Published online Jan 21, 2014. doi: 10.3748/wjg.v20.i3.673
Revised: October 25, 2013
Accepted: December 3, 2013
Published online: January 21, 2014
The combination of a proton pump inhibitor and two antibiotics (clarithromycin plus amoxicillin or metronidazole) has been the recommended first-line therapy since the first guidelines for Helicobacter pylori (H. pylori) infection in children were published. In recent years, the success of eradication therapies has declined, in part due to the development of H. pylori resistant strains. Alternative anti-H. pylori treatments are currently becoming more popular than the traditional eradication methods. Components that may be used either as a monotherapy or, in combination with antimicrobials, resulting in a more effective anti-H. pylori therapy have been investigated in depth by several researchers. One of the potential therapies is probiotic cultures; promising results have been observed in initial studies with numerous probiotic strains. Nevertheless, many questions remain unanswered. In this article, we comprehensively review the possible mechanisms of action of probiotics on H. pylori infection, and present the results of published studies using probiotics as possible agents to control H. pylori infection in children. The effect of the addition of probiotics to the standard H. pylori eradication therapy for the prevention of antibiotic associated side-effects is also discussed.
Core tip: Because of the decrease in the Helicobacter pylori (H. pylori) eradication rate after standard triple therapy with a proton pump inhibitor and two antibiotics, alternative therapies have recently received attention. In this article, we comprehensively review the possible mechanisms of action of probiotics on H. pylori infection, and present the results of the published studies using probiotics as possible agents to control H. pylori growth in children. The effect of the addition of probiotics to the standard H. pylori eradication therapy for the prevention of antibiotic associated side-effects is also discussed.
-
Citation: Pacifico L, Osborn JF, Bonci E, Romaggioli S, Baldini R, Chiesa C. Probiotics for the treatment of
Helicobacter pylori infection in children. World J Gastroenterol 2014; 20(3): 673-683 - URL: https://www.wjgnet.com/1007-9327/full/v20/i3/673.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i3.673
Helicobacter pylori (H. pylori) is a highly prevalent, serious and chronic infection that has been associated causally with a diverse spectrum of gastrointestinal disorders including chronic gastritis, peptic ulcer disease, gastric adenocarcinoma and gastric mucosa-associated lymphoid tissue lymphoma[1]. In both developed and developing countries, H. pylori is most frequently acquired during childhood, and is associated with family size, clustering in families, low socioeconomic status and low level of education[2-5]. It is commonly thought that once the H. pylori infection is acquired, it evolves toward persistent chronic infection[6] and that spontaneous clearance is relatively rare[6-8]. However, in a study of children in which prevalence by age was reported in intervals of 1 year, no increase in prevalence by age was observed[9]. This suggests that transient H. pylori infection is not uncommon in children[6,10,11]. In 6-24-mo old children in Mexico, and Texas, researchers found 80% spontaneous reversion of the infection[10,11]. In a very recent study involving 718 schoolchildren in Mexico City, Duque et al[12] found that the majority of them maintained their initial status of H. pylori infection throughout the follow-up, while 11.7% showed changes in their infection status. Variables related to health status and infection transmission, such as iron status and number of siblings, were shown to be important for the incidence of H. pylori and the spontaneous clearance of infection[12].
The combination of a proton pump inhibitor (PPI) and two antibiotics (clarithromycin plus amoxicillin or metronidazole) has been the recommended first-line therapy since the first guidelines for H. pylori infection in children were published[13-15]. In recent years, the success of eradication therapies has declined, in part due to the development of H. pylori resistant strains[16]. Several studies have documented high resistance rates to clarithromycin and metronidazole in paediatric and adult populations[17-19]. In Europe, Koletzko et al[17] showed that primary resistance to clarithromycin and metronidazole was present in 20% and 23% of H. pylori strains respectively, while secondary resistance was found in 42% and 35% of the strains recovered after at least one failed treatment for H. pylori. The use of clarithromycin for other indications, mainly for respiratory tract infections, seemed to be the major risk factor for development of primary resistance to this drug. On the other hand, the only risk factor for primary metronidazole resistance was immigration from a non-European country. In fact, the authors showed that children born in Asia, Africa or the Middle East had a 2.4 times higher risk for primary metronidazole resistance than patients of the same age and gender born in Europe. Iterative metronidazole treatments for parasitic or diarrhoeal diseases in children originating from Africa and Asia may be incriminated in the increased primary resistance rates of metronidazole recorded in these paediatric populations. A prospective United States multicentre study in adults and children also documented similar high clarithromycin resistance rates[18]. Declining eradication rates with standard triple regimens have led to the development of alternate treatment options[20,21].
Recently, ESPGHAN and NASPGHAN jointly renewed clinical guidelines for H. pylori infection in children using a standardised evidence based approach[22]. Bismuth-based triple therapy or sequential therapy was recommended as alternate first-line regimens. Quadruple therapy with PPI, metronidazole, amoxicillin, and bismuth was also suggested as second line therapy or salvage therapy in the absence of primary culture and sensitivity testing[20,22]. These regimens have the disadvantages of being expensive, risking poor compliance, causing side-effects and encouraging the emergence of resistance. Moreover, as most of the colonized children remain asymptomatic, the administration of antibiotic treatments is not ethically acceptable. Other factors limiting the administration of such treatments in developing countries is their high cost for families from low socio-economic strata (those most affected by the infection) and the relative inefficiency of the antibiotics due to the fact that children tend to be rapidly re-colonized. Alternative anti-H. pylori treatments are currently becoming more popular than the traditional eradication methods. Components that may be used either as a monotherapy or, in combination with antimicrobials, resulting in a more effective anti-H. pylori therapy have been investigated by several researchers[23]. One of the potential therapies involves probiotic cultures; promising results have been observed in initial studies with numerous probiotic strains[24-26]. Nevertheless, many questions remain unanswered. In this article, we comprehensively review the possible mechanisms of action of probiotics on H. pylori infection, followed by the outcomes of the published studies using probiotics as possible agents to control H. pylori growth in children. The effect of the addition of probiotics to the standard H. pylori eradication therapy for the prevention of antibiotic associated side-effects is also discussed.
An oral supplement or a food product that contains a sufficient number of viable micro-organisms to alter the microflora of the host and has the potential for beneficial health effects[27,28].
Probiotic micro-organisms are typically members of the genera Lactobacillus, Bifidobacterium, and Streptococcus[27-29]. These bacteria are fermentive, obligatory, or facultative anaerobic organisms, which are typically non-motile and of varying shapes. Typically they produce lactic acid. Their inherent biological features enable them to predominate and prevail over potential pathogenic microorganisms in the human digestive tract. It is currently hypothesized that these microbes generate small molecular metabolic byproducts that exert beneficial regulatory influence on host biological functions, including short-chain fatty acids such as butyrate. These metabolic byproducts are sometimes referred to as “postbiotics” and may function biologically as modulators of immune function[30]. The most studied probiotic bacteria to date belong to the genera Lactobacillus and Bifidobacterium. Some yeasts and yeast byproducts have also been studied and have been used as probiotic agents, for example the yeast Saccharomyces boulardii.
Several probiotic strains, especially lactobacilli, have exhibited antagonistic properties against H. pylori in vitro[31]. There are several putative mechanisms for probiotic efficacy against H. pylori. Lactobacilli species are commensal in the human alimentary tract and their concentrations in the normal stomach vary between 0 and 103/mL fluid[25]. Being acid resistant, they persist in the stomach longer than other bacteria. The possible role of the local microbiota in the protection against gastric lesions is suggested by the study of Elliott et al[32] who found that the level of total aerobes in the stomach of healthy rats ranged from 103 to 104 CFU/g of tissue, with Gram-negative micro-organisms representing only 5% of the population; autochthonous gastric lactobacilli were present in all rats. However, one day after the induction of gastric ulcers the total aerobe count peaked at 109-1010 CFU/g and remained high for 1 wk. At this time, Gram-negative bacteria were the majority of the total aerobes while the lactobacilli population disappeared. Colonization by Gram-negative bacteria occurred preferentially at the site of ulcer. These findings suggest that the gastroduodenal microbiota, though low numerically, could represent a first line of defense against pathogenic bacteria. Thus, the intake of exogenous lactic acid bacteria, in particular those with probiotic properties, may reinforce these protective functions in the stomach by maintaining local microbiological homeostasis, interfering with H. pylori and/or decreasing inflammatory processes[31].
Non-immunological barriers such as the acidity of the stomach and the gastric mucosal barrier also represent a first line of defense against pathogenic bacteria. Two main types of substances have been implicated in the inhibition of H. pylori by lactic acid bacteria: short chain fatty acids (SCFAs) and bacteriocins. SCFAs such as acetic, propionic, butyric, and lactic acids are produced during the metabolism of carbohydrates by probiotics and have an important role in decreasing pH[33]. Bhatia et al[34] were the first to observe an antagonistic effect of a lactobacillus strain against H. pylori and to implicate SCFAs in this effect. A dose-dependent inhibition of H. pylori growth has been observed with acetic and lactic acid, the latter demonstrating the most intense effect[35]. Lactic acid, in addition to its antimicrobial effect resulting from the lowering of the pH, could inhibit the H. pylori urease. However, the inhibitory effects of lactobacilli on H. pylori differ from strain to strain[23]. Certain lactobacilli synthesize antimicrobial compounds related to the bacteriocin family[36,37]. Bacteriocins are compounds with potential anti-H. pylori activity. They are small, heat-resistant and dialysable peptidic structures with antimicrobial activities, which are synthesized by several bacterial species including lactic acid bacteria[23].
Other possible mechanisms of protection induced by probiotics include inhibition of the adhesion of H. pylori. The adhesion of H. pylori to epithelial cells is important in determining the outcome in H. pylori-associated diseases[38]. Certain lactobacilli can exert their antiadhesion activity by secreting antimicrobial substances[23]. However, strains such as lactobacilli reuteri (L. reuteri) can inhibit H. pylori growth by competing with adhesion receptors[39]. A nonspecific rather than a specific blockage of receptor sites is the most likely mechanism because lactobacilli can inhibit adhesion of a large varieties of pathogenic bacteria, although each adheres to its particular receptor on the cells[40].
It has been suggested that intake of probiotics strengthens the mucosal barrier by stimulating mucin production. Reduced mucus secretion in a damaged epithelium is a frequent finding in H. pylori-associated gastritis. H. pylori is known to suppress MUCI and MUC5A gene expression in a human gastric cell line[41]. It has been shown in vitro that lactobacilli plantarum and lactobacilli rhamnosus increase the expression of MUC2 and MUC3 genes[42]. This property can mediate the ability of these strains to restore the mucosal permeability of gastric mucosa[43] or inhibit the adherence of pathogenic bacteria, including H. pylori[42].
Finally, modulation of immune response to pathogens should also be taken into account as a potential mechanism of probiotic efficacy. The inflammatory response to gastric H. pylori infection is characterized by the release of various inflammatory mediators such as chemokines and cytokines[23]. Probiotics could modify the immunologic response of the host by interacting with epithelial cells and modulating the secretion of antiinflammatory cytokines, which would result in a reduction of gastric activity and inflammation[44]. However, the effect of probiotics on the immune response is difficult to generalize. Distinct probiotics strains may generate different immune responses, which, in turn, depend on the host’s immune status[45].
Several studies using murine models have shown that probiotic treatment, although it is unable to clear H. pylori, is effective in reducing bacterial colonization and decreasing gastric inflammation in H. pylori-infected mice[46-50]. It has been postulated, on the basis of the results of in vitro and animal studies, that probiotics could possibly compete with and down-regulate H. pylori infection in humans[23]. Though utilization of probiotics alone does not lead to the eradication of H. pylori[51-55], a growing body of recent evidence suggests that regular intake of probiotics suppresses H. pylori infection in humans, maintaining lower levels of this pathogen in the stomach[25].
Few clinical trials evaluating the use of different probiotic strains have been reported. In some of these studies, probiotics were used alone while in others they were used as adjunctive agents in the classical treatment of H. pylori infection.
The clinical trials performed in children on the effect of probiotics on H. pylori eradication rates as an adjuvant to eradicating regimens are summarized in Table 1 and Figure 1. In the earliest study, Sýkora et al[56] found that the addition of lactobacilli casei (L. casei) DN-114001 to a standard triple therapy improved the rate of H. pylori eradication. Intention-to-treat based eradication rates for the triple therapy group supplemented with L. casei were 84.6% (95%CI: 71.2%-95.5%), and 91.6% (95%CI: 76.9%-98.2%) by per-protocol analysis. Eradication in the placebo group was 57.5% (95%CI: 42.2%-72.3%) in the intention-to-treat analysis and 61.3% (95%CI: 44.4%-75.0%) in the per protocol analysis. Reported adverse effects were infrequent and self-limiting after therapy cessation in both groups[56].
| Ref. | Study design | Therapy | Probiotic strain (product; dose; time) | Patients | Diagnosis | No. of treated patients | Eradication n (%); P value | Side effects n (%); P value | Test for confirming eradication (time after completion of therapy) |
| Sýkora et al[56] | P, R, DB, PC | A (25 mg/kg twice daily), C (7.5 mg/ kg twice daily), and O (10 or 20 mg twice daily) 1 wk + probiotic vs same eradication therapy + placebo | L. casei DN-114 001 1010 CFU in 100 mL of fermented milk (actimel, Danone); 2 wk | 86 (aged 9-15 yr) symptomatic children and adolescents | EGDS (histopathology, culture, and RUT) and HpSA | 391 362vs 471 442 | 33 (84.6)1 33 (91.6)2vs 27 (57.5)1; 0.0045 27 (61.3)2; 0.0019 | 9 (23.1) vs 10 (21.2); NS (nausea, headache, abdominal pain, recurrent vomiting, diarrhoea) | HpSA and 13C-UBT (4 wk) |
| Gotteland et al[64] | O, R | A (50 mg/kg tid), C (15 mg/kg bid), and L (1 mg/kg bid) 8 d vs Probiotic vs Symbiotic | Lactobacillus acidophilus LB (LB); capsule containing 109 heat-killed and lyophilized LB (Lacteol Forte, Laboratoire du Dr. Boucard, Paris, France); b.i.d. for 8 wk, and Saccharomyces boulardii plus inulin (SbI); sachet containing 250 mg of lyophilized Sb (Perenteryl, Merck Quìmica Chilena, Santiago, Chile); bid for 8 wk | 141 (aged 5-12 yr) asymptomatic children. 81 children were observed without any treatment | 13C-UBT | 452vs 462vs 502 | 30 (66)2vs 3 (6.5)2vs 6 (12)2; < 0.001 No spontaneous clearance was observed in children without treatment | NA | 13C-UBT (1 d) |
| Goldman et al[58] | R, DB, PC | A (50 mg/kg per day), C (20 mg/kg per day bid), and O (1 mg/kg per day) 1-wk + probiotic vs same eradication therapy + placebo | Bifidobacterium animalis and Lactobacillus casei (107 CFU/mL) in 250 mL of a commercial yogurt; once daily for 3 mo | 65 (aged 5-15 yr) symptomatic children and adolescents | EGDS and 13C-UBT (histological data NA) | 3312vs 3212 | 15 (45.5)12vs 12 (37.5)12; 0.345 at 1 mo 14 (42.4)12vs 13 (40.6)1,2; 0.542 at 3 mo | NA | 13C-UBT ( 1 and 3 mo) |
| Lionetti et al[57] | R, DB, PC | O (1 mg/kg/die) plus A (50 mg/kg/die) for 5 d followed by O (1 mg/kg/die) plus C (15 mg/kg/die)and T (20 mg/kg/die) for the next 5 d + probiotic vs same eradication therapy + placebo | L. reuteri [pill containing 108 CFU of L. reuteri ATCC 55730 (SD2112), Reuterin, Nòos]; one pill once daily for a period of 20 d | 40 (aged 3.3-18 yr) symptomatic children and adolescents | EGDS (histopathology and RUT) [pangastritis (27); antral gastritis, mild (20); antral gastritis, moderate (14); antral gastritis, severe (10)] | 2012vs 2012 | 17 (85)12vs 16 (80)12; NS | Reduction of GSRS score during eradication therapy [4.1 ± 2 (95%CI: 2.9-5.9) vs 6.2 ± 3 (95%CI: 5.2-8.3); P < 0.01] and at the end of follow-up [3.2 ± 2 (95%CI: 2.4-4) vs 5.8 ± 3.4 (95%CI: 4.8-6.9); P < 0.009]; Epigastric pain (15% vs 45%; P < 0.04); Abdominal distension (0% vs 25%; P < 0.02); Eructation (5% vs 35%; P < 0.04); Disorders of defecation (15% vs 45%; P < 0.04); Halitosis (5% vs 35%; P < 0.04) | 13C-UBT (8 wk) |
| Hurduc et al[60] | O, R | A (50 mg/kg per day, bid) and C (15 mg/kg per day, bid) 7-10 d; O or E (1 mg/kg per day, bid) 3-wk + probiotic vs same eradication therapy + placebo | Saccharomyces boulardii, Enterol, Biocodex, Gentilly Cedex; 250 mg bid; 4-wk | 90 (aged 3-18 yr) children and adolescents with dyspepsia | EGDS (histopathology and RUT) [chronic gastritis: mild (8); moderate-to-severe (82); active (32); inactive (58)] | 4812vs 4212 | 45 (93.3)12vs 34 (80.9)12; NS | 4 (8.3) vs 13 (30.9); P = 0.047 (bloating, taste disturbance, nausea, abdominal pain, diarrhoea, constipation, loss of appetite, fatigue) | EGDS (4-6 wk) (histopathology and RUT) |
| Szajewska et al[59] | R, DB, PC | A (50 mg/kg per day bid), C (20 mg/kg per day bid), and O (1 mg/kg per day) 1-wk + probiotic vs same eradication therapy + placebo | Lactobacillus GG 1 × 109 CFU; 7 d | 83 (aged 5-17 yr) symptomatic children and adolescents. Excluded from the analysis were 17 children for lack of diary and/or 13C-UBT | EGDS (2 of 3 tests - 13C-UBT, histopathology or RUT) [histological data NA] | 342vs 322 | 23 (69)2vs 22 (68); RR = 0.98 (95%CI: 0.7-1.4)2 | Therapy-related diarrhea: 2 (6) vs 6 (20); P = NS Total side effects: 18 (51.4) vs 13 (40.6); P = NS Abdominal pain: 0 vs 0 Nausea: 4 (11.4) vs 3 (9.4); P = NS Vomiting: 2 (5.7) vs 1 (3.1); P = NS Constipation: 2 (5.7) vs 2 (6.2); P = NS Flatulence: 3 (8.6) vs 1 (3.1); P = NS Taste disturbance: 4 (11.4) vs 5 (15.6); P = NS Loss of appetite: 3 (8.6) vs (3.1); P = NS Need for discontinuation of therapy: 0 vs 0 | 13C-UBT (4 wk) |
| Tolone et al[61] | R | A (50 mg/kg per day bid), C (15 mg/kg per day bid), and O (1mg/kg per day) 1-wk + probiotic vs same eradication therapy + placebo | Lactobacillus Plantarum 5 × 109, L. reuterii 2 × 109, L. casei subsp. Rhamnosus 2 × 109, Bifidobacterium infantis and B. longum 2 × 109, L. salivarius 1 × 109, L. acidoPhilus 1 × 109, Streptococcus termophilus 5 × 109, and L. sporogenes 1 × 109 + inuline as a prebiotic (5 g/dayose q.d., Probinul , Cadigroup); 7 d | 68 (mean age, 8.3 yr) children with heartburn, dyspepsia, nausea and epigastric pain | EGDS (histopathology) [histological data: NA] | 3412vs 3412 | 30 (88.2)12vs 26 (76.4)12; 0.1 | Epigastric pain: 2 (5.8) vs 6 (17.6); P < 0.05 Nausea: 1 (2.9) vs 3 (8.8); P < 0.05 Vomiting : 0 vs 2 (5.8); P < 0.05 Diarrhea: 0 vs 8 (23.5); P < 0.05 | 13C-UBT (4 wk) |
| Ahmad et al[62] | R, DB, PC | A (50 mg/kg per day bid) and F (6 mg/kg per day bid) 1-wk; O (1 mg/kg per day) 4-wk + probiotic vs same eradication therapy + placebo | Lactobacillus acidophilus, L. rhamnosus, L. bulgaricus, L. casei, Streptococcus thermophilus, Bifidobacterium infantis, B. breve; 1 × 109 CFU/1 sachet, Protexin Co; 4 wk | 66 (aged 3-14 yr) children with chronic abdominal pain, gastrointestinal bleeding, unexplained frequent vomiting and unexplained iron deficiency anemia | EGDS (positive RUT or histopathology) [Antral nodularity (57); Gastric erythema (16); Duodenal ulcer (14); Gastric ulcer (1)] | 3312vs 3312 | 30 (90.1)12vs 23 (69.7)12; 0.04 | ConstiPation: 2 (5.8) vs 2 (5.8); P = NS Nausea/vomiting: 2 (6.1) vs 9 (27.3); P = 0.02 Diarrea: 2 (6.1) vs 8 (24.2); P = 0.04 Abdominal bloating: 3 (9.1) vs 4 (12.1); P = 1 | HpSA (4-8 wk) |
In a randomized, double-blind, controlled trial conducted in Italy[57], symptomatic children with H. pylori infection were treated with 10-d sequential therapy and randomized to receive either L. reuteri ATCC 55730 or placebo for 20 d. All children (or family member) also attended an interview to recall history of gastrointestinal symptoms and the 15-item Gastrointestinal Symptom Rating Scale (GSRS) was used to assess severity and frequency of symptoms. The following symptoms were specifically investigated: epigastric burning and/or pain, abdominal pain, acid regurgitation, heartburn, sucking sensation in the epigastrium, nausea, vomiting, bloating, abdominal distension, eructation, increased flatus, disorders of defecation, inappetence, halitosis, taste disturbance and urticaria. The symptoms were scored by the child (or family member) on a four-point scale: mild (non-interfering with daily activities), moderate (slightly interfering with daily activities), severe (interfering with daily activities), very severe (continuous and if on therapy, producing treatment interruption). Stool consistency was graded from hard (0) to watery (4). Data were collected before (1 wk before intervention), during (5th and 10th day) and after completion of eradicating therapy (15th and 20th day) and patients were invited to return their diaries immediately after the intervention period. No significant difference in H. pylori eradication rates between the treated group and the control group were found. However, in all probiotic supplemented children when compared with those receiving placebo there was a significant reduction in GSRS score during eradication therapy which became markedly evident at the end of follow-up (Table 1). Children receiving L. reuteri reported less side-effects than those receiving placebo[57].
Goldman et al[58] tested the efficacy of a commercial yogurt containing B. animalis and L. casei as an adjuvant to triple therapy and found no significant difference in H. pylori eradication rates at 1 and 3 mo between probiotic and placebo group. Side effects were not assessed. Similarly, in a randomized, double-blind, controlled trial conducted in Poland[59], no difference was found with respect to H. pylori eradication rates between children who received triple therapy supplemented with Lactobacillus GC and the control group. Also, the incidence of adverse effects was not reduced. In a randomized, open trial conducted in Romania[60], children with dyspepsia and H. pylori infection were treated with eradication triple therapy and randomized to receive either Streptococcus boulardii (S. boulardii) (for 4 wk) or placebo. No significant difference in H. pylori eradication rates between the treated group and the control group were found. However, the incidence of side effects was reduced in the S. boulardii group.
Recently, Tolone et al[61] supplemented a standard triple therapy with a commercial probiotic for 7 d and showed that there was no improvement in the rate of H. pylori eradication in the probiotic group. However, the addition of probiotic to triple therapy significantly decreased the frequency of epigastric pain, nausea, vomiting, and diarrhea. In a more recent double-blind randomized placebo controlled study[62], H. pylori-positive children were treated with a triple drug treatment protocol and randomly allocated to receive either probiotic or placebo. H. pylori was eradicated in 90.09% of patients receiving probiotic and in 69.69% of those receiving placebo (P = 0.04). In probiotic supplemented children there was a lower rate of nausea/vomiting and diarrhea.
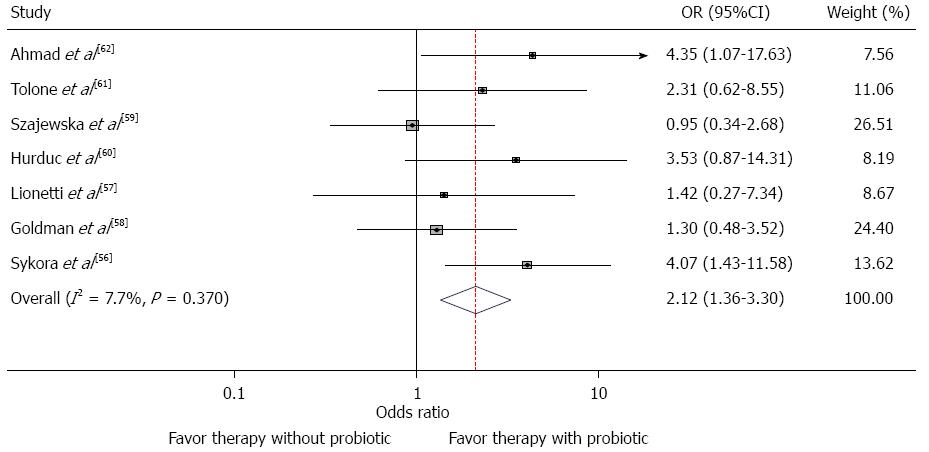
In summary, seven of the eight studies listed in Table 1 compare eradication rates for groups treated with antibiotics with those treated with antibiotics plus probiotics. The odds ratios for these seven studies are shown in the forest plot in Figure 1. Six of these seven have estimated odds ratios greater than 1.0 implying an estimated benefit for the addition of probiotics, but only two are statistically significant. The antibiotics used in the studies differ as do the treatment regimens. Similarly, the probiotics used and diagnostic techniques differ between studies. With such heterogeinity of design, even though the statistical test of the heterogeneity is not significant (χ2 = 6.5; P = 0.37), a meta-analysis of these studies would not be appropriate.
The clinical trials performed in children on the effect of probiotics on H. pylori eradication rates alone are summarized in Table 2. In a double-blind, randomized, controlled clinical trial Cruchet et al[63] evaluated the efficacy of lactobacilli johnsonii (L. johnsonii) La1 or lactobacilli paracasei (L. paracasei) ST11 as a unique intervention on H. pylori eradication in 252 asymptomatic school children screened for H. pylori by 13C-Urea breath test (UBT). Subjects were distributed into five groups to receive a product containing live L. johnsonii La1 or L. paracasei ST11, heat-killed L. johnsonii La1 or L. paracasei ST11, or just vehicle everyday for 4 wk. There was a moderate but significant difference in 13C-UBT values in children receiving live L. johnsonii La1, whereas no differences were observed in the other groups. The authors conclude that regular ingestion of a probiotic strain such as L. johnsonii La1 may interfere with H. pylori colonization in asymptomatic children and may be an effective alternative to modulate H. pylori infection and its associated gastritis in pediatric populations with high prevalences of infection by this pathogen.
| Ref. | Study design | Probiotic strain (product; dose; time) | Patients | Diagnosis | Eradication | Test for confirming eradication | Comments |
| n (%); P value | (time after completion of therapy) | ||||||
| Cruchet et al[63] | DB, R | Lactobacillus johnsonii (La1), living or heat-killed, 80 mL/die, (> 107 CFU/mL) (Chamyto, Nestlé) and L. helveticus (LH) for 4 wk; Lactobacilli paracasei ST11, living or heat-killed, (> 107 CFU/mL) and LH for 4 wk | 252 (aged 6-17 yr) asymptomatic children and adolescents: Living La1/LH, n = 511; Heat-killed La1/LH, n = 501; Living ST11/LH, n = 501; Heat-killed ST11/LH, n = 511; LH, n = 501 | 13C-UBT | A moderate but significant difference in 13C-UBT values was detected in children receiving live La1, whereas no differences were observed in the other groups | 13C-UBT (at the end of treatment) | |
| Gotteland et al[65] | R, DB, PC | Lactobacillus johnsonii La1, living or heat-killed, 80 mL/die, (> 107 CFU/mL) for 3 wk (Chamyto, Nestlé) with or without cranberry juice (CB) (200 mL) | 271 (aged 6-16 yr) asymptomatic children and adolescents: CB/La1, n = 701; Placebo juice/La1, n = 671; CB/heat-killed La1, n = 651; Placebo juice/heat-killed La1 (control), n = 691 | 13C-UBT | 16 (22.9)1 11 (16.9)1 10 (14.9)1vs 1 (1.5)1; P < 0.01 | 13C-UBT (a second 13C-UBT at the end of treatment and a third 13C-UBT after 1 mo) | The third UBT was carried out in only 19 of the 38 children found to be H. Pylori-negative in the second UBT: 12, 2, and 5 subjects from the CB/La1, placebo juice/La1, and CB/heat-killed La1 groups, respectively. Only four children (21) remained negative, after 1 mo without treatment: two from the placebo juice/La1 group and two from the CB/La1 group |
| Boonyaritichaikij et al[66] | SB, PC | Lactobacillus gasseri OLL2716 (LG21), pieces of cheese weighing 1.6-2.0 g, approximately 5 × 108 CFU/g for 1 yr | 88 (aged 3-7 yr) asymptomatic children and adolescents completed the eradication arm: LG21, n = 821; ordinary cheese, n = 61 while 222 completed the prevention arm: LG21, n = 1231; Ordinary cheese, n = 991 | HpSA | 24 (29.3)1vs 0. In the randomized prevention arm: 5 (4.1) vs 8 (8.1); P = 0.21 were HpSA positive at 12 mo | HpSA (1 yr) | A total of 440 asymptomatic children were screened by the HpSA test. Thereafter 132 H. Pylori positive and 308 H. Pylori negative children were recruited to eradication and randomized prevention arms, respectively. Eradication was defined as reversion by HpSA at 12 mo; prevention as persistently HpSA negative at 12 mo |
In a randomized open trial, Gotteland et al[64] randomized asymptomatic H. pylori-positive children to receive either 7-d triple therapy, or Saccharomyces boulardii as a symbiotic simultaneously with inulin or L. acidophilus LB daily for 8 wk. An additional group of asymptomatic H. pylori-positive children was followed for 8 wk without any treatment. A significant decrease in 13C-UBT, performed after 8 wk, was observed in the antibiotic group and in the S. boulardii group but not in the L. acidophilus LB group. No changes in 13C-UBT values were observed in untreated children. The results of this study suggest that the suppressive effect on H. pylori colonization in children depends on the probiotic strain used.
In a multicentric, randomized, controlled, double-blind trial carried out in 271 asymptomatic children who tested positive for H. pylori by 13C-UBT, Gotteland[65] evaluated whether cranberry juice and the probiotic L. johnsonii La1 could act additively or synergistically to suppress H. pylori. Subjects were allocated in four groups: cranberry juice/L. johnsonii La1, placebo juice/L. johnsonii La1, cranberry juice/heat-killed L. johnsonii La1, and placebo juice/heat-killed L. johnsonii La1 (control), given for 3 wk, after which a second UBT was carried out. A third 13C-UBT was done after one-month washout in those children who tested negative in the second 13C-UBT. H. pylori eradication rates significantly differed in the four groups: 1.5% in the control group compared with 14.9%, 16.9%, and 22.9% in the placebo juice/L. johnsonii La1, cranberry juice/heat-killed L. johnsonii La1, and cranberry juice/L. johnsonii La1, respectively; the latter group showed a slight but not significant increase when compared with the other treated groups. The third 13C-UBT was carried out only in 19 of the 38 children who tested negative in the second 13C-UBT and H. pylori was detected in 80% of them, suggesting just a temporary inhibition of the organism that disappeared once the administration of the inhibiting factors was interrupted.
In a recent study L. gasseri OLL2716 was administered in cheese to pre-school children to evaluate whether its long time administration (for one year) can eradicate H. pylori and/or prevent H. pylori infection[66]. A total of 440 children were screened by the H. pylori stool antigen (HpSA) test. Thereafter, 132 H. pylori-positive and 308 H. pylori-negative children were recruited to eradication and randomized prevention arms, respectively. Of the 132 H. pylori-positive children, 28 withdrew in the beginning because they did not like the cheese. However, 18 of the 28 subjects agreed to undergo an HpSA test again 1-year later, and were designated as the control group. Eighty-two of the remaining H. pylori-positive subjects completed the eradication arm, of which 24 (29.3%) were considered to be cured after treatment according to the HpSA test, whereas no eradication was observed in the six subjects in the placebo group consuming ordinary cheese. Spontaneous eradication was found in 1 of 18 children (5.6%) who represented the control group. The difference in the rate of eradication between the active and control groups was statistically significant. However, HpSA test was repeated in 12 of 24 subjects who were HpSA- negative after undergoing the L. gasseri treatment, but found that 5 of those 12 (41.7%) had reversed to be HpSA-positive. Therefore, a final eradication rate was around 17%. In the randomized prevention arm, 123 of 156 (79.0%) and 99 of 122 (81.0%) completed active and placebo arms, respectively, of which 4.1% and 8.1% were HpSA positive at 12 mo based on a per-protocol analysis (P = 0.21).
So far, there has been no convincing evidence on the beneficial effect of supplementation of probiotics to triple therapy for eradicating H. pylori infection in children. The very few trials performed in children on the effect of probiotics alone suggest just a temporary inhibition of H. pylori that disappears once the administration of the inhibiting factors are interrupted. Nonetheless, the majority of these studies were based on relatively small samples and, therefore, they may lack the statistical power necessary to detect an important effect of the probiotics. Finally, in most studies, the effect of probiotic treatment on H. pylori infection in children has been estimated indirectly by 13C-UBT. On the other hand, probiotic treatment seems to be able to reduce H. pylori therapy associated side effects and indirectly may help to improve the eradication rate; however it seems that the beneficial effects are strain specific. We conclude that standardized multicenter, placebo-controlled studies in larger series of children are needed to demonstrate any benefit of probiotics in the management of H. pylori infection in children, including its effect on the severity of H. pylori gastritis. Additional work is necessary to determine the strain, dose and administration to be used. Long-term studies are also needed in children to prove whether the persistent suppressive effect of probiotics on H. pylori and its associated gastritis could prevent diseases such as gastric cancer or peptic ulcer.
P- Reviewers: Mansour-Ghanaei F, MeraRM, Shibata T, SlomianyBL, Zevit N S- Editor: Zhai HH L- Editor: A E- Editor: Ma S
| 1. | Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175-1186. [PubMed] [Cited in This Article: ] |
| 2. | Staat MA, Kruszon-Moran D, McQuillan GM, Kaslow RA. A population-based serologic survey of Helicobacter pylori infection in children and adolescents in the United States. J Infect Dis. 1996;174:1120-1123. [PubMed] [Cited in This Article: ] |
| 3. | Malaty HM, Kim JG, Kim SD, Graham DY. Prevalence of Helicobacter pylori infection in Korean children: inverse relation to socioeconomic status despite a uniformly high prevalence in adults. Am J Epidemiol. 1996;143:257-262. [PubMed] [Cited in This Article: ] |
| 4. | O’Rourke K, Goodman KJ, Grazioplene M, Redlinger T, Day RS. Determinants of geographic variation in Helicobacter pylori infection among children on the US-Mexico border. Am J Epidemiol. 2003;158:816-824. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Tsai CJ, Perry S, Sanchez L, Parsonnet J. Helicobacter pylori infection in different generations of Hispanics in the San Francisco Bay Area. Am J Epidemiol. 2005;162:351-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Pérez-Pérez GI, Sack RB, Reid R, Santosham M, Croll J, Blaser MJ. Transient and persistent Helicobacter pylori colonization in Native American children. J Clin Microbiol. 2003;41:2401-2407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Malaty HM, El-Kasabany A, Graham DY, Miller CC, Reddy SG, Srinivasan SR, Yamaoka Y, Berenson GS. Age at acquisition of Helicobacter pylori infection: a follow-up study from infancy to adulthood. Lancet. 2002;359:931-935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 270] [Cited by in F6Publishing: 286] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 8. | Goodman KJ, Cockburn M. The role of epidemiology in understanding the health effects of Helicobacter pylori. Epidemiology. 2001;12:266-271. [PubMed] [Cited in This Article: ] |
| 9. | Glynn MK, Friedman CR, Gold BD, Khanna B, Hutwagner L, Iihoshi N, Revollo C, Quick R. Seroincidence of Helicobacter pylori infection in a cohort of rural Bolivian children: acquisition and analysis of possible risk factors. Clin Infect Dis. 2002;35:1059-1065. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Goodman KJ, O’rourke K, Day RS, Wang C, Nurgalieva Z, Phillips CV, Aragaki C, Campos A, de la Rosa JM. Dynamics of Helicobacter pylori infection in a US-Mexico cohort during the first two years of life. Int J Epidemiol. 2005;34:1348-1355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Phillips CV, Goodman KJ. Interpreting data in the face of competing explanations: assessing the hypothesis that observed spontaneous clearance of Helicobacter pylori was all measurement error. Int J Epidemiol. 2009;38:1110-1117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Duque X, Vilchis J, Mera R, Trejo-Valdivia B, Goodman KJ, Mendoza ME, Navarro F, Roque V, Moran S, Torres J. Natural history of Helicobacter pylori infection in Mexican schoolchildren: incidence and spontaneous clearance. J Pediatr Gastroenterol Nutr. 2012;55:209-216. [PubMed] [Cited in This Article: ] |
| 13. | Drumm B, Koletzko S, Oderda G. Helicobacter pylori infection in children: a consensus statement. European Paediatric Task Force on Helicobacter pylori. J Pediatr Gastroenterol Nutr. 2000;30:207-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 213] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 14. | Gold BD, Colletti RB, Abbott M, Czinn SJ, Elitsur Y, Hassall E, Macarthur C, Snyder J, Sherman PM. Helicobacter pylori infection in children: recommendations for diagnosis and treatment. J Pediatr Gastroenterol Nutr. 2000;31:490-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 218] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 15. | Bourke B, Ceponis P, Chiba N, Czinn S, Ferraro R, Fischbach L, Gold B, Hyunh H, Jacobson K, Jones NL. Canadian Helicobacter Study Group Consensus Conference: Update on the approach to Helicobacter pylori infection in children and adolescents--an evidence-based evaluation. Can J Gastroenterol. 2005;19:399-408. [PubMed] [Cited in This Article: ] |
| 16. | Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143-1153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 652] [Cited by in F6Publishing: 694] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 17. | Koletzko S, Richy F, Bontems P, Crone J, Kalach N, Monteiro ML, Gottrand F, Celinska-Cedro D, Roma-Giannikou E, Orderda G. Prospective multicentre study on antibiotic resistance of Helicobacter pylori strains obtained from children living in Europe. Gut. 2006;55:1711-1716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 159] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Duck WM, Sobel J, Pruckler JM, Song Q, Swerdlow D, Friedman C, Sulka A, Swaminathan B, Taylor T, Hoekstra M. Antimicrobial resistance incidence and risk factors among Helicobacter pylori-infected persons, United States. Emerg Infect Dis. 2004;10:1088-1094. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 140] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Megraud F. Helicobacter pylori and antibiotic resistance. Gut. 2007;56:1502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Malfertheiner P, Megraud F, O’Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772-781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1348] [Cited by in F6Publishing: 1286] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 21. | Gatta L, Vakil N, Leandro G, Di Mario F, Vaira D. Sequential therapy or triple therapy for Helicobacter pylori infection: systematic review and meta-analysis of randomized controlled trials in adults and children. Am J Gastroenterol. 2009;104:3069-3079; quiz 1080. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Koletzko S, Jones NL, Goodman KJ, Gold B, Rowland M, Cadranel S, Chong S, Colletti RB, Casswall T, Elitsur Y. Evidence-based guidelines from ESPGHAN and NASPGHAN for Helicobacter pylori infection in children. J Pediatr Gastroenterol Nutr. 2011;53:230-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 173] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 23. | Lesbros-Pantoflickova D, Corthésy-Theulaz I, Blum AL. Helicobacter pylori and probiotics. J Nutr. 2007;137:S812-818. [Cited in This Article: ] |
| 24. | Patel A, Shah N, Prajapati JB. Clinical appliance of probiotics in the treatment of Helicobacter pylori infection-A brief review. J Microbiol Immunol Infect. 2013;Jun 8; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Gotteland M, Brunser O, Cruchet S. Systematic review: are probiotics useful in controlling gastric colonization by Helicobacter pylori? Aliment Pharmacol Ther. 2006;23:1077-1086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 26. | Lionetti E, Francavilla R, Castellazzi AM, Arrigo T, Labò E, Leonardi S, Ciprandi G, Miraglia Del Giudice M, Salpietro V, Salpietro C. Probiotics and Helicobacter pylori infection in children. J Biol Regul Homeost Agents. 2012;26:S69-S76. [PubMed] [Cited in This Article: ] |
| 27. | Food and Agriculture Organization of the United Nations; World Health Organization. Guidelines for the evaluation of probiotics in food: joint FAO/WHO Working Group report on drafting guidelines for the evaluation of probiotics in food. Accessed October 1, 2010. Available from: http://ftp.fao.org/es/esn/food/wgreport2.pdf. [Cited in This Article: ] |
| 28. | Food and Agriculture Organization of the United Nations; World Health Organization. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria: report of a joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Accessed October 1, 2010. Available from: http://www.who.int/foodsafety/publications/fsmanagement/en/probiotics.pdf. [Cited in This Article: ] |
| 29. | Agostoni C, Axelsson I, Braegger C, Goulet O, Koletzko B, Michaelsen KF, Rigo J, Shamir R, Szajewska H, Turck D. Probiotic bacteria in dietetic products for infants: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2004;38:365-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Thomas DW, Greer FR. Probiotics and prebiotics in pediatrics. Pediatrics. 2010;126:1217-1231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 286] [Cited by in F6Publishing: 296] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 31. | Alsahli M, Michetti P. Lactobacilli for the management of Helicobacter pylori. Nutrition. 2001;17:268-269. [PubMed] [Cited in This Article: ] |
| 32. | Elliott SN, Buret A, McKnight W, Miller MJ, Wallace JL. Bacteria rapidly colonize and modulate healing of gastric ulcers in rats. Am J Physiol. 1998;275:G425-G432. [PubMed] [Cited in This Article: ] |
| 33. | Vandenbergh PA. Lactic acid bacteria, their metabolic products and interference with microbial growth. FEMS Microbiol Rev. 1993;12:221-238. [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 282] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 34. | Bhatia SJ, Kochar N, Abraham P, Nair NG, Mehta AP. Lactobacillus acidophilus inhibits growth of Campylobacter pylori in vitro. J Clin Microbiol. 1989;27:2328-2330. [PubMed] [Cited in This Article: ] |
| 35. | Midolo PD, Lambert JR, Hull R, Luo F, Grayson ML. In vitro inhibition of Helicobacter pylori NCTC 11637 by organic acids and lactic acid bacteria. J Appl Bacteriol. 1995;79:475-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 131] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | Jack RW, Tagg JR, Ray B. Bacteriocins of gram-positive bacteria. Microbiol Rev. 1995;59:171-200. [PubMed] [Cited in This Article: ] |
| 37. | Klaenhammer TR. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev. 1993;12:39-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 892] [Cited by in F6Publishing: 1098] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 38. | Guruge JL, Falk PG, Lorenz RG, Dans M, Wirth HP, Blaser MJ, Berg DE, Gordon JI. Epithelial attachment alters the outcome of Helicobacter pylori infection. Proc Natl Acad Sci USA. 1998;95:3925-3930. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 238] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 39. | Mukai T, Asasaka T, Sato E, Mori K, Matsumoto M, Ohori H. Inhibition of binding of Helicobacter pylori to the glycolipid receptors by probiotic Lactobacillus reuteri. FEMS Immunol Med Microbiol. 2002;32:105-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 178] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 40. | Bernet MF, Brassart D, Neeser JR, Servin AL. Lactobacillus acidophilus LA 1 binds to cultured human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut. 1994;35:483-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 498] [Cited by in F6Publishing: 527] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 41. | Byrd JC, Yunker CK, Xu QS, Sternberg LR, Bresalier RS. Inhibition of gastric mucin synthesis by Helicobacter pylori. Gastroenterology. 2000;118:1072-1079. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 109] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 42. | Mack DR, Michail S, Wei S, McDougall L, Hollingsworth MA. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am J Physiol. 1999;276:G941-G950. [PubMed] [Cited in This Article: ] |
| 43. | Gotteland M, Cruchet S, Verbeke S. Effect of Lactobacillus ingestion on the gastrointestinal mucosal barrier alterations induced by indometacin in humans. Aliment Pharmacol Ther. 2001;15:11-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 115] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 44. | Gill HS. Probiotics to enhance anti-infective defences in the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2003;17:755-773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 152] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 45. | Haller D, Bode C, Hammes WP, Pfeifer AM, Schiffrin EJ, Blum S. Non-pathogenic bacteria elicit a differential cytokine response by intestinal epithelial cell/leucocyte co-cultures. Gut. 2000;47:79-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 334] [Cited by in F6Publishing: 351] [Article Influence: 14.6] [Reference Citation Analysis (1)] |
| 46. | Aiba Y, Suzuki N, Kabir AM, Takagi A, Koga Y. Lactic acid-mediated suppression of Helicobacter pylori by the oral administration of Lactobacillus salivarius as a probiotic in a gnotobiotic murine model. Am J Gastroenterol. 1998;93:2097-2101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 230] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 47. | Coconnier MH, Lievin V, Hemery E, Servin AL. Antagonistic activity against Helicobacter infection in vitro and in vivo by the human Lactobacillus acidophilus strain LB. Appl Environ Microbiol. 1998;64:4573-4580. [PubMed] [Cited in This Article: ] |
| 48. | Johnson-Henry KC, Mitchell DJ, Avitzur Y, Galindo-Mata E, Jones NL, Sherman PM. Probiotics reduce bacterial colonization and gastric inflammation in H. pylori-infected mice. Dig Dis Sci. 2004;49:1095-1102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 49. | Kabir AM, Aiba Y, Takagi A, Kamiya S, Miwa T, Koga Y. Prevention of Helicobacter pylori infection by lactobacilli in a gnotobiotic murine model. Gut. 1997;41:49-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 225] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 50. | Sgouras DN, Panayotopoulou EG, Martinez-Gonzalez B, Petraki K, Michopoulos S, Mentis A. Lactobacillus johnsonii La1 attenuates Helicobacter pylori-associated gastritis and reduces levels of proinflammatory chemokines in C57BL/6 mice. Clin Diagn Lab Immunol. 2005;12:1378-1386. [PubMed] [Cited in This Article: ] |
| 51. | Cats A, Kuipers EJ, Bosschaert MA, Pot RG, Vandenbroucke-Grauls CM, Kusters JG. Effect of frequent consumption of a Lactobacillus casei-containing milk drink in Helicobacter pylori-colonized subjects. Aliment Pharmacol Ther. 2003;17:429-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 52. | Gotteland M, Cruchet S. Suppressive effect of frequent ingestion of Lactobacillus johnsonii La1 on Helicobacter pylori colonization in asymptomatic volunteers. J Antimicrob Chemother. 2003;51:1317-1319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 53. | Linsalata M, Russo F, Berloco P, Caruso ML, Matteo GD, Cifone MG, Simone CD, Ierardi E, Di Leo A. The influence of Lactobacillus brevis on ornithine decarboxylase activity and polyamine profiles in Helicobacter pylori-infected gastric mucosa. Helicobacter. 2004;9:165-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 54. | Pantoflickova D, Corthésy-Theulaz I, Dorta G, Stolte M, Isler P, Rochat F, Enslen M, Blum AL. Favourable effect of regular intake of fermented milk containing Lactobacillus johnsonii on Helicobacter pylori associated gastritis. Aliment Pharmacol Ther. 2003;18:805-813. [PubMed] [Cited in This Article: ] |
| 55. | Sakamoto I, Igarashi M, Kimura K, Takagi A, Miwa T, Koga Y. Suppressive effect of Lactobacillus gasseri OLL 2716 (LG21) on Helicobacter pylori infection in humans. J Antimicrob Chemother. 2001;47:709-710. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 222] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 56. | Sýkora J, Valecková K, Amlerová J, Siala K, Dedek P, Watkins S, Varvarovská J, Stozický F, Pazdiora P, Schwarz J. Effects of a specially designed fermented milk product containing probiotic Lactobacillus casei DN-114 001 and the eradication of H. pylori in children: a prospective randomized double-blind study. J Clin Gastroenterol. 2005;39:692-698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 57. | Lionetti E, Miniello VL, Castellaneta SP, Magistá AM, de Canio A, Maurogiovanni G, Ierardi E, Cavallo L, Francavilla R. Lactobacillus reuteri therapy to reduce side-effects during anti-Helicobacter pylori treatment in children: a randomized placebo controlled trial. Aliment Pharmacol Ther. 2006;24:1461-1468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 58. | Goldman CG, Barrado DA, Balcarce N, Rua EC, Oshiro M, Calcagno ML, Janjetic M, Fuda J, Weill R, Salgueiro MJ. Effect of a probiotic food as an adjuvant to triple therapy for eradication of Helicobacter pylori infection in children. Nutrition. 2006;22:984-988. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 59. | Szajewska H, Albrecht P, Topczewska-Cabanek A. Randomized, double-blind, placebo-controlled trial: effect of lactobacillus GG supplementation on Helicobacter pylori eradication rates and side effects during treatment in children. J Pediatr Gastroenterol Nutr. 2009;48:431-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 60. | Hurduc V, Plesca D, Dragomir D, Sajin M, Vandenplas Y. A randomized, open trial evaluating the effect of Saccharomyces boulardii on the eradication rate of Helicobacter pylori infection in children. Acta Paediatr. 2009;98:127-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 61. | Tolone S, Pellino V, Vitaliti G, Lanzafame A, Tolone C. Evaluation of Helicobacter Pylori eradication in pediatric patients by triple therapy plus lactoferrin and probiotics compared to triple therapy alone. Ital J Pediatr. 2012;38:63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 62. | Ahmad K, Fatemeh F, Mehri N, Maryam S. Probiotics for the treatment of pediatric helicobacter pylori infection: a randomized double blind clinical trial. Iran J Pediatr. 2013;23:79-84. [PubMed] [Cited in This Article: ] |
| 63. | Cruchet S, Obregon MC, Salazar G, Diaz E, Gotteland M. Effect of the ingestion of a dietary product containing Lactobacillus johnsonii La1 on Helicobacter pylori colonization in children. Nutrition. 2003;19:716-721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 123] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 64. | Gotteland M, Poliak L, Cruchet S, Brunser O. Effect of regular ingestion of Saccharomyces boulardii plus inulin or Lactobacillus acidophilus LB in children colonized by Helicobacter pylori. Acta Paediatr. 2005;94:1747-1751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 65. | Gotteland M, Andrews M, Toledo M, Muñoz L, Caceres P, Anziani A, Wittig E, Speisky H, Salazar G. Modulation of Helicobacter pylori colonization with cranberry juice and Lactobacillus johnsonii La1 in children. Nutrition. 2008;24:421-426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 66. | Boonyaritichaikij S, Kuwabara K, Nagano J, Kobayashi K, Koga Y. Long-term administration of probiotics to asymptomatic pre-school children for either the eradication or the prevention of Helicobacter pylori infection. Helicobacter. 2009;14:202-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |