Published online Aug 14, 2013. doi: 10.3748/wjg.v19.i30.4925
Revised: May 31, 2013
Accepted: June 19, 2013
Published online: August 14, 2013
AIM: To investigate whether tumor necrosis factor-α (TNF-α) mediates ischemia-reperfusion (I/R)-induced intestinal mucosal injury through c-Jun N-terminal kinase (JNK) activation.
METHODS: In this study, intestinal I/R was induced by 60-min occlusion of the superior mesenteric artery in rats followed by 60-min reperfusion, and the rats were pretreated with a TNF-α inhibitor, pentoxifylline, or the TNF-α antibody infliximab. After surgery, part of the intestine was collected for histological analysis. The mucosal layer was harvested for RNA and protein extraction, which were used for further real-time polymerase chain reaction, enzyme-linked immunosorbent assay and Western blotting analyses. The TNF-α expression, intestinal mucosal injury, cell apoptosis, activation of apoptotic protein and JNK signaling pathway were analyzed.
RESULTS: I/R significantly enhanced expression of mucosal TNF-α at both the mRNA and protein levels, induced severe mucosal injury and cell apoptosis, activated caspase-9/caspase-3, and activated the JNK signaling pathway. Pretreatment with pentoxifylline markedly downregulated TNF-α at both the mRNA and protein levels, whereas infliximab pretreatment did not affect the expression of TNF-α induced by I/R. However, pretreatment with pentoxifylline or infliximab dramatically suppressed I/R-induced mucosal injury and cell apoptosis and significantly inhibited the activation of caspase-9/3 and JNK signaling.
CONCLUSION: The results indicate there was a TNF-α-mediated JNK activation response to intestinal I/R injury.
Core tip: Ischemia-reperfusion (I/R) injury is a critical physiopathological phenomenon wherein further damage may occur when the blood supply of ischemic organs is recovered, and the mechanism of I/R remains unclear. This paper demonstrates that tumor necrosis factor-α (TNF-α) played a pivotal role in intestinal I/R injury, and pretreatment with the TNF-α inhibitor pentoxifylline or the TNF-α antibody infliximab remarkably attenuated I/R-induced injury by inhibiting TNF-α-mediated apoptosis and c-Jun N-terminal kinase (JNK) activation. The results of the study indicate there is a TNF-α-mediated JNK activation response to intestinal I/R injury.
- Citation: Yang Q, Zheng FP, Zhan YS, Tao J, Tan SW, Liu HL, Wu B. Tumor necrosis factor-α mediates JNK activation response to intestinal ischemia-reperfusion injury. World J Gastroenterol 2013; 19(30): 4925-4934
- URL: https://www.wjgnet.com/1007-9327/full/v19/i30/4925.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i30.4925
Intestinal ischemia-reperfusion (I/R) not only damages the local intestinal mucosa but also induces remote organic injury. I/R injury may occur in numerous situations, such as small bowel transplantation, strangulated hernias, neonatal necrotizing enterocolitis, cardiopulmonary bypass surgery and hypovolemic/septic shock[1]. A series of factors, including reactive oxygen species (ROS) production, calcium overload, neutrophil infiltration and cytokines release, are involved in I/R injury. Among these mediators, tumor necrosis factor-α (TNF-α), as an initial factor of the inflammatory reaction in I/R injury, is thought to play a pivotal role[2]. Prophylactic anti-TNF-α treatment may be an effective therapeutic strategy for preventing I/R-induced injury, as has been demonstrated by some studies[3]. TNF-α is thought to initiate three signaling pathways involved in cell injury and apoptosis: the apoptotic signaling pathway, the c-Jun N-terminal kinase (JNK) signaling pathway and the nuclear factor kappa-B (NF-κB) signaling pathway[4]. However, the role of TNF-α in I/R-induced injury and its mechanisms remain to be elucidated. Previous studies[5,6] demonstrated that apoptosis is a major mode of cell death caused by I/R, whereas the effect of TNF-α-mediated signaling pathways in cell apoptosis requires further exploration.
Pentoxifylline, a TNF-α inhibitor, has been investigated for a long time in I/R injury, but its effects on I/R-induced intestinal apoptosis and apoptotic pathways remain to be evaluated. Recent studies also have suggested that infliximab, a TNF-α antibody, attenuates I/R-induced injury[7]. However, it is still poorly understood whether infliximab alleviates the intestinal mucosal injury by downregulating apoptotic signaling or has acts via some other mechanisms.
The aims of this study were to determine: (1) whether inhibition of TNF-α ameliorates I/R-induced intestinal mucosal injury by suppressing cell apoptosis; (2) whether TNF-α is involved in a caspase-dependent apoptotic signaling in intestinal I/R injury; and (3) whether the JNK signaling pathways are activated by TNF-α in response to cell apoptosis in intestinal I/R injury.
The experimental protocol and design were approved by the Sun Yat-sen University Animal Experimentation Committee and performed according to the Sun Yat-sen University Guidelines for Animal Experimentation. Male Sprague-Dawley rats (approximately 200-250 g) were used in this study. The animals were housed in wire-bottomed cages placed in a room illuminated from 8:00 AM to 8:00 PM (12:12-h light-dark cycle) and maintained at 21 °C ± 1 °C. The rats were allowed access to water and chow ad libitum. The rats were anaesthetized for 3 h by intraperitoneal injection of 4% chloral hydrate (200 mg/kg). A laparotomy was performed under chloral hydras anesthesia, and the superior mesenteric artery (SMA) was occluded with a micro bulldog clamp. At the end of the ischemic period, the clamp was released, and three drops of lidocaine were applied directly onto the SMA to facilitate reperfusion. After the experiment, the animals were euthanized, and then, the entire small intestine was carefully removed and placed on ice. The oral 10-cm segment (duodenum) was removed, and the rest of the intestine was divided into two equal segments, representing the proximal (jejunum) and distal (ileum) segments. Each segment was rinsed thoroughly with physiological saline. Jejunal and ileal pieces, approximately 2 cm in length, were removed from the middle portion of each segment and fixed in 10% neutral-buffered formalin for the measurement of mucosal injury, terminal deoxynucleotidyl transferase-mediated dUDP-biotin nick-end labeling (TUNEL) assay, and immunohistochemistry of caspase-3. The remainder of the segment was opened longitudinally on the antimesenteric border to expose the intestinal mucosa. The mucosal layer was harvested by gentle scraping using a glass slide.
To investigate mucosal injury after I/R, the SMA was occluded for 60 min followed by 60-min reperfusion (I/R), and pretreatment with vehicle (1 mL of physiological saline) for 60 min prior to I/R. To evaluate the effect of pentoxifylline, a TNF-α inhibitor, on mucosal injury in the small intestine after I/R, the animals were pretreated with 50 mg/kg pentoxifylline (Sigma, St Louis, MI, United States), dissolved in 1 mL of physiological saline, by intraperitoneal injection 60 min prior to I/R. Similarly, an infliximab (Janssen Biotech, Horsham, PA) dose of 5 mg/kg dissolved in 1 mL of physiological saline was administered by intraperitoneal injection 60 min prior to I/R to evaluate the effect of infliximab pretreatment. In sham-operated (SO) rats, pretreatment was performed with vehicle for 60 min, and then, the SMA was isolated in a similar manner but was not occluded. Six rats were studied in each group.
RNA was extracted from 100 mg of mucosal scrapings using TRIzol reagent (Invitrogen, Carlsbad, CA, United States) per the manufacturer’s instructions. First-strand cDNA was synthesized from 1.5 μg of total RNA using a ReverTra Ace kit (Toyobo, Japan) per the manufacturer’s instructions. An ABI Prism 7000 sequence detection system (Applied Biosystems, Bedford, MA) was then used for real-time polymerase chain reaction (PCR) experiments to quantitate the gene expression of TNF-α and β-actin for each sample. The reactions were performed in a 20-μL volume with TaKaRa TaqTM (TaKaRa, Japan). The PCR conditions included a denaturation step at 94 °C for 5 min. Amplification was conducted for 35 cycles (denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s, and extension at 72 °C for 30 s). The quantification was performed by using 7000 SDS instrument software (Applied Biosystems) for the relative quantification of gene expression. The primer sequences used were as follows: TNF-α forward primer, 5’-CACCACGCTCTTCTGTCT ACT-3’; TNF-α reverse primer, 5’-AGATGATCTGAGTGTGAGGGTC-3’; β-actin forward primer, 5’-GAAATCGTGCGTGACATCAAAG-3’; and β-actin reverse primer, 5’-TGTAGTTTCATGGATGCCACAG-3’. The primers were supplied by Invitrogen. The results are expressed as the fold change in mRNA expression compared with the levels in sham-operated rats.
The mucosal scraping samples were immediately washed twice with ice-cold PBS (pH 7.4) and then homogenized in total protein extraction buffer. This extraction buffer consisted of 50 mmol/L Tris-HCl (pH 7.5), 150 mmol/L NaCl, 0.1% SDS, 5 mmol/L ethylenediaminetetraacetic acid (EDTA), 0.5 mmol/L phenylmethylsulfonyl fluoride, 10 μg/mL aprotinin, 10 μg/mL leuptin and 1.8 mg/mL iodoacetamide. The tissues were homogenized and lysed at 4 °C for 30 min and then centrifuged at 14000 g for 20 min at 4 °C. The resulting supernatants were purified total proteins. The supernatants were divided into multiple samples and stored at -80 °C. The protein concentrations were determined using a kit (Bio-Rad, Hercules, CA, United States).
The TNF-α concentration of intestinal mucosa was measured using a commercial kit (eBioscience, San Diego, CA, United States), according to the manufacturer’s instructions. Briefly, the enzyme-linked immunosorbent assay (ELISA) plates were coated with 100 μL/well of capture antibody diluted in coating buffer and incubated overnight at room temperature (RT). The plates were washed with wash buffer and blocked for 1 h at RT with 200 μL/well assay diluent. Then, the TNF-α standard and samples (100 μL) were pipetted into appropriate wells. Next, the plates were sealed and incubated at RT for 2 h. After washing, 100 μL of detection antibody was added to each well, sealed, and incubated for 1 h at RT. After washing, 100 μL of substrate solution was added to each well and incubated for 30 min at RT in the dark. Stop solution (2 mol/L H2SO4, 50 μL/well) was added, and the plates were read at 450 nm (570 nm correction) on a MicroPlate Reader (BioTek, Seattle, WA, United States). The results are expressed as pg TNF-α/mg protein.
After the animals were sacrificed, the tissue samples removed from the jejunum and ileum were immediately fixed in 10% neutral-buffered formalin, embedded in paraffin and sectioned. The sample sections were processed with hematoxylin-eosin staining and examined by light microscopy, according to the criteria described by Chiu et al[8] as follows: grade 0, normal mucosa; grade 1, development of subepithelial (Gruenhagen) spaces near the tips of the villi with capillary congestion; grade 2, extension of the subepithelial space with moderate epithelial lifting from the lamina propria; grade 3, significant epithelial lifting along the length of the villi with a few denuded villous tips; grade 4, denuded villi with exposed lamina propria and dilated capillaries; and grade 5, disintegration of the lamina propria, hemorrhage, and ulceration. Twenty visual fields at × 100 magnification were evaluated for each sample slide, and the final injury scoring was a gross assessment of the degree of mucosal damage. All slides were evaluated by two examiners in a blinded fashion.
The sample sections were used to detect cell apoptosis. The fragmented DNA of apoptotic cells was stained via the TUNEL method by using an in situ cell death detection kit (Roche, Switzerland). The apoptotic index was calculated in a minimum of 20 randomly selected crypts and analyzed in six separate samples. The apoptotic index was determined by dividing the number of apoptotic cells by the total number of cells in the crypt column and multiplying by 100.
Sample sections were used for caspase-3 immunohistochemical staining. The sections were deparaffinized and rehydrated prior to antigen retrieval by using EDTA (pH 8.0) for 3 min at 130 °C. The sections were incubated for 10 min with 5% BSA prior to incubation with caspase-3 antibody (1:400, Cell signaling technology, Danvers, MA, United States) at 4 °C overnight. Subsequently, the sections were incubated at 37 °C for 30 min with anti-rabbit IgG (1:400; Santa Cruz Biotechnology, Santa Cruz, CA). Antigen-antibody complexes were visualized by staining with a DAB kit (Dako, Denmark). The slides were then counter-stained with hematoxylin for 1 min and mounted. The negative controls were created by omitting the primary antibody.
Apoptotic proteins (caspase-9 and caspase-3) were analyzed by Western blotting. Equal quantities (20 μg) of lysates were electrophoresed in an SDS-PAGE gel and then transferred onto a nitrocellulose membrane (Bio-Rad). After blocking with PBS containing 0.1% polyoxyethylene sorbitan monolaurate (Tween 20, Sigma) and 5% skim milk for 1 h, the membrane was incubated with a rabbit polyclonal anti-TNF-α antibody (1:500; cell signaling technology), a mouse polyclonal anti-caspase-9 antibody (1:1000; cell signaling technology), a rabbit polyclonal anti-caspase-3 antibody (1:1000; cell signaling technology), a rabbit polyclonal anti-JNK antibody (1:1000; cell signaling technology), a rabbit polyclonal anti-p-JNK antibody (1:500; cell signaling technology), a rabbit polyclonal anti-c-Jun antibody (1:1000; Cell signaling technology), and a rabbit polyclonal anti-p-c-Jun antibody (1:500; Cell signaling technology) at 4 °C overnight. Antigen-antibody complexes were detected with horseradish peroxidase-conjugated anti-rabbit IgG (1:6000; Santa Cruz Biotechnology) or anti-mouse IgG (1:6000; Santa Cruz Biotechnology). Detection of chemiluminescence was performed using ECL Western blotting detection reagents (Amersham Pharmacia Biotech, Piscataway, NJ, United States). The densitometric assessment of bands from the autoradiogram was performed using Image Gauge VDS (Fujifilm, Tokyo, Japan). Band intensities were quantified by measuring the absolute integrated optical intensity, which estimates the band in the lane profile. The results are expressed as the ratios of β-actin densitometry units.
Ranked data (mucosal injury scoring) are presented as the median and range, and the significance between groups was tested with the Wilcoxon rank test. Other results are expressed as the mean ± SE. Data were evaluated by one-way Analysis of Variance, and multiple comparisons were performed using the method of least significant difference t test. Differences were considered significant if P < 0.05.
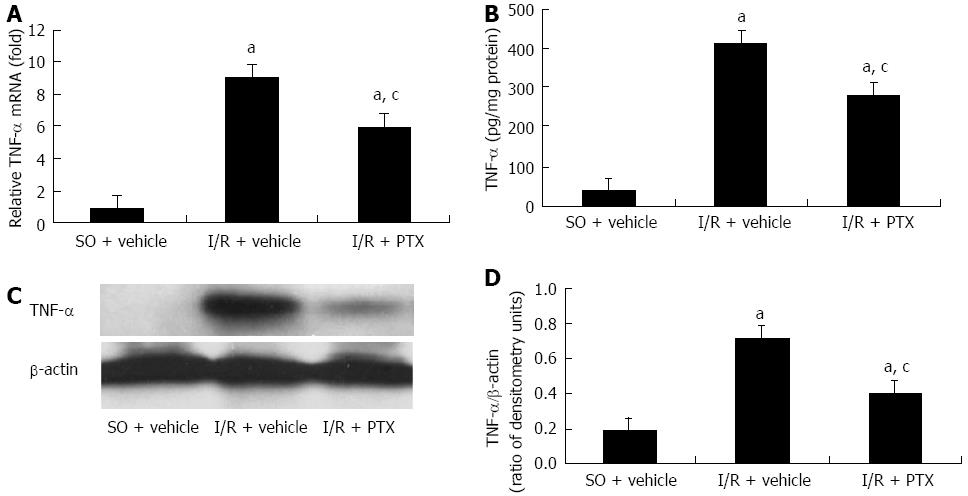
Real-time PCR, ELISA and Western blotting analysis were conducted to evaluate the expression of TNF-α after intestinal I/R and the effect of pretreatment with pentoxifylline on the expression of TNF-α. The results are shown in Figure 1. A small amount of TNF-α was detected in sham-operated rats. Compared with the sham-operated rats, the amount of intestinal mucosal TNF-α at both the mRNA and protein levels was significantly increased after I/R, and this increase of TNF-α was markedly inhibited by pretreatment with pentoxifylline.
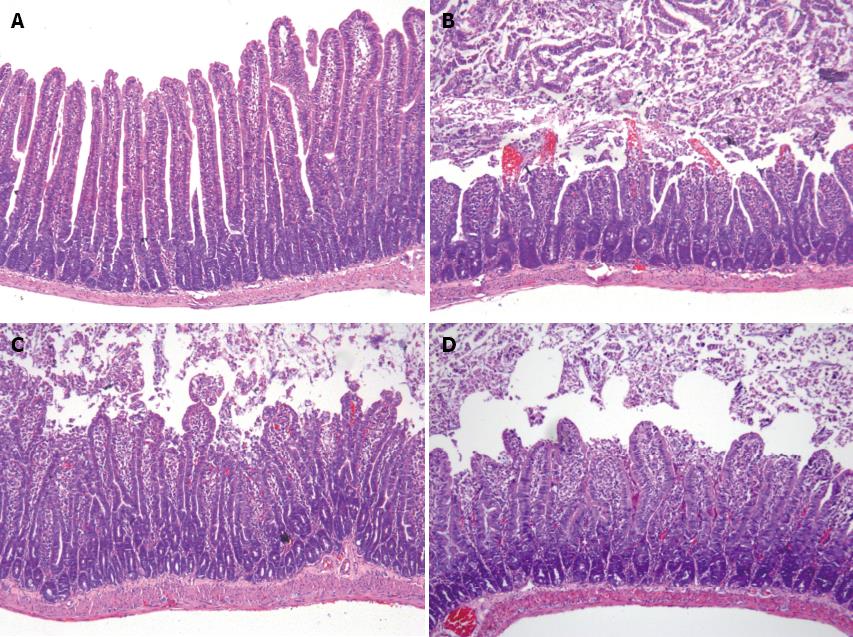
The hematoxylin-eosin staining of jejunum sections is shown in Figure 2. Samples from sham-operated rats pretreated with the vehicle alone displayed an intact mucosal structure, whereas the intestinal I/R induced apparent mucosal damage, extensive epithelial layer damage, disintegration of lamina propria and hemorrhage. In contrast, pretreatment with pentoxifylline or infliximab attenuated the I/R-induced injury. In the ileum, the results were similar to those observed in the jejunum (data not shown). The mucosal injury score is shown in Table 1, and the results indicated that pretreatment with pentoxifylline or infliximab markedly attenuated I/R-induced intestinal mucosal injury, compared with I/R pretreatment with vehicle.
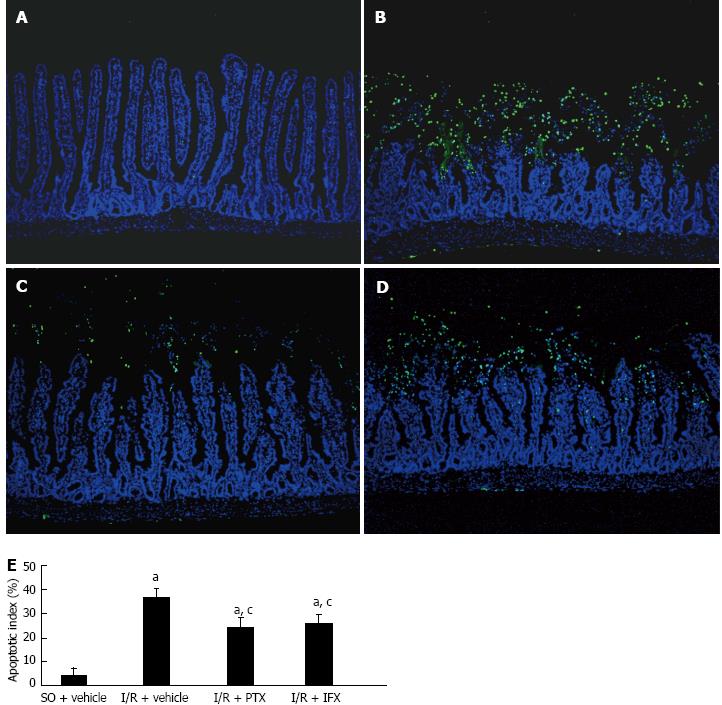
The effect of TNF-α on mucosal cell apoptosis was investigated. The results of TUNEL staining of the jejunum indicated that few apoptotic cells were observed at the villus tips in the sham-operated rats, which is consistent with physiological apoptosis during the renewal of intestinal epithelium. Compared with the sham-operated rats, marked destruction of the jejunum structure and increased staining signal of apoptotic cells were observed in I/R rats. Pretreatment with either pentoxifylline or infliximab reduced the destruction of the structure in the jejunum and decreased the number of apoptotic cells (Figure 3A-D). The jejunum mucosal apoptotic index in each group is shown in Figure 3E. Pretreatment with pentoxifylline or infliximab significantly attenuated the apoptotic index after intestinal I/R. The results in the ileum were similar to those observed in the jejunum (data not show).
To confirm the cell apoptotic death, immunohistochemistry staining of caspase-3 was performed. The results are shown in Figure 4. In the sections from the sham-operated rats, few caspase-3-positive cells were observed at the villus tips. Samples from I/R-treated rats displayed intense and extensive positive staining for caspase-3. The number of caspase-3-positive cells was markedly reduced in I/R rats pretreated with pentoxifylline or infliximab.
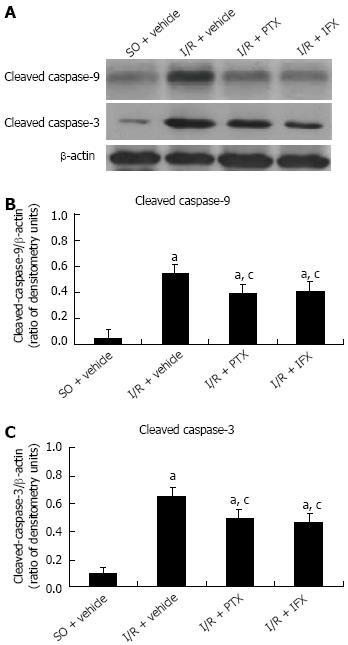
Intestinal apoptotic proteins were analyzed by Western blotting assay, and the results are shown in Figure 5. In the sham-operated rats, only small amounts of cleaved caspase-9 and caspase-3 were detected in the small intestinal mucosa. In contrast, the expression of cleaved activated caspase-9 and caspase-3 were significantly increased after intestinal I/R. However, pretreatment with pentoxifylline or infliximab significantly suppressed the activation of those caspase proteins.
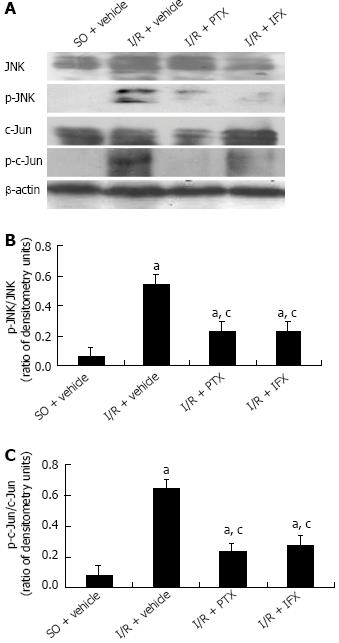
As shown in Figure 6, after intestinal I/R, the phosphorylation of JNK/c-Jun was significantly enhanced compared with that in sham-operated rats pretreated with the vehicle alone. Pretreatment with pentoxifylline markedly inhibited the phosphorylation of JNK/c-Jun, and pretreatment with infliximab also significantly decreased the activation of JNK/c-Jun. These results suggest that TNF-α mediated a JNK activation response to intestinal ischemia-reperfusion injury.
In tissue I/R injury, TNF-α is believed to be an early mediator. TNF-α is produced by a variety of cells, including macrophages, neutrophils, endothelia cells, nature killer cells and T/B lymphocytes[9,10]. The increased TNF-α may, in turn, augment the activation and action of those cells in I/R injury. TNF-α is involved in ROS production and the release of inflammation factors, such as interleukin-1, platelet-activating factor, and intercellular adhesion molecule[11]. TNF-α also mediates the injury of endothelia cells and the infiltration of neutrophils[12,13] and plays a pivotal role in I/R injury[14]. Subsequent studies have indicated that TNF-α mediates the injury induced by I/R, while inhibiting its function or expression supplied a protective effect[3]. However, some studies have demonstrated that TNF-α might be a protective factor in I/R-induced injury[15-17]. Because TNF-α has pleiotropic functions, researchers believe that after its interaction with TNF-receptor-1 and TNF-receptor-2, TNF-α activates pathways that favor both cell survival and apoptosis depending on the cell type and biological context.
Currently, two classic signal pathways are believed to mediate apoptotic signals: the caspase-8-mediated type-I apoptotic pathway and caspase-9-mediated type-II pathway. Caspase-3 ultimately executes the apoptotic signal. In a study on hepatic ischemia-reperfusion injury, the researchers demonstrated that TNF-α, but not Fas, mediated apoptosis in hepatocytes[18]. Our previous study indicated that TNF-α mediated mucosal cell apoptosis in rat intestines that suffered venous congestion[19]. However, the effect of TNF-α is poorly understood in I/R-induced intestinal cell apoptosis.
TNF-α can activate both the stress-activated protein kinase cascade, including JNK[20,21], and the NF-κB signaling pathway[22,23]. Therefore, the stimulation of cells with TNF-α may lead to both pro-apoptotic and anti-apoptotic consequences. JNK is a member of the mitogen-activated protein kinases (MAPKs). It has been shown that JNK mediates inflammatory processes by inducing the expression of adhesion molecules and inflammatory chemokines. The activation of JNK is closely related to cell apoptosis in I/R-induced injury, and TNF-α is a strong stimulating factor[24,25]. Under I/R conditions, JNK is activated by the dual phosphorylation of threonine (Thr) and tyrosine (Tyr), and the phosphorylated-JNK translocates to the nucleus, where it phosphorylates and activates different transcription factors and transactivates target genes, including c-Jun[26]. Phosphorylation of c-Jun leads to the formation of AP-1, which is involved in the transcription of a wide variety of proteins, and some of them are known to be pro-apoptotic proteins[27].
Pentoxifylline has been proven to be a potent inhibitor of TNF-α production[28]. Recently, several studies have suggested that pretreatment with pentoxifylline in intestinal I/R not only attenuates the local intestinal injury but also improves the tolerance of the remote organ to I/R[29,30]. However, the specific effects of pentoxifylline on the cell apoptosis and apoptotic signal pathways in intestinal I/R are not clear. Infliximab, a chimeric TNF-α monoclonal antibody, has been shown to inhibit the function of TNF-α in a variety of studies[31]. Because infliximab is a potent antibody against TNF-α, capable of neutralizing all forms (extracellular, transmembrane, and receptor-bound) of TNF-α[32], it may also attenuate TNF-α-mediated injury in I/R. Few studies have been conducted that examine the effect of infliximab on I/R injury. Guven et al[33] suggested that infliximab exerted neuroprotective effects in a study involving an experimental spinal cord ischemic injury. A recent study indicated that infliximab might have protective effects in intestinal I/R injury because of its anti-inflammatory and antioxidant properties[7].
In this study, our data identified that I/R dramatically induced TNF-α expression at both the mRNA and protein levels using real time-PCR, ELISA and Western bloting analysis, and pretreatment with pentoxifylline significantly downregulated TNF-α expression. Morphological analysis indicated that I/R induced apparent intestinal mucosal injury, which was characterized by the appearance of hemorrhage, extensive epithelial disruption and disintegration of lamina propria. Pretreatment with pentoxifylline or infliximab significantly alleviated the injury, which was confirmed by mucosal injury scoring. TUNEL assay and caspase-3 immunohistochemical staining demonstrated that I/R remarkably induced mucosal apoptosis, and pretreatment with pentoxifylline or infliximab suppressed cell apoptosis, thus contributing to the alleviation of injury. The observed apoptotic index values were also consistent with these results.
The Western blotting results showed that apoptotic proteins, including caspase-9 and caspase-3, were significantly activated after intestinal I/R, and the activation of caspases was suppressed by pretreatment with pentoxifylline or infliximab. The present data suggest that TNF-α mediates caspase-dependent mitochondrial apoptotic signaling. In addition, our data showed that the phosphorylation of JNK and c-Jun increased after intestinal I/R. When TNF-α was suppressed by pretreatment with pentoxifylline or infliximab, the phosphorylation of JNK and c-Jun, as well as intestinal apoptosis, were also significantly inhibited. The data suggest that the activation of the JNK signaling pathway is involved in TNF-α-mediated caspase-dependent apoptosis.
In summary, intestinal I/R dramatically induced TNF-α expression, and TNF-α activated the JNK signaling response to caspase-dependent apoptosis, resulting in intestinal injury. Clinically, a treatment involving inhibition of TNF-α by pentoxifylline or infliximab might ameliorate intestinal injury in I/R patients, and pentoxifylline has potential as a low-cost and efficacious anti-TNF-α compound.
Intestinal ischemia-reperfusion (I/R) injury may occur in numerous situations, such as small bowel transplantation, strangulated hernias, neonatal necrotizing enterocolitis, cardiopulmonary bypass surgery and hypovolemic/septic shock. A series of factors, including reactive oxygen species production, calcium overload, neutrophil infiltration and cytokine release, are involved in I/R injury. Among these mediators, tumor necrosis factor-α (TNF-α) acts as an initial inflammation factor in I/R injury and is believed to play a pivotal role. However, the exact mechanism of TNF-α in intestinal I/R is still not clearly understood.
TNF-α is believed to initiate three signaling pathways: the apoptotic signaling pathway, c-Jun N-terminal kinase (JNK) signaling pathway and nuclear factor kappa-B signaling pathway. These pathways are all involved in cell injury and apoptosis. However, the role of TNF-α in I/R-induced injury and its mechanisms remain to be elucidated. The effect of TNF-α-mediated JNK signaling pathways on cell apoptosis requires further exploration.
The study demonstrated that I/R significantly enhanced expression of mucosal TNF-α at both the mRNA and protein levels, induced severe mucosal injury and cell apoptosis, and activated JNK. Pretreatment with the TNF-α inhibitor pentoxifylline or the TNF-α antibody infliximab dramatically suppressed I/R-induced mucosal injury and cell apoptosis and significantly inhibited the activation of caspase-9/3 and JNK. The results of this study indicate that TNF-α played a pivotal role in intestinal I/R injury, and TNF-α mediated a JNK activation response to intestinal I/R injury.
In the present study, the authors investigated the mechanism of TNF-α in I/R-induced intestinal injury, which will provide new insight into the pathogenesis of I/R injury. Moreover, the study indicated that treatment via inhibiting TNF-α by pentoxifylline or infliximab might ameliorate intestinal injury in I/R patients.
TNF-α is a cytokine involved in systemic inflammation and is a member of a group of cytokines that stimulate the acute phase reaction. It is produced chiefly by activated macrophages (M1), although it can be produced by many other cell types such as CD4+ lymphocytes, NK cells and neurons. JNK were originally identified as kinases that bind and phosphorylate c-Jun on Ser-63 and Ser-73 within its transcriptional activation domain. They belong to the mitogen-activated protein kinase family and are responsive to stress stimuli, such as cytokines, ultraviolet irradiation, heat shock, and osmotic shock. They also play a role in T-cell differentiation and the cellular apoptosis pathway.
The authors have done some intriguing studies in rats in TNF-α mediated JNK activation response to intestinal ischemic reperfusion injury. This is very interesting and well written paper.
P- Reviewers Hanazaki K, Jin S S- Editor Zhai HH L- Editor A E- Editor Li JY
| 1. | Mallick IH, Yang W, Winslet MC, Seifalian AM. Ischemia-reperfusion injury of the intestine and protective strategies against injury. Dig Dis Sci. 2004;49:1359-1377. [PubMed] [Cited in This Article: ] |
| 2. | Esposito E, Cuzzocrea S. TNF-alpha as a therapeutic target in inflammatory diseases, ischemia-reperfusion injury and trauma. Curr Med Chem. 2009;16:3152-3167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 191] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 3. | Kostopanagiotou G, Avgerinos ED, Markidou E, Voiniadis P, Chondros C, Theodoraki K, Smyrniotis V, Arkadopoulos N. Protective effect of NAC preconditioning against ischemia-reperfusion injury in piglet small bowel transplantation: effects on plasma TNF, IL-8, hyaluronic acid, and NO. J Surg Res. 2011;168:301-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Deng Y, Ren X, Yang L, Lin Y, Wu X. A JNK-dependent pathway is required for TNFalpha-induced apoptosis. Cell. 2003;115:61-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 457] [Cited by in F6Publishing: 454] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 5. | Ikeda H, Suzuki Y, Suzuki M, Koike M, Tamura J, Tong J, Nomura M, Itoh G. Apoptosis is a major mode of cell death caused by ischaemia and ischaemia/reperfusion injury to the rat intestinal epithelium. Gut. 1998;42:530-537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 225] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Noda T, Iwakiri R, Fujimoto K, Matsuo S, Aw TY. Programmed cell death induced by ischemia-reperfusion in rat intestinal mucosa. Am J Physiol. 1998;274:G270-G276. [PubMed] [Cited in This Article: ] |
| 7. | Pergel A, Kanter M, Yucel AF, Aydin I, Erboga M, Guzel A. Anti-inflammatory and antioxidant effects of infliximab in a rat model of intestinal ischemia/reperfusion injury. Toxicol Ind Health. 2012;28:923-932. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478-483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1258] [Cited by in F6Publishing: 1334] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 9. | Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1713] [Cited by in F6Publishing: 1692] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 10. | Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745-756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1944] [Cited by in F6Publishing: 1938] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 11. | Chen XL, Zhang Q, Zhao R, Ding X, Tummala PE, Medford RM. Rac1 and superoxide are required for the expression of cell adhesion molecules induced by tumor necrosis factor-alpha in endothelial cells. J Pharmacol Exp Ther. 2003;305:573-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Esposito E, Mazzon E, Muià C, Meli R, Sessa E, Cuzzocrea S. Splanchnic ischemia and reperfusion injury is reduced by genetic or pharmacological inhibition of TNF-alpha. J Leukoc Biol. 2007;81:1032-1043. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Zhang C, Xu X, Potter BJ, Wang W, Kuo L, Michael L, Bagby GJ, Chilian WM. TNF-alpha contributes to endothelial dysfunction in ischemia/reperfusion injury. Arterioscler Thromb Vasc Biol. 2006;26:475-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 14. | Souza DG, Teixeira MM. The balance between the production of tumor necrosis factor-alpha and interleukin-10 determines tissue injury and lethality during intestinal ischemia and reperfusion. Mem Inst Oswaldo Cruz. 2005;100 Suppl 1:59-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Teoh N, Field J, Sutton J, Farrell G. Dual role of tumor necrosis factor-alpha in hepatic ischemia-reperfusion injury: studies in tumor necrosis factor-alpha gene knockout mice. Hepatology. 2004;39:412-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Teoh N, Leclercq I, Pena AD, Farrell G. Low-dose TNF-alpha protects against hepatic ischemia-reperfusion injury in mice: implications for preconditioning. Hepatology. 2003;37:118-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Helewski KJ, Kowalczyk-Ziomek GI, Czecior E, Swietochowska E, Wielkoszynski T, Czuba ZP, Szliszka E, Krol W. Administration of low doses of tumor necrosis factor-alpha protects rat liver from ischaemic damage and reperfusion injury. J Physiol Pharmacol. 2010;61:273-278. [PubMed] [Cited in This Article: ] |
| 18. | Rüdiger HA, Clavien PA. Tumor necrosis factor alpha, but not Fas, mediates hepatocellular apoptosis in the murine ischemic liver. Gastroenterology. 2002;122:202-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 187] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Wu B, Fujise T, Iwakiri R, Ootani A, Amemori S, Tsunada S, Toda S, Fujimoto K. Venous congestion induces mucosal apoptosis via tumor necrosis factor-alpha-mediated cell death in the rat small intestine. J Gastroenterol. 2004;39:1056-1062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Dérijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, Karin M, Davis RJ. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025-1037. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2509] [Cited by in F6Publishing: 2579] [Article Influence: 86.0] [Reference Citation Analysis (0)] |
| 21. | Kyriakis JM, Banerjee P, Nikolakaki E, Dai T, Rubie EA, Ahmad MF, Avruch J, Woodgett JR. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2021] [Cited by in F6Publishing: 2075] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 22. | Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305-1308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4271] [Cited by in F6Publishing: 4145] [Article Influence: 159.4] [Reference Citation Analysis (0)] |
| 23. | Reinhard C, Shamoon B, Shyamala V, Williams LT. Tumor necrosis factor alpha-induced activation of c-jun N-terminal kinase is mediated by TRAF2. EMBO J. 1997;16:1080-1092. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 229] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 24. | King LA, Toledo AH, Rivera-Chavez FA, Toledo-Pereyra LH. Role of p38 and JNK in liver ischemia and reperfusion. J Hepatobiliary Pancreat Surg. 2009;16:763-770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Won M, Park KA, Byun HS, Sohn KC, Kim YR, Jeon J, Hong JH, Park J, Seok JH, Kim JM. Novel anti-apoptotic mechanism of A20 through targeting ASK1 to suppress TNF-induced JNK activation. Cell Death Differ. 2010;17:1830-1841. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3924] [Cited by in F6Publishing: 3898] [Article Influence: 169.5] [Reference Citation Analysis (0)] |
| 27. | Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27:6245-6251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1012] [Cited by in F6Publishing: 1120] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 28. | Deree J, Martins JO, Melbostad H, Loomis WH, Coimbra R. Insights into the regulation of TNF-alpha production in human mononuclear cells: the effects of non-specific phosphodiesterase inhibition. Clinics (Sao Paulo). 2008;63:321-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Marqui CE, Silva HC, Ferez D, Cavassani SS, Moraes JB, Silva DA, Simões RS, Lopes CA, Taha MO, Oliveira-Júnior IS. Pretreatment with pentoxifylline attenuates lung injury induced by intestinal ischemia/reperfusion in rats. Acta Cir Bras. 2011;26:438-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Cámara-Lemarroy CR, Guzmán-de la Garza FJ, Alarcón-Galván G, Cordero-Pérez P, Muñoz-Espinosa LE, Fernández-Garza NE. Effects of thalidomide and pentoxyphylline over local and remote organ injury after intestinal ischemia/reperfusion. Transplant Proc. 2010;42:1624-1626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Di Sabatino A, Ciccocioppo R, Benazzato L, Sturniolo GC, Corazza GR. Infliximab downregulates basic fibroblast growth factor and vascular endothelial growth factor in Crohn’s disease patients. Aliment Pharmacol Ther. 2004;19:1019-1024. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907-916. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1552] [Cited by in F6Publishing: 1519] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 33. | Guven C, Borcek AO, Cemil B, Kurt G, Yildirim Z, Ucankus NL, Kilic N, Ceviker N. Neuroprotective effects of infliximab in experimental spinal cord ischemic injury. J Clin Neurosci. 2010;17:1563-1567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |