Published online Oct 21, 2014. doi: 10.3748/wjg.v20.i39.14420
Revised: April 17, 2014
Accepted: May 25, 2014
Published online: October 21, 2014
AIM: To examine whether commensal bacteria are a contributing cause of stress-related mucosal inflammation.
METHODS: Human peripheral blood monocyte-derived dendritic cells (MoDCs) were stimulated by commensal bacterial strains, including Escherichia coli, Clostridium clostridioforme, Bacteroides vulgatus (B. vulgatus), Fusobacterium varium (F. varium), and Lactobacillus delbrueckii subsp. bulgaricus. After incubation, corticotropin-releasing factor (CRF) and urocortin 1 (UCN1) mRNA in the cells was examined by real-time reverse transcription polymerase chain reaction. Supernatants from the cells were tested for CRF and UCN1 using an enzyme-linked immunosorbent assay.
RESULTS: Both CRF and UCN1 were significantly augmented by B. vulgatus and F. varium at both the mRNA and protein levels. In particular, B. vulgatus stimulated human MoDCs, resulting in extremely high levels of CRF and UCN1.
CONCLUSION: Stimulation of MoDCs by B. vulgatus and F. varium may be associated with CRF/UCN1-related intestinal disorders, such as irritable bowel syndrome and inflammatory bowel disease.
Core tip: Corticotropin-releasing factor (CRF) and urocortin 1 (UCN1) play critical roles in many stress-related intestinal disorders, such as irritable bowel syndrome and inflammatory bowel disease. However, little is known about the pathophysiology of these diseases. To examine whether commensal bacteria are a contributing cause of stress-related mucosal inflammation, human peripheral blood monocyte-derived dendritic cells were stimulated by commensal bacterial strains. Both CRF and UCN1 were significantly augmented by Bacteroides vulgatus (B. vulgatus) and Fusobacterium varium (F. varium) at both the mRNA and protein levels. Thus, B. vulgatus and F. varium may be associated with stress-related intestinal disorders.
- Citation: Koido S, Ohkusa T, Kan S, Takakura K, Saito K, Komita H, Ito Z, Kobayashi H, Takami S, Uchiyama K, Arakawa H, Ito M, Okamoto M, Kajihara M, Homma S, Tajiri H. Production of corticotropin-releasing factor and urocortin from human monocyte-derived dendritic cells is stimulated by commensal bacteria in intestine. World J Gastroenterol 2014; 20(39): 14420-14429
- URL: https://www.wjgnet.com/1007-9327/full/v20/i39/14420.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i39.14420
Psychological stress affects all people. The individual stress hormone response is mediated by corticotropin-releasing factor (CRF); this factor is produced mainly in the paraventricular nucleus of the hypothalamus system and is highly likely to affect the features of many stress-released disorders[1-3]. Therefore, CRF plays a critical role in stress-released stimulation of the hypothalamic-pituitary-adrenal axis through activation of the pituitary CRF1 receptor and, in association with urocortin 1 (UCN1), acts as a neuromodulator to coordinate visceral hypersensitivity[1,4]. Both CRF and UCN1 act within the brain stress network to increase anxiety-like behavior, abdominal pain, colon secretions, and muscle motility[5-7]. Interestingly, both CRF and UCN1 are also expressed in peripheral tissues and act directly within the colon, where they stimulate secretion and motor activity, leading to the development of watery stools/diarrhea[5-7]. These events are more common in patients with irritable bowel syndrome (IBS) or inflammatory bowel disease (IBD)[5-7].
The permeability of the bowel lining can allow the passage of bacteria through the intestine into the bowel wall[5-7]. Therefore, patients with intestinal disorders, such as IBS and IBD, experience quantitative changes in the indigenous microbiota[8,9]. Moreover, bacterial overgrowth in the small intestine is associated with increased severity of stress-related IBS and IBD symptoms and with increased intestinal gas and immune responses, and antibiotic therapy has been shown to attenuate these symptoms in human patients[10]. In animal models, the presence of intestinal flora is also essentially required for the development of colitis because colitis fails to develop under germ-free conditions[11,12].
An increased density of mast cells in the colonic mucosa and intraepithelial lymphocytosis is also observed in IBS patients[13]. Accumulating evidence suggests a close association between commensal bacteria and the proximity of immune cells to neural elements in patients with IBS or IBD. Dendritic cells (DCs) are the most potent professional antigen-presenting cells and are usually located at surveillance interfaces of the human body, such as the skin or mucosa. These cells are thought to play an important role in the generation and regulation of immune responses[14]. The relationship between hosts and their microbiota is only just beginning to be studied in detail, and given the major role of DCs in bacterial-associated antigen presentation in the gut, it would not be surprising if DC activity was altered in patients with IBS or IBD[15-17]. Indeed, DCs are considered to represent the link between allergen uptake and the clinical manifestations of intestinal inflammation[16]. However, little is known about the effects of commensal bacteria on human DCs in relation to stress-induced mucosal inflammatory disease. Previously, we reported that certain commensal bacteria, such as Clostridium clostridioforme (C. clostridioforme), Bacteroides vulgatus (B. vulgatus), Escherichia coli (E. coli), and Fusobacterium varium (F. varium), can invade colonic epithelial cells, thereby activating early intracellular signaling systems to trigger host inflammatory reactions[17]. Therefore, we assessed the relationship between these bacterial strains and CRF/UCN1 expression at both the mRNA and protein levels in human DCs in this study.
We show that significantly increased levels of CRF and UCN1 are detected at both the mRNA and protein levels in human monocyte-derived DCs (MoDCs) upon stimulation with either B. vulgatus or F. varium. In particular, B. vulgatus stimulated human MoDCs, resulting in extremely high levels of CRF and UCN1 production. Moreover, the stimulation of human MoDCs with these bacterial strains resulted in the up-regulation of the expression of HLA-ABC, HLA-DR, CD80, CD86, and CD83. Understanding how CRF and UCN1 function will help us to find improved ways to treat stress-related intestinal disorders.
The study protocol was reviewed and approved by the ethics committee of the Jikei Institutional Review Board, Jikei University School of Medicine and by the Clinical Study Committee of Jikei University Kashiwa Hospital [No. 23-278 (6739)]. Peripheral blood mononuclear cells (PBMCs) were obtained from 5 healthy donors, and individual written informed consent was obtained.
PBMCs were prepared using Ficoll density-gradient centrifugation and incubated in tissue culture flasks at 37 °C for 30 min in AIM V medium (Life Technologies Japan Ltd., Tokyo, Japan) without serum supplementation or antibiotics. After incubation to enable adherence, nonadherent cells were removed, and adherent cells were cultured for 6 d in AIM V medium supplemented with 1000 U/mL recombinant human (rh)GM-CSF (PeproTech, Rocky Hill, NJ, United States) and 500 U/mL rhIL-4 (Diaclone Research, Besancon, France) to generate human MoDCs. On day 6 of culturing, nonadherent and loosely adherent cells were collected and cultured in 100-mm tissue culture dishes (106 cells/mL; 10 mL/dish) for 30 min. Nonadherent cells were then removed and further enriched using the repeated adherence method to purify MoDCs[18].
We used four commensal bacterial strains, E. coli (JCM1649), C. clostridioforme (JCM1219; Japan Collection of Microorganisms, RIKEN, Wako, Japan), B. vulgatus (JCM5826), and F. varium (ATCC8501; ATCC, Rockville, MD, United States). These strains have been reported to be pathogens for inflammatory intestine disorders[17,19]. In addition, a probiotic, Lactobacillus delbrueckii subsp. bulgaricus (L. bulgaricus) (LB-021001; Meiji Dairies), was used as a control. The bacterial strains were harvested from GAM agar plates (Nissui Pharmaceutical Co. Ltd., Tokyo, Japan) that had been cultured at 37 °C for 72 h. Aerobic bacteria (E. coli) were incubated under 5% CO2 in a humidified incubator. Anaerobic bacteria (C. clostridioforme, B. vulgatus, F. varium, and L. bulgaricus) were incubated in an anaerobic chamber (Forma Scientific, Marietta, OH) containing 10% CO2, 10% H2, and 80% N2. After the colonies were collected using a disposable plastic loop, they were suspended at 1 × 105 cells/mL in RPMI-1640 medium supplemented with 1000 U/mL rhGM-CSF and 500 U/mL rhIL-4 in the absence of antibiotics and serum.
Purified human MoDCs (1.5 × 105/500 μL) from 5 healthy donors were incubated with each commensal bacteria strain (E. coli, C. clostridioforme, B. vulgatus, F. varium, or L. bulgaricus) (1 × 108/500 μL) in 1 mL of RPMI-1640 supplemented with 1000 U/mL rhGM-CSF and 500 U/mL rhIL-4 in the absence of antibiotics and serum in a 24-well plate under 5% CO2 at 37 °C.
Cells were incubated with FITC-conjugated monoclonal antibodies (mAbs) against HLA-ABC, HLA-DR, CD80, CD86, CD83, or matched isotype control IgG (BD Pharmingen, San Jose, CA, United States). Purified MoDC populations were gated based on their forward- vs side-scatter profile and then analyzed for HLA-ABC, HLA-DR, CD80, CD86, and CD83 expression. The mean fluorescence intensity (MFI) of the indicated molecules, which were expressed by MoDCs derived from 5 healthy donors, was analyzed.
Purified human MoDCs (1.5 × 105/500 μL) from healthy donors were incubated with the commensal bacteria (1 × 108/500 μL) in RPMI-1640 medium (total volume, 1 mL) without antibiotics or serum for 0.5, 1, 1.5, and 24 h under 5% CO2 at 37 °C. After incubation, the cells were collected, resuspended in 200 μL of Isogen (Nippon Gene Inc., Tokyo, Japan), and frozen at -80 °C. Total RNA was isolated from the stored cells using an RNeasy plus Mini Kit (QIAGEN, Inc., Hilden, Germany) following the manufacturer’s instructions. Reverse transcription (RT) was carried out to synthesize first-strand cDNA using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions. The resultant cDNA (corresponding to 100 ng of total RNA) was examined using qRT-PCR in an ABI 7300 instrument (Applied Biosystems) with the TaqMan Gene Expression Master Mix (Applied Biosystems). TaqMan primers and non-fluorescent quencher probes complementary to CRF (Assay ID: Hs01921237_s1), UCN1 (Assay ID: Hs01849155_s1), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH, Assay ID: Hs99999905_m1) genes were purchased from Applied Biosystems. GAPDH gene expression was used as an internal reference. CRF or UCN1 gene expression was examined using the comparative CT method. CT values reflect the PCR cycle number at which the amount of amplified target reaches a fixed threshold. The values of CT for the target and reference genes were calculated for each sample. ΔCT is calculated as the difference in CT between the target and reference genes. ΔΔCT is calculated as the difference between the ΔCT of the sample and the ΔCT of the calibrator (control DC 0 min sample). CRF and UCN1 mRNA expressions normalized to an internal reference and expressed relative to the control sample treated without bacteria are given by 2-ΔΔCT. mRNA expression levels are expressed as the fold-change from human MoDCs without bacterial treatment (0 min). Data represent the means ± SD of 3 independent experiments.
To examine whether human MoDCs produce CRF and UCN1, purified human MoDCs derived from 5 healthy donors (1.5 × 105/500 μL) were incubated with each sample of commensal bacteria (1 × 108/500 μL) in RPMI-1640 medium with neither antibiotics nor serum for 24 h under 5% CO2 at 37 °C. Supernatants from these cells were collected and tested for CRF (Phoenix Pharmaceuticals Inc., Belmont, CA) and UCN1 (Phoenix Pharmaceuticals Inc.) using an enzyme-linked immunosorbent assay (ELISA) following the manufacturer’s instructions. Data represent the averages of 5 healthy donors from the same sets of experiments. Background cytokine levels in conditioned medium were subtracted from each sample.
Results are expressed as the mean ± SD. One-way analysis of variance was used to determine significance. Differences were considered statistically significant when P-values were 0.05 or less.
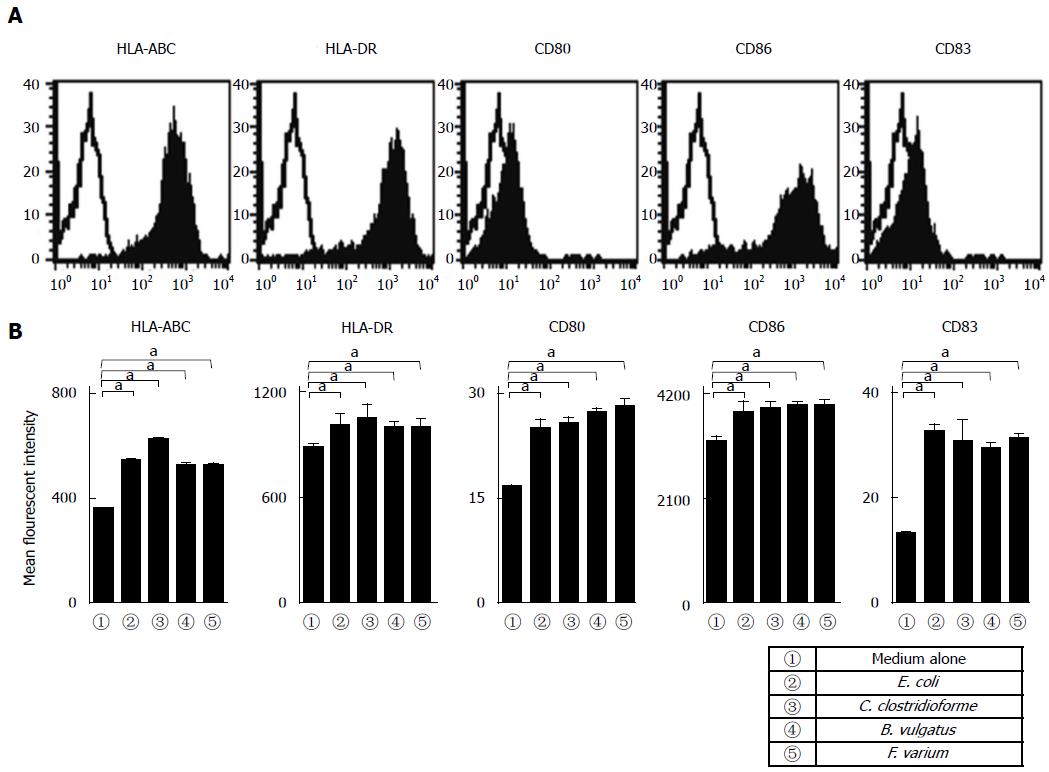
Purified MoDCs derived from 5 healthy donors (mean age: 41.2 ± 7.5 years; 5 males) were analyzed in the same sets of experiments. Human MoDCs exhibited a characteristic phenotype regarding their expressions of HLA-ABC, HLA-DR, and CD86, but low levels of CD80 and CD83 were noted (Figure 1). Moreover, these expression levels were not significantly different from those of healthy donors (Figure 1A, B). Next, to assess the effects of commensal bacteria on human MoDCs, MoDCs were stimulated with commensal bacterial strains for 24 h, after which their phenotypes were analyzed. We selected certain commensal bacterial strains (E. coli, C. clostridioforme, B. vulgatus, and F. varium) that have been reported as pathogens for inflammatory intestinal disorders[17,19,20]. The stimulation of MoDCs with each commensal bacterial strain significantly up-regulated the MFI of HLA-ABC, HLA-DR, CD80, CD86, and CD83 compared with unstimulated MoDCs (Figure 1B). In addition, no difference was observed in the MFI between MoDCs stimulated with each of the commensal bacterial strains (Figure 1B).
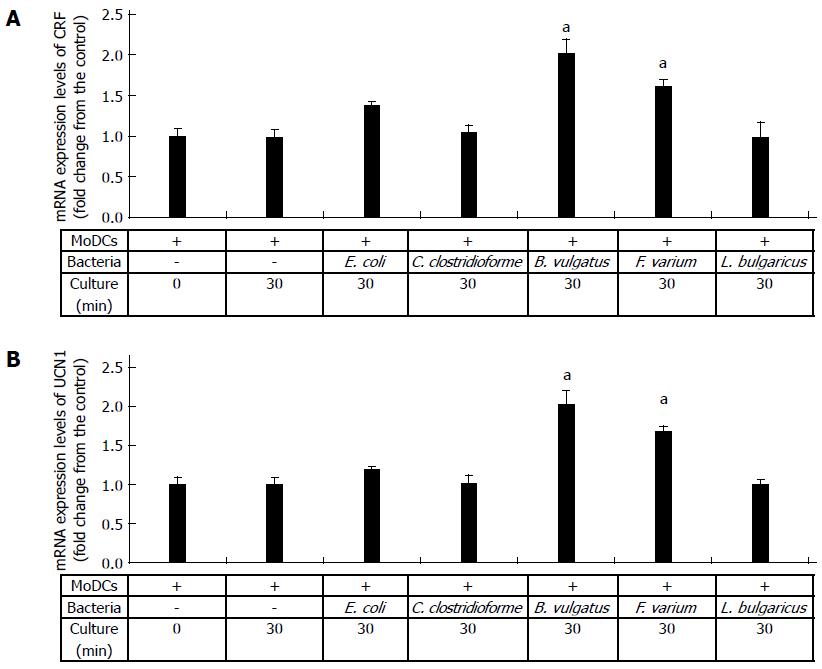
To assess the effects of commensal bacteria on human MoDCs, we analyzed the CRF and UCN1 mRNA levels in human MoDCs upon stimulation with each bacterial strain. CRF and UCN1 mRNA levels in bacteria alone and in uninfected and L. bulgaricus-infected human MoDCs were used as controls. Significant increases in CRF and UCN1 mRNA levels were observed in human MoDCs after 30 min of B. vulgatus or F. varium stimulation; thereafter, the levels decreased (data not shown). Interestingly, the expression levels of CRF and UCN1 mRNA were significantly higher in B. vulgatus and F. varium than in C. clostridioforme and E. coli (Figure 2A, B). In particular, the co-culture of human MoDCs with B. vulgatus was associated with high expression levels of both CRF and UCN1 mRNA (Figure 2A, B). In addition, CRF and UCN1 mRNA levels in bacteria alone and in uninfected and L. bulgaricus-infected human MoDCs were not increased until at least 24 h (Figure 2A, B). These results indicate that stimulation of human MoDCs with B. vulgatus and F. varium is associated with the expression of both CRF and UCN1 mRNA in MoDCs.
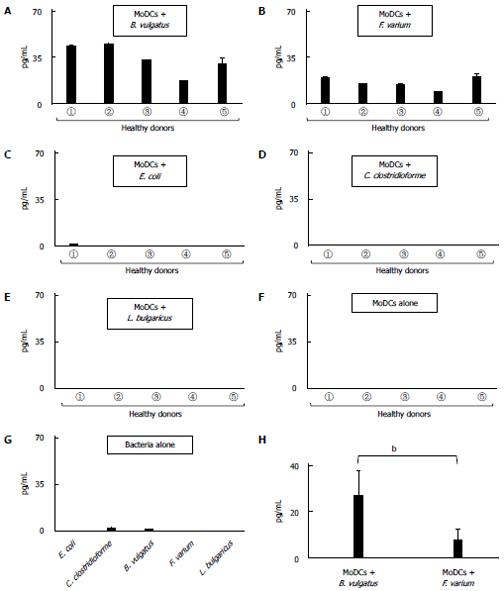
To assess the effects of commensal bacteria on human MoDCs, human MoDCs generated from 5 healthy donors were cultured with each commensal bacterial strain for 24 h. When human MoDCs were stimulated with B. vulgatus or F. varium, CRF production was significantly increased (Figure 3A, B). However, there was little, if any, production of CRF after stimulation with E. coli, C. clostridioforme, or L. bulgaricus or after no stimulation in this experimental assay (Figure 3C, D, E, F and G). Interestingly, B. vulgatus significantly stimulated human MoDCs, resulting in a much higher production of CRF than in F. varium (P < 0.01) (Figure 3H).
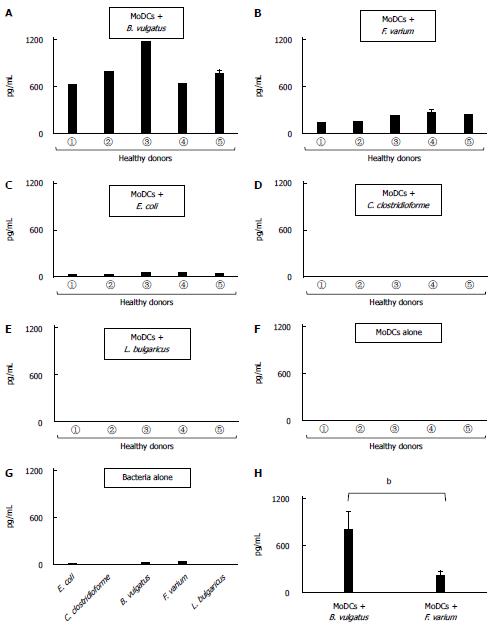
Human MoDCs, when stimulated with B. vulgatus or F. varium, produced high levels of UCN1 (Figure 4A, B). Moreover, UCN1 up-regulation was also detected in E. coli-stimulated MoDCs; however, the level of up-regulation was much lower than with B. vulgatus or F. varium (Figure 4C). In addition, there was little, if any, production of UCN1 after stimulation with C. clostridioforme or L. bulgaricus or after no stimulation (Figure 4D, E, F, and G). In particular, stimulation of human MoDCs with B. vulgatus resulted in a much higher production of UCN1 than stimulation with F. varium (P < 0.001) (Figure 4H).
The present study demonstrates for the first time that human MoDCs can produce the CRF and UCN1 proteins upon stimulation with commensal bacteria, such as B. vulgatus and F. varium. In particular, B. vulgatus significantly stimulated human MoDCs, resulting in extremely high production of both CRF and UCN1.
CRF and UCN1 are expressed in the central nervous system and in peripheral tissues and can be released by regional sensory and sympathetic nerves, immune cells, and gut enteroendocrine and enteric cells to act locally in response to stress[13,19,21-24]. Thus, CRF and UCN1 play a central role in the pathophysiology of stress-induced intestinal disturbances, such as IBS and IBD[13,19,21-24]. Moreover, it has been reported that intestinal microbiota appear to play an important role in IBS and IBD; qualitative and quantitative changes of intestinal microbiota occur in IBS and IBD subtypes[17,25]. The evidence suggests that crosstalk between bacteria and the production of CRF and UCN1 in humans might be involved in the exacerbation of inflammatory conditions by stress, but the mechanism by which this occurs remains unclear at present. DCs are potent APCs that can recognize luminal exogenous antigens, such as common intestinal microbes, and can activate innate and acquired immunity[14]. Therefore, the immunogenicity of bacteria strongly depends on the character of its interaction with DCs. Of note, evidence exists that various subsets of DCs differ in their antigen processing capacities in vivo[26]. Previously, we have shown that JAWS II mouse cells, a GM-CSF-dependent DC line established from bone marrow cells of a p53-knockout C57BL/6 mouse, produce CRF after stimulation with B. vulgatus or F. varium at equal levels[27]. The JAWS II mouse cells contain lysosomal endo- and exo-peptidases, which have been shown to be involved in antigen processing, thus providing a reliable tool for in vitro estimation of protein immunogenicity[28,29]. Therefore, we speculated that human DCs also react to commensal bacteria, resulting in the production of CRF and UCN1. However, differences should exist in the capacities for antigen processing between human DCs and bone marrow derived JAWS II from a p53-knockout C57BL/6 mouse. As it is difficult to obtain large amounts of DCs from the colonic mucosa in human samples, MoDCs were generated from 5 healthy male donors with no previous history of acute bacterial gastroenteritis caused by C. clostridioforme, B. vulgatus, E. coli, or F. varium. Our findings demonstrated that CRF and UCN1 were up-regulated in MoDCs after stimulation with B. vulgatus or F. varium; however, differing from the results from mice, the levels were much higher after stimulation with B. vulgatus than with F. varium. Moreover, low levels of UCN1 production were detected in E. coli-stimulated MoDCs, but this was not observed in a previous mouse study[27]. In this study, human MoDCs were generated using rhGM-CSF and rhIL-4, as previously described[18]. However, JAWS II was generated with murine GM-CSF alone. The origin and differentiation of DCs may be, at least in part, associated with the extremely high levels of CRF and UCN1 produced upon stimulation with B. vulgatus in human MoDCs. The amount of crosstalk between B. vulgatus and human MoDCs was previously unknown. Using electron microscopy, we detected B. vulgates in the cytoplasm of human MoDCs when co-cultured with B. vulgates (data not shown). Therefore, we speculated that B. vulgates stimulated molecules associated with CRF and UCN1 production. Recently, it was reported that CRF induced the release of proteases and TNF-αvia mast cells, all of which are associated with CRF-induced intestinal paracellular permeability; these processes are regulated by the enteric nervous system[30]. However, the molecular signaling basis and function of CRF and UCN1 production by MoDCs remain unclear. Previously, it has been reported that CRF-producing cells in the central nervous system[31] and colon[32] of rats are activated by bacterial endotoxins, particularly lipopolysaccharides (LPS). In this experimental setting, CRF production was not detected in E. coli-stimulated MoDCs. The apparent conflict between these results might be associated with the concentration of LPS used in vivo. Moreover, the binding of pathogen-associated molecular patterns to Toll-like receptors may trigger a complex series of events, leading to increased expression of proinflammatory genes in human MoDCs[33]; therefore, differences in the activation of such pathways by commensal bacteria may lead to different levels of CRF and UCN1 production.
Interestingly, a significant increase in CRF and UCN1 mRNA levels was observed in human MoDCs after 30 min of B. vulgatus and F. varium stimulation, although the levels decreased after that point. These findings may imply an immunomodulatory role for CRF and UCN1 by peripheral DCs that could be due to autocrine and/or paracrine interactions. Although mental stress is implicated in the development of IBS and IBD[34], peripheral CRF and UCN1 production by certain commensal bacteria, such as B. vulgatus and F. varium, may also be involved in gastrointestinal sensorimotor and immune cell activation within the gut. Moreover, mental stress may be not directly connected to the local release of CRF neuropeptides. Indeed, the activation of peripheral CRF receptor signaling is mostly considered to be caused by inflammatory processes in the gut[34]. Colonic permeability was increased after local immune cell activation by CRF and UCN1, resulting in increased antigenic challenge in the gut, which might be associated with intestinal disorders, such as IBS and IBD. Given the clinical significance of these pathways in bacteria-related intestinal disorders, a greater understanding of how these pathways are integrated during health and colonic disorders is needed.
Our results show that the production of CRF and UCN1 in human MoDCs is strongly augmented by commensal bacteria, such as F. varium and B. vulgatus. Although the biological significance of these observations remains obscure, these common intestinal microbes may be, at least in part, associated with the pathogenesis of disease through the increased production of CRF and/or UCN1. Further studies are needed to investigate how commensal bacteria are involved in CRF/UCN1-related intestinal inflammation triggered by gut-associated DCs.
Corticotropin-releasing factor (CRF) and urocortin 1 (UCN1) play critical roles in many stress-related intestinal disorders, such as irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD). Moreover, a close association between commensal bacteria and the proximity of human dendritic cells (DCs) to neural elements in patients with IBS or IBD has been reported. However, the pathophysiology of these diseases remains unclear.
DCs are thought to play an important role in the generation and regulation of immune responses. The relationship between hosts and their microbiota is only just beginning to be studied in detail, and given the major role of DCs in bacterial-associated antigen presentation in the gut, it would not be surprising if DC activity was altered in patients with IBS or IBD. However, little is known about the effects of commensal bacteria on human DCs in relation to stress-induced mucosal inflammatory disease.
Both CRF and UCN1 were significantly augmented by Bacteroides vulgatus (B. vulgatus) and Fusobacterium varium (F. varium) at both the mRNA and protein levels. Infection of B. vulgatus and F. varium may be associated with stress-related intestinal disorders.
To examine whether commensal bacteria are a contributing cause of stress-related mucosal inflammation, human peripheral blood monocyte-derived dendritic cells (MoDCs) were stimulated by commensal bacterial strains. The authors assessed the relationship between these bacterial strains and CRF/UCN1 expression at both the mRNA and protein levels in human DCs in this study.
Both CRF and UCN1 are expressed primarily in the paraventricular nucleus of the hypothalamus but are also expressed in peripheral tissues, and they act directly within the colon, where they stimulate secretion and motor activity, leading to the development of watery stools/diarrhea. These events are more common in patients with IBS or IBD.
The manuscript reports the effect on CRF and UCN1 production by MoDCs following stimulation with four different commensal bacterial strains. Overall, the manuscript is very interesting and well written. The conclusion that both CRF and UCN1 were significantly augmented by B. vulgatus and F. varium at both the mRNA and protein levels is straightforward. Future studies are needed to investigate whether commensal bacteria are involved in CRF/UCN1-related intestinal inflammation triggered by gut-associated DCs.
P- Reviewer: Currie PJ S- Editor: Nan J L- Editor: A E- Editor: Liu XM
| 1. | Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394-1397. [PubMed] [Cited in This Article: ] |
| 2. | Shibahara S, Morimoto Y, Furutani Y, Notake M, Takahashi H, Shimizu S, Horikawa S, Numa S. Isolation and sequence analysis of the human corticotropin-releasing factor precursor gene. EMBO J. 1983;2:775-779. [PubMed] [Cited in This Article: ] |
| 3. | Ottaway CA. Role of the neuroendocrine system in cytokine pathways in inflammatory bowel disease. Aliment Pharmacol Ther. 1996;10 Suppl 2:10-15. [PubMed] [Cited in This Article: ] |
| 4. | de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3042] [Cited by in F6Publishing: 3028] [Article Influence: 159.4] [Reference Citation Analysis (0)] |
| 5. | Taché Y, Mönnikes H, Bonaz B, Rivier J. Role of CRF in stress-related alterations of gastric and colonic motor function. Ann N Y Acad Sci. 1993;697:233-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 147] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Greenwood-Van Meerveld B, Johnson AC, Cochrane S, Schulkin J, Myers DA. Corticotropin-releasing factor 1 receptor-mediated mechanisms inhibit colonic hypersensitivity in rats. Neurogastroenterol Motil. 2005;17:415-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Mönnikes H, Schmidt BG, Taché Y. Psychological stress-induced accelerated colonic transit in rats involves hypothalamic corticotropin-releasing factor. Gastroenterology. 1993;104:716-723. [PubMed] [Cited in This Article: ] |
| 8. | Malinen E, Rinttilä T, Kajander K, Mättö J, Kassinen A, Krogius L, Saarela M, Korpela R, Palva A. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am J Gastroenterol. 2005;100:373-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 502] [Cited by in F6Publishing: 479] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 9. | Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol. 2000;95:3503-3506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 508] [Cited by in F6Publishing: 469] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 10. | Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98:412-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 160] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 11. | Kishi D, Takahashi I, Kai Y, Tamagawa H, Iijima H, Obunai S, Nezu R, Ito T, Matsuda H, Kiyono H. Alteration of V beta usage and cytokine production of CD4+ TCR beta beta homodimer T cells by elimination of Bacteroides vulgatus prevents colitis in TCR alpha-chain-deficient mice. J Immunol. 2000;165:5891-5899. [PubMed] [Cited in This Article: ] |
| 12. | Balish E, Warner T. Enterococcus faecalis induces inflammatory bowel disease in interleukin-10 knockout mice. Am J Pathol. 2002;160:2253-2257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 232] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 13. | Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693-702. [PubMed] [Cited in This Article: ] |
| 14. | Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3311] [Cited by in F6Publishing: 3359] [Article Influence: 101.8] [Reference Citation Analysis (0)] |
| 15. | Hughes PA, Zola H, Penttila IA, Blackshaw LA, Andrews JM, Krumbiegel D. Immune activation in irritable bowel syndrome: can neuroimmune interactions explain symptoms? Am J Gastroenterol. 2013;108:1066-1074. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Adams S, O’Neill DW, Bhardwaj N. Recent advances in dendritic cell biology. J Clin Immunol. 2005;25:177-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Ohkusa T, Yoshida T, Sato N, Watanabe S, Tajiri H, Okayasu I. Commensal bacteria can enter colonic epithelial cells and induce proinflammatory cytokine secretion: a possible pathogenic mechanism of ulcerative colitis. J Med Microbiol. 2009;58:535-545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | Weng D, Song B, Durfee J, Sugiyama V, Wu Z, Koido S, Calderwood SK, Gong J. Induction of cytotoxic T lymphocytes against ovarian cancer-initiating cells. Int J Cancer. 2011;129:1990-2001. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Ohkusa T, Okayasu I, Ogihara T, Morita K, Ogawa M, Sato N. Induction of experimental ulcerative colitis by Fusobacterium varium isolated from colonic mucosa of patients with ulcerative colitis. Gut. 2003;52:79-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 187] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 20. | Törnblom H, Lindberg G, Nyberg B, Veress B. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. 2002;123:1972-1979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 342] [Cited by in F6Publishing: 344] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 21. | Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351-1362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1784] [Cited by in F6Publishing: 1629] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 22. | Ekman R, Servenius B, Castro MG, Lowry PJ, Cederlund AS, Bergman O, Sjögren HO. Biosynthesis of corticotropin-releasing hormone in human T-lymphocytes. J Neuroimmunol. 1993;44:7-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 80] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Kravchenco IV, Furalev VA. Secretion of immunoreactive corticotropin releasing factor and adrenocorticotropic hormone by T- and B-lymphocytes in response to cellular stress factors. Biochem Biophys Res Commun. 1994;204:828-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Lee HJ, Kwon YS, Park CO, Oh SH, Lee JH, Wu WH, Chang NS, Lee MG, Lee KH. Corticotropin-releasing factor decreases IL-18 in the monocyte-derived dendritic cell. Exp Dermatol. 2009;18:199-204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Bonfrate L, Tack J, Grattagliano I, Cuomo R, Portincasa P. Microbiota in health and irritable bowel syndrome: current knowledge, perspectives and therapeutic options. Scand J Gastroenterol. 2013;48:995-1009. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 51] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 26. | Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1020] [Cited by in F6Publishing: 1050] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 27. | Hojo M, Ohkusa T, Tomeoku H, Koido S, Asaoka D, Nagahara A, Watanabe S. Corticotropin-releasing factor secretion from dendritic cells stimulated by commensal bacteria. World J Gastroenterol. 2011;17:4017-4022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 10] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Egger M, Jürets A, Wallner M, Briza P, Ruzek S, Hainzl S, Pichler U, Kitzmüller C, Bohle B, Huber CG. Assessing protein immunogenicity with a dendritic cell line-derived endolysosomal degradome. PLoS One. 2011;6:e17278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Jiang X, Shen C, Rey-Ladino J, Yu H, Brunham RC. Characterization of murine dendritic cell line JAWS II and primary bone marrow-derived dendritic cells in Chlamydia muridarum antigen presentation and induction of protective immunity. Infect Immun. 2008;76:2392-2401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 30. | Overman EL, Rivier JE, Moeser AJ. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-α. PLoS One. 2012;7:e39935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 31. | Ait-Belgnaoui A, Durand H, Cartier C, Chaumaz G, Eutamene H, Ferrier L, Houdeau E, Fioramonti J, Bueno L, Theodorou V. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology. 2012;37:1885-1895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 402] [Cited by in F6Publishing: 404] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 32. | Yuan PQ, Wu SV, Wang L, Taché Y. Corticotropin releasing factor in the rat colon: expression, localization and upregulation by endotoxin. Peptides. 2010;31:322-331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675-680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3474] [Cited by in F6Publishing: 3378] [Article Influence: 146.9] [Reference Citation Analysis (0)] |
| 34. | Paschos KA, Kolios G, Chatzaki E. The corticotropin-releasing factor system in inflammatory bowel disease: prospects for new therapeutic approaches. Drug Discov Today. 2009;14:713-720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |