Published online Nov 14, 2014. doi: 10.3748/wjg.v20.i42.15797
Revised: May 17, 2014
Accepted: June 26, 2014
Published online: November 14, 2014
Processing time: 241 Days and 18.7 Hours
AIM: To investigate the causes and intraoperative detection of endoscopic retrograde cholangiopancreatography (ERCP)-related perforations to support immediate or early diagnosis.
METHODS: Consecutive patients who underwent ERCP procedures at our hospital between January 2008 and June 2013 were retrospectively enrolled in the study (n = 2674). All procedures had been carried out using digital fluoroscopic assistance with the patient under conscious sedation. For patients showing alterations in the gastrointestinal anatomy, a short-type double balloon enteroscope had been applied. Cases of perforation had been identified by the presence of air in or leakage of contrast medium into the retroperitoneal space, or upon endoscopic detection of an abdominal cavity related to the perforated lumen. For patients with ERCP-related perforations, the data on medical history, endoscopic findings, radiologic findings, diagnostic methods, management, and clinical outcomes were used for descriptive analysis.
RESULTS: Of the 2674 ERCP procedures performed during the 71-mo study period, only six (0.22%) resulted in perforations (male/female, 2/4; median age: 84 years; age range: 57-97 years). The cases included an endoscope-related duodenal perforation, two periampullary perforations related to endoscopic sphincterotomy, two periampullary perforations related to endoscopic papillary balloon dilation, and a periampullary or bile duct perforation secondary to endoscopic instrument trauma. No cases of guidewire-related perforation occurred. The video endoscope system employed in all procedures was only able to immediately detect the endoscope-related perforation; the other five perforation cases were all detected by subsequent digital fluoroscope applied intraoperatively (at a median post-ERCP intervention time of 15 min). Three out of the six total perforation cases, including the single case of endoscope-related duodenal injury, were surgically treated; the remaining three cases were treated with conservative management, including trans-arterial embolization to control the bleeding in one of the cases. All patients recovered without further incident.
CONCLUSION: ERCP-related perforations may be difficult to diagnose by video endoscope and digital fluoroscope detection of retroperitoneal free air or contrast medium leakage can facilitate diagnosis.
Core tip: Duodenal perforation is a rare complication of endoscopic retrograde cholangiopancreatography (ERCP) with potentially life-threatening consequences. Early diagnosis and management is key to reducing patient morbidity and mortality, yet intraoperative detection of periampullary perforations is difficult even with video-equipped endoscope systems. In our 71-mo experience of performing ERCP, only six perforation cases occurred, five of which were undetected by intraoperative video endoscopy and required digital fluoroscopy for detection. Thus, fluoroscopic detection of retroperitoneal free air or contrast medium leakage can aid in intraoperative diagnosis and timely management of ERCP-related perforations.
- Citation: Motomura Y, Akahoshi K, Gibo J, Kanayama K, Fukuda S, Hamada S, Otsuka Y, Kubokawa M, Kajiyama K, Nakamura K. Immediate detection of endoscopic retrograde cholangiopancreatography-related periampullary perforation: Fluoroscopy or endoscopy? World J Gastroenterol 2014; 20(42): 15797-15804
- URL: https://www.wjgnet.com/1007-9327/full/v20/i42/15797.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i42.15797
Endoscopic retrograde cholangiopancreatography (ERCP)-related perforation is a relatively rare surgical complication, but has life-threatening consequences if undetected or unresolved in a timely manner[1,2]. ERCP-related perforations are classified according to the involved tissue regions, including the bowel wall, the retroperitoneal duodenum, and the pancreatic or bile ducts[3-5]. In theory, endoscope-related injuries to the duodenum can be detected immediately via the endoscope itself, and surgery to resolve the complication can be initiated to resolve the situation before ending the surgical period[3-6]. In contrast, endoscopic sphincterotomy (EST)-related periampullary injuries are more difficult to detect via endoscope and may be successfully resolved using conservative (nonoperative) treatment strategies[3-7]. Diagnosis of EST-related periampullary injuries is usually made by fluoroscopy or computed tomography (CT) detection of retroperitoneal free air and/or contrast media leakage in the involved abdominal space[3-5,7-9].
The overall incidence of ERCP-related perforations has been reported to range from 0.14% to 1.3%[1-5,7-20], with the EST-related perforations being the most frequent with incidences ranging from 0.03% to 1.3% and mortality rates ranging from 0% to 36%[1-5,7-9,11,12,14-19]. The immediate/early detection rate of EST-related perforation ranges widely, from 36% to 100% (for an average of 78%), from the relevant reports in the publicly available medical literature[3-5,7,8,11,12,16-19]. In order to determine the rates of incidence and characteristics of detection for EST-related perforations in our hospital, which is representative of a large public healthcare institution that routinely performs ERCP and serves a densely populous metropolitan region in Japan, we undertook a retrospective study of the cases of ERCP-related perforations experienced over a 71-mo period. Our findings identify the endoscopic and radiologic procedures and results leading to immediate/early diagnosis that can facilitate initiation of appropriate clinical management (both conservative and surgical) to successfully resolve ERCP-related perforations in a timely manner.
Consecutive patients who underwent ERCP procedures in the endoscopy center of Aso Iizuka Hospital (Iizuka, Japan) between January 2008 and June 2013 were retrospectively enrolled in the study. Among the total 2674 procedures performed, six resulted in ERCP-related perforations. Perforation was defined as the presence of air or contrast medium in the retroperitoneal space detected by radiological imaging, or as the presence of a cavity in the abdomen related to the perforated lumen detected by video or non-video endoscopy. For patients with ERCP-related perforations, the data on medical history, endoscopic findings, radiologic findings, diagnostic methods, management, and clinical outcomes were collected and used for descriptive analysis.
Each perforation case was assessed using a modified version[17] of the classification strategy described by Stapfer et al[4], so that endoscope-related perforations involving the lateral or medial duodenal wall were classified as type I, EST- or endoscopic papillary balloon dilation (EPBD)-related periampullary perforations were classified as type II, endoscopic instrument/non-guidewire-related ductal or duodenal perforations were classified as type III, and guidewire-related perforations (with X-ray detected retroperitoneal air) were classified as type IV.
All patients were placed under conscious sedation (achieved with intravenous delivery of 35 mg pethidine hydrochloride and 0.5-2.0 mg flunitrazepam) for the duration of the surgical procedure. All ERCP procedures were carried out with digital fluoroscope assistance using the C-vision Safire® videofluoroscope system or the Sonialvision Safire® volumetric X-ray digital linear tomosynthesis system (Shimadzu Corporation, Kyoto, Japan). For video-assisted endoscopy the JF-260 or TJF-240 video endoscopes (Olympus, Tokyo, Japan) were used. For patients with alterations in gastrointestinal anatomy, such as Roux-en-Y total gastrectomy or Billroth II gastrectomy, double balloon (DB)-ERCP was performed with the EC-450B15 short-type double balloon enteroscope (DBE) (Fujifilm, Saitama, Japan)[21]. For procedures performed prior to April 2009, standard air insufflation was used to create the intraluminal space; procedures performed from April 2009 onwards used carbon dioxide (CO2) to create the intraluminal space.
During the 71-mo study period, at total of 2,674 ERCP procedures were carried out and only six (0.22%) resulted in duodenal perforations (male:female, 2:4; median age: 84 years; age range: 57-97 years). As shown in Table 1, the cases included one an endoscope-related duodenal perforation (type I), two EST-related periampullary perforations (type II), two EPBD-related periampullary perforations (type II), and one periampullary or bile duct perforation considered to be secondary to endoscopic instrument trauma (type III). No cases of guidewire-related perforation (type IV) occurred in our study population.
| No | Age/Sex | Indications | Perforation site | Type | Mechanisms of perforation | Insufflation | Detection (Time in minutes) | Diagnostic modality | Management | Length of hospital stay after perforation, in days |
| 1 | 70/M | CBD stone | Afferent loop (Billroth-II) | I | Endoscope | Room air | Immediate (0) | Endoscope | Immediate surgery | 16 |
| 2 | 84/F | CBD stone | Periampullary | II | EST | Room air | Immediate (15) | Fluoroscope | Surgery | 56 |
| 3 | 97/F | CBD stone | Periampullary | III | Endoscopic instrument | Room air | Immediate (46) | Fluoroscope | ERBD | 64 |
| 4 | 70/F | CBD stone | Periampullary | II | EST/EPBD | CO2 | Immediate (20) | Fluoroscope | Surgery | 41 |
| 5 | 57/M | CBD stone | Periampullary | II | EST | CO2 | Immediate (4) | Fluoroscope | ENBD | 13 |
| 6 | 90/F | CBD stone | Periampullary | II | EPLBD | CO2 | Immediate (3) | Fluoroscope | ENBD | 22 |
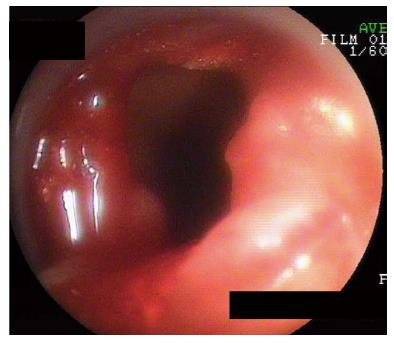
In Case 1, a perforation of the long limb with Billroth II anastomosis, the perforation occurred upon DBE insertion during the second session of ERCP and was detected immediately by endoscopic view (Figure 1). Endoscopic closure of this perforation was initially attempted using an endoclip, but when this attempt failed surgical suturing was performed. The patient recovered without incident and was discharged on post-operative day 16.
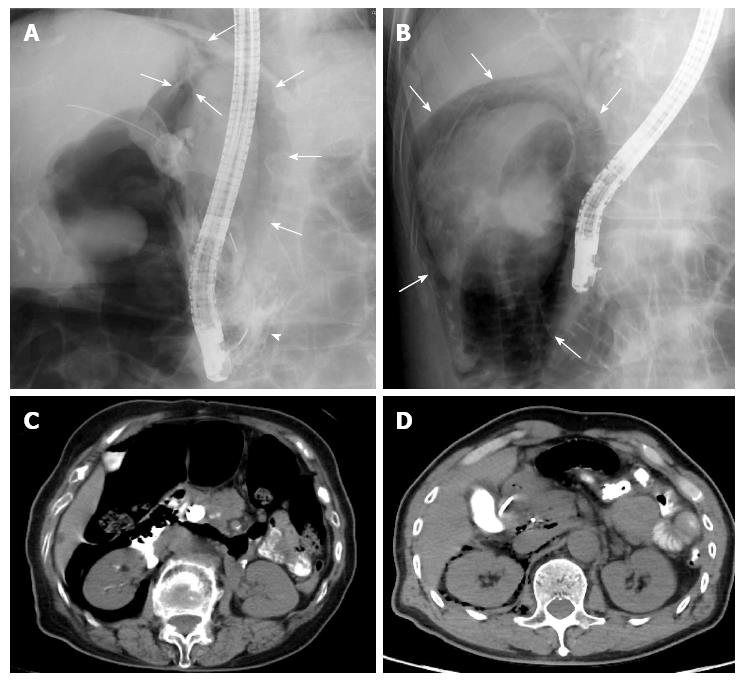
Both of the cases of EST-related perforations (type II) (Cases 2 and 5) were detected during the ERCP procedure via fluoroscope-mediated discovery of free air in the retroperitoneal space (Figure 2A and B) at 15 min (Case 2) and 4 minutes (Case 5) post-EST. Contrast media leakage was also seen in Case 2 (Figure 2A). However, in neither case was the perforation detected by endoscopic view. Both patients were subjected to an emergency CT scan immediately following the surgical procedure, and the presence of retroperitoneal free air was verified for both cases (Figure 2C and D) along with contrast media leakage in Case 2. Both cases were treated with a conservative approach, which included endoscopic drainage (endoscopic retrograde biliary drainage (ERBD) for Case 2 and endoscopic nasobiliary drainage (ENBD) for Case 5) and broad-spectrum parenteral antibiotic therapy with duration according to daily observation of each patient’s condition. Although Case 2 showed a reduction in the volume of retroperitoneal free air at post-EST day 6, fluid collection in the retroperitoneal space was found to have increased and the patient continued to experience abdominal pain. Results from laboratory tests indicated a high white blood cell (WBC) count and a high C-reactive protein level at that time. Therefore, surgery was performed on post-EST day 8. The patient recovered without incident and was discharged on post-EST day 56. For Case 5, the conservative treatment approach was effective, without need for surgical intervention; the patient was discharged on post-EST day 13.
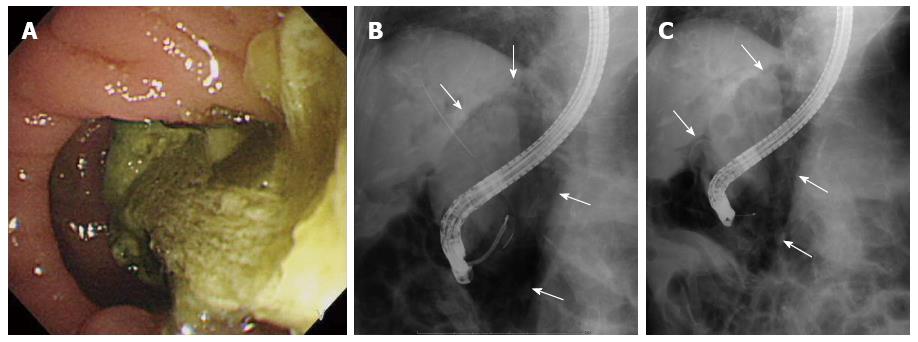
For Case 3, an endoscopic instrument-related perforation (type III), the patient had presented with a substantial mass of food residue in the periampullary diverticula prior to the ERCP procedure (Figure 3A); the residue was successfully removed by endoscopic forceps prior to the ERCP procedure. The perforation was diagnosed when free air was discovered in the retroperitoneal space by intraoperative fluoroscopy (Figure 3B). The ERCP procedure was immediately abandoned and a plastic ERBD stent was inserted. As the retroperitoneal free air was present prior to EST (Figure 3C), the perforation was considered to be an injury caused by an endoscopic instrument. Conservative treatment was effective, and the patient recovered without the need for surgical intervention. The patient was discharged on post-operative day 64.
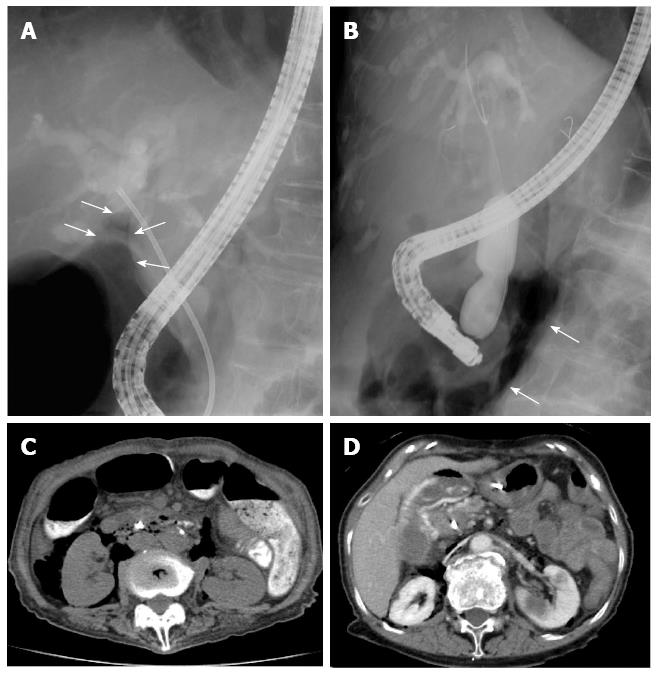
Both of the cases of EPBD-related perforations (type II) (Cases 4 and 6) were detected during the ERCP procedure via fluoroscope-mediated discovery of free air in the retroperitoneal space (Figure 4A and B) at 20 min (Case 4) and 3 min (Case 6) post-EPBD. Neither case of perforation was detected by endoscopic view during the surgery, and both were confirmed upon finding retroperitoneal free air by emergency CT scan performed immediately after the surgical procedure (Figure 4C and D). Although EST had been performed in Case 4 prior to the EPBD, the perforation was considered to be related to EPBD as the EST incision was short and the free air was not present prior to the performance of EPBD. In Case 6, a recurrent bile duct stone was found and endoscopic papillary large-balloon dilation (EPLBD) was attempted without EST, which led to periampullary massive hemorrhage that obstructed endoscopic visualization of the perforation site. Both cases were treated with a conservative approach, which included endoscopic drainage (ERBD for Case 4 and ENBD for Case 6) and broad-spectrum parenteral antibiotic therapy with duration according to daily observation of each patient’s condition. Although the perforation in Case 4 was considered to be manageable without surgery, the huge bile duct stone remained and was deemed difficult to remove endoscopically. Since the patient was already scheduled to undergo cholecystectomy for cholelithiasis in another two days from the current time, the decision was made to follow a conservative treatment plan and assess the perforation during the upcoming surgery; however, the perforation was not detected during surgery and appeared to have resolved. The patient recovered without incident and was discharged on post-ERCP day 41. Conservative treatment, including trans-arterial embolization to control the bleeding, was effective in Case 6; the patient recovered without need for surgical intervention and was discharged on post-operative day 22.
Rates of duodenal perforation, which is a rare but serious complication of ERCP, have been reported in the range of 0.14%-1.3%[1-5,7-20]. In the current study of 2674 consecutive patients who underwent ERCP in the endoscopy center at our hospital, the incidence rate (0.22%) fell within this range and represented three of the four types of the general ERCP-related perforation classifications [types I-III, with no type IV (guidewire-related) cases][3-7].
For diagnosis of the scope-related injuries (type I), neither fluoroscopy nor CT was necessary; the perforation site was large enough to be readily identified by endoscopy, as reported in previous studies[3-6]. The large size of the injury necessitates immediate surgery, and a conservative (nonsurgical) treatment approach is less likely to be effective or safe. In our single case of endoscope-related perforation, we detected the perforation endoscopically, almost immediately after it occurred, by viewing the retroperitoneal cavity directly. Our use of DBE, a forward-looking endoscope, in this procedure likely facilitated the nearly immediate detection of the injury. It is important to note that these injuries may not always be detectable by a duodenal scope, which is used frequently in ERCP procedures. Therefore, fluoroscopic detection of free air (an indicator of perforation) could be critical for early detection of the procedure-related injury.
EST- or EPBD-related perforations (type II) may be more difficult to detect by endoscopy alone. In the current study, four of the ERCP procedures resulted in EST-related perforations (n = 2) and EPBD-related perforations (n = 2), none of which were detected by the video endoscope, even in retrospective assessment of the endoscopic findings. Several reports in the publicly available literature have addressed the detection of EST-related perforations; the relevant reports identified by a simple keyword search of the PubMed database are summarized in Table 2[3-5,7,8,11,12,16-19]. The cases in these other reports were most frequently diagnosed by fluoroscopy or CT scan, and the corresponding detection rates ranged from 36% to 100%. In addition, some of the studies reported mortality rates for these previous cases, and the rates ranged widely from 0 to 36%, for an average of 5.2%[1-5,7-9,11,12,14-19]. Although a low rate of immediate detection does not necessarily equate to a high rate of mortality (Table 2), the patient cohort studied by Wu et al[12] did have a relatively high mortality rate that may have been related to the low immediate/early detection rate. On the other hand, the mean detection rate with no mortality in these previous reports is 77.8% (60%-100%)[4,5,8,16,17,19]. Thus, immediate/early detection is considered to be one of the most important factors, and not the only factor, contributing to the successful management of ERCP-related duodenal perforation.
| Ref. | Publication year | Total perforation rate, % | EST-related perforation | |||
| Rate, % | Surgery, % | Mortality, % | Immediate/early diagnosis, % | |||
| Cotton et al[1] | 1991 | NR | 1.30 | 26.8 | 16.3 | NR |
| Loperfido et al[2] | 1998 | 1.01 | 0.43 | 50.0 | 8.3 | NR |
| Howard et al[3] | 1999 | 0.60 | 0.36 | 4.5 | 4.5 | 90.9 |
| Stapfer et al[4] | 2000 | 1.00 | 0.42 | 50.0 | 0 | 66.7 |
| Masci et al[10] | 2001 | 0.64 | 0.57 | 36.0 | NR | NR |
| Enns et al[5] | 2002 | 0.35 | 0.14 | 15.4 | 0 | 76.9 |
| Christensen et al[11] | 2004 | 1.27 | 0.85 | NR | 10.0 | 100 |
| Wu et al[12] | 2006 | 0.45 | 0.17 | 45.0 | 36.0 | 36.0 |
| Fatima et al[13] | 2007 | 0.60 | 0.09 | 36.4 | NR | NR |
| Assalia et al[7] | 2007 | 0.71 | 0.55 | 11.8 | 5.9 | 94.1 |
| Kapral et al[14] | 2008 | 0.50 | 0.38 | 8.3 | 8.3 | NR |
| Morgan et al[8] | 2009 | 0.20 | 0.10 | 0 | 0 | 66.7 |
| Cotton et al[15] | 2009 | 0.14 | 0.03 | 75.0 | 0 | NR |
| Shi et al[23] | 2009 | NR | 0.26 | NR | NR | NR |
| Kim et al[9] | 2009 | 0.89 | 0.40 | 11.1 | 0 | NR |
| Kim et al[16] | 2011 | 0.16 | 0.07 | 60.0 | 0 | 60.0 |
| Polydorou et al[17] | 2011 | 0.40 | 0.30 | 20.0 | 0 | 96.7 |
| Kwon et al[18] | 2012 | 0.63 | 0.29 | 3.1 | 3.1 | 70.0 |
| Li et al[19] | 2012 | 0.19 | 0.11 | 0 | 0 | 100 |
| Kim et al[20] | 2012 | 0.62 | 0.23 | NR | NR | NR |
| Average | 0.58 | 0.35 | 26.7 | 5.15 | 78.0 | |
In the current study, five of the six perforations [with the exception of the endoscope-related (type I) case] were undetected by endoscopy, but all six of the perforations were detected by intraoperative fluoroscopy (with a median time of 15 min following the injury-inducing endoscopic intervention). It is not difficult to identify the presence of retroperitoneal free air by fluoroscopy, if the typical findings are known. For our five type II and type III cases, the presence of free air was detected below the liver (n = 4) as well as around the right kidney (n = 5). For two of these cases (Cases 5 and 6), the detection was made within 4 minutes of the injury-inducing endoscopic intervention. During surgery, endoscopists tend to concentrate their attention on the endoscopic view and/or the narrow area of the cholangio/pancreatogram. This focus may prove detrimental, however, in that it may delay the warning signs of injury, such as the presence of free air in the retroperitoneal space. To address this potential pitfall, our hospital’s standard process requires obtainment of one radiographic image of the entire abdominal area prior to the start of the ERCP procedure; this image is then compared with a similar image taken immediately following the procedure, as suggested by Li et al[19]. Moreover, our process requires the participation of an additional expert-level endoscopist to actively observe the X-ray monitor used during the procedure. In most cases the X-ray monitor is zoomed in to a specific relevant area, such as the bile duct and/or pancreatic duct, but the image is also observed in its zoomed out form in order to monitor the wider and entire abdominal area, with special attention paid to the area below the liver or around the right kidney to ensure our chances of detecting any retroperitoneal free air. We believe that the routine monitoring of the zoomed out image during procedures is one of the best ways to improve the rate of immediate detection of perforation.
A distinguishing feature of the ERCP procedures performed on three cases in the current study is the use of CO2 insufflation, as opposed to the room air insufflation that was used for the other three cases. The practice of CO2 insufflation for ERCP was instituted with the expectation of obtaining better X-ray images, based upon previous studies showing that CO2 insufflation during ERCP reduces the bowel gas volume which can otherwise occlude the image[22,23]. On the other hand, this technique may increase the risk of failing to notice the presence free air because of the quick absorbance rate of CO2. However, we did detect the presence of retroperitoneal free air in all of our cases for which CO2 insufflation was used. If the case had been that immediate detection could not be made, it might have been impossible to diagnose the perforation by fluoroscopy after a couple of hours had passed following the injury. Indeed, careful observation of the X-ray monitor during the procedure could make the detection of free air possible even when CO2 is used for the insufflation, and because of the rapid absorbance rate immediate detection during the procedure is even more critical when CO2 insufflation is used.
Another important factor in the management of ERCP-related duodenal perforation is the timing of the surgical intervention performed to resolve the complication. It has been reported that delay in surgical intervention leads to poor outcomes[4,8,12,13,18-20]. Although most of the type II periampullary perforations are considered to be manageable by conservative (nonsurgical) approaches[3-7], surgical intervention is recommended when the accumulation of fluid in the peritoneal or retroperitoneal cavity persists[4,12,16]. In the current study, one patient (Case 2) fit this indication for surgical intervention; additionally, this was the only case that showed contrast media leakage, suggesting that the presence of contrast leakage may correlate with indication of surgery[4].
In conclusion, this study suggests that retroperitoneal free air (a diagnostic indicator of bowel perforation) can be detected immediately during ERCP by fluoroscopy alone. An immediate post-surgical CT scan should also be performed for patients in whom perforation is suspected, even in the absence of fluoroscopic evidence of retroperitoneal free air. Nonetheless, it is impossible to avoid ERCP-related perforation completely, and the possibility of perforation should be carefully monitored during every procedure.
The authors wish to thank Mr. Stephen Cotton for English language editing.
Endoscopic retrograde cholangiopancreatography (ERCP) is one of the most technically challenging endoscopic procedures, with successful and safe application requiring expertise, high-level training, and experience of the endoscopist and their support team. Although ERCP is routinely applied as a diagnostic and therapeutic surgical approach in clinics across the globe, rates of procedure-related complications remain high. Duodenal perforation, in particular, is a serious ERCP-related complication requiring immediate or early detection in order to avoid the life-threatening consequences. Yet immediate or early detection is difficult and may require additional imaging modalities and techniques besides the endoscope or videoendoscope alone.
ERCP-related perforations of the duodenum and related ducts are classified according to the mode of injury and generally include scope-related duodenal wall injury, sphincterotomy- or balloon dilation-related periampullary injury, and endoscopic instrumentation (including guidewire)-related injury. Fluoroscopy and/or computed tomography are usually performed during the ERCP procedure to detect retroperitoneal free air or contrast media leakage, which suggests the existence of a duodenal perforation. Endoscopy itself has also been described as capable of detecting the perforation site.
The relatively few studies in the publicly available literature that have addressed diagnosis of endoscopic sphincterotomy- and endoscopic papillary balloon dilation-related perforations by fluoroscopy or computed tomography report a very wide range of detection rates, between 36% and 100%. Moreover, few studies have sought to determine the characteristics of perforation-related retroperitoneal free air, such as how quickly the free air appears after a perforation has occurred and which technologies are most useful for its detection. In this study, the authors demonstrated that it is possible to diagnose retroperitoneal free air by fluoroscopy-assisted endoscopy within a couple of minutes of the perforation occurrence; expertise in monitoring these technologies and interpreting their findings will be key to the success of applying this approach.
This study suggests that retroperitoneal free air can be detected immediately during the ERCP procedure by fluoroscopy alone. Intraoperative monitoring of the entire abdominal area using a videofluoroscope system or a volumetric X-ray digital linear tomosynthesis system may help to increase rates of diagnosis for ERCP-related perforations and initiation of timely therapies. Such an approach may help to decrease the rates of unfavorable outcomes due to delayed diagnosis.
Endoscopic retrograde cholangiopancreatography is a surgical technique by which a catheter is inserted into the duodenal papilla of Vater in order to infuse a contrast media into the bile and/or pancreatic duct by means of fluoroscopy-assisted endoscopy for diagnosis of cholangiopancreato-related abnormalities, such as bile duct stones or cancers.
This report of a series of six cases of ERCP-related perforation provides clinically relevant insights into the endoscopic and radiologic findings that can aid in immediate/early diagnosis and timely initiation of appropriate surgical or conservative therapy. The results from this single-center study using a Japanese cohort of patients suggest that retroperitoneal free air can be detected immediately during the ERCP procedure by fluoroscopy.
P- Reviewer: Kogure H S- Editor: Qi Y L- Editor: A E- Editor: Ma S
| 1. | Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1890] [Cited by in RCA: 2037] [Article Influence: 59.9] [Reference Citation Analysis (1)] |
| 2. | Loperfido S, Angelini G, Benedetti G, Chilovi F, Costan F, De Berardinis F, De Bernardin M, Ederle A, Fina P, Fratton A. Major early complications from diagnostic and therapeutic ERCP: a prospective multicenter study. Gastrointest Endosc. 1998;48:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 801] [Cited by in RCA: 779] [Article Influence: 28.9] [Reference Citation Analysis (1)] |
| 3. | Howard TJ, Tan T, Lehman GA, Sherman S, Madura JA, Fogel E, Swack ML, Kopecky KK. Classification and management of perforations complicating endoscopic sphincterotomy. Surgery. 1999;126:658-63; discussion 664-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 114] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Stapfer M, Selby RR, Stain SC, Katkhouda N, Parekh D, Jabbour N, Garry D. Management of duodenal perforation after endoscopic retrograde cholangiopancreatography and sphincterotomy. Ann Surg. 2000;232:191-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 239] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 5. | Enns R, Eloubeidi MA, Mergener K, Jowell PS, Branch MS, Pappas TM, Baillie J. ERCP-related perforations: risk factors and management. Endoscopy. 2002;34:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 179] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 6. | Freeman ML. Complications of endoscopic retrograde cholangiopancreatography: avoidance and management. Gastrointest Endosc Clin N Am. 2012;22:567-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Assalia A, Suissa A, Ilivitzki A, Mahajna A, Yassin K, Hashmonai M, Krausz MM. Validity of clinical criteria in the management of endoscopic retrograde cholangiopancreatography related duodenal perforations. Arch Surg. 2007;142:1059-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Morgan KA, Fontenot BB, Ruddy JM, Mickey S, Adams DB. Endoscopic retrograde cholangiopancreatography gut perforations: when to wait! When to operate! Am Surg. 2009;75:477-83; discussion 483-4. [PubMed] |
| 9. | Kim JH, Yoo BM, Kim JH, Kim MW, Kim WH. Management of ERCP-related perforations: outcomes of single institution in Korea. J Gastrointest Surg. 2009;13:728-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Masci E, Toti G, Mariani A, Curioni S, Lomazzi A, Dinelli M, Minoli G, Crosta C, Comin U, Fertitta A. Complications of diagnostic and therapeutic ERCP: a prospective multicenter study. Am J Gastroenterol. 2001;96:417-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 613] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 11. | Christensen M, Matzen P, Schulze S, Rosenberg J. Complications of ERCP: a prospective study. Gastrointest Endosc. 2004;60:721-731. [PubMed] |
| 12. | Wu HM, Dixon E, May GR, Sutherland FR. Management of perforation after endoscopic retrograde cholangiopancreatography (ERCP): a population-based review. HPB (Oxford). 2006;8:393-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Fatima J, Baron TH, Topazian MD, Houghton SG, Iqbal CW, Ott BJ, Farley DR, Farnell MB, Sarr MG. Pancreaticobiliary and duodenal perforations after periampullary endoscopic procedures: diagnosis and management. Arch Surg. 2007;142:448-54; discussion 454-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Kapral C, Duller C, Wewalka F, Kerstan E, Vogel W, Schreiber F. Case volume and outcome of endoscopic retrograde cholangiopancreatography: results of a nationwide Austrian benchmarking project. Endoscopy. 2008;40:625-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 15. | Cotton PB, Garrow DA, Gallagher J, Romagnuolo J. Risk factors for complications after ERCP: a multivariate analysis of 11,497 procedures over 12 years. Gastrointest Endosc. 2009;70:80-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 468] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 16. | Kim BS, Kim IG, Ryu BY, Kim JH, Yoo KS, Baik GH, Kim JB, Jeon JY. Management of endoscopic retrograde cholangiopancreatography-related perforations. J Korean Surg Soc. 2011;81:195-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Polydorou A, Vezakis A, Fragulidis G, Katsarelias D, Vagianos C, Polymeneas G. A tailored approach to the management of perforations following endoscopic retrograde cholangiopancreatography and sphincterotomy. J Gastrointest Surg. 2011;15:2211-2217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Kwon W, Jang JY, Ryu JK, Kim YT, Yoon YB, Kang MJ, Kim SW. Proposal of an endoscopic retrograde cholangiopancreatography-related perforation management guideline based on perforation type. J Korean Surg Soc. 2012;83:218-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Li G, Chen Y, Zhou X, Lv N. Early management experience of perforation after ERCP. Gastroenterol Res Pract. 2012;2012:657418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Kim J, Lee SH, Paik WH, Song BJ, Hwang JH, Ryu JK, Kim YT, Yoon YB. Clinical outcomes of patients who experienced perforation associated with endoscopic retrograde cholangiopancreatography. Surg Endosc. 2012;26:3293-3300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Osoegawa T, Motomura Y, Akahoshi K, Higuchi N, Tanaka Y, Hisano T, Itaba S, Gibo J, Yamada M, Kubokawa M. Improved techniques for double-balloon-enteroscopy-assisted endoscopic retrograde cholangiopancreatography. World J Gastroenterol. 2012;18:6843-6849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Kuwatani M, Kawakami H, Hayashi T, Ishiwatari H, Kudo T, Yamato H, Ehira N, Haba S, Eto K, Kato M. Carbon dioxide insufflation during endoscopic retrograde cholangiopancreatography reduces bowel gas volume but does not affect visual analogue scale scores of suffering: a prospective, double-blind, randomized, controlled trial. Surg Endosc. 2011;25:3784-3790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Shi H, Chen S, Swar G, Wang Y, Ying M. Carbon dioxide insufflation during endoscopic retrograde cholangiopancreatography: a review and meta-analysis. Pancreas. 2013;42:1093-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |