Published online Apr 21, 2015. doi: 10.3748/wjg.v21.i15.4750
Peer-review started: August 12, 2014
First decision: October 14, 2014
Revised: November 5, 2014
Accepted: January 5, 2015
Article in press: January 5, 2015
Published online: April 21, 2015
AIM: To investigate the relationship between Helicobacter pylori infection and inflammatory bowel disease (IBD) in an Asian population.
METHODS: The PubMed, EMBASE, and Cochrane Library databases were searched for observational studies published up until June 2014, without language restrictions. Additional references were obtained from reviewed articles.
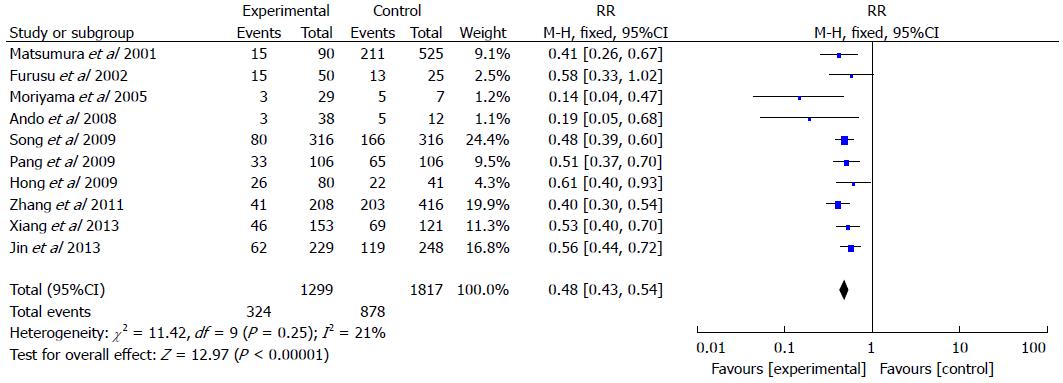
RESULTS: Ten studies involving 1299 IBD patients and 1817 controls were included in the meta-analysis (24.9% of IBD patients had H. pylori infection vs 48.3% of the controls). The pooled risk ratio for H. pylori infection in IBD patients compared with controls was 0.48 (95%CI: 0.43-0.54; P < 0.001). There was no significant heterogeneity in the included studies (I2 = 21%). Egger’s linear regression indicated that there was no significant publication bias (P = 0.203).
CONCLUSION: The H. pylori infection rate in Asian IBD patients is significantly lower than in non-IBD patients, indicating that infection protects against the development of IBD.
Core tip: A meta-analysis was carried out to investigate the relationship between Helicobacter pylori (H. pylori) infection and inflammatory bowel disease (IBD) in an Asian population. A search of PubMed, EMBASE, and Cochrane Library databases identified ten studies involving 1299 IBD patients and 1817 controls that were included in the meta-analysis (24.9% of IBD patients had H. pylori infection vs 48.3% of the controls). This meta-analysis showed that in an Asian population, the H. pylori infection rate was significantly lower in IBD patients than in non-IBD patients, indicating a protective effect of H. pylori infection against IBD.
-
Citation: Wu XW, Ji HZ, Yang MF, Wu L, Wang FY.
Helicobacter pylori infection and inflammatory bowel disease in Asians: A meta-analysis. World J Gastroenterol 2015; 21(15): 4750-4756 - URL: https://www.wjgnet.com/1007-9327/full/v21/i15/4750.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i15.4750
Inflammatory bowel disease (IBD) is described as chronic inflammation of the gastrointestinal tract in genetically susceptible individuals exposed to environmental risk factors, and involves alternating active and quiescent phases leading to an increased health burden worldwide[1,2]. Crohn’s disease (CD) and ulcerative colitis (UC) are the two primary subtypes of IBD. These diseases may result in intestinal damage, complications, and surgical interventions[3,4]. Commensal enteric bacteria are considered to have a critical role in the development of IBD. Continuous microbial antigenic stimulation can activate pathogenic immune responses and result in damage to intestinal mucosal barrier function and immunoregulation[5]. Genetic polymorphisms in the host most likely interact with intestinal bacteria to stimulate aggressive immunoreactions that cause chronic tissue injury. It is necessary to identify these host and microbial changes in individual patients in order to treat IBD[6,7].
Helicobacter species are characterized by microaerophilic metabolism, spiral shape, and peculiar motility, which contribute to their colonization of the gastrointestinal mucosal surface[8]. Helicobacter pylori (H. pylori) is a gram-negative pathogenic bacterium that is associated with chronic gastritis and is usually located on the surface of the stomach epithelium. Interestingly, H. pylori has also been identified in the normal colonic mucosa, colorectal neoplasms[9-11], and the intestinal mucosa of IBD patients[12,13]. Furthermore, H. pylori has been confirmed as a risk factor for colonic neoplasms[14,15], however, there is insufficient evidence to conclude that H. pylori has an important role in the pathogenesis of IBD.
Many observational studies have investigated the association between H. pylori infection and IBD. A meta-analysis involving 23 studies suggested that the H. pylori infection rate was lower in IBD patients than in non-IBD patients, and H. pylori was beneficial in preventing the development of IBD[16]. However, this meta-analysis only included one study that investigated the prevalence of H. pylori in an Asian population, and the conclusion may not be suitable for Asian populations. Therefore, we carried out an updated meta-analysis including only studies that determined the prevalence of H. pylori in IBD patients from Asian countries.
Studies in line with the following criteria were included: (1) investigated the relationship between H. pylori infection and IBD; (2) used a case-control, cross-sectional, or cohort design; and (3) specifically included an Asian population. Studies were excluded if: (1) data from a previously published study were used; and (2) included a pediatric population.
We performed a search of PubMed, EMBASE, and the Cochrane Library for studies published up until June 2014. A search strategy was constructed using a combination of the following words: (Helicobacter pylori or H. pylori) and (IBD or CD or UC). Articles published in any language were included. A manual search of the references listed by studies retrieved from the online databases and from previously published systematic reviews was also performed to identify additional relevant studies.
Two investigators (Wu XW and Ji HZ) extracted data. Any differences regarding study inclusion, data extraction, and interpretation were resolved by consensus before the final analysis. Study variables were collected in the following categories: year of publication, country of origin, study center, characteristics of the patients, and H. pylori detection method. To avoid inclusion of duplicated data in the final analysis, retrieved studies were carefully evaluated and checked by comparing author names, geographic locations, and period of study.
Meta-analysis was carried out by combining the risk ratio (RR) in the IBD and control groups of the individual studies in a global RR. Statistical heterogeneity testing was performed using the χ2 statistic and I2, and an I2 > 50% was considered to represent substantial heterogeneity[17]. A fixed effects model was selected when the heterogeneity test showed an I2 < 50%, otherwise a random effects model was used. A funnel plot was used to determine publication bias[18]. Analyses were conducted using Review Manager software (RevMan version 5.3; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). The statistical methods of this study were reviewed by Liu from Department of Medical Statistics, School of Medicine, Nanjing University.
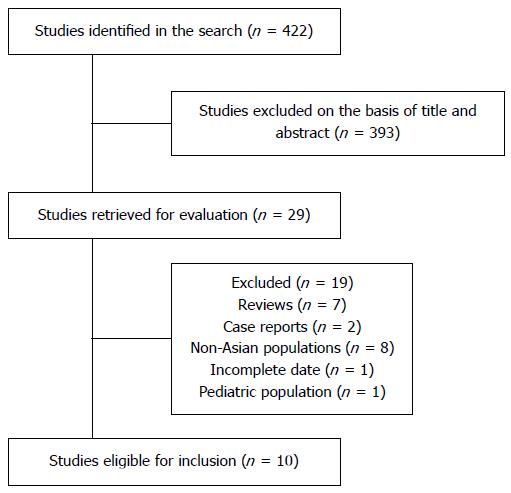
Our search identified 422 potentially relevant studies, of which 393 were excluded after title and abstract screening. Twenty-nine articles were retained for full-text review. Seven review articles, two case reports, eight studies including non-Asian populations, one study with incomplete data, and one study which included a pediatric population were subsequently excluded. We identified ten studies which fulfilled the inclusion criteria[13,19-27]. These studies included a total of 1299 cases of IBD and 1817 controls. Figure 1 shows the study flow diagram.
The characteristics of the included studies and patients are presented in Table 1. Four studies were from Japan, four studies were from China, and two studies were from South Korea. All were case-control studies. Two of them were multicenter studies, and eight were single-center studies. The mean age of IBD patients ranged from 28.9 to 44.7 years. Two studies used serologic tests (immunoglobulin G) and eight studies used non-serologic tests (C-urea breath test, biopsy specimen histology, or biopsy sample culture) to detect H. pylori.
| Ref. | Country | Study center | CD/UC, | Control, | Mean age, yr | Male, % | Helicobacter pylori detection |
| n | n | (CD/UC) | (CD/UC) | ||||
| Matsumura et al[19] 2001 | Japan | Multiple | 90/NR | 525 | 31.7/NR | 70.0/NR | IgG |
| Furusu et al[20] 2002 | Japan | Single | 25/25 | 25 | NR | NR | IgG/Histology |
| Moriyama et al[21] 2005 | Japan | Single | 29/NR | 7 | 31.6/NR | 59.0/NR | UBT |
| Ando et al[22] 2008 | Japan | Single | 38/NR | 12 | 28.9/NR | 74.0/NR | UBT |
| Hong et al[23] 2009 | South Korea | Single | 37/43 | 41 | 38.2/44.2 | 73.0/67.4 | Histology |
| Pang et al[24] 2009 | China | Single | 52/54 | 106 | 36.7/42.3 | 57.7/55.6 | IgG |
| Song et al[25] 2009 | South Korea | Multiple | 147/169 | 316 | 33.5/44.7 | 67.3/63.3 | UBT |
| Zhang et al[13] 2011 | China | Single | 104/104 | 416 | 31.0/40.9 | 66.3/57.7 | UBT |
| Jin et al[26] 2013 | China | Single | NR/153 | 121 | NR/44.6 | NR/51.6 | UBT/Culture |
| Xiang et al[27] 2013 | China | Single | 229/NR | 248 | 46.2/NR | 58.1/NR | UBT/Culture |
Of these studies, which included 1299 IBD patients and 1817 controls (Figure 2), 24.9% of patients in the IBD groups were found to have H. pylori infection, while 48.3% of patients in the control groups had H. pylori infection. The pooled RR of H. pylori infection rate in IBD patients compared to controls was 0.48 (95%CI: 0.43-0.54; P < 0.001). A fixed effects model was used for the meta-analysis as no significant heterogeneity in the included studies was observed (I2 = 21%).
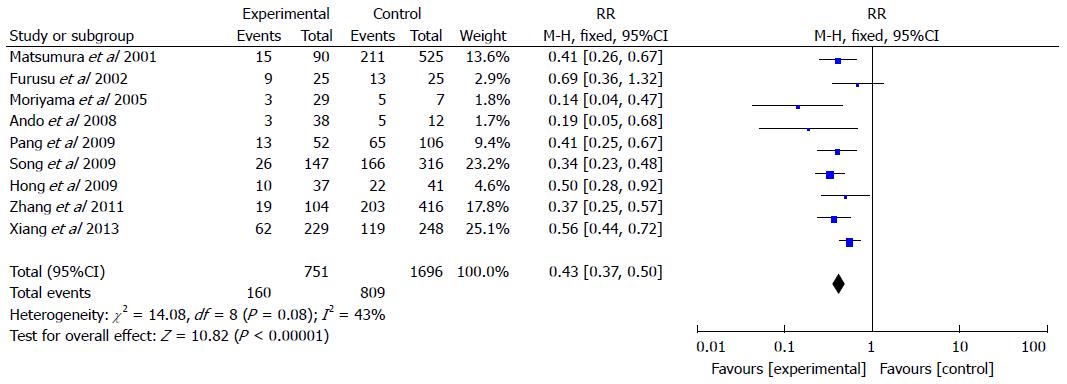
Nine studies included 751 CD patients and 1696 controls (Figure 3). The rate of H. pylori infection in CD patients was 21.3% compared with 47.7% in the control groups (RR = 0.43, 95%CI: 0.37-0.50; P < 0.001). A fixed effects model was selected for the meta-analysis as significant heterogeneity in the included studies was not observed (I2 = 43%).
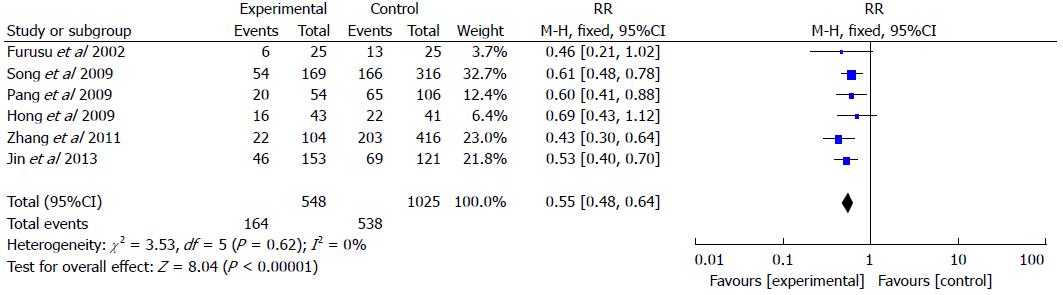
Six studies included 548 UC patients and 1025 controls (Figure 4). The rate of H. pylori infection was 29.9% in UC patients vs 52.5% in the control groups (RR = 0.55, 95%CI: 0.48-0.64; P < 0.001). We performed the meta-analysis with a fixed effects model as no significant heterogeneity was found in the included studies (I2 = 0%).
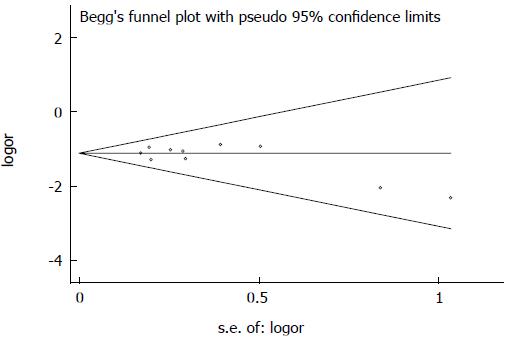
The funnel plot revealed a reasonably symmetrical distribution of the included studies examining the association between H. pylori infection and IBD (Figure 5). Egger’s linear regression indicated that there was no statistically significant evidence of publication bias (P = 0.203).
The prevalence of H. pylori infection varies markedly in different countries and regions. Higher prevalence rates are seen in some developing Asian countries[28,29], while lower rates have been found in many developed countries in Europe and North America[30,31]. Moreover, the prevalence rates may vary significantly in different geographic regions or ethnic populations of the same country[32,33]. However, the variation in H. pylori infection rate in IBD patients of different race or region has not been completely clarified.
Our meta-analysis identified ten studies focused on the association between H. pylori infection and IBD in an Asian population. The included cases were from three east-Asian countries (China, Japan, and South Korea) that are considered to have similar ethnic origin, whereas the previous meta-analysis published in 2010 involved populations mostly from European and American countries[16]. By specifically including an Asian population, the heterogeneity and publication bias were both lower than those in the previous meta-analysis. The pooled rate of H. pylori infection in the IBD groups was a little lower than that reported in the previous meta-analysis (24.9% vs 27.1%), but the pooled rate of H. pylori infection in the control groups was higher than that previously reported (48.3% vs 40.9%). These discordant results suggest that ethnic origin may have a potential impact on the relationship between H. pylori infection and IBD.
The mechanism of H. pylori infection in preventing IBD is unclear. It has been hypothesized that H. pylori may downregulate proinflammatory immune responses in the host in order to promote its own survival, resulting in beneficial effects to the host. An animal experiment showed that long-term H. pylori infection can lead to distinct changes in microbiota composition in the large intestine, indicating that H. pylori may modulate the intestinal flora to affect the development of IBD[34]. Other research in animal colitis models suggested that H. pylori infection can regulate the immune responses, resulting in benefit to the host against other chronic inflammatory conditions such as IBD[35,36]. Papamichael et al[37] showed that H. pylori infection may play a protective role against IBD via the following mechanisms: increasing the levels of some cytokines, activating dendritic cells and T cells, downregulating the Th1/Th17 pathway, and inducing the generation of antibodies against H. pylori. In our meta-analysis, the lower pooled RR of H. pylori infection in IBD patients compared to controls suggests a protective effect of infection against the development of IBD. Subgroup analyses showed a tendency toward a more pronounced effect for CD when compared to UC. One of the included studies revealed a more evident association between H. pylori infection and IBD in patients < 60 years-old, which suggests that H. pylori infection may reduce the risk of IBD in younger adults[25]. Two studies found that the rate of H. pylori infection in IBD patients treated with antibiotics was lower than untreated patients. However, IBD patients without antibiotic treatment still showed a significantly lower rate of H. pylori infection than controls[24,25]. In a study with only 153 UC patients, the H. pylori infection rates in patients with diverse severity or extent of UC were significantly lower than those in the controls[26]. Another study showed that the H. pylori infection rates in patients with colonic, small intestine and ileocolonic CD were significantly lower than that in the control group[27]. We did not find any obvious correlation between the phenotypic characteristics of IBD patients and H. pylori infection rate in the included studies.
In conclusion, our meta-analysis shows that the rate of H. pylori infection in IBD patients from Asian countries is significantly lower than in non-IBD patients. However, there are some limitations in our meta-analysis, such as an insufficient number of included studies and potential heterogeneity. More prospective high-quality controlled studies are required to confirm the results of this meta-analysis.
Epidemiologic data show that Helicobacter pylori (H. pylori) infection has a protective effect against the development of autoimmune disease. Laboratory data suggest that H. pylori can induce immune tolerance and suppress the inflammatory response. Many observational studies have investigated the association between H. pylori infection and inflammatory bowel disease (IBD). Most of these studies found that the H. pylori infection rate in IBD patients was lower than that in non-IBD patients. However, conflicting outcomes have been observed and the exact mechanism of the protective effect of H. pylori in IBD development is still unclear.
Numerous studies describing the H. pylori infection rates in IBD patients compared with healthy controls have been published in the past twenty years. A previous meta-analysis involving 23 studies suggested a lower H. pylori infection rate in IBD patients. H. pylori infection rate is related to ethnicity and region, however, the previous meta-analysis only included one study from an Asian country. This study investigated the relationship between H. pylori infection and IBD in an Asian population.
The authors demonstrated that the H. pylori infection rate in Asian IBD patients was significantly lower than in non-IBD Asian patients. The pooled H. pylori infection rate of IBD patients in this study was a little lower than the previous meta-analysis (24.9% vs 27.1%), but the pooled rate in the control group was higher than that previously reported (48.3% vs 40.9%). These results suggest that ethnic origin may have an impact on H. pylori infection rate, providing powerful evidence that H. pylori infection is a potential protective factor in the development of IBD.
The results of this meta-analysis show that IBD patients with a history of taking antibiotics had a lower H. pylori infection rate in two of the included studies, however, there were no available data for the controls. Antibiotic use may be partly responsible for the lower H. pylori infection rate in IBD patients. The exact mechanism of this interesting phenomenon should be investigated further. Large-sample and well-designed cohort studies with stringent disease and H. pylori infection definitions are required in order to delineate the protective effect of H. pylori infection in IBD development.
H. pylori is a gram-negative, microaerophilic bacterium. It is present in patients with chronic gastritis and gastric ulcers. It is also linked to the development of duodenal ulcers and stomach cancer. H. pylori may be implicated in the pathogenesis of autoimmune diseases.
This is a well-designed and performed meta-analysis that confirms the results recently published for non-Asian cohorts. The results are interesting and are worthy of publication.
P- Reviewer: Chen Z, Engin AB, Kopylov U S- Editor: Qi Y L- Editor: AmEditor E- Editor: Liu XM
| 1. | Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066-2078. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Nikolaus S, Schreiber S. Diagnostics of inflammatory bowel disease. Gastroenterology. 2007;133:1670-1689. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Larson DW, Pemberton JH. Current concepts and controversies in surgery for IBD. Gastroenterology. 2004;126:1611-1619. [PubMed] [Cited in This Article: ] |
| 4. | Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Rook GA, Brunet LR. Microbes, immunoregulation, and the gut. Gut. 2005;54:317-320. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577-594. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology. 2014;146:1489-1499. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Sonnenberg A. Review article: historic changes of Helicobacter pylori-associated diseases. Aliment Pharmacol Ther. 2013;38:329-342. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Keenan JI, Beaugie CR, Jasmann B, Potter HC, Collett JA, Frizelle FA. Helicobacter species in the human colon. Colorectal Dis. 2010;12:48-53. [PubMed] [Cited in This Article: ] |
| 10. | Bulajic M, Stimec B, Ille T, Jesenofsky R, Kecmanovic D, Pavlov M, Ceranic M, Schneider-Brachert W, Lowenfels A, Maisonneuve P. PCR detection of helicobacter pylori genome in colonic mucosa: normal and malignant. Prilozi. 2007;28:25-38. [PubMed] [Cited in This Article: ] |
| 11. | Jones M, Helliwell P, Pritchard C, Tharakan J, Mathew J. Helicobacter pylori in colorectal neoplasms: is there an aetiological relationship? World J Surg Oncol. 2007;5:51. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Oliveira AG, Rocha GA, Rocha AM, Sanna Md, Moura SB, Dani R, Marinho FP, Moreira LS, Ferrari Mde L, Castro LP. Isolation of Helicobacter pylori from the intestinal mucosa of patients with Crohn’s disease. Helicobacter. 2006;11:2-9. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Zhang S, Zhong B, Chao K, Xiao Y, Cui Y, Gao X, Chen B, He Y, Hu P, Chen M. Role of Helicobacter species in Chinese patients with inflammatory bowel disease. J Clin Microbiol. 2011;49:1987-1989. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Sonnenberg A, Genta RM. Helicobacter pylori is a risk factor for colonic neoplasms. Am J Gastroenterol. 2013;108:208-215. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Selgrad M, Bornschein J, Kandulski A, Hille C, Weigt J, Roessner A, Wex T, Malfertheiner P. Helicobacter pylori but not gastrin is associated with the development of colonic neoplasms. Int J Cancer. 2014;135:1127-1131. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Luther J, Dave M, Higgins PD, Kao JY. Association between Helicobacter pylori infection and inflammatory bowel disease: a meta-analysis and systematic review of the literature. Inflamm Bowel Dis. 2010;16:1077-1084. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [PubMed] [Cited in This Article: ] |
| 19. | Matsumura M, Matsui T, Hatakeyama S, Matake H, Uno H, Sakurai T, Yao T, Oishi T, Iwashita A, Fujioka T. Prevalence of Helicobacter pylori infection and correlation between severity of upper gastrointestinal lesions and H. pylori infection in Japanese patients with Crohn’s disease. J Gastroenterol. 2001;36:740-747. [PubMed] [Cited in This Article: ] |
| 20. | Furusu H, Murase K, Nishida Y, Isomoto H, Takeshima F, Mizuta Y, Hewlett BR, Riddell RH, Kohno S. Accumulation of mast cells and macrophages in focal active gastritis of patients with Crohn’s disease. Hepatogastroenterology. 2002;49:639-643. [PubMed] [Cited in This Article: ] |
| 21. | Moriyama T, Matsumoto T, Jo Y, Yada S, Hirahashi M, Yao T, Iida M. Mucosal proinflammatory cytokine and chemokine expression of gastroduodenal lesions in Crohn’s disease. Aliment Pharmacol Ther. 2005;21 Suppl 2:85-91. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Ando T, Watanabe O, Ishiguro K, Maeda O, Ishikawa D, Minami M, Hasegawa M, Kondo S, Goto Y, Ohmiya N. Relationships between Helicobacter pylori infection status, endoscopic, histopathological findings, and cytokine production in the duodenum of Crohn’s disease patients. J Gastroenterol Hepatol. 2008;23 Suppl 2:S193-S197. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Hong CH, Park DI, Choi WH, Park JH, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI, Kim DH. [The clinical usefulness of focally enhanced gastritis in Korean patients with Crohn’s disease]. Korean J Gastroenterol. 2009;53:23-28. [PubMed] [Cited in This Article: ] |
| 24. | Pang Z, Li MF, Huangfu Z, Zhou CL, Shen BW. Low prevalence of Helicobacter pylori infection in Chinese Han patients with inflammatory bowel disease. Shijie Huaren Xiaohua Zazhi. 2009;17:3661-3665. [Cited in This Article: ] |
| 25. | Song MJ, Park DI, Hwang SJ, Kim ER, Kim YH, Jang BI, Lee SH, Ji JS, Shin SJ. [The prevalence of Helicobacter pylori infection in Korean patients with inflammatory bowel disease, a multicenter study]. Korean J Gastroenterol. 2009;53:341-347. [PubMed] [Cited in This Article: ] |
| 26. | Jin X, Chen YP, Chen SH, Xiang Z. Association between Helicobacter Pylori infection and ulcerative colitis--a case control study from China. Int J Med Sci. 2013;10:1479-1484. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Xiang Z, Chen YP, Ye YF, Ma KF, Chen SH, Zheng L, Yang YD, Jin X. Helicobacter pylori and Crohn’s disease: a retrospective single-center study from China. World J Gastroenterol. 2013;19:4576-4581. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Shi R, Xu S, Zhang H, Ding Y, Sun G, Huang X, Chen X, Li X, Yan Z, Zhang G. Prevalence and risk factors for Helicobacter pylori infection in Chinese populations. Helicobacter. 2008;13:157-165. [PubMed] [DOI] [Cited in This Article: ] |
| 29. | Singh V, Trikha B, Nain CK, Singh K, Vaiphei K. Epidemiology of Helicobacter pylori and peptic ulcer in India. J Gastroenterol Hepatol. 2002;17:659-665. [PubMed] [Cited in This Article: ] |
| 30. | Gasbarrini G, Pretolani S, Bonvicini F, Gatto MR, Tonelli E, Mégraud F, Mayo K, Ghironzi G, Giulianelli G, Grassi M. A population based study of Helicobacter pylori infection in a European country: the San Marino Study. Relations with gastrointestinal diseases. Gut. 1995;36:838-844. [PubMed] [Cited in This Article: ] |
| 31. | Graham DY, Malaty HM, Evans DG, Evans DJ, Klein PD, Adam E. Epidemiology of Helicobacter pylori in an asymptomatic population in the United States. Effect of age, race, and socioeconomic status. Gastroenterology. 1991;100:1495-1501. [PubMed] [Cited in This Article: ] |
| 32. | Perez-Perez GI, Olivares AZ, Foo FY, Foo S, Neusy AJ, Ng C, Holzman RS, Marmor M, Blaser MJ. Seroprevalence of Helicobacter pylori in New York City populations originating in East Asia. J Urban Health. 2005;82:510-516. [PubMed] [DOI] [Cited in This Article: ] |
| 33. | Lanciers S, Hauser B, Vandenplas Y, Blecker U. The prevalence of Helicobacter pylori positivity in asymptomatic children of different ethnic backgrounds living in the same country. Ethn Health. 1996;1:169-173. [PubMed] [DOI] [Cited in This Article: ] |
| 34. | Heimesaat MM, Fischer A, Plickert R, Wiedemann T, Loddenkemper C, Göbel UB, Bereswill S, Rieder G. Helicobacter pylori induced gastric immunopathology is associated with distinct microbiota changes in the large intestines of long-term infected Mongolian gerbils. PLoS One. 2014;9:e100362. [PubMed] [DOI] [Cited in This Article: ] |
| 35. | Luther J, Owyang SY, Takeuchi T, Cole TS, Zhang M, Liu M, Erb-Downward J, Rubenstein JH, Chen CC, Pierzchala AV. Helicobacter pylori DNA decreases pro-inflammatory cytokine production by dendritic cells and attenuates dextran sodium sulphate-induced colitis. Gut. 2011;60:1479-1486. [PubMed] [DOI] [Cited in This Article: ] |
| 36. | Higgins PD, Johnson LA, Luther J, Zhang M, Sauder KL, Blanco LP, Kao JY. Prior Helicobacter pylori infection ameliorates Salmonella typhimurium-induced colitis: mucosal crosstalk between stomach and distal intestine. Inflamm Bowel Dis. 2011;17:1398-1408. [PubMed] [DOI] [Cited in This Article: ] |
| 37. | Papamichael K, Konstantopoulos P, Mantzaris GJ. Helicobacter pylori infection and inflammatory bowel disease: is there a link? World J Gastroenterol. 2014;20:6374-6385. [PubMed] [DOI] [Cited in This Article: ] |