Published online Aug 14, 2013. doi: 10.3748/wjg.v19.i30.5016
Revised: May 14, 2013
Accepted: June 1, 2013
Published online: August 14, 2013
A 67-year-old male underwent endoscopic submucosal dissection (ESD) to treat early gastric cancer (EGC) in 2001. The lesion (50 mm × 25 mm diameter) was histologically diagnosed as poorly differentiated adenocarcinoma, with an ulcer finding. Although the tumor was confined to the mucosa with no evidence of lymphovascular involvement, the ESD was regarded as a non-curative resection due to the histological type, tumor size, and existence of an ulcer finding (as indicated by the 2010 Japanese gastric cancer treatment guidelines, ver. 3). Despite strong recommendation for subsequent gastrectomy, the patient refused surgery. An alternative follow-up routine was designed, which included five years of biannual clinical examinations to detect and measure serum tumor markers and perform visual assessment of recurrence by endoscopy and computed tomography scan after which the examinations were performed annually. The patient’s condition remained stable for eight years, until a complaint of back pain in 2010 prompted further clinical investigation. Bone scintigraphy indicated increased uptake. Histological examination of biopsy specimens taken from the lumbar spine revealed adenocarcinoma resembling the carcinoma cells from the EGC that had been treated previously by ESD, and which was consistent with immunohistochemical findings of gastrointestinal tract cancer. Thus, the diagnosis of bone metastasis from EGC was made. The reported rates of EGC recurrence in surgically resected cases range 1.4%-3.4%, but among these bone metastasis is very rare. To our knowledge, this is the first reported case of bone metastasis from EGC following a non-curative ESD and occurring after an eight-year disease-free interval.
Core tip: This case report provides the first description of an adult male with bone metastasis from early gastric cancer following an eight-year disease-free interval after endoscopic submucosal dissection (ESD). Although the original tumor was confined to the mucosa, with no evidence of lymphovascular involvement, the ESD was regarded as a non-curative resection based upon the tumor’s histological type and size, and existence of an ulcer finding. If any patient, who is otherwise fit, initially refuses surgery and requests a contraindicated ESD, efforts should be made to persuade the patient to undergo a gastrectomy with lymph-node dissection.
- Citation: Kawabata H, Oda I, Suzuki H, Nonaka S, Yoshinaga S, Katai H, Taniguchi H, Kushima R, Saito Y. Bone metastasis from early gastric cancer following non-curative endoscopic submucosal dissection. World J Gastroenterol 2013; 19(30): 5016-5020
- URL: https://www.wjgnet.com/1007-9327/full/v19/i30/5016.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i30.5016
Endoscopic resection remains the preferred treatment modality for cases of early gastric cancer (EGC) with a negligible risk of lymph-node metastasis[1-3]. The 2010 Japanese gastric cancer treatment guidelines (ver. 3) provide absolute indications and expanded indications for performing endoscopic resections, including endoscopic submucosal dissection (ESD), to treat these cases[4]. In contrast, EGC cases with possible risk of lymph-node metastasis generally require surgical treatment.
Here, we describe our clinical experience with a case of bone metastasis from EGC that developed after an eight-year disease-free interval following non-curative ESD. To our knowledge, this case represents the first of its kind to be reported in the literature. In this particular case, ESD was clinically contraindicated because the EGC consisted of an undifferentiated type adenocarcinoma with an ulcer finding, but was performed in accordance with the patient refusing surgical intervention despite our strong recommendation to the contrary.
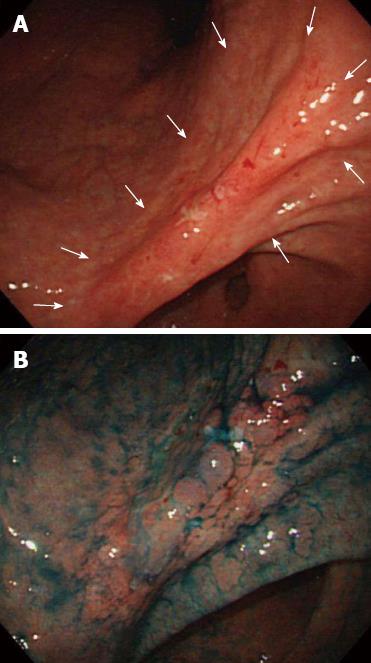
In 2001, a 58-year-old man was admitted to our hospital upon detection of EGC by screening endoscopy. Physical examination and laboratory data revealed no abnormalities, and the patient had no relevant personal or family history. The endoscopic findings indicated a superficial depressed type (0-IIc) EGC confined to the mucosa (in terms of invasion depth), along with an ulcer (50 mm) located in the gastric angle (Figure 1). Histological examination of the biopsied specimens indicated poorly- to moderately-differentiated adenocarcinoma. Ultrasound and computed tomography (CT) examinations revealed no metastasis.
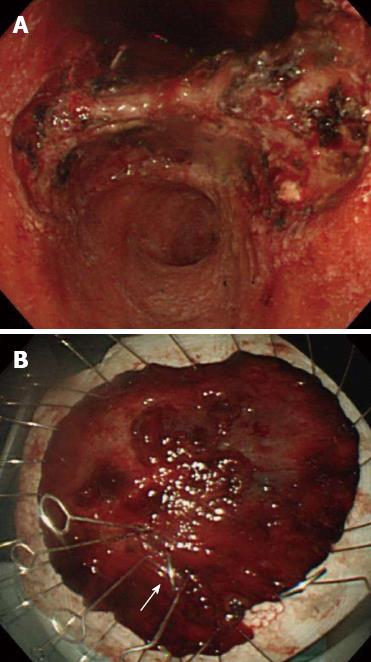
Based on the 2010 Japanese gastric cancer treatment guidelines[4], endoscopic resection was not indicated for this lesion. The patient was informed of the possible risk of lymph-node metastasis and the need for a surgical resection; however, the patient refused surgery and the ESD was performed in October 2001 (Figure 2). Histological examination of the resected tissues revealed the tumor (50 mm × 25 mm diameter) to be primary composed of poorly-differentiated adenocarcinoma and confirmed the proximal ulcer (Figure 3). The tumor was confined to the mucosa, with no evidence of lymphovascular involvement. Both the vertical and horizontal margins were free of tumor cells. However, a slight tear was observed inside the lesion and was considered to have occurred incidentally during the ESD.
Since the ESD was regarded as a non-curative resection, we repeated our strong recommendations for gastrectomy, but the patient continued to refuse. As a result, we designed a monitoring strategy in which the patient would present to clinic biannually for testing of serum tumor markers and endoscopic and CT examinations; after five years with no remarkable findings, the clinical follow-ups were decreased to once a year. The patient’s condition remained stable, with no evidence of recurrence, for eight years after the ESD.
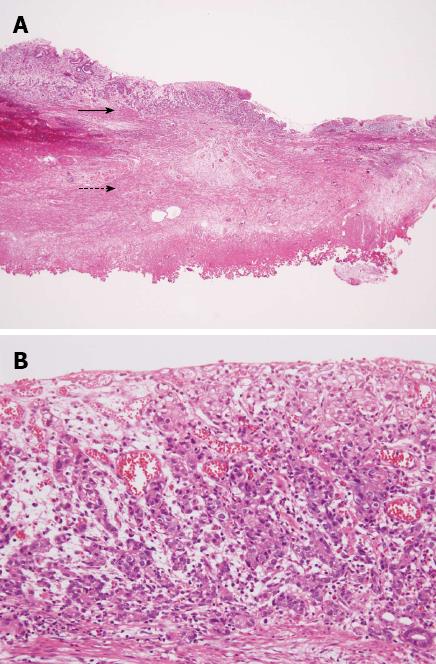
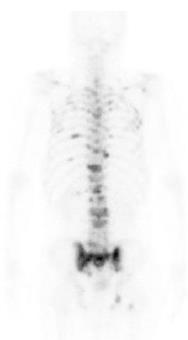
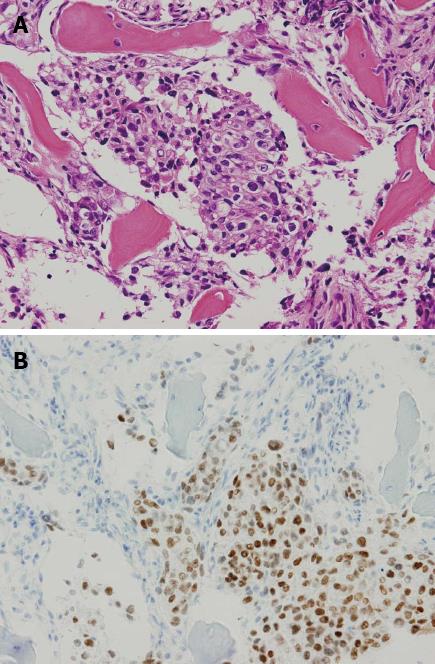
In April 2010, however, the patient presented with a complaint of back pain. Serum testing revealed a remarkably enhanced level of alkaline phosphatase (1172 IU/L, normal range: 120-340 IU/L), but endoscopic examination provided no indications of recurrence at the ESD site (Figure 4). Bone scintigraphy indicated increased uptake in the spine and pelvic regions (Figure 5). Histological examination of biopsied specimens taken from the lumbar spine revealed a poorly-differentiated adenocarcinoma that resembled the carcinoma cells in the original ESD-treated EGC (Figure 6A). Immunohistochemical examination showed the tumor cells were positive for caudal-type homeobox transcription factor 2, which is consistent with gastrointestinal tract cancer (Figure 6B). There were no findings of malignancy in any other organs. Thus, the diagnosis was made of bone metastasis from the EGC following a non-curative ESD after an eight-year disease-free interval.
The patient received chemotherapy (methotrexate + fluorouracil), but developed disseminated intravascular coagulopathy and died in August 2011.
This is the first report of bone metastasis from EGC after an eight-year disease-free interval following non-curative ESD. This case presents several aspects for discussion, including contraindicated ESD, metastasis limited to bone, and late recurrence.
A recent report of EGC indicated a lower survival rate after contraindicated ESD because of clinically diagnosed submucosal invasion and concluded that ESD was ineffective in such cases[5]. Although the case described herein did not involve clinically diagnosed submucosal invasion, ESD was contraindicated because of a possible risk of lymph-node metastasis due to the undifferentiated type adenocarcinoma with an ulcer finding. The risk of lymph-node metastasis has been reported as 13.4% for undifferentiated type EGC with ulcer finding, and as 5.9% in undifferentiated intramucosal cancer[6]. Bone metastasis is a hematogenous metastasis, so it may not have been avoided in the present case even if the patient had undergone a gastrectomy with lymph-node dissection. Nonetheless, we believe that gastrectomy would likely have provided some benefit to the patient, who was fit for surgery.
Bone metastasis is relatively common in patients with advanced breast, lung and prostate cancer, but not in gastric cancer patients (occurring in only 0.99%-2.1% of advanced cases)[7,8]. Recurrence of EGC has been reported to be in the range of 1.4%-3.4% for surgically resected cases, but among these bone metastasis is very rare[9]. In a previous investigation of 1452 EGC patients treated with curative resection, Lee et al[10] found that 21 patients (1.4%) experienced recurrence, including 4 (19.0%) with local lymph-node recurrence, 2 (9.5%) with peritoneal dissemination, 9 (42.9%) with distant metastasis, and 6 (28.6%) with mixed type recurrence. Similarly, Kobayashi et al[9] reviewed Japanese case reports to determine the characteristics of bone metastasis in EGC for this patient population. Among the tumors, 51.4% had infiltrated the mucosa and 48.6% had infiltrated the submucosa, 65.0% of the metastases were metachronous and 35.0% were synchronous, 18.8% of the primary tumors were differentiated and 81.2% were undifferentiated, and lymph-node metastasis was present in 55.2% of the patients.
In our case, the metastasis that was diagnosed after a lengthy interval (eight years) following non-curative ESD was only found in bone. One possible explanation of such a late recurrence involves the concept of tumor dormancy, a state in which cancer cells exist with no to low-level activity concomitant to normal, healthy tissues until the dormancy is disturbed by an instigating event, such as infection or immunosuppression[11]. In such a case, the activated cancer cells will have a negative impact on the prognosis of the cancer patient, regardless of the duration of the disease-free interval. Recurrence of gastric cancer is generally believed to occur within five years of the primary surgery, but there are some reports of very late recurrence, including bone metastasis after surgery, and particularly involving EGC cases[9,12-14]. It may be necessary, therefore, to follow-up EGC patients for more than five years, especially those who were treated by ESD in lieu of surgical resection or those with expanded indications.
The number of patients who undergo ESD instead of surgery has increased in recent years, in part because the indications have been expanded[3,5,15,16]; however, it is important to apply the indications properly. If any patient, who is otherwise fit, initially refuses surgery and requests a contraindicated ESD, all efforts should be made to persuade the patient to undergo a gastrectomy with lymph-node resection in order to optimize the patient prognosis.
P- Reviewers Kakizaki S, Komatsu K, Palma GD, Yoshi N S- Editor Gou SX L- Editor A E- Editor Li JY
| 1. | Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219-225. [PubMed] [Cited in This Article: ] |
| 2. | Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229. [PubMed] [Cited in This Article: ] |
| 3. | Oda I, Saito D, Tada M, Iishi H, Tanabe S, Oyama T, Doi T, Otani Y, Fujisaki J, Ajioka Y. A multicenter retrospective study of endoscopic resection for early gastric cancer. Gastric Cancer. 2006;9:262-270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 317] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 4. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1723] [Cited by in F6Publishing: 1844] [Article Influence: 141.8] [Reference Citation Analysis (0)] |
| 5. | Suzuki H, Oda I, Nonaka S, Yoshinaga S, Saito Y. Is endoscopic submucosal dissection an effective treatment for operable patients with clinical submucosal invasive early gastric cancer. Endoscopy. 2013;45:93-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Hirasawa T, Gotoda T, Miyata S, Kato Y, Shimoda T, Taniguchi H, Fujisaki J, Sano T, Yamaguchi T. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer. 2009;12:148-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 344] [Cited by in F6Publishing: 368] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 7. | Yoshikawa K, Kitaoka H. Bone metastasis of gastric cancer. Jpn J Surg. 1983;13:173-176. [PubMed] [Cited in This Article: ] |
| 8. | Guadagni S, Catarci M, Kinoshitá T, Valenti M, De Bernardinis G, Carboni M. Causes of death and recurrence after surgery for early gastric cancer. World J Surg. 1997;21:434-439. [PubMed] [Cited in This Article: ] |
| 9. | Kobayashi M, Okabayashi T, Sano T, Araki K. Metastatic bone cancer as a recurrence of early gastric cancer -- characteristics and possible mechanisms. World J Gastroenterol. 2005;11:5587-5591. [PubMed] [Cited in This Article: ] |
| 10. | Lee HJ, Kim YH, Kim WH, Lee KU, Choe KJ, Kim JP, Yang HK. Clinicopathological analysis for recurrence of early gastric cancer. Jpn J Clin Oncol. 2003;33:209-214. [PubMed] [Cited in This Article: ] |
| 11. | Holmgren L, O’Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med. 1995;1:149-153. [PubMed] [Cited in This Article: ] |
| 12. | Noda N, Sano T, Shirao K, Ono H, Katai H, Sasako M, Maruyama K. A case of bone marrow recurrence from gastric carcinoma after a nine-year disease-free interval. Jpn J Clin Oncol. 1996;26:472-475. [PubMed] [Cited in This Article: ] |
| 13. | Kammori M, Seto Y, Haniuda N, Kawahara M, Takubo K, Endo H, Kaminishi M. A case of bone metastasis from gastric carcinoma after a nine-year disease-free interval. Jpn J Clin Oncol. 2001;31:407-409. [PubMed] [Cited in This Article: ] |
| 14. | Nashimoto A, Yabusaki H, Nakagawa S. Proper follow-up schedule after curative gastric surgery. Gan To Kagaku Ryoho. 2009;36:1402-1407. [PubMed] [Cited in This Article: ] |
| 15. | Gotoda T, Iwasaki M, Kusano C, Seewald S, Oda I. Endoscopic resection of early gastric cancer treated by guideline and expanded National Cancer Centre criteria. Br J Surg. 2010;97:868-871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 217] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 16. | Hoteya S, Yamashita S, Kikuchi D, Nakamura M, Fujimoto A, Matsui A, Nishida N, Mitani T, Kuroki Y, Iizuka T. Endoscopic submucosal dissection for submucosal invasive gastric cancer and curability criteria. Dig Endosc. 2011;23:30-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |