Published online Jun 21, 2014. doi: 10.3748/wjg.v20.i23.7089
Revised: January 17, 2014
Accepted: April 1, 2014
Published online: June 21, 2014
Hepatitis C virus (HCV) is a successful pathogen on the grounds that it exploits its host’s metabolism to build up viral particles; moreover it favours its own survival by inducing chronic disease and the development of specific anatomic changes in the infected organ. Steatosis, therefore, is associated with HCV infection by necessity rather than by chance alone. Approximately 6% of HCV patients have steatohepatitis. Interestingly, HCV steatosis occurs in the setting of multiple metabolic abnormalities (hyperuricemia, reversible hypocholesterolemia, insulin resistance, arterial hypertension and expansion of visceral adipose tissue) collectively referred to as “hepatitis C-associated dysmetabolic syndrome” (HCADS). General, nonalcoholic fatty liver disease (NAFLD)-like, mechanisms of steatogenesis (including increased availability of lipogenic substrates and de novo lipogenesis; decreased oxidation of fatty substrates and export of fatty substrates) are shared by all HCV genotypes. However, genotype 3 seemingly amplifies such steatogenic molecular mechanisms reported to occur in NAFLD via more profound changes in microsomal triglyceride transfer protein; peroxisome proliferator-activated receptor alpha; sterol regulatory element-binding proteins and phosphatase and tensin homologue. HCV steatosis has a remarkable clinical impact in as much as it is an acknowledged risk factor for accelerated fibrogenesis; for impaired treatment response to interferon and ribavirin; and development of hepatocellular carcinoma. Recent data, moreover, suggest that HCV-steatosis contributes to premature atherogenesis via both direct and indirect mechanisms. In conclusion, HCV steatosis fulfills all expected requirements necessary to perpetuate the HCV life cycle. A better understanding of the physiology of HCADS will likely result in a more successful handling of disease with improved antiviral success rates.
Core tip: Hepatitis C virus (HCV) steatosis occurs in the setting of multiple abnormalities collectively referred to as “hepatitis C-associated dysmetabolic syndrome”. General, nonalcoholic fatty liver disease-like, mechanisms of steatogenesis are shared by all HCV genotypes. However, genotype 3 seemingly amplifies such steatogenic molecular mechanisms. HCV steatosis has a remarkable clinical impact in accelerating fibrogenesis; impairing treatment response to interferons and ribavirin; and favouring the development of hepatocellular carcinoma and atherosclerosis. In conclusion, steatosis fulfills all expected requirements necessary to perpetuate the HCV life cycle and is associated with HCV infection by necessity rather than by chance.
- Citation: Lonardo A, Adinolfi LE, Restivo L, Ballestri S, Romagnoli D, Baldelli E, Nascimbeni F, Loria P. Pathogenesis and significance of hepatitis C virus steatosis: An update on survival strategy of a successful pathogen. World J Gastroenterol 2014; 20(23): 7089-7103
- URL: https://www.wjgnet.com/1007-9327/full/v20/i23/7089.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i23.7089
Steatosis was reported to occur in acute and chronic “non-A, non-B hepatitis” several years before hepatitis C virus (HCV) testing was available[1,2] suggesting a direct steatogenic role of HCV. Over time, a large body of evidence has exhaustively demonstrated HCV to be strongly associated with both metabolic derangements and steatosis.
The biological significance of such metabolic derangements and histological changes is unknown. However, it is intriguing to speculate that they occur as a result of an evolutionary strategy developed by HCV to ensure its survival. Indeed, in order to be successful in its struggle for life, a pathogen virus may be expected to: (1) exploit its host’s metabolic pathways to build up viral particles; (2) favour its own survival by inducing chronic disease as opposed to self-limiting infection; and (3) induce the development of specific anatomic changes in the infected organ more often than by chance.
The aim of this article is to review the evidence that steatosis associated with HCV infection fulfills each and all the above theoretical requirements for HCV to be a successful pathogen. The existence of such a survival strategy suggests that steatosis is associated with HCV infection by necessity rather than by chance.
Steatosis is a common finding in many hepatic and extra-hepatic disorders notably including visceral obesity, a component of metabolic syndrome (MS). Over time, the initial theory that steatosis represents the hepatic manifestation of MS has evolved into the paradigm that non-alcoholic fatty liver disease (NAFLD) is an essential requirement for the development of the MS[3]. MS is a cluster of cardio-metabolic risk factors which, triggered by the expansion of adipose tissue, tend to self aggregate and to self perpetuate[4].
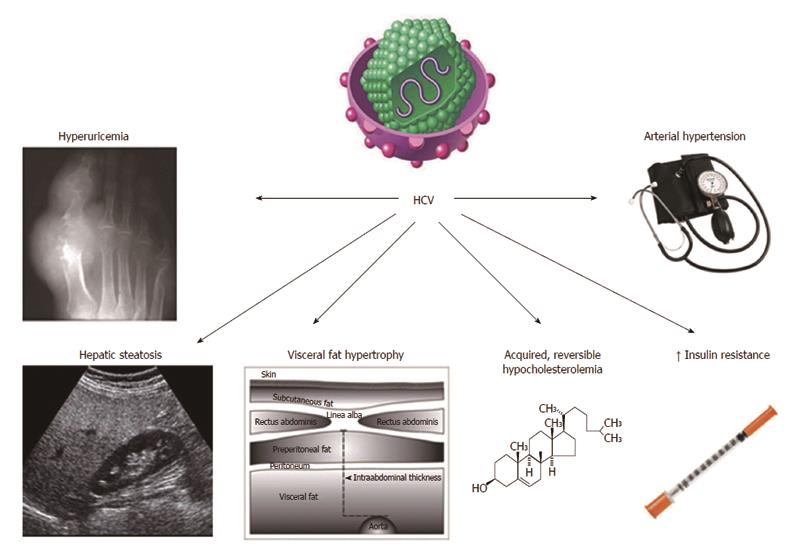
The prevalence of steatosis, in patients with chronic hepatitis C, is higher than expected by simple chance association[5]. When our group first suggested that HCV-related steatosis should probably be interpreted as an equivalent of NAFLD[5], the extent of such a similarity remained poorly defined. Since then, increasing evidence has been reinforcing the view that steatosis is only one of the many analogies linking these two conditions. In particular, HCV infection is associated with multiple metabolic derangements which, collectively, are referred to as hepatitis C associated dysmetabolic syndrome (HCADS)[6-10]. Figure 1 illustrates our present understanding of HCADS (modified from[10]). Some of such metabolic derangements, e.g., insulin resistance (IR) and hypocholesterolemia had been earlier identified[11], others (e.g., hyperuricemia and altered body fat distribution) have been recognized more recently.
Viral (including genotype, HCV RNA load and gene mutations) and host features [such as body mass index (BMI), type 2 diabetes (T2D) and alcohol consumption] affect the risk of steatosis[9-11]. Little is known about the impact of HCADS on HCV life cycle. However, it is increasingly being recognized that the individual components of the HCADS tend to facilitate HCV survival, as discussed below.
In the transgenic mouse model HCV core protein induces IR which predates the development of steatosis[12]. The specific cellular and molecular bases of IR associated with HCV infection have been extensively reviewed elsewhere[13-15]. Given that HCV infected patients are at greater risk of developing IR, it is not surprising that HCV should be considered a diabetogenic virus in predisposed individuals[16]. In their meta-analytic review, White et al[17] reported that the presence of HCV infection significantly increased the risk of T2D compared to both HCV-negative [odds ratio (OR) 1.68 and 1.67 in retrospective and prospective studies, respectively] and HBV-infected controls (OR 1.80). Such findings indicate that HCV infection per se, irrespective of chronic liver disease, directly increases T2D risk. Further evidence for such a diabetogenic action of HCV comes from the finding that human immunodeficiency virus (HIV)-HCV co-infected patients showed a higher risk of T2D when compared with HIV mono-infection (OR 1.82)[18], a condition which is often associated with hepatic steatosis and other metabolic derangements[19]. Similar results were obtained by our group by evaluating IR in different groups of mono- and co-infected patients (HIV, HCV, and HIV-HCV) with virus-associated steatosis, compared to NAFLD cases. We found that IR was predicted by HCV infection, a finding representing direct evidence for HCV infection per se to be associated with increased IR[20].
On these grounds, it is plausible to hypothesize that successful HCV eradication should be associated with the prevention of T2D. Although studies on this topic have yielded conflicting results (reviewed in[14]), recent research on a large cohort of HCV patients showed that sustained virological response (SVR) to treatment with interferon based regimen prevents the development of IR, whereas treatment failure was an independent risk factor for the de novo development of IR[21].
Taken collectively, these findings support the view that HCV is a strong risk factor for IR, as well as T2D in predisposed individuals. Accordingly, HCV eradication may potentially reverse IR thus helping to prevent the development of T2D in these patients.
In a previous study from our group, both cirrhotic and chronic hepatitis C patients showed lower serum levels of cholesterol than those observed in both healthy controls and NAFLD individuals[8]. Of interest, normalization of serum cholesterol levels occurs following achievement of SVR[22] suggesting that HCV itself reversibly perturbs the cholesterol biosynthetic pathway.
Recent research has promoted our understanding of the fine molecular mechanisms eventually leading to the development of hypocholesterolemia in the setting of HCV infection. Lipids play a key role on virion structure, target membrane, cell receptor recognition, viral membrane fusion, viral replication, assembly and egress[23]. Research has particularly focused on the key steps of HCV entry into and egress from infected hepatocytes[23,24] which represent potential targets for antiviral strategies. HCV-associated hypocholesterolemia results from the inhibition of apolipoprotein B100 secretion and, selectively, by the perturbed distal cholesterol synthesis pathway[25,26].
Contrary to common belief, HCV infects and replicates not only in hepatocytes but in many extra-hepatic sites (reviewed in[27]). Nevertheless, evidence for adipocyte infection by HCV is still lacking, although clinical data seem to suggest an interaction between HCV and adipose tissue. Evidence for such an interaction comes from two recent studies[28,29].
Mostafa et al[28] showed that mesenteric fat was significantly thicker in HCV-infected patients than in those never infected. Although, these authors did not provide direct evidence for mesenteric fat colonization by HCV, these findings seem to suggest a possible interaction of HCV with mesenteric fat. A possible interaction of HCV with visceral adipose tissue is also suggested by the data published by Zampino et al[29]. These Authors reported that, in chronic hepatitis C patients, an increased amount of abdominal fat is associated with patatin-like phospholipase domain-containing 3 gene (PNPLA3) p.I148M and with hepatic steatosis).
Although the full pathogenic scenario is far from being elucidated, the above pioneer studies[28,29] envisage the possibility of an interaction of HCV with visceral fat accumulation and host genetics.
This recent line of research has been successfully conducted exploiting in depth the concept that HCV infection closely recapitulates naturally occurring MS in humans. Petta et al[30], showed that hyperuricemia, defined as serum uric acid either > 7 mg/dL or > 6 mg/dL in men and woman, respectively, was present in 7.5% of 496 consecutive patients with biopsy-proven chronic hepatitis C. Not surprisingly, such a metabolic feature tended to cluster with low HDL cholesterol, high blood pressure, estimated glomerular filtration rate and severity of steatosis. Moreover, hyperuricemia was among the independent predictors of steatosis severity.
Younossi et al[31] in their United States population study reported that at multivariate analysis HCV-RNA positivity (found in in 173 individuals out of 19741 eligible participants) was independently associated with the presence of arterial hypertension (further to IR and T2D).
The findings in HCADS of hyperuricemia and arterial hypertension are closely reminiscent of those occurring in NAFLD[32-34].
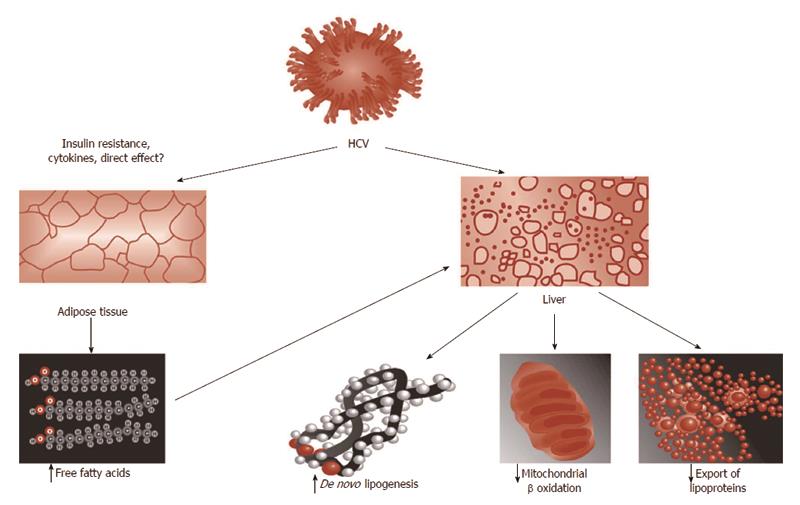
Based on the analogy of HCV-steatosis with NAFLD paradigm, it may be anticipated that HCV-related steatogenesis occurs via the following four steps, illustrated in Figure 2[9]: increased availability of lipogenic substrates; increased de novo lipogenesis; decreased oxidation of fatty substrates and decreased export of fatty substrates from the hepatocyte into the blood stream.
Increased availability of lipogenic substrates: The state of IR which is a typical feature of HCV infection[14-16,35] represents the biological background providing the hepatocyte with abundance of lipogenic substrates (such as glucose and non-esterified fatty acids) and hormones (hyperinsulinemia).
Increased de novo lipogenesis: It is well demonstrated that elevated fasting lipogenesis occurs in HCV in humans[36] as a result of up-regulated genes mediating fatty acid de novo synthesis and uptake[37]. Coupled with impaired cholesterol synthesis, therefore, HCV has been reported to induce a paradoxical state in which, while cholesterol synthetic pathways are up-regulated, synthesis of the end product cholesterol is actually impaired as a result of diversion of the intermediate geranylpyrophospate, which is necessary to HCV life cycle[36,38,39].
Decreased oxidation of fatty substrates: The seminal study by Okuda et al[40] provided the first evidence for perturbed mitochondrial function as a direct result of HCV core protein. These Authors found that expression of HCV core protein uniformly increased ROS in 3 different cell lines and also increased lipid peroxidation products in 2 out of the 3 in vitro systems. Interestingly, inhibition of mitochondrial electron transport prevented such an oxidative stress induced by core protein[40]. These findings were confirmed in HCV transgenic mice indicating that oxidative injury occurs as a direct result of HCV core protein expression both in vitro and in vivo[40].
Evidence for disruption of fatty acids metabolism in patients with chronic HCV infection has been reported by an elegant metabolomic profile analysis study by Roe et al[41] Moreover, Sato et al[42] reported that the rate of change in total ketone body concentration between 12 and 15 h after the start of fasting (an indirect index of decreased oxidation of fatty substrates) was significantly lower in chronic hepatitis C patients than in healthy volunteers and such a reduction was associated with increasing viral load and IR.
Decreased export of fatty substrates from the hepatocyte into the bloodstream: Using the HCV subgenomic replicon expression system, Domitrovich et al[25] have demonstrated that interaction with the nonstructural protein 5A and apoB 100 results in the inhibition of apolipoprotein B100 secretion.
In conclusion, in order to induce steatogenic pathways, HCV infection affects each and all the four chief classical steps involved in NAFLD steatogenesis[9,43].
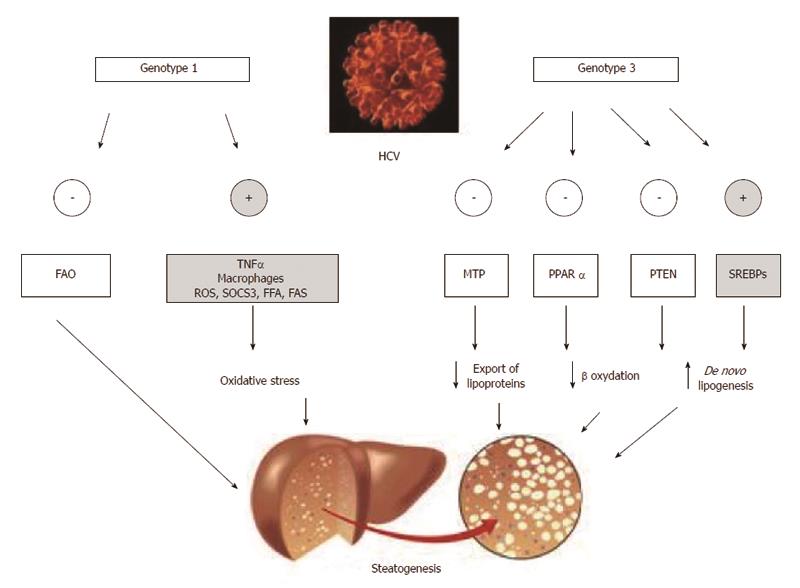
This topic has recently been analyzed in depth by Roingeard et al[44]. Data suggest that, with the notable exception of phosphatase and tensin homologue (PTEN) down-regulation, rather than having specific “steatogenic strategies”, HCV genotype 3 seemingly amplifies steatogenic mechanisms reported to occur in NAFLD[45]. Specific differences in the core protein aminoacid sequence have been reported to account for the more elevated steatogenic activity featured by HCV genotype 3[46]. Moreover, alcohol abuse might be another, spurious, mechanism accounting for the finding that HCV genotype 3 is more frequently associated with steatosis and that such steatosis is more severe[9,11]. HCV genotype 3, indeed, tends to be more common among intravenous drug addicts, a population typically featuring high alcohol consumption[47-49]. Finally, the theoretical possibility that genotype 3 HCV infection might exert a more pronounced steatogenic activity via more increased IR remains controversial[14,44].
Microsomal triglyceride transfer protein (MTP) is effective in stabilizing apoB lipidation, a key step in the assembly of VLDL particles and thus in the export capacity of triglycerides from the hepatocyte into the bloodstream[50]. Core and nonstructural HCV proteins promote steatosis by impairing MTP activity[25,51]. Such a pathogenic mechanism accounts for the acknowledged finding that both hypo-lipidemia and steatosis are reversed by HCV eradication[11,44]. Interestingly, compared to those infected with other HCV genotypes, patients with genotype 3 HCV infection have been reported to exhibit significantly reduced MTP activity[52]. Although not a definite evidence for a HCV genotype 3 direct inhibition of MTP, these data may circumstantially suggest such an effect[44], so accounting for the elective steatogenic potential of HCV genotype 3 even in the absence of worsened IR.
In liver cells, the highly expressed nuclear receptor PPAR-alpha promotes oxidation of fatty acids by stimulating their entry into mitochondria, where they undergo oxidation. Lipid-lowering fibrates, are ligands of PPAR-alpha[53].
Conversely, decreased PPAR-alpha activity will result in increased fatty deposition mediated by impaired fatty acid uptake and decreased mitochondrial oxidation of lipidic substrates[44].
Interestingly, genotype 3 HCV displays PPAR-alpha mRNAs lower than those observed in patients with genotype 1 HCV infection[54] suggesting this to be one more pathogenic mechanism accounting specifically for genotype-3 steatogenesis[44]. Indirectly confirming that steatosis represents a selective advantage for HCV survival, a pilot study has suggested the potential usefulness of PPAR-alpha agonist bezafibrate in the treatment of CHC patients[55]. Unfortunately, long-term treatment of transgenic mice expressing HCV core protein with clofibrate, induced hepatocellular carcinoma (HCC) with mitochondrial abnormalities and hepatic steatosis[2,56].
Sterol regulatory element-binding proteins (SREBPs), SREBP-1c being the hepatic isoform, are a family of transcription factors which, following activation, are translocated intranuclearly, eventually up-regulating the transcription of lipogenic genes, including fatty acid synthase (FAS)[57]. The relationships between SREBP-1c and HCV proteins are unclear. Of interest, FAS serum levels predict the development of steatosis in patients with viral hepatitis due to HCV[58]. It remains controversial whether HCV genotype 3 core proteins are capable of inducing FAS more efficiently than HCV genotype 1 proteins[59,60]. However, it is possible that HCV genotype 3 activates SREBPs more efficiently via increased oxidative stress (PI-3-K-Akt pathway and inactivation of PTEN)[61].
Studies in vitro have reported intra-hepatic down-regulation of PTEN to be another, NAFLD-like[24], genotype 3-core protein specific steatogenic mechanism which does not occur in HCV genotype 1 infection[62].
In conclusion, Figure 3 recapitulates the principles of genotype-specific molecular mechanisms of steatogenesis which exploit the host’s metabolic machinery[63-69].
Cross-sectional and prospective studies have shown that fibrosis progression risk is increased in the presence of HCV-steatosis (reviewed in[11]). Several published studies, summarized in Table 1[68,70-101], have evaluated the association of steatosis and fibrosis in either cross-sectional or prospective studies.
| Method and findings | Conclusion | Ref. |
| 98 CHC patients who had undergone repeat liver biopsies before antiviral treatment (median follow-up 5.8 yr) | In HCV patients with genotype 3 infection, steatosis was a risk factor for fibrosis progression | [68] |
| 297 consecutive patients with HCV | Steatosis and inflammation scores were the only parameters independently predicting fibrosis | [70] |
| 96 non-cirrhotic treatment-naive CHC patients | In untreated CHC patients fibrosis progression was strongly associated with worsening of steatosis | [71] |
| 1428 CHC treatment-naïve patients included in a French therapeutic trial | The variables independently associated with steatosis were genotype 3 , higher age, triglycerides and body mass index (BMI) values and septal fibrosis | [72] |
| 131 biopsy-proven CHC individuals | Hepatic steatosis was related to genotype, fibrosis degree, and serum leptin level | [73] |
| 160 CHC patients | Irrespective of viral genotype, patients who had steatosis showed significantly more fibrosis than non-steatosic | [74] |
| Cross sectional study evaluating: 233 hepatic biopsies from 219 CHC patients and hepatectomy specimens from 65 patients transplanted for HCV-related cirrhosis. Longitudinal study: 41 patients with two biopsies and 10 patients with three biopsies performed over 2-8 yr | Steatosis was associated with fibrosis independently of necroinflammation, but declined in cirrhosis | [75] |
| Retrospective study conducted on 324 US patients with CHC from a university medical center and a regional VA medical center | Steatosis was independently associated with advanced fibrosis stage | [76] |
| 135 treatment-naive CHC patients who had undergone repeat liver biopsies after a median interval of 61 months after the baseline biopsy | Irrespective of HCV genotype, steatosis was a chief contributor to fibrosis progression in mild CHC and the probability of such a fibrosis worsening is directly dependent on the proportion of steatotic hepatocytes | [77] |
| 116 CHC patients undergoing liver biopsy | The MTHFR C677T polymorphism, responsible for hyperhomocysteinemia, contributed to increasing steatogenesis and steatosis which in its turn, hastened hepatic fibrosis progression | [78] |
| Meta-analysis on individual data from 3068 patients with biopsy- proven CHC recruited from 10 centers in Europe, Australia, and United States | Steatosis was significantly and independently associated with fibrosis in CHC. Hepatic inflammation may mediate fibrogenesis in patients with liver steatosis | [79] |
| 180 patients infected with genotype 1b HCV | At multivariate analysis, fibrosis was significantly related to age, alanine transaminase, diabetes, hepatitis B core antibody, steatohepatitis and grading | [80] |
| Overall, 600 consecutive individuals: 500 with CHC; and 100 with CHB | IR, was associated with 1 and 4 HCV genotypes and high viral load. The association of significant fibrosis with IR occurs independent of steatosis | [81] |
| 153 chronic hepatitis C patients enrolled in the Swiss hepatitis c cohort study and for whom a liver biopsy and plasma samples were available | By multiple regression analysis, CTGF levels were independently associated with steatosis, a past history of alcohol abuse, plasma leptin and HCV RNA levels; when only patients with genotypes non-3 were considered, CTGF levels were independently associated with a past history of alcohol abuse, plasma leptin levels and steatosis | [82] |
| 107 consecutive CHC patients | Multiple regression analysis revealed that, HOMA-IR, fibrosis and oxidative stress were independently associated with steatosis, whereas steatosis was independently associated with oxidative stress and HOMA-IR. Steatosis and HAI were also independent predictors of fibrosis | [83] |
| 143 AA and 157 CA adults with untreated chronic HCV genotype 1 infection | In 3-variable models including race and biopsy adequacy, the factors significantly associated with fibrosis progression were age when infected, steatosis, ALT level, and necroinflammatory score | [84] |
| 228 HCV treatment-naive patients who met the inclusion/exclusion criteria | Genotype 1 and presence of steatosis were found to be associated significantly with MS. After adjusting for confounding variables, MS remained independently associated with a lack of SVR | [85] |
| 346 untreated, nondiabetic patients solely infected with either genotype 1 or 3 | HOMA-IR rather than steatosis was independently associated with fibrosis for both HCV genotype 1 and genotype 3. Exclusion of cirrhotic subjects did not alter the findings with respect to the greater contribution of IR compared to hepatic steatosis, as a predictor of fibrosis | [86] |
| Retrospective study of 460 patients with CHC | Elevated AST, alpha fetoprotein, and presence of grade 2 and 3 steatosis are independent parameters associated with stage 3 and 4 fibrosis in patients with CHC | [87] |
| 112 HCV RNA positive subjects who had two liver biopsies performed | On multivariate analysis, only baseline steatosis was significantly associated with fibrosis. Kaplan-Meier analysis demonstrated that steatosis impacted on time to progression to both significant fibrosis and cirrhosis | [88] |
| Of 253 HCV RNA-positive persons who underwent at least one liver biopsy | The presence of T2D, steatosis and duration of HCV infection were independent predictors of advanced fibrosis | [89] |
| Metanalysis of 12 published studies, including 1989 HIV/HCV co-infected patients | In co-infected patients, HS was associated with higher body mass index, diabetes mellitus, elevated alanine aminotransferase, necroinflammatory activity and fibrosis | [90] |
| Liver biopsy samples were collected from 59 patients with HCV without a sustained virologic response (SVR) or cirrhosis | There were no associations between fibrosis progression and histologic features including inflammation, fibrosis, or steatosis | [91] |
| 170 genotype 1 CHC patients | At multivariate analysis Severe (F3-F4 fibrosis), , was independently associated with older age, IR, steatosis > 10%, and moderate-severe necroinflammatory activity in CHC patients | [92] |
| 92 untreated consecutive adults with chronic HCV infection admitted for liver biopsy | In multivariate analyses, fibrosis was associated with high AST level, age ≥ 40 yr, and steatosis | [93] |
| 152 LT recipients with HCV were followed up with repeated liver biopsies for a median of 2.09 yr after index biopsy | In the multivariate analysis, steatosis at 1 yr was an independent predictor of subsequent F2 to F4 fibrosis. Steatosis was a stronger predictor of fibrosis in the setting of sirolimus use | [94] |
| 755 consecutive chronic hepatitis C patients (178 with genotype 3), admitted to three referral hospitals in Switzerland | Fibrosis was associated with steatosis in genotype 3 infected individuals alone | [95] |
| 574 CHC patients with chronic hepatitis C from a single United States center | In CHC owing to genotype 1 infection HCV, fibrosis was associated with steatosis severity | [96] |
| Clinical data and liver histology findings in 510 HCV patients were analysed | Age at liver biopsy, BMI and duration of HCV were independent risk factors for increased fibrosis in HCV patients. Steatosis as a risk factor for fibrosis is evident in HCV genotype-1 | [97] |
| 60 HCV patients compared to 41 NASH patients and 18 CHB individuals | Compared to patients who had mild steatosis, those CHC individuals with moderate steatosis exhibited higher fibrosis stages | [98] |
| 251 CHC women | Severity of fibrosis was associated with a longer duration of infection, a higher BMI, advanced steatosis and the menopause | [99] |
| Ninety HCV infected patients undergoing liver biopsy | Steatosis was not found to be independently associated with fibrosis | [100] |
| Liver biopsies from 66 out of 306 HCV/HIV non-cirrhotic patients without cirrhosis at baseline who underwent a second biopsy were case-matched with a control group selected from a cohort of 233 HCV mono-infected patients | Progression of fibrosis was similar in HIV/HCV-co-infected compared to HCV mono-infected individuals and no clinical or laboratory predictor of worsening liver disease was identified | [101] |
The vast majority of these reports have consistently provided robust evidence for steatosis being associated with fibrosis. Among such studies, probably the most single conclusive evidence that steatosis represents a risk factor for fibrosis progression comes from the study by Leandro et al[79]. These Authors conducted a meta-analysis on data belonging to 3068 CHC patients recruited at 10 centers in Europe, Australia, and north America. Steatosis and fibrosis were found in 50.9% and 87.6% of cases, respectively. At logistic regression analysis, fibrosis was independently associated with steatosis and other variables. Based on the finding that, in the subgroup analyses, the association between steatosis and fibrosis was invariably dependent upon a concurrent association of steatosis with liver inflammatory changes, the Authors reasonably speculated that the association of steatosis and fibrosis is likely mediated by hepatic inflammation.
A more restricted number of studies, however, have identified a genotype-dependency of the association of steatosis with fibrosis[68,95-97]. Finally, only in a minority of studies fibrosis and steatosis were not associated[91,98-101].
In conclusion, an impressive body of evidence supports the strong association of steatosis with fibrosis in chronic HCV infection. It is logical to hypothesize that inflammatory hepatic changes represent the candidate lesion mediating the progression, in the liver, from fatty to fibrotic changes.
Several studies, reported in Table 2, have identified steatosis as a predictor of poor treatment outcome in chronic HCV infection since the early 2000s[96,102-111].
| Method and findings | Conclusion | Ref. |
| 574 patients with chronic hepatitis C | Steatosis reduces the likelihood of achieving early and SVR in genotype 1 infected patients | [96] |
| HCV genotype 2 and 3 patients | Steatosis is the independent predictor of relapse | [102] |
| 148 consecutive adults with HCV admitted for liver biopsy | Steatosis in chronic hepatitis C is not a negative prognostic factor of response to combined antiviral therapy | [103] |
| 932 patients infected with HCV genotype 2 or 3 | Steatosis was associated with significantly higher rates of relapse, irrespective of viral load, in patients infected with HCV genotype 3 who had an RVR | [104] |
| A total of 116 patients [HCV-G4 85 (73.3%); HCV-G1 31 (26.7%)] were included | The NAS steatosis score correlates with response to antiviral therapy | [105] |
| 885 HCV patients | Steatosis did not influence the efficacy of treatment in our study population. Baseline viral load is a confounding factor, particularly in patients infected with genotype 3 and once baseline viral load was accounted for, the association between steatosis and SVR was not relevant | [106] |
| 250 patients with genotype 4 chronic hepatitis C, treated with different regimens of combined interferon | Among genotype 4 chronic hepatitis C patients, severe fibrosis, severe steatosis, treatment with standard interferon and a high serum AFP level were all negatively associated with SVR | [107] |
| 207 HCV patients | Features of the metabolic syndrome are associated with hepatic steatosis in most of these patients. Steatosis is significantly more common in genotype 3 compared with other genotypes, and in these patients, an SVR is associated with steatosis clearance | [108] |
| 357 HCV-infected US veterans | Steatosis is independently associated with stage III-IV fibrosis. However, only HCV genotype, and not steatosis, obesity, or stage III-IV fibrosis, was associated with SVR to interferon alpha-2b and ribavirin treatment | [109] |
| 80 Japanese patients with CHC. treated with IFN alpha-2b and ribavirin for 24 wk evaluated retrospectively | HS is an important predictor of poor response to therapy of IFN-alpha-2b and ribavirin in patients with CHC | [110] |
| Liver specimens with both CHC and significant steatosis (> 33%) or SH were categorized as group 1 (84 specimens). A control group (group 2) of 231 CHC patients without evidence of steatosis > 33% or SH | Overall SVR for patients with HCV and significant steatosis or SH is considerably lower than for HCV and steatosis less than 33% and no SH | [111] |
These findings appear to be consistent across different HCV genotypes. It may be argued that steatosis, in patients with HCV infection, is closely associated with hepatic fibrosis and multiple metabolic derangements (HCADS). Therefore steatosis, further to directly impairing SVR per se, may also act as a marker of dysmetabolic milieu (HCADS) and that it is the latter that actually impairs SVR.
Published studies on steatosis as a risk factor for HCC in those with HCV infection are summarized in Table 3[112-120]. With reference to such studies, it is of note that evidence linking steatosis and an increased risk of developing HCC in those with HCV infection is not limited to case-reports alone.
| Method and findings | Conclusion | Ref. |
| 1818 patients with histologically proven CHC treated with IFN Cumulative incidence and HCC risk were analyzed over a mean follow-up period of 6.1 yr HCC developed in 179 study subjects | Severe steatosis, is an independent factor significantly associated with HCC | [112] |
| The 5-yr occurrence rate of HCC in 353 consecutive patients with histologically proven HCV cirrhosis and persistent viral replication prospectively followed and screened for HCC was 34% in genotype 3 and 17% in non-3 genotype group | For patients with HCV cirrhosis and ongoing infection, infection with genotype 3 (a potent steatogenic virus) is independently associated with an increased risk of HCC development | [113] |
| The steatohepatitis-HCC variant was found in 35.5% of 62 HCC cases In 14 of the 22 cases (63.6%) of SH-HCC, the non-neoplastic liver showed changes of NAFLD/NASH superimposed on otherwise typical features of HCV-C | This study suggests a possible NAFLD/NASH pathway leading to SH-HCC in the setting of HCV-C | [114] |
| This retrospective study investigated the features of 5 patients who developed HCC after 10 yr of achieving SVR | In 3 patients, liver tissues were obtained at the treatment of HCC. These tissues showed marked improvement in both activities and fibroses, but severe steatosis in 1 patient | [115] |
| Two-hundred and sixty-six patients, who achieved SVR, were enrolled in this retrospective study | Age, hepatic fibrosis, and hepatic steatosis at pre-interferon treatment might be risk factors for developing HCC after SVR | [116] |
| A retrospective study was conducted in 88 patients undergoing curative resection of HCV-associated HCC | Hepatic steatosis is a useful predictor of postoperative recurrence of HCV-related HCC | [117] |
| 94 consecutive patients with cirrhosis due to HCV who underwent liver transplantation and had pathology available for review were retrospectively identified | In patients with HCV-related cirrhosis, the presence of hepatic steatosis is independently associated with the development of HCC | [118] |
| The histological severity of steatosis in the index liver biopsies of 25 patients with chronic hepatitis C who subsequently developed HCC was compared with matched controls who did not. As determined by percentage area of biopsy core occupied by steatosis on computer assisted morphometric evaluation, and graded semiquantitatively, steatosis was comparable among cases and controls | The odds of developing HCC among those with steatosis grades 1 and 2 did not differ significantly from those without steatosis. There was no association between increasing morphometric percentage area occupied by steatosis and the subsequent development of HCC. Neither steatosis grade or percent area of steatosis on biopsy were selected in multivariate regression analysis as independent predictors for the development of HCC | [119] |
| 161 patients with chronic HCV infection | At multivariate analysis hepatic steatosis, (together with aging, cirrhosis, and no IFN treatment) was an independent, significant risk factor for HCC | [120] |
Data appear to be consistent and most studies support this conclusion while a single case-control study has provided negative results concerning such HCV-steatosis HCC association[119]. In this limited case-control study patients with a SVR to antiviral therapy were excluded.
Based on data reported in Table 2, given that steatosis poses an additional risk for HCC, increased surveillance is necessary in HCV patients with steatosis. According to Nkontchou et al[113] such a caution needs to be particularly exercised in those with HCV genotype 3. Moreover, a possible NAFLD/NASH pathway leading to steatohepatitic-HCC in the setting of HCV-cirrhosis requires further investigation[114].
Hepatocarcinogenesis in those with HCV steatosis involves both IR and, particularly, deranged lipid metabolism. The molecular bases underlying the pathogenesis of HCV-HCC have been accurately detailed elsewhere[14,15,37,121,122].
Steatohepatitis is the stereotypical histological hallmark of a wide spectrum of etiologically diverse liver conditions which invariably display concurrent steatosis and inflammatory-fibrotic changes occurring to a variable extent[123].
Compared to simple steatosis, less has been published concerning steatohepatitis occurring in the setting of HCV infection. Probably this occurs as a result of steatohepatitis being a much more uncommon finding which occurs in 4% to 10% of cases, averaging 6.13 % of 2316 published cases as shown in Table 4[70,124-127].
| Series | Steatohepatitis | Ref. |
| 297 patients | 17 (6) | [70] |
| 170 patients | 17 (10) | [124] |
| 1458 liver biopsies | 80 (5.5) | [125] |
| 95 patients | 4 (4) | [126] |
| 296 liver biopsies | 24 (9) | [127] |
| 2316 biopsies/patients | 142 (6,13) | Cumulative |
Acknowledged risks for the development of steatohepatitis in patients with chronic hepatitis C include BMI[128]; either trigliceride and HDL cholesterol (genotype 1) or AST (genotpe 3)[127].
Similar to steatosis, steatohepatitis has been associated with advanced fibrosis[124] and impaired SVR[111] suggesting that the biological significance of steatohepatitis closely overlaps with that of high-grade steatosis. However, whether steatohepatitis is an independent risk factor for HCC in HCV-infected individuals, remains to be shown.
In the past, individuals with chronic liver disease were deemed to be spared from atherosclerosis but this paradigm has been changed by an ever increasing body of literature concerning hepatitis C[129]. HCV infection is not a unique model of atherosclerosis and numerous reports document an association of atherosclerosis with infections due to a large variety of pathogens[130].
Data reviewed elsewhere[129] indicate that HCV is associated with excess cardiovascular mortality and that, possibly, achieving SVR might prevent at least a fraction of such deaths. Studies evaluating the association of HCV sero-positivity (either HCV-Ab or HCV-RNA) with surrogate markers of atherosclerotic burden (Carotid Intima Media Thickening or plaques) have been reviewed recently elsewhere[129]. Data from our and other groups indicate that viral (HCV viral load) and host features (liver histology changes) are likely to contribute to increased vascular risk in these individuals[131,132]. Interestingly, Adinolfi et al[133] have recently reported that HCV infection is a risk factor for human ischemic stroke.
The mechanisms underlying excess cardiovascular risk include both HCV direct vessel colonization and indirect mechanism associated with HCV infection such as pro-atherogenic cardiometabolic derangement, i.e., HCADS per se, increased expression of pro-inflammatory cytokines and adhesion molecules, hyperomocysteinemia and steatosis[129,134].
We have discussed data favoring the interpretation of steatosis as an example of successful viral strategy. HCV steatosis fulfills all requirements expected to occur in order to perpetuate HCV life cycle.
Indeed, for steatosis to develop, the host’s metabolic pathways are engaged giving life to a variant (HCADS) of the commonly occurring metabolic syndrome. Such an HCADS will typically display, further to steatosis, the corollary of ordinary metabolic syndrome including IR, hyperuricemia and expanded visceral adipose tissue. However, given that intermediate products of the cholesterol biosynthetic pathway rather than cholesterol itself are necessary to HCV replication, HCV will perturb the distal biosynthetic pathway, eventually leading to hypocholesterolemia.
A better understanding of the physiology of HCADS will likely result in a more successful handling of disease with improved antiviral success rates.
The authors are indebted to Ms. Jacqueline Mole for English language editing.
P- Reviewers: Cao GW, Delladetsima IK, Feo F S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
| 1. | Kryger P, Christoffersen P. Light microscopic morphology of acute hepatitis non-A, non-B. A comparison with hepatitis type A and B. Liver. 1982;2:200-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Wiese M, Haupt R. [Histomorphologic picture of chronic non-A, non-B hepatitis]. Dtsch Z Verdau Stoffwechselkr. 1985;45:101-110. [PubMed] [Cited in This Article: ] |
| 3. | Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 764] [Cited by in F6Publishing: 809] [Article Influence: 80.9] [Reference Citation Analysis (0)] |
| 4. | Lonardo A, Caldwell SH, Loria P. Clinical physiology of NAFLD: a critical overview of pathogenesis and treatment. Expert Rev Endocrinol Metab. 2010;5:403-423. [DOI] [Cited in This Article: ] |
| 5. | Lonardo A, Adinolfi LE, Loria P, Carulli N, Ruggiero G, Day CP. Steatosis and hepatitis C virus: mechanisms and significance for hepatic and extrahepatic disease. Gastroenterology. 2004;126:586-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 336] [Cited by in F6Publishing: 325] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 6. | Lonardo A, Loria P. The hepatitis C virus-associated dysmetabolic syndrome. Hepatology. 2008;48:1018-1019; author reply 1019. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Lonardo A, Loria P, Carulli N. Dysmetabolic changes associated with HCV: a distinct syndrome? Intern Emerg Med. 2008;3:99-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Lonardo A, Ballestri S, Adinolfi LE, Violi E, Carulli L, Lombardini S, Scaglioni F, Ricchi M, Ruggiero G, Loria P. Hepatitis C virus-infected patients are ‘spared’ from the metabolic syndrome but not from insulin resistance. A comparative study of nonalcoholic fatty liver disease and hepatitis C virus-related steatosis. Can J Gastroenterol. 2009;23:273-278. [PubMed] [Cited in This Article: ] |
| 9. | Lonardo A, Adinolfi LE, Loria P. Mechanisms of hepatitis C virus-induced fatty liver disease. Hot Topics Viral Hep. 2009;5:23-28. [Cited in This Article: ] |
| 10. | Adinolfi LE, Restivo L, Zampino R, Lonardo A, Loria P. Metabolic alterations and chronic hepatitis C: treatment strategies. Expert Opin Pharmacother. 2011;12:2215-2234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Lonardo A, Loria P, Adinolfi LE, Carulli N, Ruggiero G. Hepatitis C and steatosis: a reappraisal. J Viral Hepat. 2006;13:73-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, Moriya K, Koike K. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840-848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 559] [Cited by in F6Publishing: 590] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 13. | Serfaty L, Capeau J. Hepatitis C, insulin resistance and diabetes: clinical and pathogenic data. Liver Int. 2009;29 Suppl 2:13-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Lonardo A, Adinolfi LE, Petta S, Craxì A, Loria P. Hepatitis C and diabetes: the inevitable coincidence? Expert Rev Anti Infect Ther. 2009;7:293-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Bugianesi E, Salamone F, Negro F. The interaction of metabolic factors with HCV infection: does it matter? J Hepatol. 2012;56 Suppl 1:S56-S65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 137] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 16. | Lonardo A, Carulli N, Loria P. HCV and diabetes. A two-question-based reappraisal. Dig Liver Dis. 2007;39:753-761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | White DL, Ratziu V, El-Serag HB. Hepatitis C infection and risk of diabetes: a systematic review and meta-analysis. J Hepatol. 2008;49:831-844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 299] [Cited by in F6Publishing: 317] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 18. | Machado MV, Oliveira AG, Cortez-Pinto H. Hepatic steatosis in hepatitis B virus infected patients: meta-analysis of risk factors and comparison with hepatitis C infected patients. J Gastroenterol Hepatol. 2011;26:1361-1367. [PubMed] [Cited in This Article: ] |
| 19. | Balasubramanyam A, Sekhar RV, Jahoor F, Jones PH, Pownall HJ. Pathophysiology of dyslipidemia and increased cardiovascular risk in HIV lipodystrophy: a model of ‘systemic steatosis’. Curr Opin Lipidol. 2004;15:59-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Guaraldi G, Lonardo A, Ballestri S, Zona S, Stentarelli C, Orlando G, Carli F, Carulli L, Roverato A, Loria P. Human immunodeficiency virus is the major determinant of steatosis and hepatitis C virus of insulin resistance in virus-associated fatty liver disease. Arch Med Res. 2011;42:690-697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Aghemo A, Prati GM, Rumi MG, Soffredini R, D’Ambrosio R, Orsi E, De Nicola S, Degasperi E, Grancini V, Colombo M. Sustained virological response prevents the development of insulin resistance in patients with chronic hepatitis C. Hepatology. 2012;56:1681-1687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Kuo YH, Chuang TW, Hung CH, Chen CH, Wang JH, Hu TH, Lu SN, Lee CM. Reversal of hypolipidemia in chronic hepatitis C patients after successful antiviral therapy. J Formos Med Assoc. 2011;110:363-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Blaising J, Pécheur EI. Lipids: a key for hepatitis C virus entry and a potential target for antiviral strategies. Biochimie. 2013;95:96-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Peyrou M, Bourgoin L, Foti M. PTEN in non-alcoholic fatty liver disease/non-alcoholic steatohepatitis and cancer. Dig Dis. 2010;28:236-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Domitrovich AM, Felmlee DJ, Siddiqui A. Hepatitis C virus nonstructural proteins inhibit apolipoprotein B100 secretion. J Biol Chem. 2005;280:39802-39808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Clark PJ, Thompson AJ, Vock DM, Kratz LE, Tolun AA, Muir AJ, McHutchison JG, Subramanian M, Millington DM, Kelley RI. Hepatitis C virus selectively perturbs the distal cholesterol synthesis pathway in a genotype-specific manner. Hepatology. 2012;56:49-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Revie D, Salahuddin SZ. Human cell types important for hepatitis C virus replication in vivo and in vitro: old assertions and current evidence. Virol J. 2011;8:346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Mostafa A, Mohamed MK, Saeed M, Hasan A, Fontanet A, Godsland I, Coady E, Esmat G, El-Hoseiny M, Abdul-Hamid M. Hepatitis C infection and clearance: impact on atherosclerosis and cardiometabolic risk factors. Gut. 2010;59:1135-1140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 29. | Zampino R, Coppola N, Cirillo G, Boemio A, Pisaturo M, Marrone A, Macera M, Sagnelli E, Perrone L, Adinolfi LE. Abdominal fat interacts with PNPLA3 I148M, but not with the APOC3 variant in the pathogenesis of liver steatosis in chronic hepatitis C. J Viral Hepat. 2013;20:517-523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Petta S, Macaluso FS, Cammà C, Marco VD, Cabibi D, Craxì A. Hyperuricaemia: another metabolic feature affecting the severity of chronic hepatitis because of HCV infection. Liver Int. 2012;32:1443-1450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Younossi ZM, Stepanova M, Nader F, Younossi Z, Elsheikh E. Associations of chronic hepatitis C with metabolic and cardiac outcomes. Aliment Pharmacol Ther. 2013;37:647-652. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 32. | Lonardo A, Loria P, Leonardi F, Borsatti A, Neri P, Pulvirenti M, Verrone AM, Bagni A, Bertolotti M, Ganazzi D. ST.E.N.A. Study Group. Policentrica Steatosi Epatica Non Alcolica. Fasting insulin and uric acid levels but not indices of iron metabolism are independent predictors of non-alcoholic fatty liver disease. A case-control study. Dig Liver Dis. 2002;34:204-211. [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 91] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Petta S, Cammà C, Cabibi D, Di Marco V, Craxì A. Hyperuricemia is associated with histological liver damage in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2011;34:757-766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 34. | Sirota JC, McFann K, Targher G, Johnson RJ, Chonchol M, Jalal DI. Elevated serum uric acid levels are associated with non-alcoholic fatty liver disease independently of metabolic syndrome features in the United States: Liver ultrasound data from the National Health and Nutrition Examination Survey. Metabolism. 2013;62:392-399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 35. | Bugianesi E. Mechanisms of insulin resistance associated with hepatitis C virus infection. Hot Topics Viral Hep. 2012;8:13-17. [Cited in This Article: ] |
| 36. | Lambert JE, Bain VG, Ryan EA, Thomson AB, Clandinin MT. Elevated lipogenesis and diminished cholesterol synthesis in patients with hepatitis C viral infection compared to healthy humans. Hepatology. 2013;57:1697-1704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | Wu JM, Skill NJ, Maluccio MA. Evidence of aberrant lipid metabolism in hepatitis C and hepatocellular carcinoma. HPB (Oxford). 2010;12:625-636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 38. | Ye J, Wang C, Sumpter R, Brown MS, Goldstein JL, Gale M. Disruption of hepatitis C virus RNA replication through inhibition of host protein geranylgeranylation. Proc Natl Acad Sci USA. 2003;100:15865-15870. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 301] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 39. | Kapadia SB, Chisari FV. Hepatitis C virus RNA replication is regulated by host geranylgeranylation and fatty acids. Proc Natl Acad Sci USA. 2005;102:2561-2566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 386] [Cited by in F6Publishing: 399] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 40. | Okuda M, Li K, Beard MR, Showalter LA, Scholle F, Lemon SM, Weinman SA. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002;122:366-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 690] [Cited by in F6Publishing: 667] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 41. | Roe B, Kensicki E, Mohney R, Hall WW. Metabolomic profile of hepatitis C virus-infected hepatocytes. PLoS One. 2011;6:e23641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 42. | Sato C, Saito T, Misawa K, Katsumi T, Tomita K, Ishii R, Haga H, Okumoto K, Nishise Y, Watanabe H. Impaired mitochondrial β-oxidation in patients with chronic hepatitis C: relation with viral load and insulin resistance. BMC Gastroenterol. 2013;13:112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 43. | Lonardo A, Loria P, Argo C, Caldwell S. Perspectives on cellular dysfunction in nonalcoholic steatohepatitis: a case of ‘multiorganelle failure’? Proceedings of a virtual workshop on nonalcoholic steatohepatitis. Expert Rev Gastroenterol Hepatol. 2011;5:135-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Roingeard P. Hepatitis C virus diversity and hepatic steatosis. J Viral Hepat. 2013;20:77-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Bechmann LP, Hannivoort RA, Gerken G, Hotamisligil GS, Trauner M, Canbay A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol. 2012;56:952-964. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 593] [Cited by in F6Publishing: 633] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 46. | Hourioux C, Patient R, Morin A, Blanchard E, Moreau A, Trassard S, Giraudeau B, Roingeard P. The genotype 3-specific hepatitis C virus core protein residue phenylalanine 164 increases steatosis in an in vitro cellular model. Gut. 2007;56:1302-1308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 47. | Pawlotsky JM, Tsakiris L, Roudot-Thoraval F, Pellet C, Stuyver L, Duval J, Dhumeaux D. Relationship between hepatitis C virus genotypes and sources of infection in patients with chronic hepatitis C. J Infect Dis. 1995;171:1607-1610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 248] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 48. | Nalpas B, Thiers V, Pol S, Driss F, Thepot V, Berthelot P, Brechot C. Hepatitis C viremia and anti-HCV antibodies in alcoholics. J Hepatol. 1992;14:381-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 66] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 49. | Verbaan H, Andersson K, Eriksson S. Intravenous drug abuse--the major route of hepatitis C virus transmission among alcohol-dependent individuals? Scand J Gastroenterol. 1993;28:714-718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 50. | Pereira IV, Stefano JT, Oliveira CP. Microsomal triglyceride transfer protein and nonalcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2011;5:245-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | Perlemuter G, Sabile A, Letteron P, Vona G, Topilco A, Chrétien Y, Koike K, Pessayre D, Chapman J, Barba G. Hepatitis C virus core protein inhibits microsomal triglyceride transfer protein activity and very low density lipoprotein secretion: a model of viral-related steatosis. FASEB J. 2002;16:185-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 419] [Cited by in F6Publishing: 445] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 52. | Mirandola S, Realdon S, Iqbal J, Gerotto M, Dal Pero F, Bortoletto G, Marcolongo M, Vario A, Datz C, Hussain MM. Liver microsomal triglyceride transfer protein is involved in hepatitis C liver steatosis. Gastroenterology. 2006;130:1661-1669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 53. | Ferré P. The biology of peroxisome proliferator-activated receptors: relationship with lipid metabolism and insulin sensitivity. Diabetes. 2004;53 Suppl 1:S43-S50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 556] [Cited by in F6Publishing: 544] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 54. | de Gottardi A, Pazienza V, Pugnale P, Bruttin F, Rubbia-Brandt L, Juge-Aubry CE, Meier CA, Hadengue A, Negro F. Peroxisome proliferator-activated receptor-alpha and -gamma mRNA levels are reduced in chronic hepatitis C with steatosis and genotype 3 infection. Aliment Pharmacol Ther. 2006;23:107-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 55. | Fujita N, Kaito M, Kai M, Sugimoto R, Tanaka H, Horiike S, Konishi M, Iwasa M, Watanabe S, Adachi Y. Effects of bezafibrate in patients with chronic hepatitis C virus infection: combination with interferon and ribavirin. J Viral Hepat. 2006;13:441-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 56. | Tanaka N, Moriya K, Kiyosawa K, Koike K, Gonzalez FJ, Aoyama T. PPARalpha activation is essential for HCV core protein-induced hepatic steatosis and hepatocellular carcinoma in mice. J Clin Invest. 2008;118:683-694. [PubMed] [Cited in This Article: ] |
| 57. | Vluggens A, Reddy JK. Nuclear receptors and transcription factors in the development of fatty liver disease. Curr Drug Metab. 2012;13:1422-1435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 58. | Joven J, Espinel E, Rull A, Beltrán-Debón R, Aragonès G, Rodríguez-Gallego E, Camps J, Pedro-Botet J, Sans T, Menéndez JA. Serum fatty acid synthase concentration is increased in patients with hepatitis viral infection and may assist in the prediction of liver steatosis. J Clin Virol. 2011;51:199-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 59. | Jackel-Cram C, Babiuk LA, Liu Q. Up-regulation of fatty acid synthase promoter by hepatitis C virus core protein: genotype-3a core has a stronger effect than genotype-1b core. J Hepatol. 2007;46:999-1008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 60. | Ryan MC, Desmond PV, Slavin JL, Congiu M. Expression of genes involved in lipogenesis is not increased in patients with HCV genotype 3 in human liver. J Viral Hepat. 2011;18:53-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 61. | Oem JK, Jackel-Cram C, Li YP, Zhou Y, Zhong J, Shimano H, Babiuk LA, Liu Q. Activation of sterol regulatory element-binding protein 1c and fatty acid synthase transcription by hepatitis C virus non-structural protein 2. J Gen Virol. 2008;89:1225-1230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 62. | Clément S, Peyrou M, Sanchez-Pareja A, Bourgoin L, Ramadori P, Suter D, Vinciguerra M, Guilloux K, Pascarella S, Rubbia-Brandt L. Down-regulation of phosphatase and tensin homolog by hepatitis C virus core 3a in hepatocytes triggers the formation of large lipid droplets. Hepatology. 2011;54:38-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 63. | Hourigan LF, Macdonald GA, Purdie D, Whitehall VH, Shorthouse C, Clouston A, Powell EE. Fibrosis in chronic hepatitis C correlates significantly with body mass index and steatosis. Hepatology. 1999;29:1215-1219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 481] [Cited by in F6Publishing: 466] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 64. | Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33:1358-1364. [PubMed] [Cited in This Article: ] |
| 65. | Clouston AD, Jonsson JR, Purdie DM, Macdonald GA, Pandeya N, Shorthouse C, Powell EE. Steatosis and chronic hepatitis C: analysis of fibrosis and stellate cell activation. J Hepatol. 2001;34:314-320. [PubMed] [Cited in This Article: ] |
| 66. | Hwang SJ, Luo JC, Chu CW, Lai CR, Lu CL, Tsay SH, Wu JC, Chang FY, Lee SD. Hepatic steatosis in chronic hepatitis C virus infection: prevalence and clinical correlation. J Gastroenterol Hepatol. 2001;16:190-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 123] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 67. | Adinolfi LE, Utili R, Andreana A, Tripodi MF, Marracino M, Gambardella M, Giordano M, Ruggiero G. Serum HCV RNA levels correlate with histological liver damage and concur with steatosis in progression of chronic hepatitis C. Dig Dis Sci. 2001;46:1677-1683. [PubMed] [Cited in This Article: ] |
| 68. | Westin J, Nordlinder H, Lagging M, Norkrans G, Wejstål R. Steatosis accelerates fibrosis development over time in hepatitis C virus genotype 3 infected patients. J Hepatol. 2002;37:837-842. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 217] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 69. | Hui JM, Kench J, Farrell GC, Lin R, Samarasinghe D, Liddle C, Byth K, George J. Genotype-specific mechanisms for hepatic steatosis in chronic hepatitis C infection. J Gastroenterol Hepatol. 2002;17:873-881. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 70. | Monto A, Alonzo J, Watson JJ, Grunfeld C, Wright TL. Steatosis in chronic hepatitis C: relative contributions of obesity, diabetes mellitus, and alcohol. Hepatology. 2002;36:729-736. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 256] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 71. | Castéra L, Pawlotsky JM, Dhumeaux D. Worsening of steatosis and fibrosis progression in hepatitis C. Gut. 2003;52:1531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 72. | Poynard T, Ratziu V, McHutchison J, Manns M, Goodman Z, Zeuzem S, Younossi Z, Albrecht J. Effect of treatment with peginterferon or interferon alfa-2b and ribavirin on steatosis in patients infected with hepatitis C. Hepatology. 2003;38:75-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 446] [Cited by in F6Publishing: 422] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 73. | Romero-Gómez M, Castellano-Megias VM, Grande L, Irles JA, Cruz M, Nogales MC, Alcón JC, Robles A. Serum leptin levels correlate with hepatic steatosis in chronic hepatitis C. Am J Gastroenterol. 2003;98:1135-1141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 74. | Hickman IJ, Powell EE, Prins JB, Clouston AD, Ash S, Purdie DM, Jonsson JR. In overweight patients with chronic hepatitis C, circulating insulin is associated with hepatic fibrosis: implications for therapy. J Hepatol. 2003;39:1042-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 135] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 75. | Wyatt J, Baker H, Prasad P, Gong YY, Millson C. Steatosis and fibrosis in patients with chronic hepatitis C. J Clin Pathol. 2004;5:402-406. [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 76. | Hu KQ, Kyulo NL, Esrailian E, Thompson K, Chase R, Hillebrand DJ, Runyon BA. Overweight and obesity, hepatic steatosis, and progression of chronic hepatitis C: a retrospective study on a large cohort of patients in the United States. J Hepatol. 2004;40:147-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 77. | Fartoux L, Chazouillères O, Wendum D, Poupon R, Serfaty L. Impact of steatosis on progression of fibrosis in patients with mild hepatitis C. Hepatology. 2005;41:82-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 140] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 78. | Adinolfi LE, Ingrosso D, Cesaro G, Cimmino A, D’Antò M, Capasso R, Zappia V, Ruggiero G. Hyperhomocysteinemia and the MTHFR C677T polymorphism promote steatosis and fibrosis in chronic hepatitis C patients. Hepatology. 2005;41:995-1003. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 79. | Leandro G, Mangia A, Hui J, Fabris P, Rubbia-Brandt L, Colloredo G, Adinolfi LE, Asselah T, Jonsson JR, Smedile A. Relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: a meta-analysis of individual patient data. Gastroenterology. 2006;130:1636-1642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 416] [Cited by in F6Publishing: 435] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 80. | Masarone M, La Mura V, Bruno S, Gaeta GB, Vecchione R, Carrino F, Moschella F, Torella R, Persico M. Steatohepatitis is associated with diabetes and fibrosis in genotype 1b HCV-related chronic liver disease. J Viral Hepat. 2007;14:714-720. [PubMed] [Cited in This Article: ] |
| 81. | Moucari R, Asselah T, Cazals-Hatem D, Voitot H, Boyer N, Ripault MP, Sobesky R, Martinot-Peignoux M, Maylin S, Nicolas-Chanoine MH. Insulin resistance in chronic hepatitis C: association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology. 2008;134:416-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 375] [Cited by in F6Publishing: 360] [Article Influence: 22.5] [Reference Citation Analysis (1)] |
| 82. | Hora C, Negro F, Leandro G, Oneta CM, Rubbia-Brandt L, Muellhaupt B, Helbling B, Malinverni R, Gonvers JJ, Dufour JF. Connective tissue growth factor, steatosis and fibrosis in patients with chronic hepatitis C. Liver Int. 2008;28:370-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 83. | Vidali M, Tripodi MF, Ivaldi A, Zampino R, Occhino G, Restivo L, Sutti S, Marrone A, Ruggiero G, Albano E. Interplay between oxidative stress and hepatic steatosis in the progression of chronic hepatitis C. J Hepatol. 2008;48:399-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 84. | Terrault NA, Im K, Boylan R, Bacchetti P, Kleiner DE, Fontana RJ, Hoofnagle JH, Belle SH. Fibrosis progression in African Americans and Caucasian Americans with chronic hepatitis C. Clin Gastroenterol Hepatol. 2008;6:1403-1411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 85. | Hanouneh IA, Feldstein AE, Lopez R, Yerian L, Pillai A, Zein CO, Zein NN. Clinical significance of metabolic syndrome in the setting of chronic hepatitis C virus infection. Clin Gastroenterol Hepatol. 2008;6:584-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 86. | Cua IH, Hui JM, Kench JG, George J. Genotype-specific interactions of insulin resistance, steatosis, and fibrosis in chronic hepatitis C. Hepatology. 2008;48:723-731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 87. | Hu SX, Kyulo NL, Xia VW, Hillebrand DJ, Hu KQ. Factors associated with hepatic fibrosis in patients with chronic hepatitis C: a retrospective study of a large cohort of U.S. patients. J Clin Gastroenterol. 2009;43:758-764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 88. | Cross TJ, Quaglia A, Hughes S, Joshi D, Harrison PM. The impact of hepatic steatosis on the natural history of chronic hepatitis C infection. J Viral Hepat. 2009;16:492-499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 89. | Livingston SE, Deubner H, Bruden DL, McMahon BJ, Homan CE, Townshend-Bulson LJ, Bruce MG, Hennessy TW, Williams JL, Gretch DR. Factors associated with the progression of fibrosis on liver biopsy in Alaska Native and American Indian persons with chronic hepatitis C. Can J Gastroenterol. 2010;24:445-451. [PubMed] [Cited in This Article: ] |
| 90. | Machado MV, Oliveira AG, Cortez-Pinto H. Hepatic steatosis in patients coinfected with human immunodeficiency virus/hepatitis C virus: a meta-analysis of the risk factors. Hepatology. 2010;52:71-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 91. | Sterling RK, Wegelin JA, Smith PG, Stravitz RT, Luketic VA, Fuchs M, Puri P, Shiffman ML, Contos MA, Mills AS. Similar progression of fibrosis between HIV/HCV-infected and HCV-infected patients: Analysis of paired liver biopsy samples. Clin Gastroenterol Hepatol. 2010;8:1070-1076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 92. | Petta S, Cammà C, Di Marco V, Macaluso FS, Maida M, Pizzolanti G, Belmonte B, Cabibi D, Di Stefano R, Ferraro D. Hepatic steatosis and insulin resistance are associated with severe fibrosis in patients with chronic hepatitis caused by HBV or HCV infection. Liver Int. 2011;31:507-515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 93. | Ahmed AM, Hassan MS, Abd-Elsayed A, Hassan H, Hasanain AF, Helmy A. Insulin resistance, steatosis, and fibrosis in Egyptian patients with chronic Hepatitis C virus infection. Saudi J Gastroenterol. 2011;17:245-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 94. | Brandman D, Pingitore A, Lai JC, Roberts JP, Ferrell L, Bass NM, Terrault NA. Hepatic steatosis at 1 year is an additional predictor of subsequent fibrosis severity in liver transplant recipients with recurrent hepatitis C virus. Liver Transpl. 2011;17:1380-1386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 95. | Rubbia-Brandt L, Fabris P, Paganin S, Leandro G, Male PJ, Giostra E, Carlotto A, Bozzola L, Smedile A, Negro F. Steatosis affects chronic hepatitis C progression in a genotype specific way. Gut. 2004;53:406-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 214] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 96. | Patton HM, Patel K, Behling C, Bylund D, Blatt LM, Vallée M, Heaton S, Conrad A, Pockros PJ, McHutchison JG. The impact of steatosis on disease progression and early and sustained treatment response in chronic hepatitis C patients. J Hepatol. 2004;40:484-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 282] [Cited by in F6Publishing: 298] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 97. | Nieminen U, Arkkila PE, Kärkkäinen P, Färkkilä MA. Effect of steatosis and inflammation on liver fibrosis in chronic hepatitis C. Liver Int. 2009;29:153-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 98. | Czaja AJ, Carpenter HA, Santrach PJ, Moore SB. Host- and disease-specific factors affecting steatosis in chronic hepatitis C. J Hepatol. 1998;29:198-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 177] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 99. | Asselah T, Boyer N, Guimont MC, Cazals-Hatem D, Tubach F, Nahon K, Daïkha H, Vidaud D, Martinot M, Vidaud M. Liver fibrosis is not associated with steatosis but with necroinflammation in French patients with chronic hepatitis C. Gut. 2003;52:1638-1643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 100. | Matos CA, Perez RM, Pacheco MS, Figueiredo-Mendes CG, Lopes-Neto E, Oliveira EB, Lanzoni VP, Silva AE, Ferraz ML. Steatosis in chronic hepatitis C: relationship to the virus and host risk factors. J Gastroenterol Hepatol. 2006;21:1236-1239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 101. | Macías J, Berenguer J, Japón MA, Girón JA, Rivero A, López-Cortés LF, Moreno A, González-Serrano M, Iribarren JA, Ortega E. Fast fibrosis progression between repeated liver biopsies in patients coinfected with human immunodeficiency virus/hepatitis C virus. Hepatology. 2009;50:1056-1063. [PubMed] [Cited in This Article: ] |
| 102. | Restivo L, Zampino R, Guerrera B, Ruggiero L, Adinolfi LE. Steatosis is the predictor of relapse in HCV genotype 3- but not 2-infected patients treated with 12 weeks of pegylated interferon-α-2a plus ribavirin and RVR. J Viral Hepat. 2012;19:346-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 103. | Rafi H, Kabbaj N, Salihoun M, Amrani L, Acharki M, Guedira M, Nya M, Amrani N. Influence of steatosis on progression of fibrosis and virological response in chronic hepatitis C cases. Arab J Gastroenterol. 2011;12:136-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 104. | Shah SR, Patel K, Marcellin P, Foster GR, Manns M, Kottilil S, Healey L, Pulkstenis E, Subramanian GM, McHutchison JG. Steatosis is an independent predictor of relapse following rapid virologic response in patients with HCV genotype 3. Clin Gastroenterol Hepatol. 2011;9:688-693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 105. | AlQaraawi AM, Sanai FM, Al-Husseini H, Albenmousa A, AlSheikh A, Ahmed LR, Hersi A, Al-Otaibi MM, Syed M, Ali SM. Prevalence and impact of hepatic steatosis on the response to antiviral therapy in Saudi patients with genotypes 1 and 4 chronic hepatitis C. Dig Dis Sci. 2011;56:1222-1228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 106. | Rodriguez-Torres M, Govindarajan S, Diago M, Morgan T, Anand B, Barange K, Suter F, Lin A, Hooper G, Shiffman M. Hepatic steatosis in patients with chronic hepatitis C virus genotype 2 or 3 does not affect viral response in patients treated with peginterferon alpha-2a (40KD) (PEGASYS) plus ribavirin (COPEGUS) for 16 or 24 weeks. Liver Int. 2009;29:237-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 107. | Gad RR, Males S, El Makhzangy H, Shouman S, Hasan A, Attala M, El Hoseiny M, Zalata K, Abdel-Hamid M, Fontanet A. Predictors of a sustained virological response in patients with genotype 4 chronic hepatitis C. Liver Int. 2008;28:1112-1119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 108. | Reddy KR, Govindarajan S, Marcellin P, Bernstein D, Dienstag JL, Bodenheimer H, Rakela J, Messinger D, Schmidt G, Ackrill A. Hepatic steatosis in chronic hepatitis C: baseline host and viral characteristics and influence on response to therapy with peginterferon alpha-2a plus ribavirin. J Viral Hepat. 2008;15:129-136. [PubMed] [Cited in This Article: ] |
| 109. | Hu KQ, Currie SL, Shen H, Cheung RC, Ho SB, Bini EJ, McCracken JD, Morgan T, Bräu N, Schmidt WN. Clinical implications of hepatic steatosis in patients with chronic hepatitis C: a multicenter study of U.S. veterans. Dig Dis Sci. 2007;52:570-578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 110. | Yaginuma R, Ikejima K, Okumura K, Kon K, Suzuki S, Takei Y, Sato N. Hepatic steatosis is a predictor of poor response to interferon alpha-2b and ribavirin combination therapy in Japanese patients with chronic hepatitis C. Hepatol Res. 2006;35:19-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 111. | Harrison SA, Brunt EM, Qazi RA, Oliver DA, Neuschwander-Tetri BA, Di Bisceglie AM, Bacon BR. Effect of significant histologic steatosis or steatohepatitis on response to antiviral therapy in patients with chronic hepatitis C. Clin Gastroenterol Hepatol. 2005;3:604-609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 112. | Asahina Y, Tsuchiya K, Nishimura T, Muraoka M, Suzuki Y, Tamaki N, Yasui Y, Hosokawa T, Ueda K, Nakanishi H. α-fetoprotein levels after interferon therapy and risk of hepatocarcinogenesis in chronic hepatitis C. Hepatology. 2013;58:1253-1262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 204] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 113. | Nkontchou G, Ziol M, Aout M, Lhabadie M, Baazia Y, Mahmoudi A, Roulot D, Ganne-Carrie N, Grando-Lemaire V, Trinchet JC. HCV genotype 3 is associated with a higher hepatocellular carcinoma incidence in patients with ongoing viral C cirrhosis. J Viral Hepat. 2011;18:e516-e522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 114. | Salomao M, Yu WM, Brown RS, Emond JC, Lefkowitch JH. Steatohepatitic hepatocellular carcinoma (SH-HCC): a distinctive histological variant of HCC in hepatitis C virus-related cirrhosis with associated NAFLD/NASH. Am J Surg Pathol. 2010;34:1630-1636. [PubMed] [Cited in This Article: ] |
| 115. | Nojiri K, Sugimoto K, Shiraki K, Kusagawa S, Tanaka J, Beppu T, Yamamoto N, Takei Y, Hashimoto A, Shimizu A. Development of hepatocellular carcinoma in patients with chronic hepatitis C more than 10 years after sustained virological response to interferon therapy. Oncol Lett. 2010;1:427-430. [PubMed] [Cited in This Article: ] |
| 116. | Tanaka A, Uegaki S, Kurihara H, Aida K, Mikami M, Nagashima I, Shiga J, Takikawa H. Hepatic steatosis as a possible risk factor for the development of hepatocellular carcinoma after eradication of hepatitis C virus with antiviral therapy in patients with chronic hepatitis C. World J Gastroenterol. 2007;13:5180-5187. [PubMed] [Cited in This Article: ] |
| 117. | Takuma Y, Nouso K, Makino Y, Saito S, Takayama H, Takahara M, Takahashi H, Murakami I, Takeuchi H. Hepatic steatosis correlates with the postoperative recurrence of hepatitis C virus-associated hepatocellular carcinoma. Liver Int. 2007;27:620-626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 118. | Pekow JR, Bhan AK, Zheng H, Chung RT. Hepatic steatosis is associated with increased frequency of hepatocellular carcinoma in patients with hepatitis C-related cirrhosis. Cancer. 2007;109:2490-2496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 155] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 119. | Kumar D, Farrell GC, Kench J, George J. Hepatic steatosis and the risk of hepatocellular carcinoma in chronic hepatitis C. J Gastroenterol Hepatol. 2005;20:1395-1400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 120. | Ohata K, Hamasaki K, Toriyama K, Matsumoto K, Saeki A, Yanagi K, Abiru S, Nakagawa Y, Shigeno M, Miyazoe S. Hepatic steatosis is a risk factor for hepatocellular carcinoma in patients with chronic hepatitis C virus infection. Cancer. 2003;97:3036-3043. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 239] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 121. | Koike K. Hepatitis C virus contributes to hepatocarcinogenesis by modulating metabolic and intracellular signaling pathways. J Gastroenterol Hepatol. 2007;22 Suppl 1:S108-S111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 122. | Lonardo A, Loria P. Potential for statins in the chemoprevention and management of hepatocellular carcinoma. J Gastroenterol Hepatol. 2012;27:1654-1664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 123. | Lonardo A, Bagni A, Tarugi P, Loria P. The wide spectrum of steatohepatitis: a report of four cases and a review of the literature. Eur J Gastroenterol Hepatol. 2004;16:1043-1050. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 124. | Ong JP, Younossi ZM, Speer C, Olano A, Gramlich T, Boparai N. Chronic hepatitis C and superimposed nonalcoholic fatty liver disease. Liver. 2001;21:266-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 111] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 125. | Brunt EM, Ramrakhiani S, Cordes BG, Neuschwander-Tetri BA, Janney CG, Bacon BR, Di Bisceglie AM. Concurrence of histologic features of steatohepatitis with other forms of chronic liver disease. Mod Pathol. 2003;16:49-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 126. | Liu CJ, Jeng YM, Chen PJ, Lai MY, Yang HC, Huang WL, Kao JH, Chen DS. Influence of metabolic syndrome, viral genotype and antiviral therapy on superimposed fatty liver disease in chronic hepatitis C. Antivir Ther. 2005;10:405-415. [PubMed] [Cited in This Article: ] |
| 127. | Bedossa P, Moucari R, Chelbi E, Asselah T, Paradis V, Vidaud M, Cazals-Hatem D, Boyer N, Valla D, Marcellin P. Evidence for a role of nonalcoholic steatohepatitis in hepatitis C: a prospective study. Hepatology. 2007;46:380-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 128. | Solis-Herruzo JA, Pérez-Carreras M, Rivas E, Fernández-Vázquez I, Garfia C, Bernardos E, Castellano G, Colina F. Factors associated with the presence of nonalcoholic steatohepatitis in patients with chronic hepatitis C. Am J Gastroenterol. 2005;100:1091-1098. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 129. | Lonardo A, Ballestri S, Restivo L, Adinolfi LE, Loria P. Hepatitis C and cardiovascular risk: facts and controversies. Hot Topics Viral Hep. 2012;8:27-35. [Cited in This Article: ] |
| 130. | Stassen FR, Vainas T, Bruggeman CA. Infection and atherosclerosis. An alternative view on an outdated hypothesis. Pharmacol Rep. 2008;60:85-92. [PubMed] [Cited in This Article: ] |
| 131. | Petta S, Torres D, Fazio G, Cammà C, Cabibi D, Di Marco V, Licata A, Marchesini G, Mazzola A, Parrinello G. Carotid atherosclerosis and chronic hepatitis C: a prospective study of risk associations. Hepatology. 2012;55:1317-1323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 132. | Adinolfi LE, Restivo L, Zampino R, Guerrera B, Lonardo A, Ruggiero L, Riello F, Loria P, Florio A. Chronic HCV infection is a risk of atherosclerosis. Role of HCV and HCV-related steatosis. Atherosclerosis. 2012;221:496-502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 133. | Adinolfi LE, Restivo L, Guerrera B, Sellitto A, Ciervo A, Iuliano N, Rinaldi L, Santoro A, Li Vigni G, Marrone A. Chronic HCV infection is a risk factor of ischemic stroke. Atherosclerosis. 2013;231:22-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 134. | Loria P, Marchesini G, Nascimbeni F, Ballestri S, Maurantonio M, Carubbi F, Ratziu V, Lonardo A. Cardiovascular risk, lipidemic phenotype and steatosis. A comparative analysis of cirrhotic and non-cirrhotic liver disease due to varying etiology. Atherosclerosis. 2014;232:99-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |