Published online Jan 21, 2014. doi: 10.3748/wjg.v20.i3.745
Revised: October 17, 2013
Accepted: October 19, 2013
Published online: January 21, 2014
AIM: To evaluate the clinical characteristics of patients with Barcelona Clinic Liver Cancer (BCLC) stage 0 and A hepatocellular carcinoma (HCC) after transarterial chemoembolization (TACE).
METHODS: Between January 2001 and September 2011, 129 patients with BCLC stage 0 and stage A HCC who underwent TACE were retrospectively enrolled. Patient characteristics, routine computed tomography and TACE findings, survival time and 1-, 5-, and 10-year survival rates, risk factors for mortality, and survival rates according to the number of risk factors were assessed.
RESULTS: The mean size of HCC tumors was 2.4 ± 1.1 cm, and the mean number of TACE procedures performed was 2.5 ± 2.1. The mean overall survival time and 1-, 5-, and 10-year survival rates were 80.6 ± 4.9 mo and 91%, 63% and 49%, respectively. In the Cox regression analysis, a Child-Pugh score > 5 (P = 0.005, OR = 3.86), presence of arterio-venous shunt (P = 0.032, OR = 4.41), amount of lipiodol used (> 7 mL; P = 0.013, OR = 3.51), and female gender (P = 0.008, OR = 3.47) were risk factors for mortality. The 1-, 5-, and 10-year survival rates according to the number of risk factors present were 96%, 87% and 87% (no risk factors), 89%, 65%, and 35% (1 risk factor), 96%, 48% and unavailable (2 risk factors), and 63%, 17%, and 0% (3 risk factors), respectively (P < 0.001).
CONCLUSION: TACE may be used as curative-intent therapy in patients with BCLC stage 0 and stage A HCC. The Child-Pugh score, arterio-venous shunt, amount of lipiodol used, and gender were related to mortality after TACE.
Core tip: In this study, transarterial chemoembolization (TACE) was associated with a relatively good survival rate in patients with stage 0 and stage A Barcelona Clinic Liver Cancer (BCLC). The Child-Pugh score, presence of arterio-venous shunt, amount of lipiodol used during TACE, and female gender were correlated with mortality in patients with BCLC stage 0 and stage A hepatocellular carcinoma who underwent TACE. Patients with more than 2 risk factors should be treated by other curative-intent treatments after the first TACE.
- Citation: Kim HC, Suk KT, Kim DJ, Yoon JH, Kim YS, Baik GH, Kim JB, Kim CH, Sung H, Choi JY, Han KH, Park SH. Transarterial chemoembolization in Barcelona Clinic Liver Cancer Stage 0/A hepatocellular carcinoma. World J Gastroenterol 2014; 20(3): 745-754
- URL: https://www.wjgnet.com/1007-9327/full/v20/i3/745.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i3.745
Hepatocellular carcinoma (HCC) is a major health problem and is the sixth most common neoplasm in the world[1]. The incidence of HCC is increasing in Europe and the United States, and it is currently the leading cause of death among cirrhotic patients[2-4]. Eighty percent of patients with HCC have cirrhosis. The annual incidence of HCC in patients with cirrhosis is 3%-5%[2]. Hepatitis B virus infection is the main risk factor in Asia and Africa[5]. In Western countries, hepatitis C virus infection is the main risk factor[6,7]. The rising incidence of HCC has sparked widespread interest in research regarding the clinical management of HCC.
The Barcelona Clinic Liver Cancer (BCLC) staging system was constructed based on the results obtained in several randomized controlled and cohort studies[8-10]. The BCLC staging system has been validated by several groups in Europe and the United States[11-13], and the treatment of HCC has dramatically changed in the last few years[14]. Patients with BCLC stage 0 and stage A HCC are candidates for curative therapies such as resection, transplantation or radiofrequency ablation. The survival rate of patients with BCLC stage 0 and stage A HCC approaches 50%-70% at 5 years after curative therapy[15]. Five-year recurrence rates vary according to the therapeutic modality[16-18].
Transarterial chemoembolization (TACE) is the only intervention recommended by current HCC treatment guidelines for intermediate-stage patients[19]. In general, TACE has not been recommended as a first-line therapy for patients with BCLC stage 0 and stage A HCC, based on the results of a single retrospective study[20]. However, this study reported the results of transarterial embolization, not TACE, and little evidence comparing TACE with other curative therapies in patients with early stage HCC is available[21,22]. Thus, considerable controversy still remains regarding appropriate patient selection for TACE. In this 10-year retrospective study, we evaluated the clinical characteristics of patients with BCLC stage 0 and stage A HCC after TACE.
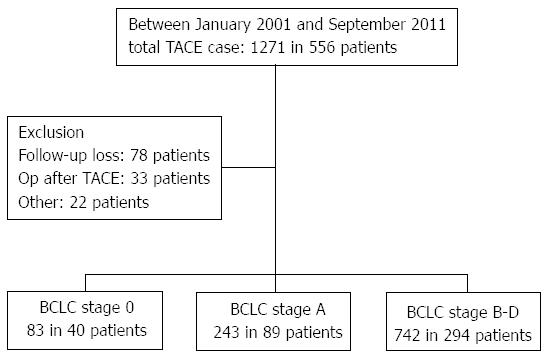
Between January 2001 and September 2011, a total of 129 patients with BCLC stage 0 (n = 40) and stage A HCC (n = 89) who underwent TACE were retrospectively enrolled (Figure 1). All of these patients were either unable to or refused to undergo resection, transplantation, or ablative therapy. Contraindications for these modalities included an unacceptably high risk of surgery, unacceptably high risk of ablation due to the location of mass (close to the gallbladder, liver hilum, liver capsule, diaphragm or pericardium), financial constraints, or patient refusal. Thirty-three patients who were treated with surgery after TACE were excluded. Patient characteristics, routine pre- and post-treatment computed tomography (CT) findings, TACE findings, and 1, 5 and 10-year survival rates were reviewed and assessed. The study protocol conformed to the ethical guidelines established by the 1975 Declaration of Helsinki and received a priori approval by the participating hospitals’ institutional review boards for human research.
Baseline evaluations were conducted including family and alcohol history, X-ray, electrocardiography, electrolyte panels, liver function tests, and viral markers. The diagnosis of HCC was established by α-fetoprotein (AFP) > 200 ng/mL, liver biopsy, and imaging tests, which included magnetic resonance image or contrast-enhanced CT scanning in the arterial and portal venous phases. Reviews of CT and TACE imaging were performed by a single radiologist (H.C.K.) who had over 15 years of experience with the TACE procedure. The clinical characteristics and medical reports were reviewed by 1 hepatologist (K.T.S.). The diagnosis of liver cirrhosis was established by liver biopsy and/or imaging tests such as ultrasound and/or contrast-enhanced CT in conjunction with laboratory data and by observing clinical complications of cirrhosis (presence of ascites, hepatic encephalopathy, and esophageal varices). Kaplan-Meier survival analysis and Cox regression analysis were used to investigate risk factors for mortality. Survival rates, as classified by the number of risk factors, were also evaluated and compared.
All patients gave informed consent prior to the procedure. All TACE procedures were performed using the Seldinger technique by a board-certified attending interventional radiologist who specialized in interventional oncology (H.C.K.)[23]. After arterial access was obtained via the common femoral artery, a 5-French catheter (RH, ANA MD Company, Seoul, South Korea) was introduced, and diagnostic angiography was performed of the celiac axis and superior mesenteric artery to assess arterial anatomy and to confirm patency of the portal vein. After the tumor feeder vessel was identified, a 2.9-French microcatheter (ASAHI Stride Microcatheter, Vascular Perspectives Ltd, Manchester, United Kingdom) was coaxially inserted through a 5-French catheter and advanced into the hepatic artery supplying the targeted tumor. Depending on the size, location, and blood supply, the tip of the catheter was advanced into the hepatic artery and the feeding branch.
After appropriate catheter placement, a chemotherapeutic emulsion of 2-10 mL of iodized oil (Lipiodol Ultra-Fluide, Andre Guerbet Laboratories, Anlney-Sous-Bois, France) and 10-50 mg of doxorubicin (Adriamycin, Ildong pharmaceutical CO. LTD, Seoul, South Korea) was injected. The doxorubicin-iodized oil emulsion was prepared by dissolving doxorubicin into a solution of nonionic water-soluble contrast medium and saline solution and mixed with lipiodol by shaking manually approximately 10 times. The dose of anticancer agent used for the TACE procedure was determined by the radiologist based on the size, number, and blood supply of the target tumors. The maximum dose of doxorubicin for a single TACE session was 50 mg. The injection was performed under fluoroscopy. If the slowing of antegrade blood flow was achieved, the chemotherapeutic infusion was discontinued, and subsequent embolization was performed using a gelatin sponge (Cutanplast, Mascia-Brunelli, Spa, Italy). In patients with decompensated liver function (classified as patients with Child-Pugh class C disease), if a large amount of intratumoral liver tissue was at risk for infarction due to reflux of emboli or segmental TACE, gelatin sponge embolization was not performed. TACE was terminated when the hepatic vein was visualized, the tumor vessels were completely filled with drug, and the tumor blush disappeared on subsequent angiographic imaging.
After the initial TACE treatment, patients underwent CT scans at 1, 3 and 6 mo after the procedure to evaluate the status of their tumors. In the case of an incomplete TACE, as evidenced by tumor recurrence or progression, a second TACE was performed after the follow-up CT scan.
Imaging response was classified according to the Response Evaluation Criteria in Solid Tumors (RECIST), the European Association for the Study of the Liver (EASL), and the Modified RECIST (mRECIST) criteria[24,25]. According to RECIST, a complete response (CR) is defined as the disappearance of all target lesions. A partial response (PR) is defined as a 30% minimum decrease in the sum of the longest diameter of the target lesions, taking as reference the baseline sum of the longest diameter. Progressive disease (PD) is designated as a 20% minimum increase in the sum of the longest diameter of the target lesions, taking as reference either the smallest sum of the longest diameter recorded since the start of treatment or the appearance of one or more new lesions. Stable disease (SD) is indicated by insufficient tumor shrinkage to qualify for PR. According to the EASL guidelines, CR is defined as the absence of enhancing tumor areas, reflecting complete tissue necrosis. PR is defined as a > 50% decrease in the enhancing areas, reflecting partial tissue necrosis. PD is defined as a > 25% increase in the size of a single, measurable lesion or the appearance of new lesions, and SD is defined as a tumor response between PR and PD. The response categories, according to the criteria of mRECIST, are as follows: CR is defined as a disappearance of any intra-tumoral arterial enhancement in all target lesions; PR is defined as at least a 30% decrease in the sum of the diameters of viable target lesions; SD is defined as any cases that do not qualify for either CR or PD; and PD is defined as an increase of at least 20% in the sum of the diameters of viable target lesions.
Time to recurrence was defined as time from treatment to recurrence. Time to progression was defined as the time between treatment and radiological progression. The definitions of recurrence and progression were based on the mRECIST amendments. Patients alive and free of recurrence or progression at the end of follow-up were censored. Progression-free survival time was defined as the time between treatment and either radiological progression or death[24].
Quantitative data were expressed as the mean ± SD, unless otherwise stated. Numerical differences between the groups classified by categorical variables were assessed using the Pearson χ2 test. To evaluate differences between the continuous variables among the groups, the independent t test was used. Survival was expressed as the mean (SE). Mean survival times were obtained using the Kaplan-Meier method and the Log-rank test. Cox regression analysis was used to investigate independent predictors of mortality. Age, gender and other plausible risk factors from the results of the univariate analysis (P < 0.500) were used in the Cox regression analysis. For the Cox regression analysis of lipiodol and doxorubicin, an area under the receiver operating characteristic was used to select the appropriate amount (lipiodol 7 cc and doxorubicin 27 cc). Subsequently, the Enter method was used to determine the OR and the risk factors for the Cox regression analysis. Risk factors are presented in terms of the OR and 95%CI. The times to recurrence and progression, recurrence-free survival, and progression-free survival were estimated using the Kaplan-Meier method. Data were analyzed using statistical software (SPSS, version 13.0, SPSS Inc., Chicago, IL, United States). A P value < 0.05 was considered significant for all tests.
A total of 129 patients were enrolled in this study (40 with BCLC stage 0 and 89 with BCLC stage A). The mean size of the HCC tumors was 2.4 ± 1.1 cm, and the mean number of TACE procedures performed was 2.5 ± 2.1. There were no significant differences among the continuous variables between the BCLC stage 0 and BCLC stage A groups (P > 0.050), with the exception of AFP (P = 0.018) and the greatest diameter of a nodule (P < 0.001). There were no significant differences in the distribution of etiology between the BCLC stage 0 and BCLC stage A groups (P = 0.822). There were 111 patients (38 in the BCLC stage 0 group and 73 in the BCLC stage A group) who showed evidence of cirrhosis in this study (Table 1).
| Variables | BCLC stage 0 (n = 40) | BCLC stage A (n = 89) | P value |
| Age (yr) | 59.5 ± 10.0 | 61.7 ± 11.0 | 0.208 |
| Male | 28 (73) | 64 (67) | 0.836 |
| Etiology | |||
| HBV | 18 (45) | 40 (45) | 0.822 |
| HCV | 5 (13) | 9 (10) | |
| Alcohol | 10 (25) | 21 (24) | |
| Others | 7 (17) | 19 (21) | |
| Child-Pugh score | 5.4 ± 1.0 | 5.9 ± 0.9 | 0.976 |
| Cirrhosis | 38 (95) | 73 (85) | 0.146 |
| Albumin (g/dL) | 3.9 ± 0.7 | 3.9 ± 2.2 | 0.904 |
| AST (IU/L) | 67.7 ± 68.3 | 91.4 ± 217.2 | 0.503 |
| ALT (IU/L) | 43.2 ± 40.1 | 62.2 ± 133.1 | 0.378 |
| TB (mg/dL) | 1.2 ± 1.3 | 1.2 ± 0.9 | 0.971 |
| GGT (IU/L) | 98.9 ± 98.9 | 136.3 ± 244.9 | 0.398 |
| N of TACE treatment | 2.1 ± 1.5 | 2.7 ± 2.5 | 0.066 |
| AFP (ng/mL) | 75.6 ± 159.6 | 490.7 ± 1582.4 | 0.018 |
| Greatest diameter of mass (cm) | 1.4 ± 0.3 | 2.8 ± 1.0 | < 0.001 |
An analysis of CT and TACE imaging revealed that 115 patients had 1 nodule, 10 patients had 2 nodules and 4 patients had 3 nodules. Tumors were located in the left lobe of the liver in 18 patients, in the right lobe in 103 patients, and in both the left and right lobes in 8 patients. The greatest tumor diameter was 2.4 ± 1.1 cm. In 4 patients, the nodule was associated with a capsule in the CT scan. The amounts of doxorubicin and lipiodol used were 26.2 ± 12.5 mg and 6.1 ± 3.5 mL, respectively. Complete lipiodol uptake was observed in 101 patients, and a gelatin sponge was used in 56 patients. Ninety-four patients had 1 feeding vessel, 5 patients had 2 feeding vessels, and 30 patients had more than 3 feeding vessels. Eighteen patients had arterio-venous shunting, while 3 patients presented with an extra-hepatic collateral blood supply.
In the univariate analysis, the Child-Pugh score, BCLC class, number of TACE procedures, and amount of lipiodol used were correlated with mortality in patients with BCLC stage 0 and stage A HCC (P < 0.05). The other variables were not associated with mortality, as shown in Table 2.
| Variables | Mortality (n = 41) | Survival (n = 88) | P value |
| Male | 27 (66) | 65 (74) | 0.405 |
| Age (yr) | 60.9 ± 9.8 | 60.9 ± 11.2 | 0.956 |
| Alcohol history | 23 (56) | 52 (59) | 0.846 |
| Smoking history | 29 (71) | 59 (67) | 0.835 |
| Presence of cirrhosis | 37 (90) | 77 (88) | 0.772 |
| BCLC class (A0/A1) | 7/34 | 33/55 | 0.024 |
| Number of nodule | 36/2/3 | 79/8/1 | 0.129 |
| Number of feeding vessel (1/2/≥ 3) | 34/0/7 | 64/1/23 | 0.471 |
| Child-Pugh score | 6.1 ± 1.0 | 5.6 ± 0.9 | 0.013 |
| Greatest diameter of nodule (cm) | 2.5 ± 1.0 | 2.3 ± 1.1 | 0.383 |
| Number of TACE treatment | 3.3 ± 2.5 | 2.2 ± 2.0 | 0.010 |
| Amount of doxorubicin used (mg) | 29.4 ± 13.2 | 25.9 ± 12.4 | 0.156 |
| Amount of lipiodol used (mL) | 7.3 ± 3.7 | 5.9 ± 3.4 | 0.041 |
| Presence of arterio-venous shunt | 9 (20) | 9 (10) | 0.284 |
| Use of gelatin sponge | 21 (51) | 35 (40) | 0.239 |
| Complete lipiodol uptake | 31 (76) | 63 (72) | 0.492 |
| Sub-segmental approach of catheter | 32 (78) | 66 (75) | 0.624 |
| Extrahepatic collateral supply | 1 (2) | 2 (2) | 1.000 |
| Visible hepatic vein during TACE | 34 (83) | 73 (83) | 0.711 |
In the Cox regression analysis, the Child-Pugh score > 5 (n = 67, P = 0.005, and OR = 3.86), the presence of an arterio-venous shunt (n = 18, P = 0.032, and OR = 4.41), the amount of lipiodol used (> 7 cc; n = 32, P = 0.013, and OR = 3.51) during TACE, and female gender (n = 92, P = 0.008, and OR = 3.47) were risk factors for mortality (Table 3). There was a positive correlation between nodule diameter and the amount of lipiodol used (r = 0.343, P < 0.001).
| Variables | Cox-regression analysis | ||
| P value | HR | 95%CI | |
| Age (yr) | 0.42 | 0.99 | 0.95-1.02 |
| Gender (female/male1) | 0.008 | 3.47 | 1.39-8.68 |
| Child-Pugh score (> 5/51) | 0.005 | 3.86 | 1.50-9.91 |
| Amount of lipiodol used (> 7 cc/ ≤ 7 cc1) | 0.013 | 3.51 | 1.30-9.44 |
| Arterio-venous shunt (yes/no1) | 0.032 | 4.41 | 1.14-17.11 |
| Number of nodules (> 1/11) | 0.530 | 1.63 | 0.36-7.51 |
| Amount of doxorubicin used (> 27 cc/ ≤ 27 cc1) | 0.318 | 0.98 | 0.93-1.02 |
| BCLC class (A1/A01) | 0.766 | 1.17 | 0.41-3.36 |
| Lipiodol uptake (complete/incomplete1) | 0.174 | 2.47 | 0.67-9.08 |
| Number of feeding vessel (≥ 2/11) | 0.086 | 0.32 | 0.09-1.17 |
| Gelfoam use (yes/no1) | 0.054 | 2.38 | 0.98-5.76 |
| Greatest diameter of nodule (cm) | 0.395 | 0.85 | 0.57-1.25 |
| AFP (ng/mL) | 0.065 | 1.00 | 1.00-1.01 |
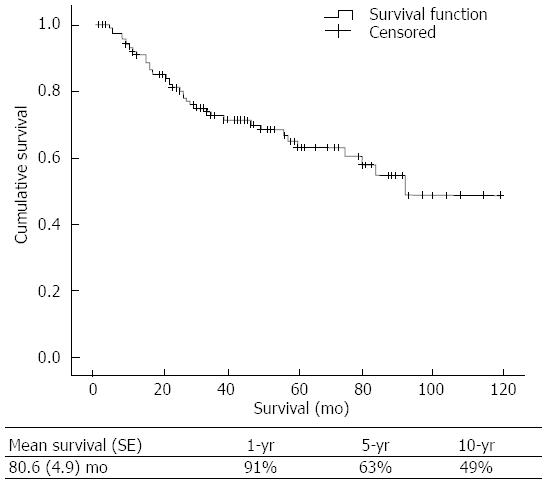
The mean overall survival time and 1, 5, and 10-year survival rates were 80.6 ± 4.9 mo and 91%, 63% and 49%, respectively (Figure 2). Of the risk factors studied, only a Child-Pugh score of 5 was a statistically significant predictor of survival (P = 0.002). Other risk factors were not associated with a survival difference between groups (P > 0.050; Figure 3).
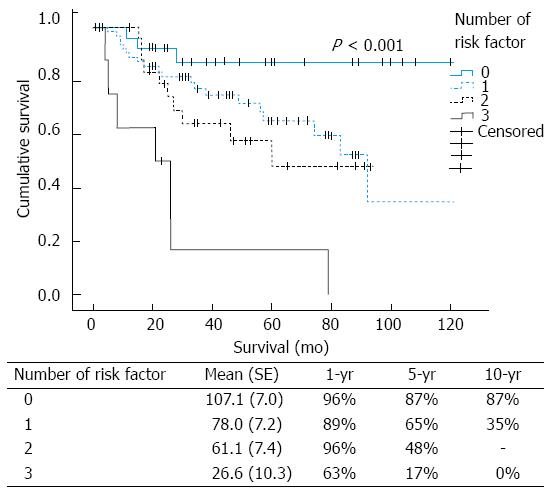
The mean survival times of patients with 0, 1, 2 and 3 risk factors were 107.1 ± 7.0, 78.0 ± 7.2, 61.1 ± 7.4 and 26.6 ± 10.3 mo, respectively. The 1, 5 and 10-year survival rates according to the number of risk factors were 96%, 87% and 87%, respectively, for patients with no risk factors (n = 29). For patients with 1 risk factor (n = 67), the corresponding rates were 89%, 65%, and 35%, respectively; and for patients with 2 risk factors (n = 25), the survival rates were 96%, 48%, and not available. For patients with 3 risk factors (n = 8), the 1-, 5- and 10-year survival rates were 63%, 17%, and 0%, respectively (P < 0.001; Figure 4).
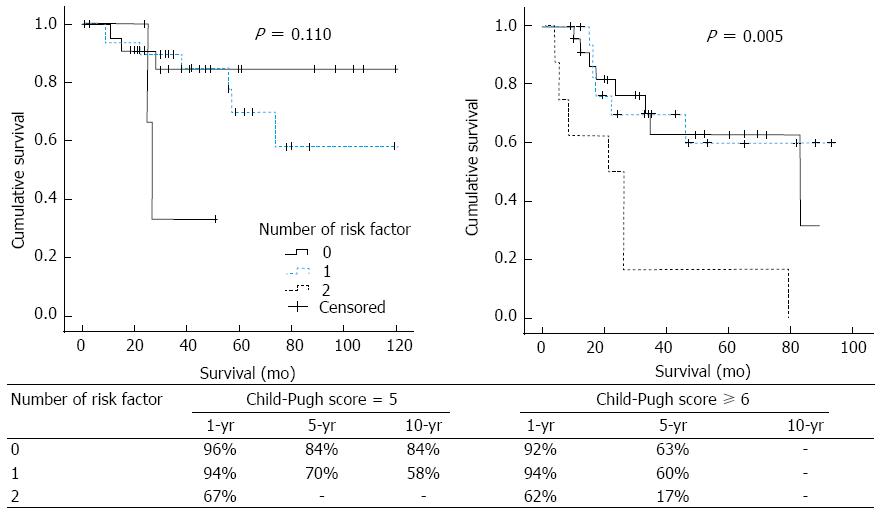
The 1-year survival rates classified according to the Child-Pugh score (5 or ≥ 6) were similar. Patients with a Child-Pugh score of 5 had 1-year survival rates of 96%, 94%, and 67% for patients with no, 1, and 2 risk factors, respectively; however, patients with a Child-Pugh score ≥ 6 had 1-year survival rates of 92%, 94% and 62% for patients with no, 1, and 2 risk factors, respectively (Figure 5).
Follow-up CT after the first TACE showed that the numbers of CR, PR, SD and PD were as follows: 4, 7, 107 and 0, respectively, according to the RECIST criteria; 99, 12, 4 and 3, respectively, according to the EASL criteria; and 100, 14, 3, and 2, respectively, according to the mRECIST criteria. Follow-up CT after the last TACE showed that the numbers of CR, PR, SD and PD were 16, 65, 16 and 10, respectively, according to the RECIST criteria; 40, 15, 17 and 40, respectively, according to the EASL criteria; and 49, 7, 7 and 44, respectively, according to the mRECIST criteria.
Forty-four patients (34.1%) had disease progression at the time of the follow-up CT scan. The average time to progression was 77.3 (5.1) mo, and the progression-free survival time was 33.8 (30.6) mo (Tables 4 and 5).
| Numbers of patients with recurrence | 77 (59.70%) |
| Numbers of patients with progression | 44 (34.10%) |
| Time to recurrence and progression1 | 47.0 (4.6) and 77.3 (5.1) |
| 1-yr recurrence and progression rate | 33% and 22% |
| 2-yr recurrence and progression rate | 55% and 33% |
| 3-yr recurrence and progression rate | 61% and 39% |
| Recurrence-free survival1 | 27.8 (29.2) |
| Progression-free survival1 | 33.8 (30.6) |
TACE is a widely used primary treatment for unresectable HCC and significantly delays tumor progression and vascular invasion[26]. For early-stage HCC, TACE is not indicated as a first-line option. In this study, which evaluated the efficacy of TACE as a first-line therapy for patients with BCLC stage 0 and stage A HCC, TACE had overall 1-, 5- and 10-year survival rates of 91%, 63% and 49%, respectively; these results are comparable to the results of other curative therapies (5-year survival of 50%-70%), including resection, transplantation, or percutaneous treatment[15,27,28]. Kinugasa et al[29] suggested that palliative TACE could be effective for treating HCC with 3 tumors or fewer (each up to 3 cm in diameter). Other data have also suggested that TACE provides a survival benefit for patients with early stage HCC[21,22]. Therefore, the efficacy of TACE in the treatment of BCLC stage 0 and stage A HCC might be comparable to that of other curative treatments.
Our data also suggest that improvements in TACE technique, early diagnosis using precise imaging modalities, and a regular follow-up schedule according to standard guidelines could improve the survival rate of patients with BCLC stage 0 and stage A HCC.
In general, resection, transplantation, and ablation have been considered superior to TACE for very-early-stage or early-stage HCC. However, few studies comparing TACE and other curative therapies in patients with early-stage HCC are available. Therefore, prospective studies comparing the efficacy of TACE, surgery and ablation are needed in the future.
Although this study provides a rationale for the use of TACE in the treatment of patients with BCLC stage 0 and stage A HCC (particularly in patients in whom surgery or ablation is risky due to tumor location), further research comparing the efficacy of TACE with curative therapies is needed because these retrospective data do not provide enough evidence to fully support a potential survival benefit.
Previously reported risk factors for mortality following TACE in patients with intermediate or advanced HCC include the extent of lipiodol uptake, tumor location, number, and size, tumor marker levels, viral marker levels, patient age, and liver function[29-31]. The present study examined risk factors for mortality in patients with early-stage HCC and demonstrated that the Child-Pugh score, presence of arterio-venous shunt, amount of lipiodol used during TACE, and female gender were correlated with mortality.
The present study is the first report that discusses the outcome of TACE classified by the number of clinical risk factors among patients with BCLC stage 0 and stage A HCC. In the study, the number of risk factors was shown to be negatively correlated with survival. Overall, it seems likely that TACE could be a curative therapy for patients with fewer than 2 risk factors because our data showed that the 5-year survival rate in such patients was more than 50%. However, the results of this study suggest that TACE should remain a palliative treatment option for patients with ≥ 2 risk factors; these patients should be treated with another curative-intent treatment following the first TACE.
Child-Pugh scores were a major risk factor for mortality in this study. Survival in patients with a Child-Pugh score of 5 was excellent (1, 5 and 10-year survival rates of 95%, 76% and 70%, respectively). Twenty-eight patients (70%) who died had a Child-Pugh score > 5. Sala et al[32] reported that Child-Pugh class A was the strongest prognostic variable in patients undergoing percutaneous treatments. Shi et al[33] showed that the Child-Pugh class was significantly correlated with survival following TACE in patients with unresectable HCC. In addition, another study documented that the degree of Child-Pugh class was a risk factor for mortality[34]. However, among patients undergoing transplantation, the presence of a single HCC ≤ 5 cm or up to 3 nodules < 3 cm is an important risk factor for mortality[28]. Taken together, it can be concluded that the underlying Child-Pugh score is the primary risk factor for mortality following treatment of HCC, except in the case of transplantation.
TACE-related factors, including the presence of arterio-venous shunt and the amount of lipiodol used during TACE, were also important in predicting patient survival following the procedure. In early-stage HCC, the majority of the tumors are likely to be well differentiated and less invasive; as a result, such tumors can often be controlled by the complete obstruction of their blood supply. In theory, the presence of an arterio-venous shunt might cause the premature wash-out of gelatin sponge or lipiodol, eventually interrupting the complete obstruction of hepatic artery and the delivery of doxorubicin-based intra-arterial chemotherapy. Conversely, the amount of lipiodol used was positively correlated with tumor size, which is the key variable in staging systems[35]. A previous report demonstrated that heterogeneous lipiodol uptake was significantly correlated with local recurrence[29]. In addition, female gender was also a definite risk factor for mortality after TACE. Various factors might contribute to this result. By identifying these risk factors, an earlier prediction of overall survival may be possible, thereby improving the outcome of TACE by refining its delivery and technique.
In this study, the RECIST criteria, in comparison with the EASL or mRECIST criteria, appeared to overlook many cases of complete response. Another study likewise demonstrated that RECIST often overlooked complete responses and mistakenly assessed the therapeutic efficacy of loco-regional therapies. Therefore, it is recommended that the response evaluation following TACE be estimated by the EASL or mRECIST criteria instead of the RECIST criteria.
A meta-analysis demonstrated that chemoembolization could improve the survival of carefully selected patients with unresectable HCC[36]. However, the efficacy of TACE for treating early stage HCC has not been thoroughly studied. In this study, time to progression was shown to be 77.3 ± 5.1 mo and progression-free survival time was 33.8 ± 30.6 mo. Forty-four (34.1%) patients in the study showed progression. The mean number of TACE procedures was 2.5 ± 2.1. Therefore, repeat TACE procedures based on surveillance imaging could increase progression-free survival in patients with BCLC stage 0 and BCLC stage A HCC.
The limitations of this study include the retrospective study design. Numerous other studies have reported on the TACE technique, including its advantages, methods, drug dosing, therapy protocol, scoring system, and cost[37-39]. Therefore, new approaches for reducing mortality in HCC should be available in the future.
In conclusion, TACE demonstrated a relatively good survival rate in patients with BCLC stage 0 and stage A HCC in this study. The Child-Pugh score, presence of arterio-venous shunt, amount of lipiodol used during TACE, and female gender were correlated with mortality in patients with BCLC stage 0 and stage A HCC who were treated with TACE. Patients with more than 2 risk factors should be treated by other curative-intent treatments after the first TACE.
In general, transarterial chemoembolization (TACE) has not been recommended as a first-line therapy for patients with Barcelona Clinic Liver Cancer (BCLC) stage 0 and stage A hepatocellular carcinoma (HCC), based on the results of a single retrospective study. However, this study analyzed the results of transarterial embolization, not TACE, and few studies comparing TACE with other curative therapies in patients with early stage HCC are available. Thus, considerable controversy remains regarding patient selection for TACE.
TACE demonstrated a relatively good survival rate in patients with BCLC stage 0 and stage A HCC in this study. The Child-Pugh score, presence of an arterio-venous shunt, amount of lipiodol used during TACE, and female gender were correlated with mortality in patients with BCLC stage 0 and stage A HCC who were treated with TACE.
Patients with more than 2 risk factors should be treated by other curative-intent treatments after the first TACE.
According to RECIST, complete response (CR) is defined as the disappearance of all target lesions. Partial response (PR) is defined as a 30% minimum decrease in the sum of the longest diameter of target lesions, taking as reference the baseline sum of the longest diameter. Progressive disease (PD) is designated as a 20% minimum increase in the sum of the longest diameter of target lesions, taking as reference either the smallest sum of the longest diameter recorded since the beginning of treatment or the appearance of one or more new lesions. Stable disease (SD) is indicated by insufficient shrinkage to qualify for partial response. According to the European Association for the Study of the Liver guidelines, CR is defined as the absence of enhancing tumor areas, reflecting complete tissue necrosis. PR is defined as a > 50% decrease in the enhancing areas, reflecting partial tissue necrosis. PD is defined as a > 25% increase in the size of a single, measurable lesion or as the appearance of new lesions. SD is defined as a tumor response classified between PR and PD. The response categories, according to the criteria of mRECIST, are defined as follows. CR is defined as the disappearance of any intra-tumoral arterial enhancement in all target lesions; PR is defined as at least a 30% decrease in the sum of the diameters of viable target lesions; SD is defined as any cases that do not qualify for either PR or PD; and PD is defined as an increase of at least 20% in the sum of the diameters of viable target lesions.
In this paper, the authors retrospectively investigate the effect of TACE on early stage HCC survival. Because the role of TACE in this setting is still under debate, this paper is of interest to the readers of the Journal. The sample size is adequate, and the methods are appropriately chosen. A 10-year retrospective study design was used. If promising results were obtained, the clinical importance is potentially significant.
P- Reviewer: Driscoll D S- Editor: Wen LL L- Editor: A E- Editor: Wu HL
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2002;55:74-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13286] [Cited by in F6Publishing: 13416] [Article Influence: 706.1] [Reference Citation Analysis (1)] |
| 2. | Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M, Del Ninno E, Morabito A, Colombo M. The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology. 2006;43:1303-1310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 433] [Cited by in F6Publishing: 413] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 3. | Shaw JJ, Shah SA. Rising incidence and demographics of hepatocellular carcinoma in the USA: what does it mean? Expert Rev Gastroenterol Hepatol. 2011;5:365-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Thomas MB, Jaffe D, Choti MM, Belghiti J, Curley S, Fong Y, Gores G, Kerlan R, Merle P, O’Neil B. Hepatocellular carcinoma: consensus recommendations of the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol. 2010;28:3994-4005. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 280] [Cited by in F6Publishing: 306] [Article Influence: 21.9] [Reference Citation Analysis (1)] |
| 5. | Jin SY, Choi IH. Early hepatocellular carcinoma. Korean J Hepatol. 2011;17:238-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Lee MH, Yang HI, Lu SN, Jen CL, Yeh SH, Liu CJ, Chen PJ, You SL, Wang LY, Chen WJ. Hepatitis C virus seromarkers and subsequent risk of hepatocellular carcinoma: long-term predictors from a community-based cohort study. J Clin Oncol. 2010;28:4587-4593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 7. | Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485-1491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1196] [Cited by in F6Publishing: 1285] [Article Influence: 85.7] [Reference Citation Analysis (0)] |
| 8. | Llovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol. 2005;40:225-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 388] [Cited by in F6Publishing: 383] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 9. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5972] [Cited by in F6Publishing: 6338] [Article Influence: 487.5] [Reference Citation Analysis (1)] |
| 10. | Papatheodoridis GV, Manolakopoulos S, Touloumi G, Vourli G, Raptopoulou-Gigi M, Vafiadis-Zoumbouli I, Vasiliadis T, Mimidis K, Gogos C, Ketikoglou I. Virological suppression does not prevent the development of hepatocellular carcinoma in HBeAg-negative chronic hepatitis B patients with cirrhosis receiving oral antiviral(s) starting with lamivudine monotherapy: results of the nationwide HEPNET. Greece cohort study. Gut. 2011;60:1109-1116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 11. | Cillo U, Bassanello M, Vitale A, Grigoletto FA, Burra P, Fagiuoli S, D’Amico F, Ciarleglio FA, Boccagni P, Brolese A. The critical issue of hepatocellular carcinoma prognostic classification: which is the best tool available? J Hepatol. 2004;40:124-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | D’Avola D, Iñarrairaegui M, Pardo F, Rotellar F, Marti P, Bilbao JI, Martinez-Cuesta A, Benito A, Alegre F, Mauleón E. Prognosis of hepatocellular carcinoma in relation to treatment across BCLC stages. Ann Surg Oncol. 2011;18:1964-1971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Cho YK. Comparison of seven staging systems in cirrhotic patients with hepatocellular carcinoma in a cohort of patients who underwent radiofrequency ablation with complete response. Am J Gastroenterol. 2008;103:1835-1836; author reply 1836-1837. [PubMed] [Cited in This Article: ] |
| 14. | Reig M, Matilla A, Bustamante J, Castells L, de La Mata M, Delgado M, Moreno JM, Forner A, Varela M. Recommendations for the management of Sorafenib in patients with hepatocellular carcinoma. Gastroenterol Hepatol. 2010;33:741-752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3241] [Cited by in F6Publishing: 3212] [Article Influence: 153.0] [Reference Citation Analysis (0)] |
| 16. | Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, Rossi S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 774] [Cited by in F6Publishing: 783] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 17. | Onaca N, Davis GL, Jennings LW, Goldstein RM, Klintmalm GB. Improved results of transplantation for hepatocellular carcinoma: a report from the International Registry of Hepatic Tumors in Liver Transplantation. Liver Transpl. 2009;15:574-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Zavaglia C, De Carlis L, Alberti AB, Minola E, Belli LS, Slim AO, Airoldi A, Giacomoni A, Rondinara G, Tinelli C. Predictors of long-term survival after liver transplantation for hepatocellular carcinoma. Am J Gastroenterol. 2005;100:2708-2716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 187] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 19. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2207] [Cited by in F6Publishing: 2190] [Article Influence: 104.3] [Reference Citation Analysis (0)] |
| 20. | Arii S, Yamaoka Y, Futagawa S, Inoue K, Kobayashi K, Kojiro M, Makuuchi M, Nakamura Y, Okita K, Yamada R. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology. 2000;32:1224-1229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 568] [Cited by in F6Publishing: 532] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 21. | Hsu KF, Chu CH, Chan DC, Yu JC, Shih ML, Hsieh HF, Hsieh TY, Yu CY, Hsieh CB. Superselective transarterial chemoembolization vs hepatic resection for resectable early-stage hepatocellular carcinoma in patients with Child-Pugh class a liver function. Eur J Radiol. 2012;81:466-471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Bargellini I, Sacco R, Bozzi E, Bertini M, Ginanni B, Romano A, Cicorelli A, Tumino E, Federici G, Cioni R. Transarterial chemoembolization in very early and early-stage hepatocellular carcinoma patients excluded from curative treatment: a prospective cohort study. Eur J Radiol. 2012;81:1173-1178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Higgs ZC, Macafee DA, Braithwaite BD, Maxwell-Armstrong CA. The Seldinger technique: 50 years on. Lancet. 2005;366:1407-1409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M, Talwalkar J. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698-711. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1232] [Cited by in F6Publishing: 1298] [Article Influence: 81.1] [Reference Citation Analysis (0)] |
| 25. | Forner A, Ayuso C, Varela M, Rimola J, Hessheimer AJ, de Lope CR, Reig M, Bianchi L, Llovet JM, Bruix J. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer. 2009;115:616-623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 323] [Cited by in F6Publishing: 352] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 26. | Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127:S179-S188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 397] [Cited by in F6Publishing: 386] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 27. | Kudo M. Radiofrequency ablation for hepatocellular carcinoma: updated review in 2010. Oncology. 2010;78 Suppl 1:113-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 28. | Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 662] [Cited by in F6Publishing: 643] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 29. | Kinugasa H, Nouso K, Takeuchi Y, Yasunaka T, Onishi H, Nakamura SI, Shiraha H, Kuwaki K, Hagihara H, Ikeda F. Risk factors for recurrence after transarterial chemoembolization for early-stage hepatocellular carcinoma. J Gastroenterology. 2011;4:421-426. [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Lee JK, Chung YH, Song BC, Shin JW, Choi WB, Yang SH, Yoon HK, Sung KB, Lee YS, Suh DJ. Recurrences of hepatocellular carcinoma following initial remission by transcatheter arterial chemoembolization. J Gastroenterol Hepatol. 2002;17:52-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Nouso K, Ito Y, Kuwaki K, Kobayashi Y, Nakamura S, Ohashi Y, Yamamoto K. Prognostic factors and treatment effects for hepatocellular carcinoma in Child C cirrhosis. Br J Cancer. 2008;98:1161-1165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Sala M, Llovet JM, Vilana R, Bianchi L, Solé M, Ayuso C, Brú C, Bruix J. Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology. 2004;40:1352-1360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 348] [Cited by in F6Publishing: 329] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 33. | Shi M, Chen JA, Lin XJ, Guo RP, Yuan YF, Chen MS, Zhang YQ, Li JQ. Transarterial chemoembolization as initial treatment for unresectable hepatocellular carcinoma in southern China. World J Gastroenterol. 2010;16:264-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 35] [Cited by in F6Publishing: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Ikai I, Arii S, Kojiro M, Ichida T, Makuuchi M, Matsuyama Y, Nakanuma Y, Okita K, Omata M, Takayasu K. Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer. 2004;101:796-802. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 346] [Cited by in F6Publishing: 339] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 35. | Kondo K, Chijiiwa K, Nagano M, Hiyoshi M, Kai M, Maehara N, Ohuchida J, Nakao H, Ohkuwa Y. Comparison of seven prognostic staging systems in patients who undergo hepatectomy for hepatocellular carcinoma. Hepatogastroenterology. 2007;54:1534-1538. [PubMed] [Cited in This Article: ] |
| 36. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2502] [Cited by in F6Publishing: 2483] [Article Influence: 112.9] [Reference Citation Analysis (0)] |
| 37. | Wu KT, Wang CC, Lu LG, Zhang WD, Zhang FJ, Shi F, Li CX. Hepatocellular carcinoma: clinical study of long-term survival and choice of treatment modalities. World J Gastroenterol. 2013;19:3649-3657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 23] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 38. | Ni JY, Liu SS, Xu LF, Sun HL, Chen YT. Meta-analysis of radiofrequency ablation in combination with transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2013;19:3872-3882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 112] [Cited by in F6Publishing: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 39. | Jun CH, Ki HS, Lee KH, Park KJ, Park SY, Cho SB, Park CH, Joo YE, Kim HS, Choi SK. Impact of serum C-reactive protein level on the prognosis of patients with hepatocellular carcinoma undergoing TACE. Clin Mol Hepatol. 2013;19:70-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |