Published online Dec 21, 2014. doi: 10.3748/wjg.v20.i47.17993
Revised: April 3, 2014
Accepted: July 15, 2014
Published online: December 21, 2014
AIM: To compare the interpretation of probe-based confocal laser endomicroscopy (pCLE) findings between endoscopists and gastrointestinal (GI)-pathologists.
METHODS: All pCLE procedures were undertaken and the endoscopist rendered assessment. The same pCLE videos were then viewed offline by an expert GI pathologist. Histopathology was considered the gold standard for definitive diagnosis. The sensitivity, specificity and accuracy for diagnosis of dysplastic/ neoplastic GI lesions and interobserver agreement between endoscopists and experienced gastrointestinal pathologist for pCLE findings were analyzed.
RESULTS: Of the 66 included patients, 40 (60.6%) had lesions in the esophagus, 7 (10.6%) in the stomach, 15 (22.7%) in the biliary tract, 3 (4.5%) in the ampulla and 1 (1.5%) in the colon. The overall sensitivity, specificity and accuracy for diagnosing dysplastic/neoplastic lesions using pCLE were higher for endoscopists than pathologist at 87.0% vs 69.6%, 80.0% vs 40.0% and 84.8% vs 60.6% (P = 0.0003), respectively. Area under the ROC curve (AUC) was greater for endoscopists than the pathologist (0.83 vs 0.55, P = 0.0001). Overall agreement between endoscopists and pathologist was moderate for all GI lesions (K = 0.43; 95%CI: 0.26-0.61), luminal lesions (K = 0.40; 95%CI: 0.20-0.60) and those of dysplastic/neoplastic pathology (K = 0.55; 95%CI: 0.37-0.72), the agreement was poor for benign (K = 0.13; 95%CI: -0.097-0.36) and pancreaticobiliary lesions (K = 0.19; 95%CI: -0.26-0.63).
CONCLUSION: There is a wide discrepancy in the interpretation of pCLE findings between endoscopists and pathologist, particularly for benign and malignant pancreaticobiliary lesions. Further studies are needed to identify the cause of this poor agreement.
Core tip: Probe-based confocal endomicroscopy (pCLE) has emerged as a valuable tool in the diagnosis and management of gastrointestinal disorders. It has helped the endoscopist to make real time decisions and targeted biopsies. Histopathology still remains the gold standard. We compared the interpretation of pCLE findings between an endoscopist and a dedicated gastrointestinal pathologist and found there was a discrepency in the intepretation of the same findings between them. This is interesting as the endoscopist has a different approach of intepreting real time endomicroscopy compared to that of a pathologist.
- Citation: Peter S, Council L, Bang JY, Neumann H, Mönkemüller K, Varadarajulu S, Wilcox CM. Poor agreement between endoscopists and gastrointestinal pathologists for the interpretation of probe-based confocal laser endomicroscopy findings. World J Gastroenterol 2014; 20(47): 17993-18000
- URL: https://www.wjgnet.com/1007-9327/full/v20/i47/17993.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i47.17993
Endoscopic tissue sampling with histopathology is considered the gold standard for diagnosis and management of most gastrointestinal (GI) disorders. The differentiation between benign and malignant lesions is vital to further management. Even though random biopsies are considered the norm, they are also involved in flaws such as sampling errors along with an incremental cost that may be incurred[1,2]. Confocal laser endoscopy (CLE) is a new endoscopic technology developed to obtain high resolution images of the gastrointestinal mucosa allowing in vivo and real time endomicroscopic analysis of the targeted tissue[3-5]. It enables differentiation between malignant and benign lesions crucial for clinical decision making. Based on defined criteria, the interpreter is able to make distinguishing decisions on the nature of the lesion for subsequent therapy[6].
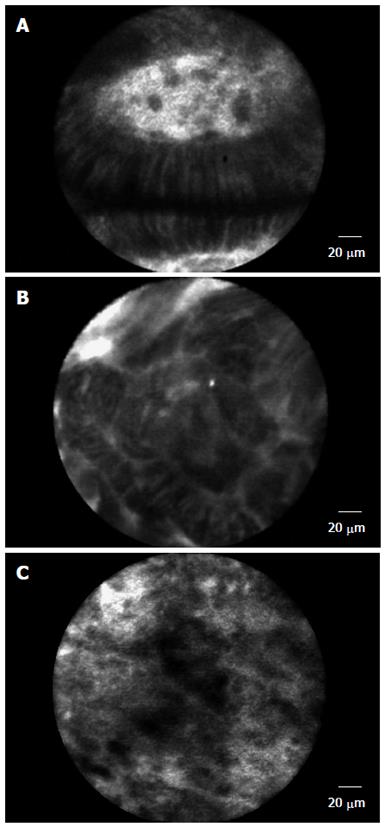
The principle of CLE is based on tissue reflectance or tissue fluorescence after application of fluorescence agents (e.g., fluorescein sodium) generating images that demonstrate cellular architecture and microvasculature that is comparable with traditional histology. Several studies have shown the usefulness of this technology in determining pathology in a wide range of GI tissue sites such as Barrett’s esophagus (BE) (Figure 1), duodenum, colonic mucosa and pancreatic biliary lesions[7-16]. From these studies it has been shown that CLE can be performed and interpreted accurately after adequate training. However, it is still not clear what the learning curve for adequate diagnosis and interpretation using this new technology will be in predicting better outcomes. Understanding this will have long term effects on operating costs while enhancing the benefit to the patient.
In this context, we postulated that an endoscopist had the capability of real time imaging while a pathologist would have the inherent advantage of histopathological cellular differentiation. We therefore aimed to evaluate the differences in interpretation of probe-based CLE (pCLE) findings between them.
Consecutive patients undergoing endoscopy with pCLE at a tertiary medical center were identified. Eligibility for this study consisted of an indication for endoscopy with pCLE such as Barrett esophagus, undetermined gastric or colonic polyps, ampullary neoplasms or bile duct stricture. Exclusion criteria were the following: age < 18 years, inability to give written informed consent, coagulopathy, renal failure or known allergy to fluorescein sodium. The study was approved by the Institutional Review Board of the University of Alabama at Birmingham (UAB).
Patients underwent endoscopy (GIF; Olympus America, Center Valley, PA, United States) and were followed by pCLE (Cellvizio; Mauna Kea Technologies, Paris, France). pCLE was performed using 3 different probes appropriate for the area of pathology studied: (1) GastroFlex UHD; (2) CholangioFlex UHD; and (3) ColoFlex UHD. The probe (diameter 2.5 mm) was inserted through the accessory channel and gently approached to the mucosa as previously descripted[5]. The depth of imaging was 40-70 mm for CholangioFlex probes, and 55-65 mm for GastroFlex and ColoFlex probes. The maximal field of view is 325 mm for Cholangio-Flex probes, 600 mm for GastroFlex and ColoFlex probes, and 240 mm for GastroFlex UHD and ColoFlex UHD. The lateral resolution is 3.5 mm for CholangioFlex and 1 mm for GastroFlex UHD and ColoFlex UHD. The images were scanned with a rate of 12 frames per second, hence demonstrating a real-time video on a second screen that is positioned next to the endoscopy monitor. For tissue contrast, 5 mL intravenous fluorescein (10%; Alcon Pharma, Novartis, E Hanover, NJ, United States) was used, which has been shown to be safe in ophthalmology and previous CLE studies[17].
Real time pCLE interpretation was rendered for all lesions after endoscopic evaluation of the area and lesion and images were stored as video sequences as well as images. pCLE image interpretation was performed according to updated Miami criteria for pCLE[6]. The results were recorded in Excel worksheets. Subsequent biopsies were taken from all studied areas; these were collected after detection of the lesion, after interpretation of the image via pCLE. Histologic samples were processed by using standard procedures and evaluated by an expert pathologist specialized in gastroenterology. Biopsies were classified at histology according to the type of epithelium (inflammatory or hyperplastic polyp, tubular, tubulovillous, or villous adenoma) and degree of dysplasia (none, low-grade, high-grade, or cancer) according to the updated Vienna criteria for the diagnosis of GI neoplasia, omitting the category moderate dysplasia[18]. The histologic slides were separately reviewed by a dedicated gastrointestinal pathologist. Histology was considered the gold standard for diagnosis.
Learning phase: In order to standardize image interpretation the gastroenterologists (and the GI pathologist underwent special training with formal certification in defining criteria of lesions using pCLE according to updated Miami classification[6]. Therefore, a total of 20 videos and image sets were used to train the participants using a standardized training set from Cellvizio, Mauna Kea Technologies. Some of the topics of the videos included lesions from the esophagus e.g., Barrett’s (normal or neoplastic), colonic lesions (hyperplastic vs neoplastic) or pancreaticobiliary lesions. The training included didactic teaching involving approximately 6 h and culminating in an exam format. All endoscopists were naïve to this tool similarly the pathologist had no previous experience.
Practice phase: The three endoscopists performed a total of 70 cases of pCLE during the study period.
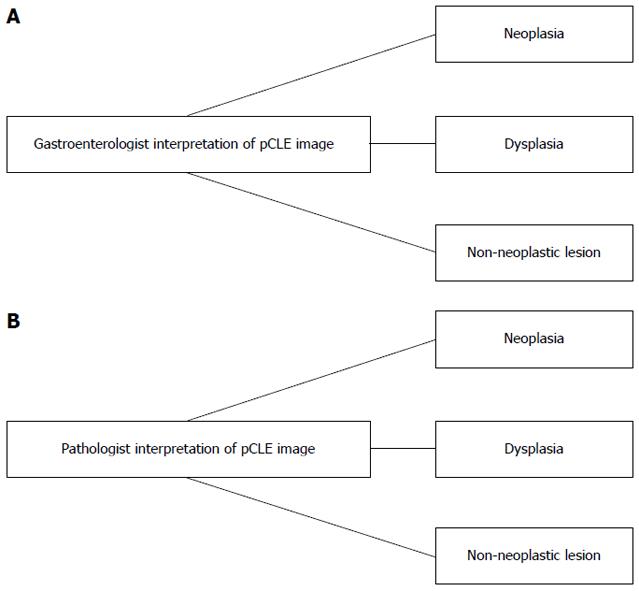
Study phase: Image selection and image evaluation was done and a total of 70 video clips with images of consecutive patients were selected. First, the endoscopist rated the histological diagnosis based on pCLE findings and results were entered in Excel worksheets. Accordingly, the pCLE videos were viewed offline by the expert GI pathologist who was blinded to the image interpretation of the endoscopist. The videos were defined as “benign” or “dysplasia/neoplasia” if they contained dysplasia or cancer. From the selected consecutive pCLE videos, the overall impression of the interpreters was evaluated for any of the above changes (Figure 2). If images were rendered not clear or difficult to interpret, this was also included in the analysis.
Data from the study were entered into a Microsoft Excel (Microsoft Corporation, Washington, United States) spreadsheet. Information from the GI physicians as well as the GI pathologist was entered onto a case report form and further entered into the Excel database. The accuracy, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for prediction of benign/normal vs dysplasia/neoplasia was calculated between them. Interobserver variability was calculated using the K statistic and results based on this were defined as: poor < 0.2, fair 0.21-0.4, moderate 0.41-0.6, substantial 0.61- 0.8 and excellent 0.81-1[19]. Statistical analysis was performed using SAS 9.2 (SAS Institute, Cary, NC, United States).
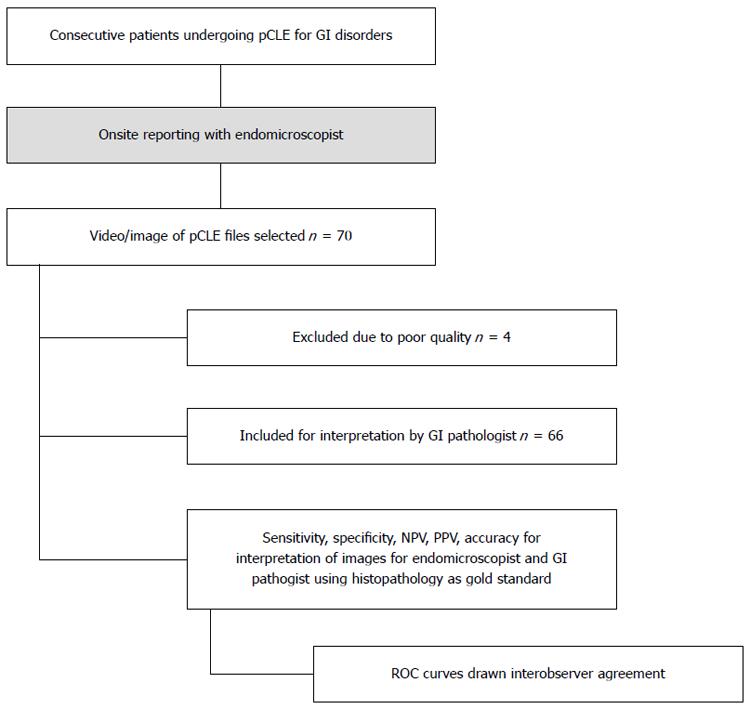
A total of 70 consecutive patients undergoing pCLE imaging were included in the study, 4, patients were excluded because of incomplete information or poor image files and quality (Figure 3). Sixty-six patients were finally included, male 66.7%, female 33.3%, mean age were 60.3 ± 13.6 years. Majority of patients underwent pCLE for determination of indeterminate lesions 34 (51.5%) and surveillance 18 (27.3%) (Table 1). Of 66 patients, 40 (60.6%) had lesions in the esophagus, 7 (10.6%) in the stomach, 15 (22.7%) in the biliary tract, 3 (4.5%) in the ampulla and 1 (1.5%) in the colon (Table 1). The pathologies included Barrett’s esophagus, colonic and gastric polyps with indeterminate pre-pCLE diagnosis, indeterminate biliary strictures, and ampullary lesions.
| Characteristics | |||
| Age (yr) | Mean ± SD | 60.3 ± 13.6 | |
| Median | 61.5 | ||
| IQR | 51-71 | ||
| Range | 17-86 | ||
| Gender | Female | 22 (33.3) | |
| Male | 44 (66.7) | ||
| Race | Black | 7 (10.6) | |
| White | 54 (81.8) | ||
| Other | 5 (7.6) | ||
| Lesion type | Esophagus | All | 40 (60.6) |
| Normal/Benign | 12 (30) | ||
| LGD | 13 (32.5) | ||
| HGD | 8 (20) | ||
| Neoplastic | 7 (17.5) | ||
| Gastric | All | 7 (10.6) | |
| Normal/Benign | 2 (28.6) | ||
| LGD | 1 (14.3) | ||
| HGD | 2 (28.6) | ||
| Neoplastic | 2 (28.6) | ||
| Colonic | All | 1 (1.5) | |
| Normal/Benign | 0 | ||
| LGD | 0 | ||
| HGD | 1 (100) | ||
| Neoplastic | 0 | ||
| Ampulla | All | 3 (4.5) | |
| Normal/Benign | 2 (66.7) | ||
| LGD | 0 | ||
| HGD | 1 (33.3) | ||
| Neoplastic | 0 | ||
| Pancreaticobiliary | All | 15 (22.7) | |
| Normal/Benign | 4 (26.7) | ||
| LGD | 0 | ||
| HGD | 1 (6.7) | ||
| Neoplastic | 10 (66.7) | ||
| Indication for pCLE | Indeterminate lesion | 34 (51.5) | |
| Surveillance | 18 (27.3) | ||
| Targeted biopsy ± therapy | 13 (19.7) | ||
| Other | 1 (1.5) |
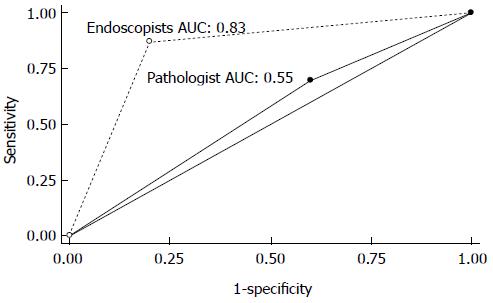
The overall sensitivity, specificity and accuracy for diagnosing dysplastic/neoplastic lesions using pCLE were higher for endoscopists than pathologist at 87.0% vs 69.6%, 80.0% vs 40.0% and 84.8% vs 60.6% (P = 0.0003), respectively. For luminal lesions (esophagus, stomach and colon) they were 82.4% vs 64.7%, 92.9% vs 42.9% and 85.4% vs 58.3%. For ampullary and pancreaticobiliary lesions the results were 100% vs 83.3%, 50% vs 33.3% and 100% vs 50% (Table 2). Also, the area under the ROC curve (AUC) was greater for endoscopists than the pathologist (0.83 vs 0.55, P = 0.0001) (Figure 4).
| Lesion site | Sensitivity (95%CI) | Specificity (95%CI) | PPV(95%CI) | NPV(95%CI) | Accuracy (95%CI) | |
| Endoscopist | All lesion types | 87.0 | 80.0 | 90.9 | 72.7 | 84.8 |
| (77.2-96.7) | (62.4-97.5) | (82.4-99.4) | (54.1-91.3) | (76.2-93.5) | ||
| Luminal1 | 82.4 | 92.9 | 96.6 | 68.4 | 85.4 | |
| (69.5-95.2) | (66.1-99.8) | (82.2-99.9) | (47.5-89.3) | (75.4-95.4) | ||
| Ampulla/PB | 100 | 50.0 | 80.0 | 100 | 83.3 | |
| (73.5-100) | (11.8-88.2) | (51.9-95.7) | (29.2-100) | (58.6-96.4) | ||
| Pathologist | All lesion types | 69.6 | 40.0 | 72.7 | 36.4 | 60.6 |
| (56.3-82.9) | (18.5-61.5) | (59.6-85.9) | (16.3-56.5) | (48.8-72.4) | ||
| Luminal1 | 64.7 | 42.9 | 73.3 | 33.3 | 58.3 | |
| (48.6-80.8) | (16.9-68.8) | (57.5-89.2) | (11.6-55.1) | (44.4-72.2) | ||
| Ampulla/PB | 83.3 | 33.3 | 71.4 | 50.0 | 66.7 | |
| (51.6-97.9) | (4.3-77.7) | (41.9-91.6) | (6.8-93.2) | (41.0-86.7) | ||
| P value for overall rates: | - | - | - | - | < 0.0012 | |
| P value for luminal lesions: | - | - | - | - | < 0.0012 | |
| P value for PB lesions: | - | - | - | - | 0. 3752 | |
While the overall agreement between endoscopists and pathologist was moderate for all GI lesions (K = 0.43; 95%CI: 0.26-0.61), luminal lesions (K = 0.40; 95%CI: 0.20-0.60) and those of dysplastic/neoplastic pathology (K = 0.55; 95%CI: 0.37-0.72), the agreement was poor for benign (K = 0.13; 95%CI: -0.097-0.36) and pancreatico-biliary lesions (K = 0.19; 95%CI: -0.26-0.63) (Table 3).
| Agreement | kappa | P value | ||
| Overall (all lesion types) | 73.7% | 0.43 | < 0.001 | |
| Lesion site: | Luminal | 75.0% | 0.40 | < 0.001 |
| Ampulla/PB | 70.4% | 0.19 | 0.191 | |
| Degree of abnormality | Benign | 60.0% | 0.13 | 0.102 |
| Dysplasia | 84.0% | 0.58 | < 0.001 | |
| Neoplasia | 73.7% | 0.07 | 0.114 | |
| Dysplasia/neoplasia combined | 79.7% | 0.55 | < 0.001 |
In this study we found that there was inconsistent agreement between endoscopists and an experienced GI pathologist for the diagnosis of GI lesions using pCLE. While the agreement for the diagnosis of dysplastic or malignant lesions was moderate the concordance for benign lesions was suboptimal.
There are several potential reasons that may explain these findings. It could be argued that endoscopists have more practice reading endomicroscopy. This assumption is unlikely, as pCLE is a relatively new technology and its interpretation requires knowledge of microstructural (i.e., pathological) changes. We hypothesized that the pathologist will have the “natural” advantage of understanding the cell structure given their expertise in cyto-pathological interpretation. This is one of the aspects that make our study important, as we have shown that interpretation of images is not solely based on ultra-structural knowledge. In addition, the interpretation is not only based on still photos, but relies on video sequences as well. This could be a challenge for a pathologist, who loses the ability to “control” the slide specimen. Therefore, our study has clinical implications as it emphasizes the necessity to implement and focus on training in interpretation of pCLE images and videos.
A crucial aspect of standard pathology is the ability to archive tissue and slides for future re-analysis and processing. With pCLE there is also a possibility of storing the “specimen” (i.e., imaged sequences) for future analysis. This feature is of paramount importance as often a clear-cut diagnosis will not be established during live endoscopy and review of the data will clarify or allow the clinician to reach a diagnosis. It is also possible that the endoscopist has an inherent advantage of achieving a diagnosis while performing the endoscopy. In clinical practice there is usually a flood of clinical, laboratory and radiological data that aids the physician in reaching a diagnosis. In addition, during endoscopy, there are additional features observed that can improve the diagnostic yield. It could be argued that endoscopists had an advantage as they were performing doing regular endomicroscopy and thus had more skills to interpret the images when confronted with them during the study phase. In contrast, the GI pathologist could be considered relatively a “non-expert” given the experience with documented didactic training on confocal endomicroscopy and had no onsite endoscopic real time expertise. Of note, we did not try to replace their individual potential roles however aimed to see how best these results were in relevance to enhance the overall reading performance of confocal endomicroscopy and in effect reduce the overall biopsy burden by specific targeting of relevant areas. We theorized that such an analysis would help in tailoring and understanding their specific roles within the multidisciplinary approach to further treatment.
Also, in our study we also wanted to reflect real practice and therefore selected to have a wide variety of pathologies to review. Thus, our study was unique such that images designated and studied were well dispersed among a varied spectrum of disorders including benign to malignant gastrointestinal lesions such as Barrett’s esophagus, gastric polyps, colonic polyp, ampulla tumors and intra-biliary indeterminate strictures.
From the results, the endomicroscopists scored significantly better in overall interpretation of lesions than the pathologist with higher accuracy, sensitivity and specificity. It seems to suggest that despite the advantage of having cellular interpretation the pathologist wasn’t good for interpreting confocal images. This could be related to the tangential view as to how the cells are oriented while imaging with the probe base laser device as compared to routine cross sectional microscopy for routine histopathology. Secondly, the specific cell HE staining for cellular morphology is not possible during endomicroscopy. Thirdly, there is a disadvantage of not able to visualize these in real - time with close details of the lesions within sight which would have resulted in higher accuracy reported in the endomicroscopy group.
In a previous study Dunbar et al[20] evaluated whether the combined use of CLE and pathology improved the diagnostic yield of Barrett esophagus-associated neoplasia as compared to standard endoscopy with four-quadrant biopsy protocol. Although the authors found that targeted biopsy using CLE reduces the amount of biopsies needed to make a diagnosis and significantly improved the diagnostic yield for endoscopically in and apparent BE neoplasia, the overall k value for all participants was also moderate at 0.56 (95%CI: 0.50-0.62). Their study aimed primarily at evaluating the utility of CLE for predicting mucosal histopathology. They could not assess accuracy because mucosal biopsy was not routinely performed during the CLE procedure and in vivo CLE imaging did not show HGD or cancer.
In another study by Gaddam et al[21] predefined pCLE criteria were tested to evaluate the difference in interpretation of Barrett’s esophagus between experts and non-experts consisting of all gastroenterologists. They found that the accuracy in diagnosing dysplasia was 81.5 % (95%CI: 77.5%-81%), with no difference between experts vs non-experts k = 0.61 (0.53-0.69), suggesting that both groups could interpret these images after a short learning curve. Based on the results from our study we believe that the skills to learn pCLE may be acquired more slowly and depend on real-life endoscopy. The endoscopist has the benefit to control the scope, the angles of visualization and the knowledge of what they saw. Also the pathologist did not view the endoscopic images enabling the endoscopist to have more elements for the diagnosis as mentioned. These findings are of additional importance as it would imply that regular experience of live cases or continuous exposure to pCLE would be important for a pathologist to acquire more skills in the interpretation of images. The availability of images and/or clips could enhance the pathologist’s ability to make a specific diagnosis. This demonstrates the importance of joint collaboration and work of endoscopists and GI pathologists. It is well known that close collaboration during EUS-guided fine needle aspiration (FNA) and on-site interpretation of cytological specimens improves the diagnostic accuracy. This concept may need to be applied to the training phase of CLE, thus inviting the pathologist to participate during live endoscopy and acquire skills in CLE.
We want to acknowledge potential limitations of our study. Firstly, the images were not selected and subsequently blinded for interpretation. This was because we wanted to extrapolate or replicate a real time endomicroscopy scenario whereby onsite interpretation and diagnosis was done live by the endomicroscopist with further biopsies sent to the pathologist. Nevertheless, the final histopathology reports were blinded till the end of the study of the study for final image reporting comparison and analysis of reporting groups. Secondly, we did not analyze differences in different criteria based on glandular structures or microvasculature of lesions as we wanted the interpretation to be simple as benign vs dysplastic/ neoplastic, also the fact there is a variation in interpretation between grades of dysplasia and neoplasia for routine histopathological samples. Thirdly, we did not aim to look at differences between the endomicroscopists (i.e., interobserver agreement) rather compare this group with a pathologist as we recognized each had a different role and approach to interpretation and therefore were interested in bringing this out in this study. This is the topic for further, ongoing studies. Also we acknowledge there was heterogeneity in the lesions that were studied, however it seem to reflect the pCLE application in real time clinical practice. Lastly we included one single pathologist for the study and one might argue that the accuracy interpretation results might be improved with more expert GI pathologists. We acknowledge this as a major limitation as assessment made by a single pathologist without a consolidated experience may be affected by subjective elements and therefore, the data has little value as indicator and cannot be generalized.
In summary, from our study we are able to show that reporting can be done substantially between both the gastroenterologist and the pathologist. However, there seems to be discrepancy in the interpretation of pCLE findings between them particularly for benign and pancreaticobiliary lesions. Given the unique roles of both the endomicroscopist and the pathologist, it will be interesting to see if combining them helps in improving the overall accuracy of pCLE interpretation. Further studies are needed to identify how well endomicoscopic and histopathological criteria can be molded together by merging both distinguishable features. This will be relevant especially in situations when results are indeterminate or the degree of dysplasia vs neoplasia is unclear.
Endoscopic tissue sampling with histopathology is considered the gold standard for diagnosis and management of most gastrointestinal (GI) disorders. Even though random biopsies are considered the norm, they are also involved in flaws such as sampling errors along with an incremental cost that may be incurred.
Newer imaging technology such as the confocal laser endoscopy (CLE) has been developed to obtain high resolution images of the gastrointestinal mucosa allowing in vivo and real time endomicroscopic analysis of the targeted tissue.
Studies have shown that CLE can be performed and interpreted accurately after adequate training. However, it is still not clear what the learning curve for adequate diagnosis and interpretation using this new technology will be in predicting better outcomes. Understanding this will have long term effects on operating costs while enhancing the benefit to the patients.
This paper aims to compare the interpretation of CLE findings between endoscopists and GI pathologists, in order to achieve a better diagnostic reproducibility and confirmation of interpretation of in vivo images in situations where results are indeterminate or the degree of dysplasia vs neoplasia is unclear. The study is broad and covers much different pathology, not choosing to focus on specific pathologic locations or lesions but assessing the sensitivity, specificity and accuracy of confocal microscopy for endoscopists and pathologists, then comparing those rates. Endomicroscopy probe-based CLE (pCLE) procedures were undertaken and the endoscopist rendered assessment. And then images viewed offline by a GI pathologist. Using histopathology as a gold standard for definitive diagnosis the study showed that the endoscopist was able to better define the nature of the lesion in real time compared with the pathologist.
The study brings to light the “real world” differences one might encounter with the interpretation of images using the confocal laser pCLE technology if the pathologist is not present in the room during the procedure. This is an initial study and further, larger studies are needed to clarify the sensitivity and specificity of this technology in specific lesions and pathologies based upon the results.
CLE is based on tissue reflectance or tissue fluorescence after application of fluorescence agents (e.g., fluorescein sodium) generating images that demonstrate cellular architecture and microvasculature that is comparable with traditional histology. The pCLE is the device consisting of several fiber light bundles (> 10000 optical fibers) with distal lens through which the laser beam is transmitted while being connected to a laser-scanning unit and light source. This is passed through the working channel of the endoscope and can be approximated to the targeted mucosal area in the gastrointestinal tract for further visualization.
The authors have conducted a well thought out study bringing to light the “real world” differences one might encounter with the interpretation of images using the confocal laser pCLE. The topic is of potential interest in clinical practice to define and improve the outcome in endoscopic diagnosis. The paper is only focused on the analysis of the differences in interpretation by the two specialists. Although the study is planned so correct enough, it contains limitations that make the paper a pilot study.
P- Reviewer: Feuerstadt P, Morini S S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
| 1. | Sharma P, Montgomery E. Gastrointestinal dysplasia. Pathology. 2013;45:273-285. [PubMed] [Cited in This Article: ] |
| 2. | Calhoun BC, Gomes F, Robert ME, Jain D. Sampling error in the standard evaluation of endoscopic colonic biopsies. Am J Surg Pathol. 2003;27:254-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Kiesslich R, Gossner L, Goetz M, Dahlmann A, Vieth M, Stolte M, Hoffman A, Jung M, Nafe B, Galle PR. In vivo histology of Barrett’s esophagus and associated neoplasia by confocal laser endomicroscopy. Clin Gastroenterol Hepatol. 2006;4:979-987. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 362] [Cited by in F6Publishing: 385] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 4. | Kiesslich R, Goetz M, Vieth M, Galle PR, Neurath MF. Confocal laser endomicroscopy. Gastrointest Endosc Clin N Am. 2005;15:715-731. [PubMed] [Cited in This Article: ] |
| 5. | Neumann H, Kiesslich R, Wallace MB, Neurath MF. Confocal laser endomicroscopy: technical advances and clinical applications. Gastroenterology. 2010;139:388-392, 392.e1-e2. [PubMed] [Cited in This Article: ] |
| 6. | Wallace M, Lauwers GY, Chen Y, Dekker E, Fockens P, Sharma P, Meining A. Miami classification for probe-based confocal laser endomicroscopy. Endoscopy. 2011;43:882-891. [PubMed] [Cited in This Article: ] |
| 7. | Bertani H, Frazzoni M, Dabizzi E, Pigò F, Losi L, Manno M, Manta R, Bassotti G, Conigliaro R. Improved detection of incident dysplasia by probe-based confocal laser endomicroscopy in a Barrett’s esophagus surveillance program. Dig Dis Sci. 2013;58:188-193. [PubMed] [Cited in This Article: ] |
| 8. | Pittayanon R, Rerknimitr R. Role of digital chromoendoscopy and confocal laser endomicroscopy for gastric intestinal metaplasia and cancer surveillance. World J Gastrointest Endosc. 2012;4:472-478. [PubMed] [Cited in This Article: ] |
| 9. | Neumann H, Kudo S, Vieth M, Neurath MF. Real-time in vivo histologic examination using a probe-based endocytoscopy system for differentiating duodenal polyps. Endoscopy. 2013;45 Suppl 2 UCTN:E53-E54. [PubMed] [Cited in This Article: ] |
| 10. | Li WB, Zuo XL, Li CQ, Zuo F, Gu XM, Yu T, Chu CL, Zhang TG, Li YQ. Diagnostic value of confocal laser endomicroscopy for gastric superficial cancerous lesions. Gut. 2011;60:299-306. [PubMed] [Cited in This Article: ] |
| 11. | De Palma GD, Wallace MB, Giovannini M. Confocal laser endomicroscopy. Gastroenterol Res Pract. 2012;2012:216209. [PubMed] [Cited in This Article: ] |
| 12. | Peter S, Bang JY, Mönkemuller K, Varardarajulu S, Wilcox CM. Endomicroscopy of the pancreaticobiliary system. Diagn Ther Endosc. 2013;2013:310105. [PubMed] [Cited in This Article: ] |
| 13. | Kiesslich R, Burg J, Vieth M, Gnaendiger J, Enders M, Delaney P, Polglase A, McLaren W, Janell D, Thomas S. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology. 2004;127:706-713. [PubMed] [Cited in This Article: ] |
| 14. | Shahid MW, Buchner AM, Hasan MK, Gomez V, Wallace MB. The Role of Probe-Based Confocal Laser Endomicroscopy (pCLE) in Detection of Dysplasia in Duodenal Polyps. Gastrointest Endosc. 2009;69:AB369. [DOI] [Cited in This Article: ] |
| 15. | Meining A, Chen YK, Pleskow D, Stevens P, Shah RJ, Chuttani R, Michalek J, Slivka A. Direct visualization of indeterminate pancreaticobiliary strictures with probe-based confocal laser endomicroscopy: a multicenter experience. Gastrointest Endosc. 2011;74:961-968. [PubMed] [Cited in This Article: ] |
| 16. | Sharma P, Meining AR, Coron E, Lightdale CJ, Wolfsen HC, Bansal A, Bajbouj M, Galmiche JP, Abrams JA, Rastogi A. Real-time increased detection of neoplastic tissue in Barrett’s esophagus with probe-based confocal laser endomicroscopy: final results of an international multicenter, prospective, randomized, controlled trial. Gastrointest Endosc. 2011;74:465-472. [PubMed] [Cited in This Article: ] |
| 17. | Wallace MB, Meining A, Canto MI, Fockens P, Miehlke S, Roesch T, Lightdale CJ, Pohl H, Carr-Locke D, Löhr M. The safety of intravenous fluorescein for confocal laser endomicroscopy in the gastrointestinal tract. Aliment Pharmacol Ther. 2010;31:548-552. [PubMed] [Cited in This Article: ] |
| 18. | Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Fléjou JF, Geboes K. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251-255. [PubMed] [Cited in This Article: ] |
| 19. | Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159-174. [PubMed] [Cited in This Article: ] |
| 20. | Dunbar KB, Kiesslich R, Deinert K, Goetz M, Maitra A, Montgomery EA, Vieth M, Zhang Z, Canto MI. Confocal Laser Endomicroscopy Image Interpretation: Interobserver Agreement Among Gastroenterologists and Pathologists. Gastrointest Endosc. 2007;65:AB348. [DOI] [Cited in This Article: ] |
| 21. | Gaddam S, Mathur SC, Singh M, Arora J, Wani SB, Gupta N, Overhiser A, Rastogi A, Singh V, Desai N. Novel probe-based confocal laser endomicroscopy criteria and interobserver agreement for the detection of dysplasia in Barrett’s esophagus. Am J Gastroenterol. 2011;106:1961-1969. [PubMed] [Cited in This Article: ] |