Published online Dec 28, 2014. doi: 10.3748/wjg.v20.i48.18131
Revised: September 22, 2014
Accepted: November 7, 2014
Published online: December 28, 2014
Liver cirrhosis (LC), the end stage of many forms of chronic hepatitis of different etiologies is a diffuse process characterized by fibrosis and the conversion of normal liver architecture into structurally abnormal nodules surrounded by annular fibrosis. This chronic progressive clinical condition, leads to liver cell failure and portal hypertension, which can favour the onset of hepatocellular carcinoma. Defining the phase of the natural history is crucial for therapeutic choice and prognosis. Liver biopsy is currently considered the best available standard of reference but it has some limits, so alternative tools have been developed to substitute liver biopsy when assessing liver fibrosis. Serum markers offer a cost-effective alternative to liver biopsy being less invasive and theoretically without complications. They can be classified into direct and indirect markers which may be used alone or in combination to produce composite scores. Diagnostic imaging includes a number of instruments and techniques to estimate liver fibrosis and cirrhosis like ultrasound (US), US Doppler, contrast enhanced US and Elastography. US could be used for the diagnosis of advanced LC while is not able to evaluate progression of fibrosis, in this case Elastography is more reliable. This review aims to revise the most recent data from the literature about non invasive methods useful in defining liver fibrosis.
Core tip: Liver biopsy is the current best available standard of reference to diagnose liver fibrosis, but it has several limits. Non-invasive methods to detect and follow up liver fibrosis (direct and indirect serum markers, ultrasound, ultrasound doppler, contrast enhanced ultrasound and Elastography) have been extensively studied in the last years but strong evidences of their usefulness in the real practice are still lacking. This work aims to review the most recent literature about the use of these non-invasive markers in defining liver fibrosis.
- Citation: Soresi M, Giannitrapani L, Cervello M, Licata A, Montalto G. Non invasive tools for the diagnosis of liver cirrhosis. World J Gastroenterol 2014; 20(48): 18131-18150
- URL: https://www.wjgnet.com/1007-9327/full/v20/i48/18131.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i48.18131
Liver cirrhosis (LC) represents the end stage of many forms of chronic hepatitis of different etiologies and with quite variable courses[1-6]. In chronic liver disease (CLD), the wound-healing process progresses towards fibrosis due to the persistence of a noxa patogena[7].
The accumulation of collagen fibers and non-collagenous components in the extracellular matrix (ECM) determines a progressive distortion of liver structure until a clear picture emerges of LC, defined as a diffuse process characterized by fibrosis and the conversion of normal liver architecture into structurally abnormal nodules surrounded by annular fibrosis[8].
Cirrhosis can be either micro- and macro-nodular. In macro-nodular cirrhosis, nodules are greater than 3 mm in size[9]. It is a chronic progressive clinical condition, leading to liver cell failure and portal hypertension, which in turn create a micro-environment that favours the onset of hepatocellular carcinoma (HCC). This new condition therefore modifies the natural history of CLD[2].
Progression towards fibrosis and cirrhosis is a heterogeneous process influenced in many ways: by etiological or host factors (sex, genetics, immunosuppression, race, obesity, etc.) and by treatments.
Clinically, LC may be divided into two phases: (1) compensated; and (2) decompensated[10]. Decompensated LC is easily diagnosed as it presents with a series of clinical and laboratory signs (ascites, spider naevi, encephalopathy, esophageal varices, gastrointestinal bleeding, thrombocytopenia, hypoalbuminemia), while compensated LC may be difficult to differentiate from chronic hepatitis. Again, LC may be divided into two forms according to the presence/absence of portal hypertension[10]. Consequently, accurate evaluation of the fibrosis stage or of the appearance of overt cirrhosis is fundamental not only to reach a correct diagnosis but also to commence correct treatment or screening protocols to permit an early diagnosis of the frequent and fatal complications of LC, such as esophageal varices, HCC, etc.[11-13].
Fibrosis consists of the accumulation over time of collagen and non-collagenous material in the ECM of interstitial liver as a consequence of necro-inflammatory phenomena due to various etiological factors (viral, alcohol, autoimmune, toxic, etc.).
Tools to estimate liver fibrosis may be invasive, such as liver biopsy, or non-invasive, which may be divided into serological tests and imaging instruments.
In the evaluation of chronic hepatitis, liver biopsy is considered as the “reference standard” to determine both the necroinflammatory (grading) and the fibrosis (staging) components.
After the discovery of hepatitis C virus (HCV) in 1989, the old qualitative histological classification of chronic hepatitis, (which codified the terms: chronic persistent, chronic aggressive and chronic lobular hepatitis) was replaced by semiquantitative and reproducible histological scoring systems[14,15].
Knodell et al[16] were the first to attribute weighted numeric values to lesions, creating a score defined as the Histology Activity Index (HAI), which evaluated Grading and Staging. Since then, several histological classification systems have been proposed to stage fibrosis and grade necroinflammation in CHC (Table 1).
| No fibrosis | Fibrous portal expansion | Portal/periportal fibrosis | Portal fibrosis with rare septa | Abundant bridging fibrosis | Marked bridging with occasional nodules | Cirrhosis | |
| Knodell et al[16] | F0 | F1 | F1 | F3 | F3 | F4 | F4 |
| Scheuer et al[17] | F0 | F1 | F2 | F2 | F3 | F3 | F4 |
| IASL[18] | F0 | F1 | F2 | F2 | F3 | F3 | F4 |
| Ishak et al[19] | F0 | F1 | F2 | F3 | F4 | F5 | F6 |
| METAVIR[20] | F0 | F1 | F1 | F2 | F3 | F4 | F4 |
The Sheuer, Ishak, METAVIR and other scoring systems have been used above all for viral liver diseases, while for non-alcoholic fatty liver disease (NAFLD) the Brunt score has been used[17-21]. The METAVIR and Ishak scores are the ones currently most adopted. METAVIR divides fibrosis into 5 stages, progressing from absent (F0) to mild (F1), significant (F2), advanced (or severe) (F3) fibrosis and cirrhosis (F4). The Ishak score is more detailed, dividing the disease evolution process into six stages. However, although liver biopsy is currently considered the gold standard for determining the stage of fibrosis, it has some limitations in correctly defining liver disease and, above all, in evaluating its prognosis.
Sampling error: It is well known that liver biopsy can over- or under-estimate the degree of liver disease progression in 1/3 cases as it samples only a tiny portion of the liver (about 1/50000). The quality of liver biopsy is determined by the length, width, fragmentation and number of complete portal tracts. A small sample size may be inadequate for staging fibrosis, especially in pathologies in which histological damage is not uniformly distributed[22,23] and cirrhosis can go undetected on a single passage in 10%-30% of cases[24]. For this reason Scheuer[24] suggested that “bigger is better”. In the literature, the work of Colleredo clearly demonstrates this limitation, and introduces the concept of a “minimum number of complete portal tracts” necessary for a correct diagnosis[25]. However, although the American Association for the Study of Liver Diseases has recommended a biopsy sample of at least 20-30 mm in length and containing at least 11 complete portal tracts[26], this recommendation is not always followed[27].
Inter-observer variability: Despite the introduction of the HAI score, inter-observer variability is a reality among hepatopathologists, reaching 20% when categorizing the degree of fibrosis[21,28].
Invasiveness: Although quite safe, liver biopsy may lead to some moderate (20%) or severe (0.5%) complications and mortality (0.03%)[29,30].
Dynamic process of fibrosis: One of the main limitations of liver biopsy is that fibrosis is a dynamic process, thus making this tool of little use for estimating the evolution of fibrosis and the effect of therapeutic agents on it over time.
Staging cirrhosis: Even when histology shows a picture of cirrhosis, liver biopsy is of no help in staging cirrhosis, and other tools (Hepatic venous pressure gradient-HVPG- or esophago-gastroduodenoscopy) are necessary to determine the presence and degree of portal hypertension.
As liver fibrosis implies morphological damage, liver biopsy has become the natural gold standard for staging the disease. However, because of the above-mentioned limitations, some authors believe that liver biopsy should rather be considered the best available standard of reference[31]. For this reason, alternative tools have been developed to substitute liver biopsy when assessing liver fibrosis.
Serum markers: Serum markers of liver fibrosis generally offer a cost-effective alternative to liver biopsy, they are less invasive and theoretically without complications. Thanks to these features they could therefore be performed repeatedly and used in monitoring the fibrotic process dynamically, for example in clinical practice, to follow up the efficacy of an antiviral-therapy in the regression of fibrosis.
Serum markers of liver fibrosis can be classified into two kinds: (1) direct markers: molecules which derive directly from the ECM or are produced by activated hepatic stellate cells (HSC), such as hyaluronic acid; and (2) indirect markers: molecules produced by the liver parenchyma following chronic damage either of hepatocyte (AST) or cholangiocyte (GGT) origin, or those which indicate compromised hepatic synthesis (bilirubin, INR) or the presence of portal hypertension (platelets, gamma globulin). Direct and indirect markers may be used alone or in combination to produce composite scores which can be relatively simple to calculate or based on complex formulas.
Hyaluronan: Hyaluronan (formerly termed hyaluronic acid) is an anionic, non-sulfated glycosaminoglycan widely distributed throughout connective, epithelial, and neural tissues. It is currently considered one of the best direct markers of fibrosis, having sensitivity and specificity values of 86%-100% and 88% respectively in NAFLD[32] and in fibrosis related to other etiologies[33]. Moreover, at a cut-off concentration of 60 μg/L it has been shown to have a high negative predictive value (98%-100%), which is significantly higher than the positive predictive value (61%). This is probably because its synthesis is stimulated in the activated HSC[34], then it is secreted into the sinusoidal bloodstream where it normally has a short half-life, but this is prolonged in disease conditions[34]. Consequently, its main usefulness could be for ruling out advanced fibrosis and cirrhosis.
Procollagen Type I Carboxy terminal peptide and Procollagen type III amino-terminal peptide: A procollagen peptide contains an additional peptide sequence at its amino- and carboxy-terminal end, known as the propeptide. Collagen types I, II, III, IV and V are synthesized as precursor molecules called procollagens. These propeptides are cleaved from the collagen molecule during its secretion, after which the collagens polymerize into extracellular collagen fibrils. The amount of free propeptides thus directly correlates with collagen molecule synthesis. Procollagen Type I C-peptide (PICP) has been extensively referenced in studies correlating collagen levels and health disorders such as liver fibrosis. For example, PICP levels have been shown to be normal in patients with mild chronic hepatitis C and elevated in 50% of patients with moderately advanced or advanced chronic hepatitis C, including patients with LC[35].
However, among the several pro-collagen and collagen fragments proposed as biomarkers[36] only the aminoterminal propeptide of type III pro-collagen (PIIINP) has had a limited clinical application[37], with sensitivity and specificity values varying considerably (around 76%-78% and 71%-81%, respectively). These specificity values are unconvincing because PIIINP is not only specific for liver fibrosis as it is also elevated in lung fibrosis, chronic pancreatitis and rheumatologic diseases[38].
Metalloproteinases: Matrix metalloproteinases (MMPs) are zinc-dependent endopeptidases which are capable of degrading all kinds of extracellular matrix proteins, but can also process a number of bioactive molecules. The three most commonly studied human MMPs are: MMP-2 (gelatinase-A), MMP-3 (stromelysin) and MMP-9 (gelatinase-B). MMP-2 circulating levels studied in patients with chronic hepatitis C were not significantly related to the degree of liver fibrosis[39]. In another study, serum MMP-9 levels were shown to decrease during the progression of chronic hepatitis to cirrhosis, with the lowest level in the cirrhosis group[40].
Tissue inhibitors of matrix metalloproteinases: MMPs are inhibited by specific endogenous tissue inhibitors of matrix metalloproteinases (TIMPs), which comprise a family of four protease inhibitors: TIMP1, TIMP2, TIMP3 and TIMP4. TIMP-dependent inhibition of ECM degradation may promote liver fibrosis, and an elevation in TIMP levels has been described in chronic liver diseases. In detail, in a group of patients with chronic hepatitis C plasma TIMP-1 levels were shown to significantly correlate with histological activity index, portal inflammation and periportal and focal necrosis. Moreover, plasma TIMP-2 levels significantly correlated with fibrosis and confluent necrosis. Using ROC analysis both TIMP-1 and TIMP-2 had a significant diagnostic ability in detecting advanced liver disease (AUC = 0.73 for both, P = 0.015 and 0.036 respectively), while normal plasma TIMP-1 excluded advanced liver disease[39]. Badra et al[40] also found a positive correlation between TIMP-1 levels and degree of fibrosis when studying patients with chronic hepatitis and LC.
Laminin: Laminins are a major ECM protein component of the basal lamina. In general, they are heterotrimeric glycoproteins with binding regions for collagen, integrins, cellular domains and proteoglycans. In the liver they are synthesized by Ito cells and for this reason considered a marker of fibrogenesis. The concentration of laminin has been reported to be correlated with portal venous pressure, thus being a potentially useful biochemical marker of portal hypertension. In detail, at a cut-off concentration of 1.45 U/mL, sensitivity was 0.87, specificity 0.74, diagnostic efficiency 0.81 and positive and negative predictive values at the same cut-off were 0.77 and 0.85, respectively[41]. In a subsequent study, an increased concentration of laminin was described in a group of cirrhotic patients and significant differences in laminin concentrations were found between the various Child’s grades and between patients and controls[42].
Transforming growth factor-beta1: Transforming growth factor beta 1 (TGF-β1) is a polypeptide member of the transforming growth factor beta superfamily of cytokines. It is a secreted protein that performs many cellular functions and has been identified as a pro-fibrogenic cytokine. For this reason, TGF-β1 blood concentrations have been studied as markers of liver fibrosis. In a study on the prediction of progressive liver fibrosis in hepatitis C infection a close correlation was found between TGF-β serum levels and the rate of fibrosis progression. Patients with no progression of fibrosis had significantly lower TGF-β serum levels than patients with progressive disease, and a TGF-β level below 75 ng/mL was predictive of stable disease[43]. Furthermore, in a previous study on 88 patients with chronic HCV, a correlation was found between TGF-β levels and severity of fibrosis[44].
Connective tissue growth factor: Connective tissue growth factor (CTGF), also known as CCN2, is a matricellular protein of the CCN family of extracellular matrix-associated heparin-binding proteins and it is associated with wound healing and virtually all fibrotic pathologies. CTGF is thought to cooperate with TGF-β to induce sustained fibrosis and to exacerbate extracellular matrix production in fibrosis-inducing conditions. In a recent study the diagnostic performance of CTGF was assessed by comparing the area under the receiver operating characteristic (ROC) curves (AUC) with a panel of fibrosis markers. The correlation coefficient between serum CTGF levels and fibrosis stages was 0.689 and the AUC of CTGF was 0.841 (95%CI: 0.762-0.920) in distinguishing mild fibrosis from significant fibrosis[45]. Values were even better in a previous study where the AUCs for fibrosis vs controls and for cirrhosis patients vs controls were 0.955 and 0.887, respectively, with 100% and 84% sensitivity, respectively, and 89% and 85% specificity, respectively[46].
YKL-40: YKL-40, also called human cartilage glycoprotein-39 (HC gp-39), is a member of the 18 glycosyl hydrolase family. The pattern of its expression in normal and disease states suggests that it could have a function in remodeling or facilitating the degradation of the extracellular matrix. Elevated YKL-40 concentrations have been found in the sera of liver disease patients. In particular, sensitivity and specificity values around 80% and an AUC of 0.81 for fibrosis were reported in a study on HCV-related chronic hepatitis patients[47], and 88% specificity and 51% sensitivity were reported in alcoholic liver disease patients[48]. Moreover, the CHI3L1 promoter polymorphism -131G > C, which determines YKL-40 serum levels, was associated with the severity of HCV-induced liver fibrosis[49].
Microfibril-associated glycoprotein 4: To identify new candidate biomarkers for hepatic fibrosis, a proteomic approach of microdissected cirrhotic septa and liver parenchyma cells was performed. In the cirrhotic septa, an increase was detected in the expression of cell-structure-associated proteins, including human microfibril-associated protein 4 (MFAP-4). A subsequent quantitative analysis of MFAP-4 serum levels in a large number of patients showed a high diagnostic accuracy for predicting non-diseased liver vs cirrhosis: AUC = 0.97; for stage 0 vs stage 4 fibrosis: AUC = 0.84; and for stages 0 to 3 vs stage 4 fibrosis: AUC = 0.76[50]. This consequently represents a novel approach to study new markers of liver fibrosis.
Cytokeratin-18 fragments: Cytokeratin-18 (CK18) is an intermediate filament representing about 5% of total protein in various epithelial and parenchymal cells[51]. Different caspases induce the apoptosis mechanism via the cleavage of CK 18 in different positions, forming CK18 fragments (CK18Fs)[52]. In the study of Bantel et al[53], raised concentrations of CK18 fragments were evident in HCV patients with advanced liver fibrosis. Yilmaz et al[54] showed that in NAFLD patients levels of M30 antigen (a neoepitope in cytokeratin 18) and M65 (the cytosolic pool of CK18) distinguished between advanced fibrosis and early-stage fibrosis, with 64% and 70% sensitivity, and 77% and 71% specificity, respectively.
Indirect serum markers: Several scores have been proposed to attempt to overcome the need for liver biopsy in diagnosing and monitoring the fibrotic process in chronic liver diseases. Most of the literature data, however, have been obtained in mainly HCV-infected patient case series. Table 2 summarizes the principal scoring systems, taking into account the parameters used in calculating the scores, etiology of liver disease and sensitivity and specificity values of the tests.
| Test | Parameters | Etiology | Sensitivity | Specificity |
| Age-platelet index (AP)[55] | Age, platelet count | HCV | 52% | 93% |
| APRI[56] | AST/platelet count | HCV | 57% | 93% |
| ASPRI[57] | Age, spleen diameter, platelet count | HBV | 75% | 90% |
| AST/ALT ratio[58] | Aspartate aminotransferase, alanine aminotransferase | HBV | 51% | 71% |
| BARD score[59] | BMI, AST/ALT ratio, diabetes | NAFLD | 62% | 66% |
| Bonacini-index (Cirrhosis discriminant score - CDS)[60] | ALT/AST ratio, INR, platelet count | HCV | 46% | 98% |
| ELF and simplified ELF index[61] | Age, Hyaluronic acid, N-terminal propeptide of type II collagen, and TIMP-1 levels | Mixed | 90% | 69% |
| FIB-4[62] | Platelet count, AST, ALT, age | HIV/HCV | 65% | 97% |
| Fibro-α score[63] | Platelet count, AST, ALT, α-fetoprotein level | HCV | 90% | 57% |
| Fibroindex[64] | Platelet count, AST, γ-globulin | HCV | 35% | 97% |
| Fibrometer test[65] | Platelet count, prothrombin index, AST, α2-macro-globulin, hyaluronan,urea, age | VirusAlcohol | 80%91% | 84%92% |
| Fibrometer NAFLD[66] | Glucose, AST, ALT, ferritin, platelet count, body weight, age | NAFLD | 79% | 96% |
| Fibronectin discriminant score[67] | Platelet count, AST, Albumin and fibronectin levels | HCV | 87% | 75% |
| FibroQ[68] | Age, platelet count, AST, ALT, Prothrombin index | HBV/HCV | 79% | 71% |
| Fibrosis-cirrhosis index[69] | Platelet count, Alkaline phosphatase, bilirubin, albumin levels | HCV | 86% | 80% |
| Fibrosis index[70] | Platelet count, Albumin level | HCV | 67% | 97% |
| Fibrosis probability index(Sud index)[71] | Age, AST, Total cholesterol level, insulin resistance and alcohol intake | HCV | 73% | 74% |
| Fibrosis Routine Test[72] | Age, platelet count, AST, α-fetoprotein and albumin levels | HCV | 83% | 73% |
| FibroSpect II[73] | hyaluronan, TIMP-1,α2-macroglobulin | HCV | 76% | 73% |
| Fibrotest[74] | Haptoglobin, α2-macroglobulin, apolipoprotein A1, γGT, bilirubin, gender | HCV | 75% | 85% |
| Forns-index[75] | Age, platelet count, γGT, cholesterol | HCV | 30% | 95% |
| Globulin-albumin ratio[76] | Globulin and albumin levels | HCV | 43% | 98% |
| GUCI[77] | Platelet count, AST, Prothrombin index | HCV | 80% | 78% |
| HALT-C model[78] | Platelet count, TIMP-1 and hyaluronic acid levels | HCV | 71% | 80% |
| Hepascore[79] | Bilirubin, γGT, hyaluronan, α2-macroglobulin, age, gender | HCV | 84% | 71% |
| King’s score[80] | Age, platelet count, AST, INR | HCV | 86% | 80% |
| Lok index[81] | Platelet count, AST, ALT, INR | HCV | 68% | 72% |
| MP3 score[82] | MMP-1 and PIIIP levels | HCV | 60% | 92% |
| NAFLD fibrosis score[83] | Age, hyperglycemia, BMI, platelet count, albumin, AST/ALT ratio (dual cut-offs) | NAFLD | 77% | 96% |
| Pohl index[84] | Platelet count, AST, ALT | HCV | 41% | 99% |
| Sabadell NIHCED index[85] | Age, platelet count, AST, ALT, Prothrombin time, right hepatic lobe atrophy, splenomegaly, and caudate lobe hypertrophy | HCV | 80% | 96% |
| Significant fibrosis index[86] | Haptoglobin, α2-macroglobulin, TIMP-1, MMP-2, and GGT levels | HBV/HCV | 71% | 80% |
| VITRO score[87] | vWF-Ag, platelet count | HCV | 83% | 79% |
| Zeng index[88] | Age, α2-Macroglobulin, GGT, and hyaluronic acid levels | HBV | 40% | 90% |
The first diagnostic index was proposed by Williams et al[58], who suggested that an AST/ALT ratio of > 1.0 in a patient with non-alcoholic liver disease should be related to the presence of cirrhosis. Poynard et al[55] then proposed a simple score combining age and platelet count, referred to as AP, which had 93% specificity and 52% sensitivity in identifying histological disease in hepatitis C-infected patients. In the same year, a paper by Bonacini et al[60] assessed the utility of a modified three-parameter cirrhosis discriminant score (CDS) for diagnosing advanced fibrosis or cirrhosis in patients with evidence of chronic hepatitis C, taking into account three laboratory parameters (platelets, ALT/AST ratio and PT). It showed a positive correlation between the CDS and histological fibrosis score which was 46% sensitive and 98% specific for the diagnosis of histological fibrosis scores of 3 or 4.
Since then, a great number of papers have been published with novel scores whose sensitivity and specificity values were announced as being better than the previous ones, but actually none have been adopted in clinical practice. The principal ones are Fibrotest[75], Forns[76], APRI[56] and FIB-4[62].
As regards the Fibrotest, its authors concluded that a combination of simple serum tests could be used to reduce the need for liver biopsy, the most informative markers being alpha2-macroglobulin, alpha2-globulin (or haptoglobin), gamma globulin, apolipoprotein A1, gamma glutamyltranspeptidase and total bilirubin. With the best index, a high negative predictive value (100% certainty of absence of F2, F3, or F4) was obtained for scores ranging from zero to 0.10 (12% of all patients), and a high positive predictive value (> 90% certainty of presence of F2, F3, or F4) for scores ranging from 0.60 to 1.00 (34% of all patients)[75]. Subsequently, Forns et al[75] constructed a model and a score system combining age, GGT, cholesterol and platelet count which aimed to identify patients without significant liver fibrosis. Using the best cut-off score, the presence of significant fibrosis could be excluded with high accuracy (negative predictive value of 96%) in 36% of the patients.
The APRI score was then developed to amplify the opposing effects of liver fibrosis on AST and platelet count. Using optimized cut-off values, the authors showed that significant fibrosis could be accurately predicted in 51% and cirrhosis in 81% of patients with chronic HCV infection[56].
The FIB-4 index was developed to predict liver fibrosis in patients with HIV/HCV coinfection. It was calculated taking into account simple parameters such as age, AST and ALT levels and PLT count, and the authors proved that 87% of the 198 patients with FIB-4 values outside the cut off-values would be correctly classified, thus liver biopsy could be avoided in 71% of the validation group[62].
Diagnostic imaging includes a number of instruments and techniques to estimate liver fibrosis and cirrhosis.
Ultrasound (US) is routinely used in the diagnosis and monitoring of subjects with chronic liver disease. It is inexpensive, non-invasive, readily available and acceptable to patients. This tool is also recommended by various international guidelines for evaluating the large spectrum of CLD. It provides useful data on the morphologic changes taking place in the liver, as well as on CLD complications (such as portal hypertension), and it also enables the early detection of HCC[12,13,89]. Indeed, its performance in screening programs has changed the clinical and laboratory presentation of HCC in the new millennium[90].
The introduction of US has made the liver a very easy organ to study and during the last few decades ultrasound imaging, which ranges from gray-scale to real time gray-scale US, color-Doppler and, more recently, contrast-enhanced US (CEUS), has provided further, important information on the diagnosis and progression of CLD[90-92].
Gray-scale US: Gray-scale US (B-Mode) imaging of a given organ includes an evaluation of size, echo-pattern and surface.
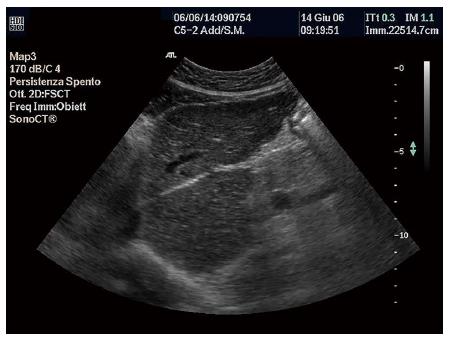
Size: Classically, in LC there is an atrophy of the right lobe associated with hypertrophy of the left lobe, while the caudate lobe and lateral segment of the left lobe occupy a larger proportion of the liver volume. Hypertrophy of the caudate lobe is a highly specific finding of LC (Figure 1), while in the advanced phase atrophy is complete. Various studies have reported that this asymmetry can be clearly seen at US, in the raised volume of the caudate lobe alone or in the increased ratio between the transverse diameter of the caudate lobe and the transverse diameter of the right lobe. Values > 0.65 are abnormal. This ratio, however, indicates an advanced LC with a good specificity (95%), but a low sensitivity (43%), therefore, it is inaccurate for the early diagnosis of LC[93].
Echo-patterns: The definition of echo-patterns in diffuse liver diseases has been debated in the literature, studies often reporting contrasting results. The echo-pattern of cirrhosis has been defined as a coarse pattern, which is characterized by ‘‘pinhead’’ echoes which are coarse and not homogeneously distributed, without posterior beam attenuation (Figure 1)[94,95]. Sensitivity and specificity are estimated as 57% and 88%, respectively[96].
This sub-optimal diagnostic reliability is due to different factors: (1) the same pattern may also be found in CLD without cirrhosis and it may be lacking in cirrhosis, especially in the early phase; (2) inter-observer variability: according to some authors, inter-observer agreement (assessed by the kappa value k) in defining the echo-pattern of diffuse liver disease is insufficient (around 0.4)[97,98]; and (3) high echogenicity: the coarse pattern increases liver echogenicity, causing some difficulty in differentiating between cirrhosis and steatosis. The many papers published on this issue have reported that the presence of attenuation beam and of focal steatosis, which are lacking in cirrhosis, helps to distinguish between the two forms[94,97,99,100].
The mechanism of attenuation depends on physical phenomena defined as: scatter, absorption and refraction. These are enhanced by the large interfaces of lipidic drops typical of steatosis, while the cirrhotic liver, which has few interfaces, does not favour these phenomena[101]. However, the attenuation beam and focal steatosis are not always found in steatosis and many forms of NAFLD present both fatty and fibrotic liver during their course, thus making differentiation difficult[102].
Liver surface: Micro- or macro-nodularity in LC are seen at US as liver surface irregularities. In the micronodular forms the liver presents only a superficial irregularity, which is much more evident in macronodular LC (Figure 1). This is considered one of the most sensitive and more reproducible US signs (k of agreement range 0.77- 0.9)[103,104].
Gaiani et al[105] reported 82% sensitivity and 79% specificity for this sign when associated with reduction in portal velocity. Further, Colli et al[106] and Iacobellis et al[107] reported a sensitivity of 54% and 46%, and a specificity of 93% and 95% for surface irregularity alone. Iacobellis reported a sensitivity of 90% and specificity of 83% when combining surface irregularity with a platelet count < 140000/mmc. To confirm that the association between US and serum data improves LC diagnosis, Colli et al[104] used the model based on the sequential combination of the Bonacini score (ALT/AST ratio, platelet count and INR) associated with US liver surface characteristics in 176 patients with chronic HCV infection. They were able to define the presence/absence of severe fibrosis or cirrhosis in 67%; only in 33% of the patients was a biopsy required.
Recently, Berzigotti et al[103] compared left lobe liver surface irregularity with transient elastography (TE) to assess the diagnostic value of these methods in patients with a suspected but not definite diagnosis of cirrhosis. They found that liver surface irregularity evaluation was better for LC diagnosis, while TE was preferable to rule it out. The combination of both offered the best diagnostic accuracy.
Other gray-scale US findings are useful in the staging and follow-up of LC, but much less so in early diagnosis.
Portal vein dilatation: A diameter greater than 1.2 cm has a sensitivity of less than 50% and a 90% specificity for the diagnosis of portal hypertension[108].
Dilatation and reduction in the respiratory variations of splenic and mesenteric vein diameters have 79.7% sensitivity and 100% specificity for the diagnosis of portal hypertension[108].
Splenomegaly: Spleen bipolar diameter > 12 cm or largest splenic cross-sectional area passing through the hilum > 45 cm2 may indicate portal hypertension[92]; Berzigotti et al[109] reported that spleen diameter was the only US sign showing a significant association with clinically significant portal hypertension (hepatic venous pressure gradient ≥ 10 mmHg) at univariate analysis. However, splenomegaly may be induced by many other causes and therefore is not specific for portal hypertension.
Ultrasound is also a very useful and reliable method in the estimation of liver fibrosis and portal hypertension in patients with hepato-splenic schistosomiasis, especially in countries where this infection is endemic. In fact, the World Health Organization believes that the diagnosis of liver fibrosis, in developing countries, may reliably be based on ultrasound and proposes guidelines for schistosomiasis, which are modified for S. japonicum infection, in which the parenchymal and periportal fibrosis are graded separately[110,111]. The knowledge of these guidelines is very useful also in Western countries where recent migration flows have brought this disease uncommon in the past.
The Doppler US signs for portal hypertension are: (1) reduced portal vein blood flow velocity (time-averaged mean vel. < 14-16 cm/s2), with sensitivity and specificity between 80-88% and 80-96%, respectively[92]; (2) portal vein congestion index > 0.08. This parameter[92], however, is not used routinely, probably due to its being difficult to measure and its lower reproducibility; and (3) measurements of the hepatic arterial Resistive Index are less useful[92].
In the course of LC, the appearance of clinically significant portal hypertension worsens prognosis and Doppler US is very useful in evaluating portal hypertension. However, it is not sufficient without the integration of gray-scale US and serological and clinical data. Furthermore, Doppler US alone is not able to predict the presence of esophageal varices[92].
CEUS is prevalently used in the study of liver tumors. However, in recent years it has also been used in the evaluation of liver fibrosis[112,113]. The rationale for its application stems from the changes in intrahepatic microcirculation that occur in chronic liver diseases with fibrotic evolution.
Normally, the blood flows from the portal branches to the sinusoids and hepatic veins. In patients with severe fibrosis or cirrhosis, there are severe changes in the microcirculation (arteriovenous shunting and arterialisation of capillary beds) which lead to a by-pass of the sinusoids, with blood passing directly into the hepatic veins, and this determines a reduction in transit time (hyperdynamic flow). By using CEUS and calculating intrahepatic transit time, hyperdynamic flow may be estimated. Contrast arrival time and baseline and peak intensity in the hepatic artery, portal vein, right hepatic vein and liver parenchyma have been used to calculate intrahepatic transit time, hepatic artery to hepatic vein transit time (HA-HVTT) and portal vein to hepatic vein transit time (PV-HVTT). To diagnose severe fibrosis, Staub et al[112] found for a transit time of under 13 s, 78.5% of specificity, 78.9% of sensitivity, 78.3% of positive predictive value, 83.3% of negative predictive value and a 78.8% performance accuracy. Transit time was computed as the difference between the arrival of microbubbles in the portal vein and in the hepatic vein (PV-HATT). HA-HVTT and PV-HVTT gradually shortened with the progression of liver fibrosis[113]. Recently, however, the EFSUMB (European Federation of Societies for Ultrasound in Medicine and Biology) considered that its use in staging chronic hepatitis is also limited by the substantial overlap between the different stages of fibrosis[92].
In conclusion, gray-scale US has an limited sensitivity and specificity in LC diagnosis, although diagnostic reliability improves when Doppler US or laboratory data are added. In fact, the most accurate sign, i.e., liver surface irregularity alone, has a 55% sensitivity, while in association with platelet count this reaches 90%[107,108]. Finally, US is not able to evaluate either the progression of fibrosis or the more advanced stage of the disease (F3).
In the last two decades new diagnostic tools have been developed which, when supported by ultrasound, permit the estimation of fibrosis. They are based on the hypothesis that fibrosis in a given tissue determines a reduction in elasticity or an increase in stiffness[114-116].
During the course of chronic hepatitis, the liver becomes more fibrotic and its stiffness therefore increases. This may be recorded by transient elastometry (TE), which measures the degree of stiffness using ultrasounds. TE is therefore the equivalent of palpation, and for this reason it has been defined “palpation imaging”[114-116].
Two concepts are fundamental for elastography: (1) the evaluation of strain which deforms the tissue (due to a force that deforms the tissue) namely static or quasi-static methods, which are defined as strain imaging techniques; and (2) the speed analysis of a shear wave induced by a mechanical vibrator or other techniques, is defined as the shear-wave technique[114].
The stiffer a tissue is, the higher the propagation speed of US waves will be; their evaluation will allow an estimation of stiffness.
Strain imaging: In this group, the distortions generated by compressions created by hand, with a probe, by the heartbeat or by arterial pulse on adjacent tissues, are measured.
Strain imaging may measure stiffness as follows: (1) qualitative: stiffness is given in the gray or colour scale; this is the method used in real-time elastography (RTE); and (2) semi-quantitative, based on the ratio strain: two regions of interest (ROIs) are selected: the surface to be examined and the adjacent one, which serves as a reference. The ratio between the two strains gives a semi-quantitative measure.
Shear wave technique: It evaluates the speed of propagation of a shear wave induced by a mechanical vibrator or other methods. The greater the hardness of a tissue, the greater the speed of propagation of the ultrasonic waves will be and the greater its stiffness. The propagation speed of the shear wave is expressed in m/s or Kilopascals (kPa) (using Young’s modulus)[115].
The quantitative measure is more useful in the study of liver stiffness. The stiffer a tissue is, the greater its strain.
For liver study there are several elastography techniques, the best known are: TE and acoustic radiation force impulse (ARFI).
The proliferation of all these methods complicates the interpretation of results because they are often not equivalent; the cut-off of one method, even if expressed in the same unit of measure is often not comparable with another[117].
Moreover, all these techniques have some limitations in common: (1) liver stiffness is not only determined by the degree of fibrosis, but also by the degree of necrosis and inflammation and is influenced by cholestasis. Therefore, stiffness values may be high without being an expression of fibrosis, i.e., in acute hepatitis, during the viral flare of chronic hepatitis, biliary tree obstruction etc.[118-120]; (2) measures are less accurate if there is venous insufficiency or when they are performed near the hepatic capsule[120,121], and (3) the reference values of stiffness may have different cut-offs due to the different etiologies of liver disease[121].
TE (FibroScan TM® EchoSensTM, Paris, France), uses an M ultrasound probe (5 MHz) with a dedicated vibrating system that produces mechanical waves with a low frequency and amplitude (50 MHz). A mechanical impulse generates shear waves in the liver which come back to the transducer mounted on the end of the probe, which functions as both a generator and receiver; the shear wave velocity measured (in meters per second) can then be converted into liver stiffness.
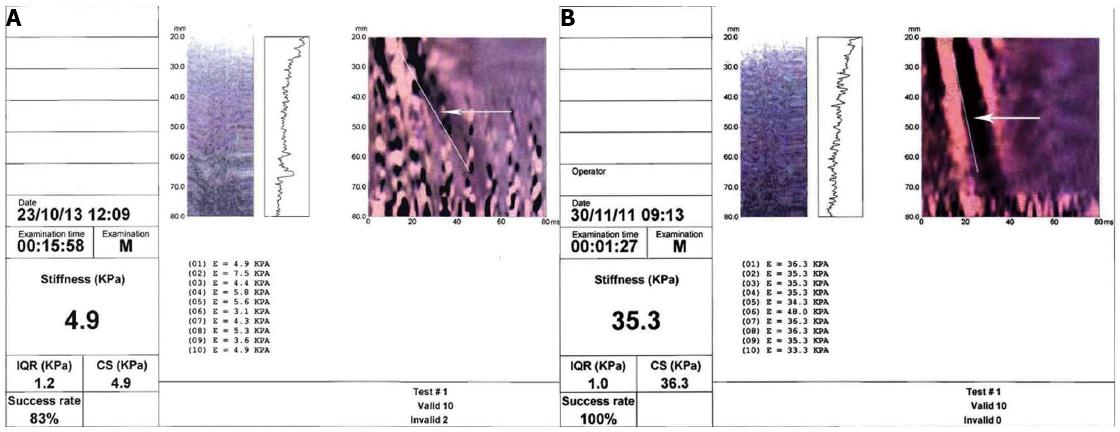
Liver stiffness measurement is proportional to the speed of propagation and is expressed in kiloPascals (kPa), in accordance with Young’s modulus. The result ranges from 2.5 to 75 kPa. The measurement is performed at a depth below the skin surface of between 2.5 cm and 7.5 cm. The final value obtained is the mean of ten measurements. The liver sample evaluated is around 100 times greater than that of a liver biopsy[122]. Besides the liver stiffness value, another two values are given, i.e., the success rate (SR), which corresponds to the rate of the number of measurements/total number of valid results (corresponding to the number of measurements obtained/number impulses applied) and the interquartile range (IQR). The test is not reliable if the SR is less than 60% or the IQR is higher than 30%[122] (Figure 2A and B).
This tool is the one most frequently applied in liver diseases, it is easy to use and only a short training period (50 tests) is necessary. However, it has a high interobserver variability 0.98 (95%CI: 0.977-0.987), but when fibrosis is at the lowest stage (minimal) the intra-observer and inter-observer variability is even greater[122,124]. The normality threshold in Roulot’s study was 5.8 ± 1.54 in men and 5.23 ± 1.59 kPa in women[125].
TE has been largely studied for HCV-associated CLD, but in recent years many studies have estimated stiffness in HBV-associated liver disease, in co-infected HBV/HCV patients, as well as in nonalcoholic steatohepatitis (NASH) and autoimmune cholestatic liver diseases. In most of these cases TE has been compared to the METAVIR score scoring system[20]. All these studies have demonstrated that there is no specific cut-off to discriminate LC and that it varies according to the etiology of liver disease.
HCV liver diseases: Most studies have been performed on HCV liver diseases and different cut-off values have been used by the various authors to define fibrosis stages. Recently the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) issued guidelines in which values above 6.8-7.6 kPa may indicate the presence of significant fibrosis (F ≥ 2 according to METAVIR score) with a high probability, while the range 11-13.6 kPa may indicate a cirrhotic stage (F = 4 according to METAVIR score) (Figure 2B). All these studies have shown that TE is able to define the presence of cirrhosis with a good diagnostic reliability, while it is less able to define the minor stages of fibrosis. Meta-analyses performed to date have indicated that in HCV-associated CLD sensitivity and specificity in defining a stage F ≥ 2 are 78% and 79%, respectively; for an F = 4 stage sensitivity is 83% and specificity 89%[117,124-129]. EASL guidelines indicate that TE can be used to assess liver fibrosis in patients with chronic hepatitis C (level of recommendation A2)[130].
HBV liver diseases: In chronic hepatitis B many studies have indicated that values of 7 kPa parallel an F ≥ 2 stage and 11 kPa an F4, according to the METAVIR score; a recent meta-analysis at these cut-offs estimated an Area Under ROC of 0.887 and 0.92, respectively[131-134]. In chronic hepatitis B the frequent ALT flares (the expression of necro-inflammatory processes) influence stiffness values independently of fibrosis[122-133]. Further, in the guidelines for the management of chronic HBV infection, the EASL advises TE to estimate liver fibrosis (level of recommendation B1 and C2)[135].
HCV/HIV co-infection: Also in HCV/HIV co-infected patients the reliability of FibroScan TMTM is good; de Leidighen reported a sensitivity of 100% and specificity of 83% to define the F4 stage at a cut-off level of 11.8[136,137].
Alcoholic liver diseases: In patients with chronic alcoholic liver diseases, higher cut-off values indicating cirrhosis (19.5 kPa) are reported, with a sensitivity and specificity of 85.7% and 84.2%, respectively. However, it has been reported recently that stiffness values decrease after alcohol withdrawal[138,139].
NAFLD/NASH: The studies on NAFLD and NASH are very interesting. This is the most common liver disease in the Western world, largely associated to the metabolic syndrome[6]; progression to LC is frequent[5] and often there are no signs or symptoms. Due to the high number of patients suffering from NAFLD, TE could be an ideal tool to monitor these patients and to select candidates for liver biopsy. However, TE is less reliable in patients with BMI > 30, which is common in NAFLD patients. In fact, subcutaneous fat increases the distance between the probe and the liver and does not allow a correct estimate of liver stiffness[123,140,141]. The recent introduction of the XL probe should allow a more accurate study of liver stiffness in patients with a skin-to-liver capsule distance > 2.5 cm. A limitation of the XP probe would be a skin to-liver capsule distance > 3.5 cm, a thickness corresponding to a BMI > 40[139-144]. Studies carried out on fibrosis in NASH have reported different cut-off levels. Wong et al[145], in a study with the highest number of patients so far performed reported a 91.1% sensitivity and 75.3% specificity at a cut-off of 7.9 kPa for a F ≥ 3 and a 92% sensitivity and 91% specificity, respectively[145] at a cut-off of 10.3 for F4.
In patients with cholestatic liver diseases (PBC and PSC) higher cut-off levels than for viral LC have been reported (17.3 kPa)[146].
Portal hypertension: Various studies have been performed using TE to evaluate its reliability in defining the presence of portal hypertension and esophageal varices. A wide range of cut-off levels have been reported to date and TE cannot therefore be considered reliable in defining portal hypertension[117].
Recently, a combination of liver stiffness and spleen diameter (measured using ultrasound) and platelet count evaluation was proposed to identify patients with portal hypertension[147].
Transplant patients: TE has also been proposed in evaluating fibrosis progression in patients with post-transplant recurrent hepatitis C. In fact, an increase in stiffness values has been associated to an increase in the stage of liver fibrosis[148]. In the study by Adebajo a sensitivity and specificity of 83% for significant fibrosis (F ≥ 2) and a sensitivity of 98% and a specificity of 84% for cirrhosis (F4), were reported[149].
Assessment of fibrosis during antiviral therapy: Studies performed to date do not give definitive indications on its utility, as the reduction in liver stiffness is at least in part due to the decrease in necro-inflammation[150-153]. The EASL guidelines do not advise TE in the follow-up of treated patients[130-135].
A very recent study by D’Ambrosio raises some doubts on the ability of TE to exclude a diagnosis of cirrhosis in patients with SVR[153]. In this study, sensitivity for the diagnosis of cirrhosis in SVR, 61 mo after the end of treatment, using 12 kPa as a cut-off level, was 61% (lower than before therapy) while specificity was 95%. The authors reported that after treatment in patients with SVR, LC also persisted when fibrosis quantity decreased, but a nodular architecture associated with annular fibrosis (cirrhosis sign) remained. A non-invasive tool cannot estimate this morphometric parameter which is essential for the diagnosis of cirrhosis, as it only calculates the quantity of fibrosis and therefore is of little use.
TE has been shown to be a useful tool in defining LC (F4), although it is less reliable in defining lower degrees of fibrosis[117]. In particular settings, recent international guidelines advise it[130,135]. It is easy to use, reproducible and well-tolerated by patients. Its disadvantages are that a dedicated tool is needed, it is inadequate in patients with ascites or tight intercostal spaces, reliability is limited in obese and diabetic patients using a standard probe and its values are influenced by phenomena such as necro-inflammation, extra-hepatic cholestasis, venous stasis, etc.
ARFI is a new ultrasound technique which estimates tissue stiffness by measuring the shear wave velocity induced by acoustic radiation, an application of which is Virtual Touch™ which provides: (1) tissue imaging; and (2) tissue quantification.
Virtual Touch™ tissue imaging estimates stiffness in gray-scale, and therefore gives a qualitative measure. Virtual Touch™ tissue quantification gives a quantitative measure of stiffness[115].
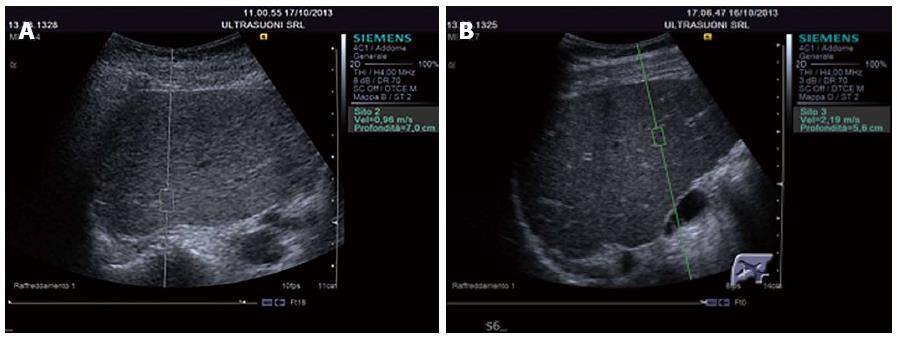
The greater the stiffness of a tissue (expression of fibrosis), the higher the speed is. ARFI has the advantage of offering an elastographic measurement given by an US machine[115]. The US probe automatically produces a brief acoustic impulse at around 2.6 MHz in a region of interest (ROI) of 5 mm × 10 mm in size. Using B-Mode US, the operator may position the ROI anywhere within the liver, 2-8-cm down the Glisson’s capsule (Figure 3A and B). Passing through the tissue the acoustic impulses generate shear waves spreading perpendicularly to the impulses and the stiffness value is obtained by measuring the speed. This technique has been developed by Siemens on Acuson S2000 and is also available on the Philips iU22 ultrasound system.
Similarly to FibroScanTM, the patient is examined supine with the right arm abducted, and the probe is positioned in the right intercostal space (usually the 5th). The number of measurements required reported in the literature varies between 6 and 10[154,155].
A greater diagnostic accuracy in stiffness measurement has been reported in the right lobe than in the left, above all in the deeper areas of the right lobe (5th segment), owing to less manual compression[156]. When used together with gray-scale US, which allows a choice of the area to be measured, ARFI may also be performed in subjects with ascites to assess the stiffness of focal lesions and of steatosis areas. It may avoid vessels, the gallbladder or biliary tree which may influence stiffness values; it may allow a thorough study of the liver by using the various types of US technology (B-Mode, Doppler, CEUS, elastography). Measurements have a good inter- and intra-observer variability both in healthy subjects and in CLD patients[124,157]. However, they have some limitations because they are not performed in real time, the ROI cannot be modified and the ideal range of measurements to be performed is not yet known[157,158].
As in the case of TE, there are more ARFI studies on patients with HCV-associated liver disease, although more recently studies on NAFLD or HBV-associated liver disease have also been carried out[154,156,159-167]. Table 3 reports the cut-offs and diagnostic reliability for liver fibrosis at the various stages according to the METAVIR score.
| Ref. | Patients analyzed by ARFI (n) | Etiology | F≥2 | F≥3 | F = 4 | |||||||||
| Cut-off m/s | Se% | Sp% | AUROC | Cut-off m/s | Se% | Sp% | AUROC | Cut-off m/s | Se% | Sp% | AUROC | |||
| Fierbinteanu-Braticevici et al[159] | 74 | HCV | 1.22 | 100 | 71 | 0.91 | 1.54 | 97 | 100 | 0.99 | 1.94 | 100 | 98 | 0.99 |
| Friedrich-Rust et al[160] | 81 | HCV/HBV | 1.37 | 69 | 92 | 0.82 | 1.45 | 84 | 86 | 0.91 | 1.75 | 82 | 91 | 0.91 |
| Lupsor et al[161] | 102 | HCV | 1.34 | 68 | 93 | 0.86 | 1.61 | 79 | 95 | 0.91 | 2.00 | 80 | 95 | 0.94 |
| Yoneda et al[162] | 54 | NASH | 1.77 | 100 | 91 | 0.97 | 1.91 | 100 | 96 | 0.98 | ||||
| Piscaglia et al[156] | 70 | Mixed | 1.63 | 59 | 100 | 0.79 | 1.67 | 75 | 97 | 0.91 | 1.87 | 81 | 91 | 0.91 |
| Rizzo et al[163] | 139 | HCV | 1.31 | 81 | 70 | 0.86 | 1.71 | 91 | 86 | 0.94 | 2.11 | 83 | 86 | 0.89 |
| Sporea et al[164] | 199 | Mixed | 1.27 | 89 | 68 | 0.89 | 1.56 | 80 | 89 | 0.88 | 1.71 | 93 | 87 | 0.93 |
| Sporea et al[154] | 93 | Mixed | 1.41 | 71 | 78 | 0.77 | 1.69 | 73 | 88 | 0.79 | 1.81 | 100 | 88 | 0.92 |
| Sporea et al[165] | 911 | HCV | 1.33 | 69 | 80 | 0.80 | 1.43 | 75 | 81 | 0.82 | 1.55 | 84 | 76 | 0.84 |
Table 4 reports the results of two meta-analyses; in 8 studies Friedrich found that the best cut-offs for the diagnosis of fibrosis were: significant (F ≥ 2), 1.34 m/s; severe (F ≥ 3) 1.55 m/s; cirrhosis (F ≥ 4) 1.8 m/s; he concluded that ARFI is a good tool for the diagnosis of significant fibrosis and excellent for severe fibrosis[166]. More recently, Nierhoff et al[167], analysing a greater number of studies, confirmed the diagnostic reliability data for significant and severe fibrosis and cirrhosis with similar cut-off values to those of the previous meta-analyses.
Table 5 reports the sensitivity, specificity and AUROC at various cut-offs of ARFI and TE in the diagnosis of fibrosis according to the METAVIR score[160-165,168]. The results are not unequivocal: according to some studies ARFI and TE have a similar accuracy in the diagnosis of F ≥ 3 and F ≥ 4, and FibroScanTM should be more reliable in the earlier stages[162-164], while according to others ARFI should have a greater accuracy[163]. In a recent meta-analysis comparing ARFI and FibroScanTM, Bota reported a similar performance, suggesting that the accuracy of the two tools is similar for the diagnosis of severe fibrosis[169].
| Ref. | Etiology | Transient elastography | ARFI | ||||||||||
| F≥2 | F≥3 | F = 4 | F≥2 | F≥3 | F = 4 | ||||||||
| AUROC | Cu-off kPa | AUROC | Cut-off kPa | AUROC | Cut-off kPa | AUROC | Cut-off m/s | AUROC | Cut-offm/s | AUROC | Cut-offm/s | ||
| Friedrich-Rust et al[160] | HCV | 0.84 | 0.91 | 0.910 | 0.82 | 1.37 | 0.91 | 1.45 | 0.91 | 1.75 | |||
| Lupsor et al[161] | HCV | 0.96 | 8.1 | 0.96 | 0.6 | 0.97 | 13.1 | 0.86 | 1.34 | 0.90 | 1.61 | 0.94 | 2.00 |
| Yoneda et al[162] | NASH | - | - | 0.99 | 9.9 | 0.998 | 16.0 | 0.97 | 1.77 | 0.97 | 1.99 | ||
| Rizzo et al[163] | HCV | 0.78 | 6.5 | 0.80 | 8.8 | 0.8 | 11.0 | 0.86 | 1.3 | 0.94 | 1.70 | 0.89 | 2.00 |
| Sporea et al[164] | Mixed | 0.91 | 0.99 | 0.77 | 1.41 | 0.79 | 1.69 | 0.92 | 1.81 | ||||
| Sporea et al[165] | HCV | 0.82 | 6.7 | 0.87 | 9.6 | 0.93 | 11.9 | 0.81 | 1.36 | 0.86 | 1.47 | 0.885 | 1.69 |
| Bota et al[168] | Mixed | 0.871 | - | - | - | 0.931 | - | 0.851 | 1.31 | - | - | 0.931 | 1.80 |
When compared with the FibroScanTM M Probe, ARFI had a better reliability in obese patients; however, the introduction of the new XL probe will probably reduce this gap.
ARFI and transaminases: ARFI values are also influenced by necro-inflammatory activity, albeit to a lesser extent than in TE. In patients with normal ALT, ARFI values are significantly lower than in patients with increased ALT values[169].
In conclusion, all these studies show that ARFI, as well as TE, are able to diagnose severe fibrosis (F ≥ 3) or cirrhosis (F ≥ 4) with a higher accuracy, while for lower levels of fibrosis reliability is reduced, due to the data overlap.
Portal hypertension: To date, there are no results giving precise information on the ability of this tool to predict portal hypertension[170].
MRE is similar to ultrasound elastography, it uses a vibration device to induce a shear wave in the liver. The system consists of an active driver, located outside the magnet room, which generates continuous low frequency vibrations (60 MHz), lower than TE. These vibrations are transmitted to a drum-like passive driver. The trasducer is positioned in the right hypochondrium (or last posterior ribs). The acoustic vibrations, which then transmits into the body, produce shear wave motion within the liver. The shear wave propagation within the liver together with a modified phase-contrast MR sequence, and processing the wave images with an inversion algorithm obtain a quantitative image of shear stiffness (elastogram)[171].
Advantages of MRE includes: (1) It can be used in obese or ascites patients; (2) It is not limited by narrow intercostal space; and (3) It has a higher sensitivity than the elastographic methods in defining mild fibrosis and it has a better reproducibility.
Recently, Guo et al[172] has reported AUROC for MRE staging fibrosis of 0.94, 0.97, 0.96, and 0.97 for F1-F4, respectively, whereas AUROC for ARFI staging of 0.82, 0.85, 0.94, and 0.94 for F1-F4, respectively. Huwart et al[173] found that MRE has a better diagnostic accuracy than ultrasound elastography for staging liver fibrosis.
In MRE different cut-offs of cirrhosis have been reported. Venkatesh proposes 4.13 kPa as cut-off for LC, while values between 2.84 and 2.93 would be able to distinguish a normal liver from a fibrotic one[174].
Moreover, Loomba has reported, in liver steatosis of very obese patients (BMI: 50.07 ± 13.4), AUROC values for MRE, discriminating advanced fibrosis from mild fibrosis, of 0.924; a threshold greater than 3.63 kPa had a sensitivity of 0.86, specificity of 0.91, PPV of 0.68, and NPV of 0.97[175].
To improve the diagnostic accuracy of the individual non-invasive methods described above, recent data from the literature have proposed their combined use. For example, a recent paper evaluated and compared the ability of serum hyaluronic acid and YKL-40 values, as well as TE findings, to predict advanced hepatic fibrosis in a cohort from a single pediatric center. It concluded that YKL-40 had no predictive value and TE was superior to HA, but the addition of HA did not improve the performance of TE[176]. In another study fibrosis staging and biochemical data to calculate APRI, FIB-4, and AST/ALT-ratio (AAR) were analyzed. Logistic regression was performed to investigate whether biochemical scores were significant predictors of advanced fibrosis independent of TE. The authors concluded that the combination of TE and FIB-4 was useful in the prediction of advanced fibrosis, even if the effect of this combination was marginal when only asymptomatic patients were included[177].
In 2005, Castéra et al[178] prospectively assessed the performance of FibroScanTM in patients with chronic hepatitis C combined with Fibrotest and APRI in predicting the stage of liver fibrosis. The authors found that the best performance was obtained by combining the FibroScanTM and Fibrotest, with areas under the ROC curve of 0.88 for F > 2, 0.95 for F > 3, and 0.95 for F = 4. When the FibroScanTM and Fibrotest results agreed, liver biopsy examination confirmed them in 84% of cases for F > 2, 95% for F > 3, and 94% for F = 4. Their conclusion was that the combined use of FibroScanTM and Fibrotest to evaluate liver fibrosis could avoid a biopsy procedure in most patients with chronic hepatitis C. Previous studies, however, did not find any improvement in the accuracy of predicting cirrhosis when TE and other serum markers (in particular platelet count and procollagen III N-terminal peptide) were combined[179].
Apart from the methods here described, in recent years new elastographic methods are emerging for the study of liver fibrosis: the Supersonic Shear Imaging[180], also named ShearWave™ elastography, which is based on the measurement of the velocity of a local shear wave through soft tissues and a recently proposed method in China, the Fibrotouch®.
During the progression of CLD evaluating liver fibrosis is of paramount importance. Liver biopsy cannot be performed in all patients for the series of reasons mentioned above. Consequently, in the last few years other non-invasive serological tests and imaging techniques have been proposed as reliable indicators of fibrosis.
From the literature data it is evident that no single non-invasive test can substitute liver biopsy in any phase of fibrosis progression. However, it can now be affirmed that both serological tests and imaging techniques are quite reliable for diagnosing LC as well as for excluding the presence of fibrosis. In contrast, in the intermediate stages their reliability is limited.
Sequential flow charts, using serological tests and imaging techniques, could be used to help improve diagnostic reliability.
The authors thank Dr. S. Madonia, Internal Medicine Unit, Ospedale “Cervello”, Palermo, Italy and Dr. L. Rizzo, Ultrasuoni Diagnostic Medical Center, Catania, Italy for kindly providing images of TE and ARFI.
P- Reviewer: He JY, Kaya M, Ristic-Medic D, Sazci A, Shimizu Y, Wong GLH, Zhu X S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
| 1. | Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013;58:593-608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 879] [Cited by in F6Publishing: 868] [Article Influence: 78.9] [Reference Citation Analysis (0)] |
| 2. | Crawford JM. Liver cirrhosis. Pathology of the Liver. 4th ed. London, England: Churchill Livingstone 2002; 575-619. [Cited in This Article: ] |
| 3. | Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359:1486-1500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 689] [Cited by in F6Publishing: 663] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 4. | Seeff LB, Hoofnagle JH. National Institutes of Health Consensus Development Conference: management of hepatitis C: 2002. Hepatology. 2002;36:S1-S2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649-1657. [PubMed] [Cited in This Article: ] |
| 6. | Soresi M, Noto D, Cefalù AB, Martini S, Vigna GB, Fonda M, Manzato E, Cattin L, Fellin R, Averna MR. Nonalcoholic fatty liver and metabolic syndrome in Italy: results from a multicentric study of the Italian Arteriosclerosis society. Acta Diabetol. 2013;50:241-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Hytiroglou P, Snover DC, Alves V, Balabaud C, Bhathal PS, Bioulac-Sage P, Crawford JM, Dhillon AP, Ferrell L, Guido M. Beyond “cirrhosis”: a proposal from the International Liver Pathology Study Group. Am J Clin Pathol. 2012;137:5-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Anthony PP, Ishak KG, Nayak NC, Poulsen HE, Scheuer PJ, Sobin LH. The morphology of cirrhosis. Recommendations on definition, nomenclature, and classification by a working group sponsored by the World Health Organization. J Clin Pathol. 1978;31:395-414. [PubMed] [Cited in This Article: ] |
| 9. | Anthony PP, Ishak KG, Nayak NC, Poulsen HE, Scheuer PJ, Sobin LH. The morphology of cirrhosis: definition, nomenclature, and classification. Bull World Health Organ. 1977;55:521-540. [PubMed] [Cited in This Article: ] |
| 10. | Garcia-Tsao G, Friedman S, Iredale J, Pinzani M. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology. 2010;51:1445-1449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 359] [Cited by in F6Publishing: 361] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 11. | de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005;43:167-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 794] [Cited by in F6Publishing: 710] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 12. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5972] [Cited by in F6Publishing: 6338] [Article Influence: 487.5] [Reference Citation Analysis (1)] |
| 13. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4059] [Cited by in F6Publishing: 4338] [Article Influence: 361.5] [Reference Citation Analysis (2)] |
| 14. | De Groote J, Desmet VJ, Gedigk P, Korb G, Popper H, Poulsen H, Scheuer PJ, Schmid M, Thaler H, Uehlinger E. A classification of chronic hepatitis. Lancet. 1968;2:626-628. [PubMed] [Cited in This Article: ] |
| 15. | Popper H, Schaffner F. The vocabulary of chronic hepatitis. N Engl J Med. 1971;284:1154-1156. [PubMed] [Cited in This Article: ] |
| 16. | Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431-435. [PubMed] [Cited in This Article: ] |
| 17. | Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13:372-374. [PubMed] [Cited in This Article: ] |
| 18. | Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513-1520. [PubMed] [Cited in This Article: ] |
| 19. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [PubMed] [Cited in This Article: ] |
| 20. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [PubMed] [Cited in This Article: ] |
| 21. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6807] [Cited by in F6Publishing: 7387] [Article Influence: 388.8] [Reference Citation Analysis (5)] |
| 22. | Maharaj B, Maharaj RJ, Leary WP, Cooppan RM, Naran AD, Pirie D, Pudifin DJ. Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet. 1986;1:523-525. [PubMed] [Cited in This Article: ] |
| 23. | Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898-1906. [PubMed] [Cited in This Article: ] |
| 24. | Scheuer PJ. Liver biopsy size matters in chronic hepatitis: bigger is better. Hepatology. 2003;38:1356-1358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol. 2003;39:239-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 623] [Cited by in F6Publishing: 643] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 26. | Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49:1017-1044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1449] [Cited by in F6Publishing: 1419] [Article Influence: 94.6] [Reference Citation Analysis (0)] |
| 27. | Duarte-Rojo A, Altamirano JT, Feld JJ. Noninvasive markers of fibrosis: key concepts for improving accuracy in daily clinical practice. Ann Hepatol. 2012;11:426-439. [PubMed] [Cited in This Article: ] |
| 28. | Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614-2618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1504] [Cited by in F6Publishing: 1504] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 29. | Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol. 1986;2:165-173. [PubMed] [Cited in This Article: ] |
| 30. | Montalto G, Soresi M, Carroccio A, Bascone F, Tripi S, Aragona F, Di Gaetano G, Notarbartolo A. Percutaneous liver biopsy: a safe outpatient procedure? Digestion. 2001;63:55-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Mehta SH, Lau B, Afdhal NH, Thomas DL. Exceeding the limits of liver histology markers. J Hepatol. 2009;50:36-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 32. | Lydatakis H, Hager IP, Kostadelou E, Mpousmpoulas S, Pappas S, Diamantis I. Non-invasive markers to predict the liver fibrosis in non-alcoholic fatty liver disease. Liver Int. 2006;26:864-871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Guéchot J, Laudat A, Loria A, Serfaty L, Poupon R, Giboudeau J. Diagnostic accuracy of hyaluronan and type III procollagen amino-terminal peptide serum assays as markers of liver fibrosis in chronic viral hepatitis C evaluated by ROC curve analysis. Clin Chem. 1996;42:558-563. [PubMed] [Cited in This Article: ] |
| 34. | Gressner AM, Haarmann R. Hyaluronic acid synthesis and secretion by rat liver fat storing cells (perisinusoidal lipocytes) in culture. Biochem Biophys Res Commun. 1988;151:222-229. [PubMed] [Cited in This Article: ] |
| 35. | Jarcuska P, Janicko M, Veselíny E, Jarcuska P, Skladaný L. Circulating markers of liver fibrosis progression. Clin Chim Acta. 2010;411:1009-1017. [PubMed] [Cited in This Article: ] |
| 36. | Schuppan D, Stölzel U, Oesterling C, Somasundaram R. Serum assays for liver fibrosis. J Hepatol. 1995;22:82-88. [PubMed] [Cited in This Article: ] |
| 37. | Collazos J, Díaz F. Role of the measurement of serum procollagen type III N-terminal peptide in the evaluation of liver diseases. Clin Chim Acta. 1994;227:37-43. [PubMed] [Cited in This Article: ] |
| 38. | Gressner OA, Weiskirchen R, Gressner AM. Biomarkers of liver fibrosis: clinical translation of molecular pathogenesis or based on liver-dependent malfunction tests. Clin Chim Acta. 2007;381:107-113. [PubMed] [Cited in This Article: ] |
| 39. | Walsh KM, Timms P, Campbell S, MacSween RN, Morris AJ. Plasma levels of matrix metalloproteinase-2 (MMP-2) and tissue inhibitors of metalloproteinases -1 and -2 (TIMP-1 and TIMP-2) as noninvasive markers of liver disease in chronic hepatitis C: comparison using ROC analysis. Dig Dis Sci. 1999;44:624-630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 113] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 40. | Badra G, Lotfy M, El-Refaie A, Obada M, Abdelmonem E, Kandeel S, Fathy A. Significance of serum matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in chronic hepatitis C patients. Acta Microbiol Immunol Hung. 2010;57:29-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Gressner AM, Tittor W, Kropf J. The predictive value of serum laminin for portal hypertension in chronic liver diseases. Hepatogastroenterology. 1988;35:95-100. [PubMed] [Cited in This Article: ] |
| 42. | Collazos J, Díaz F, Genollá J. Serum concentrations of laminin in cirrhosis of the liver. Gut. 1993;34:974-976. [PubMed] [Cited in This Article: ] |
| 43. | Kanzler S, Baumann M, Schirmacher P, Dries V, Bayer E, Gerken G, Dienes HP, Lohse AW. Prediction of progressive liver fibrosis in hepatitis C infection by serum and tissue levels of transforming growth factor-beta. J Viral Hepat. 2001;8:430-437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 44. | Nelson DR, Gonzalez-Peralta RP, Qian K, Xu Y, Marousis CG, Davis GL, Lau JY. Transforming growth factor-beta 1 in chronic hepatitis C. J Viral Hepat. 1997;4:29-35. [PubMed] [Cited in This Article: ] |
| 45. | Zhang D, Wang NY, Yang CB, Fang GX, Liu W, Wen J, Luo C. The clinical value of serum connective tissue growth factor in the assessment of liver fibrosis. Dig Dis Sci. 2010;55:767-774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Gressner AM, Yagmur E, Lahme B, Gressner O, Stanzel S. Connective tissue growth factor in serum as a new candidate test for assessment of hepatic fibrosis. Clin Chem. 2006;52:1815-1817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 47. | Saitou Y, Shiraki K, Yamanaka Y, Yamaguchi Y, Kawakita T, Yamamoto N, Sugimoto K, Murata K, Nakano T. Noninvasive estimation of liver fibrosis and response to interferon therapy by a serum fibrogenesis marker, YKL-40, in patients with HCV-associated liver disease. World J Gastroenterol. 2005;11:476-481. [PubMed] [Cited in This Article: ] |
| 48. | Tran A, Benzaken S, Saint-Paul MC, Guzman-Granier E, Hastier P, Pradier C, Barjoan EM, Demuth N, Longo F, Rampal P. Chondrex (YKL-40), a potential new serum fibrosis marker in patients with alcoholic liver disease. Eur J Gastroenterol Hepatol. 2000;12:989-993. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 49. | Berres ML, Papen S, Pauels K, Schmitz P, Zaldivar MM, Hellerbrand C, Mueller T, Berg T, Weiskirchen R, Trautwein C. A functional variation in CHI3L1 is associated with severity of liver fibrosis and YKL-40 serum levels in chronic hepatitis C infection. J Hepatol. 2009;50:370-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 50. | Mölleken C, Sitek B, Henkel C, Poschmann G, Sipos B, Wiese S, Warscheid B, Broelsch C, Reiser M, Friedman SL. Detection of novel biomarkers of liver cirrhosis by proteomic analysis. Hepatology. 2009;49:1257-1266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 51. | Chu PG, Weiss LM. Keratin expression in human tissues and neoplasms. Histopathology. 2002;40:403-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 613] [Cited by in F6Publishing: 556] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 52. | Leers MP, Kölgen W, Björklund V, Bergman T, Tribbick G, Persson B, Björklund P, Ramaekers FC, Björklund B, Nap M. Immunocytochemical detection and mapping of a cytokeratin 18 neo-epitope exposed during early apoptosis. J Pathol. 1999;187:567-572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 53. | Bantel H, Lügering A, Heidemann J, Volkmann X, Poremba C, Strassburg CP, Manns MP, Schulze-Osthoff K. Detection of apoptotic caspase activation in sera from patients with chronic HCV infection is associated with fibrotic liver injury. Hepatology. 2004;40:1078-1087. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 214] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 54. | Yilmaz Y, Dolar E, Ulukaya E, Akgoz S, Keskin M, Kiyici M, Aker S, Yilmaztepe A, Gurel S, Gulten M. Soluble forms of extracellular cytokeratin 18 may differentiate simple steatosis from nonalcoholic steatohepatitis. World J Gastroenterol. 2007;13:837-844. [PubMed] [Cited in This Article: ] |
| 55. | Poynard T, Bedossa P. Age and platelet count: a simple index for predicting the presence of histological lesions in patients with antibodies to hepatitis C virus. METAVIR and CLINIVIR Cooperative Study Groups. J Viral Hepat. 1997;4:199-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 191] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 56. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2762] [Cited by in F6Publishing: 2976] [Article Influence: 141.7] [Reference Citation Analysis (0)] |
| 57. | Kim BK, Kim SA, Park YN, Cheong JY, Kim HS, Park JY, Cho SW, Han KH, Chon CY, Moon YM. Noninvasive models to predict liver cirrhosis in patients with chronic hepatitis B. Liver Int. 2007;27:969-976. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 58. | Williams AL, Hoofnagle JH. Ratio of serum aspartate to alanine aminotransferase in chronic hepatitis. Relationship to cirrhosis. Gastroenterology. 1988;95:734-739. [PubMed] [Cited in This Article: ] |
| 59. | Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57:1441-1447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 545] [Cited by in F6Publishing: 553] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 60. | Bonacini M, Hadi G, Govindarajan S, Lindsay KL. Utility of a discriminant score for diagnosing advanced fibrosis or cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1997;92:1302-1304. [PubMed] [Cited in This Article: ] |
| 61. | Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, Hubscher S, Roskams T, Pinzani M, Arthur MJ. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004;127:1704-1713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 756] [Cited by in F6Publishing: 706] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 62. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2633] [Cited by in F6Publishing: 2989] [Article Influence: 166.1] [Reference Citation Analysis (0)] |
| 63. | Omran MM, Farid K, Emran TM, Attallah AA. Fibro-α score as a simple and useful non-invasive test for predicting significant liver fibrosis in chronic hepatitis C patients. Arab J Gastroenterol. 2011;12:74-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 64. | Koda M, Matunaga Y, Kawakami M, Kishimoto Y, Suou T, Murawaki Y. FibroIndex, a practical index for predicting significant fibrosis in patients with chronic hepatitis C. Hepatology. 2007;45:297-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 192] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 65. | Calès P, Oberti F, Michalak S, Hubert-Fouchard I, Rousselet MC, Konaté A, Gallois Y, Ternisien C, Chevailler A, Lunel F. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology. 2005;42:1373-1381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 392] [Cited by in F6Publishing: 358] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 66. | Calès P, Lainé F, Boursier J, Deugnier Y, Moal V, Oberti F, Hunault G, Rousselet MC, Hubert I, Laafi J. Comparison of blood tests for liver fibrosis specific or not to NAFLD. J Hepatol. 2009;50:165-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 189] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 67. | Attallah AM, Abdallah SO, Attallah AA, Omran MM, Farid K, Nasif WA, Shiha GE, Abdel-Aziz AA, Rasafy N, Shaker YM. Diagnostic value of fibronectin discriminant score for predicting liver fibrosis stages in chronic hepatitis C virus patients. Ann Hepatol. 2013;12:44-53. [PubMed] [Cited in This Article: ] |
| 68. | Hsieh YY, Tung SY, Lee IL, Lee K, Shen CH, Wei KL, Chang TS, Chuang CS, Wu CS, Lin YH. FibroQ: an easy and useful noninvasive test for predicting liver fibrosis in patients with chronic viral hepatitis. Chang Gung Med J. 2009;32:614-622. [PubMed] [Cited in This Article: ] |
| 69. | Ahmad W, Ijaz B, Javed FT, Gull S, Kausar H, Sarwar MT, Asad S, Shahid I, Sumrin A, Khaliq S. A comparison of four fibrosis indexes in chronic HCV: development of new fibrosis-cirrhosis index (FCI). BMC Gastroenterol. 2011;11:44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 70. | Ohta T, Sakaguchi K, Fujiwara A, Fujioka S, Iwasaki Y, Makino Y, Araki Y, Shiratori Y. Simple surrogate index of the fibrosis stage in chronic hepatitis C patients using platelet count and serum albumin level. Acta Med Okayama. 2006;60:77-84. [PubMed] [Cited in This Article: ] |
| 71. | Sud A, Hui JM, Farrell GC, Bandara P, Kench JG, Fung C, Lin R, Samarasinghe D, Liddle C, McCaughan GW. Improved prediction of fibrosis in chronic hepatitis C using measures of insulin resistance in a probability index. Hepatology. 2004;39:1239-1247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 140] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 72. | Attallah AM, Omran MM, Farid K, El-Bendary M, Emran TM, Albannan MS, El-Dosoky I. Development of a novel score for liver fibrosis staging and comparison with eight simple laboratory scores in large numbers of HCV-monoinfected patients. Clin Chim Acta. 2012;413:1725-1730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 73. | Patel K, Gordon SC, Jacobson I, Hézode C, Oh E, Smith KM, Pawlotsky JM, McHutchison JG. Evaluation of a panel of non-invasive serum markers to differentiate mild from moderate-to-advanced liver fibrosis in chronic hepatitis C patients. J Hepatol. 2004;41:935-942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 251] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 74. | Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069-1075. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1066] [Cited by in F6Publishing: 1113] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 75. | Forns X, Ampurdanès S, Llovet JM, Aponte J, Quintó L, Martínez-Bauer E, Bruguera M, Sánchez-Tapias JM, Rodés J. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36:986-992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 672] [Cited by in F6Publishing: 751] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 76. | Luo JC, Hwang SJ, Chang FY, Chu CW, Lai CR, Wang YJ, Lee PC, Tsay SH, Lee SD. Simple blood tests can predict compensated liver cirrhosis in patients with chronic hepatitis C. Hepatogastroenterology. 2002;49:478-481. [PubMed] [Cited in This Article: ] |
| 77. | Islam S, Antonsson L, Westin J, Lagging M. Cirrhosis in hepatitis C virus-infected patients can be excluded using an index of standard biochemical serum markers. Scand J Gastroenterol. 2005;40:867-872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 109] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 78. | Fontana RJ, Goodman ZD, Dienstag JL, Bonkovsky HL, Naishadham D, Sterling RK, Su GL, Ghosh M, Wright EC. Relationship of serum fibrosis markers with liver fibrosis stage and collagen content in patients with advanced chronic hepatitis C. Hepatology. 2008;47:789-798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 79. | Adams LA, Bulsara M, Rossi E, DeBoer B, Speers D, George J, Kench J, Farrell G, McCaughan GW, Jeffrey GP. Hepascore: an accurate validated predictor of liver fibrosis in chronic hepatitis C infection. Clin Chem. 2005;51:1867-1873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 387] [Cited by in F6Publishing: 368] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 80. | Cross TJ, Rizzi P, Berry PA, Bruce M, Portmann B, Harrison PM. King’s Score: an accurate marker of cirrhosis in chronic hepatitis C. Eur J Gastroenterol Hepatol. 2009;21:730-738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 81. | Lok AS, Ghany MG, Goodman ZD, Wright EC, Everson GT, Sterling RK, Everhart JE, Lindsay KL, Bonkovsky HL, Di Bisceglie AM. Predicting cirrhosis in patients with hepatitis C based on standard laboratory tests: results of the HALT-C cohort. Hepatology. 2005;42:282-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 230] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 82. | Leroy V, Monier F, Bottari S, Trocme C, Sturm N, Hilleret MN, Morel F, Zarski JP. Circulating matrix metalloproteinases 1, 2, 9 and their inhibitors TIMP-1 and TIMP-2 as serum markers of liver fibrosis in patients with chronic hepatitis C: comparison with PIIINP and hyaluronic acid. Am J Gastroenterol. 2004;99:271-279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 182] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 83. | Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846-854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1917] [Cited by in F6Publishing: 2018] [Article Influence: 118.7] [Reference Citation Analysis (1)] |
| 84. | Pohl A, Behling C, Oliver D, Kilani M, Monson P, Hassanein T. Serum aminotransferase levels and platelet counts as predictors of degree of fibrosis in chronic hepatitis C virus infection. Am J Gastroenterol. 2001;96:3142-3146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 194] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 85. | Obrador BD, Prades MG, Gómez MV, Domingo JP, Cueto RB, Rué M, Real J, Guiteras PM. A predictive index for the diagnosis of cirrhosis in hepatitis C based on clinical, laboratory, and ultrasound findings. Eur J Gastroenterol Hepatol. 2006;18:57-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 86. | Cheong JY, Um SH, Seo YS, Kim DJ, Hwang SG, Lee YJ, Cho M, Yang JM, Kim YB, Park YN. Non-invasive index for predicting significant liver fibrosis: comparison of diagnostic performances in patients with chronic hepatitis B and C. Dig Dis Sci. 2011;56:555-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 87. | Maieron A, Salzl P, Peck-Radosavljevic M, Trauner M, Hametner S, Schöfl R, Ferenci P, Ferlitsch M. Von Willebrand Factor as a new marker for non-invasive assessment of liver fibrosis and cirrhosis in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2014;39:331-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 88. | Zeng MD, Lu LG, Mao YM, Qiu DK, Li JQ, Wan MB, Chen CW, Wang JY, Cai X, Gao CF. Prediction of significant fibrosis in HBeAg-positive patients with chronic hepatitis B by a noninvasive model. Hepatology. 2005;42:1437-1445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 89. | Soresi M, La Spada E, Giannitrapani L, Campagna E, Di Gesaro V, Granà W, Sandonato L, Brancatelli G, Rotolo G, Affronti A. Hepatocellular carcinoma: comparison of two different periods at the same center. Eur J Intern Med. 2010;21:127-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 90. | Berzigotti A, Piscaglia F. Ultrasound in portal hypertension--part 1. Ultraschall Med. 2011;32:548-68; quiz 569-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 91. | Berzigotti A, Piscaglia F. Ultrasound in portal hypertension--part 2--and EFSUMB recommendations for the performance and reporting of ultrasound examinations in portal hypertension. Ultraschall Med. 2012;33:8-32; quiz 30-1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 92. | Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, Piscaglia F, Wilson SR, Barr RG, Chammas MC. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver--update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med. 2013;34:11-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 210] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 93. | Giorgio A, Amoroso P, Lettieri G, Fico P, de Stefano G, Finelli L, Scala V, Tarantino L, Pierri P, Pesce G. Cirrhosis: value of caudate to right lobe ratio in diagnosis with US. Radiology. 1986;161:443-445. [PubMed] [Cited in This Article: ] |
| 94. | Taylor KJ, Riely CA, Hammers L, Flax S, Weltin G, Garcia-Tsao G, Conn HO, Kuc R, Barwick KW. Quantitative US attenuation in normal liver and in patients with diffuse liver disease: importance of fat. Radiology. 1986;160:65-71. [PubMed] [Cited in This Article: ] |
| 95. | Joseph AE, Saverymuttu SH, al-Sam S, Cook MG, Maxwell JD. Comparison of liver histology with ultrasonography in assessing diffuse parenchymal liver disease. Clin Radiol. 1991;43:26-31. [PubMed] [Cited in This Article: ] |
| 96. | Saverymuttu SH, Joseph AE, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J (Clin Res Ed). 1986;292:13-15. [PubMed] [Cited in This Article: ] |
| 97. | Sanford NL, Walsh P, Matis C, Baddeley H, Powell LW. Is ultrasonography useful in the assessment of diffuse parenchymal liver disease? Gastroenterology. 1985;89:186-191. [PubMed] [Cited in This Article: ] |
| 98. | Strauss S, Gavish E, Gottlieb P, Katsnelson L. Interobserver and intraobserver variability in the sonographic assessment of fatty liver. AJR Am J Roentgenol. 2007;189:W320-W323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 292] [Article Influence: 17.2] [Reference Citation Analysis (1)] |
| 99. | Palmentieri B, de Sio I, La Mura V, Masarone M, Vecchione R, Bruno S, Torella R, Persico M. The role of bright liver echo pattern on ultrasound B-mode examination in the diagnosis of liver steatosis. Dig Liver Dis. 2006;38:485-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 224] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 100. | Soresi M, Giannitrapani L, Florena AM, La Spada E, Di Gesaro V, Rappa F, Alessandri A, Tripi S, Romano M, Montalto G. Reliability of the bright liver echo pattern in diagnosing steatosis in patients with cryptogenic and HCV-related hypertransaminasaemia. Clin Radiol. 2009;64:1181-1187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 101. | Joseph AE, Saverymuttu SH. Ultrasound in the assessment of diffuse parenchymal liver disease. Clin Radiol. 1991;44:219-221. [PubMed] [Cited in This Article: ] |
| 102. | Zardi EM, Caturelli E. May sonography distinguish between liver fibrosis and liver steatosis? Dig Liver Dis. 2007;39:790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 103. | Berzigotti A, Abraldes JG, Tandon P, Erice E, Gilabert R, García-Pagan JC, Bosch J. Ultrasonographic evaluation of liver surface and transient elastography in clinically doubtful cirrhosis. J Hepatol. 2010;52:846-853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 104. | Colli A, Colucci A, Paggi S, Fraquelli M, Massironi S, Andreoletti M, Michela V, Conte D. Accuracy of a predictive model for severe hepatic fibrosis or cirrhosis in chronic hepatitis C. World J Gastroenterol. 2005;11:7318-7322. [PubMed] [Cited in This Article: ] |
| 105. | Gaiani S, Gramantieri L, Venturoli N, Piscaglia F, Siringo S, D’Errico A, Zironi G, Grigioni W, Bolondi L. What is the criterion for differentiating chronic hepatitis from compensated cirrhosis? A prospective study comparing ultrasonography and percutaneous liver biopsy. J Hepatol. 1997;27:979-985. [PubMed] [Cited in This Article: ] |
| 106. | Colli A, Fraquelli M, Andreoletti M, Marino B, Zuccoli E, Conte D. Severe liver fibrosis or cirrhosis: accuracy of US for detection--analysis of 300 cases. Radiology. 2003;227:89-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 218] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 107. | Iacobellis A, Fusilli S, Mangia A, Clemente R, Festa V, Giacobbe A, Facciorusso D, Niro G, Conoscitore P, Andriulli A. Ultrasonographic and biochemical parameters in the non-invasive evaluation of liver fibrosis in hepatitis C virus chronic hepatitis. Aliment Pharmacol Ther. 2005;22:769-774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 108. | Bolondi L, Gandolfi L, Arienti V, Caletti GC, Corcioni E, Gasbarrini G, Labò G. Ultrasonography in the diagnosis of portal hypertension: diminished response of portal vessels to respiration. Radiology. 1982;142:167-172. [PubMed] [Cited in This Article: ] |
| 109. | Berzigotti A, Gilabert R, Abraldes JG, Nicolau C, Bru C, Bosch J, García-Pagan JC. Noninvasive prediction of clinically significant portal hypertension and esophageal varices in patients with compensated liver cirrhosis. Am J Gastroenterol. 2008;103:1159-1167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 110. | World Health Organization. Richter J, Hatz C, Campagne G, Bergquist N, Jenkins J (October 22–26, 1996) Ultrasound in Schistosomiasis: A Pratical Guide to the Standardized use of Ultrasonography for the assessment of Schistosomiasisrelated morbidity. Second International Workshop, Niamey, Niger TDR/STR/SCH/00.1. Available from: http://www.who.int/tdr/publications/documents/ultrasound-schistosomiasis.pdf. [Cited in This Article: ] |
| 111. | Zhu X, Zhang J, Fan W, Gong Y, Yan J, Yuan Z, Wu L, Cui H, Luo H, Kong Q. MAPKAP1 rs10118570 polymorphism is associated with anti-infection and anti-hepatic fibrogenesis in schistosomiasis japonica. PLoS One. 2014;9:e105995. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 112. | Staub F, Tournoux-Facon C, Roumy J, Chaigneau C, Morichaut-Beauchant M, Levillain P, Prevost C, Aubé C, Lebigot J, Oberti F. Liver fibrosis staging with contrast-enhanced ultrasonography: prospective multicenter study compared with METAVIR scoring. Eur Radiol. 2009;19:1991-1997. [PubMed] [Cited in This Article: ] |
| 113. | Li N, Ding H, Fan P, Lin X, Xu C, Wang W, Xu Z, Wang J. Intrahepatic transit time predicts liver fibrosis in patients with chronic hepatitis B: quantitative assessment with contrast-enhanced ultrasonography. Ultrasound Med Biol. 2010;36:1066-1075. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 114. | Gennisson JL, Deffieux T, Fink M, Tanter M. Ultrasound elastography: principles and techniques. Diagn Interv Imaging. 2013;94:487-495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 525] [Cited by in F6Publishing: 517] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 115. | Bamber J, Cosgrove D, Dietrich CF, Fromageau J, Bojunga J, Calliada F, Cantisani V, Correas JM, D’Onofrio M, Drakonaki EE. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013;34:169-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 756] [Cited by in F6Publishing: 690] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 116. | Dietrich CF, Cantisani V. Current status and perspectives of elastography. Eur J Radiol. 2014;83:403-404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 117. | Cosgrove D, Piscaglia F, Bamber J, Bojunga J, Correas JM, Gilja OH, Klauser AS, Sporea I, Calliada F, Cantisani V. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: Clinical applications. Ultraschall Med. 2013;34:238-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 621] [Cited by in F6Publishing: 461] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 118. | Coco B, Oliveri F, Maina AM, Ciccorossi P, Sacco R, Colombatto P, Bonino F, Brunetto MR. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat. 2007;14:360-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 484] [Cited by in F6Publishing: 441] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 119. | Millonig G, Reimann FM, Friedrich S, Fonouni H, Mehrabi A, Büchler MW, Seitz HK, Mueller S. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718-1723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 440] [Cited by in F6Publishing: 425] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 120. | Millonig G, Friedrich S, Adolf S, Fonouni H, Golriz M, Mehrabi A, Stiefel P, Pöschl G, Büchler MW, Seitz HK. Liver stiffness is directly influenced by central venous pressure. J Hepatol. 2010;52:206-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 121. | D’Onofrio M, Gallotti A, Mucelli RP. Tissue quantification with acoustic radiation force impulse imaging: Measurement repeatability and normal values in the healthy liver. AJR Am J Roentgenol. 2010;195:132-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 122. | Castéra L, Foucher J, Bernard PH, Carvalho F, Allaix D, Merrouche W, Couzigou P, de Lédinghen V. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology. 2010;51:828-835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 365] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 123. | Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G, Colombo M. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968-973. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 629] [Cited by in F6Publishing: 618] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 124. | Wong VW, Chan HL. Transient elastography. J Gastroenterol Hepatol. 2010;25:1726-1731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 125. | Roulot D, Czernichow S, Le Clésiau H, Costes JL, Vergnaud AC, Beaugrand M. Liver stiffness values in apparently healthy subjects: influence of gender and metabolic syndrome. J Hepatol. 2008;48:606-613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 315] [Cited by in F6Publishing: 303] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 126. | Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Lédinghen V, Marcellin P, Dhumeaux D, Trinchet JC. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1090] [Cited by in F6Publishing: 1062] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 127. | Talwalkar JA, Kurtz DM, Schoenleber SJ, West CP, Montori VM. Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2007;5:1214-1220. [PubMed] [Cited in This Article: ] |
| 128. | Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960-974. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1046] [Cited by in F6Publishing: 1011] [Article Influence: 63.2] [Reference Citation Analysis (1)] |
| 129. | Tsochatzis EA, Gurusamy KS, Ntaoula S, Cholongitas E, Davidson BR, Burroughs AK. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol. 2011;54:650-659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 490] [Cited by in F6Publishing: 484] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 130. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55:245-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 889] [Cited by in F6Publishing: 968] [Article Influence: 74.5] [Reference Citation Analysis (0)] |
| 131. | Oliveri F, Coco B, Ciccorossi P, Colombatto P, Romagnoli V, Cherubini B, Bonino F, Brunetto MR. Liver stiffness in the hepatitis B virus carrier: a non-invasive marker of liver disease influenced by the pattern of transaminases. World J Gastroenterol. 2008;14:6154-6162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 97] [Cited by in F6Publishing: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 132. | Arena U, Vizzutti F, Corti G, Ambu S, Stasi C, Bresci S, Moscarella S, Boddi V, Petrarca A, Laffi G. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology. 2008;47:380-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 557] [Cited by in F6Publishing: 543] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 133. | Marcellin P, Ziol M, Bedossa P, Douvin C, Poupon R, de Lédinghen V, Beaugrand M. Non-invasive assessment of liver fibrosis by stiffness measurement in patients with chronic hepatitis B. Liver Int. 2009;29:242-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 364] [Cited by in F6Publishing: 400] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 134. | Chon YE, Choi EH, Song KJ, Park JY, Kim do Y, Han KH, Chon CY, Ahn SH, Kim SU. Performance of transient elastography for the staging of liver fibrosis in patients with chronic hepatitis B: a meta-analysis. PLoS One. 2012;7:e44930. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 213] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 135. | European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2323] [Cited by in F6Publishing: 2338] [Article Influence: 194.8] [Reference Citation Analysis (0)] |
| 136. | de Lédinghen V, Douvin C, Kettaneh A, Ziol M, Roulot D, Marcellin P, Dhumeaux D, Beaugrand M. Diagnosis of hepatic fibrosis and cirrhosis by transient elastography in HIV/hepatitis C virus-coinfected patients. J Acquir Immune Defic Syndr. 2006;41:175-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 260] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 137. | Li Vecchi V, Soresi M, Colomba C, Mazzola G, Colletti P, Mineo M, Di Carlo P, La Spada E, Vizzini G, Montalto G. Transient elastography: a non-invasive tool for assessing liver fibrosis in HIV/HCV patients. World J Gastroenterol. 2010;16:5225-5232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 9] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 138. | Nguyen-Khac E, Chatelain D, Tramier B, Decrombecque C, Robert B, Joly JP, Brevet M, Grignon P, Lion S, Le Page L. Assessment of asymptomatic liver fibrosis in alcoholic patients using fibroscan: prospective comparison with seven non-invasive laboratory tests. Aliment Pharmacol Ther. 2008;28:1188-1198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 139. | Bardou-Jacquet E, Legros L, Soro D, Latournerie M, Guillygomarc’h A, Le Lan C, Brissot P, Guyader D, Moirand R. Effect of alcohol consumption on liver stiffness measured by transient elastography. World J Gastroenterol. 2013;19:516-522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 51] [Cited by in F6Publishing: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 140. | de Lédinghen V, Vergniol J, Foucher J, Merrouche W, le Bail B. Non-invasive diagnosis of liver steatosis using controlled attenuation parameter (CAP) and transient elastography. Liver Int. 2012;32:911-918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 248] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 141. | Festi D, Schiumerini R, Marzi L, Di Biase AR, Mandolesi D, Montrone L, Scaioli E, Bonato G, Marchesini-Reggiani G, Colecchia A. Review article: the diagnosis of non-alcoholic fatty liver disease -- availability and accuracy of non-invasive methods. Aliment Pharmacol Ther. 2013;37:392-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 142. | Friedrich-Rust M, Hadji-Hosseini H, Kriener S, Herrmann E, Sircar I, Kau A, Zeuzem S, Bojunga J. Transient elastography with a new probe for obese patients for non-invasive staging of non-alcoholic steatohepatitis. Eur Radiol. 2010;20:2390-2396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 143. | Myers RP, Pomier-Layrargues G, Kirsch R, Pollett A, Duarte-Rojo A, Wong D, Beaton M, Levstik M, Crotty P, Elkashab M. Feasibility and diagnostic performance of the FibroScan XL probe for liver stiffness measurement in overweight and obese patients. Hepatology. 2012;55:199-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 349] [Cited by in F6Publishing: 343] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 144. | de Lédinghen V, Vergniol J, Foucher J, El-Hajbi F, Merrouche W, Rigalleau V. Feasibility of liver transient elastography with FibroScan using a new probe for obese patients. Liver Int. 2010;30:1043-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 159] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 145. | Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, Choi PC, Kowo M, Chan AW, Merrouche W. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 876] [Cited by in F6Publishing: 874] [Article Influence: 62.4] [Reference Citation Analysis (1)] |
| 146. | Corpechot C, El Naggar A, Poujol-Robert A, Ziol M, Wendum D, Chazouillères O, de Lédinghen V, Dhumeaux D, Marcellin P, Beaugrand M. Assessment of biliary fibrosis by transient elastography in patients with PBC and PSC. Hepatology. 2006;43:1118-1124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 319] [Cited by in F6Publishing: 311] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 147. | Berzigotti A, Seijo S, Arena U, Abraldes JG, Vizzutti F, García-Pagán JC, Pinzani M, Bosch J. Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology. 2013;144:102-111.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 349] [Cited by in F6Publishing: 342] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 148. | Corradi F, Piscaglia F, Flori S, D’Errico-Grigioni A, Vasuri F, Tamé MR, Andreone P, Boni P, Gianstefani A, Bolondi L. Assessment of liver fibrosis in transplant recipients with recurrent HCV infection: usefulness of transient elastography. Dig Liver Dis. 2009;41:217-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 149. | Adebajo CO, Talwalkar JA, Poterucha JJ, Kim WR, Charlton MR. Ultrasound-based transient elastography for the detection of hepatic fibrosis in patients with recurrent hepatitis C virus after liver transplantation: a systematic review and meta-analysis. Liver Transpl. 2012;18:323-331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 150. | Vergniol J, Foucher J, Castéra L, Bernard PH, Tournan R, Terrebonne E, Chanteloup E, Merrouche W, Couzigou P, de Lédinghen V. Changes of non-invasive markers and FibroScan values during HCV treatment. J Viral Hepat. 2009;16:132-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 151. | Ogawa E, Furusyo N, Toyoda K, Takeoka H, Maeda S, Hayashi J. The longitudinal quantitative assessment by transient elastography of chronic hepatitis C patients treated with pegylated interferon alpha-2b and ribavirin. Antiviral Res. 2009;83:127-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 152. | Ogawa E, Furusyo N, Murata M, Ohnishi H, Toyoda K, Taniai H, Ihara T, Ikezaki H, Hayashi T, Kainuma M. Longitudinal assessment of liver stiffness by transient elastography for chronic hepatitis B patients treated with nucleoside analog. Hepatol Res. 2011;41:1178-1188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 153. | D’Ambrosio R, Aghemo A, Fraquelli M, Rumi MG, Donato MF, Paradis V, Bedossa P, Colombo M. The diagnostic accuracy of Fibroscan for cirrhosis is influenced by liver morphometry in HCV patients with a sustained virological response. J Hepatol. 2013;59:251-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 106] [Article Influence: 9.6] [Reference Citation Analysis (1)] |
| 154. | Sporea I, Sirli RL, Deleanu A, Popescu A, Focsa M, Danila M, Tudora A. Acoustic radiation force impulse elastography as compared to transient elastography and liver biopsy in patients with chronic hepatopathies. Ultraschall Med. 2011;32 Suppl 1:S46-S52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 155. | Karlas T, Pfrepper C, Wiegand J, Wittekind C, Neuschulz M, Mössner J, Berg T, Tröltzsch M, Keim V. Acoustic radiation force impulse imaging (ARFI) for non-invasive detection of liver fibrosis: examination standards and evaluation of interlobe differences in healthy subjects and chronic liver disease. Scand J Gastroenterol. 2011;46:1458-1467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 156. | Piscaglia F, Salvatore V, Di Donato R, D’Onofrio M, Gualandi S, Gallotti A, Peri E, Borghi A, Conti F, Fattovich G. Accuracy of VirtualTouch Acoustic Radiation Force Impulse (ARFI) imaging for the diagnosis of cirrhosis during liver ultrasonography. Ultraschall Med. 2011;32:167-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 157. | Bota S, Sporea I, Sirli R, Popescu A, Danila M, Costachescu D. Intra- and interoperator reproducibility of acoustic radiation force impulse (ARFI) elastography--preliminary results. Ultrasound Med Biol. 2012;38:1103-1108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 158. | Frulio N, Trillaud H. Ultrasound elastography in liver. Diagn Interv Imaging. 2013;94:515-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 159. | Fierbinteanu-Braticevici C, Andronescu D, Usvat R, Cretoiu D, Baicus C, Marinoschi G. Acoustic radiation force imaging sonoelastography for noninvasive staging of liver fibrosis. World J Gastroenterol. 2009;15:5525-5532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 133] [Cited by in F6Publishing: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 160. | Friedrich-Rust M, Wunder K, Kriener S, Sotoudeh F, Richter S, Bojunga J, Herrmann E, Poynard T, Dietrich CF, Vermehren J. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology. 2009;252:595-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 452] [Cited by in F6Publishing: 451] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 161. | Lupsor M, Badea R, Stefanescu H, Sparchez Z, Branda H, Serban A, Maniu A. Performance of a new elastographic method (ARFI technology) compared to unidimensional transient elastography in the noninvasive assessment of chronic hepatitis C. Preliminary results. J Gastrointestin Liver Dis. 2009;18:303-310. [PubMed] [Cited in This Article: ] |
| 162. | Yoneda M, Suzuki K, Kato S, Fujita K, Nozaki Y, Hosono K, Saito S, Nakajima A. Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology. 2010;256:640-647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 290] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 163. | Rizzo L, Calvaruso V, Cacopardo B, Alessi N, Attanasio M, Petta S, Fatuzzo F, Montineri A, Mazzola A, L’abbate L. Comparison of transient elastography and acoustic radiation force impulse for non-invasive staging of liver fibrosis in patients with chronic hepatitis C. Am J Gastroenterol. 2011;106:2112-2120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 164. | Sporea I, Badea R, Sirli R, Lupsor M, Popescu A, Danila M, Focsa M, Deleanu A. How efficient is acoustic radiation force impulse elastography for the evaluation of liver stiffness? Hepat Mon. 2011;11:532-538. [PubMed] [Cited in This Article: ] |
| 165. | Sporea I, Bota S, Peck-Radosavljevic M, Sirli R, Tanaka H, Iijima H, Badea R, Lupsor M, Fierbinteanu-Braticevici C, Petrisor A. Acoustic Radiation Force Impulse elastography for fibrosis evaluation in patients with chronic hepatitis C: an international multicenter study. Eur J Radiol. 2012;81:4112-4118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 108] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 166. | Friedrich-Rust M, Nierhoff J, Lupsor M, Sporea I, Fierbinteanu-Braticevici C, Strobel D, Takahashi H, Yoneda M, Suda T, Zeuzem S. Performance of Acoustic Radiation Force Impulse imaging for the staging of liver fibrosis: a pooled meta-analysis. J Viral Hepat. 2012;19:e212-e219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 343] [Cited by in F6Publishing: 329] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 167. | Nierhoff J, Chávez Ortiz AA, Herrmann E, Zeuzem S, Friedrich-Rust M. The efficiency of acoustic radiation force impulse imaging for the staging of liver fibrosis: a meta-analysis. Eur Radiol. 2013;23:3040-3053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 168. | Bota S, Sporea I, Peck-Radosavljevic M, Sirli R, Tanaka H, Iijima H, Saito H, Ebinuma H, Lupsor M, Badea R. The influence of aminotransferase levels on liver stiffness assessed by Acoustic Radiation Force Impulse Elastography: a retrospective multicentre study. Dig Liver Dis. 2013;45:762-768. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 169. | Bota S, Herkner H, Sporea I, Salzl P, Sirli R, Neghina AM, Peck-Radosavljevic M. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int. 2013;33:1138-1147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 311] [Cited by in F6Publishing: 292] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 170. | Vermehren J, Polta A, Zimmermann O, Herrmann E, Poynard T, Hofmann WP, Bojunga J, Sarrazin C, Zeuzem S, Friedrich-Rust M. Comparison of acoustic radiation force impulse imaging with transient elastography for the detection of complications in patients with cirrhosis. Liver Int. 2012;32:852-858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 171. | Yin M, Talwalkar JA, Glaser KJ, Manduca A, Grimm RC, Rossman PJ, Fidler JL, Ehman RL. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastroenterol Hepatol. 2007;5:1207-1213.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 703] [Cited by in F6Publishing: 668] [Article Influence: 39.3] [Reference Citation Analysis (1)] |
| 172. | Guo Y, Parthasarathy S, Goyal P, McCarthy RJ, Larson AC, Miller FH. Magnetic resonance elastography and acoustic radiation force impulse for staging hepatic fibrosis: a meta-analysis. Abdom Imaging. 2014;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 173. | Huwart L, Sempoux C, Vicaut E, Salameh N, Annet L, Danse E, Peeters F, ter Beek LC, Rahier J, Sinkus R. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008;135:32-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 547] [Cited by in F6Publishing: 510] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 174. | Venkatesh SK, Yin M, Ehman RL. Magnetic resonance elastography of liver: clinical applications. J Comput Assist Tomogr. 2013;37:887-896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 175. | Loomba R, Wolfson T, Ang B, Hooker J, Behling C, Peterson M, Valasek M, Lin G, Brenner D, Gamst A. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: A prospective study. Hepatology. 2014;60:1920-1928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 313] [Cited by in F6Publishing: 338] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 176. | Lee CK, Perez-Atayde AR, Mitchell PD, Raza R, Afdhal NH, Jonas MM. Serum biomarkers and transient elastography as predictors of advanced liver fibrosis in a United States cohort: the Boston children’s hospital experience. J Pediatr. 2013;163:1058-64.e2. [PubMed] [Cited in This Article: ] |
| 177. | Lannerstedt H, Konopski Z, Sandvik L, Haaland T, Løberg EM, Haukeland JW. Combining transient elastography with FIB4 enhances sensitivity in detecting advanced fibrosis of the liver. Scand J Gastroenterol. 2013;48:93-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 178. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [PubMed] [Cited in This Article: ] |
| 179. | Lee MH, Cheong JY, Um SH, Seo YS, Kim DJ, Hwang SG, Yang JM, Han KH, Cho SW. Comparison of surrogate serum markers and transient elastography (Fibroscan) for assessing cirrhosis in patients with chronic viral hepatitis. Dig Dis Sci. 2010;55:3552-3560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 180. | Cassinotto C, Lapuyade B, Mouries A, Hiriart JB, Vergniol J, Gaye D, Castain C, Le Bail B, Chermak F, Foucher J. Non-invasive assessment of liver fibrosis with impulse elastography: comparison of Supersonic Shear Imaging with ARFI and FibroScan®. J Hepatol. 2014;61:550-557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 227] [Article Influence: 22.7] [Reference Citation Analysis (0)] |