Published online Aug 14, 2014. doi: 10.3748/wjg.v20.i30.10331
Revised: January 24, 2014
Accepted: April 27, 2014
Published online: August 14, 2014
Drug absorption represents an important factor affecting the efficacy of oral drug treatment. Gastric secretion and motility seem to be critical for drug absorption. A causal relationship between impaired absorption of orally administered drugs and Helicobacter pylori (H. pylori) infection has been proposed. Associations have been reported between poor bioavailability of l-thyroxine and l-dopa and H. pylori infection. According to the Maastricht Florence Consensus Report on the management of H. pylori infection, H. pylori treatment improves the bioavailability of both these drugs, whereas the direct clinical benefits to patients still await to be established. Less strong seems the association between H. pylori infection and other drugs malabsorption, such as delavirdine and ketoconazole. The exact mechanisms forming the basis of the relationship between H. pylori infection and impaired drugs absorption and/or bioavailability are not fully elucidated. H. pylori infection may trigger a chronic inflammation of the gastric mucosa, and impaired gastric acid secretion often follows. The reduction of acid secretion closely relates with the wideness and the severity of the damage and may affect drug absorption. This minireview focuses on the evidence of H. pylori infection associated with impaired drug absorption.
Core tip: Drug absorption is a critical factor affecting the efficacy of orally administered therapies. A causal relationship between impaired absorption of orally administered drugs and Helicobacter pylori (H. pylori) infection has been proposed. Previous studies have observed that H. pylori infection and poor bioavailability of l-dopa and l-thyroxine are associated. Less strong seems the association between H. pylori infection and delavirdine and ketoconazole malabsorption. The absorption of oral drugs may potentially be influenced by gastric pH. When a treatment with an oral drug fails, this may be due to a H. pylori-related gastritis and its associated gastric hypochlorhydria, which may partially or totally be reversible.
-
Citation: Lahner E, Virili C, Santaguida MG, Annibale B, Centanni M.
Helicobacter pylori infection and drugs malabsorption. World J Gastroenterol 2014; 20(30): 10331-10337 - URL: https://www.wjgnet.com/1007-9327/full/v20/i30/10331.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i30.10331
Helicobacter pylori (H. pylori) is a widely distributed pathogen of humans infecting about one half of the world population. H. pylori infection is associated with divergent outcomes ranging from symptom-free gastritis to peptic ulcer and gastric cancer[1,2]. The chronic inflammatory response to H. pylori infection may impair some of the physiological gastric functions, i.e., acid secretion. The severity and distribution of gastritis determine the effect of H. pylori infection on gastric acid secretion: when the inflammatory response involves the acid secreting corporal mucosa harbouring the oxyntic glands, hypochlorhydria may occur[3].
Drug absorption is an important factor affecting the efficacy of oral drug treatment. In turn, drug absorption may be affected by gastric secretion and motility[4,5]. A causal relationship between impaired absorption of orally administered drugs and H. pylori infection has been proposed. Previous studies linked the poor bioavailability of both thyroxine and l-dopa to H. pylori infection[6]. According to the Maastricht Florence Consensus Report on the management of H. pylori infection, the bioavailability of both these drugs was improved by H. pylori treatment, whereas the direct clinical benefits to patients still await to be established[7]. Less strong seems the association between H. pylori infection and other drugs, such as delavirdine and ketoconazole malabsorption[6]. This minireview focuses on the evidence of H. pylori infection associated with impaired drug absorption.
L-dopa is the mainstream drug in Parkinson’s disease treatment[8]. The effect of eradication treatment on the clinical and pharmacokinetic response to L-dopa was investigated in advanced Parkinson’s disease patients with high IgG antibody titer against H. pylori[9]. From a pilot study (conducted on six patients only) it emerged that eradication treatment increased by 21%; the L-dopa plasma concentration also improving the clinical benefit. A RCT[10] performed on 34 Parkinson’s disease patients with active H. pylori-gastritis diagnosed by histology, confirmed these results. Patients were randomized to eradication treatment or an antioxidant treatment (allopurinol). In the group of patients treated with eradication therapy, L-dopa absorption increased by 54%, the clinical disability of patients significantly improved and a significant decrease of gastritis scores kept in step with improved L-dopa pharmacokinetics. The positive effect of H. pylori eradication treatment on motor fluctuations was confirmed in a more recent study conducted in South Korea in 2008[11]: in 34 H. pylori-positive patients with Parkinson’s disease, the l-dopa “onset” time and the l-dopa “on-time” significantly decreased by 25.9% and increased by 11.9% after successful cure of infection, while the symptoms score assessed by UPDRS-III scale did not differ before and after cure. The authors therefore concluded that H. pylori eradication may prevent and improve the clinical status of H. pylori infected patients with Parkinson’s disease and motor fluctuations (Table 1).
| Ref. | Study design (level of evidence)1 | n | Helicobacter pylori positivity | Intervention | Outcome measure | Effect of eradicaction treatment |
| Benvenga et al[20] | Crossover study (4) | 6 | Serology | Eradication tx vs placebo | AUC of | 21% increase after eradication treatment |
| L-dopa | No variation after placebo | |||||
| Pierantozzi et al[10] | RCT (1) | 34 | Serology, stool test, CP-test or culture | Eradication tx vs antioxidant tx (allopurinol) | AUC of | 54% increase after eradication treatment |
| L-dopa | No variation after antioxidant tx | |||||
| Lee et al[11] | Case series (3) | 65 | Urea breath test | Eradication tx | UPDRS-III | No variation |
| L-dopa "onset" and "on-time" | 25.9% decrease and 11.9% increase after eradication tx |
The results of a very recent study[12] showed that, compared to age- and gender-matched H. pylori-negative patients, 20 H. pylori positive patients with Parkinson’s disease had decreased “complications of therapy” with average total UPDRS-IV symptoms score of 4.8 ± 3.0 vs 7.7 ± 3.8 (P < 0.05), despite no significant difference in l-dopa equivalent dose. Wearing-off and sleep disturbance were significantly less common in the H. pylori group. The authors explain the observed decreased occurrence of symptoms fluctuations with a likely altered absorption of l-dopa due to the presence of H. pylori infection, but unfortunately they did not assess l-dopa plasma levels neither eradication treatment to support their hypothesis.
Levothyroxine sodium (T4) is the drug of choice in the treatment of thyroid failure as well as of differentiated thyroid carcinoma[13]. A proper T4 treatment requires both the identification of the optimal daily dosage, calculated on the basis of the single patient anthropometric characteristics, and an efficient intestinal absorption of the hormone. It has been proven that both gastric[14-16] and intestinal disorders[17,18] may adversely affect the absorption of the hormone although it actually takes place at the small intestine level[19,20].
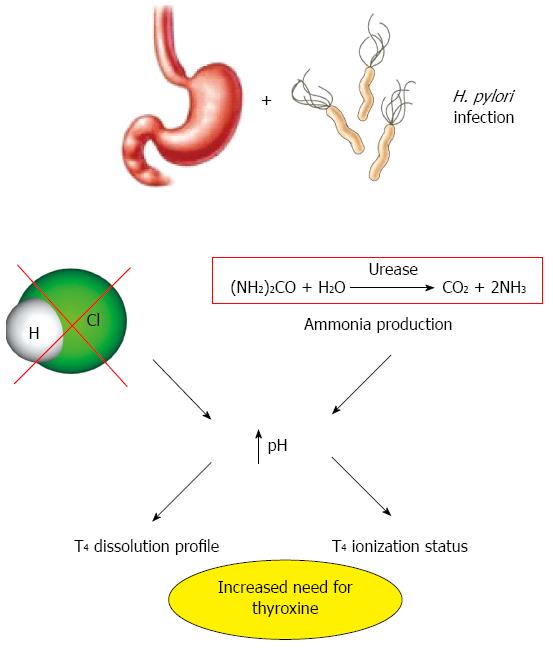
In 2006 the first study was published describing that at least three different conditions of impaired gastric acid secretion may affect the daily requirement of thyroxine in patients with multinodular goiter. H. pylori infection, gastric atrophy and a chronic PPI treatment were, in fact, all associated with a significant increase of T4 dose. Gastric atrophy required a stable increase of about one third of daily T4 dose whereas the effect of H. pylori infection on T4 treatment was reversed upon bacterial eradication; however, in this latter case, the dose had to be increased to reach the therapeutic target[14]. This part of the work was confirmed five years later in a different and less accurate setting[21] and criticized by the same authors in that the potential presence of additional intestinal disorders was not quoted. However, the pleiotropic characteristics of H. pylori infection/host interaction account for the different drug absorption impairment as well[22].
Autoimmune disorders affecting the stomach have been also proven to be responsible for T4 therapy failure. Patients with chronic atrophic gastritis exhibited an increased T4 requirement and these data have been confirmed in a study from Checchi et al[16] who have shown a positive correlation between the increased need for T4 and the high titre of serum parietal cell antibodies (PCA). These antibodies, directed against H+/K+ ATPase, are considered as a marker of gastric autoimmunity and a suspicious flag for the presence of gastric atrophy[23]. However, the increase of T4 requirement was greater in patients with histologically proven gastric atrophy than in PCA-positive subjects[16], suggesting that the presence of PCA does not necessarily imply the presence of atrophic gastritis[23]. More controversial is the interference of proton pump inhibitor (PPI) administration on the levothyroxine sodium treatment[24]. This is relevant in that patients with H. pylori infection often use PPI for treatment. A clear-cut effect of chronic PPI treatment on the elevation of TSH and the consequent need to increase thyroxine dose has been reported in two different studies[14,25]. These findings, however, have not been confirmed in the case of short periods of PPI administration: two pharmacokinetic trials in subjects treated at the same time with T4 and PPI for 1 wk failed to reveal significant differences in peak T4 levels or in the mean Area Under the Curve (AUC) for T4 absorption[24]. The discrepancy has been attributed to the different length of PPI treatment as suggested in a minded review by Liwanpo and Hershman[24].
Delavirdine, a reverse transcriptase inhibitor, is approved by the Food and Drug Administration as combination therapy with appropriate antiretroviral drugs for human immunodeficiency virus (HIV)-1 infection treatment[26]. So far, in only two studies performed by the same authors[27,28] the role of hypochlorhydria in absorption of delavirdine was evaluated in H. pylori-positive patients with concomitant HIV-seropositivity. In a case-series of five HIV-positive patients with gastric hypoacidity (intragastric pH > 3) and positivity to H. pylori serology, it was shown that eradication treatment was able to reverse hypochlorhydria and to significantly increase the absorption of delavirdine[27]. A randomized crossover study on 21 HIV-positive with (n = 11) and without (n = 10) gastric hypoacidity, showed that delavirdine absorption is decreased by 47% in hypoacidic subjects and can be increased by the administration of delavirdine together with orange juice (increase by 57%)[28]. These findings suggest that in subjects with HIV-infection, absorption of delavirdine can be increased by an acid intragastric pH. In 62% of the investigated patients, positive H. pylori serology was reported, but unfortunately eradication treatment was not performed. Despite the similar prevalence of H. pylori infection in both HIV-positive and -negative patients[29,30], a higher histological score of H. pylori-gastritis has been described in HIV-positive patients[31].
In HIV-positive subjects, ketoconazole absorption also seems to be impaired. In a small case series conducted in 1988 by Lake-Bakaar et al[32], the absorption of oral ketoconazole was correlated with gastric acidity in AIDS patients. In this study, the area under the curve of ketoconazole concentration decreased and was normalized by hydrochloric acid in all 7 hypochlorhydric patients, whereas the 3 normochlorhydric patients had normal levels of ketoconazole absorption. This study, however, did not even consider the presence of H. pylori infection, but concluded that in AIDS patients ketoconazole tablets given with acid may be more efficacious because of its pH-dependent bioavailability.
The exact mechanisms forming the basis of the relationship between H. pylori infection and impaired drugs absorption and/or bioavailability are not yet established. In general, characteristics of the drug, as molecular size and shape, ionization degree, and lipid solubility of its ionized and nonionized forms may affect drug absorption[4,5]. Noticeably, motility of the stomach as well as volume and modifications of gastric juice may influence the pharmacokinetics of a drug[33].
H. pylori infection acts as a trigger to chronic inflammation of gastric mucosa, causing an impairment of gastric physiology[3]: gastric motility may be affected by inducing myoelectrical activity variations which in turn bring about an impaired gastric emptying[34-36]. Also an impaired gastric acid secretion often follows H. pylori-induced gastritis, being the degree of impairment closely related with the wideness and the severity of the damage[3]. In fact, when H. pylori gastritis involves the corporal mucosa, which contains the oxyntic glands, a diminished acid secretion may ensue. At an early stage of infection, a direct inhibition of the parietal cells secretion may be exerted by products of inflammation[3,37]. Interleukin 1β, a proinflammatory cytokine and a key mediator in H. pylori-associated disease, inhibits gastric acid secretion in vitro and in vivo[38-40]. Gastric hypoaciditiy may be partly or fully reversed upon H. pylori eradication, without losing the bulk of parietal cells[41-43]. A massive loss of parietal cells, as a consequence of long-standing H. pylori injury, may ensue atrophy of oxyntic glands and, thus, hypoacidity of gastric juice[3,37]. In such a case, whether the cure of infection might reverse the impaired acid secretion is, as yet, controversial[42].
The process of drug absorption may be potentially affected by these pathological changes of the intragastric environment during H. pylori infection. The consequences of this infection may explain the proposed link between H. pylori-related gastritis and reduced bioavailability of some drugs. In particular, the reduced gastric acid secretion seems to be relevant in the process of impaired drug absorption associated with H. pylori infection (Figure 1). In this condition, the process of drug absorption may be affected by the different ionization degree of the molecules as well as by the lesser solubility of many drugs[5].
As far as concern with l-dopa, the hypotheses on the interfering effect of H. pylori on its absorption are still debated. The solubility of l-dopa is pH-sensitive[44], and the putative restoration of normal gastric acidity upon eradication may account for the improved absorption of this drug and the related clinical response[3]. Unluckily, gastric acidity was not assessed in the abovementioned studies. In one of them[10], however, the reported scores of inflammation suggest corporal gastritis and, thus, potential hypoacidity in most of the Parkinson’s disease patients studied[10]. More recently, an interesting hypothesis was proposed and supported by experimental data by Lyte et al[45]: they suggested a direct utilization of l-dopa by H. pylori to preserve its ecological milieu in the gastric mucosa. Evidence that l-dopa may affect the in vitro growth of H. pylori in an iron-restricted minimal medium strengthen this hypothesis[45]. In fact, H. pylori seems to reduce the amount of available oral l-dopa for the treatment of Parkinson’s disease, utilizing l-dopa for its own survival and growth. An association between the incidence of H. pylori infection and occurrence of Parkinsons’ disease has been observed, and a possible cause/effect relationship has been proposed suggesting that H. pylori-induced autoimmunity may act as a trigger for the neuronal damage leading to Parkinsonism[46]. A recent review proposed, through statistical models, a linkage between idiopathic Parkinsonism and infectious/inflammatory status of the gastrointestinal mucosa, mainly due to H. pylori infection[47]. This association between defective absorption of l-dopa and H. pylori-gastritis may be relevant from an epidemiological point of view, since a high incidence of H. pylori infection has been reported in the ten years of highest prevalence of Parkinson’s disease[8,48]. On this ground, a specific diagnostic work-up to detect H. pylori should be proposed in elderly Parkinson’s disease patients, in whom the response to l-dopa treatment is often poor[48].
With regard to T4 malabsorption in subjects with gastric disease or undergoing therapy with PPI, the exact mechanism is incompletely understood, but the variations of gastric acid secretion are a distinctive feature in this situation. The mechanisms underlying the reduction of gastric acidity are shown in Figure 1. Gastric pH variations may change ionization of thyroxine molecule leading to a different ability of this hormone to cross the lipid bilayer of enterocytes apostrophe plasma membrane. In fact, levothyroxine is a low permeability molecule with three ionizable groups and, depending on the solution pH, it can exist as a cation, zwitterion, anion or dianion[49,50]. Sodium- and pH-sensitivity of the iodothyronines cellular uptake in several tissues has been already shown about 30 years ago[51-53]. The pH-dependent variability may or may not deal with a further clue parameter: the dissolution profile of pharmaceutical thyroxine preparation[54,55]. The water solubility of thyroxine sodium salt is reduced in environmental pH from 1 to 3, remains constant between 3 and 7 and increases in pH of about 7[54]. The dissolution of T4 is one of most limiting phases for its absorption but it is not needed when using a liquid preparation of thyroxine[54]. A better pH-dependent dissolution profile of glycerol-dissolved preparations (e.g., softgel) has also been demonstrated, as compared with the traditional tablets[55]. These new formulations may help to improve T4 treatment efficacy in patients with H. pylori infections or other causes of altered gastric secretion[56].
A causative role of hypochlorhydria in impaired delavirdine absorption has been proposed, even taking into account the small sample size and the uncontrolled nature of the study design. In HIV-positive subjects, hypochlorhydria has been frequently reported[57-59] and the most significant predictor for increased intragastric pH has been reported to be H. pylori infection[29]. Delavirdine is a weakly basic drug, whose solubility is strongly reduced when median pH increases. This underlines the sustainability of a biochemical role of intragastric pH on the absorption of delavirdine[30]. Finally, impaired oral ketoconazole absorption in HIV-positive patients was correlated with reduced gastric acid secretion, corrected by administration of hydrochloric acid[32].
The impact of H. pylori infection on drug malabsorption has been focused in this review despite the limited number of studies addressing that issue. To date, four drugs used for the treatment of very different diseases have been investigated, l-dopa, thyroxine, delavirdine and ketoconazole. The issue of drug malabsorption may be particularly important when the implicated drug is generally used for long-term treatments as occurs for l-dopa and thyroxine used for the treatment of chronic conditions such as Parkinon’s disease and thyroid disease.
The common denominator of the impaired absorption of these very different drugs and H. pylori infection seems to be decreased gastric acid secretion.
However, the limit of this review is that the single studies of this issue are very dyshomogeneous and the overall level of evidence is poor. Furthermore, specific pathophysiological studies investigating the probable link between hypochlorhydria and impaired drugs absorption are lacking. Thus, the exact role of H. pylori infection in drug malabsorption remains, to date, speculative and further studies are needed. Despite the constrained evidence of available studies, when an oral drug therapy fails, one of the possible reasons may be a H. pylori-related gastritis and its associated gastric hypoacidity which may be partially or totally reversible. So far, when a treatment is prescribed in general practice, physicians should be aware of the possibility that changes in gastric mucosa and/or gastric acidity status may impair the kinetics of certain drugs. H. pylori infection in this process may be of paramount importance due to its huge prevalence all over the world.
P- Reviewer: Ekmektzoglou KA, Pawaiya RVS, Zubarik R S- Editor: Qi Y L- Editor: O’Neill M E- Editor: Zhang DN
| 1. | Tonkic A, Tonkic M, Lehours P, Mégraud F. Epidemiology and diagnosis of Helicobacter pylori infection. Helicobacter. 2012;17 Suppl 1:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 90] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 2. | Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175-1186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1848] [Cited by in F6Publishing: 1816] [Article Influence: 82.5] [Reference Citation Analysis (3)] |
| 3. | El-Omar EM. Mechanisms of increased acid secretion after eradication of Helicobacter pylori infection. Gut. 2006;55:144-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Hirtz J. The gastrointestinal absorption of drugs in man: a review of current concepts and methods of investigation. Br J Clin Pharmacol. 1985;19 Suppl 2:77S-83S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 74] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Prescott LF. Gastrointestinal absorption of drugs. Med Clin North Am. 1974;58:907-916. [PubMed] [Cited in This Article: ] |
| 6. | Lahner E, Annibale B, Delle Fave G. Systematic review: impaired drug absorption related to the co-administration of antisecretory therapy. Aliment Pharmacol Ther. 2009;29:1219-1229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1541] [Cited by in F6Publishing: 1494] [Article Influence: 124.5] [Reference Citation Analysis (3)] |
| 8. | Lang AE, Lozano AM. Parkinson’s disease. First of two parts. N Engl J Med. 1998;339:1044-1053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1460] [Cited by in F6Publishing: 1361] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 9. | Pierantozzi M, Pietroiusti A, Sancesario G, Lunardi G, Fedele E, Giacomini P, Frasca S, Galante A, Marciani MG, Stanzione P. Reduced L-dopa absorption and increased clinical fluctuations in Helicobacter pylori-infected Parkinson’s disease patients. Neurol Sci. 2001;22:89-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Pierantozzi M, Pietroiusti A, Brusa L, Galati S, Stefani A, Lunardi G, Fedele E, Sancesario G, Bernardi G, Bergamaschi A. Helicobacter pylori eradication and l-dopa absorption in patients with PD and motor fluctuations. Neurology. 2006;66:1824-1829. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 135] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Lee WY, Yoon WT, Shin HY, Jeon SH, Rhee PL. Helicobacter pylori infection and motor fluctuations in patients with Parkinson’s disease. Mov Disord. 2008;23:1696-1700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Rahne KE, Tagesson C, Nyholm D. Motor fluctuations and Helicobacter pylori in Parkinson’s disease. J Neurol. 2013;260:2974-2980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Wiersinga WM. Thyroid hormone replacement therapy. Horm Res. 2001;56 Suppl 1:74-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Centanni M, Gargano L, Canettieri G, Viceconti N, Franchi A, Delle Fave G, Annibale B. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N Engl J Med. 2006;354:1787-1795. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in F6Publishing: 195] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 15. | Centanni M, Santaguida MG, Gargano L. Malabsorption of T4: new insights on oral thyroxine treatment. hotthyroidology.com, Official Journal of ETA 2007, n.169. Available from: http://www.hotthyroidology.com/editorial_169.html. [Cited in This Article: ] |
| 16. | Checchi S, Montanaro A, Pasqui L, Ciuoli C, De Palo V, Chiappetta MC, Pacini F. L-thyroxine requirement in patients with autoimmune hypothyroidism and parietal cell antibodies. J Clin Endocrinol Metab. 2008;93:465-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Virili C, Bassotti G, Santaguida MG, Iuorio R, Del Duca SC, Mercuri V, Picarelli A, Gargiulo P, Gargano L, Centanni M. Atypical celiac disease as cause of increased need for thyroxine: a systematic study. J Clin Endocrinol Metab. 2012;97:E419-E422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 18. | Ruchała M, Szczepanek-Parulska E, Zybek A. The influence of lactose intolerance and other gastro-intestinal tract disorders on L-thyroxine absorption. Endokrynol Pol. 2012;63:318-323. [PubMed] [Cited in This Article: ] |
| 19. | Hays MT. Localization of human thyroxine absorption. Thyroid. 1991;1:241-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 98] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Benvenga S, Bartolone L, Squadrito S, Lo Giudice F, Trimarchi F. Delayed intestinal absorption of levothyroxine. Thyroid. 1995;5:249-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 114] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Bugdaci MS, Zuhur SS, Sokmen M, Toksoy B, Bayraktar B, Altuntas Y. The role of Helicobacter pylori in patients with hypothyroidism in whom could not be achieved normal thyrotropin levels despite treatment with high doses of thyroxine. Helicobacter. 2011;16:124-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Smolka AJ, Backert S. How Helicobacter pylori infection controls gastric acid secretion. J Gastroenterol. 2012;47:609-618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 60] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 23. | Centanni M, Marignani M, Gargano L, Corleto VD, Casini A, Delle Fave G, Andreoli M, Annibale B. Atrophic body gastritis in patients with autoimmune thyroid disease: an underdiagnosed association. Arch Intern Med. 1999;159:1726-1730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 88] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Liwanpo L, Hershman JM. Conditions and drugs interfering with thyroxine absorption. Best Pract Res Clin Endocrinol Metab. 2009;23:781-792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 25. | Sachmechi I, Reich DM, Aninyei M, Wibowo F, Gupta G, Kim PJ. Effect of proton pump inhibitors on serum thyroid-stimulating hormone level in euthyroid patients treated with levothyroxine for hypothyroidism. Endocr Pract. 2007;13:345-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Friedland GH, Pollard R, Griffith B, Hughes M, Morse G, Bassett R, Freimuth W, Demeter L, Connick E, Nevin T. Efficacy and safety of delavirdine mesylate with zidovudine and didanosine compared with two-drug combinations of these agents in persons with HIV disease with CD4 counts of 100 to 500 cells/mm3 (ACTG 261). ACTG 261 Team. J Acquir Immune Defic Syndr. 1999;21:281-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Shelton MJ, Akbari B, Hewitt RG, Adams JM, Morse GD. Eradication of Helicobacter pylori is associated with increased exposure to delavirdine in hypochlorhydric HIV-positive patients. J Acquir Immune Defic Syndr. 2000;24:79-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Shelton MJ, Hewitt RG, Adams JM, Cox SR, Chambers JH, Morse GD. Delavirdine malabsorption in HIV-infected subjects with spontaneous gastric hypoacidity. J Clin Pharmacol. 2003;43:171-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Tran JQ, Gerber JG, Kerr BM. Delavirdine: clinical pharmacokinetics and drug interactions. Clin Pharmacokinet. 2001;40:207-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Panos GZ, Xirouchakis E, Tzias V, Charatsis G, Bliziotis IA, Doulgeroglou V, Margetis N, Falagas ME. Helicobacter pylori infection in symptomatic HIV-seropositive and -seronegative patients: a case-control study. AIDS Res Hum Retroviruses. 2007;23:709-712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Olmos M, Araya V, Pskorz E, Quesada EC, Concetti H, Perez H, Cahn P. Coinfection: Helicobacter pylori/human immunodeficiency virus. Dig Dis Sci. 2004;49:1836-1839. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Lake-Bakaar G, Tom W, Lake-Bakaar D, Gupta N, Beidas S, Elsakr M, Straus E. Gastropathy and ketoconazole malabsorption in the acquired immunodeficiency syndrome (AIDS). Ann Intern Med. 1988;109:471-473. [PubMed] [Cited in This Article: ] |
| 33. | Golub AL, Frost RW, Betlach CJ, Gonzalez MA. Physiologic considerations in drug absorption from the gastrointestinal tract. J Allergy Clin Immunol. 1986;78:689-694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 34] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | McColl KE, el-Omar E, Gillen D. Helicobacter pylori gastritis and gastric physiology. Gastroenterol Clin North Am. 2000;29:687-703, viii. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Thor P, Lorens K, Tabor S, Herman R, Konturek JW, Konturek SJ. Dysfunction in gastric myoelectric and motor activity in Helicobacter pylori positive gastritis patients with non-ulcer dyspesia. J Physiol Pharmacol. 1996;47:469-476. [PubMed] [Cited in This Article: ] |
| 36. | Miyaji H, Azuma T, Ito S, Abe Y, Ono H, Suto H, Ito Y, Yamazaki Y, Kohli Y, Kuriyama M. The effect of helicobacter pylori eradication therapy on gastric antral myoelectrical activity and gastric emptying in patients with non-ulcer dyspepsia. Aliment Pharmacol Ther. 1999;13:1473-1480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Annibale B, Capurso G, Delle Fave G. The stomach and iron deficiency anaemia: a forgotten link. Dig Liver Dis. 2003;35:288-295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | McNamara D, El-Omar E. Helicobacter pylori infection and the pathogenesis of gastric cancer: a paradigm for host-bacterial interactions. Dig Liver Dis. 2008;40:504-509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 39. | Beales IL, Calam J. Interleukin 1 beta and tumour necrosis factor alpha inhibit acid secretion in cultured rabbit parietal cells by multiple pathways. Gut. 1998;42:227-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 221] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 40. | Takashima M, Furuta T, Hanai H, Sugimura H, Kaneko E. Effects of Helicobacter pylori infection on gastric acid secretion and serum gastrin levels in Mongolian gerbils. Gut. 2001;48:765-773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 41. | El-Omar EM. The importance of interleukin 1beta in Helicobacter pylori associated disease. Gut. 2001;48:743-747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 242] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 42. | Annibale B, Di Giulio E, Caruana P, Lahner E, Capurso G, Bordi C, Delle Fave G. The long-term effects of cure of Helicobacter pylori infection on patients with atrophic body gastritis. Aliment Pharmacol Ther. 2002;16:1723-1731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Osawa H, Kita H, Ohnishi H, Hoshino H, Mutoh H, Ishino Y, Watanabe E, Satoh K, Sugano K. Helicobacter pylori eradication induces marked increase in H+/K+-adenosine triphosphatase expression without altering parietal cell number in human gastric mucosa. Gut. 2006;55:152-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Standaert DG, Young AB. Treatment of central nervous system degenerative disorders. The pharmacological basis of therapeutics. IXth ed. New York: McGraw-Hill 1996; 503-519. [Cited in This Article: ] |
| 45. | Lyte M. Microbial endocrinology as a basis for improved L-DOPA bioavailability in Parkinson’s patients treated for Helicobacter pylori. Med Hypotheses. 2010;74:895-897. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Dobbs SM, Dobbs RJ, Weller C, Charlett A. Link between Helicobacter pylori infection and idiopathic parkinsonism. Med Hypotheses. 2000;55:93-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Weller C, Oxlade N, Dobbs SM, Dobbs RJ, Charlett A, Bjarnason IT. Role of inflammation in gastrointestinal tract in aetiology and pathogenesis of idiopathic parkinsonism. FEMS Immunol Med Microbiol. 2005;44:129-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 48. | Dobbs RJ, Charlett A, Dobbs SM, Weller C, Peterson DW. Parkinsonism: differential age-trend in Helicobacter pylori antibody. Aliment Pharmacol Ther. 2000;14:1199-1205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 49. | Lindenberg M, Kopp S, Dressman JB. Classification of orally administered drugs on the World Health Organization Model list of Essential Medicines according to the biopharmaceutics classification system. Eur J Pharm Biopharm. 2004;58:265-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 522] [Cited by in F6Publishing: 493] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 50. | Chemburkar SR, Deming KC, Reddy RE. Chemistry of thyroxine: an historical perspective and recent progress on its synthesis. Tetrahedron. 2010;66:1955-1962. [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 51. | Hennemann G, Docter R, Friesema EC, de Jong M, Krenning EP, Visser TJ. Plasma membrane transport of thyroid hormones and its role in thyroid hormone metabolism and bioavailability. Endocr Rev. 2001;22:451-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 193] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 52. | Centanni M, Sapone A, Taglienti A, Andreoli M. Effect of extracellular sodium on thyroid hormone uptake by mouse thymocytes. Endocrinology. 1991;129:2175-2179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 53. | Centanni M, Robbins J. Role of sodium in thyroid hormone uptake by rat skeletal muscle. J Clin Invest. 1987;80:1068-1072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 54. | Yue CS, Scarsi C, Ducharme MP. Pharmacokinetics and potential advantages of a new oral solution of levothyroxine vs. other available dosage forms. Arzneimittelforschung. 2012;62:631-636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 55. | Pabla D, Akhlaghi F, Zia H. A comparative pH-dissolution profile study of selected commercial levothyroxine products using inductively coupled plasma mass spectrometry. Eur J Pharm Biopharm. 2009;72:105-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 56. | Centanni M. Thyroxine treatment: absorption, malabsorption, and novel therapeutic approaches. Endocrine. 2013;43:8-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 57. | Shaffer RT, LaHatte LJ, Kelly JW, Kadakia S, Carrougher JG, Keate RF, Starnes EC. Gastric acid secretion in HIV-1 infection. Am J Gastroenterol. 1992;87:1777-1780. [PubMed] [Cited in This Article: ] |
| 58. | Welage LS, Carver PL, Revankar S, Pierson C, Kauffman CA. Alterations in gastric acidity in patients infected with human immunodeficiency virus. Clin Infect Dis. 1995;21:1431-1438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 64] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 59. | Shelton MJ, Adams JM, Hewitt RG, Morse GD. Previous infection with Helicobacter pylori is the primary determinant of spontaneous gastric hypoacidity in human immunodeficiency virus-infected outpatients. Clin Infect Dis. 1998;27:739-745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |