis: a retrospective study of 115 cases of autoimmune liver disease. Gastroenterol Clin Biol. 2007;31:17-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 256. | Kim WR, Lindor KD, Locke GR, Therneau TM, Homburger HA, Batts KP, Yawn BP, Petz JL, Melton LJ, Dickson ER. Epidemiology and natural history of primary biliary cirrhosis in a US community. Gastroenterology. 2000;119:1631-1636. [PubMed] [Cited in This Article: ] |
| 257. | Poupon RE, Lindor KD, Cauch-Dudek K, Dickson ER, Poupon R, Heathcote EJ. Combined analysis of randomized controlled trials of ursodeoxycholic acid in primary biliary cirrhosis. Gastroenterology. 1997;113:884-890. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 477] [Cited by in F6Publishing: 408] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 258. | Shi J, Wu C, Lin Y, Chen YX, Zhu L, Xie WF. Long-term effects of mid-dose ursodeoxycholic acid in primary biliary cirrhosis: a meta-analysis of randomized controlled trials. Am J Gastroenterol. 2006;101:1529-1538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 259. | Poupon RE, Bonnand AM, Chrétien Y, Poupon R. Ten-year survival in ursodeoxycholic acid-treated patients with primary biliary cirrhosis. The UDCA-PBC Study Group. Hepatology. 1999;29:1668-1671. [PubMed] [Cited in This Article: ] |
| 260. | Corpechot C, Poupon R. Geotherapeutics of primary biliary cirrhosis: bright and sunny around the Mediterranean but still cloudy and foggy in the United Kingdom. Hepatology. 2007;46:963-965. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 261. | Christensen E, Crowe J, Doniach D, Popper H, Ranek L, Rodés J, Tygstrup N, Williams R. Clinical pattern and course of disease in primary biliary cirrhosis based on an analysis of 236 patients. Gastroenterology. 1980;78:236-246. [PubMed] [Cited in This Article: ] |
| 262. | Gershwin ME, Nishio A, Isibashi H, Lindor K. Primary biliary cirrhosis. Liver immunology. Philadelphia: Hanley & Belfus Inc 2003; 311-328. [Cited in This Article: ] |