Published online May 21, 2015. doi: 10.3748/wjg.v21.i19.5823
Peer-review started: October 13, 2014
First decision: November 14, 2014
Revised: December 4, 2014
Accepted: January 21, 2015
Article in press: January 21, 2015
Published online: May 21, 2015
Inflammatory bowel diseases (IBDs) are chronic disorders of modern society, requiring management strategies aimed at prolonging an active life and establishing the exact etiology and pathogenesis. These idiopathic diseases have environmental, genetic, immunologic, inflammatory, and oxidative stress components. On the one hand, recent advances have shown that abnormal immune reactions against the microorganisms of the intestinal flora are responsible for the inflammation in genetically susceptible individuals. On the other hand, in addition to T helper cell-type (Th) 1 and Th2 immune responses, other subsets of T cells, namely regulatory T cells and Th17 maintained by IL-23 are likely to develop IBD. IL-23 acts on innate immune system members and also facilitates the expansion and maintenance of Th17 cells. The IL-17/IL-23 axis is relevant in IBD pathogenesis both in human and experimental studies. Novel biomarkers of IBD could be calprotectin, microRNAs, and serum proinflammatory cytokines. An efficient strategy for IBD therapy is represented by the combination of IL-17A and IL-17F in acute IL-17A knockout TNBS-induced colitis, and also definite decrease of the inflammatory process in IL-17F knockout, DSS-induced colitis have been observed. Studying the correlation between innate and adaptive immune systems, we hope to obtain a focused review in order to facilitate future approaches aimed at elucidating the immunological mechanisms that control gut inflammation.
Core tip: The pathogenesis of inflammatory bowel diseases (Crohn’s disease and ulcerative colitis) is multifactorial and still not completely understood. There is a need to identify new diagnostic biomarkers as well as an efficient therapy for these diseases. A better understanding of the immunological mechanisms that control gut inflammation, is of high clinical importance because it offers the possibility to develop new drugs, which attack the key pro-inflammatory pathways in chronic intestinal inflammation.
- Citation: Cătană CS, Berindan-Neagoe I, Cozma V, Magdaş C, Tăbăran F, Dumitraşcu DL. Contribution of the IL-17/IL-23 axis to the pathogenesis of inflammatory bowel disease. World J Gastroenterol 2015; 21(19): 5823-5830
- URL: https://www.wjgnet.com/1007-9327/full/v21/i19/5823.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i19.5823
Inflammatory bowel diseases (IBD) including ulcerative colitis (UC) and Crohn’s disease (CD) require a better knowledge of the exact autoimmune mechanisms involved in their pathogenesis[1]. A better understanding of the pathogenesis of IBD will allow the better control of the inappropriate and continuing inflammatory response to commensal microbes in a genetically susceptible host[2-5] . In addition, in CD pathogenesis the “dialogue” between the host and intestinal microbiota includes the defensins and dysbiosis as well as the hemostatic system[6]. The pathways for intestinal homeostasis include: epithelial restitution, barrier function, antimicrobial defense, innate and adaptive immunity, “reactive oxygen species” (ROS) generation and the metabolic pathway[4].
Immune tolerance, inflammation, mucosal pathways and/or epithelial restitution are promoted by intra- and intercellular networks using plastic cellular programs responsible for intestinal homeostasis[7].
UC is characterized by inflammation limited to the colon as a result of a disturbance of the normal immune control of the gut symbiotic bacteria. By contrast, CD involves any part of the gastrointestinal tract. Chronic inflammation involves the submucosal and mucosal layers leading to bleeding, abdominal pain, diarrhea and malnourishment. The gut wall permeability is also increased. The Peyer’s patches of the small intestine could be the site of the immune tolerance breakdown to the microorganisms of the intestinal flora[4].
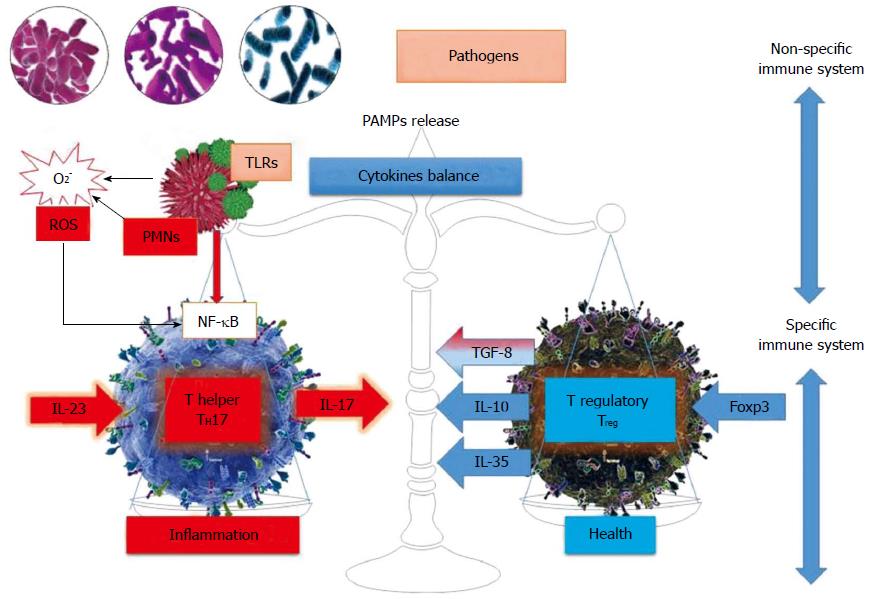
The immune system is made of two compartments: innate and acquired. The function of the non-specific system is to recognize all external agents and also act against them without specificity and memory. The mucosa is the first place where pathogens/allergens encounter polymorphonuclear neutrophils, which directly recognize the PAMP (pathogen associated molecular pattern) through pattern recognition receptors such as Toll-like receptors (TLRs). The key factor between TLRs and neutrophils interactions are ROS. There are at least 10 variants of TLRs expressed on the surface of neutrophils, macrophages, dendritic cells and, to a lesser extent, lymphocytes. Through these types of receptors, the innate immune response has a certain specificity compared to the high degree of variability present on the surface molecules of the pathogenic agents[8].
First, abnormalities of innate immunity in association with epithelial barrier dysfunction could be the “key” point to the initiation of the mucosal inflammation[9,10]. Besides the recognition role, the innate immune response mediated by neutrophils, natural killer (NK) cells, monocytes/macrophages, dendritic cells and the complement system has other important functions such as phagocytosis and direct cell cytotoxicity or Antibody-Dependent Cell Cytotoxicity (ADCC). Moreover, NK cells and NKT cells exhibit cytotoxic activity in cancerous cells. Although the innate immune system is extremely effective as a first line of defense against many aggressors, it also causes multiple collateral effects by producing antibacterial free radicals. This is the point where the specific adaptive immune system intervenes[8].
Second, the adaptive immunity has classically been considered to play a crucial role in the pathogenesis of IBD. The acquired immune response is ensured by T and B lymphocytes. The adaptive immune response eliminates specific pathogens through humoral and cellular response[8]. However, recent research in immunology and genetics has shown that the innate immune response is equally important in inducing inflammation in IBD patients. It has been highlighted that an altered epithelial barrier function contributes to the intestinal inflammation in UC, while aberrant innate immune responses, such as autophagy, innate microbial sensing and antimicrobial peptide production are associated with CD pathogenesis[3].
The immunology of IBD represents an imbalance between two types of T cells populations: regulatory T cells (Treg) and pro- inflammatory T cells (Figure 1).
Besides Th1 and Th2, two other subsets have recently been described: CD4+CD25+T regulatory cells and Th17 cells (Table 1). Th17 cells expressing RORγ1 favor the occurrence of allergic reactions and autoimmune phenomena by producing IL-17 cytokines. Therefore, the Th17/Treg balance prevents or promotes inflammation and autoimmune diseases. After activation, naïve CD4-T cells differentiate into three subsets of Th effector cells, each subset having a unique cytotoxic profile and specific biological functions. Treg cells that differentiate in the presence of the Foxp3 transcription factor (90-100 aminoacids that form a DNA binding motif) produce anti-inflammatory mediators such as IL-10 and TGF-β, whose function is to preserve immune tolerance and homeostasis[8]. In addition to natural thymic Treg cells, Treg could be induced in the periphery under specific conditions. Th17 and iTregs are closely related so that they can generate one another under the control of TGF-β maintaining their balance in the context of IBD[10,11].
| CD4 T-cell subsets | Surface expression | Polarizing Cytokines | Master regulator transcription factor | STAT regulators | Effector profile/signature cytokines |
| Th1 | IL-12RB2 | IL-12, | STAT1, | IFN-γ, | |
| IFN-γR | IFN-γ, | T-bet | STAT4 | IL-2, | |
| Tim3 | IL-27 | TNF-α, | |||
| IL-10 | |||||
| Th2 | IL-17RB | IL-4, | |||
| Tim1 | IL-25, | GATA-3 | STAT6 | IL-4, IL-5, | |
| IL-6 | MAF | IL-13, IL-25, | |||
| IL-2, IL-10 | |||||
| Th17 | IL-1R1 | TGF-β, | RORγt, | IL-17A, | |
| IL-12RB | IL-6, | RORα, | IL-17F, | ||
| IL-23R | IL-1β, | STAT3 | Il-21, | ||
| CCR6 | IL-21, | IL-22 | |||
| CD161 | IL-23 | ||||
| IL-13Ra1 | |||||
| TREG | CD25 | ||||
| CD39 | TGF-β | Foxp3 | STAT5 | TGF-β, | |
| CD73 | IL-10 | ||||
| CD101 | |||||
| CD127 | |||||
| TFh | CD84 | IL-6, | Bcl6 | IL-6, | |
| CXCR5 | IL-1β, TNFα | IRF4, | IL-2, | ||
| IL-6R | IL-21 | c-Maf, | STAT3/5 | IL-10, | |
| IL-21R | CXCL13 | Batf, | IL-21 | ||
| gp130 |
The most commonly described effector T cells are Th1, Th17 in CD and Th2 in UC, Th17 being responsible for the IL-17 production, a key pro- inflammatory cytokine that is increased in IBD[12]. Conversely, given the redundancy of effector and regulatory pathways leading to IBD lesions and the Th17 cells protective functions, the neutralization of IL-17A did not diminish the inflammatory process in CD[3]. In addition, effector T-cells and cytokines have been targets of many recently developed treatment agents for CD[13].
The first interaction between the innate and the acquired systems occurs when antigen-presenting cells (APCs), which are part of the innate system, present antigens to the T cells belonging to the innate system[8]. “Polarization” of adaptive immune responses was firstly presented in 1986[14]. This hypothesis was that on encountering an antigen presented by APC, naïve CD4+T-cells acquired a unique effector immunophenotype, representing the final stage of non-reversible differentiation and being defined by specific effector cytokines[15,16].
The Th cell polarization model is promoted by cytokines which derive from the innate immune system recognizing microbe-associated molecular patterns (MAMPs) and establishing proinflammatory antimicrobial responses[11].
The Th1 vs Th2 “polarization model” was presented twenty-five years ago in the context of responses against infectious agents. It is generally accepted that a deregulation in the immune response towards the gut flora is responsible for the intestinal chronic inflammation in genetically predisposed individuals. Caspase recruitment domain 9 (CARD9) is a nonredundant adapter protein that conveys signal information downstream of pattern recognition receptors. CARD9 has also been associated with autoinflammatory disorders members and with the activation of NF-κB family, enhancing the Th17 cytokines production[17]. Chronic inflammation is coordinated by the oxidative stress manifested by the increase in pro-inflammatory cytokines encoded by NF-κB genes[8].
The “polarization model” was also used to explain the immunophenotype of non-infectious inflammatory conditions. Expression studies on the signature cytokines demonstrated the crucial role of IL-23, Il-17 and IL-6 in the development of experimental colitis, Th17 cells being involved in disease propagation. Moreover, IL-23 promotes the expansion and maintenance of Th17 cells, which secrete the proinflammatory cytokine IL-17 involved in the pathogenesis of many chronic inflammatory disorders. Other studies have shown that IL-23 acts on the cells of the innate immune system and contributes to the inflammatory cytokine production and tissue inflammation. Therefore, the role of the IL-23/IL-17 axis in the pathogenesis of IBD chronic intestinal inflammation has been highlighted both in animal and human studies, this pathway being relevant especially in the pathogenesis of Crohn’s disease[17].
The combinations of cytokines, which initiate the Th17 polarization process as well as the functional stability of Th17 lineage, have been extensively studied[18]. In humans, the initial development of Th17 cells is induced by TGF-β and IL-6 synergism as well as the presence of low levels of TGF-β and IL-1β[19]. The second stage is the expansion of the Th17 population under the activation of IL-21. This stage is followed by the stabilization phase driven by the pro-inflammatory cytokine IL-23, generating the cytokine profile for the CD4 Th17cell subset which has a unique property named plasticity[20]. In other words, the functions of Th17 cells depend on the immunological environment in which they developed.
The main pro-inflammatory function of the Th17cells is ensured via IL-17 secretion. From this point of view and according to the “polarization model”, effector lineage is generally exclusive. To be more precise, an inflammatory state can be either Th-17 or Th-1-driven. However, it has recently been shown that Th17 cells acquire the ability to upregulate t-bet (transcription factor) and produce IFN-γ, generating IL-17/ IFN-γ double positive cells, Th1/ Th17[21].
Such a population with the ability to produce both IL-17 and IFN-γ is also present in the inflamed gut mucosa[22]. Moreover, Th1- and Th17-mediated signaling may act in synergy and have deleterious effects on the gut mucosa, Th1-effects being more prominent during the chronic phase of CD as compared to the Th17 signaling pathway[23].
IL-23 is also upregulated in the active disease. However, all the necessary constituent elements for the IL-23/Th17 polarization process are to be found only in the chronic inflammatory lesions of CD[24].
In addition, this unique population may differentiate into specific Th1 IFN-γ producing cells, thus excluding the Th17 population[25-27]. The extraordinary T17 cells plasticity, confirmed in experimental and clinical studies, could be explained in at least three different ways: firstly, by inducing Th1 cells- IFN-γ secretion, secondly by expressing T-bet and producing IFN-γ in addition to IL-17, and thirdly completely trans-differentiating into Th1-subtype, IFN-γ producing cells expressing CD161, the surface marker of Th17 cells progenitors. Moreover, Th17-derived Th17/Th1 and Th1 cells, rather than Th17 cells alone, have an important function in IBD[11,20].
Th17 cells also possess a regulatory function recently described as human IL-17-producing Foxp3+/RORγt double positive T-cells[19]. In conclusion, the effector lymphocytes are not terminally differentiated. They may differentiate into regulatory pathways and alternative effectors under the local immunological pressure[28]. In patients with IBD the prevalence of circulating Foxp3 DE CD4 (+) and IL-17 T cells is increased and the coexpression of Foxp3 and RORγt in these cells requires conversion from Treg cells to Th17 cells associated with a decreased suppressive function of Foxp3 CD4(+) T lymphocytes[29].
Only three genetic polymorphisms have a pathogenetic role in IBD. The first one is related to NOD2 and mainly occurs in Caucasians[4,30]. GWAS (genome-wide association studies) for CD show the genes associated with CD risk such as ATG16L1 (autophagy 16-like 1), IRGM (immunity- related guanosine triphosphatase M) and LRRK2 (leucine-rich repeat kinase 2)[31].
GWAS have identified certain polymorphisms relevant to CD which are detected in genes encoding for IL-23/Th17 pathway proteins. The most important of IL-23r gene is the Arg381Gln polymorphism which confers protection against developing IBD. Conversely, the polymorphism associated with significantly elevated IL-17A mRNA transcripts is IL-17a variant IVS1+ 18 G>C[32]. The strategy of treatment is also affected by the gene polymorphisms, the IL-23R genotype status determining the early response to anti-TNF[33].
Inflammation is the “key” player in IBD, elevated levels of serum tumor necrosis factor alpha, TNF-α and CRP (C-reactive protein) being associated with CD[34]. Mucosal biomarkers in IBD as predictors of response to therapy and disease severity are: cytokines, adhesion molecules, intracellular markers of activation, immune and non immune cells and other factors (TLRs, mucin, MUC, G6PD, Glucose-6-phosphate dehydrogenase)[7]. Serum and colonic omentin-1, a newly discovered adipokine, acts as an anti-inflammatory agent as well as a new biomarker for the CD evolution, its correlation with the disease activity being superior to that of CRP[35].
Calprotectin is a more recently established marker for intestinal inflammation. This paper revealed the present knowledge on fecal calprotectin testing as predictor of intestinal inflammation. In inflammatory bowel disease, the calprotectin fecal test shows higher intensity values[36].
Thirty prospective studies confirmed that fecal calprotectin from granulocytes and macrophages released by activated innate immunity had been a real value in the diagnosis and disease activity evaluation, reaching a sensitivity and specificity up to 95% and 91%, respectively. Fecal calprotectin is more efficient than CRP, ESR (erythrocyte sedimentation rate), ASCAs (anti-Saccharomyces cerevisiae antibodies), ANCAs (antineutrophil cytoplasmic antibodies) and Omp C (outer membrane porin C)[37]. Fecal lactoferrin reflects the inflammation status of the intestine, the diagnostic rate being similar to that of fecal calprotectin and better than that of CRP. Another fecal marker correlated with endoscopic scores is neopterin, an index of disease activity in UC and CD[38]. A non-invasive biomarker of inflammation in IBD, which has high sensitivity and specificity and good compliance is S100A12; it stimulates the proinflammation NF-κB pathway. In addition, fecal S100A12 reflects the drug treatment response[34].
Serum microRNAs, which are small non-coded single-stranded RNAs, could be upregulated or downregulated in IBD[37]. It is very important to confirm the existence of specific expression patterns of miRNAs associated with IBD in different stages and demonstrate the utility of miRNA as ideal biomarkers[39]. Thus, it was demonstrated that miR-31, miR-206, miR-424 and miR-146a were novel biomarkers of inflammatory bowel disease[40].
The crucial role of the IL-23/Th17 axis in intestinal inflammation was demonstrated once again by eliminating certain components of this pathway and thus ameliorating the severity of the disease. Corticosteroids are classical immunosuppressive drugs of pro-inflammatory cytokine production used for the induction of clinical remission in IBD, but they may cause severe side effects. Consequently, it is of high clinical importance to define other drugs that attack the key pro-inflammatory pathways in chronic intestinal inflammation[41].
Small molecules that neutralize specific elements of the IL-23/Th17 pathway were used[42]. Drugs that target IL-17 include the anti IL-17 monoclonal antibody named secukinumab and the small molecule vidofludimus that blocks IL-17 release[43]. Apilimod mesylate inhibits IL-12 and IL-23 transcription, the anti-IL-12 ABT-874/J695 monoclonal antibodies ustekinumab and briakinumab target the p40 unit which is common to both IL-23 and IL-12; SCH-900222 targets the p19 subunit specific to IL-23[44]. Ustekinumab is efficient in moderate-to-severe CD, especially in patients in whom anti-TNF treatment had previously failed. An anti-IL-21 antibody neutralizes IL-21 and reduces the IL-17 secretion by lamina propria lymphocytes isolated from IBD patients[45]. The importance of ustekinumab is due to its simultaneous inhibition of Th17 and Th1 cells, both of them being involved in IBD etiopathology[46].
A potential strategy for IBD therapy is represented by the IL-17A and IL-17F combination, an important protection being observed against intestinal inflammation in an acute IL-17A knockout TNBS- induced colitis and also a clear improvement in the inflammatory process in IL-17F knockout DSS-induced colitis. Only IL-17A KO in the DSS model worsened the colonic inflammation[47].
A novel efficient and safe oral immunomodulatory drug, vidofludimus inhibits DHODH (dihydroorotate dehydrogenase), which is the key enzyme involved in pyrimidine biosynthesis in activated lymphocytes by reducing the IL-17A and IL-17F expression through the NF-κB pathway[48,49] .
The data presented in this review are mainly based on studies carried out on humans. Animal models of IBD are not totally relevant for this human specific condition; differences between human disease and animal models exist[50]. Animal models of intestinal inflammation may help understanding the action of inflammatory cytokines[51]. This topic was not the focus of the present review, where we looked for human data, for better accuracy. Data from clinical studies are highly relevant. A recent review shows the importance of immunological changes in the gut inflammation and these offer the background for emerging therapies[52].
This review presents the contribution of the IL-17/IL-23 axis in the pathogenesis of inflammatory bowel disease. Better understanding of the pathogenesis of this condition represent the background for the progress in therapy.
P- Reviewer: Hassan M, Hokama A, Kim SS S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
| 1. | Rubin DC, Shaker A, Levin MS. Chronic intestinal inflammation: inflammatory bowel disease and colitis-associated colon cancer. Front Immunol. 2012;3:107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 261] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 2. | Thoreson R, Cullen JJ. Pathophysiology of inflammatory bowel disease: an overview. Surg Clin North Am. 2007;87:575-585. [PubMed] [Cited in This Article: ] |
| 3. | Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev. 2014;13:3-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 484] [Cited by in F6Publishing: 596] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 4. | Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1693] [Cited by in F6Publishing: 1713] [Article Influence: 131.8] [Reference Citation Analysis (1)] |
| 5. | Nunes T, Fiorino G, Danese S, Sans M. Familial aggregation in inflammatory bowel disease: is it genes or environment? World J Gastroenterol. 2011;17:2715-2722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 30] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Danese S. What’s hot in inflammatory bowel disease in 2011? World J Gastroenterol. 2011;17:545-546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 10] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Scaldaferri F, Correale C, Gasbarrini A, Danese S. Mucosal biomarkers in inflammatory bowel disease: key pathogenic players or disease predictors? World J Gastroenterol. 2010;16:2616-2625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 28] [Cited by in F6Publishing: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Cătană CS, Berindan-Neagoe I. Aging and immunity. Age related changes in immunological, biochemical and genetic markers. Saarbrucken: Lambert Academic Publishing 2012; . [Cited in This Article: ] |
| 9. | Bamias G, Corridoni D, Pizarro TT, Cominelli F. New insights into the dichotomous role of innate cytokines in gut homeostasis and inflammation. Cytokine. 2012;59:451-459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Hansen R, Thomson JM, El-Omar EM, Hold GL. The role of infection in the aetiology of inflammatory bowel disease. J Gastroenterol. 2010;45:266-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Gálvez J. Role of Th17 Cells in the Pathogenesis of Human IBD. ISRN Inflamm. 2014;2014:928461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 215] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 12. | Himmel ME, Hardenberg G, Piccirillo CA, Steiner TS, Levings MK. The role of T-regulatory cells and Toll-like receptors in the pathogenesis of human inflammatory bowel disease. Immunology. 2008;125:145-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 13. | Plevy SE, Targan SR. Future therapeutic approaches for inflammatory bowel diseases. Gastroenterology. 2011;140:1838-1846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348-2357. [PubMed] [Cited in This Article: ] |
| 15. | Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933-944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1240] [Cited by in F6Publishing: 1205] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 16. | Jelley-Gibbs DM, Strutt TM, McKinstry KK, Swain SL. Influencing the fates of CD4 T cells on the path to memory: lessons from influenza. Immunol Cell Biol. 2008;86:343-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Geremia A, Jewell DP. The IL-23/IL-17 pathway in inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2012;6:223-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 69] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 18. | Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337-348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 787] [Cited by in F6Publishing: 810] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 19. | Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345-350. [PubMed] [Cited in This Article: ] |
| 20. | Siakavellas SI, Bamias G. Role of the IL-23/IL-17 axis in Crohn’s disease. Discov Med. 2012;14:253-262. [PubMed] [Cited in This Article: ] |
| 21. | Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 789] [Cited by in F6Publishing: 829] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 22. | Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Filì L, Ferri S, Frosali F. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849-1861. [PubMed] [Cited in This Article: ] |
| 23. | Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756-1767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 738] [Cited by in F6Publishing: 781] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 24. | Liu Z, Yadav PK, Xu X, Su J, Chen C, Tang M, Lin H, Yu J, Qian J, Yang PC. The increased expression of IL-23 in inflammatory bowel disease promotes intraepithelial and lamina propria lymphocyte inflammatory responses and cytotoxicity. J Leukoc Biol. 2011;89:597-606. [Cited in This Article: ] |
| 25. | Sallusto F, Zielinski CE, Lanzavecchia A. Human Th17 subsets. Eur J Immunol. 2012;42:2215-2220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 26. | Ahern PP, Schiering C, Buonocore S, McGeachy MJ, Cua DJ, Maloy KJ, Powrie F. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity. 2010;33:279-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 415] [Cited by in F6Publishing: 390] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 27. | McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 2006;27:17-23. [PubMed] [Cited in This Article: ] |
| 28. | Murphy KM, Stockinger B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nat Immunol. 2010;11:674-680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 357] [Cited by in F6Publishing: 358] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 29. | Ueno A, Jijon H, Chan R, Ford K, Hirota C, Kaplan GG, Beck PL, Iacucci M, Fort Gasia M, Barkema HW. Increased prevalence of circulating novel IL-17 secreting Foxp3 expressing CD4 T cells and defective suppressive function of circulating Foxp3 regulatory cells support plasticity between Th17 and regulatory T cells in inflammatory bowel disease patients. Inflamm Bowel Dis. 2013;19:2522-2534. [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 30. | Cho JH, Brant SR. Recent insights into the genetics of inflammatory bowel disease. Gastroenterology. 2011;140:1704-1712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 293] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 31. | Parkes M, Barrett JC, Prescott NJ, Tremelling M, Anderson CA, Fisher SA, Roberts RG, Nimmo ER, Cummings FR, Soars D. Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn’s disease susceptibility. Nat Genet. 2007;39:830-832. [PubMed] [Cited in This Article: ] |
| 32. | Kim SW, Kim ES, Moon CM, Park JJ, Kim TI, Kim WH, Cheon JH. Genetic polymorphisms of IL-23R and IL-17A and novel insights into their associations with inflammatory bowel disease. Gut. 2011;60:1527-1536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 33. | Jürgens M, Laubender RP, Hartl F, Weidinger M, Seiderer J, Wagner J, Wetzke M, Beigel F, Pfennig S, Stallhofer J. Disease activity, ANCA, and IL23R genotype status determine early response to infliximab in patients with ulcerative colitis. Am J Gastroenterol. 2010;105:1811-1819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 34. | van de Logt F, Day AS. S100A12: a noninvasive marker of inflammation in inflammatory bowel disease. J Dig Dis. 2013;14:62-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 35. | Lu Y, Zhou L, Liu L, Feng Y, Lu L, Ren X, Dong X, Sang W. Serum omentin-1 as a disease activity marker for Crohn’s disease. Dis Markers. 2014;2014:162517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Grad C, David L, Portincasa P, Dumitraşcu DL. Diagnostic value of calprotectin in irritable bowel syndrome and in inflammatory bowel disease. Rom J Intern Med. 2012;50:3-6. [PubMed] [Cited in This Article: ] |
| 37. | Fengming Y, Jianbing W. Biomarkers of inflammatory bowel disease. Dis Markers. 2014;2014:710915. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 38. | Husain N, Tokoro K, Popov JM, Naides SJ, Kwasny MJ, Buchman AL. Neopterin concentration as an index of disease activity in Crohn’s disease and ulcerative colitis. J Clin Gastroenterol. 2013;47:246-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 39. | Iborra M, Bernuzzi F, Correale C, Vetrano S, Fiorino G, Beltrán B, Marabita F, Locati M, Spinelli A, Nos P. Identification of serum and tissue micro-RNA expression profiles in different stages of inflammatory bowel disease. Clin Exp Immunol. 2013;173:250-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 40. | Lin J, Welker NC, Zhao Z, Li Y, Zhang J, Reuss SA, Zhang X, Lee H, Liu Y, Bronner MP. Novel specific microRNA biomarkers in idiopathic inflammatory bowel disease unrelated to disease activity. Mod Pathol. 2014;27:602-608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 41. | Neurath MF. New targets for mucosal healing and therapy in inflammatory bowel diseases. Mucosal Immunol. 2014;7:6-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 223] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 42. | Danese S. New therapies for inflammatory bowel disease: from the bench to the bedside. Gut. 2012;61:918-932. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 221] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 43. | Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PD, Wehkamp J, Feagan BG, Yao MD, Karczewski M. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693-1700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1055] [Cited by in F6Publishing: 1099] [Article Influence: 91.6] [Reference Citation Analysis (0)] |
| 44. | Sands BE, Jacobson EW, Sylwestrowicz T, Younes Z, Dryden G, Fedorak R, Greenbloom S. Randomized, double-blind, placebo-controlled trial of the oral interleukin-12/23 inhibitor apilimod mesylate for treatment of active Crohn’s disease. Inflamm Bowel Dis. 2010;16:1209-1218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 45. | Fina D, Sarra M, Fantini MC, Rizzo A, Caruso R, Caprioli F, Stolfi C, Cardolini I, Dottori M, Boirivant M. Regulation of gut inflammation and th17 cell response by interleukin-21. Gastroenterology. 2008;134:1038-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 209] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 46. | Sandborn WJ, Gasink C, Gao LL, Blank MA, Johanns J, Guzzo C, Sands BE, Hanauer SB, Targan S, Rutgeerts P, Ghosh S, de Villiers WJ, Panaccione R, Greenberg G, Schreiber S, Lichtiger S, Feagan BG, ERTIFI Study Group. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. 2012;367:1519-1528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 810] [Cited by in F6Publishing: 779] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 47. | Wedebye Schmidt EG, Larsen HL, Kristensen NN, Poulsen SS, Lynge Pedersen AM, Claesson MH, Pedersen AE. TH17 cell induction and effects of IL-17A and IL-17F blockade in experimental colitis. Inflamm Bowel Dis. 2013;19:1567-1576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 48. | Fitzpatrick LR. Novel Pharmacological Approaches for Inflammatory Bowel Disease: Targeting Key Intracellular Pathways and the IL-23/IL-17 Axis. Int J Inflam. 2012;2012:389404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 49. | Herrlinger KR, Diculescu M, Fellermann K, Hartmann H, Howaldt S, Nikolov R, Petrov A, Reindl W, Otte JM, Stoynov S. Efficacy, safety and tolerability of vidofludimus in patients with inflammatory bowel disease: the ENTRANCE study. J Crohns Colitis. 2013;7:636-643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 50. | Ray S, De Salvo C, Pizarro TT. Central role of IL-17/Th17 immune responses and the gut microbiota in the pathogenesis of intestinal fibrosis. Curr Opin Gastroenterol. 2014;30:531-538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 51. | Blumberg RS, Saubermann LJ, Strober W. Animal models of mucosal inflammation and their relation to human inflammatory bowel disease. Curr Opin Immunol. 1999;11:648-656. [PubMed] [Cited in This Article: ] |
| 52. | Plaza-Diaz J, Gomez-Llorente C, Fontana L, Gil A. Modulation of immunity and inflammatory gene expression in the gut, in inflammatory diseases of the gut and in the liver by probiotics. World J Gastroenterol. 2014;20:15632-15649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 146] [Cited by in F6Publishing: 128] [Article Influence: 12.8] [Reference Citation Analysis (0)] |