Published online Dec 21, 2014. doi: 10.3748/wjg.v20.i47.18022
Revised: October 15, 2014
Accepted: December 5, 2014
Published online: December 21, 2014
AIM: To assess the effects of 3-field lymphadenectomy for esophageal carcinoma.
METHODS: We conducted a computerized literature search of the PubMed, Cochrane Controlled Trials Register, and EMBASE databases from their inception to present. Randomized controlled trials (RCTs) or observational epidemiological studies (cohort studies) that compared the survival rates and/or postoperative complications between 2-field lymphadenectomy (2FL) and 3-field lymphadenectomy (3FL) for esophageal carcinoma with R0 resection were included. Meta-analysis was conducted using published data on 3FL vs 2FL in esophageal carcinoma patients. End points were 1-, 3-, and 5-year overall survival rates and postoperative complications, including recurrent nerve palsy, anastomosis leak, pulmonary complications, and chylothorax. Subgroup analysis was performed on the involvement of recurrent laryngeal lymph nodes.
RESULTS: Two RCTs and 18 observational studies with over 7000 patients were included. There was a clear benefit for 3FL in the 1- (RR = 1.16; 95%CI: 1.09-1.24; P < 0.01), 3- (RR = 1.44; 95%CI: 1.19-1.75; P < 0.01), and 5-year overall survival rates (RR = 1.37; 95%CI: 1.18-1.59; P < 0.01). For postoperative complications, 3FL was associated with significantly more recurrent nerve palsy (RR = 1.43; 95%CI: 1.28-1.60; P = 0.02) and anastomosis leak (RR = 1.26; 95%CI: 1.05-1.52; P = 0.09). In contrast, there was no significant difference for pulmonary complications (RR = 0.93; 95%CI: 0.75-1.16, random-effects model; P = 0.27) or chylothorax (RR = 0.77; 95%CI: 0.32-1.85; P = 0.69).
CONCLUSION: This meta-analysis shows that 3FL improves overall survival rate but has more complications. Because of the high heterogeneity among outcomes, definite conclusions are difficult to draw.
Core tip: Surgery for esophageal cancer includes removal of the primary lesion and lymph node dissection; however, there is a long-standing debate concerning the application of 3-field lymphadenectomy (3FL). The main purpose of the present meta-analysis was to present all available evidence in a systematic, quantitative, and unbiased fashion to establish the following 3 points: the effect of 3FL on the overall survival rate, identification of postoperative complications of 3FL compared to 2-field lymphadenectomy, and description of patient characteristics of those who will likely benefit from 3FL.
-
Citation: Ma GW, Situ DR, Ma QL, Long H, Zhang LJ, Lin P, Rong TH. Three-field
vs two-field lymph node dissection for esophageal cancer: A meta-analysis. World J Gastroenterol 2014; 20(47): 18022-18030 - URL: https://www.wjgnet.com/1007-9327/full/v20/i47/18022.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i47.18022
Esophageal cancer is one of the most lethal malignancies and has a long-term overall survival (OS) rate of only approximately 25% in most Western series[1], while in some Japanese series, the 5-year OS rate was reported to be approximately 50%[2]. Extensive research has been conducted to improve treatment options, especially for the optimal extent of lymph node dissection. Since 1983, several Japanese institutions[3,4] have employed radical 3-field lymphadenectomy (3FL) of the bilateral cervical, mediastinal, and abdominal regions, with the theoretical justification that relapse of cervical lymph node occurs frequently[5,6].
After almost 30 years, however, 3FL is not widely applied because its advantages and disadvantages remain controversial, resulting in an increasing amount of research focusing on identifying optimal patients and clarifying indications for 3FL recently. Nevertheless, esophagectomy remains technically demanding, and few centers can recruit a sufficient number of patients to perform clinical trials that can withstand scrutiny.
The present meta-analysis aimed to investigate the following 3 primary points: (1) the effect of 3FL on the OS rate; (2) a comparison of postoperative complications between 2FL and 3FL, and (3) identification of optimal patients who will most likely benefit from 3FL. To comprehensively and credibly answer these queries, we conducted a detailed meta-analysis using data from currently available studies that compared 3FL with 2FL, including 2 randomized controlled trials (RCTs)[7,8] and 18 observational studies[9-26]. The meta-analysis was performed on data from 1-, 3-, and 5-year OS rates, complications (recurrent nerve palsy, anastomosis leak, chylothorax, and pulmonary complications), and subgroups of recurrent laryngeal lymph node involvement. The article was arranged using a guide for reporting meta-analysis of observational studies[27].
This work has been carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. This study was approved by the Ethics Committee of Sun Yat-sen University Cancer Center.
To identify relevant studies, we conducted a computerized literature search of the PubMed, Cochrane Controlled Trials Register, and EMBASE databases from their inception to present. The search terms included the following: (1) “three-field”, “3-field”, “three field”, “3 field”, “extended cervical”, “cervical lymph node dissection”, “cervical lymphadenectomy”, “neck lymph node dissection”, “neck lymphadenectomy”, “3-F”, “3F”, and “3FL”; (2) esophageal neoplasms (MeSH); and (3) a combination of (1) and (2).
The titles and abstracts of the identified studies were scanned to exclude any study that was clearly irrelevant. The full texts of the remaining articles were read to determine whether they contained information on the topic of interest. The reference lists of articles with relevant information were reviewed to identify citations to other studies on the same topic.
The reports considered in this meta-analysis were either RCTs or observational epidemiological studies (cohort studies) that compared the survival rates and/or postoperative complications between 2FL and 3FL for esophageal carcinoma with R0 resection. There was no restriction regarding the language of articles. Articles were excluded from the analyses if they (1) contained insufficient published data for determining an estimate of relative risk (RR) or confidence interval (CI); (2) included special restrictions to types and/or stages of esophageal carcinoma (restriction to squamous carcinoma was not included because almost all tumors are of this type); (3) randomly applied 2FL or 3FL to the included patients; (4) did not apply curative resection to all included patients; and/or (5) were of poor quality and led to large biases in the analysis. In addition, for studies with multiple publications from the same population, only one with the largest data set was included. We did not assess the methodological quality of the primary studies because quality assessment in meta-analysis is controversial. Scores constructed in an ad-hoc fashion may lack demonstrated validity, and results may not be associated with quality[28].
Two reviewers independently extracted data using predefined criteria from each study, including the following: (1) Basic information comprising the first author’s last name, year of publication, journal name, study region, study design, type and stage of esophageal tumor, and inclusion and/or exclusion criteria; (2) Published data, including the 1-, 3-, and 5-year OS rates (collected by 2 methods provided by the author or measurement of the Kaplan-Meier survival curve with the software Engauge.exe), study population, operation time, complications, and subgroup data.
When more than one estimate of effect (RR) was presented in observational studies, the most adjusted estimate was chosen. Differences in data extraction were resolved by consensus and reference to the original articles.
We performed 3 comparisons between 3FL and 2FL for esophageal carcinoma-the OS rate, complications, and subgroups. For OS, 1-, 3-, and 5-year rates were compared; for complications, recurrent nerve palsy, anastomosis leak, pulmonary complications, and chylothorax were included; and for subgroup analysis, studies with recurrent laryngeal lymph node positivity/negativity were included.
Data from each study were analyzed using Review Manager software (RevMan version 5.0; http://ims.cochrane.org/revman/download). Treatment effects were expressed as RRs with 95%CIs for dichotomous outcomes. Because mortality or morbidity was not a small probability event in the participants, the Mantel-Haenszel analysis method was used[29].
We separately pooled RR estimates from each study for each outcome using random-effects meta-analysis. Statistical heterogeneity of the RRs was evaluated using the χ2-test with significance set at P < 0.01, and the I2 statistic was calculated; publication bias was assessed using funnel plots. Low, moderate, and high degrees of heterogeneity correspond to I2 values of 25%, 50%, and 75%, respectively. Sensitivity analyses were used to evaluate whether the results could have been markedly affected by a single study.
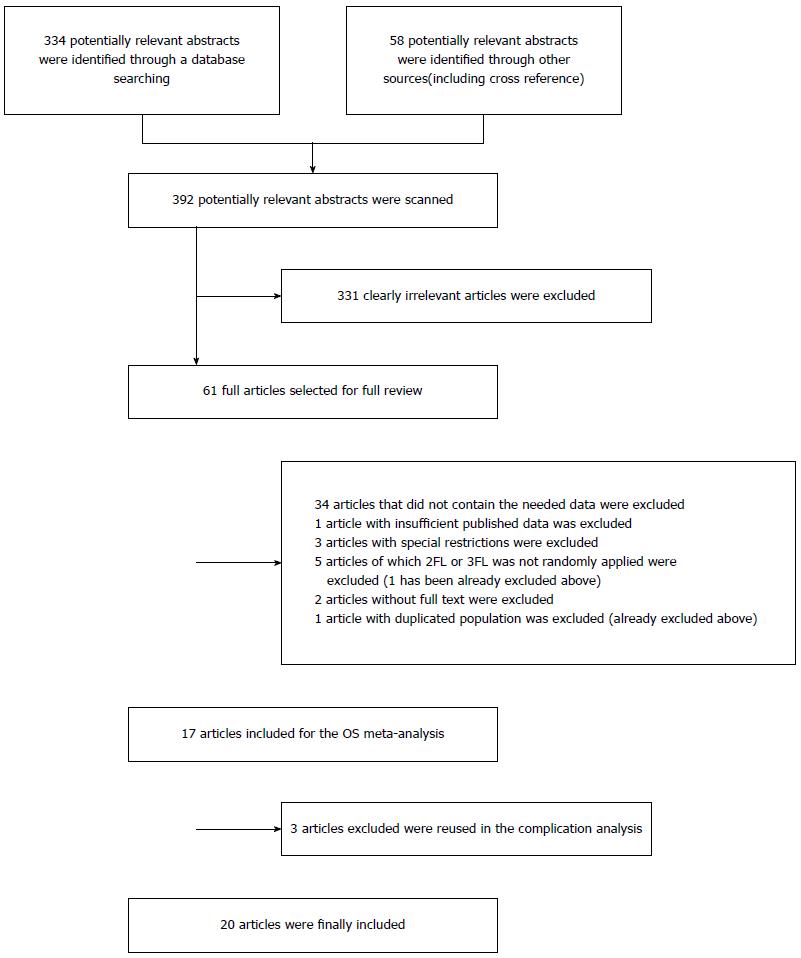
The references (n = 334) were retrieved by the original search strategy or by manual searches (n = 58). The abstracts were reviewed, and 61 articles were selected for full-text evaluation. After applying the inclusion and exclusion criteria, 20 articles were finally included (Table 1). The flow chart of the study inclusion process is shown in Figure 1.
| Study | Year | Journal | Study design | Region | Study population, n | Operation time |
| Li et al[10] | 2012 | J Surg Oncol | Obser. | China | 3FL: 136; 2FL: 227 | 2003-2010 |
| Thakur et al[9] | 2011 | Indian J Cancer | Obser. | Nepal | 3FL: 61; 2FL: 21 | 2003-2011 |
| Shim et al[12] | 2010 | J Thorac Oncol | Obser. | South Korea | 3FL: 57; 2FL: 34 | 1994-2007 |
| Zhang et al[11] | 2008 | Zhonghua Zhongliu Zazhi | Obser. | China | 3FL: 60; 2FL: 62 | 2001-2006 |
| Igaki et al[13] | 2004 | Ann Surg | Obser. | Japan | 3FL: 101; 2FL: 55 | 1988-1997 |
| Noguchi et al[14] | 2004 | Dis Esophagus | Obser. | Japan | 3FL: 68; 2FL: 78 | 1990-2001 |
| Hagry et al[15] | 2003 | Eur J Cardiothorac Surg | Obser. | Belgium | 3FL: 34; 2FL: 38 | 1975-2001 |
| Gradauskas et al[16] | 2002 | Medicina (Kaunas) | Obser. | Lithuania | 3FL: 23; 2FL: 19 | 1997-2002 |
| Shiozaki et al[17] | 2001 | Dis Esophaguss | Obser. | Japan | 3FL: 129; 2FL: 123 | 1985-1998 |
| Tabira et al[18] | 1999 | J Cardiovasc Surg | Obser. | Japan | 3FL: 66; 2FL: 86 | 1983-1996 |
| Kawahara et al[19] | 1998 | J Surg Oncol | Obser. | Japan | 3FL: 44; 2FL: 44 | 1974-1995 |
| Nishihira et al[7] | 1998 | Am J Surg | RCT. | Japan | 3FL: 32; 2FL: 30 | 1987-1994 |
| Fujita et al[20] | 1995 | Ann Surg | Obser. | Japan | 3FL: 63; 2FL: 65 | 1986-1991 |
| Kakegawa et al[21] | 1995 | Gan To Kagaku Ryoho | Obser. | Japan | 3FL: 124; 2FL: 107 | 1985-1994 |
| Kato et al[22] | 1995 | Ann Chir Gynaecol | Obser. | Japan | 3FL: 100; 2FL: 410 | 1962-1993 |
| Akiyama et al[23] | 1994 | Ann Surg | Obser. | Japan | 3FL: 324; 2FL: 393 | 1973-1993 |
| Fujita et al[24] | 1992 | Kurume Med J | Obser. | Japan | 3FL: 27; 2FL: 100 | 1982-1988 |
| Isono et al[25] | 1991 | Oncology | Obser. | Japan | 3FL: 1740; 2FL: 2671 | 1983-1989 |
| Kato et al[8] | 1991 | Ann Thorac Surg | RCT | Japan | 3FL: 77; 2FL: 73 | 1985-1989 |
| Ando et al[26] | 1989 | Nihon Geka Gakkai Zasshi | Obser. | Japan | 3FL: 22; 2FL: 56 | 1984-1988 |
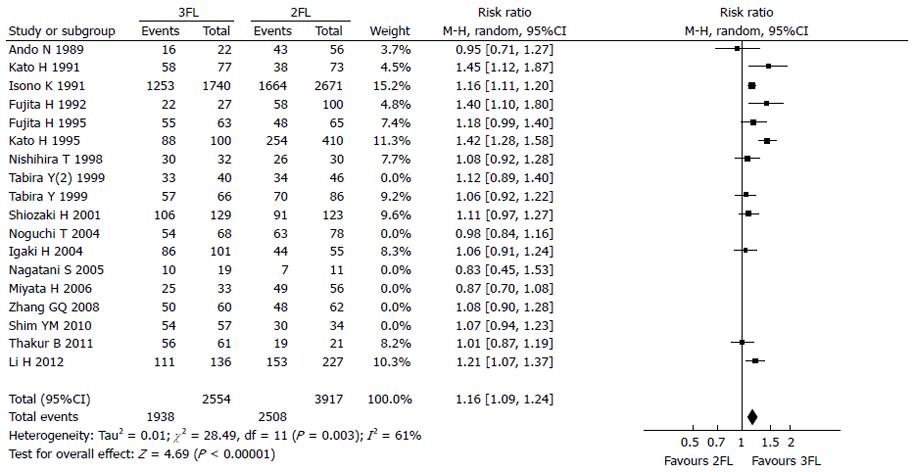
Twelve studies were used for 1-year OS rate analysis, including 2554 3FL patients and 3917 2FL patients. Only 5 studies reported a statistically significant difference between 2FL and 3FL, with a better OS rate in the 3FL group. Among studies with no statistical significance, 6 reported a higher 1-year OS rate in the 3FL group, whereas 1 reported a lower rate, which raised concerns regarding the significance of 3FL. Meta-analysis of all 12 studies showed a statistically significant difference between 3FL and 2FL, with a pooled RR of 1.16 (95%CI: 1.09-1.24; P < 0.00001; random-effects model) and statistical heterogeneity (P = 0.0003; I2 = 61%). 3FL showed a significant improvement in the 1-year OS rate.
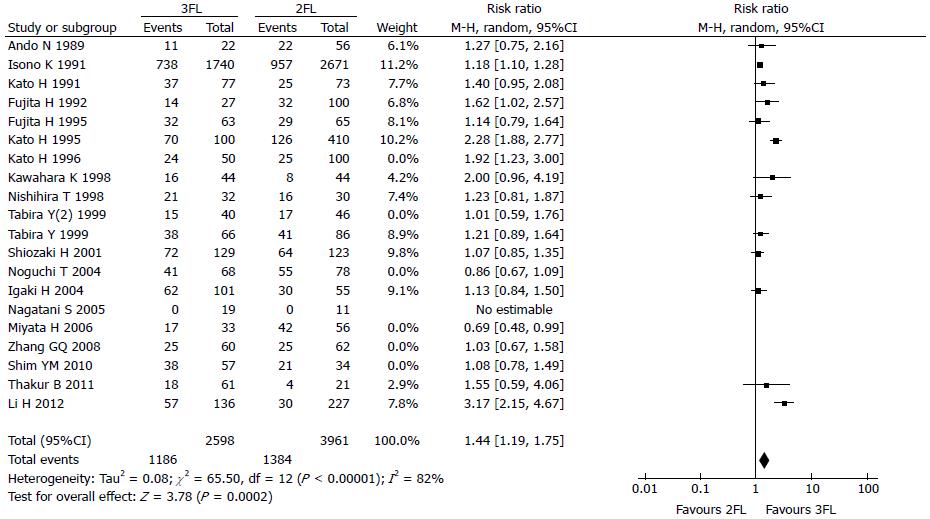
The 3-year OS rate presented in 13 studies included 2598 3FL patients and 3961 2FL patients. Four studies reported a statistically significant difference with a better OS rate in the 3FL group. Studies with no statistical significance reported a higher 3-year OS rate. Meta-analysis of all 13 studies showed statistically significant differences between 3FL and 2FL with a pooled RR of 1.44 (95%CI: 1.19-1.75, P < 0.00001, random-effects model) and statistical heterogeneity (P < 0.00001; I2 = 82%). 3FL showed a significantly higher 3-year OS rate.
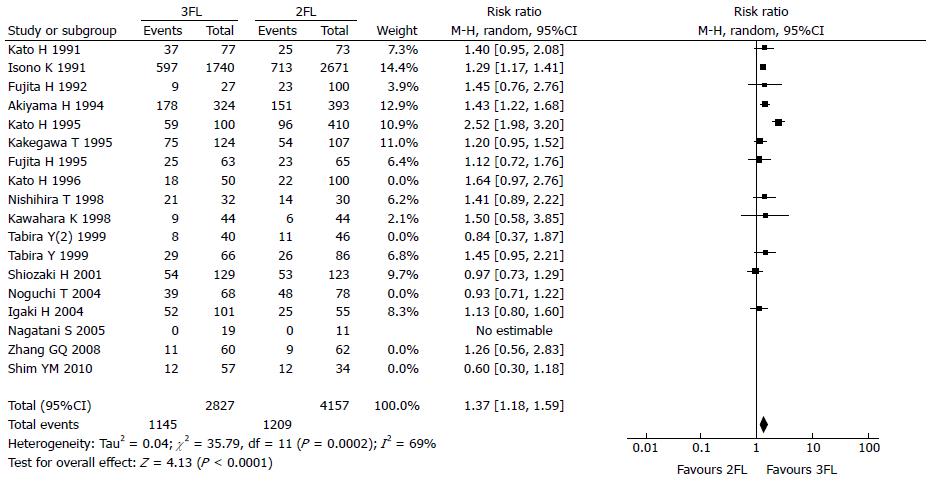
The 5-year OS rate reported in 12 studies included 2827 3FL patients and 4157 2FL patients. Only 3 studies reported a statistically significant difference with a better OS rate in the 3FL group. Among the 9 studies with no statistical significance, 8 reported a higher 5-year OS rate in the 3FL group, while 1 reported a lower rate. Meta-analysis of all 12 studies showed a statistically significant difference between 3FL and 2FL with a pooled RR of 1.37 (95%CI: 1.18-1.59; P = 0.0002; random-effects model) and statistical heterogeneity (P < 0.00001; I2 = 69%). 3FL showed a significant improvement in the 3-year OS rate. The forests plots are shown in Figure 2, Figure 3 and Figure 4.
After reviewing the postoperative complications, we included the 4 most common complications for analysis; these complications included recurrent nerve palsy, anastomosis leak, chylothorax, and pulmonary complications. The meta-analysis results from all studies demonstrated that 3FL was associated with more complications than 2FL with respect to anastomosis leakage and recurrent nerve palsy (Table 2). Chylothorax and pulmonary complications were not statistically significantly different between 3FL and 2FL.
| Complications | Studies | Participants | Fixed-effects model | Random-effects model | Tests of homogeneity | ||||||
| 3FL | 2FL | RR | 95%CI | RR | 95%CI | Q | df | P | I2(%) | ||
| Recurrent nerve palsy | 10 | 2320 | 3534 | 1.43 | 1.28 to 1.60 | 1.48 | 1.13-1.92 | 19.05 | 9 | 0.02 | 53 |
| Anastomosis leak | 10 | 608 | 926 | 1.26 | 1.05 to 1.52 | 1.32 | 0.97-1.81 | 14.53 | 9 | 0.09 | 38 |
| Pulmonary complications | 12 | 2370 | 3653 | 0.88 | 0.80 to 0.98 | 0.93 | 0.75-1.16 | 13.38 | 11 | 0.27 | 18 |
| Chylothorax | 6 | 458 | 699 | 0.77 | 0.32 to 1.85 | 0.87 | 0.33-2.26 | 3.05 | 5 | 0.69 | 0 |
There were insufficient data available for subgroup analysis; therefore, only data pertaining to recurrent laryngeal lymph node positivity/negativity were integrated for meta-analysis. Three studies were included in the positive group[10,17,18], including 107 3FL patients and 92 2FL patients. Meta-analysis of these 3 studies showed statistically significant differences between 3FL and 2FL because the OS rate in the 3FL group was superior with a pooled 1-year RR of 1.29 (95%CI: 1.08-1.53; P = 0.004; fixed-effects model) and statistical heterogeneity (P = 0.80, I2 = 0%), and a 3-year RR of 6.80 (95%CI: 2.99-15.46; P < 0.00001; fixed-effects model) and statistical heterogeneity (P = 0.98; I2 = 0%). For the negative group, 2 studies were analyzed[10,17], including 176 3FL patients and 271 2FL patients. Meta-analysis of the 2 studies showed statistically significant differences between 3FL and 2FL with a better OS rate in the 3FL group, a pooled 1-year RR of 1.14 (95%CI: 1.03-1.27; P = 0.01, fixed-effects model) with statistical heterogeneity (P = 0.86, I2 = 0%), and a 3-year RR of 1.92 (95%CI: 0.56-6.53; P = 0.30; random-effects model) with statistical heterogeneity (P = 0.003; I2 = 89%). For the other subgroups: (1) carcinoma in the upper or middle third esophagus had a survival advantage with 3FL[20,23,30]; (2) early superficial carcinoma confined to the mucosa had an equal OS rate between 3FL and 2FL[31]; and (3) poor prognostic subgroups had metastatic nodes in all 3 fields and the lower-third of tumors had positive cervical nodes with the involvement of ≥ 5 lymph nodes. The subgroups had equal OS rates between 3FL and 2FL[32].
Surgery for esophageal cancer includes removal of the primary lesion and lymph node dissection; however, there is a long-standing debate concerning application of 3FL, which was initiated at Chiba University (Chiba-shi, Japan) in 1983[25]. This method was based on the observation that the relapse rates at the cervical nodes were 30%-40%, which presented a significant obstacle in the improvement of surgical results[5,6]. After 1983, 3FL was widely employed in Japan, but not worldwide until recently because of lack of evidence. As mentioned above, esophagectomy is technically demanding, and few centers can recruit a sufficient number of patients to perform randomized clinical trials that can withstand scrutiny; thus, only 2 randomized trials to date have been published that compared 3FL with 2FL. One trial showed a survival advantage for 3FL; however, patients treated with 2FL were older and had more proximal tumors[8]. In the second trial, the 5-year OS rates were not statistically different between 3FL and 2FL (66.2% and 48%, respectively)[7]. These limited randomized trials were, however, insufficient to conclude that 3FL was advantageous. As there are many observational studies comparing 3FL and 2FL, we performed the present meta-analysis to synthesize data to yield more comprehensive and credible results.
Meta-analysis serves as a valuable tool for studying rare and unintended treatment effects and extends prior randomized and nonrandomized studies by permitting synthesis of data and providing more stable estimates of effects. To the best of our knowledge, this is the first meta-analysis of published studies to compare 3FL and 2FL for esophageal cancer and provide evidence for the comparison of OS, postoperative complications, and subgroups.
This meta-analysis brings together all currently available data from randomized trials and observational studies comparing 2FL and 3FL in esophageal carcinoma patients, thereby providing reliable assessment of the role of 3FL. Through this meta-analysis, we discussed the 3 queries mentioned above. First, regarding the effect of 3FL on the OS rate, the present study revealed that 3FL had a significant improvement in the 1-, 3-, and 5-year OS rates (RR = 1.16, 1.44, and 1.37, respectively). However, we questioned the credibility of the 3 aspects of these results. The first was the high degree heterogeneity of all the 3 outcomes. For meta-analysis of mostly observational studies, when outcome heterogeneity is particularly problematic, a single summary measure is likely inappropriate. Thus, evaluating heterogeneity becomes a key issue. Although we did our best to exclude articles that did not meet the selection criteria, the heterogeneity may be because of the following factors: (1) different types, stages, and locations of esophageal carcinoma in each observational study; (2) different patient ethnicities with different genotypes and proportion of tumor types; and (3) different institutions, surgeons with unequal skills, and different operative durations. However, the amount of information was not sufficient for stratifying or regression analysis. The second aspect was derived from theoretical justification. Although we could not perform 3FL analysis because of frequent recurrence in the cervical lymph nodes, 2 studies on recurrence patterns after esophagectomy reported recurrence rates of 7% and 1%, which were both significantly lower than the 30% incidence rate expected from cervical metastases[33,34]. Finally, on the basis of the funnel plot results, we concluded that publication bias occurred in all the 3 outcomes.
The second part of this analysis compared postoperative complications between 2FL and 3FL. Our results determined that 3FL had more complications than 2FL in anastomosis leak and recurrent nerve palsy, while the incidences of chylothorax and pulmonary complications were not significantly different. Heterogeneity was not high because less mixed factors may have affected the result. The result was much less controversial and presents an obvious disadvantage of 3FL.
Treatment complications are often detrimental. For example, recurrent laryngeal nerve palsy due to extensive dissection of the recurrent laryngeal nerve chain remains the main concern and can affect up to 70% of cases[20]. Recurrent laryngeal nerve palsy impedes not only immediate postoperative recovery but also long-term quality of life in terms of speech, swallowing, and respiratory functions.
For the last query, we identified patients who would likely benefit from 3FL. As previously mentioned, much current research has focused on identifying optimal patients through subgroup analysis. An important study that evaluated recurrent nerve nodal involvement in 86 3FL patients identified a relationship between thoracic recurrent nerve nodal involvement and cervical metastases. Only 11% of the 63 patients without thoracic recurrent nodal involvement had positive cervical nodes, in contrast to 43% of the 23 patients with recurrent thoracic nodal disease and positive cervical nodes. A “sentinel node” concept was proposed to guide the addition of cervical lymphadenectomy[35]. However, from our results, we could not conclude that 3FL benefited only positive, but not negative, cervical nodes based on the available data. As to the other subgroups, limited data were derived from published studies; thus, they were insufficient to draw definite conclusions.
The main purpose of the present meta-analysis was to present all available evidence in a systematic, quantitative, and unbiased fashion to establish the following 3 points: the effect of 3FL on the OS rate, identification of postoperative complications of 3FL compared to 2FL, and description of patient characteristics of those who will likely benefit from 3FL. However, because of limitations of the available data, only the second query was clearly answered. For the first query, we could only determine that 3FL had better 1-, 3-, and 5-year OS rates compared to 2FL, when all currently available data were integrated. However, the credibility of the results remains controversial. Clinicians can make treatment decisions based on this evidence and consultations with patients on their perceived treatment outcomes.
Debate about the application of 3-field lymphadenectomy (3FL) for esophageal cancer has been heated for a long time, because the advantages and disadvantages still remain controversial when compared to the traditional 2-field lymphadenectomy (2FL).
Over the decades, many observational studies comparing 3FL and 2FL have been performed to figure out whether 3FL was advantageous. Moreover, a few randomized trials were recently performed to investigate it. However, those are insufficient to reach a precise conclusion.
This is a meta-analysis of published studies to compare 3FL and 2FL for esophageal cancer and provide evidence for the comparison of overall survival, postoperative complications, and subgroups. Based on this meta-analysis, 3FL had better 1-, 3-, and 5-year overall survival rates compared to 2FL while 3FL had more complications than 2FL in anastomosis leak and recurrent nerve palsy.
3FL has better overall survival rates but more complications. Clinicians can make treatment decisions based on this evidence and consultations with patients on their acceptable treatment outcomes.
3FL is dissecting lymph node of the bilateral cervical, mediastinal, and abdominal regions, with the theoretical justification that relapse of cervical lymph node occur frequently.
The authors present a meta-analysis using published data on 3FL vs 2FL in esophageal carcinoma patients. End points of this meta-analysis were 1-, 3-, and 5-year overall survival rates and postoperative complications. This is an important clinical question and the results of this analysis will likely have an impact on clinical decisions in the future. The meta-analysis was conducted properly, objectively and the results are valid and significant. The conclusions of the manuscript are accurate, and supported by the data.
P- Reviewer: Amornyotin S, Dovat S, Shao R S- Editor: Yu J L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Daly JM, Fry WA, Little AG, Winchester DP, McKee RF, Stewart AK, Fremgen AM. Esophageal cancer: results of an American College of Surgeons Patient Care Evaluation Study. J Am Coll Surg. 2000;190:562-572; discussion 572-573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 342] [Cited by in F6Publishing: 378] [Article Influence: 15.8] [Reference Citation Analysis (1)] |
| 2. | Nakagawa S, Kanda T, Kosugi S, Ohashi M, Suzuki T, Hatakeyama K. Recurrence pattern of squamous cell carcinoma of the thoracic esophagus after extended radical esophagectomy with three-field lymphadenectomy. J Am Coll Surg. 2004;198:205-211. [PubMed] [Cited in This Article: ] |
| 3. | Isono K, Ochiai T, Okuyama K, Onoda S. The treatment of lymph node metastasis from esophageal cancer by extensive lymphadenectomy. Jpn J Surg. 1990;20:151-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Sannohe Y, Hiratsuka R, Doki K. Lymph node metastases in cancer of the thoracic esophagus. Am J Surg. 1981;141:216-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 71] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Isono K, Onoda S, Okuyama K, Sato H. Recurrence of intrathoracic esophageal cancer. Jpn J Clin Oncol. 1985;15:49-60. [PubMed] [Cited in This Article: ] |
| 6. | Isono K, Onoda S, Ishikawa T, Sato H, Nakayama K. Studies on the causes of deaths from esophageal carcinoma. Cancer. 1982;49:2173-2179. [PubMed] [Cited in This Article: ] |
| 7. | Nishihira T, Hirayama K, Mori S. A prospective randomized trial of extended cervical and superior mediastinal lymphadenectomy for carcinoma of the thoracic esophagus. Am J Surg. 1998;175:47-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 184] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 8. | Kato H, Watanabe H, Tachimori Y, Iizuka T. Evaluation of neck lymph node dissection for thoracic esophageal carcinoma. Ann Thorac Surg. 1991;51:931-935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 205] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Thakur B, Zhang CS, Meng XL, Bhaktaman S, Bhurtel S, Khakural P. Eight-year experience in esophageal cancer surgery. Indian J Cancer. 2011;48:34-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Li H, Yang S, Zhang Y, Xiang J, Chen H. Thoracic recurrent laryngeal lymph node metastases predict cervical node metastases and benefit from three-field dissection in selected patients with thoracic esophageal squamous cell carcinoma. J Surg Oncol. 2012;105:548-552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Zhang GQ, Han F, Sun W, Pang ZL, SiKanDaer AB, Wang HJ. [Impact of different extents of lymph node dissection on the survival in stage III esophageal cancer patients]. Zhonghua Zhongliu Zazhi. 2008;30:858-862. [PubMed] [Cited in This Article: ] |
| 12. | Shim YM, Kim HK, Kim K. Comparison of survival and recurrence pattern between two-field and three-field lymph node dissections for upper thoracic esophageal squamous cell carcinoma. J Thorac Oncol. 2010;5:707-712. [PubMed] [Cited in This Article: ] |
| 13. | Igaki H, Tachimori Y, Kato H. Improved survival for patients with upper and/or middle mediastinal lymph node metastasis of squamous cell carcinoma of the lower thoracic esophagus treated with 3-field dissection. Ann Surg. 2004;239:483-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Noguchi T, Wada S, Takeno S, Hashimoto T, Moriyama H, Uchida Y. Two-step three-field lymph node dissection is beneficial for thoracic esophageal carcinoma. Dis Esophagus. 2004;17:27-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Hagry O, Coosemans W, De Leyn P, Nafteux P, Van Raemdonck D, Van Cutsem E, Hausterman K, Lerut T. Effects of preoperative chemoradiotherapy on postsurgical morbidity and mortality in cT3-4 +/- cM1lymph cancer of the oesophagus and gastro-oesophageal junction. Eur J Cardiothorac Surg. 2003;24:179-186; discussion 186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Gradauskas P, Rubikas R, Petrauskas V. [Early results of esophageal resections, performed due to carcinoma]. Medicina (Kaunas). 2002;38 Suppl 2:61-64. [PubMed] [Cited in This Article: ] |
| 17. | Shiozaki H, Yano M, Tsujinaka T, Inoue M, Tamura S, Doki Y, Yasuda T, Fujiwara Y, Monden M. Lymph node metastasis along the recurrent nerve chain is an indication for cervical lymph node dissection in thoracic esophageal cancer. Dis Esophagus. 2001;14:191-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Tabira Y, Kitamura N, Yoshioka M, Tanaka M, Nakano K, Toyota N, Mori T. Significance of three-field lymphadenectomy for carcinoma of the thoracic esophagus based on depth of tumor infiltration, lymph nodal involvement and survival rate. J Cardiovasc Surg (Torino). 1999;40:737-740. [PubMed] [Cited in This Article: ] |
| 19. | Kawahara K, Maekawa T, Okabayashi K, Shiraishi T, Yoshinaga Y, Yoneda S, Hideshima T, Shirakusa T. The number of lymph node metastases influences survival in esophageal cancer. J Surg Oncol. 1998;67:160-163. [PubMed] [Cited in This Article: ] |
| 20. | Fujita H, Kakegawa T, Yamana H, Shima I, Toh Y, Tomita Y, Fujii T, Yamasaki K, Higaki K, Noake T. Mortality and morbidity rates, postoperative course, quality of life, and prognosis after extended radical lymphadenectomy for esophageal cancer. Comparison of three-field lymphadenectomy with two-field lymphadenectomy. Ann Surg. 1995;222:654-662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 298] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 21. | Kakegawa T, Yamana H. [Progress in surgical treatment of carcinoma of the intrathoracic esophagus]. Gan To Kagaku Ryoho. 1995;22:855-862. [PubMed] [Cited in This Article: ] |
| 22. | Kato H. Lymph node dissection for thoracic esophageal carcinoma. Two- and 3-field lymph node dissection. Ann Chir Gynaecol. 1995;84:193-199. [PubMed] [Cited in This Article: ] |
| 23. | Akiyama H, Tsurumaru M, Udagawa H, Kajiyama Y. Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg. 1994;220:364-372; discussion 372-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 691] [Cited by in F6Publishing: 620] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 24. | Fujita H, Kakegawa T, Yamana H, Shima I, Rikitake H, Hyodo M, Yokoyama T, Fujii T, Toh U, Tsugane S. Cervico-thoraco-abdominal (3-field) lymph node dissection for carcinoma in the thoracic esophagus. Kurume Med J. 1992;39:167-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Isono K, Sato H, Nakayama K. Results of a nationwide study on the three-field lymph node dissection of esophageal cancer. Oncology. 1991;48:411-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 359] [Cited by in F6Publishing: 373] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 26. | Ando N, Shinozawa Y, Kikunaga H, Koyama Y, Nagashima A, Osaku M, Abe O. [An assessment of extended lymphadenectomy including cervical node dissection for cancer of the thoracic esophagus]. Nihon Geka Gakkai Zasshi. 1989;90:1616-1618. [PubMed] [Cited in This Article: ] |
| 27. | Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008-2012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14425] [Cited by in F6Publishing: 15643] [Article Influence: 651.8] [Reference Citation Analysis (0)] |
| 28. | Jüni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA. 1999;282:1054-1060. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1390] [Cited by in F6Publishing: 1339] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 29. | Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719-748. [PubMed] [Cited in This Article: ] |
| 30. | Löhlein D. [Esophageal carcinoma: surgical treatment concepts; access and resectability]. Schweiz Med Wochenschr. 1999;129:1211-1216. [PubMed] [Cited in This Article: ] |
| 31. | Nishimaki T, Tanaka O, Suzuki T, Aizawa K, Watanabe H, Muto T. Tumor spread in superficial esophageal cancer: histopathologic basis for rational surgical treatment. World J Surg. 1993;17:766-771; discussion 771-772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 55] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Nishimaki T, Suzuki T, Suzuki S, Kuwabara S, Hatakeyama K. Outcomes of extended radical esophagectomy for thoracic esophageal cancer. J Am Coll Surg. 1998;186:306-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 126] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 33. | Law SY, Fok M, Wong J. Pattern of recurrence after oesophageal resection for cancer: clinical implications. Br J Surg. 1996;83:107-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 116] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Dresner SM, Wayman J, Shenfine J, Harris A, Hayes N, Griffin SM. Pattern of recurrence following subtotal oesophagectomy with two field lymphadenectomy. Br J Surg. 2000;87:362-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 169] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 35. | Tabira Y, Yasunaga M, Tanaka M, Nakano K, Sakaguchi T, Nagamoto N, Ogi S, Kitamura N. Recurrent nerve nodal involvement is associated with cervical nodal metastasis in thoracic esophageal carcinoma. J Am Coll Surg. 2000;191:232-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |