Published online Jun 28, 2015. doi: 10.3748/wjg.v21.i24.7478
Peer-review started: January 2, 2015
First decision: January 22, 2015
Revised: March 2, 2015
Accepted: April 28, 2015
Article in press: April 28, 2015
Published online: June 28, 2015
AIM: To investigate the association between liver markers and the risk of type 2 diabetes (T2DM) and impaired fasting glucose (IFG).
METHODS: A total of 8863 participants (3408 men and 5455 women) over 30 years of age were analyzed from the fifth Korean National Health and Nutrition Examination Survey (2010-2011). The associations of serum liver markers such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), AST/ALT, and gamma-glutamyltransferase (GGT) with T2DM and IFG were analyzed using logistic regression models. Participants were divided into sex-specific quartiles on the basis of liver markers.
RESULTS: The prevalence of T2DM and IFG were 11.3% and 18.3%. Increasing quartiles of ALT and GGT were positively and AST/ALT were negatively correlated with T2DM and IFG. Analysis of the liver marker combinations showed that if any two or more markers were in the highest risk quartile, the risks of both T2DM and IFG increased significantly. The risk was greatest when the highest ALT and GGT and lowest AST/ALT quartile were combined, with the risk of T2DM at 3.21 (95%CI: 1.829-5.622, P < 0.001) in men and 4.60 (95%CI: 3.217-6.582, P < 0.001) in women. Men and women with the highest AST and ALT and lowest AST/ALT quartile had a 1.99 and 2.40 times increased risk of IFG.
CONCLUSION: Higher levels of GGT and ALT and lower AST/ALT within the physiological range are independent, additive risk factors of T2DM and IFG.
Core tip: We investigated the association between liver markers and the risk of type 2 diabetes (T2DM) and impaired fasting glucose (IFG) in a general Korean population. Increasing quartiles of alanine aminotransferase (ALT) and gamma-glutamyltransferase (GGT) were positively and aspartate aminotransferase (AST)/ALT was negatively correlated with both T2DM and IFG. Analysis of the liver marker combinations showed that if any two or more markers were in the highest risk quartile, the risks of both T2DM and IFG increased significantly. Higher levels of GGT and ALT and lower AST/ALT within the physiological range are independent, additive risk factors of T2DM and IFG.
- Citation: Ko SH, Baeg MK, Han KD, Ko SH, Ahn YB. Increased liver markers are associated with higher risk of type 2 diabetes. World J Gastroenterol 2015; 21(24): 7478-7487
- URL: https://www.wjgnet.com/1007-9327/full/v21/i24/7478.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i24.7478
The liver has been implicated in the pathogenesis of type 2 diabetes (T2DM). It plays an important role in the maintenance of normal glucose levels, and hepatic dysfunction resulting from insulin resistance syndrome has been suggested as leading to T2DM.
Liver enzymes such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyltransferase (GGT) are considered as surrogate markers of liver function. Recently, an elevated serum GGT level has been reported as an important risk factor in the development of impaired fasting glucose (IFG), T2DM, cardiovascular disease, and metabolic syndrome (MetS)[1-6]. AST and ALT have also been reported to be associated with MetS and T2DM[4,7-11]. There have been some reports that when AST, ALT, and GGT were tested, only GGT remained associated with T2DM[7,9]. A recent meta-analysis reported that both ALT and GGT elevation were associated with increased risk of T2DM, and that GGT might be a stronger risk factor than ALT[12].
In spite of this evidence, it is not fully understood which liver enzymes are better indicators of T2DM, whether the relationships are consistent in prediabetes states such as IFG, and whether liver markers have incremental predictive effects for T2DM. Therefore, this study aimed to (1) compare the relationships of AST, ALT, and GGT with both T2DM and IFG in a nationally representative sample of Korean adults; and (2) determine whether AST, ALT, and GGT have an incremental effect on the prevalence of T2DM and IFG.
This cross-sectional study was based on the fifth Korean National Health and Nutrition Examination Survey (KNHANES V) conducted from 2010 to 2011. The KNHANES is a nationally representative, cross-sectional survey designed to estimate the health and nutritional status of the Korean population, as determined by the Korea Centers for Disease Control and Prevention (KCDCP). The KNHANES consisted of a health interview, health examination, and nutrition survey. Additional details regarding the study design and methods have been described elsewhere[13,14]. KNHANES V used a rolling sampling design with stratified multistage cluster probability sampling, which was certified as being representative of the Korean population by the Korean Department of Statistics.
Participants older than 30 years of age were included in this study. Exclusion criteria were a history of malignancy, hepatitis B or C, liver cirrhosis, alcohol intake > 30 g/d, pregnancy, or missing data for variables included in the analysis.
The survey protocol was conducted according to the Declaration of Helsinki and was approved by the Institutional Review Board of the Catholic University Medical Center (IRB No. VC14EISI0212). All participants in the survey signed informed consent forms.
Anthropometric measurements of the participants were taken by specially trained examiners. Waist circumference (WC) was measured at the narrowest point between the lower borders of the rib cage and the iliac crest. Blood pressure (BP) was measured three times using a mercury sphygmomanometer (Baumanometer; Baum, Copiague, NY, United States), and the mean value of the second and third measurements was used.
Questionnaires were used to collect demographic information such as age, sex, residential district (urban, rural), and medical history. Alcohol consumption was categorized into nondrinkers or mild-to-moderate drinkers (1.0-30.0 g alcohol/d). Cigarette smoking was classified as ‘‘yes’’ for participants who had smoked ≥ 100 cigarettes and ‘‘no’’ for those who had smoked < 100 cigarettes during their lifetime. Regular exercise was defined as moderate physical activity ≥ 30 min per day for > 5 d per week and/or strenuous physical activity for ≥ 20 min per day for > 3 d per week. Education level was classified as middle school or lower, high school, and college or higher. Household income was divided into quartiles based on per capita household income.
Blood samples were collected in the morning after ≥ 8 h of fasting. Samples were appropriately processed, refrigerated at 2-8 °C, and analyzed within 24 h at the Central Testing Institute in Seoul, Korea. Analyses of fasting glucose, AST, ALT, GGT, total cholesterol, high density lipoprotein cholesterol, low density lipoprotein cholesterol, and triglycerides (TG) were performed using a Hitachi Automatic Analyzer 7600 (Hitachi 7600, Tokyo, Japan).
Individuals with T2DM were defined as those with fasting plasma glucose (FPG) ≥ 126 mg/dL, a previous diagnosis of T2DM by a physician, or those taking antidiabetes medication. Impaired fasting glucose (IFG) was classified as FPG between 100 and 125 mg/dL. Hypertension was defined as a systolic BP ≥ 140 mmHg, a diastolic BP ≥ 90 mmHg, or self-reported current use of antihypertensive medications. Hypercholesterolemia was defined as a blood cholesterol level of ≥ 240 mg/dL or current use of lipid-lowering drugs. Subjects were classified as having MetS if they had at least three of the following: WC ≥ 90 cm in men or ≥ 80 cm in women (the modified Asian criteria), TG ≥ 150 mg/dL or TG-lowering medication, low high density lipoprotein cholesterol (< 40 mg/dL in men or < 50 mg/dL in women), systolic BP ≥ 130 mmHg, diastolic BP ≥ 85 mmHg or antihypertensive medication use, FPG ≥ 100 mg/dL or antidiabetes medication use or previously diagnosed T2DM[15].
Data were analyzed using SAS for Windows (version 9.20, SAS Institute, Cary, NC, United States). We used the stratification variables and sampling weights designated by the KCDCP, which were based on the sample design of each survey year.
Data are presented as mean ± SE for continuous variables or as proportions (SE) for categorical variables. If necessary, logarithmic transformation was performed to achieve a normal distribution. Pearson’s χ2 tests were used to test the differences in the proportion of categorical variables, and independent t-tests were used for evaluating the difference between the means of two continuous variables.
Participants were divided into sex-specific quartiles on the basis of serum liver marker levels as follows: for ALT, 15, 21, and 29 IU/L in men and 12, 15, and 20 IU/L in women; for AST, 19, 22, and 26 IU/L in men and 16, 19, and 23 IU/L in women; for AST/ALT, 0.8, 1.1, and 1.3 in men and 1.1, 1.3, and 1.5 in women; and for GGT, 21, 29, and 47 IU/L in men and 13, 17, and 23 IU/L in women. Associations of liver markers with the risk of T2DM and IFG were analyzed separately by sex using multiple logistic regression models. Risk for those in the second, third, and fourth quartiles (Q2, Q3, and Q4, respectively) of each liver marker was compared with those in the first quartile (Q1). Two models were constructed for each liver marker: in model 1, adjustments were made for age; model 2 included additional adjustments for BMI, smoking, alcohol intake, physical activity, education level, income level, hypertension, and hypercholesterolemia. When two or more liver markers were analyzed, we used the Q4 of AST, ALT, and GGT, whereas Q1 was used for AST/ALT. Results are presented as odds ratios (OR) and 95%CI. A P value < 0.05 was considered to be significant.
Of the 17476 KNHANES V participants, 11705 were eligible for this study. 8863 participants (3408 men and 5455 women) were included and 2842 subjects excluded due to a history of malignancy (n = 142), hepatitis B or C (n = 161), liver cirrhosis (n = 24), alcohol intake > 30 g per day (n = 830), pregnancy (n = 25) or missing data for variables included in the analysis (n = 1660). The prevalence of T2DM in the entire population was 11.3%, considering sampling weights and stratification. The prevalence of T2DM was higher in men (13.9%) than in women (9.8%).
Table 1 shows the clinical characteristics of the study participants. Participants with T2DM were older and had higher BMI and WC than participants without T2DM. Compared with subjects without T2DM, subjects of both sexes who had T2DM had higher rates of metabolic syndrome, hypertension, and hypercholesterolemia, and lower levels of income and education. Although levels of liver enzymes were within the normal range, subjects of both sexes who had T2DM had higher concentrations of AST, ALT, and GGT than subjects who did not. There was no difference in AST/ALT between men with and without T2DM. However, AST/ALT was lower in the T2DM group of women.
| Men | Women | |||||
| Nondiabetes | Diabetes | P value | Nondiabetes | Diabetes | P value | |
| No. | 2936 | 472 | 4923 | 532 | ||
| Age (yr) | 48.5 (0.35) | 57.1 (0.60) | < 0.001 | 49.6 (0.29) | 61.9 (0.76) | < 0.001 |
| BMI (kg/m2) | 24.1 (0.07) | 24.5 (0.18) | 0.012 | 23.4 (0.06) | 25.6 (0.18) | < 0.001 |
| Waist circumference (cm) | 84.5 (0.21) | 87.7 (0.53) | < 0.001 | 78.5 (0.20) | 86.7 (0.52) | < 0.001 |
| Systolic BP (mmHg) | 121.1 (0.40) | 125.1 (0.91) | < 0.001 | 117.2 (0.35) | 129.4 (1.03) | < 0.001 |
| Diastolic BP (mmHg) | 80.0 (0.29) | 78.3 (0.57) | 0.006 | 74.7 (0.20) | 75.5 (0.55) | 0.178 |
| Smoker, current | 40.9 (1.17) | 39.3 (2.69) | 0.576 | 4.2 (0.37) | 5.1 (1.21) | 0.488 |
| Drinking, mild to moderate | 18.8 (0.90) | 16.9 (2.14) | 0.438 | 3.3 (0.33) | 2.2 (0.89) | 0.330 |
| Regular exercise | 22.8 (0.94) | 22.7 (2.39) | 0.983 | 18.9 (0.80) | 13.7 (2.02) | 0.030 |
| Income, lowest quartile | 13.7 (0.85) | 26.7 (2.56) | < 0.001 | 18.1 (0.79) | 35.7 (2.54) | < 0.001 |
| Education, ≤ middle school | 25.1 (1.12) | 41.0 (2.88) | < 0.001 | 39.3 (1.11) | 75.7 (2.64) | < 0.001 |
| Rural area | 20.6 (2.22) | 24.5 (3.43) | 0.140 | 21.6 (2.18) | 26.5 (3.51) | 0.045 |
| Hypertension | 31.3 (1.11) | 54.3 (2.73) | < 0.001 | 24.8 (0.80) | 63.8 (2.89) | < 0.001 |
| Hypercholesterolemia | 11.5 (0.70) | 25.7 (2.50) | < 0.001 | 13.4 (0.60) | 36.50 (2.4) | < 0.001 |
| Metabolic syndrome | 25.4 (1.03) | 66.1 (2.74) | < 0.001 | 24.2 (0.68) | 80.8 (2.33) | < 0.001 |
| FPG (mg/dL) | 94.3 (0.24) | 147.4 (3.19) | < 0.001 | 91.6 (0.17) | 138.5 (2.28) | < 0.001 |
| Triglycerides (mg/dL)1 | 128.2 (124.4-132.2) | 146.1 (134.9-158.3) | 0.003 | 94.6 (92.8-96.5) | 137.3 (129.5-145.5) | < 0.001 |
| Total cholesterol (mg/dL) | 193.1 (0.81) | 181.3 (2.32) | < 0.001 | 191.9 (0.66) | 194.0 (2.18) | 0.353 |
| HDL-cholesterol (mg/dL) | 49.0 (0.29) | 45.7 (0.75) | < 0.001 | 55.5 (0.22) | 49.5 (0.67) | < 0.001 |
| LDL-cholesterol (mg/dL) | 114.7 (0.78) | 102.0 (2.02) | < 0.001 | 114.5 (0.55) | 113.0 (2.04) | 0.487 |
| ALT (IU/L)1 | 22.3 (21.8-22.8) | 25.3 (23.8-26.9) | < 0.001 | 15.3 (15.0-15.5) | 21.0 (20.0-22.1) | < 0.001 |
| AST (IU/L)1 | 22.3 (22.0-22.6) | 24.0 (22.9-25.1) | 0.003 | 19.2 (19.0-19.4) | 22.1 (21.2-23.0) | < 0.001 |
| AST/ALT | 1.1 (0.01) | 1.0 (0.03) | 0.265 | 1.3 (0.01) | 1.1 (0.02) | < 0.001 |
| GGT1 | 33.9 (32.9-34.9) | 42.5 (39.3-46.0) | < 0.001 | 18.8 (18.4-19.1) | 26.6 (25.1-28.1) | < 0.001 |
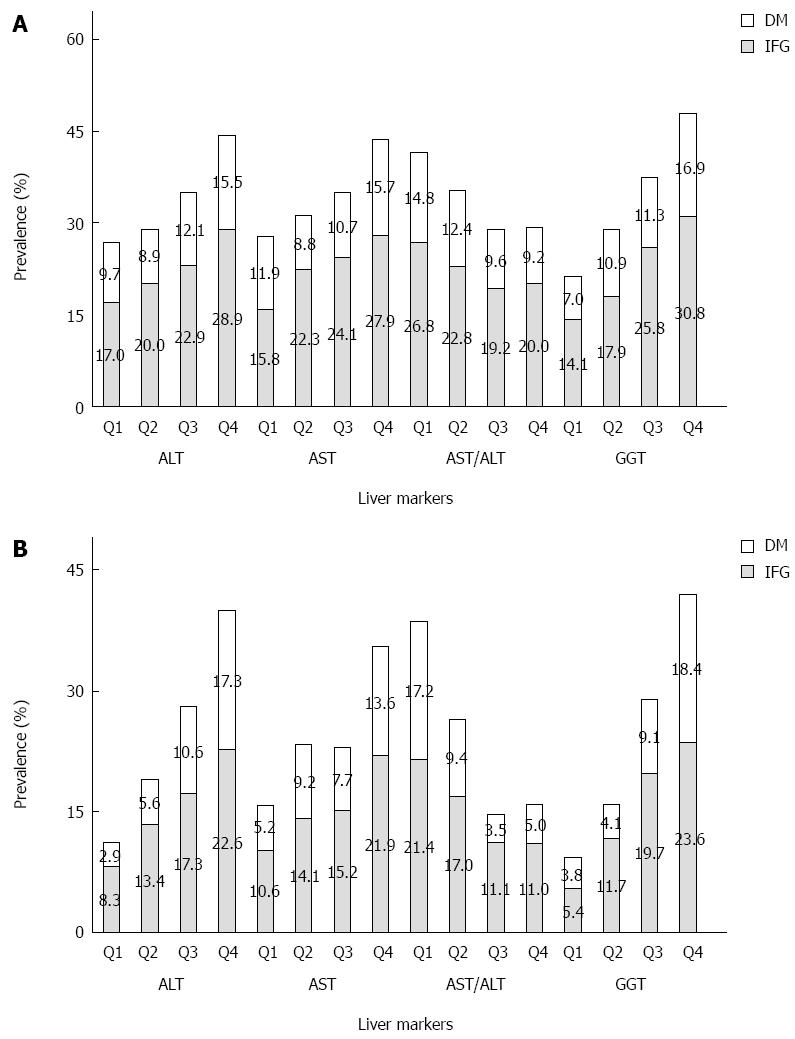
The prevalence of IFG and T2DM across quartiles of serum liver markers is presented in Figure 1. The participants without T2DM were divided into an IFG and non-IFG group. The prevalence of IFG was 18.3% (22.5% in men, 15.2% in women). The highest prevalence of T2DM occurred in the highest quartiles of serum ALT, and the prevalence of T2DM increased linearly with increasing GGT in both men and women. The prevalence of IFG increased with increasing quartiles of ALT, AST, and GGT in both sexes, and AST/ALT was negatively associated with IFG in women.
We investigated the prevalence of T2DM in those meeting at least two of the following criteria: in Q4 for AST, ALT, or GGT; or in Q1 for AST/ALT. In every case where two or more markers were combined, the prevalence of T2DM was higher than the highest quartiles of a single marker. When all four markers were combined, the prevalence of T2DM was over 20% in both sexes (data not shown).
The risk of T2DM among those in Q2, Q3, and Q4 for liver markers compared with those in Q1 (the reference category) is presented in Table 2.
| Men | Women | |||||||
| ALT | AST | AST/ALT | GGT | ALT | AST | AST/ALT | GGT | |
| Model 1 | ||||||||
| Q1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Q2 | 1.04 (0.708-1.515) | 0.62 (0.429-0.891) | 0.58 (0.416-0.812) | 1.81 (1.195-2.748) | 1.54 (0.975-2.429) | 1.16 (0.811-1.670) | 0.44 (0.338-0.569) | 0.84 (0.537-1.319) |
| Q3 | 1.61 (1.105-2.343) | 0.75 (0.525-1.068) | 0.37 (0.256-0.519) | 2.07 (1.406-3.051) | 2.74 (1.723-4.351) | 0.78 (0.527-1.141) | 0.16 (0.112-0.233) | 1.68 (1.102-2.559) |
| Q4 | 2.63 (1.820-3.797) | 1.16 (0.824-1.617) | 0.24 (0.162-0.351) | 3.47 (2.372-5.074) | 5.31 (3.528-7.991) | 1.27 (0.884-1.816) | 0.17 (0.118-0.242) | 3.99 (2.766-5.744) |
| P for trend | < 0.001 | 0.256 | < 0.001 | < 0.001 | < 0.001 | 0.396 | < 0.001 | < 0.001 |
| Model 2 | ||||||||
| Q1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Q2 | 1.07 (0.698-1.652) | 0.55 (0.366-0.836) | 0.67 (0.447-0.998) | 1.61 (1.015-2.538) | 1.27 (0.777-2.077) | 1.08 (0.741-1.577) | 0.51 (0.377-0.695) | 0.69 (0.426-1.116) |
| Q3 | 1.43 (0.899-2.274) | 0.74 (0.484-1.119) | 0.42 (0.270-0.640) | 2.03 (1.312-3.151) | 1.90 (1.158-3.120) | 0.70 (0.449-1.092) | 0.23 (0.154-0.357) | 1.11 (0.686-1.801) |
| Q4 | 2.22 (1.381-3.559) | 0.96 (0.640-1.432) | 0.30 (0.185-0.483) | 3.05 (1.913-4.877) | 3.16 (1.990-5.026) | 1.06 (0.714-1.571) | 0.28 (0.188-0.429) | 2.23(1.459-3.397) |
| P for trend | < 0.001 | 0.681 | < 0.001 | < 0.001 | < 0.001 | 0.926 | < 0.001 | < 0.001 |
In the age-adjusted logistic regression analysis, increasing quartiles of ALT and GGT had positive linear correlations with T2DM, whereas AST/ALT was negatively associated with T2DM. When adjusted for age, BMI, smoking, alcohol intake, regular physical activity, education level, income level, hypertension, hypercholesterolemia, and hypertriglyceridemia, the ORs across quartiles of AST/ALT were 1, 0.67, 0.42, and 0.30 in men and 1, 0.51, 0.23, and 0.28 in women (P for trend < 0.001). Compared with the Q1 of ALT, the ORs for T2DM were 2.22 (95%CI: 1.381-3.559; P for trend < 0.001) and 3.16 (95%CI: 1.990-5.026; P for trend < 0.001) for Q4 of men and women, respectively. The positive association between GGT and T2DM was consistently present among both men (adjusted OR = 3.05; 95%CI: 1.913-4.877; P for trend < 0.001) and women (adjusted OR = 2.23; 95%CI: 1.459-3.397; P for trend < 0.001), when Q4 of GGT was compared with Q1. There was no association of AST level with T2DM in either sex.
Table 3 shows the ORs for the prevalence of IFG by quartiles of liver markers. After adjustment for age, all liver markers were significantly associated with IFG, and trends across quartiles of liver markers were statistically significant (all P for trend < 0.05). In model 2, participants in Q4 of ALT and GGT continued to be at elevated risk of IFG, although the association between AST and IFG was no longer significant. Higher AST/ALT ratio was associated with a significantly reduced risk of IFG.
| Men | Women | |||||||
| ALT | AST | AST/ALT | GGT | ALT | AST | AST/ALT | GGT | |
| Model 1 | ||||||||
| Q1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Q2 | 1.29 (0.942-1.754) | 1.40 (1.030-1.889) | 0.65 (0.487-0.855) | 1.46 (1.066-1.998) | 1.50 (1.117-2.022) | 1.15 (0.863-1.540) | 0.63 (0.496-0.805) | 2.05 (1.452-2.902) |
| Q3 | 1.69 (1.269-2.246) | 1.57 (1.171-2.095) | 0.46 (0.342-0.612) | 2.54 (1.907-3.393) | 1.98 (1.474-2.658) | 1.09 (0.785-1.502) | 0.37 (0.285-0.475) | 3.74 (2.606-5.355) |
| Q4 | 2.76 (2.026-3.750) | 2.08 (1.532-2.815) | 0.39 (0.283-0.547) | 3.63 (2.721-4.852) | 3.24 (2.397-4.369) | 1.70 (1.265-2.274) | 0.32 (0.248-0.423) | 5.56 (3.970-7.780) |
| P for trend | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.001 | < 0.001 | < 0.001 |
| Model 2 | ||||||||
| Q1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Q2 | 1.07 (0.738-1.546) | 1.29 (0.905-1.849) | 0.77 (0.550-1.077) | 1.27 (0.871-1.843) | 1.27 (0.904-1.789) | 1.04 (0.749-1.444) | 0.72 (0.556-0.936) | 1.98 (1.333-2.946) |
| Q3 | 1.07 (0.752-1.513) | 1.33 (0.924-1.906) | 0.63 (0.446-0.890) | 1.97 (1.362-2.856) | 1.53 (1.081-2.151) | 0.92 (0.642-1.323) | 0.48 (0.360-0.642) | 2.97 (1.974-4.469) |
| Q4 | 1.69 (1.172-2.447) | 1.46 (1.009-2.111) | 0.70 (0.464-1.055) | 2.32 (1.608-3.356) | 2.00 (1.422-2.823) | 1.34 (0.980-1.838) | 0.49 (0.365-0.667) | 4.00 (2.720-5.874) |
| P for trend | 0.005 | 0.053 | 0.042 | < 0.001 | < 0.001 | 0.094 | < 0.001 | < 0.001 |
In addition, we assessed the risk of T2DM and IFG with the combination of highest-risk quartiles of liver markers: ALT (Q4), AST/ALT (Q1), and GGT (Q4) (Table 4). After adjustment for age, BMI, smoking, alcohol intake, regular physical activity, education level, income level, hypertension, hypercholesterolemia, and hypertriglyceridemia, if two or more markers were in the highest risk quartile, the risks of both T2DM and IFG were increased compared with the other quartiles (all P values < 0.05). When the combination of ALT (Q4), GGT (Q4), and AST/ALT (Q1) was compared with the other quartiles, the ORs for prevalence of T2DM were 3.21 (95%CI: 1.829-5.622; P < 0.001) in men and 4.60 (95%CI: 3.217-6.582; P < 0.001) in women. In the same adjusted models, men and women who were in Q4 of ALT and GGT and Q1 of AST/ALT had 1.99 and 2.40 times increased risks of IFG, respectively. The increase in risk was generally greater for T2DM than for IFG.
| Men | Women | |||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| In type 2 diabetes | ||||
| ALT, highest quartile, and AST/ALT, lowest quartile | 2.77 (1.749-4.371) | < 0.001 | 3.76 (2.731-5.175) | < 0.001 |
| ALT, highest quartile, and GGT, highest quartile | 2.37 (1.486-3.794) | < 0.001 | 4.03 (2.961-5.492) | < 0.001 |
| GGT, highest quartile, and AST/ALT, lowest quartile | 2.73 (1.614-4.634) | < 0.001 | 4.20 (2.987-5.903) | < 0.001 |
| ALT, GGT, highest quartile, and AST/ALT, lowest quartile | 3.21 (1.829-5.622) | < 0.001 | 4.60 (3.217-6.582) | < 0.001 |
| In IFG | ||||
| ALT, highest quartile, and AST/ALT, lowest quartile | 2.04 (1.410-2.952) | < 0.001 | 1.88 (1.439-2.458) | < 0.001 |
| ALT, highest quartile, and GGT, highest quartile | 2.10 (1.413-3.105) | < 0.001 | 2.27 (1.676-3.072) | < 0.001 |
| GGT, highest quartile, and AST/ALT, lowest quartile | 1.79 (1.182-2.716) | 0.006 | 2.38 (1.761-3.220) | < 0.001 |
| ALT, GGT, highest quartile, and AST/ALT, lowest quartile | 1.99 (1.264-3.131) | 0.003 | 2.40 (1.720-3.354) | < 0.001 |
In this study, we demonstrated that serum ALT and GGT levels were positively associated and AST/ALT inversely associated with the prevalence of T2DM. The association between AST and the prevalence of T2DM was not significant. These associations remained evident even after adjusting for potential confounding factors by multivariate analysis, thus reinforcing our conclusion that higher ALT and GGT, and lower AST/ALT levels were independent risk factors for T2DM in both sexes. When a combination of two markers was examined, the risk of T2DM increased, the highest risk combination being GGT with AST/ALT in women and ALT with AST/ALT in men. The risk of T2DM was highest when three markers were examined, which suggests that ALT, GGT, and AST/ALT have a cumulative effect on the risk of T2DM.
Several studies have reported a relationship between liver markers and diabetes. Some have reported that ALT and GGT are a risk factor for both T2DM and MetS[16,17], while others have reported that only GGT was significantly associated with the risk of T2DM[6,12,16]. A recent meta-analysis reported that incident diabetes in women is more associated with GGT than with ALT[12]. However, in our study, the ALT level appeared to be the most significant risk factor for T2DM in women, independent of potential confounders. Several studies have examined the association of serum AST with risk for T2DM, with inconsistent results[17,18]. Our results agree with a recent Japanese report[18], which found no association between AST with diabetes risk in a study of male Japanese office workers. We also assessed the risk of T2DM associated with the AST/ALT ratio which found that there was a negative correlation with the AST/ALT ratio.
When the analysis was expanded in to IFG, multiple regression analysis revealed similar results as those for T2DM, in that ALT and GGT showed a positive correlation with IFG while AST/ALT showed a negative correlation. This shows that subjects with the highest quartiles of ALT or GGT or the lowest quartile of AST/ALT have an increased risk of prediabetes. When the analysis was expanded to consider combinations of two or more liver markers, similar results as those for T2DM were obtained, although the ORs were a little lower. These results agree with previous studies that investigated the role of GGT in prediabetes[18,19]. Our study also showed an additive effect for the predictive value of liver markers in prediabetes. As prediabetes is an important risk factor for the development of overt diabetes, further studies regarding the role of the liver in the development and progression of diabetes are indicated.
In the clinical context, AST, ALT, and GGT levels are used to indicate hepatic inflammation, and AST/ALT can distinguish an alcoholic etiology in fatty liver[20]. In addition to these conventional clinical uses, our findings emphasize that highest ALT and GGT quartiles and the lowest AST/ALT quartile within their respective normal ranges can be biomarkers for T2DM.
Biological mechanisms that explain the relationships between liver markers and glucose metabolism have not been elucidated, but there are some potential candidates. One is that increased serum ALT, GGT and decreased AST/ALT levels reflect hepatic steatosis or visceral obesity[21-23]. The excess accumulation of fat in liver, known as nonalcoholic fatty liver disease (NAFLD), causes hepatic insulin resistance and is considered a feature of the MetS[24,25]. ALT has been closely associated with hepatic steatosis and is commonly utilized as an epidemiologic biomarker of NAFLD. In addition, GGT and ALT have also been reported to be correlated with hepatic insulin resistance, which may have contributed to the increase in diabetes or IFG risk[26].
Another mechanism explaining the relationship between liver markers and T2DM may be related to oxidative stress, reported to contribute to the development and progression of diabetes[27]. GGT has been reported to play a central role in the antioxidant system, especially in intracellular glutathione homeostasis, and acts as a marker of oxidative stress because it is increases during oxidative stress states[28,29]. Paradoxically, recent studies have reported that GGT may play a direct role in the generation of reactive oxygen species[30]. The rise in reactive oxygen species as a result of increased GGT may exceed the capacity of the antioxidant system, leading to oxidative stress. Inflammation that occurs through oxidative stress results in decreased responsiveness to insulin, ultimately leading to T2DM[31,32]. Oxidative stress and chronic inflammation are also contributing factors in the development and progression of NAFLD[27,31,32].
However, there are some limitations to this study. First, because of the cross-sectional nature of the data, only associations, not causality, can be examined. Second, oral glucose tolerance tests were not performed, limiting identification of participants with impaired glucose tolerance. Third, we relied on a single measurement of liver markers and fasting blood glucose, although these can vary even within individuals at various time points. This may have led to a misclassification bias. However, single measurements of fasting glucose usually lead to nondifferential misclassification of IFG or T2DM, which would probably lead to a lessening of the associations reported in this study. If we had used a more rigorous design for the sample collection, stronger associations could be expected. Fourth, though hepatic insulin resistance may have contributed to the increase in diabetes or IFG risk, we could not measure hepatic insulin resistance as currently accepted methods such as the isotope dilution methods or oral glucose tolerance tests were not done[33].
Despite these limitations, the main strength of this study is that these results can be generalized to the whole Korean population, as the population of the current study was generally representative of Koreans, and the data were collected using standardized methods.
Another strength is that we combined the markers that were confirmed to be associated with T2DM to see if a combination of two or more markers identified a higher risk of T2DM. In both men and women, the risk of T2DM was higher when two markers were combined. When these observations were expanded in a similar manner to IFG, similar conclusions were drawn, although the OR was a little lower than that for T2DM. To the best of our knowledge, this is the first study reporting an additive association between ALT, AST/ALT, GGT, and T2DM and IFG. Liver markers have not been regarded as traditional risk factors for T2DM. This may be because a single liver marker alone is inadequate as a screening tool in clinical practice. However, our results suggest that an increase in the risk of IFG or T2DM should be considered when there is an increase of two or more liver markers.
Finally, our results have potentially important clinical implications. Serum ALT, AST/ALT, and GGT, are measured by standardized methods and are readily available in routine clinical practice. As such, they may provide a simple and predictive measure for assessing the risk of T2DM and IFG. This study shows the possibility of using ALT, AST/ALT, and GGT as supplementary predictive risk factors for IFG or T2DM. At present, ALT and GGT are used as screening, diagnostic and monitoring tools for liver disease. Accordingly, the upper normal limit was determined based on the ability to discriminate persons with hepatobiliary disease[34-36]. However, as our study and others have reported, there is reasonably consistent evidence that high-normal ALT and GGT are risk factors for T2DM, independent of obesity and other known risk factors. This has led to recent studies suggesting lowering the currently accepted upper normal limit of ALT levels[37,38].
In our study, the boundary of the highest quartile of ALT was 29 IU/L in men and 20 IU/L in women, which is lower than the previously reported normal upper limits of adults with biopsy-proven normal liver histology (33 IU/L for men and 25 IU/L for women)[38]. This is also similar to the highest quartile level reported in a recent study on the risk of liver disease and MetS[37]. The boundary of the highest quartile of GGT was 47 IU/L in men and 23 IU/L in women, which is similar to a recent report which showed that GGT ≥ 22 IU/L was significantly associated with an increased risk of fatal coronary artery disease and mortality[39]. Therefore, studies investigating a new threshold for liver enzymes and considering their predictive roles in metabolic and cardiovascular diseases should be performed.
In conclusion, higher levels of serum GGT and ALT, and decreased AST/ALT levels, even within the normal range, are independent, additive risk factors for the prevalence of T2DM and IFG in both sexes. Although more population-based studies and research on the underlying pathophysiology are needed, these liver markers may be additionally useful in identifying individuals at higher risk for T2DM and IFG.
We thank the members of the Division of Chronic Disease Surveillance of the Korean Center for Disease Control and Prevention who conducted the national survey and everyone who contributed to this project.
The liver plays an important role in the maintenance of normal glucose levels, and hepatic dysfunction has been suggested as leading to type 2 diabetes (T2DM). Liver enzymes are considered as surrogate markers of liver function. Elevated serum gamma-glutamyltransferase (GGT) levels have been reported as an important risk factor in the development of impaired fasting glucose, T2DM, cardiovascular disease, and metabolic syndrome. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) have also been reported to be associated with metabolic syndrome and T2DM.
It is not fully understood which liver enzymes are better indicators of T2DM, whether the relationships are consistent in prediabetes states such as impaired fasting glucose, and whether liver markers have incremental predictive effects for T2DM.
Several studies have reported that ALT and GGT are a risk factor for both T2DM and metabolic syndrome, while others have reported that only GGT was significantly associated with the risk of T2DM. This may be because increased serum ALT, GGT and decreased AST/ALT levels reflect hepatic steatosis or visceral obesity. In addition, GGT and ALT have also been reported to be correlated with hepatic insulin resistance, which may be associated with the increase in diabetes or impaired fasting glucose risk.
Serum ALT, AST/ALT, and GGT, are measured by standardized methods and are readily available in routine clinical practice. As such, they may provide a simple and predictive measure for assessing the risk of T2DM and impaired fasting glucose. Also, as our study and others have reported, there is reasonably consistent evidence that high-normal ALT and GGT are risk factors for T2DM. This suggests that lowering the currently accepted upper normal limit of ALT levels should be considered.
The paper investigates the association between liver enzymes and the risk to have IFG or T2DM in the population of the KNHANES survey older than 30 years. They observed that higher levels of ALT and/or GGT and lower levels of AST/ALT increased the risk to have IFG and T2DM and that the levels for the increased risk are lower than that considered pathological. Ethical requirements are fulfilled.
P- Reviewer: Hu ZW, Namikawa T, Prodam F S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | Onat A, Can G, Örnek E, Çiçek G, Ayhan E, Doğan Y. Serum γ-glutamyltransferase: independent predictor of risk of diabetes, hypertension, metabolic syndrome, and coronary disease. Obesity (Silver Spring). 2012;20:842-848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 2. | Grundy SM. Gamma-glutamyl transferase: another biomarker for metabolic syndrome and cardiovascular risk. Arterioscler Thromb Vasc Biol. 2007;27:4-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Emdin M, Pompella A, Paolicchi A. Gamma-glutamyltransferase, atherosclerosis, and cardiovascular disease: triggering oxidative stress within the plaque. Circulation. 2005;112:2078-2080. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 244] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 4. | Lee DH, Ha MH, Kim JH, Christiani DC, Gross MD, Steffes M, Blomhoff R, Jacobs DR. Gamma-glutamyltransferase and diabetes--a 4 year follow-up study. Diabetologia. 2003;46:359-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 230] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 5. | Gautier A, Balkau B, Lange C, Tichet J, Bonnet F. Risk factors for incident type 2 diabetes in individuals with a BMI of & lt; 27 kg/m2: the role of gamma-glutamyltransferase. Data from an Epidemiological Study on the Insulin Resistance Syndrome (DESIR). Diabetologia. 2010;53:247-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | André P, Balkau B, Vol S, Charles MA, Eschwège E. Gamma-glutamyltransferase activity and development of the metabolic syndrome (International Diabetes Federation Definition) in middle-aged men and women: Data from the Epidemiological Study on the Insulin Resistance Syndrome (DESIR) cohort. Diabetes Care. 2007;30:2355-2361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Nakanishi N, Suzuki K, Tatara K. Serum gamma-glutamyltransferase and risk of metabolic syndrome and type 2 diabetes in middle-aged Japanese men. Diabetes Care. 2004;27:1427-1432. [PubMed] [Cited in This Article: ] |
| 8. | Wannamethee SG, Shaper AG, Lennon L, Whincup PH. Hepatic enzymes, the metabolic syndrome, and the risk of type 2 diabetes in older men. Diabetes Care. 2005;28:2913-2918. [PubMed] [Cited in This Article: ] |
| 9. | André P, Balkau B, Born C, Royer B, Wilpart E, Charles MA, Eschwège E. Hepatic markers and development of type 2 diabetes in middle aged men and women: a three-year follow-up study. The D.E.S.I.R. Study (Data from an Epidemiological Study on the Insulin Resistance syndrome). Diabetes Metab. 2005;31:542-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Vozarova B, Stefan N, Lindsay RS, Saremi A, Pratley RE, Bogardus C, Tataranni PA. High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51:1889-1895. [PubMed] [Cited in This Article: ] |
| 11. | Sattar N, Scherbakova O, Ford I, O’Reilly DS, Stanley A, Forrest E, Macfarlane PW, Packard CJ, Cobbe SM, Shepherd J. Elevated alanine aminotransferase predicts new-onset type 2 diabetes independently of classical risk factors, metabolic syndrome, and C-reactive protein in the west of Scotland coronary prevention study. Diabetes. 2004;53:2855-2860. [PubMed] [Cited in This Article: ] |
| 12. | Fraser A, Harris R, Sattar N, Ebrahim S, Davey Smith G, Lawlor DA. Alanine aminotransferase, gamma-glutamyltransferase, and incident diabetes: the British Women’s Heart and Health Study and meta-analysis. Diabetes Care. 2009;32:741-750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 285] [Cited by in F6Publishing: 283] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 13. | Lim H, Nguyen T, Choue R, Wang Y. Sociodemographic disparities in the composition of metabolic syndrome components among adults in South Korea. Diabetes Care. 2012;35:2028-2035. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Yoon JY, Park HA, Kang JH, Kim KW, Hur YI, Park JJ, Lee R, Lee HH. Prevalence of dietary supplement use in Korean children and adolescents: insights from Korea National Health and Nutrition Examination Survey 2007-2009. J Korean Med Sci. 2012;27:512-517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640-1645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8720] [Cited by in F6Publishing: 9585] [Article Influence: 639.0] [Reference Citation Analysis (0)] |
| 16. | Kim CH, Park JY, Lee KU, Kim JH, Kim HK. Association of serum gamma-glutamyltransferase and alanine aminotransferase activities with risk of type 2 diabetes mellitus independent of fatty liver. Diabetes Metab Res Rev. 2009;25:64-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Hanley AJ, Williams K, Festa A, Wagenknecht LE, D’Agostino RB, Kempf J, Zinman B, Haffner SM. Elevations in markers of liver injury and risk of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2004;53:2623-2632. [PubMed] [Cited in This Article: ] |
| 18. | Nakanishi N, Nishina K, Li W, Sato M, Suzuki K, Tatara K. Serum gamma-glutamyltransferase and development of impaired fasting glucose or type 2 diabetes in middle-aged Japanese men. J Intern Med. 2003;254:287-295. [PubMed] [Cited in This Article: ] |
| 19. | Nguyen QM, Srinivasan SR, Xu JH, Chen W, Hassig S, Rice J, Berenson GS. Elevated liver function enzymes are related to the development of prediabetes and type 2 diabetes in younger adults: the Bogalusa Heart Study. Diabetes Care. 2011;34:2603-2607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Botros M, Sikaris KA. The de ritis ratio: the test of time. Clin Biochem Rev. 2013;34:117-130. [PubMed] [Cited in This Article: ] |
| 21. | Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38:263-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 735] [Cited by in F6Publishing: 760] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 22. | Ichimori S, Shimoda S, Goto R, Matsuo Y, Maeda T, Furukawa N, Kawashima J, Kodama S, Sekigami T, Isami S. Ezetimibe improves glucose metabolism by ameliorating hepatic function in Japanese patients with type 2 diabetes. J Diabetes Investig. 2012;3:179-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Fan H, Pan Q, Xu Y, Yang X. Exenatide improves type 2 diabetes concomitant with non-alcoholic fatty liver disease. Arq Bras Endocrinol Metabol. 2013;57:702-708. [PubMed] [Cited in This Article: ] |
| 24. | Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844-1850. [PubMed] [Cited in This Article: ] |
| 25. | Marchesini G, Forlani G. NASH: from liver diseases to metabolic disorders and back to clinical hepatology. Hepatology. 2002;35:497-499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Venkatesan C, Younossi ZM. Potential mechanisms underlying the associations between liver enzymes and risk for type 2 diabetes. Hepatology. 2012;55:968-970. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Ceriello A. Oxidative stress and glycemic regulation. Metabolism. 2000;49:27-29. [PubMed] [Cited in This Article: ] |
| 28. | Lee DH, Blomhoff R, Jacobs DR. Is serum gamma glutamyltransferase a marker of oxidative stress? Free Radic Res. 2004;38:535-539. [PubMed] [Cited in This Article: ] |
| 29. | Lim JS, Yang JH, Chun BY, Kam S, Jacobs DR, Lee DH. Is serum gamma-glutamyltransferase inversely associated with serum antioxidants as a marker of oxidative stress? Free Radic Biol Med. 2004;37:1018-1023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 30. | Drozdz R, Parmentier C, Hachad H, Leroy P, Siest G, Wellman M. gamma-Glutamyltransferase dependent generation of reactive oxygen species from a glutathione/transferrin system. Free Radic Biol Med. 1998;25:786-792. [PubMed] [Cited in This Article: ] |
| 31. | Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105:141-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1141] [Cited by in F6Publishing: 1211] [Article Influence: 121.1] [Reference Citation Analysis (0)] |
| 32. | Onat A, Hergenç G. Low-grade inflammation, and dysfunction of high-density lipoprotein and its apolipoproteins as a major driver of cardiometabolic risk. Metabolism. 2011;60:499-512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Choukem SP, Gautier JF. How to measure hepatic insulin resistance? Diabetes Metab. 2008;34:664-673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Ruhl CE, Everhart JE. Upper limits of normal for alanine aminotransferase activity in the United States population. Hepatology. 2012;55:447-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 35. | Lum G, Gambino SR. Serum gamma-glutamyl transpeptidase activity as an indicator of disease of liver, pancreas, or bone. Clin Chem. 1972;18:358-362. [PubMed] [Cited in This Article: ] |
| 36. | Nalpas B, Vassault A, Charpin S, Lacour B, Berthelot P. Serum mitochondrial aspartate aminotransferase as a marker of chronic alcoholism: diagnostic value and interpretation in a liver unit. Hepatology. 1986;6:608-614. [PubMed] [Cited in This Article: ] |
| 37. | Kim HY, Kim CW, Lee CD, Choi JY, Park CH, Bae SH, Yoon SK, Han K, Park YM. Can “healthy” normal alanine aminotransferase levels identify the metabolically obese phenotype? Findings from the Korea national health and nutrition examination survey 2008-2010. Dig Dis Sci. 2014;59:1330-1337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Lee JK, Shim JH, Lee HC, Lee SH, Kim KM, Lim YS, Chung YH, Lee YS, Suh DJ. Estimation of the healthy upper limits for serum alanine aminotransferase in Asian populations with normal liver histology. Hepatology. 2010;51:1577-1583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 39. | Fraser A, Harris R, Sattar N, Ebrahim S, Smith GD, Lawlor DA. Gamma-glutamyltransferase is associated with incident vascular events independently of alcohol intake: analysis of the British Women’s Heart and Health Study and Meta-Analysis. Arterioscler Thromb Vasc Biol. 2007;27:2729-2735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 219] [Article Influence: 12.9] [Reference Citation Analysis (0)] |