Published online Dec 7, 2013. doi: 10.3748/wjg.v19.i45.8312
Revised: June 29, 2013
Accepted: July 4, 2013
Published online: December 7, 2013
AIM: To describe our patients affected with ectopic biliary tree gastrinoma and review the literature on this topic.
METHODS: Between January 1992 and June 2012, 28 patients affected by duodenopancreatic endocrine tumors in multiple endocrine neoplasia type 1 (MEN1) syndrome underwent surgery at our institution. This retrospective review article analyzes our experience regarding seventeen of these patients subjected to duodenopancreatic surgery for Zollinger-Ellison syndrome (ZES). Surgical treatment consisted of duodenopancreatectomy (DP) or total pancreatectomy (TP). Regional lymphadenectomy was always performed. Any hepatic tumoral lesions found were removed during surgery. In MEN1 patients, removal of duodenal lesions can sometimes lead to persistence or recurrence of hypergastrinemia. One possible explanation for this unfavorable outcome could be unrecognized ectopic localization of gastrin-secreting tumors. This study described three cases among the seventeen patients who were found to have an ectopic gastrinoma located in the biliary tree.
RESULTS: Seventeen MEN1 patients affected with ZES were analyzed. The mean age was 40 years. Fifteen patients underwent DP and two TP. On histopathological examination, duodeno pancreatic endocrine tumors were found in all 17 patients. Eighty-one gastrinomas were detected in the first three portions of the duodenum. Only one gastrinoma was found in the pancreas. The mean number of gastrinomas per patient was 5 (range 1-16). Malignancy was established in 12 patients (70.5%) after lymph node, liver and omental metastases were found. Three patients exhibited biliary tree gastrinomas as well as duodenal gastrinoma(s). In two cases, the ectopic gastrinoma was removed at the same time as pancreatic surgery, while in the third case, the biliary tree gastrinoma was resected one year after DP because of recurrence of ZES.
CONCLUSION: These findings suggest the importance of checking for the presence of ectopic gastrinomas in the biliary tree in MEN1 patients undergoing ZES surgery.
Core tip: Enteropancreatic endocrine neoplasms affect up to 90% of multiple endocrine neoplasia type1 (MEN1) patients. Gastrinomas are the most common functional enteropancreatic neuroendocrine tumors and were thought to be located almost exclusively in the duodenum in MEN1 patients. This study describes our experience regarding ectopic biliary tree gastrinomas in MEN1 syndrome. Our data doubles the number of cases reported in literature on this topic. Furthermore, the present study brings to light important issues that could help to establish the best biochemical and oncological cure for such cases, of which clinicians should be aware to improve the management of MEN1 patients.
- Citation: Tonelli F, Giudici F, Nesi G, Batignani G, Brandi ML. Biliary tree gastrinomas in multiple endocrine neoplasia type 1 syndrome. World J Gastroenterol 2013; 19(45): 8312-8320
- URL: https://www.wjgnet.com/1007-9327/full/v19/i45/8312.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i45.8312
Multiple endocrine neoplasia type 1 (MEN1) is a familial syndrome characterized by tumors of the parathyroids, the anterior pituitary and the enteropancreatic endocrine system. Enteropancreatic endocrine neoplasms affect up to 90% of MEN1 patients, the incidence increasing with age[1-3]. In MEN1, the most common functional enteropancreatic neuroendocrine tumors are gastrinomas, located almost exclusively in the duodenum. According to recent literature, pancreatic localization is extremely rare[4-6]. Zollinger-Ellison syndrome (ZES) and/or increased basal or stimulated plasma gastrin levels[5] often persist after surgical treatment. This could be caused by either metastatic disease or potentially unrecognized localization of gastrin-secreting tumors.
Interestingly, primary sporadic gastrinomas may arise not only in the pancreas and duodenum, but also in other organs, such as the stomach, ovary, omentum, kidney, jejunum, esophagus, extra-hepatic biliary tree or liver[7-13]. All these sites have usually been considered atypical in MEN1 syndrome, or until now, never described. Recently, the extra-hepatic biliary tree and liver were indicated as the sites of primary gastrinomas in three MEN1 patients from two different centers[14,15]. Our experience includes a further three MEN1 patients with an ectopic gastrinoma in the biliary tree, thus doubling the number of cases recognized in the international literature. The implications of this phenotype are important in the clinical management of MEN1 ZES.
Between January 1992 and June 2012, 28 MEN1 patients affected with duodenopancreatic endocrine tumors were observed and operated on at our institution, following a protocol approved by the Tuscany Region. MEN1 diagnosis was confirmed after genetic testing on germline DNA obtained from peripheral blood leukocytes. Serum gastrin, chromogranin A, pancreatic polypeptide (PP), somatostatin, glucagon and vasoactive intestinal peptide levels were assessed. A preoperative secretin stimulation test (bolus 2 μg/kg, Secrelux®, SANOCHEMIA Pharmazeutika AG) were carried out in all patients and taken as positive where gastrin increased to > 120 pg/dL 2-5 min after secretin infusion. Indications for surgery were established by the presence of hypergastrinemia, insulinoma or pancreatic nodule(s) over 1 cm in diameter. Abdominal ultrasonography (US), computed tomography (CT) and/or magnetic resonance imaging (MRI), somatostatin receptor scintigraphy (SSRS-Octreoscan), endoscopic ultrasonography (EUS) or selective pancreatic angiography with hepatic venous sampling after selective arterial secretin injection (SASI) test were performed in the majority of patients. Surgical intervention included: (1) complete duodenopancreatic mobilization, and colo-epiploic detachment; (2) inspection and palpation of the pancreas, duodenum, first jejunal loops and gallbladder; (3) abdominal intraoperative US (IOUS); (4) transillumination of the duodenum; and (5) intraoperative serum gastrin measurement by rapid immunoradiometric assay at the induction of anesthesia (basal value), 15, 30 and 45 min after pancreatic surgery. Further postoperative samples were taken after 3, 4 and 24 h. Gastrin values were measured using the rapid radioimmunoassay for serum gastrin, using specific antibodies (100% cross-reactivity) for small gastrin (G-17).
Surgery consisted of duodenopancreatectomy (DP) or total pancreatectomy (TP). Regional lymphadenectomy was performed in all patients and included pancreaticoduodenal, splenic and celiac nodes. Any hepatic tumoral lesions were removed during surgery.
All specimens were available for analysis and were confirmed as being gastrinomas by immunohistochemistry. In accordance with the World Health Organization’s criteria for endocrine tumors, we distinguished between microadenomas (< 5 mm) and macrumors (> 5 mm). Tumor size, proliferation index, immunohistochemical phenotype, angioinvasion, and evidence of metastatic spread were assessed. The diagnosis of each tumor as a gastrinoma was based on the presence of over 90% of cells displaying positive immunoreaction to gastrin.
During follow-up, fasting serum gastrin, additional entero-hormone levels and secretin provocative tests were performed 3 and 6 mo postoperatively, and yearly thereafter. Cure of hyperestrinism was defined as a normal serum gastrin concentration and a negative secretin test. Abdominal US and/or EUS, CT or MRI were carried out once every two years, or, if necessary, when hormonal levels increased.
Seventeen of the 28 operated patients (60.7%) were affected with gastrinomas, ten patients with insulinoma and one patient with vipoma. All the patients also had non-functioning tumors in the pancreas. The mean age of patients affected by ZES was 40 years (range 24-63 years): 15 patients underwent DP and two TP. On histological examination, duodenopancreatic endocrine tumors were found in all 17 patients. Immunohistochemical evaluation revealed a total of 81 gastrinomas in the first three portions of the duodenum. In our series, only one gastrinoma was located in the pancreas. The mean number of gastrinomas per patient was 5 (range 1-16). The largest gastrinoma was 1.8 cm in diameter (mean diameter 0.54 cm), the smallest less than 1 mm in diameter. One apparently primary gastrinoma was detected in a peri-pancreatic lymph node. Overall, malignancy was established in 12 patients (70.5%) after finding lymph node, liver and omental metastases. Two cases, believed to be liver metastases intraoperatively, turned out to be focal nodular hyperplasia on pathological examination.
During follow-up, the secretin stimulation test was positive in only two patients after a mean of 84.8 mo. One (case 3) is discussed below, the other developed nodal recurrence of gastrinoma.
Out of the 17 patients with gastrinoma, three were found to be located in the biliary tree, either at surgery, on histological examination of the surgical specimen or during follow-up. These three cases are detailed below.
The first case concerns a 46-year-old woman whose father had suffered from gastric ulcer and died of a myocardial infarction at the age of 61 years. Her mother had colorectal cancer at the age of 78 years. Our patient had had kidney stones since 1992, with recurrent renal colic episodes. She was also affected by type II mellitus diabetes and monoclonal gammopathy. In 1999, she experienced epigastralgia; esophago-gastro-duodenoscopy (EGDS) showed ulceration of the first and second portions of the duodenum. Genetic testing for MEN1 gene mutation was positive (Table 1). Complete biochemical assessment showed elevated levels of calcium, parathyroid hormone (PTH) and gastrin (213.2 pg/mL; VR < 108). Abdominal US revealed multiple nodules over 2 cm in diameter in the head, body and tail of the pancreas. Abdominal CT confirmed the pancreatic lesions, displaying additional smaller nodules. In December 2000, she underwent total parathyroidectomy with eight parathyroid fragments autografted in the non-dominant forearm. After surgery, she was normocalcemic and the PTH serum level was within the normal range.
| Pt | Sex/age (yr) | MEN1 mutation | Basal gastrinemia (NV < 108 pg/mL) | Previous surgical treatment | Surgical treatment | Postoperative complications | Latest complications and adverse events | Basal serum gastrin postoperative (pg/mL) | Secretin provocative test (at follow-up) |
| 1 | F/46 | Missense, Leu413Pro exon 9 | 213.2 | Total PTX | TP and removal of a bile duct gastrinoma | Insulin-dependentdiabetes mellitusCholangitis | 39.9 | NEG | |
| 2 | F/55 | Frameshift, 843del GA, exon 6 | 87.1 | Left nephrectomy for hydronephrosis, total PTX with autotransplantation | WDP | Pancreatojejunal dehiscence for acute pancreatitis→TP with splenectomy | Abdominal abscess | 24.8 | NEG |
| 3 | F/37 | Arg415 exon 9stop | 204.3 | Less than subtotal PTX | (2010) PPDP(2012) Hepatic resection (V-VIII seg) and enucleation of a pancreatic head nodule | Biliary fistula | Subocclusion | 32.4 | NEG |
Serological and radiological examination and a secretin provocative test were performed on follow-up, showing increased gastrin values after stimulation. In 2001, the patient was subjected to TP with gastroduodenal resection and splenectomy (Table 1). During surgery, a nodule was noted in the bile duct and resected. IOUS did not show any hepatic lesions. Gastrin progressively decreased, reaching 42 pg/mL at the end of the surgical procedure. Pathological examination revealed 16 non-functioning neuroendocrine tumors in the pancreas (maximum diameter 3.4 cm in the pancreatic tail) and two neuroendocrine gastrin-positive tumors reaching 1 cm, one in the duodenum and one in the bile duct. The Ki-67 index was less than 1% for all lesions. There was no evidence of lymph node metastasis (Table 2). The patient was discharged after 12 d and is currently on MEN1 follow up.
| Pt | Gastrinomas (n) | Size of largest duodenal gastrinoma (cm) | Size of largest biliary gastrinoma (cm) | Other pancreatic endocrine neoplasms (n) | Size of largest NF PEN (cm) | Total tumors (n) | Hepatic findings | Gastrinoma metastases |
| 1 | 2 (D, BD) | 1.0 | 1.2 | 16 NF | 3.4 | 18 | Negative | Negative |
| 2 | 4 (3D, 1G) | 0.2 | 0.2 | 32 NF | 1.6 | 32 | Negative | Negative |
| 3 | 3 (2D, 1BD) | 0.3 | 1.5 | 37 NF | 2.0 | 40 | Negative | Positive (N) |
The second case concerns a 55-year-old woman. Her mother had died of chronic renal failure at the age of 86 and her father at 67 of a carcinoid of the small bowel, her sister had died at the age of 46 from neurologic complications of a pituitary prolactinoma.
Our patient’s clinical history began at the age of 24 when she developed kidney stones with recurrent renal colic. In 1994, she underwent left nephrectomy for hydronephrosis and hysteroannessiectomy for uterine leiomyomatosis. In 1999, she suffered episodes of epigastric pain and EGDS showed the presence of a duodenal ulcer. In 2001, the patient was clinically diagnosed with MEN1 and was referred to our department for a complete serum biochemical assessment, which showed elevated levels of PTH and calcium. A genetic diagnosis of MEN1 was made (Table 1), and in 2002, she underwent total parathyroidectomy, with a parathyroid autograft in the non-dominant forearm. After surgery, she was normocalcemic with normal PTH serum levels.
On follow-up, a secretin provocative test showed an increase in gastrin values after stimulation. CT revealed the presence of nodules in the pancreatic head. SSRS confirmed the presence of pathological neuroendocrine tissue in the first and second portions of the duodenum, and in the head of the pancreas. In October 2003, she underwent Whipple’s duodenopancreatectomy with regional lymphadenectomy and cholecystectomy (Table 1). Intraoperative US showed no residual pancreatic or hepatic lesions, and gastrin levels decreased to 25 pg/mL at the end of the surgical procedure. Pathological examination revealed 28 non-functioning neuroendocrine tumors of the pancreas (maximum diameter 1.6 cm) and four gastrin-secreting tumors (maximum diameter 0.2 cm), three in the duodenum and one in the gallbladder fundus. The Ki-67 index was less than 1%. There was no evidence of lymph node involvement (Table 2). The postoperative course was complicated by acute pancreatitis and consequent pancreato-jejunal anastomotic dehiscence, thus requiring TP with splenectomy. The patient was discharged after 38 d and is still on follow-up for MEN1.
The third case concerns a 36-year-old woman, whose father had died of myocardial infarction at the age of 52, while her mother and two sisters were in good health. In 2004, she was subjected to left inferior parathyroidectomy for primary hyperparathyroidism, which recurred 4 mo later. The right superior parathyroid gland was removed, and partial thymectomy was carried out. Intraoperative PTH decreased from 1050 pg/mL at baseline to 150 pg/mL, 20 min after parathyroidectomy. On suspicion of MEN1, the patient was referred to our institution for a genetic test, which revealed mutation of the MEN1 gene (Table 1). She underwent complete biochemical and radiological examination with CT, but no neuroendocrine lesions of the pancreas were found. MRI was negative for pituitary lesions.
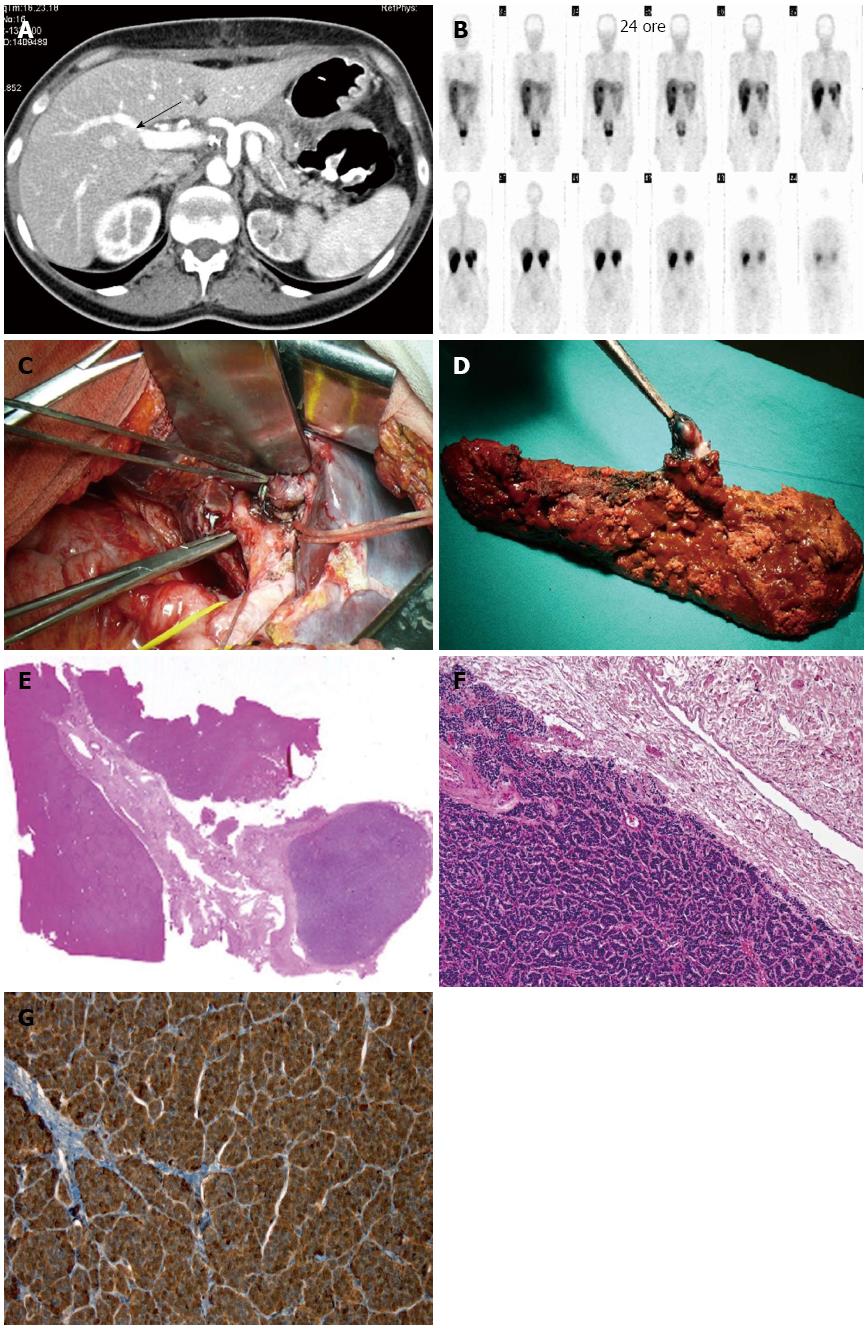
In 2010, she was admitted to our surgical unit for heartburn, abdominal pain and diarrhea. An increase in basal gastrin serum levels (184 pg/mL; NV < 108) and a secretin provocative test confirmed hypergastrinemia (9783 pg/mL). EGDS showed esophagitis and gastroduodenitis with peptic ulcers. The clinical diagnosis of ZES in MEN1 was put forward, and the patient started proton pump inhibitor (PPI) therapy. Abdominal EUS showed a 7-mm nodule in the pancreatic head, together with small submucosal lesions of the first and second parts of the duodenum. In October 2010, she underwent pylorus-preserving duodenopancreatectomy extended to the pancreatic body, with removal of the regional lymph nodes and the gallbladder (Table 1). End-to-side Wirsung-jejunal anastomosis, and common bile duct-jejunal and duodeno-jejunal anastomoses through trans-mesocolic Roux-en-Y loop were then performed. The IOUS showed no hepatic lesions. Gastrin serum levels progressively decreased, reaching 20 pg/mL at the end of the surgical procedure. Pathology revealed 37 neuroendocrine tumors of the pancreas (maximum diameter 2 cm) and three gastrin positive tumors of the duodenum (maximum diameter 2 mm). Of the 31 lymph nodes examined, two had neuroendocrine tumor tissue positive for gastrin. The postoperative course was uneventful and the patient was discharged 17 d later on PPI therapy (Omeprazole 40 mg × 2/die). One month after surgery, she had abdominal pain and vomiting, and was readmitted to our ward. An upper gastro-intestinal tract X-ray showed slow transit at the level of the anastomotic jejunal loop. Laparotomy was carried out with dissection of adhesions between the small bowel and the great omentum, and the mesocolic defect was closed, suturing it to the stomach. The day after surgery, gastrin levels were measured, revealing a significant increase in serum values (173 pg/mL). Five days later the patient was discharged and given PPI drugs and somatostatin analogs. After two mo, a 12 mm hepatic lesion, posterior to the V-VIII segment portal vein branch, was detected by CT and confirmed at US. MRI was subsequently performed to better define the hepatic lesion, which showed a 15 mm nodule in the V segment, immediately below the right portal bifurcation. SSRS confirmed the presence of the hepatic nodule with a high density of SST2 receptor (Figure 1). Basal gastrin serum levels were within the normal range (45.6 pg/mL), but a secretin stimulation test displayed an increase to 482 pg/mL. In January 2012, the patient was readmitted to our department with epigastric pain, heartburn and anemia (Hb 7.1) associated with a syncopal episode. An EGDS and a colonoscopy gave negative results. A SASI test was carried out, producing two gastrin peaks at 1541 and 1217 pg/mL 40 s after stimulation in the hepatic and superior mesenteric arteries, respectively. EUS identified three pancreatic lesions of approximately 0.5 cm in diameter. In February 2012, the patient underwent hepatic resection of the V-VIII segments to remove the lesion, which was found inside the glissonian sheet of the anterior sectional pedicle and not in the liver parenchyma (Figure 1 and Table 1). A 0.7 cm lesion of the residual pancreatic body was also enucleated (Table 2). Intraoperative gastrin levels decreased below the normal range to 32 pg/mL. A gastrin-secreting tumor in the biliary tree and a pancreatic neuroendocrine lesion were found at histology (Figure 1). Both lesions had a Ki-67 index lower than 1%. The patient developed a biliary fistula, which was then treated conservatively. She was discharged after 15 d and is still on follow-up for MEN1.
Primary gastrinomas are classified as duodenal, pancreatic and extraintestinal-extrapancreatic. The pancreas and duodenum are the most frequent sites of sporadic gastrinomas, while the duodenum is almost exclusively the localization of gastrinomas in MEN1 patients[4-6]. MEN1-associated gastrinomas occur predominantly in the first three parts of the duodenum, are usually multiple and measure less than 5 mm in diameter[3-6,16]. In some cases, duodenal gastrinomas are only found on microscopic examination. From 34% to 85% of duodenal gastrinomas are metastatic at the time of surgery, but tumor spread is usually restricted to the regional lymph nodes[4-6]. Pancreatic localization is rare in MEN1 syndrome, sometimes being associated with a more aggressive behavior of the tumor, which may metastasize to the liver[17].
Extraduodenal-extrapancreatic gastrinomas in MEN1 syndrome are rare, while they account for 22% of sporadic gastrinomas according to Norton et al[18]. The improved ability to identify gastrinomas in the duodenal wall, either preoperatively or intraoperatively, has resulted in an apparent increase in the incidence of duodenal gastrinomas and decrease in the incidence of occult lesions and primary peripancreatic lymph node gastrinomas. Wu et al[19] found extrapancreatic and extraduodenal gastrinomas in only 8 (5%) of 142 patients with ZES, none of whom had MEN1. The sites of these gastrinomas were the ovary, omentum, stomach, jejunum, kidney, biliary tract, and, in 2 cases, the liver[19].
To the best of our knowledge, only twenty cases of hormonally active gastrin-producing or immunostaining gastrin positive neuroendocrine tumors of the extrahepatic biliary tree have been published in literature, 18 in the sporadic setting and two in MEN1[14]. The majority of these patients were female and under 50 years of age. The most common symptom was jaundice and, more rarely, peptic ulceration or frank ZES. Generally, basal gastrin serum levels were high and the secretin test was positive. These gastrinomas mainly occurred in the common bile duct or common hepatic duct, less frequently at the confluence of the hepatic duct. Tumor size varied from a few millimeters to over 2 centimeters. Lymph node or liver metastases were detected in almost 50% of these cases. All these gastrinomas were resectable, and in most cases radical surgery was carried out with no evidence of disease recurrence at either short or long term follow-up.
Primary hepatic gastrinomas are even less frequent than those occurring in the extrahepatic biliary tree, with fewer than 20 cases reported in the literature and only one case described in MEN1[15,20]. These gastrinomas are generally single nodules, 1-7 cm in diameter, with high radiotracer uptake at octreoscan. Liver resection resulted in prompt normalization of gastrin serum levels with no evidence of recurrence, suggesting these tumors are primary rather than metastases of occult extrahepatic microgastrinomas. Therefore, only three MEN1 ectopic gastrinomas have been described before the present study: two in the extrahepatic biliary tree and one in the liver[14,15]. The clinicopathological characteristics of these primary ectopic gastrinomas are summarized in Table 3. All the patients were young females, with associated hyperparathyroidism, pituitary adenoma, and pancreatic functioning or non-functioning tumors. In all cases, the basal gastrin values were high and the ectopic gastrinoma was single, varying from 6 to 15 mm. The two extrahepatic biliary duct cases were detected intraoperatively, while the hepatic gastrinoma was diagnosed preoperatively at CT and SSRS. Lymph nodes displayed neuroendocrine tumor tissue positivity for gastrin in only one patient. In another, a suspected hepatic metastasis was ablated with radiofrequency and alcoholization. In all but one patient, excision of these ectopic gastrinomas was carried out during DP to remove concomitant duodenal or pancreatic neuroendocrine tumors. These patients were eugastrinemic immediately after surgery and at follow-up.
| Ref. | Sex/age | Site/size (mm) | Gastrin: basal/stimulation | Diagnostic imaging positivity | MEN1 stigmata | Surgical treatment | Metastasis | BPC gastrin |
| Price et al[14] | F/55 | CD (CBD junction)/6 mm | 4500/NR | IOUS, discovered at surgery | pHPT, PA, NF-PETs (n°21/1-6 mm) | Distal splenopancreatectomy; RFA hepatic excision BC | Liver, (solitary) | 780 (2 yr) (recurrence) |
| Price et al[14] | F/43 | CBD/15 × 12 × 1 | 580/4300 | IOUS, discovered at surgery | pHPT, PA, NF-PET (4), DG (3/1-4 mm); GC, lipoma | PPDP; enucleation; gastric excision | Lymph nodes (CBD HA) | 86 (2 yr) |
| Lee et al[15] | F/39 | Liver (II-III seg)/16 × 15 × 0.9 | 447/NR | CCT, SSRS, IOUS | pHPT, PA, insulinoma | WDP; II-III liver segmentectomy | - | 34 (po) |
Our experience regarding MEN1 ectopic gastrinomas is similar to that in the literature: gastrinomas were found incidentally during DP in two patients, and detected in the liver in another after recurrence of ZES. The most likely hypothesis explaining the development of gastrinomas in the biliary tree is the presence of stem cells with the capacity to differentiate into specific endocrine cells. Endocrine cells are widely distributed in the epithelial layer of organs that originate from the primitive gut. Argyrophil and somatostatin cells have been identified in the glandular epithelium of both the extrahepatic and intrahepatic bile ducts in adult and infant normal livers[21,22]. These cells may increase in number, and many kinds of other endocrine cells appear anew and even proliferate in hepatocytes[22]. Endocrine cells also occur in the gallbladder, where metaplastic changes of the mucosa can be induced by lithiasis. Similarly, primary gastrinomas developing in the hepatic parenchyma may originate from endocrine cells within the small peripheral intrahepatic biliary tree. In our third case, the gastrinoma was not localized in the hepatic parenchyma, but along the vascular pedicle, suggesting a possible origin from cells arranged along the bile duct.
Histological findings of gastrinomas almost invariably correspond to well-differentiated neuroendocrine tumors, according to the WHO 2010 classification[23]. Immunohistochemically, gastrin can be detected in all tumors: many duodenal gastrinomas are multihormonal, and contain single somatostatin or serotonin expressing cells. Approximately 50% of gastrinomas contain PP, glucagon and/or insulin, as well as gastrin. There is a high risk of gastrinomas behaving malignantly, irrespective of size, with frequent lymph node and liver metastases; pancreatic tumors tend to be worse than duodenal lesions. Malignancy cannot be predicted histologically, except on evidence of angioinvasion or infiltration of surrounding tissues. The proliferation fraction can be taken as an indicator of malignancy and a Ki-67 index greater than 10% is invariably associated with development of metastases[23].
Distinguishing ectopic primary gastrinomas from metastatic gastrinomas is not easy. MEN1 gastrinomas of the duodenum or pancreas can metastasize to the liver and be mistaken for ectopics if the primary tumor goes undetected. Several factors in our MEN1 patients helped to exclude the biliary gastrinomas as being metastatic rather than ectopic. First, segmentary biliary ducts and gallbladder are unusual sites for metastasis. Second, the gastrinomas in all the patients were single lesions and with no disease progression, as commonly occurs in metastatic neuroendocrine tumors. No liver or lymph node metastases were found in two cases, while in the third, diagnosed with duodenal gastrinomas metastatic to the regional lymph nodes, gastrin values normalized rapidly at the end of the first operation, but increased one month later.
Surgical treatment of MEN1 gastrinomas is a matter of debate. Some authors suggest simple enucleation of the duodenal mucosa or limited full-thickness duodenal resection, while others recommend DP[24,25]. The majority of patients after duodenal resection, are not cured definitively, and hypergastrinemia, as well as metastatic disease, can occur[26,27]. According to Thompson[28], the secretin stimulation test proved negative in only one third of patients submitted to this procedure, in spite of the fact that most of them (68%) remained eugastrinemic for a long postoperative period. DP has been claimed to promote higher curability rates[27-33]. In our experience, surgery is curative after resection of affected pancreatic tissue and enucleation of macroscopic nodules in the residual pancreas. On reviewing the literature, the curability rate of DP with regional lymphadenectomy in MEN1 gastrinomas is more than 85%[27,31,34-36]. Indeed, resection of the so-called “gastrinoma triangle”, achieved with DP, also allows the removal of lesions located in the common bile duct, the cystic duct or the gallbladder. The frequent relapse in MEN1 gastrinoma patients after duodenal excision, could be caused by residual ectopic gastrinomas that were not removed through conservative surgery, rather than to duodenal recurrence.
The possibility of primary gastrinomas arising in the biliary tree in MEN1 syndrome must always be taken into consideration to ensure that appropriate therapy is used, as this ectopic localization can explain surgical failure in MEN1 ZES. Intraoperative palpation, transillumination and IOUS of the biliary tree could be useful to locate ectopic gastrinomas. The clinical community should be made aware of these findings to improve the management of MEN1 patients.
Surgical removal of duodenal multiple endocrine neoplasia type1 (MEN1) gastrinomas can lead to persistence or recurrence of hypergastrinemia. A possible explanation for this unfavorable outcome could be unrecognized ectopic localization of gastrin-secreting tumors.
Primary gastrinomas arising in the biliary tree can explain surgical failure in the treatment of Zollinger-Ellison syndrome in MEN1 syndrome.
Only twenty cases of hormonally active gastrin-producing or immunostaining gastrin positive neuroendocrine tumors of the extrahepatic biliary tree have been published in the literature, 18 in the sporadic setting and two cases in MEN1. Primary hepatic gastrinomas are even more infrequent than those of the extrahepatic biliary tree, with fewer than 20 cases reported in the literature to date and only one case described in MEN1.
Three out of 17 MEN1 patients were found to be affected by an ectopic gastrinoma located in the biliary tree. These findings suggest that in MEN1 patients, the presence of ectopic biliary tree gastrinoma is not rare, occurring in 17.6% of cases.
MEN1 is a familial syndrome characterized by tumors of the parathyroids, the anterior pituitary and the enteropancreatic endocrine system. Enteropancreatic endocrine neoplasms affect up to 90% of MEN1 patients. Gastrinomas are the most common functional enteropancreatic neuroendocrine tumor and, according to recent literature, are located almost exclusively in the duodenum in MEN1 patients.
These results lead to doubling of the number of cases described in the literature regarding ectopic biliary tree gastrinomas in MEN1 patients. These findings should be made available to the clinical community to improve the management of MEN1 patients.
P- Reviewers: Poitras P, Pritchard M, Wilcox CM S- Editor: Gou SX L- Editor: Stewart GJ E- Editor: Ma S
| 1. | Calender A, Cadiot G, Mignon M. [Multiple endocrine neoplasia type 1: genetic and clinical aspects]. Gastroenterol Clin Biol. 2001;25:B38-B48. [PubMed] [Cited in This Article: ] |
| 2. | Thakker RV, Newey PJ, Walls GV, Bilezikian J, Dralle H, Ebeling PR, Melmed S, Sakurai A, Tonelli F, Brandi ML. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J Clin Endocrinol Metab. 2012;97:2990-3011. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 879] [Cited by in F6Publishing: 760] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 3. | Tonelli F, Giudici F, Fratini G, Brandi ML. Pancreatic endocrine tumors in multiple endocrine neoplasia type 1 syndrome: review of literature. Endocr Pract. 2011;17 Suppl 3:33-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Pipeleers-Marichal M, Somers G, Willems G, Foulis A, Imrie C, Bishop AE, Polak JM, Häcki WH, Stamm B, Heitz PU. Gastrinomas in the duodenums of patients with multiple endocrine neoplasia type 1 and the Zollinger-Ellison syndrome. N Engl J Med. 1990;322:723-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 285] [Cited by in F6Publishing: 297] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 5. | Tonelli F, Fratini G, Nesi G, Tommasi MS, Batignani G, Falchetti A, Brandi ML. Pancreatectomy in multiple endocrine neoplasia type 1-related gastrinomas and pancreatic endocrine neoplasms. Ann Surg. 2006;244:61-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Jensen RT, Cadiot G, Brandi ML, de Herder WW, Kaltsas G, Komminoth P, Scoazec JY, Salazar R, Sauvanet A, Kianmanesh R. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: functional pancreatic endocrine tumor syndromes. Neuroendocrinology. 2012;95:98-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 429] [Cited by in F6Publishing: 329] [Article Influence: 27.4] [Reference Citation Analysis (1)] |
| 7. | Goudet P, Murat A, Binquet C, Cardot-Bauters C, Costa A, Ruszniewski P, Niccoli P, Ménégaux F, Chabrier G, Borson-Chazot F. Risk factors and causes of death in MEN1 disease. A GTE (Groupe d’Etude des Tumeurs Endocrines) cohort study among 758 patients. World J Surg. 2010;34:249-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 211] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 8. | Lopez CL, Falconi M, Waldmann J, Boninsegna L, Fendrich V, Goretzki PK, Langer P, Kann PH, Partelli S, Bartsch DK. Partial pancreaticoduodenectomy can provide cure for duodenal gastrinoma associated with multiple endocrine neoplasia type 1. Ann Surg. 2013;257:308-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Dickson PV, Rich TA, Xing Y, Cote GJ, Wang H, Perrier ND, Evans DB, Lee JE, Grubbs EG. Achieving eugastrinemia in MEN1 patients: both duodenal inspection and formal lymph node dissection are important. Surgery. 2011;150:1143-1152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Glazer ES, Tseng JF, Al-Refaie W, Solorzano CC, Liu P, Willborn KA, Abdalla EK, Vauthey JN, Curley SA. Long-term survival after surgical management of neuroendocrine hepatic metastases. HPB (Oxford). 2010;12:427-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 161] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 11. | Gedaly R, Daily MF, Davenport D, McHugh PP, Koch A, Angulo P, Hundley JC. Liver transplantation for the treatment of liver metastases from neuroendocrine tumors: an analysis of the UNOS database. Arch Surg. 2011;146:953-958. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 12. | Liu DM, Kennedy A, Turner D, Rose SC, Kee ST, Whiting S, Murthy R, Nutting C, Heran M, Lewandowski R. Minimally invasive techniques in management of hepatic neuroendocrine metastatic disease. Am J Clin Oncol. 2009;32:200-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Kennedy AS, Dezarn WA, McNeillie P, Coldwell D, Nutting C, Carter D, Murthy R, Rose S, Warner RR, Liu D. Radioembolization for unresectable neuroendocrine hepatic metastases using resin 90Y-microspheres: early results in 148 patients. Am J Clin Oncol. 2008;31:271-279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 297] [Cited by in F6Publishing: 327] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 14. | Price TN, Thompson GB, Lewis JT, Lloyd RV, Young WF. Zollinger-Ellison syndrome due to primary gastrinoma of the extrahepatic biliary tree: three case reports and review of literature. Endocr Pract. 2009;15:737-749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Lee SR, Choi MC, Ahn KJ. A case of multiple endocrine neoplasia type 1 with primary liver gastrinoma. J Korean Med Sci. 2010;25:953-956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Tonelli F, Fratini G, Falchetti A, Nesi G, Brandi ML. Surgery for gastroenteropancreatic tumours in multiple endocrine neoplasia type 1: review and personal experience. J Intern Med. 2005;257:38-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Weber HC, Venzon DJ, Lin JT, Fishbein VA, Orbuch M, Strader DB, Gibril F, Metz DC, Fraker DL, Norton JA. Determinants of metastatic rate and survival in patients with Zollinger-Ellison syndrome: a prospective long-term study. Gastroenterology. 1995;108:1637-1649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 340] [Cited by in F6Publishing: 355] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 18. | Norton JA, Doppman JL, Jensen RT. Curative resection in Zollinger-Ellison syndrome. Results of a 10-year prospective study. Ann Surg. 1992;215:8-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 164] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Wu PC, Alexander HR, Bartlett DL, Doppman JL, Fraker DL, Norton JA, Gibril F, Fogt F, Jensen RT. A prospective analysis of the frequency, location, and curability of ectopic (nonpancreaticoduodenal, nonnodal) gastrinoma. Surgery. 1997;122:1176-1182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Ishikawa Y, Yoshida H, Mamada Y, Taniai N, Matsumoto S, Bando K, Mizuguchi Y, Kakinuma D, Kanda T, Akimaru K. Curative resection of primary hepatic gastrinoma. Hepatogastroenterology. 2008;55:2224-2227. [PubMed] [Cited in This Article: ] |
| 21. | Dancygier H, Klein U, Leuschner U, Hübner K, Classen M. Somatostatin-containing cells in the extrahepatic biliary tract of humans. Gastroenterology. 1984;86:892-896. [PubMed] [Cited in This Article: ] |
| 22. | Kurumaya H, Ohta G, Nakanuma Y. Endocrine cells in the intrahepatic biliary tree in normal livers and hepatolithiasis. Arch Pathol Lab Med. 1989;113:143-147. [PubMed] [Cited in This Article: ] |
| 23. | Klöppel G. Classification and pathology of gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer. 2011;18 Suppl 1:S1-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 232] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 24. | Mignon M, Ruszniewski P, Podevin P, Sabbagh L, Cadiot G, Rigaud D, Bonfils S. Current approach to the management of gastrinoma and insulinoma in adults with multiple endocrine neoplasia type I. World J Surg. 1993;17:489-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 85] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Lips CJ. Clinical management of the multiple endocrine neoplasia syndromes: results of a computerized opinion poll at the Sixth International Workshop on Multiple Endocrine Neoplasia and von Hippel-Lindau disease. J Intern Med. 1998;243:589-594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Mortellaro VE, Hochwald SN, McGuigan JE, Copeland EM, Vogel SB, Grobmyer SR. Long-term results of a selective surgical approach to management of Zollinger-Ellison syndrome in patients with MEN-1. Am Surg. 2009;75:730-733. [PubMed] [Cited in This Article: ] |
| 27. | Bartsch DK, Langer P, Wild A, Schilling T, Celik I, Rothmund M, Nies C. Pancreaticoduodenal endocrine tumors in multiple endocrine neoplasia type 1: surgery or surveillance? Surgery. 2000;128:958-966. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 105] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Thompson NW. Current concepts in the surgical management of multiple endocrine neoplasia type 1 pancreatic-duodenal disease. Results in the treatment of 40 patients with Zollinger-Ellison syndrome, hypoglycaemia or both. J Intern Med. 1998;243:495-500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 104] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Imamura M, Takahashi K. Use of selective arterial secretin injection test to guide surgery in patients with Zollinger-Ellison syndrome. World J Surg. 1993;17:433-438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 58] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Delcore R, Friesen SR. Role of pancreatoduodenectomy in the management of primary duodenal wall gastrinomas in patients with Zollinger-Ellison syndrome. Surgery. 1992;112:1016-1022; discussion 1022-1023. [PubMed] [Cited in This Article: ] |
| 31. | Stadil F. Treatment of gastrinomas with duodenopancreatectomy. Endocrine Tumors of the Pancreas: Recent Advances in Research and Management, 23vol. Frontiers of Gastrointestinal Research. Basel, Switzerland: Karger 1995; 333-341. [Cited in This Article: ] |
| 32. | Demeure MJ, Klonoff DC, Karam JH, Duh QY, Clark OH. Insulinomas associated with multiple endocrine neoplasia type I: the need for a different surgical approach. Surgery. 1991;110:998-1004; discussion 1004-1005. [PubMed] [Cited in This Article: ] |
| 33. | O'Riordain DS, O’Brien T, van Heerden JA, Service FJ, Grant CS. Surgical management of insulinoma associated with multiple endocrine neoplasia type I. World J Surg. 1994;18:488-493; discussion 493-494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 106] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Thakker RV. Multiple endocrine neoplasia type 1. Indian J Endocrinol Metab. 2012;16:S272-S274. [PubMed] [Cited in This Article: ] |
| 35. | Akerström G, Hessman O, Skogseid B. Timing and extent of surgery in symptomatic and asymptomatic neuroendocrine tumors of the pancreas in MEN 1. Langenbecks Arch Surg. 2002;386:558-569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | Thompson NW. Surgical treatment of the endocrine pancreas and Zollinger-Ellison syndrome in the MEN 1 syndrome. Henry Ford Hosp Med J. 1992;40:195-198. [PubMed] [Cited in This Article: ] |