Published online Jun 21, 2015. doi: 10.3748/wjg.v21.i23.7305
Peer-review started: November 15, 2014
First decision: December 26, 2014
Revised: January 10, 2015
Accepted: January 21, 2015
Article in press: January 21, 2015
Published online: June 21, 2015
AIM: To compare the efficacy and safety of recombinant streptokinase (rSK) vs hydrocortisone acetate-based suppositories in acute hemorrhoidal disease.
METHODS: A multicenter (11 sites), randomized (1:1:1), open, controlled trial with parallel groups was performed. All participating patients gave their written, informed consent. After inclusion, patients with acute symptoms of hemorrhoids were centrally randomized to receive, as outpatients, by the rectal route, suppositories of rSK 200000 IU of one unit every 8 h (first 3 units) and afterwards every 12 h until 8 administrations were completed (schedule A), one unit every 8 h until 6 units were completed (schedule B), or 25 mg hydrocortisone acetate once every 8 h up to a maximum of 24 administrations. Evaluations were performed at 3, 5, and 10 d post-inclusion. The main end-point was the 5th-day response (disappearance of pain and bleeding, and ≥ 70% reduction of the lesion size). Time to response and need for thrombectomy were secondary efficacy variables. Adverse events were also evaluated.
RESULTS: Groups were homogeneous with regards to demographic and baseline characteristics. Fifth day complete response rates were 156/170 (91.8%; 95%CI: 87.3-96.2), 155/170 (91.2%; 95%CI: 86.6%-95.7%), and 46/170 (27.1%; 95%CI: 20.1%-34.0%) with rSK (schedule A and B) and hydrocortisone acetate suppositories, respectively. These 64.6% and 63.9% differences (95%CI: 56.7%-72.2% and 55.7%-72.0%) were highly significant (P < 0.001). This advantage was detected since the early 3rd day evaluation (68.8% and 64.1% vs 7.1% for the rSK and active control groups, respectively; P < 0.001) and was maintained even at the late 10th day assessment (97.1% and 93.5% vs 67.1% for rSK and hydrocortisone acetate, respectively; P < 0.001). Time to response was 3 d (95%CI: 2.9-3.1) for both rSK groups and 10 d (95%CI: 9.3-10.7) in the hydrocortisone acetate group. This difference was highly significant (P < 0.001). All subgroup stratified analyses (with or without thrombosis and hemorrhoid classification) showed a statistically significant advantage for the rSK groups. Thrombectomy was necessary in 4/251 and 14/133 patients with baseline thrombosis in the rSK and hydrocortisone acetate groups, respectively (P < 0.001). There were no adverse events attributable to the experimental treatment.
CONCLUSION: rSK suppositories showed a significant advantage over a widely-used over-the-counter hydrocortisone acetate preparation for the treatment of acute hemorrhoidal illness, as well as having an adequate safety profile.
Core tip: Medical treatments for acute hemorrhoidal disease very seldom come from randomized, controlled clinical trials. The paper describes recombinant streptokinase suppositories, a candidate to a new therapeutic alternative based on thrombolysis. The results show a significant efficacy advantage with respect to hydrocortisone acetate, a widely-used, over-the-counter product.
-
Citation: Hernández-Bernal F, Castellanos-Sierra G, Valenzuela-Silva CM, Catasús-Álvarez KM, Martínez-Serrano O, Lazo-Diago OC, Bermúdez-Badell CH, Causa-García JR, Domínguez-Suárez JE, Investigators PALST4(OHWRSAGO. Recombinant streptokinase
vs hydrocortisone suppositories in acute hemorrhoids: A randomized controlled trial. World J Gastroenterol 2015; 21(23): 7305-7312 - URL: https://www.wjgnet.com/1007-9327/full/v21/i23/7305.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i23.7305
Hemorrhoid disease is one of the most common proctologic conditions affecting large numbers of people in the world and is one of the principal reasons that patients seek consultation from a colorectal surgeon. Treatments include conservative medical management, office procedures, and surgical approaches in an operating room[1-9].
The Initial treatment of the hemorrhoidal illness consists of general conservative measures (hygienic-dietetic, life style changes, and symptomatic treatment) to restore the intestinal habit and to diminish the local symptoms. Although several medicines have been tested, significant benefits have not been obtained to control this condition and very seldom do these procedures come from randomized, controlled clinical trials[4,10]. Therefore, in an important group of patients, surgery becomes the final solution[5,6,9,11-13].
Streptokinase (SK) is an indirect fibrinolytic agent that interacts with plasminogen, forming an active complex with protease action that activates plasminogen into plasmin. The local application of recombinant streptokinase (rSK) on hemorrhoids, where thrombosis and/or inflammation with microthrombi may be present was first tested in a proof-of-concept, pilot trial in 10 patients[14] and afterwards in a phase II, multicenter, randomized, double-blind, placebo-controlled trial on 80 patients. Although a 200000 IU suppository showed a beneficial effect on hemorrhoidal symptoms (36% larger response rate, 5 d faster) over excipient controls with an adequate safety profile[15], a 100000 IU rSK suppository did not. Afterwards, a randomized trial compared this product with a common phenylephrine over-the counter preparation. A 43% advantage in the 5th day response, shorter time to response, and a reduction in the need for thrombectomy were obtained[16]. Further clinical development previewed comparisons with other commonly-used products.
Corticosteroids have been used for the non-invasive treatment of acute hemorrhoids[17]. Among them, hydrocortisone acetate is widely prescribed, and over-the-counter preparations of this drug have been established in common medical practice for the past several decades. The aim of the present work was to compare the efficacy and safety of 200000 IU rSK suppositories versus a hydrocortisone acetate-based commercially-available suppository for the treatment of acute hemorrhoids in terms of clinical response, need for thrombectomy, and adverse events through a multicenter, randomized clinical trial. At the same time, alternative rSK treatment schedules were compared in order to optimize its use.
A randomized (1:1), open, controlled, phase III clinical trial with parallel groups was carried out in 11 hospitals from 10 Cuban provinces. Patients aged 18-75 years old with acute symptoms and signs of hemorrhoids (characterized by anal pain and/or bleeding), tumors of variable size and appearance (possibly colored red-violet), substantial associated edema, and who gave their written, informed consent to participate were eligible. Exclusion criteria were: SK administration in the previous 6 mo; antecedents of intracranial hemorrhage; allergy to SK, corticosteroids, or any other component of the medicament; stroke, intracranial surgery or skull trauma less than 3 mo before; and any other bleeding-risk condition. Patients with acute diarrhea in the last 12 h, hemorrhoids caused by portal hypertension, septic or severe hemorrhagic complications, associated fistula or cancer, pregnancy, puerperium, or mental disorders were also excluded. The trial was performed in proctology wards from central hospitals in province capitals throughout the country. Participant investigators were coloproctology specialists. The protocol followed the Declaration of Helsinki guidelines and was approved by the Ethics Committees of the participating hospitals and by the Cuban Regulatory Authority.
Recombinant streptokinase was produced in Escherichia coli at the Center for Genetic Engineering and Biotechnology (CIGB), Havana[18]. Suppositories of 2 g (Proctokinasa®, Heber Biotec, Havana) were prepared containing 200000 IU of rSK, 20 μg thimerosal, 20 mg sorbitan monostearate (Span 60), 10 mg sodium salicylate, and hard fat (Witepsol W25). This formulation was properly validated and its stability reached 18 mo[19,20]. Anusol-HC® suppositories (Salix Pharmaceuticals, Morrisville) contained 25 mg hydrocortisone acetate in a hydrogenated vegetable oil base. Their organoleptic characteristics, presentations, and therapeutic schedules were different, which determined that the trial could not be double-blind.
The patients included were randomly distributed to the treatment groups: I) rSK (schedule A): one unit with 200000 IU every 8 h (first 3 units) and afterwards every 12 h until completing 8 administrations were completed; II) rSK (schedule B): one unit with 200000 IU every 8 h until completing 6 units were completed; and III) hydrocortisone acetate: one suppository (25 mg) every 8 h up to a maximum of 24 administrations. Treatment was administered by the rectal route. Concomitant treatment for all groups included high-fiber diet, abundant liquid ingestion, bed rest, local hygiene, and oral analgesics if in pain. Thrombectomy was performed in cases with thrombosis if there was no improvement at all during the first 72 h and the patient’s pain was such that it was required. Treatment started immediately after confirmation of inclusion and continued as an outpatient. Compliance was monitored through a log card designed for that purpose and the recollection of empty suppository blister packs.
Diagnosis was performed clinically or verified by anoscopy, if necessary. Hemorrhoids were classified according to their origin. External hemorrhoids originate distal to the dentate line, arising from the inferior hemorrhoidal plexus. Internal hemorrhoids originate proximal to the dentate line, arising from the superior hemorrhoidal plexus, covered with mucosa. Internal hemorrhoids were further classified into four grades, according to the extent of prolapse: (1) grade I: the hemorrhoidal tissue protrudes into the lumen of the anal canal, but does not prolapse outside; (2) grade II: hemorrhoids may prolapse beyond the external sphincter and be visible during evacuation, but spontaneously return to lie within the anal canal; (3) grade III: hemorrhoids protrude outside the anal canal and require manual reduction; and (4) grade IV: hemorrhoids are irreducible and constantly prolapsed. Some hemorrhoids were regarded as mixed (internal-external), arising from the inferior and superior hemorrhoidal plexus and their anastomotic connections. Acute hemorrhoids could present with or without thrombosis[1-3,9,21].
Clinical evaluations were carried out by the specialists via outpatient visits on the 3rd, 5th, and 10th days after treatment onset. The main endpoint was the proportion of patients with complete clinical response on the 5th day, determined via disappearance of pain and bleeding, and a reduction of more than 70% of the initial lesion size (measured with calibrated millimeter rulers). Time to response and need for thrombectomy were secondary efficacy variables.
Adverse events (type, duration, severity, outcome, and causality relationship) were carefully registered. Severity of adverse events was classified into three levels: (1) mild, if no therapy was necessary; (2) moderate, if a specific treatment was needed; and (3) severe, when hospitalization or its prolongation was required, the reaction was life-threatening, or contributed to the patient’s death. A qualitative assessment was used to classify the causal relationship as definite, probable, possible, or doubtful[22]. Adverse reactions known for intravenous SK (fever, shivering, nausea, vomiting, low blood pressure, hemorrhages, and allergy)[23] were specially searched.
Central 1:1:1 randomization was done at CIGB in blocks of 6 individuals by means of a computerized random number generator. Each hospital pharmacy received a stock with the unmasked products in sufficient amounts to guarantee the treatment according to their inclusion rate. The decision to accept a participant was made by the investigators without knowledge of the group assignment. Informed consent was obtained and then the clinical researcher phoned (a 24-h line was specially set up for the trial) the central trial coordinator (FHB) who, after collecting the patient’s initials, assigned the corresponding code (site code + patient consecutive number) and treatment, which was then prescribed and requested to the hospital pharmacy. Trial on-site monitoring verified this process as well as the accurateness accuracy of all the case report forms versus the primary information, treatment compliance, and all Good Clinical Practices procedures.
The trial hypothesis was to obtain a proportion of 70% of the patients with complete response at the 5th day after treatment onset with rSK suppositories (as obtained in the previous studies) and a 20% advantage (that could be considered clinically significant) over the response rate in the active control group. Assuming type I and II errors of 0.05 and 0.20, respectively, in a superiority model, a sample size of 161 subjects was estimated using the PASS software (http://www.ncss.com). With a 5% dropout rate taken into account, the final sample size was rounded to 170 subjects per group.
After review and query resolution, data were double-entered in databases built with the OpenClinica software (http://www.openclinica.com). SPSS version 15 software was used for statistical analyses. Complete response rate comparison between groups was assessed by the likelihood ratio χ2 test (or the Fisher´s exact test) and the 95% confidence interval (CI) of the estimated proportions and their differences. Times to complete response were estimated by survival analyses (Kaplan-Meier) and compared with the log-rank test. The level of significance chosen was α = 0.05. All analyses were done on an intention-to-treat basis. Missing evaluations were imputed as the last observation carried forward.
The statistical methods of this study were reviewed by Carmen M Valenzuela-Silva (Mathematician and Master in Statistics and Probabilities) from the Center for Genetic Engineering and Biotechnology, Havana, Cuba. Statistical methods are were suitable, and adequately and appropriately described when they are used to verify the results; only homogeneous results were averaged. The number of observations and subjects (n) is given, along with and the hypothesis used to calculate it. Losses in observations, such as drop-outs from the study, are reported; response values and their differences have 95% confidence limits calculated; the word “significantly” was replaced by its synonyms if it indicated extent, instead being strictly reserved for indicating statistical significance, based on a P value or a non-overlapping 95%CI. In addition, the final report of the trial was reviewed by the biostatistics experts of the Cuban NRA.
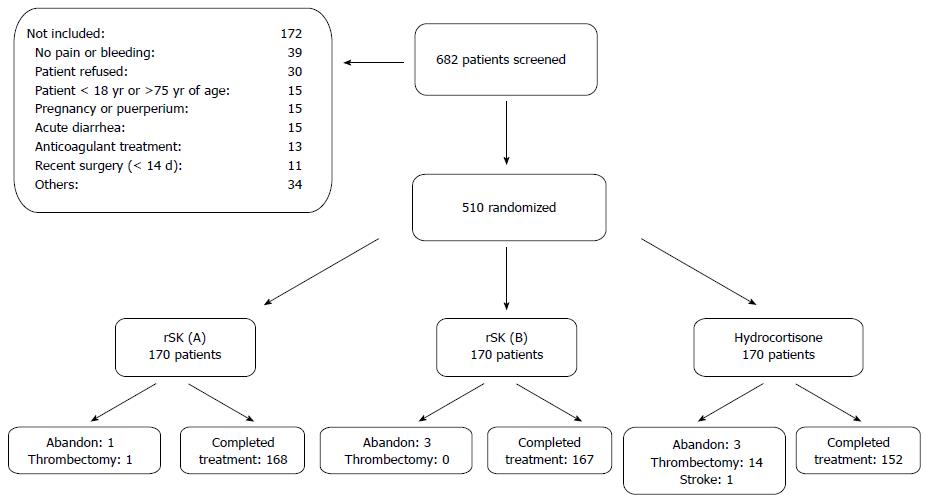
From November 2011 to January 2012, a total of 510 patients were included out of 682 that were screened. Their disposition is shown in Figure 1. The main causes for non-inclusion were no pain or bleeding, refusal to consent, age, pregnancy or puerperium, bleeding risk conditions (anti-coagulant therapy and recent surgery), other concomitant diseases (acute diarrhea), and other causes. Out of the 172 non-included subjects, 26 (15.1%) were due to possible contraindications or precautions of the rSK treatment.
Treatment was completed in 98.8% and 98.2% of the patients treated with rSK (schedule A and B, respectively), and in 89.4% of the hydrocortisone acetate group. Causes of non-compliance are described in Figure 1. Abandons and thrombectomies were due to symptom worsening and took place within the first 72 h of treatment. These patients, as well as the one with stroke, missed the response evaluation visits and were considered failures.
Table 1 shows the demographic and baseline characteristics of the patients. Most of them were males aged 18 to 75 years-old, with the ethnic distribution of the Cuban population in the participating provinces. Treatment was started within 5 d after the onset of symptoms in more than 75% of the subjects. Hemorrhoids were 64% external and 75% with thrombosis. Symptoms presented for the first time in 74% of the patients, and 7% had previous thrombectomy. No relevant imbalances between the groups can be seen.
| rSK-A | rSK-B | Hydrocortisone | Total | ||
| n | 170 | 170 | 170 | 510 | |
| Gender | Male/female (%male) | 90/80 (52.9) | 95/75 (55.9) | 92/78 (54.1) | 277/233 (54.3) |
| Ethnicity | White | 76 (44.7) | 98 (57.6) | 80 (47.1) | 254 (49.8) |
| Black | 18 (10.6) | 18 (10.6) | 20 (11.8) | 56 (11.0) | |
| Mestizo | 76 (44.7) | 54 (31.8) | 70 (41.2) | 200 (39.2) | |
| Age | Median ± QR (Min; Max) | 46 ± 19 (19; 75) | 46 ± 14 (19; 75) | 46 ± 18 (18; 74) | 46 ± 17 (18; 75) |
| Days from start of symptoms to treatment onset | Median ± QR (Min; Max) | 3 ± 2 (0; 22) | 2 ± 1 (0; 27) | 2 ± 1 (0; 15) | 3 ± 1 (0; 27) |
| Classification of the hemorrhoids | External | 103 (60.6) | 112 (65.9) | 112 (65.9) | 327 (64.1) |
| Internal | 56 (32.9) | 51 (30.0) | 48 (28.2) | 155 (30.4) | |
| Mixed | 11 (6.5) | 7 (4.1) | 10 (5.9) | 28 (5.5) | |
| Grade of prolapse, (I or II):(III or IV) | 54:13 | 37:21 | 43:15 | 134:49 | |
| Presence of thrombosis | 122 (71.8) | 129 (75.9) | 133 (78.2) | 384 (75.3) | |
| Debut of disease | 130 (76.5) | 122 (71.8) | 123 (72.4) | 375 (73.5) | |
| Previous thrombectomy | 12 (7.1) | 12 (7.1) | 11 (6.5) | 35 (6.9) | |
Table 2 shows the results of the clinical evaluations. The analyses were “intention-to-treat” and involved all patients who were randomly assigned. Dependence of the complete response rate was obtained for the main trial outcome at 5 d. rSK suppository-treated groups showed the expected 87% complete response rate and a > 60% advantage over the hydrocortisone group. A multilevel analysis did not yield relevant differences among the participating hospitals regarding this main trial outcome (results not shown). Secondary outcome evaluations at 3 and 10 d yielded highly significant differences as well. Time to response was seven days shorter in the rSK groups.
| I: rSK schedule A | II: rSK schedule B | III: Hydrocortisone | P value | |
| n | 170 | 170 | 170 | |
| Response after 3 d | 117 (68.8) | 109 (64.1) | 12 (7.1) | < 0.0011 |
| Response after 5 d (95%CI) | 156 (91.8) (87.3-96.2) | 155 (91.2) (86.6-95.7) | 46 (27.1) (20.1-34.0) | < 0.0011 |
| Difference with III (95%CI) | 64.6 (56.7-72.2) | 63.9 (55.7-72.0) | ||
| Response after 10 d | 165 (97.1) | 159 (93.5) | 114 (67.1) | < 0.0011 |
| Days to response, median (95%CI) | 3 (2.9-3.1) | 3 (2.9-3.1) | 10 (9.3-10.7) | < 0.0012 |
Complete response rate was > 85% in all subgroups treated with rSK, regardless of the hemorrhoid classification or type of acute event (Table 3). A significant advantage of the rSK treatment was found for all subgroups. Thrombectomy was needed in very few cases but dependence was detected in significant treatment.
| I: rSK schedule A | II: rSK schedule B | III: Hydrocortisone | P value1 | |
| Type of acute event | ||||
| Without thrombosis | 42/48 (87.5) | 36/41 (87.8) | 10/37 (27.0) | < 0.0001 |
| With thrombosis | 114/122 (93.4) | 119/129 (92.2) | 36/133 (27.1) | < 0.0001 |
| Thrombectomy | 2/122 (1.6) | 2/129 (1.6) | 14/133 (10.5) | < 0.001 |
| Hemorrhoid classification | ||||
| External | 96/103 (93.2) | 100/112 (89.3) | 19/112 (17.0) | < 0.0001 |
| Internal + mixed | 60/67 (89.6) | 55/58 (94.8) | 27/58 (46.6) | < 0.0001 |
| Prolapse grade | ||||
| I or II | 49/54 (91) | 35/37 (95) | 21/43 (49) | < 0.0001 |
| III or IV | 11/13 (85) | 20/21 (95) | 6/15 (40) | < 0.001 |
A total of 14/170 and 16/340 adverse events (AE) were reported in the hydrocortisone acetate and rSK groups, respectively. The AE are shown in Table 4. Most reports were mild or moderate. Only three events were severe: two patients with local pain and one stroke in the hydrocortisone acetate group. None of the AE was considered to have a definite causal relationship with treatment, since they could be explained by the underlying illness.
| I: rSK schedule A | II: rSK schedule B | III: Hydrocortisone | |
| n | 170 | 170 | 170 |
| Total subjects with adverse events | 5 (2.9) | 7 (4.1) | 11 (6.5) |
| Events | |||
| Anal pruritus | 2 (1.2) | 4 (2.4) | 2 (1.2) |
| Rectal bleeding | 2 (1.2) | 1 (0.6) | 1 (0.6) |
| Anal fissure | 1 (0.6) | 0 | 3 (1.8) |
| Headache | 1 (0.6) | 2 (1.2) | 0 |
| Anal pain | 0 | 0 | 2 (1.2) |
| Local burning sensation | 1 (0.6) | 0 | 1 (0.6) |
| Tenesmus | 1 (0.6) | 0 | 1 (0.6) |
| Constipation | 0 | 0 | 1 (0.6) |
| Diarrhea | 0 | 0 | 1 (0.6) |
| Discomfort due to mass sensation | 1 (0.6) | 0 | 0 |
| Edema | 0 | 0 | 1 (0.6) |
| Stroke | 0 | 0 | 1 (0.6) |
This work reports the therapeutic efficacy of rSK suppositories on acute hemorrhoids in a randomized trial when compared to the commonly-used treatment of hydrocortisone acetate. These active controls are preferred to a placebo, due to ethical considerations, since it is not acceptable to exclude patients from an accepted therapy. No reports of the quantitative effect of hydrocortisone acetate-based preparations on the resolution of acute hemorrhoidal illness were found, whether from controlled clinical trials or not[24,25]. The sample size of the study was enough to fulfill its aim. The multicentric character of the trial, with several provinces involved, contributes to the generalizability (external validity and applicability) of the findings. A double-blind design was not possible in this case, since the products had different presentations and schedules. However, central randomization, concealment, and active monitoring minimized this source of bias. The trial performed well, without deviations and with minimal dropouts. This was facilitated by the fact that treatment was short lasting, simple, and inclusion could be completed easily. Therefore, the internal validity of the study was adequate.
The trial hypothesis was fulfilled: a > 70% complete response rate was obtained on the 5th day in the rSK treated groups and the advantage over the control group was > 60%, which was far above the expected difference. The difference found at the 3rd day indicates that the effect begins shortly after treatment. At 10 d, more than 90% of the patients treated with rSK had responded completely and the difference with the control group was 26%, despite the fact that the natural course of the acute event leads to its resolution. The results confirm previous evidence of effect found for this product[11-13]. Similar results were obtained with six or eight administrations of rSK suppositories.
The median time-to-complete response was shortened to seven days with respect to the hydrocortisone acetate preparation. The shorter response time is an interesting result with a potential impact on patients’ quality of life. This illness frequently affects the active population that needs to return as soon as possible to their normal daily routine[4,26-28]. Other larger studies have reported longer healing periods with control standard treatments and other agents[22,24,25,29].
Subgroup analyses showed the efficacy for all clinical variants of acute hemorrhoids, including all grades of prolapse. The differences were large enough to exclude that any of these significant findings were from chance alone.
Need for thrombectomy was 10.5% in the control group, similar to other reports[9,11] and the control groups in the previous rSK studies. The rSK-treated groups showed a much smaller thrombectomy rate, in agreement with the previous trial with this product[12,13] and its proposed mechanism of action as a thrombolytic agent. Since thrombosis is a frequent complication of hemorrhoidal disease (75% in this series), the possibility of a non-surgical approach would be beneficial to a noteworthy number of patients.
The results indicate that the rSK suppository is safe and tolerable. The adverse events reported were minimal, mild, resolved spontaneously, and had a low causal relationship with the rSK. In the same sense, there were no bleeding complications in the rSK group. In fact, bleeding as a symptom of the underlying disease cleared adequately in this group. It was previously shown that rSK suppository application does not alter systemic hemostasis[11].
The results of this trial show that rSK suppository preparation has significant advantages over widely used over-the-counter hydrocortisone acetate control preparation for the treatment of acute hemorrhoidal illness, with an adequate safety profile. These results completed the requirements for product approval in Cuba by the National Regulatory Authority[30]. Further pharmacosurveillance of the use of this product in actual clinical practice should confirm its effectiveness and explore its use in other clinical settings in order to optimize the cost-benefit ratio.
The authors wish to acknowledge the National Coloproctology Group from the Public Health Ministry of Cuba for its support; specially Drs. Ahmed Guzmán-Guerrero and Francisco Llorente-Llano, who actively advised and supported this work.
Acute hemorrhoids are a worldwide health problem. Although several medicines have been tested, significant benefits have not been obtained to control this condition. When response is obtained, the result takes many days and, in an important group of patients where surgical or other invasive procedures are indicated, are not exempted from complications. Streptokinase (SK) is a fibrinolytic agent. Its local application on hemorrhoids, where thrombosis and/or inflammation with microthrombi may be present, was tested in previous trials where a 200000 IU suppository showed a beneficial effect on hemorrhoidal symptoms (36% larger response rate, 5 d faster) over excipient controls and (43% larger response rate, 5 d faster) over a phenylephrine-based over-the-counter suppository, with an adequate safety profile. The aim of the present work was to compare the efficacy and safety of 200000 IU SK suppositories vs a hydrocortisone acetate-based over-the-counter suppository for the treatment of acute hemorrhoids through a multicenter, randomized clinical trial.
Medical treatments for acute hemorrhoid episodes very seldom come from randomized, controlled clinical trials. The current paper describes that a candidate to a new therapeutic alternative, based on thrombolysis, shows significant efficacy advantage with respect to a widely-used over-the-counter product.
The results of this trial show that the SK suppository preparation has significant advantages over the widely-used over-the-counter hydrocortisone control preparation for the treatment of acute hemorrhoidal illness (91% response rate at 5 d vs 27% with the control; median time to response: 3 d vs 10 d in the control), with an adequate safety profile. Fewer thrombectomy procedures were necessary (1.6% vs 10.5% in the controls).
These results confirm the efficacy of a new first-in-class treatment for this common disease. Further pharmacosurveillance of the use of this product in actual clinical practice should confirm its effectiveness and explore its use in other clinical settings in order to optimize the cost-benefit ratio.
Fibrinolysis: breakdown of fibrin that forms the clots that occlude blood flow in veins or arteries. Streptokinase: indirect fibrinolytic agent that interacts with plasminogen, forming an active complex with protease action that activates plasminogen into plasmin, which is the direct fibrinolytic enzyme.
In this paper, the authors investigated the efficacy and safety of recombinant streptokinase suppositories in acute hemorrhoidal disease. Previous studies showed that the local application of streptokinase in patients with thrombosed hemorrhoids had a significantly beneficial effect on hemorrhoidal symptoms. This is a topic of interest to researchers in related areas.
P- Reviewer: Peng JS S- Editor: Yu J L- Editor: Rutherford A E- Editor: Zhang DN
| 1. | Altomare DF, Giannini I. Pharmacological treatment of hemorrhoids: a narrative review. Expert Opin Pharmacother. 2013;14:2343-2349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Ganz RA. The evaluation and treatment of hemorrhoids: a guide for the gastroenterologist. Clin Gastroenterol Hepatol. 2013;11:593-603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Lohsiriwat V. Hemorrhoids: from basic pathophysiology to clinical management. World J Gastroenterol. 2012;18:2009-2017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 270] [Cited by in F6Publishing: 207] [Article Influence: 17.3] [Reference Citation Analysis (10)] |
| 4. | Sanchez C, Chinn BT. Hemorrhoids. Clin Colon Rectal Surg. 2011;24:5-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Song SG, Kim SH. Optimal treatment of symptomatic hemorrhoids. J Korean Soc Coloproctol. 2011;27:277-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Fox A, Tietze PH, Ramakrishnan K. Anorectal conditions: hemorrhoids. FP Essent. 2014;419:11-19. [PubMed] [Cited in This Article: ] |
| 7. | Lohsiriwat V. Approach to hemorrhoids. Curr Gastroenterol Rep. 2013;15:332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Jacobs D. Clinical practice. Hemorrhoids. N Engl J Med. 2014;371:944-951. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 9. | Wald A, Bharucha AE, Cosman BC, Whitehead WE. ACG clinical guideline: management of benign anorectal disorders. Am J Gastroenterol. 2014;109:1141-1157; (Quiz) 1058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 177] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 10. | Cintron J, Abacarian H. Benign anorectal: hemorrhoids. The ASCRS of Colon and Rectal Surgery. New York, NY: Springer-Verlag 2007; 156-177. [Cited in This Article: ] |
| 11. | de Miguel M, Oteiza F, Ciga MA, Ortiz H. [The surgical treatment of hemorrhoids]. Cir Esp. 2005;78 Suppl 3:15-23. [PubMed] [Cited in This Article: ] |
| 12. | Herold A, Joos A, Bussen D. [Operations for hemorrhoids: indications and techniques]. Chirurg. 2012;83:1040-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Ratto C, Parello A, Veronese E, Cudazzo E, D’Agostino E, Pagano C, Cavazzoni E, Brugnano L, Litta F. Doppler-guided transanal haemorrhoidal dearterialization for haemorrhoids: results from a multicentre trial. Colorectal Dis. 2015;17:O10-O19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Quintero L, Hernández-Bernal F, Marrero MA, Valenzuela CM, López M, Barcelona S, Ibargollín R, Bobillo H, Aguilera A, Bermúdez Y. Initial evidence of safety and clinical effect of recombinant streptokinase suppository in acute hemorrhoidal disease. Open, proof-of-concept, pilot trial. BA. 2010;27:277-280 Available from: http://www.elfosscientiae.cigb.edu.cu/PDFs\BA\2010\27\4BA002704OL277-280.pdf. [Cited in This Article: ] |
| 15. | Hernández-Bernal F, Valenzuela-Silva CM, Quintero-Tabío L, Castellanos-Sierra G, Monterrey-Cao D, Aguilera-Barreto A, López-Saura P. Recombinant streptokinase suppositories in the treatment of acute haemorrhoidal disease. Multicentre randomized double-blind placebo-controlled trial (THERESA-2). Colorectal Dis. 2013;15:1423-1428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Hernández-Bernal F, Castellanos-Sierra G, Valenzuela-Silva CM, Catasús-Álvarez KM, Valle-Cabrera R, Aguilera-Barreto A, López-Saura PA. Recombinant streptokinase vs phenylephrine-based suppositories in acute hemorrhoids, randomized, controlled trial (THERESA-3). World J Gastroenterol. 2014;20:1594-1601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 11] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Gupta PJ. Suppositories in anal disorders: a review. Eur Rev Med Pharmacol Sci. 2007;11:165-170. [PubMed] [Cited in This Article: ] |
| 18. | Estrada MP, Hernández L, Pérez A, Rodríguez P, Serrano R, Rubiera R, Pedraza A, Padrón G, Antuch W, de la Fuente J. High level expression of streptokinase in Escherichia coli. Biotechnology (N Y). 1992;10:1138-1142. [PubMed] [Cited in This Article: ] |
| 19. | Aguilera A, Muñoz L, Bermúdez Y, Arias D, Martínez Y, García G, Hernández L, Martínez E, Valdés R. Validation of a Chromogenic Substrate Method for Biological Activity Quantification of Streptokinase. BioProcess J. 2014;13:49-59. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Aguilera A, Bermudez Y, Martínez E, Marrero MA, Muñoz L, Páez R, Tamargo B, Hernández LF, García O. Formulation development of a recombinant Streptokinase suppository for hemorrhoids treatment. Biotecnología Aplicada. 2013;30:182-186. [Cited in This Article: ] |
| 21. | Kaidar-Person O, Person B, Wexner SD. Hemorrhoidal disease: A comprehensive review. J Am Coll Surg. 2007;204:102-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 22. | Naranjo CA, Shear NH, Busto U. Adverse drug reactions. Principles of medical pharmacology. 6th ed. New York: Oxford University Press 1998; 791-800. [Cited in This Article: ] |
| 23. | Betancourt BY, Marrero-Miragaya MA, Jiménez-López G, Valenzuela-Silva C, García-Iglesias E, Hernández-Bernal F, Debesa-García F, González-López T, Alvarez-Falcón L, López-Saura PA. Pharmacovigilance program to monitor adverse reactions of recombinant streptokinase in acute myocardial infarction. BMC Clin Pharmacol. 2005;5:5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Chan KK, Arthur JD. External haemorrhoidal thrombosis: evidence for current management. Tech Coloproctol. 2013;17:21-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Misra MC. Drug treatment of haemorrhoids. Drugs. 2005;65:1481-1491. [PubMed] [Cited in This Article: ] |
| 26. | Greenspon J, Williams SB, Young HA, Orkin BA. Thrombosed external hemorrhoids: outcome after conservative or surgical management. Dis Colon Rectum. 2004;47:1493-1498. [PubMed] [Cited in This Article: ] |
| 27. | Menteş BB, Görgül A, Tatlicioğlu E, Ayoğlu F, Unal S. Efficacy of calcium dobesilate in treating acute attacks of hemorrhoidal disease. Dis Colon Rectum. 2001;44:1489-1495. [PubMed] [Cited in This Article: ] |
| 28. | Perrotti P, Antropoli C, Molino D, De Stefano G, Antropoli M. Conservative treatment of acute thrombosed external hemorrhoids with topical nifedipine. Dis Colon Rectum. 2001;44:405-409. [PubMed] [Cited in This Article: ] |
| 29. | Herold A, Dietrich J, Aitchison R. Intra-anal Iferanserin 10 mg BID for hemorrhoid disease: a prospective, randomized, double-blind, placebo-controlled trial. Clin Ther. 2012;34:329-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | CECMED (Centro para el Control Estatal de Medicamentos, Equipos y Dispositivos Médicos)/Ministry of Public Health, Cuba. Approval Certificate Proctokinasa® (recombinant Streptokinase). No. B-12-129-B01, 2012-08-23. Available from: http://www.cecmed.cu/Pages/RegSan.htm. [Cited in This Article: ] |