Published online Jan 14, 2015. doi: 10.3748/wjg.v21.i2.439
Peer-review started: April 2, 2014
First decision: April 28, 2014
Revised: May 15, 2014
Accepted: July 22, 2014
Article in press: July 22, 2014
Published online: January 14, 2015
AIM: To investigate the densities of dendritic cells (DCs) and FOXP3+ regulatory T cells (Tregs) and their interrelations in the small bowel mucosa in untreated celiac disease (CD) patients with and without type 1 diabetes (T1D).
METHODS: Seventy-four patients (45 female, 29 male, mean age 11.1 ± 6.8 years) who underwent small bowel biopsy were studied. CD without T1D was diagnosed in 18 patients, and CD with T1D was diagnosed in 15 patients. Normal small bowel mucosa was found in two T1D patients. Thirty-nine patients (mean age 12.8 ± 4.9 years) with other diagnoses (functional dyspepsia, duodenal ulcer, erosive gastritis, etc.) formed the control group. All CD patients had partial or subtotal villous atrophy according to the Marsh classification: Marsh grade IIIa in 9, grade IIIb in 21 and grade IIIc in 3 cases. Thirty-nine patients without CD and 2 with T1D had normal small bowel mucosa (Marsh grade 0). The densities of CD11c+, IDO+, CD103+, Langerin (CD207+) DCs and FOXP3+ Tregs were investigated by immunohistochemistry (on paraffin-embedded specimens) and immunofluorescence (on cryostat sections) methods using a combination of mono- and double-staining. Sixty-six serum samples were tested for IgA-tissue transglutaminase (tTG) using a fully automated EliA™ Celikey® IgA assay (Pharmacia Diagnostics, Freiburg, Germany).
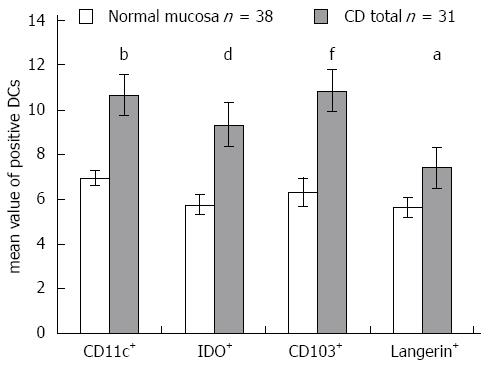
RESULTS: The density of CD11c+ DCs was significantly increased in CD patients compared with patients with normal mucosa (21.67 ± 2.49 vs 13.58 ± 1.51, P = 0.007). The numbers of FOXP3+ cells were significantly higher in CD patients (10.66 ± 1.50 vs 1.92 ± 0.37, P = 0.0002) and in patients with CD and coexisting T1D (8.11 ± 1.64 vs 1.92 ± 0.37, P = 0.002) compared with patients with normal mucosa. The density of FOXP3+ cells significantly correlated with the histological grade of atrophic changes in the small bowel mucosa according to the March classification (r = 0.62; P < 0.0001) and with levels of IgA antibody (r = 0.55; P < 0.0001). The densities of IDO+ DCs were significantly higher in CD patients (21.6 ± 2.67 vs 6.26 ± 0.84, P = 0.00003) and in patients with CD and coexisting T1D (19.08 ± 3.61 vs 6.26 ± 0.84, P = 0.004) compared with patients with normal mucosa. A significant correlation was identified between the densities of IDO+ DCs and FOXP3+ T cells (r = 0.76; P = 0.0001). The mean values of CD103+ DCs were significantly higher in CD patients (10.66 ± 1.53 vs 6.34 ± 0.61, P = 0.01) and in patients with CD and associated T1D (11.13 ± 0.72 vs 6.34 ± 0.61, P = 0.00002) compared with subjects with normal small bowel mucosa. The mean value of Langerin+ DCs was higher in CD patients compared with persons with normal mucosa (7.4 ± 0.92 vs 5.64 ± 0.46, P = 0.04).
CONCLUSION: The participation of diverse DC subsets in the pathological processes of CD and the possible involvement of tolerogenic DCs in Tregs development to maintain intestinal immunological tolerance in CD patients are revealed.
Core tip: Significantly higher densities of CD11c+ dendritic cells (DCs) and of tolerogenic IDO+, CD103+ and Langerin+ DCs in the small bowel mucosa of patients with celiac disease (CD) compared with subjects with normal small bowel mucosa were revealed using immunohistochemistry in 74 patients. This article highlights the participation of diverse DC subsets in the pathological processes in the small bowel mucosa, pointing out the importance of Langerin+ DCs in untreated CD patients with and without type 1 diabetes and indicating the possible involvement of tolerogenic DCs in regulatory T cells development to maintain intestinal immunological tolerance in CD patients.
- Citation: Vorobjova T, Uibo O, Heilman K, Uibo R. Increased density of tolerogenic dendritic cells in the small bowel mucosa of celiac patients. World J Gastroenterol 2015; 21(2): 439-452
- URL: https://www.wjgnet.com/1007-9327/full/v21/i2/439.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i2.439
Celiac disease (CD) is characterized by an altered immune response to ingested wheat gluten and related prolamins in rye and barley, which leads to inflammation, villous atrophy and crypt hyperplasia in the small bowel mucosa, accompanied by an increased number of infiltrating lymphocytes in the epithelium and in the lamina propria (LP)[1]. The generation of inflammatory and damaging T cells is governed by several control mechanisms, while intestinal dendritic cells (DCs) are the key cells that regulate which T cells become activated or deleted. In this process, regulatory T cells (Tregs) also play an important role in intestinal homeostasis[2,3].
The role of the gut immune system is also thought to be of crucial importance in the pathogenesis of type 1 diabetes (T1D)[4-6]. Several research groups have demonstrated a marked association between the development of T1D and preceding alterations in the small bowel mucosa, including altered gut microbiota in these patients[4,7,8]. As a result, CD and T1D coexist more frequently compared with chance occurrence, with an average prevalence of CD among children with T1D of 4.5% (0.97%-16.4%) in 26 reports[9].
DCs have received much attention in CD due to their strategic role in gut homeostasis by processing external antigens (including wheat proteins) and by determining tolerance to self-antigens[10,11]. The important role of CD11c+CD103+ DCs in the induction of Tregs differentiation has been established[12]. CD103+ DCs are required for the induction of tolerogenic immune responses and contribute to the control of inflammatory responses and homeostasis in the intestinal mucosa by the incremental conversion of naive T cells into FOXP3+ Treg cells[13,14]. Functionally specialized CD103+ DCs derived from the small intestinal LP appear to be the only cells able to regulate T cells homing[15].
In addition to the role of CD103+ DCs, that of indoleamine 2,3-dioxygenase (IDO) for Tregs induction was also demonstrated[16-18]. IDO is an immunomodulatory enzyme involved in tryptophan catabolism with immunosuppressive effects that has been implicated in the control of intestinal inflammation[17]. Higher IDO expression has been measured in intestinal biopsies from CD patients[19].
Among the DCs covering body surfaces, either mucosa or skin, DCs carrying Langerin (CD207) proteins have received considerable interest[20]. Langerin was originally identified as a Langerhans cell (LC)-specific C-type lectin receptor involved in antigen capture[21]. Langerin expression is predominant in skin DCs, but Langerin-expressing DCs are also present in the mucosal tissue and can be induced by immunization and sometimes by nutrient deficiency[22]. The expression of Langerin by CD103+CD11b+ LP DCs in the human ileum has recently been reported[23]. However, the presence of Langerin+ DCs in the small bowel mucosa in pathological conditions such as CD and T1D has not yet been studied. Still, one could preclude that Langerin+ cells might be involved in CD pathogenesis, taking into account the fact that interleukin (IL)-15, a central cytokine in the CD mucosa[24,25], can skew DC precursors to differentiate into Langerin+ cells[26]. In addition, we do not know how Langerin+ DCs are related to other DC subsets and Tregs in the human small intestinal mucosa. Because Langerin+ DCs are significant modulators of events in the skin, another important immunological barrier of the organism, knowledge of the function of these cells in the small intestinal mucosa may be of general importance.
In the present study, we aimed to investigate the densities of CD11c+ DCs, CD103+ DCs, IDO+ DCs and Langerin+ DCs, along with FOXP3+ Treg cells, in the small bowel mucosa in CD patients with and without coexisting T1D and to compare these densities with the those found in histologically normal intestinal mucosa in persons with functional dyspepsia using immunohistochemical and immunofluorescence methods.
Seventy-four patients (45 female, 29 male, mean age 11.1 ± 6.8 years) who were admitted to the Children’s Clinic of Tartu University Hospital and underwent small bowel biopsy were studied. All patients were recruited at the time of CD diagnosis. CD without T1D was diagnosed in 18 (mean age 9.9 ± 10.7 years) patients, and CD with T1D was diagnosed in 15 (mean age 8.5 ± 3.7 years) patients. Normal small bowel mucosa was found in two T1D patients with equivocal values of IgA antibodies to tissue transglutaminase (tTG, 7.0 and 9.8 U/mL, respectively; both boys, 13 and 4 years old). Because healthy persons could not be included in the control group due to ethical constraints (gastroduodenoscopy), we selected thirty-nine patients (mean age 12.8 ± 4.9 years) with other diagnoses (mainly with functional dyspepsia, duodenal ulcer and erosive gastritis) for the control group (Table 1). Diagnosis of CD was established on the basis of the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) criteria[27]. Morphologically, the small bowel mucosa was assessed according to the Marsh classification[28] on the basis of biopsy samples taken by gastroduodenoscopy from distal duodenum.
| Study group | Number of persons | Age1 (yr) | Male | Female | IgA-tTG-positive (> 10 U/mL) | ||
| n | Age1 (yr) | n | Age1 (yr) | ||||
| CD | 18 | 9.9 ± 10.7 | 5 (28)b | 8.3 ± 3.1 | 13 (72)b | 10.5 ± 12.5 | 18/18b |
| CD with T1D | 15 | 8.5 ± 3.7 | 8 (53) | 8.6 ± 4.8 | 7 (47) | 8.5 ± 2.1 | 11/11 |
| T1D | 2 | 8.5 ± 6.4 | 2 (100) | 8.5 ± 6.4 | 0 | - | 0/2 |
| Control group | 39 | 12.8 ± 4.9 | 14 (36)b | 11.4 ± 5.9 | 25 (64)b | 13.5 ± 4.3 | 2/35b |
| Total | 74 | 11.1 ± 6.8 | 29 (39)b | 9.9 ± 5.2 | 45 (61)b | 11.9 ± 7.6 | 31/66 |
According to this classification, all CD patients had partial or subtotal villous atrophy: a Marsh grade of IIIa was observed in 9 cases, grade IIIb in 21 cases and grade IIIc in 3 cases. Thirty-nine patients without CD and 2 with T1D had normal small bowel mucosa (Marsh grade 0).
All patients with CD had IgA antibodies to tTG as assessed using an EliA™ Celikey® IgA assay (Pharmacia Diagnostics, Freiburg, Germany) (mean value 414.5 ± 130.5 U/mL). In the control group, two persons with normal small bowel mucosa (a 16-year-old boy and an 8-year-old girl, both with functional dyspepsia) were positive for IgA antibodies to tTG (515.0 and 58.3 U/mL, respectively). The mean value of IgA antibodies to tTG in the control group was 17.9 ± 14.7 U/mL.
This study complies with the Declaration of Helsinki and was approved by the Ethics Review Committee for Human Research of the University of Tartu. All studied children and/or their parents gave written informed consent to participate in the study.
Small bowel biopsy from the distal duodenum was performed by gastroduodenoscopy. Two specimens were used for morphological and immunohistochemical examinations, and the third specimen was immediately quick-frozen in Tissue Tek OCT Compound (Sakura, Finetek, Finland) and stored at -80 °C for further use in immunofluorescence studies.
The following antibodies were used: monoclonal mouse anti-CD11c (NCL-L-CD11c-563) Novocastra™ Liquid, diluted 1:80; monoclonal mouse anti-human FOXP3 antibodies (clone 236A/E7, Abcam, Cambridge, United States), diluted 1:60 (16.6 μg/mL in 1% normal horse serum); and anti-IDO (mouse monoclonal-anti-human indoleamine 2,3-dioxygenase), clone 1F8.2 (Chemicon, Millipore Corporation), diluted 1:50.
For the detection of CD11c+ DCs and FOXP3+ T cells in the small bowel mucosa of 63 patients, double staining was used; mono-staining for IDO+ DCs was performed in 58 patients.
Formalin-fixed biopsy specimens of the small bowel mucosa were studied using the Avidin-Biotin method. Paraffin slides were deparaffinized, and antigen retrieval was achieved by microwave treatment in 1 mmol/L EDTA (Scharlau Chemie S.A., pH 8.0), once at 900 W for 7 min and twice at 440 W for 5 min. After cooling for 20 min at room temperature (rt), endogenous peroxidase activity was quenched by incubating the slides for 30 min at rt in 0.5% H2O2-methanol. To avoid nonspecific reactions, slides were treated with 2.5% normal horse serum (Vectastain ABC Kit, Vector Laboratories, Burlingame, CA, United States) for 10 min at rt. Additionally, to block the binding of antibodies to the Fc receptor, the FcR Blocking Reagent (human, Miltenyi Biotec GmbH, Germany), diluted 1:100, was used for 10 min at 4 °C. The sections were incubated with monoclonal mouse anti-CD11c antibody for 15 min at rt, then overnight at 4 °C. We used biotinylated anti-mouse IgG (Vectastain ABC Kit, Vector Laboratories, Burlingame, CA, United States), diluted 1:200 (incubation for 30 min at rt), as the secondary antibody. The bound antibody was detected with a commercial avidin-biotin immunoperoxidase system (Vectastain ABC Kit, Vector Laboratories, Burlingame, CA, United States) according to the manufacturer’s instructions, using the Vector VIP Peroxidase substrate kit (SK-4600) (incubation for 10 min) (purple-red staining). The reaction was stopped by rinsing the sections in cold water, after which staining for FOXP3 was performed using monoclonal mouse anti-human FOXP3 antibodies for 1 h at rt, followed by incubation overnight at 4 °C. After washing in 1 × Tris-buffered saline (Tris-HCI, pH 7.5), the sections were incubated with the above-mentioned secondary antibodies using the Vectastain ABC Kit (Vector Laboratories, Burlingame, CA, United States). The bound antibody was visualized using Vector SG (Vector Laboratories, Burlingame, CA, United States) as the substrate (incubation for 10 min) (blue staining). Next, the sections were immersed in cold water to stop the reaction, after which the stained tissue sections were dehydrated and mounted in Canada balsam. We used tissue sections without primary antibody (incubation with 1% horse serum) for the negative control and human tonsil sections for the positive control.
Staining for IDO+ DCs on paraffin sections was performed as described above; primary antibodies were incubated with the sections overnight at 4 °C. The bound antibody was detected with a commercial avidin-biotin immunoperoxidase system (Vectastain ABC Kit, Vector Laboratories, Burlingame, CA, United States) according to the manufacturer’s instructions, using the Vector VIP Peroxidase substrate kit (SK-4600) (incubation for 10 min) (purple-red staining).
Double-staining for IDO and CD103 and for CD11c and CD103 was performed on frozen sections of the small bowel mucosa of 71 patients. Mono-staining for Langerin (CD207) on sections from the same frozen biopsies was performed for 70 patients. The 4 μm frozen sections cut with a cryomicrotome (Leica CM1950, Leica Microsystems, Germany) were mounted on SuperFrost Plus slides (Menzel GmbH and Co KG, Braunschweig, Germany), air-dried for 1 h and used immediately. Tissue sections were fixed in 4% paraformaldehyde for 10 min at rt, followed by permeabilization with 0.3% Triton-X 100 (SERVA, Feinbiochemica, Heidelberg, Germany) in Tris buffer for 30 min at rt.
Before incubation with primary anti-IDO antibodies, endogenous biotin was blocked using the Biotin Blocking System Kit (DAKO, Cytomation, Carpinteria, United States) for 10 min at rt. Additionally, to block the binding of antibodies to the Fc receptor, the FcR Blocking Reagent (human from Miltenyi Biotec GmbH, Germany), diluted 1:100, was used on all studied sections for 10 min at 4 °C. This reagent was also used before incubation with anti-CD11c, anti-CD103 and anti-Langerin primary antibodies.
The sections were incubated with anti-IDO clone 1F8.2 (Chemicon, Millipore Corporation) monoclonal mouse-anti human IDO antibody, diluted 1:50, in TRIS buffer overnight at 4 °C in a humid chamber under coverslips. For the secondary antibody, we used biotinylated anti-mouse IgG (in horse), diluted 1:150 (Vector Laboratories, Burlingame, CA, United States), and a positive reaction was visualized after the incubation with Streptavidin-Alexa blue 350 (Invitrogen, by Life Technologies), diluted 1:100, for 1 h at rt. After that, the slides were double-stained with anti-CD103 [Integrin αE (N-19): sc-6606, Santa Cruz Biotechnology] polyclonal goat-anti-human antibody, diluted 1:50, overnight at 4 °C, followed by incubation with donkey-anti-goat-Alexa 488 (Invitrogen, by Life Technologies), diluted 1:100 and incubated for one hour at rt, as the secondary antibody.
Cryostat sections cut from the same frozen biopsies were incubated in parallel with anti-CD11c [Integrin alpha X (H-68): sc-28663, Santa Cruz Biotechnology] and polyclonal rabbit-anti-human (diluted 1:50 and incubated overnight at 4 °C), followed by incubation with anti-rabbit IgG (whole molecule) conjugated with a Cy3 (Fab’) fragment sheep antibody (SIGMA-Aldrich, United States), diluted 1:100 and incubated for one hour at rt. After washing in TRIS buffer, the slides were double-stained with anti-CD103 [Integrin αE (N-19): sc-6606. Santa Cruz Biotechnology] polyclonal goat-anti-human (diluted 1:50 and incubated overnight at 4 °C), followed by incubation with donkey-anti-goat-Alexa 488 (Invitrogen, by Life Technologies), diluted 1:100 and incubated for one hour at rt, as the secondary antibody.
A separate cryostat section from the same frozen biopsy specimen was incubated with anti-Langerin (CD207) (N-14) goat-anti-human polyclonal antibody (Santa Cruz Biotechnology), diluted 1:50 and incubated overnight at 4 °C, followed by incubation with donkey-anti-Goat-Alexa 488 (Invitrogen, by Life Technologies), diluted 1:100 and incubated for one hour at rt, as the secondary antibody. After washing in TRIS buffer, the slides were mounted in TRIS-glycerol solution. The negative control was performed by omitting the primary antibody (incubation with TRIS buffer alone).
The paraffin sections were microscopically examined using a objective × 40 and a eyepiece × 10 in a Zeiss KF 2 transmitted light microscope (Carl Zeiss, Jena, Germany). The cryosections were examined under an immunofluorescence microscope (Olympus BX50, Japan) using objectives with magnification × 40 and an eyepiece with magnification × 10. The micro-photos were obtained using a Leica DM5500 B (Leica Microsystems CMS GmbH, Germany) under the same magnifications.
All sections were studied with the investigator blinded to the diagnosis data. In each of the 5 different microscopic fields, DCs positive for CD11c, CD103, IDO and Langerin, along with lymphocytes positive for FOXP3, were counted. Cell densities were expressed as the mean number of positively stained cells per field. The results of double-staining for IDO and CD11c and for CD11c and CD103 were analyzed in a similar manner.
Sixty-six sera samples were tested for IgA antibody (IgA-tTG) using a fully automated EliA™ Celikey® IgA assay (Pharmacia Diagnostics, Freiburg, Germany) according to the manufacturer’s instructions. According to the manufacturer’s suggestions, IgA-tTG values higher than 10 EliA U/mL were considered positive.
The results obtained for the different study groups are presented as mean ± SE. Statistical calculations were performed using the Graph Pad Prism 5.0 software using the t-test, Mann-Whitney U test and Spearman’s rank correlation test. The χ2 or Fisher exact test was used for nominal variables.
Differences were considered statistically significant at P < 0.05. Sensitivity, specificity and receiver operating characteristic (ROC) curves with the areas under the curve (AUC) were calculated for different DC markers and FOXP3 using StatsDirect software.
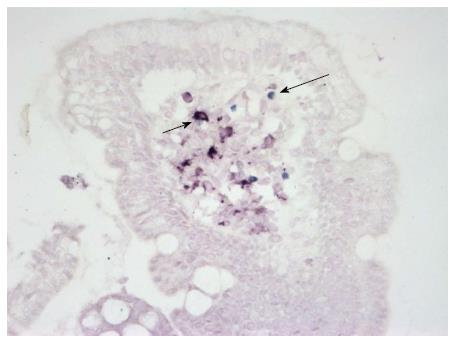
Using double-staining on paraffin sections, both CD11c+ DCs and FOXP3+ Treg cells were detected in the small bowel mucosa (Figure 1).
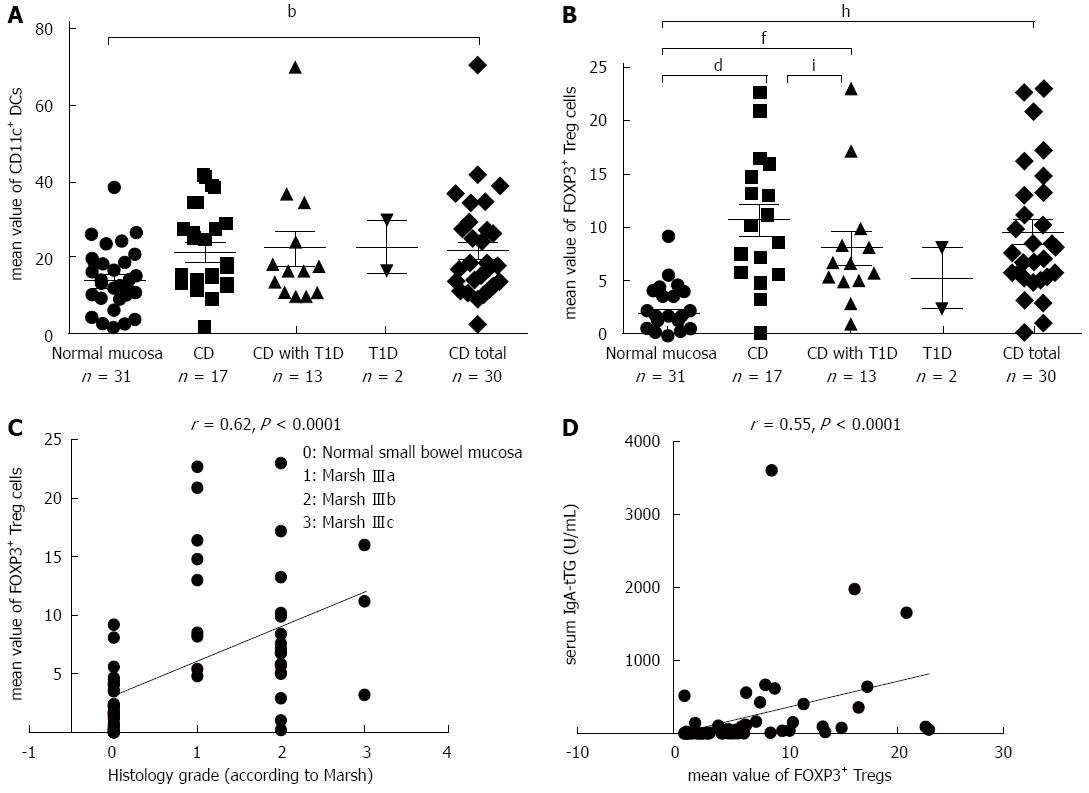
The density of CD11c+ DCs was significantly increased in CD patients compared with patients with normal mucosa (P = 0.007) (Figure 2A). The numbers of FOXP3+ Tregs were significantly higher in CD patients (P = 0.0002) and in patients with CD and coexisting T1D (P = 0.002) compared with patients with normal mucosa (Figure 2B). The density of FOXP3+ Tregs significantly correlated with the histological grade of atrophic changes in the small bowel mucosa according to the March classification (r = 0.62; P < 0.0001) and with levels of IgA-tTG (r = 0.55; P < 0.0001) (Figure 2C and D).
IDO+ DCs were detected on paraffin sections by mono-staining using the avidin-biotin immunohistochemical method (Figure 3).
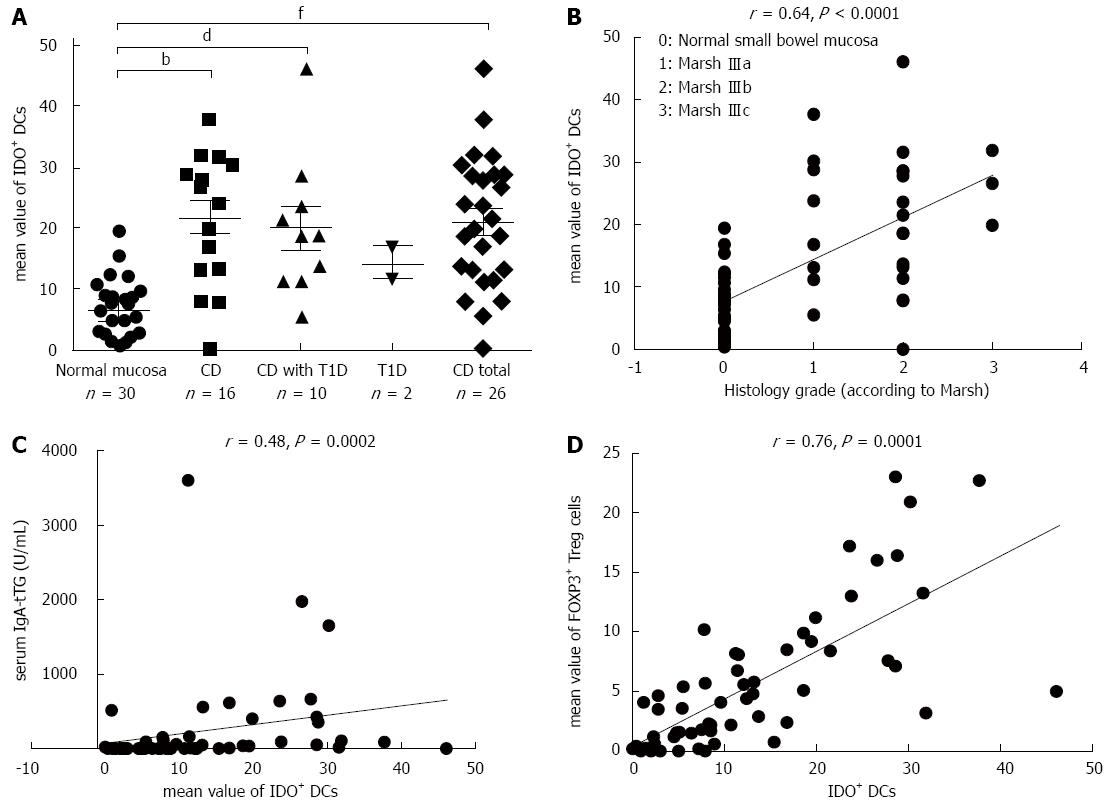
The densities of IDO+ DCs were significantly higher in CD patients (P = 0.00003) and in patients with CD and coexisting T1D (P = 0.004) compared with patients with normal mucosa (Figure 4A). This difference was dependent on the grade of atrophic and inflammatory changes in the small bowel mucosa, e.g., in subtotal villous atrophy (grade IIIb, according to Marsh), the mean value of IDO+ DCs was 10.37 ± 1.24 vs 6.40 ± 1.75 in partial villous atrophy (grade IIIa; P = 0.03). This finding is also supported by analysis of the correlation of density of IDO+ DCs on paraffin sections with the histological grade of the small bowel mucosa (r = 0.64; P < 0.0001) and with levels of IgA-tTG (r = 0.48; P = 0.0002) (Figure 4B and C). A significant correlation was established between the densities of IDO+ DCs and FOXP3+ Treg cells on paraffin sections for the entire study group (r = 0.76; P = 0.0001) (Figure 4D).
To simultaneously study the densities of CD11c+, CD103+ and IDO+ DCs in the same tissue section, we used double immunofluorescence staining on two consecutive cryostat serial sections from the same biopsy specimen of the small bowel mucosa - one for CD103 and IDO (Figure 5A) and the other for CD103 and CD11c (Figure 5B). The mean values of CD103+ DCs were significantly higher in CD patients (P = 0.01) and in patients with CD and associated T1D (P = 0.00002) compared with subjects with normal small bowel mucosa (Figure 6A). The density of CD103+ DCs was correlated with the histological grade of small bowel mucosa atrophy (r = 0.39; P = 0.0008) (Figure 6B). A significant correlation was identified between the densities of CD103+ DCs and FOXP3+ Tregs for the entire study group (r = 0.33; P = 0.0087) (Figure 6C). The mean values of CD11c+, IDO+ and CD103+ DCs were significantly higher in CD patients compared with persons with normal small bowel mucosa (Figure 7).
The IDO+ and CD103+ markers were simultaneously expressed in 30% of the DCs in the LP of the bowel mucosa of CD patients and in 25% of the DCs in the normal mucosa. Both markers, CD11c and CD103, were expressed in 5% of the DCs in the LP of the bowel mucosa of the studied persons, with CD11c accounting for 47.5% and CD103 accounting for 47%. Double expression of CD11c and CD103 was observed in 6.5% of the DCs in the CD group compared with 4.2% of the visualized DCs in persons with normal mucosa.
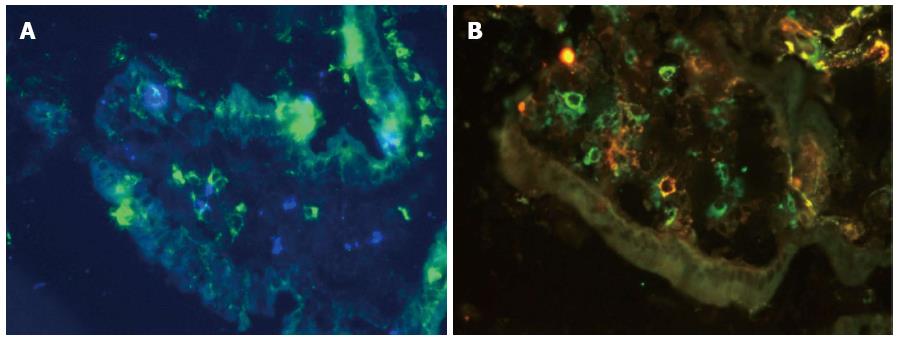
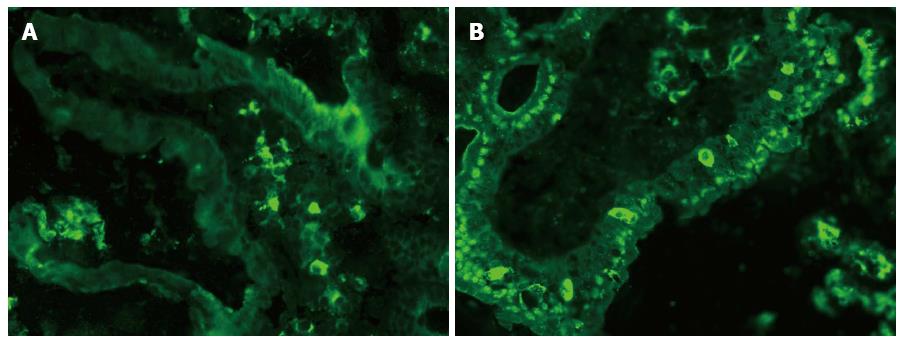
Immunofluorescence staining for Langerin on a cryostat section of the small bowel mucosa is presented in Figure 8. Langerin+ DCs were identified in both the LP (Figure 8A) and the epithelium of the villus of the small bowel mucosa (Figure 8B).
The mean value of Langerin+ DCs was higher in CD patients compared with persons with normal mucosa (P = 0.04; Figures 7 and 9). The correlations between Langerin+ DCs and CD11c+ DCs (r = 0.57; P = 0.04) and between Langerin+ DCs and CD103+ DCs were more pronounced in female CD patients (r = 0.77; P = 0.002).
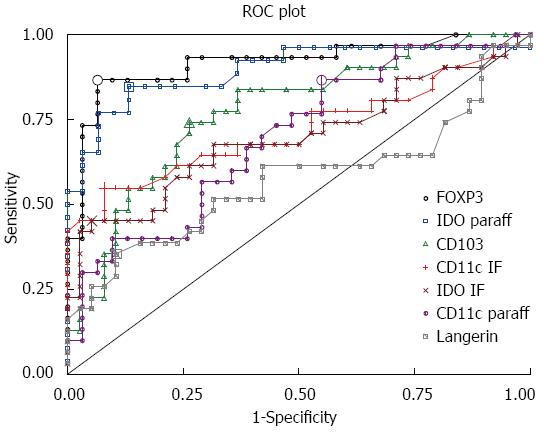
According to the analysis of the ROC curves, FOXP3 and IDO positivity had the highest discriminative power (AUC) for both CD (AUC = 0.92 for FOXP3 and 0.88 for IDO) and CD with coexisting T1D (AUC = 0.91 for FOXP3 and 0.91 for IDO), while CD103 had also a high discriminative power for CD associated with T1D (AUC = 0.86) (Table 2 and Figure 10).
| Markers | Entire study group | Patients with CD | Patients with CD and T1D | |||||||||
| AUC | Cut-off | Sensitivity | Specificity | AUC | Cut-off | Sensitivity | Specificity | AUC | Cut-off | Sensitivity | Specificity | |
| FOXP3 (paraff) | 0.92 | 4.8 | 0.87 | 0.94 | 0.92 | 4.8 | 0.88 | 0.94 | 0.91 | 5.0 | 0.85 | 0.94 |
| IDO (paraff) | 0.89 | 11.2 | 0.85 | 0.87 | 0.88 | 13.1 | 0.81 | 0.93 | 0.91 | 11.2 | 0.90 | 0.87 |
| CD11c (IF) | 0.72 | 10.1 | 0.55 | 0.92 | 0.79 | 10.1 | 0.61 | 0.92 | 0.62 | 12.1 | 0.38 | 1.00 |
| IDO (IF) | 0.70 | 9.7 | 0.45 | 0.95 | 0.72 | 10.1 | 0.50 | 0.97 | 0.67 | 9.7 | 0.39 | 0.95 |
| CD11c (paraff) | 0.70 | 10.6 | 0.87 | 0.45 | 0.71 | 24.8 | 0.47 | 0.90 | 0.68 | 16.6 | 0.62 | 0.71 |
| CD103 (IF) | 0.77 | 7.8 | 0.74 | 0.74 | 0.71 | 8.8 | 0.61 | 0.76 | 0.86 | 7.3 | 1.00 | 0.68 |
| Langerin (IF) | 0.57 | 8.8 | 0.36 | 0.90 | 0.59 | 8.6 | 0.44 | 0.84 | 0.55 | 7.4 | 0.54 | 0.68 |
The densities of IDO+ DCs (6.55 ± 0.51 vs 4.67 + 0.72), CD103+ (7.16 ± 0.85 vs 4.93 ± 0.65) and CD11c+ DCs (7.62 ± 0.43 vs 5.83 + 0.37) were significantly higher in females compared with males (P = 0.042, P = 0.04 and P = 0.003, respectively). The number of Langerin+ DCs (6.27 ± 0.61 vs 4.56 ± 0.62) was also higher in females than in males (P = 0.01). Among all persons studied, the density of IDO+ DCs was significantly higher in females compared with males (8.41 ± 0.70 and 5.41 ± 0.73, respectively; P = 0.004).
The main finding of this study was the significantly higher densities of CD11c+, tolerogenic IDO+, CD103+ and Langerin+ DCs in the small bowel mucosa of patients with CD compared with subjects with normal small bowel mucosa. The densities of IDO+ and particularly of CD103+ DCs were high in CD patients with coexisting T1D, possibly demonstrating the strongest pressure on the local immune system in these patients. These results are consistent with our finding that FOXP3+ Tregs are present at higher densities in CD patients with and without coexisting T1D. A significant correlation between the densities of tolerogenic DCs and FOXP3+ Tregs in the small bowel mucosa of the persons studied might confirm the involvement of these DCs in enhanced FOXP3+ Tregs development.
Accumulation of CD11c+ DCs in celiac lesions was also observed in a study by Ráki et al[29], who showed that CD11c+ DCs are able to activate gluten-reactive T cells. In a subsequent study, the same group of authors established that rapid accumulation of CD14+CD11c+ DCs occurred in the LP of the gut mucosa of treated patients with CD after a three-day gluten challenge and asserted that gluten-induced recruitment of these cells is specific for CD[30]. Another study by this group demonstrated the increased density of CD163+CD11c+ DCs in celiac lesions[31]. However, according to their results, the density of CD103+ DCs was decreased in CD patients. Importantly, in these studies, the study group consisted of older patients (mean age 39 years) compared with the patients in our CD group (mean age 11.1 ± 6.8 years), which might at least partly explain the discrepancies between the results of their study and ours. Thus, we believe that the increased density of CD103+ DCs in the proximal part of the small intestinal mucosa of CD patients, particularly those with coexisting T1D, is of pathogenic relevance.
Another important finding in our study was the significant correlation between densities of IDO+ DCs and FOXP3+ Tregs, indicating the tolerogenic capacity of IDO+ DCs. Moreover, 29.7% of the LP DCs were double-positive for both IDO and CD103 markers in the CD patient group. Thus, our data are in agreement with those of Matteoli et al[18], who reported that IDO expression was particularly associated with CD11c+ CD103+ DCs, both in mouse and human LP. According to these authors, IDO is involved in the capacity of CD103+ DCs to drive FOXP3+ Tregs development.
However, the study of Badami et al[32] showed that T1D patients had a reduced number of FOXP3+ Treg cells due to both impaired differentiation by gut-associated CD103+CD11c+ DC and their need to maintain immune tolerance for pancreatic cells. These authors did not find any difference in the amount of intestinal CD103+CD11c DCs in the LP between T1D patients, CD patients and controls. The discrepancy between the results in the above-mentioned study and ours could be partly explained by the different methods used in these two studies. Badami et al[32] used multiparametric fluorescent-activated sorter analysis of the cell subsets in the biopsy specimens of the studied persons and a conversion assay for the assessment of tolerogenic function of the LP DCs or blood monocyte-derived DCs in vitro. We performed immunohistochemical evaluations of a subpopulation of DCs and T cells in duodenal biopsy specimens. It should also be noted that their T1D group was significantly older (mean age 29 years) than our patient and control populations, which might also have influenced the results.
Similarly to other authors, we observed increased expression levels of FOXP3 mRNA and protein in the small bowel mucosa of patients with CD with and without associated T1D in our previous study, which might indicate an imbalance between regulatory and effector mechanisms in the pathogenesis of these diseases[33,34].
The results of the present study regarding the higher density of FOXP3+ Tregs in CD patients with and without coexisting T1D are consistent with the relevant results of other studies. Cianci et al[35] reported increased Tregs in the peripheral blood and the duodenal mucosa of patients with active CD; they regarded this phenomenon as an example of an “immunological niche”, where naive T cells recruited by the gluten trigger from the peripheral blood to local mucosa tissue will differentiate into Tregs in the presence of anti-inflammatory cytokines, such as transforming growth factor (TGF)-beta and IL-10. A significant increase in the density of FOXP3+ T regs in the LP of CD patients, correlated with both the histological Marsh grade and the serum levels of transglutaminase type 2 autoantibodies, was demonstrated by Brazowski et al[36]. The increased expression of CD4+CD25+FOXP3+ circulating Tregs in untreated CD patients can be explained as an attempt to quench intestinal inflammation and the immune response to dietary gluten[37]. Borrelli et al[38] reported an increased density of FOXP3+ Tregs in the duodenal mucosa and in the peripheral blood of patients with potential CD and interpreted this phenomenon as an effort by the immune system to down-regulate current inflammation and to restrict its progression toward mucosal damage through either the redistribution of FOXP3+ Tregs or their local proliferation.
In a study by Kivling et al[39], children with CD with or without associated T1D had significantly higher FOXP3 mRNA expression levels compared with children with only T1D. This difference could indicate increased Tregs-associated activity in the case of two autoimmune disorders (CD and coexisting T1D) in contrast to a single disorder of the immune system (children with only T1D). However, some studies have established impairment of the regulatory activity of both intestinal and peripheral blood FOXP3+ Treg cells in patients with active CD[40-42]. This phenomenon could be explained by the overproduction of IL-15, a cytokine preventing the response of effector T cells to the suppressive effects of Tregs, and partly by the overexpression of IL-15Rα in CD patients[41,43].
There is evidence that IDO plays an important role as a suppressor of lymphocyte-mediated inflammatory responses[17]. In our study, the number of IDO+ DCs was greater in the small bowel mucosa of patients with higher-grade CD, i.e., grade IIIb according to the Marsh classification, compared with patients with grade IIIa small bowel mucosa. The high discriminative power of the IDO marker in CD patients with and without T1D established in our study also supports the above statement. This finding is in good agreement with the results of Torres et al[19], who revealed high expression levels of IDO in intestinal biopsies of CD patients. Moreover, these authors observed that increased levels of interferon-gamma and tumor necrosis factor-alpha were potent inducers of IDO expression levels in CD patients. The increase in IDO activity is considered to be an attempt to control chronic antigen stimulation via the down-regulation of T cell-mediated autoimmunity[19].
A central novel finding in our study was the presence of variable densities of Langerin+ (CD207+) cells in patients with normal or atrophic small bowel mucosa, pointing to their role among the other DCs of the intestinal mucosa. The presence of Langerin+ cells in the duodenal epithelium (Figure 8) could indicate the possibility that these cells actively participate in the transportation of antigenic material through the epithelial layer, as has been demonstrated earlier for CD11c+ DCs[44]. The presence of different types of DCs in both the LP and the villous epithelium has also been shown by Farache et al[45]. Because the density of Langerin+ DCs in the small bowel mucosa was significantly higher in CD patients compared with persons with normal intestinal mucosa, these cells might indeed play a specific role in CD. This difference may be due to the capacity of Langerin+ DCs to take up and route the antigen(s) from the epithelium into the organelles[44]. Rochereau et al[46] confirmed the presence of CD11c+/Langerin+ DCs in mouse Peyer’s Patches, located predominantly in the dome region, which supports their function in antigen uptake. However, Langerin+ DCs could be actively recruited to the CD mucosa through their binding to different sugar residues on microorganisms, and some of these interactions might be directly connected to CD development[47]. The binding of Langerin+ DCs to heparin[48], which is reactive to the disease-specific autoantigen tTG, might have an additional impact, namely because the heparin-binding residues of tTG are strong autoantigenic epitopes[49].
One of the limitations of the study is related to a gender imbalance between the study groups, which might have an influence on the results. In female patients, the density of DCs in normal small bowel mucosa was significantly higher compared with male patients. This difference indicates that some results may have been skewed due to divergences in the composition of the study groups. However, Sankaran-Walters et al[50] showed that women have higher levels of T cell proliferation and activation and up-regulation in gene expression-related immune functions in the gut microenvironment in the absence of disease, all of which can predispose women to inflammation-associated diseases. Moreover, DC differentiation and function are regulated by the estrogen receptor ligands[51]. Mao et al[52] reported that estrogen-dependent CD11c+CD11b Ly6C- DCs express Langerin (CD207). Our result regarding the significant correlation between the densities of CD11c+ and Langerin+ DCs, particularly in female patients with CD, is in agreement with the finding of the above authors. Xiao et al[53] demonstrated that estrogen can induce IDO expression by DCs through suppression of T cell function via the IDO pathway. In our study, the higher prevalence of IDO+ DCs in female compared with male patients, especially in the group with normal mucosa and a mean age of 13.5 ± 4.3 years, could have already been affected by the female hormonal status, although the mean age of male subjects with normal mucosa (11.4 ± 5.8) did not differ significantly from female subjects of similar age (P = 0.25). Despite the gender influences, we still believe that differences in the distribution of the DC subsets in the small intestinal mucosa of CD patients and controls are significant.
Another limitation of our study is that we did not know the microbiota status of the small intestines of the studied persons. We agree that the microbiota can strongly influence and regulate the homeostasis of effector immune cells, including the distribution of different subtypes of DCs and Treg cells, along with other immunoregulatory events, in the small bowel mucosa[3,54]. However, this factor is known to be one of the major limitations of other similar studies, unless the intestinal microbiota is studied specifically[55]. Even in cases where it is studied, the interpretation of study results is difficult because the intestinal microbiota is dependent on different nutritional factors[7,8].
In conclusion, we established that CD patients expressed higher densities of CD11c+, IDO+ and CD103+ DCs and Langerin+ DCs in the small bowel mucosa compared with the control persons. The densities of both FOXP3+ Tregs and IDO+ DCs were significantly increased in CD patients with and without coexisting T1D. A significant correlation was identified between the densities of CD103+ DCs and FOXP3+ Tregs and between the densities of IDO+ DCs and FOXP3+ Tregs. This finding highlights the participation of diverse DC subsets in the pathological processes of CD and indicates the possible involvement of tolerogenic DCs in Tregs development to maintain intestinal immunological tolerance in CD patients. Our results demonstrate the diversity of the mechanisms of immunoregulation and the various types of DC involvement in CD, emphasizing the importance of Langerin+ DCs in the small intestinal mucosa.
We would like to thank Dr. Tiina Rägo from the Children’s Clinic of Tartu University Hospital, Tartu, for part of the clinical material and Mrs. Anu Kaldmaa from the Department of Immunology, Institute of Biomedicine and Translational Medicine, University of Tartu, and Mrs. Merje Jakobson from the Department of Pathology, Tartu University Hospital, for their assistance with the laboratory procedures. We are grateful to Mrs. Kristi Alnek and Helis Janson from the Department of Immunology, Institute of Biomedicine and Translational Medicine, University of Tartu, for performing the tissue transglutaminase IgA-tTG immunoassay. We thank Mrs. ülle Kirsimägi from the Surgery Department, University of Tartu, and the medical student, Helerin Raikerus, for their help with statistical analysis.
The role of the gut immune system is thought to be of crucial importance in the pathogenesis of celiac disease (CD) and type 1 diabetes (T1D). Several research groups have demonstrated a marked association between the development of T1D and preceding alterations in the small bowel mucosa. Dendritic cells (DCs) have received much attention in both diseases due to their strategic role in gut homeostasis by processing external antigens (including wheat proteins) and by determining tolerance to self-antigens.
The important role of CD11c+CD103+ DCs and indoleamine 2,3-dioxygenase (IDO) in the induction of regulatory T cells (Tregs) differentiation have been established. In addition, DCs carrying Langerin (CD207) proteins have received considerable interest. The expression of Langerin by CD103+CD11b+ lamina propria DCs in the human ileum has recently been reported. However, the presence of Langerin+ DCs in the small bowel mucosa in pathological conditions such as CD and T1D has not yet been studied. We also do not know how Langerin+ DCs are related to other DC subsets and Tregs in the human small intestinal mucosa. Because Langerin+ DCs are significant modulators of events in the skin, another important immunological barrier of the organism, knowledge of the function of these cells in the small intestinal mucosa may be of general importance. This research aims to investigate the densities of CD11c+ DCs, CD103+ DCs, IDO+ DCs and Langerin+ DCs, along with FOXP3+ Tregs, in the small bowel mucosa in CD patients with and without coexisting T1D and to compare these densities with those found in histologically normal intestinal mucosa in persons with functional dyspepsia using immunohistochemical and immunofluorescence methods.
The main finding of this study was the significantly higher densities of CD11c+, tolerogenic IDO+, CD103+ and Langerin+ DCs in the small bowel mucosa of patients with CD compared with subjects with normal small bowel mucosa. The densities of IDO+ and particularly of CD103+ DCs were high in CD patients with coexisting T1D, possibly demonstrating the strongest pressure on the local immune system in these patients. A significant correlation between the densities of tolerogenic DCs and FOXP3+ Tregs in the small bowel mucosa of the persons studied might confirm the involvement of these DCs in enhanced FOXP3+ Tregs development.
This finding highlights the participation of diverse DC subsets in the pathological processes of CD and indicates the possible involvement of tolerogenic DCs in Tregs development to maintain intestinal immunological tolerance in CD patients. This results demonstrate the diversity of the mechanisms of immunoregulation and the various types of DC involvement in CD, emphasizing the importance of Langerin+ DCs in the small intestinal mucosa.
IDO is an immunomodulatory enzyme involved in tryptophan catabolism with immunosuppressive effects that has been implicated in the control of intestinal inflammation. Higher IDO expression has been measured in intestinal biopsies from CD patients. Langerin was originally identified as a Langerhans cell -specific C-type lectin receptor involved in antigen capture. Tolerogenic DCs are dendritic cells, predominantly CD103+, that are isolated from lamina propria and mesenteric lymph nodes and are able to drive the development of CD4+FOXP3+ Tregs. Tregs are a subpopulation of CD4+CD25+FOXP3+ T cells that modulate the immune system, mainly by immunosuppressive activity, and play an important role in intestinal homeostasis.
The current manuscript studied DCs and FOXP3-positive Tregs and their interactions in the small bowel mucosa of patients with CD with or without T1D.
P- Reviewer: Pessi T S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Gujral N, Freeman HJ, Thomson AB. Celiac disease: prevalence, diagnosis, pathogenesis and treatment. World J Gastroenterol. 2012;18:6036-6059. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 412] [Cited by in F6Publishing: 345] [Article Influence: 28.8] [Reference Citation Analysis (4)] |
| 2. | Jabri B, Sollid LM. Tissue-mediated control of immunopathology in coeliac disease. Nat Rev Immunol. 2009;9:858-870. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 230] [Cited by in F6Publishing: 224] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 3. | Bollrath J, Powrie FM. Controlling the frontier: regulatory T-cells and intestinal homeostasis. Semin Immunol. 2013;25:352-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Vaarala O. Gut and the induction of immune tolerance in type 1 diabetes. Diabetes Metab Res Rev. 1999;15:353-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 5. | Auricchio R, Paparo F, Maglio M, Franzese A, Lombardi F, Valerio G, Nardone G, Percopo S, Greco L, Troncone R. In vitro-deranged intestinal immune response to gliadin in type 1 diabetes. Diabetes. 2004;53:1680-1683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Uibo R, Panarina M, Teesalu K, Talja I, Sepp E, Utt M, Mikelsaar M, Heilman K, Uibo O, Vorobjova T. Celiac disease in patients with type 1 diabetes: a condition with distinct changes in intestinal immunity? Cell Mol Immunol. 2011;8:150-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Visser J, Rozing J, Sapone A, Lammers K, Fasano A. Tight junctions, intestinal permeability, and autoimmunity: celiac disease and type 1 diabetes paradigms. Ann N Y Acad Sci. 2009;1165:195-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 159] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 8. | Sorini C, Falcone M. Shaping the (auto)immune response in the gut: the role of intestinal immune regulation in the prevention of type 1 diabetes. Am J Clin Exp Immunol. 2013;2:156-171. [PubMed] [Cited in This Article: ] |
| 9. | Holmes GK. Screening for coeliac disease in type 1 diabetes. Arch Dis Child. 2002;87:495-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 101] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Rescigno M, Di Sabatino A. Dendritic cells in intestinal homeostasis and disease. J Clin Invest. 2009;119:2441-2450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 230] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 11. | Lewis KL, Reizis B. Dendritic cells: arbiters of immunity and immunological tolerance. Cold Spring Harb Perspect Biol. 2012;4:a007401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Iliev ID, Spadoni I, Mileti E, Matteoli G, Sonzogni A, Sampietro GM, Foschi D, Caprioli F, Viale G, Rescigno M. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut. 2009;58:1481-1489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 307] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 13. | Scott CL, Aumeunier AM, Mowat AM. Intestinal CD103+ dendritic cells: master regulators of tolerance? Trends Immunol. 2011;32:412-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 240] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 14. | del Rio ML, Bernhardt G, Rodriguez-Barbosa JI, Förster R. Development and functional specialization of CD103+ dendritic cells. Immunol Rev. 2010;234:268-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 203] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 15. | Stock A, Napolitani G, Cerundolo V. Intestinal DC in migrational imprinting of immune cells. Immunol Cell Biol. 2013;91:240-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, Marshall B, Chandler P, Antonia SJ, Burgess R. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867-1870. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 767] [Cited by in F6Publishing: 734] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 17. | Cherayil BJ. Indoleamine 2,3-dioxygenase in intestinal immunity and inflammation. Inflamm Bowel Dis. 2009;15:1391-1396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Matteoli G, Mazzini E, Iliev ID, Mileti E, Fallarino F, Puccetti P, Chieppa M, Rescigno M. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut. 2010;59:595-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 272] [Cited by in F6Publishing: 267] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 19. | Torres MI, López-Casado MA, Lorite P, Ríos A. Tryptophan metabolism and indoleamine 2,3-dioxygenase expression in coeliac disease. Clin Exp Immunol. 2007;148:419-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8:935-947. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 580] [Cited by in F6Publishing: 564] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 21. | Valladeau J, Ravel O, Dezutter-Dambuyant C, Moore K, Kleijmeer M, Liu Y, Duvert-Frances V, Vincent C, Schmitt D, Davoust J. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71-81. [PubMed] [Cited in This Article: ] |
| 22. | Chang SY, Kweon MN. Langerin-expressing dendritic cells in gut-associated lymphoid tissues. Immunol Rev. 2010;234:233-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Welty NE, Staley C, Ghilardi N, Sadowsky MJ, Igyártó BZ, Kaplan DH. Intestinal lamina propria dendritic cells maintain T cell homeostasis but do not affect commensalism. J Exp Med. 2013;210:2011-2024. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 24. | Mention JJ, Ben Ahmed M, Bègue B, Barbe U, Verkarre V, Asnafi V, Colombel JF, Cugnenc PH, Ruemmele FM, McIntyre E. Interleukin 15: a key to disrupted intraepithelial lymphocyte homeostasis and lymphomagenesis in celiac disease. Gastroenterology. 2003;125:730-745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 326] [Cited by in F6Publishing: 334] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 25. | De Nitto D, Monteleone I, Franzè E, Pallone F, Monteleone G. Involvement of interleukin-15 and interleukin-21, two gamma-chain-related cytokines, in celiac disease. World J Gastroenterol. 2009;15:4609-4614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Mohamadzadeh M, Berard F, Essert G, Chalouni C, Pulendran B, Davoust J, Bridges G, Palucka AK, Banchereau J. Interleukin 15 skews monocyte differentiation into dendritic cells with features of Langerhans cells. J Exp Med. 2001;194:1013-1020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 203] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 27. | Revised criteria for diagnosis of coeliac disease. Report of Working Group of European Society of Paediatric Gastroenterology and Nutrition. Arch Dis Child. 1990;65:909-911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1014] [Cited by in F6Publishing: 1058] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 28. | Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’). Gastroenterology. 1992;102:330-354. [PubMed] [Cited in This Article: ] |
| 29. | Ráki M, Tollefsen S, Molberg Ø, Lundin KE, Sollid LM, Jahnsen FL. A unique dendritic cell subset accumulates in the celiac lesion and efficiently activates gluten-reactive T cells. Gastroenterology. 2006;131:428-438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Beitnes AC, Ráki M, Brottveit M, Lundin KE, Jahnsen FL, Sollid LM. Rapid accumulation of CD14+CD11c+ dendritic cells in gut mucosa of celiac disease after in vivo gluten challenge. PLoS One. 2012;7:e33556. [PubMed] [Cited in This Article: ] |
| 31. | Beitnes AC, Ráki M, Lundin KE, Jahnsen J, Sollid LM, Jahnsen FL. Density of CD163+ CD11c+ dendritic cells increases and CD103+ dendritic cells decreases in the coeliac lesion. Scand J Immunol. 2011;74:186-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Badami E, Sorini C, Coccia M, Usuelli V, Molteni L, Bolla AM, Scavini M, Mariani A, King C, Bosi E. Defective differentiation of regulatory FoxP3+ T cells by small-intestinal dendritic cells in patients with type 1 diabetes. Diabetes. 2011;60:2120-2124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 33. | Tiittanen M, Westerholm-Ormio M, Verkasalo M, Savilahti E, Vaarala O. Infiltration of forkhead box P3-expressing cells in small intestinal mucosa in coeliac disease but not in type 1 diabetes. Clin Exp Immunol. 2008;152:498-507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 34. | Vorobjova T, Uibo O, Heilman K, Rägo T, Honkanen J, Vaarala O, Tillmann V, Ojakivi I, Uibo R. Increased FOXP3 expression in small-bowel mucosa of children with coeliac disease and type I diabetes mellitus. Scand J Gastroenterol. 2009;44:422-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Cianci R, Cammarota G, Frisullo G, Pagliari D, Ianiro G, Martini M, Frosali S, Plantone D, Damato V, Casciano F. Tissue-infiltrating lymphocytes analysis reveals large modifications of the duodenal “immunological niche” in coeliac disease after gluten-free diet. Clin Transl Gastroenterol. 2012;3:e28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Brazowski E, Cohen S, Yaron A, Filip I, Eisenthal A. FOXP3 expression in duodenal mucosa in pediatric patients with celiac disease. Pathobiology. 2010;77:328-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Frisullo G, Nociti V, Iorio R, Patanella AK, Marti A, Assunta B, Plantone D, Cammarota G, Tonali PA, Batocchi AP. Increased CD4+CD25+Foxp3+ T cells in peripheral blood of celiac disease patients: correlation with dietary treatment. Hum Immunol. 2009;70:430-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Borrelli M, Salvati VM, Maglio M, Zanzi D, Ferrara K, Santagata S, Ponticelli D, Aitoro R, Mazzarella G, Lania G. Immunoregulatory pathways are active in the small intestinal mucosa of patients with potential celiac disease. Am J Gastroenterol. 2013;108:1775-1784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Kivling A, Nilsson L, Fälth-Magnusson K, Söllvander S, Johanson C, Faresjö M. Diverse foxp3 expression in children with type 1 diabetes and celiac disease. Ann N Y Acad Sci. 2008;1150:273-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Granzotto M, dal Bo S, Quaglia S, Tommasini A, Piscianz E, Valencic E, Ferrara F, Martelossi S, Ventura A, Not T. Regulatory T-cell function is impaired in celiac disease. Dig Dis Sci. 2009;54:1513-1519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 41. | Zanzi D, Stefanile R, Santagata S, Iaffaldano L, Iaquinto G, Giardullo N, Lania G, Vigliano I, Vera AR, Ferrara K. IL-15 interferes with suppressive activity of intestinal regulatory T cells expanded in Celiac disease. Am J Gastroenterol. 2011;106:1308-1317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 42. | Hmida NB, Ben Ahmed M, Moussa A, Rejeb MB, Said Y, Kourda N, Meresse B, Abdeladhim M, Louzir H, Cerf-Bensussan N. Impaired control of effector T cells by regulatory T cells: a clue to loss of oral tolerance and autoimmunity in celiac disease? Am J Gastroenterol. 2012;107:604-611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 43. | Ben Ahmed M, Belhadj Hmida N, Moes N, Buyse S, Abdeladhim M, Louzir H, Cerf-Bensussan N. IL-15 renders conventional lymphocytes resistant to suppressive functions of regulatory T cells through activation of the phosphatidylinositol 3-kinase pathway. J Immunol. 2009;182:6763-6770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 44. | Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1870] [Cited by in F6Publishing: 1767] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 45. | Farache J, Zigmond E, Shakhar G, Jung S. Contributions of dendritic cells and macrophages to intestinal homeostasis and immune defense. Immunol Cell Biol. 2013;91:232-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 46. | Rochereau N, Verrier B, Pin JJ, Genin C, Paul S. Phenotypic localization of distinct DC subsets in mouse Peyer Patch. Vaccine. 2011;29:3655-3661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Feinberg H, Rowntree TJ, Tan SL, Drickamer K, Weis WI, Taylor ME. Common polymorphisms in human langerin change specificity for glycan ligands. J Biol Chem. 2013;288:36762-36771. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 48. | Chabrol E, Nurisso A, Daina A, Vassal-Stermann E, Thepaut M, Girard E, Vivès RR, Fieschi F. Glycosaminoglycans are interactants of Langerin: comparison with gp120 highlights an unexpected calcium-independent binding mode. PLoS One. 2012;7:e50722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 49. | Teesalu K, Uibo O, Uibo R, Utt M. Kinetic and functional characterisation of the heparin-binding peptides from human transglutaminase 2. J Pept Sci. 2012;18:350-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 50. | Sankaran-Walters S, Macal M, Grishina I, Nagy L, Goulart L, Coolidge K, Li J, Fenton A, Williams T, Miller MK. Sex differences matter in the gut: effect on mucosal immune activation and inflammation. Biol Sex Differ. 2013;4:10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 51. | Kovats S. Estrogen receptors regulate an inflammatory pathway of dendritic cell differentiation: mechanisms and implications for immunity. Horm Behav. 2012;62:254-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 52. | Mao A, Paharkova-Vatchkova V, Hardy J, Miller MM, Kovats S. Estrogen selectively promotes the differentiation of dendritic cells with characteristics of Langerhans cells. J Immunol. 2005;175:5146-5151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 53. | Xiao BG, Liu X, Link H. Antigen-specific T cell functions are suppressed over the estrogen-dendritic cell-indoleamine 2,3-dioxygenase axis. Steroids. 2004;69:653-659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 54. | Goto Y, Ivanov II. Intestinal epithelial cells as mediators of the commensal-host immune crosstalk. Immunol Cell Biol. 2013;91:204-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 55. | Sjöberg V, Sandström O, Hedberg M, Hammarström S, Hernell O, Hammarström ML. Intestinal T-cell responses in celiac disease - impact of celiac disease associated bacteria. PLoS One. 2013;8:e53414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |