Published online Jan 21, 2014. doi: 10.3748/wjg.v20.i3.857
Revised: September 2, 2013
Accepted: September 16, 2013
Published online: January 21, 2014
A 77-year-old man with inflammatory bowel disease (IBD) and who was treated with anti-tumor necrosis factor (TNF), 6-mercaptopurine and corticosteroids, presented with primary effusion lymphoma-like lymphoma (PEL-like lymphoma) with massive ascites. The patient’s clinical course was complicated by acute renal insufficiency and hypotension, which led to death within 2 wk. In general, patients with IBD may have an increased risk for development of lymphoma, which is frequently associated with immunosuppressive and/or anti-TNF antibody therapies. PEL is a rare subset of lymphoma localized to serous body cavities, lacks tumor mass or nodal involvement, and is associated with infection by human herpes virus 8 (HHV-8). Primary neoplastic effusion may also be present in patients with large B-cell lymphoma without evidence of human immunodeficiency virus or HHV-8 infections. This type of lymphoma is classified as PEL-like lymphoma. Both PEL and PEL-like lymphoma types have been reported in patients undergoing immunosuppressive therapy, but to the best of our knowledge, the case described herein represents the first PEL-like lymphoma occurring in a patient with IBD.
Core tip: We report a case of primary effusion lymphoma (PEL)-like lymphoma in an elderly, male inflammatory bowel disease (IBD) patient on 6-mercaptopurine (6-MP) treatment. This rare lymphoma subtype is localized to serous body cavities without tumor mass formation or nodal involvement, and has been previously reported in individuals with hepatitis C virus or Epstein-Barr virus infection, in patients who underwent organ transplantation, and elderly patients This novel case in an IBD patient illustrates the importance of considering PEL-like lymphoma in IBD patients treated with 6-MP and anti-tumor necrosis factor antibodies who subsequently develop serous body cavity effusion.
- Citation: Nussinson E, Shibli F, Shahbari A, Rock W, Elias M, Elmalah I. Primary effusion lymphoma-like lymphoma in a patient with inflammatory bowel disease. World J Gastroenterol 2014; 20(3): 857-862
- URL: https://www.wjgnet.com/1007-9327/full/v20/i3/857.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i3.857
Primary effusion lymphoma (PEL) and PEL-like lymphoma are rare subsets of B cell lymphoma. The infrequent cases described in the literature have involved immunodeficient individuals [including patients positive for human immunodeficiency virus (HIV), who underwent transplant, or were undergoing immunosuppressive treatment][1,2], individuals with concomitant infection [including Epstein-Barr virus (EBV)[2-5] or hepatitis C virus (HCV)][2,4-10], cirrhotic[4,6,11] and elderly[4,6,12] individuals. To date however, no case of these rare lymphomas has involved a patient with inflammatory bowel disease (IBD).
Since its initial description as a distinct clinical entity in 1996[13], PEL has been classified as a subtype of lymphoma and characterized as strongly associated with human herpes virus 8 (HHV-8) infection. In contrast, the PEL-like lymphoma subtype, first described in 2008, appears unrelated to HHV-8 infection[1]. Lymphoma is a recognized complication of IBD, most frequently occurring in the form of diffuse large B cell lymphoma. Yet, according to cohort population studies it is debatable whether IBD patients have an increased risk of lymphoma compared with the general population[14-27]. Thiopurine immunosuppressive therapy alone or in combination with anti-tumor necrosis factor (TNF) antibody has rarely been associated with the development of lymphoma in IBD[18-20,26-33]. In young IBD patients, anti-TNF treatment may be associated with rare forms of T cell lymphoma, such as hepatosplenic lymphoma and natural killer T cell lymphoma[34-37].
Herein, we report the occurrence of PEL-like lymphoma in an elderly IBD patient on immunosuppressive therapy. The patient developed massive ascites, fluid cytology of which showed CD20-, BCL2, and vimentin positive large lymphocytes consistent with PEL-like lymphoma.
A 77-year-old man with a 3-year history of IBD presented with fever (peak at 39.5 °C) and dyspnea. The on-going IBD condition had been originally diagnosed following a sustained bout of severe and frequent bloody diarrhea. The patient’s medical history included ischemic heart disease and mitral valve annuloplasty due to mitral valve thickening with myxomatous changes and significant regurgitation. At the initial admission, the patient’s serum C-reactive protein (CRP) levels were markedly elevated (78 mg/dL) and the hemoglobin level was slightly below the normal range (13.1 g/dL; normal: 13.8-17.2 g/dL). Colonoscopy showed active left-sided colitis and diverticulosis, and the colonoscopic biopsies showed chronic ulcerative colitis.
An initial treatment of 5-aminosalicylate (4 g/d) had been administered, but the patient showed no response and oral corticosteroid therapy was initiated and provided good results. However, steroid dependency was observed. A tuberculin test was normal and serological tests for hepatitis C antibodies and hepatitis B surface antigen were negative. Anti-TNF therapy (infliximab) had then been initiated but produced only a moderate response and was discontinued after the third dose due to an allergic reaction and only a moderate response.
A second colonoscopy performed 1 year after the initial diagnosis and treatment of IBD showed no signs of active endoscopic colitis, although diverticulosis of the left colon and two small sporadic adenomas of the right colon were observed, together with normal terminal ileum. Histopathological analysis of current biopsies taken from the hepatic flexure to the rectum showed chronic inflammation, crypt abscesses, and multinucleated giant cells. Two years after the initial diagnosis of IBD (and 1 year before the most recent admission), treatment with 6-mercaptopurine (6-MP; 50 mg/d) was administered.
At the patient’s most recent clinic presentation, physical examination revealed diminished inspiratory breath sounds on auscultation and increased dullness on percussion of the left lung field. Heart auscultation revealed a mild systolic murmur in the mitral valve area. The patient’s abdomen was distended and mildly tender in the left lower quadrant, with slightly increased peristaltic sounds. Shifting dullness consistent with moderate ascites was observed. No palpable organomegaly, masses, or enlarged lymph nodes were detected.
The laboratory test results were as follows: elevated CRP level (68 mg/dL); decreased hemoglobin level (11.6 g/dL); low hematocrit (34%; normal range: 38.8%- 50.0%); low white blood cell (WBC) count (2.09 × 103/μL with 1400 neutrophils/μL; normal range: 3.50-10.50 × 103/μL); normal platelet count (232000/μL; normal range: 150000-450000/μL); high erythrocyte sedimentation rate (20 mm/h; normal range: 0-17 mm/h); high creatinine level (1.7 mg/dL; normal range: 0.5-1.1 mg/dL); low serum protein level (5.02 g/dL; normal range: 6-8 g/dL); and low albumin level (2.7 g/dL; normal range: 3.4-5.6 g/dL). Serum levels of alkaline phosphatase, glutamic oxaloacetic transaminase, glutamic pyruvic transaminase, and lactate dehydrogenase were all within normal range. Serum was negative for EBV (IgM), HCV, hepatitis B virus (HBV) and HIV antibodies, and positive for cytomegalic virus IgM antibody with high avidity. Serologic tests for Brucella, Mycoplasma, and varicella-zoster virus were negative. Carbohydrate antigen (CA) 19-9, carcinoembryonic antigen, and alpha-fetoprotein levels were within normal range, whereas the CA125 level was high (516 U/mL; normal limit: < 35 U/mL). An echocardiogram revealed suspicious vegetation on the mitral valve, although this finding was later determined to be incorrect. Urine culture identified Enterococcus bacteria and antibiotic therapy (amoxicillin and gentamicin) was administered.
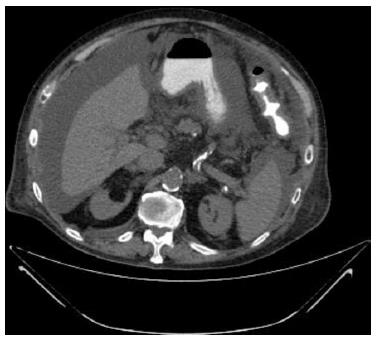
Over the next two weeks, the patient’s overall condition deteriorated. Increasing amounts of pleural effusion and massive ascites developed. Chest and abdominal computed tomography showed bilateral pleural effusion, ascites and omental infiltration without enlarged masses or lymph nodes (Figure 1). Doppler ultrasonography of the portal, hepatic and femoral veins showed normal flow without venous thrombosis. Ascites fluid analysis yielded the following results: elevated WBC count (580 × 103/μL; normal limit: < 500 × 103/μL); normal neutrophils count (30 × 103/μL; normal limit: < 250 × 103/μL); elevated monocytes count (180 × 103/μL; normal limit: < 9% of WBC); elevated atypical lymphocytes count (140 × 103/μL; normal value: 0); normal glucose (86 mg/dL; normal limit: > 50 mg/dL); near normal total protein level (2.6 g/dL; normal limit: < 2.5 g/dL); albumin level 1.5 g/dL; high lactate dehydrogenase level (1260 U/L; normal limit: 0.6 of the serum level); normal amylase level (26 U/L; normal limit: < 100 U/L); and normal triglyceride level (16 mg/dL; normal limit: < 200 mg/dL).
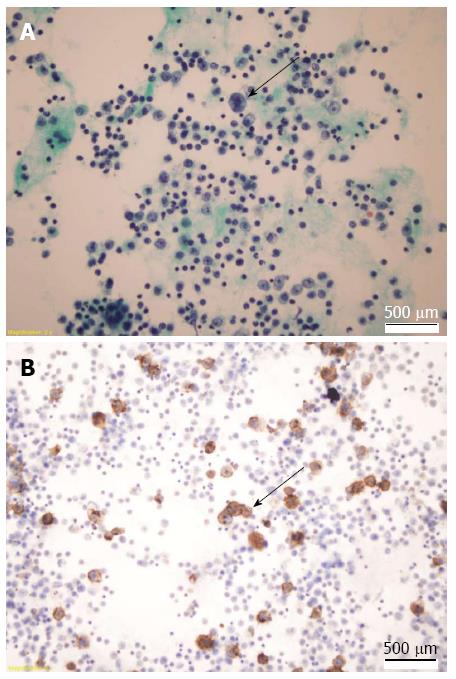
Bacterial culturing of ascites fluid provided negative results for all species tested, and polymerase chain reaction for Mycobacterium tuberculosis was negative. Cytologic examination of the clear yellow ascites fluid showed enlarged cells with large nuclei, macronucleoli, and abundant cytoplasm (Figure 2A). Immunohistochemical analysis showed negativity for HHV-8 latent nuclear antigen expression. Immunophenotypically, the cells were positive for CD20 (Figure 2B), BCL-2 and vimentin. Flow cytometry revealed CD20- and CD19-positive and CD10-, CD38-, CD56-negative large B cells.
Collectively, these data were consistent with a diagnosis of large B cell lymphoma. After 10 d of admission the patient developed hypotension with acute renal failure, which was attributed to the gentamicin treatment. Despite treatment with intravenous norepinephrine and ascites fluid drainage with intravenous albumin infusion the renal failure became aggravated. The patient underwent hemodialysis but succumbed to the lethal disease course at 14 d after the most recent admission.
An increased risk of lymphoma in IBD patients has been reported in several studies[14-20,33,38,39]; in contrast, more recent studies did not show a significantly increased risk of lymphoma in IBD patients compared with the general population[16,17,20-27,38]. Thus, the high risk of lymphoma in IBD patients compared with the general population is still debated. However, the use of thiopurine and anti-TNF alone or in combination is known to be associated with a 2.6- to 5.28-fold increased risk of lymphoma in IBD patients[18,19,29,30]. The standardized incidence ratio (relative to the normal population) for lymphoma in IBD patients who were prescribed anti-TNF[32] was shown to be 5.5, and in another study, a 3-fold higher frequency of lymphoma was found amongst IBD patients given anti-TNF[30]. However, even with the increased risk of lymphoma in patients with IBD on thiopurine immunosuppression and anti-TNF therapy, the overall incidence of lymphoma is low[19,29].
Several cases of drug-induced lymphomas in IBD patients are present in the literature, providing precedence for the current case of 6-MP-related PEL-like lymphoma. Indeed, IBD patients over the age of 65 have been characterized as having higher risk of lymphoma due to thiopurine treatment[18,19]. IBD patients under the age of 50 who received thiopurine have shown less frequent rates of lymphoma, and these cases have been suggested to be associated with infectious mononucleosis (EBV)[18,19,26,30]. Anti-TNF therapy in adolescent male IBD patients has also been suggested as associated with development of the rare hepatosplenic T cell lymphoma[34,36,37]; these T cell-derived tumors are EBV-negative in IBD patients and associated with very poor prognosis[19]. In addition, hepatosplenic T cell lymphoma has been reported as a rare complication in IBD patients and attributed to long-term thiopurine exposure[36]. Finally, a single case of infliximab-induced natural killer T cell lymphoma (CD3-, CD56-, CD30- and EBV-positive) in a young IBD patient was reported recently[35].
PEL is a relatively rare subtype of B cell lymphoma, accounting for approximately 0.3% of non-Hodgkin’s lymphoma in HIV-negative individuals and approximately 4% of non-Hodgkin’s lymphoma in HIV-positive patients. Generally, PEL develops in HHV-8-positive patients and may be associated with HIV infection[40]. It is characterized by the involvement of serous body cavities and presents as pleural, peritoneal, and pericardial effusion with no tumor mass or nodal involvement[2,11]. Its phenotypic characterization includes negative expression of B cell- and T cell-associated antigens (such as the classic B-cell markers CD20 and BCL-2[1,41,42]) and positive expression of activation markers (such as CD30, CD38 and CD71) and epithelial membrane antigen and plasma cell markers (such as CD138)[2,5]. However, immunophenotyping studies have shown that it is represented by a monoclonal B cell population[42].
HHV-8 and EBV viral infections are considered etiological factors for the development of PEL. Several possible mechanisms may explain how HHV-8 promotes oncogenesis in PEL. First, expression of the viral homologue of cyclin D, a latent gene product of the HHV-8 genome in infected cells, can lead to uncontrolled cell division[3]. Second, the HHV-8 encoded protein latency associated nuclear antigen (LANA)-1 exerts its oncogenic activities by binding to the tumor suppressor protein, p53[43]. Third, LANA-1 is also known to block the transforming growth factor-beta signaling pathway[44]. Finally, another HHV-8 encoded protein, LANA-2, is known to inhibit apoptosis[44].
EBV may also induce B cell proliferation and post-transplantation lymphoproliferative disorder through the EBV-associated protein latent membrane protein-1, which causes cell growth and transformation[45,46]. While EBV-related lymphoma has been described in IBD patients treated with thiopurines[46,47] and the majority of PEL cases in the literature show evidence of EBV infection, the precise role of this virus in PEL oncogenesis remains unclear.
According to the World Health Organization, PEL is only related to HHV-8-positive primary lymphomatous effusion[1]. However, primary neoplastic effusion has also been demonstrated in cases of Burkitt’s lymphoma (CD10-positive) and of large B cell lymphoma (HIV-negative and HHV-8-independent)[1]. In the present case, the lymphomatous effusion cells were found to be devoid of HHV-8 infection and displayed morphological and immunophenotypic features of large B cell lymphoma, consistent with the diagnosis of PEL-like lymphoma[1,41].
The majority of PEL-like lymphoma cases reported in the literature mainly involve elderly or immunocompromised patients[1,2,11,40]. Those cases involving immunocompetent patients show a trend of concomitant HCV or EBV infection (19%-42% of patients)[1,2,5,7-11]. HCV infection may cause PEL-like lymphoma by involvement of the CD81 antigen on the cell surface of B cells, which binds to HCV and triggers polyclonal B cell expansion. Subsequent genetic changes may contribute to the development of B cell lymphoma; for example, overexpression of BCL-2 (an anti-apoptotic factor) and HCV-induced translocation can result in deregulation of PAX5 gene transcription (which encodes the B cell specific antigen)[4,7-10,48]. However, the present case showed no evidence of infection with HCV or EBV.
PEL-like lymphoma accounts for 20% of PEL cases[12]. The definite molecular pathways that underlie the development of malignant lymphomatous effusion in PEL-like lymphoma are still unknown, but are probably similar to those of B cell lymphoma and may include genetic changes such as immunoglobulin and cMYC gene rearrangement (translocations), mutations in the BCL-6 and P53 genes (pro-apoptotic factor)[2,49-51], and trisomy 8[2,4].
The outcome of HIV-negative, HHV-8-unrelated PEL-like lymphoma is better (median survival: 6-10 mo; 1-year survival rate: 35%) than that of HIV-positive PEL (median survival: 4 mo; 1-year survival rate: 17%)[2]. Treatment for PEL includes combination chemotherapy with cyclophosphamide, doxorubicin, vincristine and prednisone, to which adjunctive treatment with rituximab (anti-CD20 antibody) may be added for PEL-like lymphoma to achieve improved outcomes[3,52,53]. Our patient did not receive chemotherapy because he was critically ill with hypotension and renal failure at the time of diagnosis, which was established only a few days before his death.
In conclusion, we have described the first case of PEL-like lymphoma in an elderly IBD patient on immunosuppressive and biological therapy. We suggest that PEL-like lymphoma can be considered as an additional subset of lymphoma that may rarely complicate the course of IBD treated with immunosuppressive and biologic agents.
P- Reviewer: Libra M S- Editor: Zhai HH L- Editor: A E- Editor: Liu XM
| 1. | Carbone A, Gloghini A. PEL and HHV8-unrelated effusion lymphomas: classification and diagnosis. Cancer. 2008;114:225-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Adiguzel C, Bozkurt SU, Kaygusuz I, Uzay A, Tecimer T, Bayik M. Human herpes virus 8-unrelated primary effusion lymphoma-like lymphoma: report of a rare case and review of the literature. APMIS. 2009;117:222-229. [PubMed] [Cited in This Article: ] |
| 3. | Chen YB, Rahemtullah A, Hochberg E. Primary effusion lymphoma. Oncologist. 2007;12:569-576. [PubMed] [Cited in This Article: ] |
| 4. | Alexanian S, Said J, Lones M, Pullarkat ST. KSHV/HHV8-negative effusion-based lymphoma, a distinct entity associated with fluid overload states. Am J Surg Pathol. 2013;37:241-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Brimo F, Popradi G, Michel RP, Auger M. Primary effusion lymphoma involving three body cavities. Cytojournal. 2009;6:21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Kim KH, Lee JH, Jeong HC, Kim GW, Song SH, Jung SY, Kim GI, Kim EK. A case of human herpes virus-8 unrelated primary effusion lymphoma-like lymphoma presented as pleural effusion. Tuberc Respir Dis (Seoul). 2012;73:336-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Ichinohasama R, Miura I, Kobayashi N, Saitoh Y, DeCoteau JF, Saiki Y, Mori S, Kadin ME, Ooya K. Herpes virus type 8-negative primary effusion lymphoma associated with PAX-5 gene rearrangement and hepatitis C virus: a case report and review of the literature. Am J Surg Pathol. 1998;22:1528-1537. [PubMed] [Cited in This Article: ] |
| 8. | Paner GP, Jensen J, Foreman KE, Reyes CV. HIV and HHV-8 negative primary effusion lymphoma in a patient with hepatitis C virus-related liver cirrhosis. Leuk Lymphoma. 2003;44:1811-1814. [PubMed] [Cited in This Article: ] |
| 9. | Ascoli V, Lo Coco F, Artini M, Levrero M, Fruscalzo A, Mecucci C. Primary effusion Burkitt’s lymphoma with t(8; 22) in a patient with hepatitis C virus-related cirrhosis. Hum Pathol. 1997;28:101-104. [PubMed] [Cited in This Article: ] |
| 10. | Takao T, Kobayashi Y, Kuroda J, Omoto A, Nishimura T, Kamitsuji Y, Fukiya E, Nakamura C, Kimura S, Yoshikawa T. Rituximab is effective for human herpesvirus-8-negative primary effusion lymphoma with CD20 phenotype associated hepatitis C virus-related liver cirrhosis. Am J Hematol. 2004;77:419-420. [PubMed] [Cited in This Article: ] |
| 11. | Gandhi SA, Mufti G, Devereux S, Ireland R. Primary effusion lymphoma in an HIV-negative man. Br J Haematol. 2011;155:411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Kobayashi Y, Kamitsuji Y, Kuroda J, Tsunoda S, Uoshima N, Kimura S, Wada K, Matsumoto Y, Nomura K, Horiike S. Comparison of human herpes virus 8 related primary effusion lymphoma with human herpes virus 8 unrelated primary effusion lymphoma-like lymphoma on the basis of HIV: report of 2 cases and review of 212 cases in the literature. Acta Haematol. 2007;117:132-144. [PubMed] [Cited in This Article: ] |
| 13. | Nador RG, Cesarman E, Chadburn A, Dawson DB, Ansari MQ, Sald J, Knowles DM. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi’s sarcoma-associated herpes virus. Blood. 1996;88:645-656. [PubMed] [Cited in This Article: ] |
| 14. | Greenstein AJ, Gennuso R, Sachar DB, Heimann T, Smith H, Janowitz HD, Aufses AH. Extraintestinal cancers in inflammatory bowel disease. Cancer. 1985;56:2914-2921. [PubMed] [Cited in This Article: ] |
| 15. | Van Domselaar M, López San Román A, Bastos Oreiro M, Garrido Gómez E. [Lymphoproliferative disorders in an inflammatory bowel disease unit]. Gastroenterol Hepatol. 2010;33:12-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Bewtra M, Lewis JD. Safety profile of IBD: lymphoma risks. Gastroenterol Clin North Am. 2009;38:669-689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Palli D, Trallori G, Bagnoli S, Saieva C, Tarantino O, Ceroti M, d’Albasio G, Pacini F, Amorosi A, Masala G. Hodgkin’s disease risk is increased in patients with ulcerative colitis. Gastroenterology. 2000;119:647-653. [PubMed] [Cited in This Article: ] |
| 18. | Beaugerie L, Brousse N, Bouvier AM, Colombel JF, Lémann M, Cosnes J, Hébuterne X, Cortot A, Bouhnik Y, Gendre JP. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009;374:1617-1625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 774] [Cited by in F6Publishing: 748] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 19. | Weinstock DM. Epstein-Barr virus, lymphoma risk and the potential role of HIV infection in IBD patients undergoing immunosuppression. Dig Dis. 2010;28:519-524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Holubar SD, Dozois EJ, Loftus EV, Teh SH, Benavente LA, Harmsen WS, Wolff BG, Cima RR, Larson DW. Primary intestinal lymphoma in patients with inflammatory bowel disease: a descriptive series from the prebiologic therapy era. Inflamm Bowel Dis. 2011;17:1557-1563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Sultan K, Korelitz BI, Present D, Katz S, Sunday S, Shapira I. Prognosis of lymphoma in patients following treatment with 6-mercaptopurine/azathioprine for inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:1855-1858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Askling J, Brandt L, Lapidus A, Karlén P, Björkholm M, Löfberg R, Ekbom A. Risk of haematopoietic cancer in patients with inflammatory bowel disease. Gut. 2005;54:617-622. [PubMed] [Cited in This Article: ] |
| 23. | Lewis JD, Bilker WB, Brensinger C, Deren JJ, Vaughn DJ, Strom BL. Inflammatory bowel disease is not associated with an increased risk of lymphoma. Gastroenterology. 2001;121:1080-1087. [PubMed] [Cited in This Article: ] |
| 24. | Bebb JR, Logan RP. Review article: does the use of immunosuppressive therapy in inflammatory bowel disease increase the risk of developing lymphoma? Aliment Pharmacol Ther. 2001;15:1843-1849. [PubMed] [Cited in This Article: ] |
| 25. | Lakatos PL, Lovasz BD, David G, Pandur T, Erdelyi Z, Mester G, Balogh M, Szipocs I, Molnar C, Komaromi E. The risk of lymphoma and immunomodulators in patients with inflammatory bowel diseases: results from a population-based cohort in Eastern Europe. J Crohns Colitis. 2013;7:385-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Vos AC, Bakkal N, Minnee RC, Casparie MK, de Jong DJ, Dijkstra G, Stokkers P, van Bodegraven AA, Pierik M, van der Woude CJ. Risk of malignant lymphoma in patients with inflammatory bowel diseases: a Dutch nationwide study. Inflamm Bowel Dis. 2011;17:1837-1845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Loftus EV, Tremaine WJ, Habermann TM, Harmsen WS, Zinsmeister AR, Sandborn WJ. Risk of lymphoma in inflammatory bowel disease. Am J Gastroenterol. 2000;95:2308-2312. [PubMed] [Cited in This Article: ] |
| 28. | Jones JL, Loftus EV. Lymphoma risk in inflammatory bowel disease: is it the disease or its treatment? Inflamm Bowel Dis. 2007;13:1299-1307. [PubMed] [Cited in This Article: ] |
| 29. | Smith MA, Irving PM, Marinaki AM, Sanderson JD. Review article: malignancy on thiopurine treatment with special reference to inflammatory bowel disease. Aliment Pharmacol Ther. 2010;32:119-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Siegel CA, Marden SM, Persing SM, Larson RJ, Sands BE. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn’s disease: a meta-analysis. Clin Gastroenterol Hepatol. 2009;7:874-881. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 367] [Cited by in F6Publishing: 368] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 31. | Kandiel A, Fraser AG, Korelitz BI, Brensinger C, Lewis JD. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut. 2005;54:1121-1125. [PubMed] [Cited in This Article: ] |
| 32. | Herrinton LJ, Liu L, Weng X, Lewis JD, Hutfless S, Allison JE. Role of thiopurine and anti-TNF therapy in lymphoma in inflammatory bowel disease. Am J Gastroenterol. 2011;106:2146-2153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 33. | Farrell RJ, Ang Y, Kileen P, O’Briain DS, Kelleher D, Keeling PW, Weir DG. Increased incidence of non-Hodgkin’s lymphoma in inflammatory bowel disease patients on immunosuppressive therapy but overall risk is low. Gut. 2000;47:514-519. [PubMed] [Cited in This Article: ] |
| 34. | Thai A, Prindiville T. Hepatosplenic T-cell lymphoma and inflammatory bowel disease. J Crohns Colitis. 2010;4:511-522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 35. | Deneau M, Wallentine J, Guthery S, O’Gorman M, Bohnsack J, Fluchel M, Bezzant J, Pohl JF. Natural killer cell lymphoma in a pediatric patient with inflammatory bowel disease. Pediatrics. 2010;126:e977-e981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Kotlyar DS, Blonski W, Diamond RH, Wasik M, Lichtenstein GR. Hepatosplenic T-cell lymphoma in inflammatory bowel disease: a possible thiopurine-induced chromosomal abnormality. Am J Gastroenterol. 2010;105:2299-2301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Molnár T, Farkas K, Nagy F, Szepes Z, Wittmann T. Lymphomas in two IBD patients treated with anti-TNF-α mono or combination therapy: is hepatosplenic lymphoma really the “old maid”? Inflamm Bowel Dis. 2011;17:2025-2026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Claessen MM, Siersema PD, Vleggaar FP. IBD-related carcinoma and lymphoma. Best Pract Res Clin Gastroenterol. 2011;25 Suppl 1:S27-S38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 39. | Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854-862. [PubMed] [Cited in This Article: ] |
| 40. | Yiakoumis X, Pangalis GA, Kyrtsonis MC, Vassilakopoulos TP, Kontopidou FN, Kalpadakis C, Korkolopoulou P, Levidou G, Androulaki A, Siakantaris MP. Primary effusion lymphoma in two HIV-negative patients successfully treated with pleurodesis as first-line therapy. Anticancer Res. 2010;30:271-276. [PubMed] [Cited in This Article: ] |
| 41. | Matsumoto Y, Nomura K, Ueda K, Satoh K, Yasuda N, Taki T, Yokota S, Horiike S, Okanoue T, Taniwaki M. Human herpesvirus 8-negative malignant effusion lymphoma: a distinct clinical entity and successful treatment with rituximab. Leuk Lymphoma. 2005;46:415-419. [PubMed] [Cited in This Article: ] |
| 42. | Carbone A, Gloghini A. AIDS-related lymphomas: from pathogenesis to pathology. Br J Haematol. 2005;130:662-670. [PubMed] [Cited in This Article: ] |
| 43. | Moore PS. KSHV manipulation of the cell cycle and apoptosis. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: Cambridge University Press 2007; Chapter 30. [PubMed] [Cited in This Article: ] |
| 44. | Sunil M, Reid E, Lechowicz MJ. Update on HHV-8-Associated Malignancies. Curr Infect Dis Rep. 2010;12:147-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 45. | Shair KH, Bendt KM, Edwards RH, Bedford EC, Nielsen JN, Raab-Traub N. EBV latent membrane protein 1 activates Akt, NFkappaB, and Stat3 in B cell lymphomas. PLoS Pathog. 2007;3:e166. [PubMed] [Cited in This Article: ] |
| 46. | Van Biervliet S, Velde SV, De Bruyne R, De Looze D, De Vos M, Van Winckel M. Epstein-Barr virus related lymphoma in inflammatory bowel disease. Acta Gastroenterol Belg. 2008;71:33-35. [PubMed] [Cited in This Article: ] |
| 47. | Dayharsh GA, Loftus EV, Sandborn WJ, Tremaine WJ, Zinsmeister AR, Witzig TE, Macon WR, Burgart LJ. Epstein-Barr virus-positive lymphoma in patients with inflammatory bowel disease treated with azathioprine or 6-mercaptopurine. Gastroenterology. 2002;122:72-77. [PubMed] [Cited in This Article: ] |
| 48. | Libra M, Polesel J, Russo AE, De Re V, Cinà D, Serraino D, Nicoletti F, Spandidos DA, Stivala F, Talamini R. Extrahepatic disorders of HCV infection: a distinct entity of B-cell neoplasia? Int J Oncol. 2010;36:1331-1340. [PubMed] [Cited in This Article: ] |
| 49. | Ohno H. Pathogenetic and clinical implications of non-immunoglobulin; BCL6 translocations in B-cell non-Hodgkin’s lymphoma. J Clin Exp Hematop. 2006;46:43-53. [PubMed] [Cited in This Article: ] |
| 50. | Willis TG, Dyer MJ. The role of immunoglobulin translocations in the pathogenesis of B-cell malignancies. Blood. 2000;96:808-822. [PubMed] [Cited in This Article: ] |
| 51. | Shimazaki M, Fujita M, Tsukamoto K, Matsuki T, Iwata M, Takahashi H, Doi A, Hyakkoku M, Yamauchi K, Genda S. An unusual case of primary effusion lymphoma in a HIV-negative patient not pathogenetically associated with HHV8. Eur J Haematol. 2003;71:62-67. [PubMed] [Cited in This Article: ] |
| 52. | Terasaki Y, Okumura H, Saito K, Sato Y, Yoshino T, Ichinohasama R, Ishida Y. HHV-8/KSHV-negative and CD20-positive primary effusion lymphoma successfully treated by pleural drainage followed by chemotherapy containing rituximab. Intern Med. 2008;47:2175-2178. [PubMed] [Cited in This Article: ] |
| 53. | Wang T, Nava VE, Schechter GP, Lichy JH, Liu ML. Human herpes virus 8-unrelated primary effusion lymphoma-like lymphoma: a patient successfully treated with pleurodesis. J Clin Oncol. 2011;29:e747-e750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |