Published online Aug 14, 2013. doi: 10.3748/wjg.v19.i30.4850
Revised: April 4, 2013
Accepted: May 7, 2013
Published online: August 14, 2013
Conventional ultrasound (US) is the recommended imaging method for lymph node (LN) diseases with the advantages of high resolution, real time evaluation and relative low costs. Current indications of transcutaneous ultrasound and endoscopic ultrasound include the detection and characterization of lymph nodes and the guidance for LN biopsy. Recent advances in US technology, such as contrast enhanced ultrasound (CEUS), contrast enhanced endoscopic ultrasound (CE-EUS), and real time elastography show potential to improve the accuracy of US for the differential diagnosis of benign and malignant lymph nodes. In addition, CEUS and CE-EUS have been also used for the guidance of fine needle aspiration and assessment of treatment response. Complementary to size criteria, CEUS could also be used to evaluate response of tumor angiogenesis to anti-angiogenic therapies. In this paper we review current literature regarding evaluation of lymphadenopathy by new and innovative US techniques.
Core tip: The differentiation of malignant from benign lymph nodes by ultrasound, computed tomography and magnetic resonance imaging traditionally relies mainly on size measurements and topographic distribution. However, sensitivity and specificity in the differentiation of benign and malignant lymph nodes are disappointing using only size parameters. The presented paper is intended to discuss, comment and illustrate the clinical important work-up of lymphadenopathy with respect of recently introduced imaging techniques including contrast enhanced ultrasound and elastography.
- Citation: Cui XW, Jenssen C, Saftoiu A, Ignee A, Dietrich CF. New ultrasound techniques for lymph node evaluation. World J Gastroenterol 2013; 19(30): 4850-4860
- URL: https://www.wjgnet.com/1007-9327/full/v19/i30/4850.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i30.4850
The differentiation of malignant from benign lymph nodes by ultrasound (US), computed tomography (CT) and magnetic resonance imaging (MRI) traditionally relies mainly on size measurements and topographic distribution[1-3]. However, sensitivity and specificity in the differentiation of benign and malignant lymph nodes are disappointing using only size parameters. Reasons for the low accuracy include that malignant lymph node infiltration occurs in up to 30% in lymph nodes of less than 5 mm which has been shown for lung, esophageal, gastric, pancreatic and rectal carcinoma[4-10]. The evaluation of shape and border often adds no or only little more information to exclude malignancy[11,12]. New imaging methods should be able to delineate the early and circumscribed malignant infiltration and to improve ultrasound guided biopsy.
Colour Doppler ultrasound (CDI) adds value for the differentiation of malignant from normal or reactive nodes by displaying the macrovessel architecture. Normal LNs generally show hilar predominant normal vascularity. Inflammatory lymph nodes are typically more vascularised without changes of the predominant hilar vessel architecture. In contrast metastatic lymph nodes present peripheral or mixed vascularity and loss of hilar type of vascularisation[13].
Contrast enhanced CDI has improved the viusalisation of macrovessels (angioarchitecture) but does not allow evaluation of microvessels[14]. Demonstration of malignant neovascularisation, e.g., vessels penetrating the LN capsule, has been used as the characteristic feature of lymph node metastases.
Spectral Doppler ultrasound contributes to differentiation of malignant and benign solid neoplasia[15]. Likewise, normal and inflammatory lymph nodes show lower vascular resistance [resistive index (RI)] as compared to malignant lymph nodes[16] but overall results are disappointing.
Although Doppler ultrasound techniques have extended the opportunities for the differentiation of malignant from benign lymph nodes by displaying changes of macrovascularity and the vascular resistance[13,17,18], they do not improve lymph node detection rate and vascularity is often not detected in small lymph nodes[19]. Therefore, Doppler techniques and contrast enhanced Doppler techniques in general have not significantly improved the diagnostic work up of lymphadenopathy. There is a need for new imaging techniques for better characterisation of lymph nodes with the opportunity to assess also the internal microvessel architecture of lymph nodes and tissue elasticity for detection of early circumscribed malignant infiltration.
In the presented paper we discuss current knowledge about recent advances in ultrasound technology for improved lymph node evaluation.
Contrast enhanced ultrasound (CEUS) is the application of ultrasound contrast agents (UCA) to traditional sonography. The currently used UCA are microbubbles stabilized by a shell which has a high degree echogenicity. Since their physical size is just 1-4 micrometres in diameter (equal to or smaller than red blood cells), UCA allow depiction of both the macrovasculature and the microvasculature[20]. CEUS has been introduced more than ten years ago and guidelines have been published for the liver[20,21] and non-liver indications[22]. Currently 4.8 mL SonoVue® is recommended for imaging superficial LNs with a high frequency probe and for imaging the mediastinal and abdominal LNs with a high frequency endoscopic probe in CE-EUS.
CEUS techniques provide information on vascularisation and perfusion patterns, and exploit the differences in blood flow characteristics between normal and pathological tissue but knowledge about lymph node evaluation is limited[22]. CEUS could be helpful by identifying changes in vascular architecture of macro- and micro-vessels and avascular areas as signs of malignant infiltration.
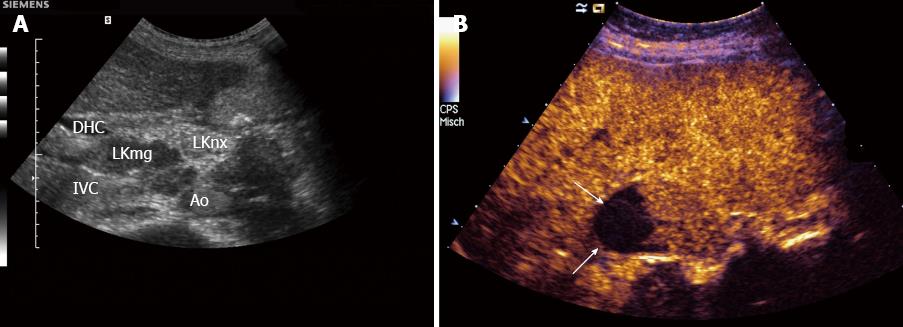
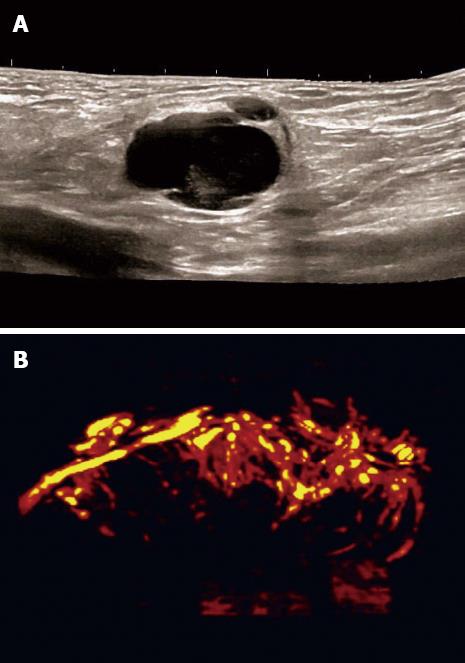
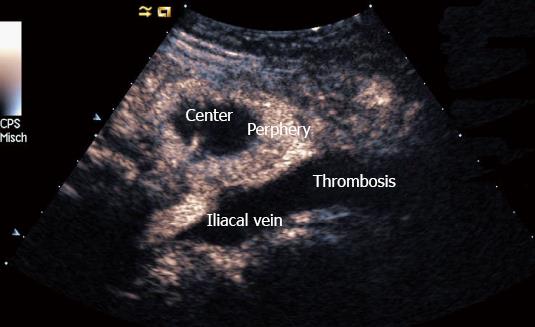
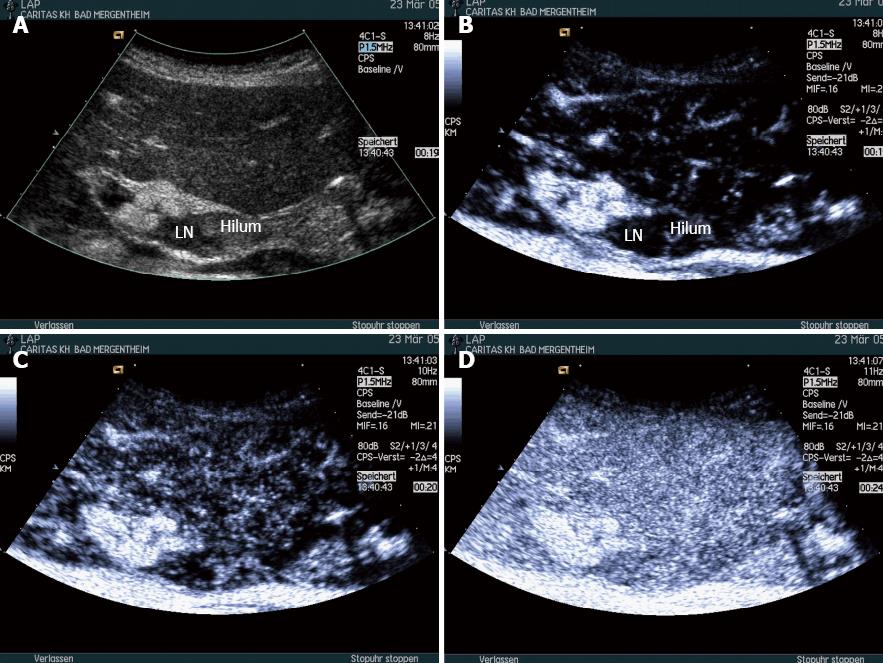
Carcinoma infiltration causes the development of pathological vessels (neoangiogenesis) and, therefore, a change of the perfusion pattern with heterogeneous enhancement due to the presence of caliber changes of the neoplastic vessels and arteriovenous shunts[23-27]. Focal hypoenhancement may result from the partial insufficiency of blood-supply due to overpressure in the LN caused by the neoplastic infiltration. Malignant lymph nodes not only have a greater number of peripheral vessels, but also longer contrast enhancement duration than benign lymph nodes[28]. Destructive avascular necroses are an important imaging sign for malignant infiltration (Figures 1, 2 and 3). Avascular areas are detected by the lack of contrast agent uptake in the necrotic zones and the peripherally located pronounced hyperenhancement (rim enhancement)[29,30]. The contrast enhancement pattern of focal cortical thickening has been also identified as an important sign to differentiate benign and malignant lymphadenopathy. In benign lymph nodes contrast enhancement within the cortex is homogeneous, whereas in malignant lymph the cortical thickening is less well vascularized than the adjacent normal lymph node parenchyma[24].
In conclusion, criteria for carcinomatous lymph node infiltration on CEUS are centripetal inhomogeneous enhancement and perfusion defects.
It is essential to consider lymphoma separately because some of its features are different from other LN disease[13,31]. The very few studies published so far showed that in lymphoma contrast enhancement patterns are highly variable. The most often observed pattern is intense homogeneous enhancement, which is not different from reactive inflammatory lymph nodes[25,31] (Figure 4).
In conclusion, there is evidence that the vascular pattern of lymphomatous lymph node infiltration resembles that of non-malignant nodes.
Most inflammatory processes do not change the hilum predominant vessel architecture of lymph nodes. According to the majority of published papers, normal and inflammatory LNs are characterized by a centrifugal and homogeneous enhancement pattern[23-26] (Figure 5). Therefore, inflammation changes the enhancement pattern only by the amount (peak) enhancement but not by changes of distribution. It is worth mentioning that non-destructive necrosis, which is reflected in avascular areas on CEUS, can be also found in granulomatous lymphadenitis, e.g., cat-scratch disease (bartonellosis), tuberculosis and sarcoidosis.
Changes and reduction of intranodal vascularity may be the first sign of response to antineoplastic treatment as shown for gastrointestinal stromal tumors and renal cell carcinoma[22,32]. Since tumour growth depends on neovascularization, CEUS can also help to detect focal nodular tumour recurrence in scars and to guide biopsy[33]. In Hodgkin’s disease well demarcated avascular areas have been described as a typical sign of treatment response[34,35].
Quantification software (time intensity curve analysis) has been used for the differential diagnosis of benign and malignant LNs but results so far are conflicting[36]. There are two studies showing that the difference of intensity between the hypervascular and hypovascular regions was significantly higher in metastatic than in non-metastatic LNs[26,37]. Steppan et al[38] reported that malignant compared to benign lymph nodes showed higher maximum intensity and duration of enhancement while Yu et al[25] reported no significant differences on maximum intensity. Time to peak intensity and area under the curve of malignant lymph nodes and lymphomas were less than that of benign LNs. Ahuja could demonstrate a reduction of vessel density (vascularity) and delay in the time to peak enhancement after treatment. It has to be mentioned that the changes in peak enhancement were operator dependent[33]. Since evidence is inconsistent quantification techniques cannot be generally recommended for clinical use. European Federation for Ultrasound in Medicine and Biology (EFSUMB) has published recommendations on the use of dynamic contrast enhanced ultrasound (DCE-US) discussing the current use and limitations in detail[39].
In conclusion, contrast enhanced techniques compared to conventional ultrasound may improve the differential diagnosis of benign LNs from malignant LNs and provide a more accurate selection of nodes to be submitted to fine-needle aspiration biopsy[25,28].
Elastography is a non-invasive method in which the stiffness of the tissue can be imaged as colour map or shear wave velocity. Two main forms of elastography have been studied for the evaluation of lymph nodes. One form is strain elastography (SE). The ultrasound probe is used to palpate the tissue[40] usually transcutaneously but optionally also intra-operatively or via an endoscope[41-45]. The tissue deformation produced (i.e., strain) is assessed by following the way the speckle in the image moves, usually with a tracking algorithm working on the radio-frequency data. The data can then be used to form an image that is coded in colour or grey-scale to show the pattern of strain, which is inversely related to tissue stiffness. Therefore, SE allows assessment and visualization of relative elasticity differences. The area to be evaluated is defined by a ROI in a similar way to CDI[44,46]. New technical developments allow for averaging over several frames to calculate the mean histogram value which corresponds to overall elasticity within a selected area[47]. Comparing two different areas within the ROI allows calculation of the strain ratio. SE is the most commonly used method for the evaluation of lymph nodes. The other forms of elastography are shear wave elastography techniques (SWE) which include transient elastography (TE) (e.g., Fibroscan™, Echosens, France), Acoustic Radiation Force Impulse imaging (e.g., ARFI, Siemens, Germany) and Supersonic Shear Wave Imaging (SSI) (Supersonic, France). In shear wave elastography the “pushing” ultrasound beam causes minute displacements in soft tissue, which depend on the magnitude of tissue stiffness. Using tracking algorithms, the resulting shear waves can be detected sonographically. So far only SSI has been studied for the evaluation of LN. In elastographic images of normal lymph nodes the nodal cortex is significantly harder than the medulla and the hilum[48,49].
EFSUMB has prepared recommendations on the use of elastography. In two sets of papers the techniques are explained in more detail[50,51].
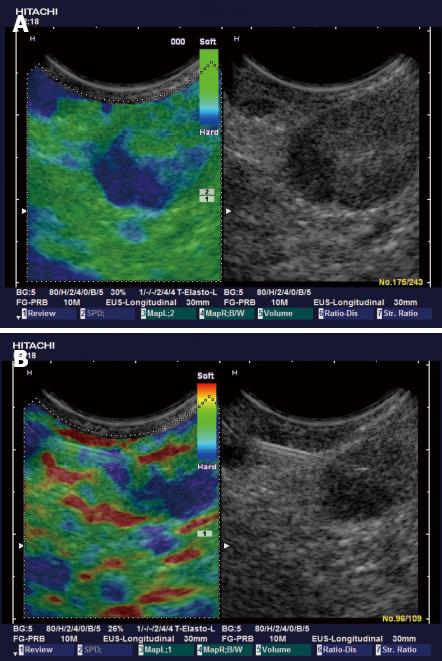
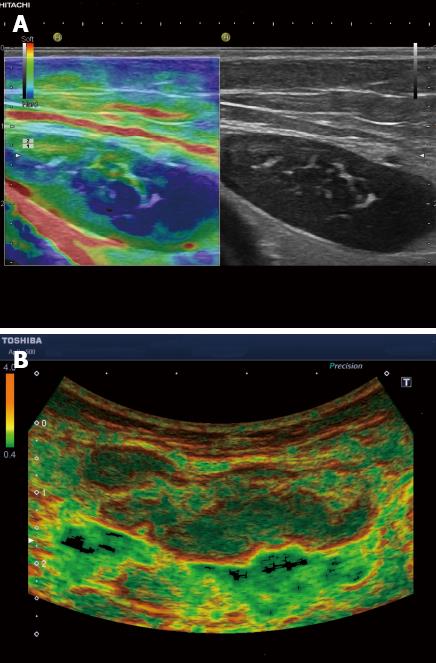
Typically the well differentiated carcinoma at least initially infiltrates lymph nodes in a circumscribed manner (focally stiffer, harder) (Figure 6), whereas the undifferentiated carcinoma leads to a diffuse (stiffer, harder) infiltration.
Suspected cervical LN metastases from hypopharyngeal and thyroid carcinomas have been recently investigated using SE (real time elastography)[39,52]. An elasticity index has been created by comparing the elasticity of the LN with the surrounding head and neck muscle tissue (muscle to LN strain ratio). Using a ratio of > 1.5 as an indicator of malignant infiltration, sensitivity was 82% and specificity 98% which is superior to the best B-mode criteria[52]. These data have been reproduced by Tan et al[53]. Moreover, interobserver agreement with SE was very high (kappa 0.828-0.946)[53].
Applying a higher cut-off value for strain ratio (1.78) Teng et al[54] at the cost of an only moderate specificity (65%) reported a very high sensitivity (98%) for discriminating malignant from benign suspicious cervical lymph nodes.
Other authors used a scoring system (percentage of blue-coding lymph node area) to differentiate malignant from benign lymph nodes in head and neck cancer patients. A blue coded (hard) area of > 50% of total lymph node area (score 3 and 4) or observation of a central necrosis (score 5) predicted malignant infiltration with high accuracy and added value to traditional ultrasound criteria[55-57].
So far two papers are published for the differential diagnosis of lymph nodes using shear wave elastography on 55 cervical enlarged lymph nodes using SSI. Malignant nodes were homogeneously stiffer than benign lymph nodes. The sensitivity (42%), specificity (100%) and accuracy (62%) were promising defining a cut-off level of 30.2 kPa[58]. The intra- and inter-observer reliability of shear wave elastography using SSI was shown to be fair to excellent for 176 neck lesions according to the intraclass correlation coefficients (0.78-0.85)[59].
In conclusion, elastography seems to be a very promising diagnostic tool for the differentiation between benign and malignant lymph nodes. This is reflected by a recent meta-analysis which reported a pooled sensitivity and specificity for the diagnosis of malignant lymph nodes of 74% and 90% for elasticity scores, and 88% and 81% for strain ratio, respectively[60]. However, to date studies comparing the two techniques of elastography (SE and SWE) are lacking.
Knowledge of strain imaging in lymphoma is very limited. So far different lymphoma cannot be differentiated. Initial experience suggests that focal lymph node infiltration (Figure 7A) is indicative for low grading of follicular lymphoma whereas diffuse and homogenous lymph node infiltration is typically found in high grade lymphoma (Figure 7B).
Most inflammatory processes do not change the elastographic architecture of lymph nodes. The hilum in normal lymph nodes remains softer than the stiffer cortex also in inflammatory lymph nodes. Circumscribed softer (and stage dependent also stiffer) lymph node areas are found in tuberculosis but this has only been shown in few cases.
Diagnostic and therapeutic endoscopic ultrasound has been established in the last thirty years[61,62]. The technique can be also applied with colour Doppler imaging as discussed above. Recently CE-EUS and real time endoscopic elastography (RTE-EUS) have been introduced.
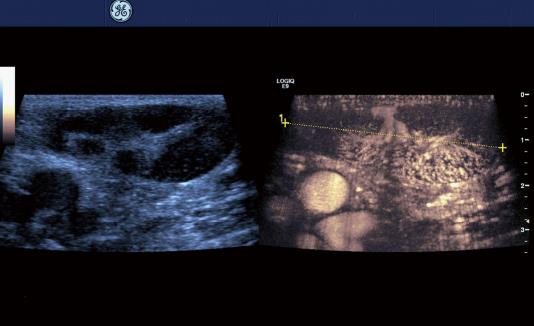
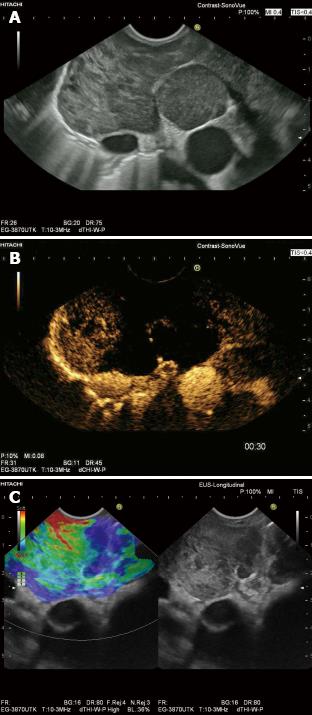
Contrast enhanced endoscopic ultrasound (CE-EUS) is CEUS performed with an endoscopic probe, which can be performed on both Doppler mode with high MI and contrast specific mode with low MI[63] to also guide therapeutic procedures. The dose of the ultrasound contrast agent (UCA) should be 4.8 mL for SonoVue®. CE-EUS can improve the detection of small intranodal vessels and thus could be useful in characterization of LNs[3,64] (Figure 8). CE-EUS has improved our understanding of gastrointestinal (subepithelial) tumors[65-67], differential diagnosis of pancreatic neoplasia[15,68-75] and other organ infiltration[74,75] through analysis of perfusion patterns.
There are only a few reports about the usefulness of contrast enhanced endoscopic Doppler ultrasound in the differentiation between malignant and benign lymphadenopathy. Kanamori et al[64] performed CE-EUS with high MI on 46 patients in whom EUS revealed LN in the mediastinum or abdominal cavity and suggested that CE-EUS is useful for differentiating benign from malignant LNs by detecting defects of enhancement in malignant nodes. The sensitivity, specificity, and accuracy rate of CE-EUS were 100%, 86.4% and 92.3%, respectively. In another study by Hocke et al[3], high MI CE-EUS was performed in 122 patients, and it was found that CE-EUS improved the specificity in diagnosing benign LNs as compared to B-mode EUS by analysing arteries and veins. However, it did not improve the accurate identification of malignant LNs and therefore could not replace EUS-guided fine-needle aspiration[3].
To the best of our knowledge, there is only one report on the application of low MI CE-EUS for the discrimination of benign and malignant abdominal lymph nodes. A Japanese group investigated 43 patients with intra-abdominal lesions of undetermined origin, which were suspected to be malignant lymph nodes, and evaluated the enhancement pattern after injection of the UCA Sonazoid®. Final pathological examination revealed that 35 lesions in fact were lymph nodes. All but one of the malignant lesions showed a heterogeneous enhancement pattern, whereas none of the benign lesion displayed heterogeneous enhancement. Most interestingly the interobserver agreement was very high (kappa 0.953)[78].
Endoscopic elastography is real time elastography performed with an endoscopic probe, which has led to further improvement in B mode imaging results for classification of benign and malignant LNs (Figure 8), particularly by targeting LNs for needle sampling. Janssen et al[79] reported on 50 patients, 66 LNs were described elastographically (dominant colour/tissue hardness and guidance for tissue samples) and the elastogram data later compared with the histological findings obtained in the same session from fine needle biopsy. This study revealed that benign LNs exhibited predominantly intermediate homogeneous deformation (yellow/green), while malignant LNs were characterized by a quantitative dominance of hard (blue) units. The accuracy, which could be consistently reproduced by two more reviewers (kappa 0.84), for benign vs malignant LNs was about 85%. Intra- and interobserver agreement was also high in one recent study using visual assessment of the elastography image to differentiate between malignant and benign lymph nodes[80]. However, the same group found that EUS elastography did not perform better than EUS morphology in differentiating between malignant and benign lymph nodes in patients with resectable upper gastrointestinal cancer[45]. These findings conflict with the results of two other groups, which showed superior accuracy of EUS elastography strain ratio and histogram analysis, respectively, in comparison with conventional EUS criteria in differentiating malignant and benign lymph nodes in the nodal staging of esophageal cancer[81,82].
Sǎftoiu et al[41] used similar criteria for qualitative analysis in their study. In computer analysis, accuracy for differential diagnosis of malignant vs benign LNs increased slightly from 93% to 95%. In a follow-up study[47], they reached an accuracy for differentiation between benign and malignant LNs of 89%, using the computer based histogram analysis of video sequences, while this was significantly superior to the B-Mode image analysis (accuracy 53%). Another recent study with pathological confirmation yielded however lower values for sensitivity, specificity and accuracy, based on strain ratio calculations[45].
A recent meta-analysis calculated a sensitivity of 88% and a specificity of 85%, respectively, of EUS elastography for differentiating between benign and malignant lymph nodes[83].
In conclusion, the sensitivity of an imaging procedure critically depends on spatial resolution, which in elastography is as good as in conventional ultrasound since both depend on the same physical rules. The smallest LN metastases may escape both B-mode diagnosis and endosonographic fine needle biopsy. Elastography can detect the smallest metastasis-related changes in tissue hardness and it is considered to be potentially useful for target selection prior to endosonographic guided tissue sampling[10].
RTE can be recommended for discrimination of benign and malignant lymph nodes by identifying malignant regions that should be targeted for EUS-FNA (Figure 8).
The detection or exclusion of sentinel lymph node (SLN) micrometastases is critical in staging cancer, especially breast cancer and melanoma, because it directly affects patient’s prognosis and surgical management. It is well known that conventional US is not able to detect SLN in most cases. However, studies showed that low MI CEUS can be used for detecting SLN, which may become a potential application in clinical routine, like lymphoscintigraphy[32,84-89]. The application of CEUS for the investigation of SLN has shown promising results in animal models but the technique has not been sufficiently evaluated in humans. About 1 mL of contrast agent (e.g., SonoVue®) is injected subcutaneously (intralymphatic) near the tumour site and the enhanced lymphatics are traced to the sentinel lymph node. Initial experience indicates that the method is not toxic and performs as well as blue dye or radioisotope methods. The current literature has been recently reviewed[90] and the topic is not the subject of this paper.
Panoramic imaging, 3D[91] and 3D-CEUS[92,93] have been used for improved anatomic and topographic description of lymphadenopathy but have not gained additional information except improved presentation of results to clinicians.
The currently possible lymph node detection rate is limited by a minimal required lymph node size which is between 5-10 mm. Since about one third of malignant infiltrations occur in lymph nodes which are not detectable by all imaging methods, reliable exclusion of malignant lymph node infiltration is almost impossible. Therefore, current imaging methods mainly focus on the improved detection of early malignant infiltration in detectable lymph nodes, e.g., to guide neoadjuvant treatment strategies.
Ultrasound techniques (CEUS, CE-EUS and elastography) demonstrate high spatial resolution which is important for early detection of malignant lymph node infiltration (Table 1). CEUS compared with conventional CDI could improve the visualization of vessels in LNs which is essential for the evaluation of vessel distribution. The visualization of avascular necrotic deposits of neoplastic cells is helpful for the differentiation of benign and malignant lymphadenopathy. The identification of hypoenhancing areas in malignant lymph nodes may guide biopsy for improved early detection of malignant infiltration.
| Lymphadenopathy more (most) likely | B-mode | (Contrast enhanced) Colour Doppler | Vascular resistance | CEUS (contrast special imaging mode) | Elastography |
| Inflammatory | Preserved architectur, homogeneous, thin cortex | Preserved vessel architecture, hilar vascularity with or without tree like branching. | Lower, RI < 0.8, PI < 1.6 | Homogeneous enhancement from the hilum, centrifugal enhancement | No data, most often normal architecture (except tuberculosis) |
| Malignant infiltration (metastasis) | Destroyed architecture (capsule), eccentric hypoechoic cortical thickening, | Peripheral or mixed vascularity, inhomogeneous vessel density, split arteries, torturous course of vessels | Higher, RI > 0.8, PI > 1.6, often variableat different sites | Centripetal enhancement, different intra-nodal enhancement levels, inhomogeneous wash-out, perfusion defects | Initially circumscribed. SR in diffuse infiltration > 1.5 (1.78) |
| inhomogeneity of the internal structure, loss of echogenic hilum, surrounding edema | |||||
| Lymphoma | Focal or global hypoechoic cortical thickening, usually without echogenic hilum, peri-nodular edema, pseudocystic appearance | Often but not always preserved vessel architecture, rich vascularity | Intermediate RI and PI | Intense homogeneous enhancement, starts with diffuse bright spots, peripheral hypo-or non-enhancement | No data; wide range of appearance applying qualitative criteria |
In addition, the strictly intravascular distribution of intravenously injected contrast agents (e.g., SonoVue®) allows the assessment of neoangiogenesis which is of importance for treatment evaluation under antiangiogenetic treatment.
CEUS cannot be recommended for the diagnosis of lymphoma so far. However, CEUS may be a tool to assess the treatment response by indentifying the reduction of vascularisation, e.g., in Hodgkin’s disease.
Elastography is mainly helpful in delineating the very early circumscribed malignant infiltration for improved US- and EUS-guided fine needle aspiration (biopsy). Additionally, normal elastographic architecture of enlarged inflammatory lymph nodes can be helpful to prove a benign inflammatory disease, e.g., sarcoidosis.
P- Reviewers Levent D, Korpanty G S- Editor Song XX L- Editor A E- Editor Li JY
| 1. | Sharma A, Fidias P, Hayman LA, Loomis SL, Taber KH, Aquino SL. Patterns of lymphadenopathy in thoracic malignancies. Radiographics. 2004;24:419-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Sumi M, Ohki M, Nakamura T. Comparison of sonography and CT for differentiating benign from malignant cervical lymph nodes in patients with squamous cell carcinoma of the head and neck. AJR Am J Roentgenol. 2001;176:1019-1024. [PubMed] [Cited in This Article: ] |
| 3. | Hocke M, Menges M, Topalidis T, Dietrich CF, Stallmach A. Contrast-enhanced endoscopic ultrasound in discrimination between benign and malignant mediastinal and abdominal lymph nodes. J Cancer Res Clin Oncol. 2008;134:473-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Prenzel KL, Hölscher AH, Drebber U, Agavonova M, Gutschow CA, Bollschweiler E. Prognostic impact of nodal micrometastasis in early esophageal cancer. Eur J Surg Oncol. 2012;38:314-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Prenzel KL, Mönig SP, Sinning JM, Baldus SE, Brochhagen HG, Schneider PM, Hölscher AH. Lymph node size and metastatic infiltration in non-small cell lung cancer. Chest. 2003;123:463-467. [PubMed] [Cited in This Article: ] |
| 6. | Prenzel KL, Hölscher AH, Vallböhmer D, Drebber U, Gutschow CA, Mönig SP, Stippel DL. Lymph node size and metastatic infiltration in adenocarcinoma of the pancreatic head. Eur J Surg Oncol. 2010;36:993-996. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Jenssen C, Dietrich CF, Burmester E. [Malignant neoplasias of the gastrointestinal tract--endosonographic staging revisited]. Z Gastroenterol. 2011;49:357-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Moehler M, Al-Batran SE, Andus T, Anthuber M, Arends J, Arnold D, Aust D, Baier P, Baretton G, Bernhardt J. German S3-guideline “Diagnosis and treatment of esophagogastric cancer”. Z Gastroenterol. 2011;49:461-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 9. | Jürgensen C, Dietrich CF. Role of endoscopic ultrasound (EUS) in the staging of rectal cancer. Z Gastroenterol. 2008;46:580-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Jenssen C, Dietrich CF. Endoscopic ultrasound-guided fine-needle aspiration biopsy and trucut biopsy in gastroenterology - An overview. Best Pract Res Clin Gastroenterol. 2009;23:743-759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Ying M, Ahuja A, Brook F, Brown B, Metreweli C. Nodal shape (S/L) and its combination with size for assessment of cervical lymphadenopathy: which cut-off should be used. Ultrasound Med Biol. 1999;25:1169-1175. [PubMed] [Cited in This Article: ] |
| 12. | Vassallo P, Wernecke K, Roos N, Peters PE. Differentiation of benign from malignant superficial lymphadenopathy: the role of high-resolution US. Radiology. 1992;183:215-220. [PubMed] [Cited in This Article: ] |
| 13. | Ahuja AT, Ying M. Sonographic evaluation of cervical lymph nodes. AJR Am J Roentgenol. 2005;184:1691-1699. [PubMed] [Cited in This Article: ] |
| 14. | Schmid-Wendtner MH, Partscht K, Korting HC, Volkenandt M. Improved differentiation of benign and malignant lymphadenopathy in patients with cutaneous melanoma by contrast-enhanced color Doppler sonography. Arch Dermatol. 2002;138:491-497. [PubMed] [Cited in This Article: ] |
| 15. | Hocke M, Schulze E, Gottschalk P, Topalidis T, Dietrich CF. Contrast-enhanced endoscopic ultrasound in discrimination between focal pancreatitis and pancreatic cancer. World J Gastroenterol. 2006;12:246-250. [PubMed] [Cited in This Article: ] |
| 16. | Ying M, Ahuja A. Sonography of neck lymph nodes. Part I: normal lymph nodes. Clin Radiol. 2003;58:351-358. [PubMed] [Cited in This Article: ] |
| 17. | Ahuja A, Ying M. Sonographic evaluation of cervical lymphadenopathy: is power Doppler sonography routinely indicated. Ultrasound Med Biol. 2003;29:353-359. [PubMed] [Cited in This Article: ] |
| 18. | Tschammler A, Heuser B, Ott G, Schmitt S, Hahn D. Pathological angioarchitecture in lymph nodes: underlying histopathologic findings. Ultrasound Med Biol. 2000;26:1089-1097. [PubMed] [Cited in This Article: ] |
| 19. | Moritz JD, Ludwig A, Oestmann JW. Contrast-enhanced color Doppler sonography for evaluation of enlarged cervical lymph nodes in head and neck tumors. AJR Am J Roentgenol. 2000;174:1279-1284. [PubMed] [Cited in This Article: ] |
| 20. | Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, Piscaglia F, Wilson SR, Barr RG, Chammas MC. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver--update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med. 2013;34:11-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 210] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 21. | Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, Piscaglia F, Wilson SR, Barr RG, Chammas MC. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver - update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol. 2013;39:187-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 473] [Cited by in F6Publishing: 479] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 22. | Piscaglia F, Nolsøe C, Dietrich CF, Cosgrove DO, Gilja OH, Bachmann Nielsen M, Albrecht T, Barozzi L, Bertolotto M, Catalano O. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med. 2012;33:33-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 663] [Cited by in F6Publishing: 659] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 23. | Rubaltelli L, Khadivi Y, Tregnaghi A, Stramare R, Ferro F, Borsato S, Fiocco U, Adami F, Rossi CR. Evaluation of lymph node perfusion using continuous mode harmonic ultrasonography with a second-generation contrast agent. J Ultrasound Med. 2004;23:829-836. [PubMed] [Cited in This Article: ] |
| 24. | Rubaltelli L, Beltrame V, Tregnaghi A, Scagliori E, Frigo AC, Stramare R. Contrast-enhanced ultrasound for characterizing lymph nodes with focal cortical thickening in patients with cutaneous melanoma. AJR Am J Roentgenol. 2011;196:W8-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Yu M, Liu Q, Song HP, Han ZH, Su HL, He GB, Zhou XD. Clinical application of contrast-enhanced ultrasonography in diagnosis of superficial lymphadenopathy. J Ultrasound Med. 2010;29:735-740. [PubMed] [Cited in This Article: ] |
| 26. | Ouyang Q, Chen L, Zhao H, Xu R, Lin Q. Detecting metastasis of lymph nodes and predicting aggressiveness in patients with breast carcinomas. J Ultrasound Med. 2010;29:343-352. [PubMed] [Cited in This Article: ] |
| 27. | Wan CF, Du J, Fang H, Li FH, Zhu JS, Liu Q. Enhancement patterns and parameters of breast cancers at contrast-enhanced US: correlation with prognostic factors. Radiology. 2012;262:450-459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 28. | Yang WT, Metreweli C, Lam PK, Chang J. Benign and malignant breast masses and axillary nodes: evaluation with echo-enhanced color power Doppler US. Radiology. 2001;220:795-802. [PubMed] [Cited in This Article: ] |
| 29. | Sakaguchi T, Yamashita Y, Katahira K, Nishimura R, Baba Y, Arakawa A, Takahashi M, Yumoto E, Shinohara M. Differential diagnosis of small round cervical lymph nodes: comparison of power Doppler US with contrast-enhanced CT and pathologic results. Radiat Med. 2001;19:119-125. [PubMed] [Cited in This Article: ] |
| 30. | King AD, Tse GM, Ahuja AT, Yuen EH, Vlantis AC, To EW, van Hasselt AC. Necrosis in metastatic neck nodes: diagnostic accuracy of CT, MR imaging, and US. Radiology. 2004;230:720-726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 185] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 31. | Nakase K, Yamamoto K, Hiasa A, Tawara I, Yamaguchi M, Shiku H. Contrast-enhanced ultrasound examination of lymph nodes in different types of lymphoma. Cancer Detect Prev. 2006;30:188-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Berho M, Oviedo M, Stone E, Chen C, Nogueras J, Weiss E, Sands D, Wexner S. The correlation between tumour regression grade and lymph node status after chemoradiation in rectal cancer. Colorectal Dis. 2009;11:254-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Ahuja AT, Ying M, Ho SY, Antonio G, Lee YP, King AD, Wong KT. Ultrasound of malignant cervical lymph nodes. Cancer Imaging. 2008;8:48-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 156] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 34. | Eich HT, Müller RP, Engenhart-Cabillic R, Lukas P, Schmidberger H, Staar S, Willich N. Involved-node radiotherapy in early-stage Hodgkin’s lymphoma. Definition and guidelines of the German Hodgkin Study Group (GHSG). Strahlenther Onkol. 2008;184:406-410. [PubMed] [Cited in This Article: ] |
| 35. | Engert A, Eichenauer DA, Dreyling M. Hodgkin’s lymphoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20 Suppl 4:108-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Ignee A, Jedrejczyk M, Schuessler G, Jakubowski W, Dietrich CF. Quantitative contrast enhanced ultrasound of the liver for time intensity curves-Reliability and potential sources of errors. Eur J Radiol. 2010;73:153-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Rubaltelli L, Corradin S, Dorigo A, Tregnaghi A, Adami F, Rossi CR, Stramare R. Automated quantitative evaluation of lymph node perfusion on contrast-enhanced sonography. AJR Am J Roentgenol. 2007;188:977-983. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Steppan I, Reimer D, Müller-Holzner E, Marth C, Aigner F, Frauscher F, Frede T, Zeimet AG. Breast cancer in women: evaluation of benign and malignant axillary lymph nodes with contrast-enhanced ultrasound. Ultraschall Med. 2010;31:63-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Dietrich CF, Averkiou MA, Correas JM, Lassau N, Leen E, Piscaglia F. An EFSUMB introduction into Dynamic Contrast-Enhanced Ultrasound (DCE-US) for quantification of tumour perfusion. Ultraschall Med. 2012;33:344-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 228] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 40. | Dietrich CF. Elastography, the new dimension in ultrasonography. Praxis (Bern 1994). 2011;100:1533-1542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Săftoiu A, Vilmann P, Hassan H, Gorunescu F. Analysis of endoscopic ultrasound elastography used for characterisation and differentiation of benign and malignant lymph nodes. Ultraschall Med. 2006;27:535-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 42. | Janssen J. [(E)US elastography: current status and perspectives]. Z Gastroenterol. 2008;46:572-579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Giovannini M, Thomas B, Erwan B, Christian P, Fabrice C, Benjamin E, Geneviève M, Paolo A, Pierre D, Robert Y. Endoscopic ultrasound elastography for evaluation of lymph nodes and pancreatic masses: a multicenter study. World J Gastroenterol. 2009;15:1587-1593. [PubMed] [Cited in This Article: ] |
| 44. | Dietrich CF. Elastography Applications. Endo heute. 2011;24:177-212. [Cited in This Article: ] |
| 45. | Larsen MH, Fristrup C, Hansen TP, Hovendal CP, Mortensen MB. Endoscopic ultrasound, endoscopic sonoelastography, and strain ratio evaluation of lymph nodes with histology as gold standard. Endoscopy. 2012;44:759-766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 46. | Bachmann-Nielsen M, Săftoiu A. [Elastography - true or false. Ultraschall Med. 2011;32:5-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 47. | Săftoiu A, Vilmann P, Ciurea T, Popescu GL, Iordache A, Hassan H, Gorunescu F, Iordache S. Dynamic analysis of EUS used for the differentiation of benign and malignant lymph nodes. Gastrointest Endosc. 2007;66:291-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 48. | Wojcinski S, Dupont J, Schmidt W, Cassel M, Hillemanns P. Real-time ultrasound elastography in 180 axillary lymph nodes: elasticity distribution in healthy lymph nodes and prediction of breast cancer metastases. BMC Med Imaging. 2012;12:35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 49. | Wing-Han Yuen Q, Zheng YP, Huang YP, He JF, Chung-Wai Cheung J, Ying M. In-vitro Strain and Modulus Measurements in Porcine Cervical Lymph Nodes. Open Biomed Eng J. 2011;5:39-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Bamber J, C1 Bamber J, Cosgrove D, Dietrich CF, Fromageau J, Bojunga J, Calliada F, Cantisani V, Correas JM, D'Onofrio M. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013;34:169-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 756] [Cited by in F6Publishing: 690] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 51. | Cosgrove D, Piscaglia F, Bamber J, Bojunga J, Correas JM, Gilja OH, Klauser AS, Sporea I, Calliada F, Cantisani V. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: Clinical applications. Ultraschall Med. 2013;34:238-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 621] [Cited by in F6Publishing: 461] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 52. | Lyshchik A, Higashi T, Asato R, Tanaka S, Ito J, Hiraoka M, Insana MF, Brill AB, Saga T, Togashi K. Cervical lymph node metastases: diagnosis at sonoelastography--initial experience. Radiology. 2007;243:258-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 187] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 53. | Tan R, Xiao Y, He Q. Ultrasound elastography: Its potential role in assessment of cervical lymphadenopathy. Acad Radiol. 2010;17:849-855. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 54. | Teng DK, Wang H, Lin YQ, Sui GQ, Guo F, Sun LN. Value of ultrasound elastography in assessment of enlarged cervical lymph nodes. Asian Pac J Cancer Prev. 2012;13:2081-2085. [PubMed] [Cited in This Article: ] |
| 55. | Ishibashi N, Yamagata K, Sasaki H, Seto K, Shinya Y, Ito H, Shinozuka K, Yanagawa T, Onizawa K, Bukawa H. Real-time tissue elastography for the diagnosis of lymph node metastasis in oral squamous cell carcinoma. Ultrasound Med Biol. 2012;38:389-395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Choi JJ, Kang BJ, Kim SH, Lee JH, Jeong SH, Yim HW, Song BJ, Jung SS. Role of sonographic elastography in the differential diagnosis of axillary lymph nodes in breast cancer. J Ultrasound Med. 2011;30:429-436. [PubMed] [Cited in This Article: ] |
| 57. | Taylor K, O’Keeffe S, Britton PD, Wallis MG, Treece GM, Housden J, Parashar D, Bond S, Sinnatamby R. Ultrasound elastography as an adjuvant to conventional ultrasound in the preoperative assessment of axillary lymph nodes in suspected breast cancer: a pilot study. Clin Radiol. 2011;66:1064-1071. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 58. | Bhatia KS, Cho CC, Tong CS, Yuen EH, Ahuja AT. Shear wave elasticity imaging of cervical lymph nodes. Ultrasound Med Biol. 2012;38:195-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 59. | Bhatia K, Tong CS, Cho CC, Yuen EH, Lee J, Ahuja AT. Reliability of shear wave ultrasound elastography for neck lesions identified in routine clinical practice. Ultraschall Med. 2012;33:463-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 60. | Ying L, Hou Y, Zheng HM, Lin X, Xie ZL, Hu YP. Real-time elastography for the differentiation of benign and malignant superficial lymph nodes: a meta-analysis. Eur J Radiol. 2012;81:2576-2584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 61. | Dietrich CF, Jenssen C. Evidence based endoscopic ultrasound. Z Gastroenterol. 2011;49:599-621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 62. | Dietrich CF, Hocke M, Jenssen C. Interventional endosonography. Ultraschall Med. 2011;32:8-22, quiz 23-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 63. | Dietrich CF. Contrast-enhanced low mechanical index endoscopic ultrasound (CELMI-EUS). Endoscopy. 2009;41 Suppl 2:E43-E44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 64. | Kanamori A, Hirooka Y, Itoh A, Hashimoto S, Kawashima H, Hara K, Uchida H, Goto J, Ohmiya N, Niwa Y. Usefulness of contrast-enhanced endoscopic ultrasonography in the differentiation between malignant and benign lymphadenopathy. Am J Gastroenterol. 2006;101:45-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 65. | Sakamoto H, Kitano M, Matsui S, Kamata K, Komaki T, Imai H, Dote K, Kudo M. Estimation of malignant potential of GI stromal tumors by contrast-enhanced harmonic EUS (with videos). Gastrointest Endosc. 2011;73:227-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 66. | Kannengiesser K, Mahlke R, Petersen F, Peters A, Ross M, Kucharzik T, Maaser C. Contrast-enhanced harmonic endoscopic ultrasound is able to discriminate benign submucosal lesions from gastrointestinal stromal tumors. Scand J Gastroenterol. 2012;47:1515-1520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 67. | Dietrich CF, Jenssen C, Hocke M, Cui XW, Woenckhaus M, Ignee A. Imaging of gastrointestinal stromal tumours with modern ultrasound techniques - a pictorial essay. Z Gastroenterol. 2012;50:457-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 68. | Gong TT, Hu DM, Zhu Q. Contrast-enhanced EUS for differential diagnosis of pancreatic mass lesions: a meta-analysis. Gastrointest Endosc. 2012;76:301-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 69. | Napoleon B, Alvarez-Sanchez MV, Gincoul R, Pujol B, Lefort C, Lepilliez V, Labadie M, Souquet JC, Queneau PE, Scoazec JY. Contrast-enhanced harmonic endoscopic ultrasound in solid lesions of the pancreas: results of a pilot study. Endoscopy. 2010;42:564-570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 70. | Kitano M, Kudo M, Yamao K, Takagi T, Sakamoto H, Komaki T, Kamata K, Imai H, Chiba Y, Okada M. Characterization of small solid tumors in the pancreas: the value of contrast-enhanced harmonic endoscopic ultrasonography. Am J Gastroenterol. 2012;107:303-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 230] [Cited by in F6Publishing: 211] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 71. | Reddy NK, Ioncică AM, Săftoiu A, Vilmann P, Bhutani MS. Contrast-enhanced endoscopic ultrasonography. World J Gastroenterol. 2011;17:42-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 47] [Cited by in F6Publishing: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 72. | Săftoiu A, Vilmann P, Gorunescu F, Janssen J, Hocke M, Larsen M, Iglesias-Garcia J, Arcidiacono P, Will U, Giovannini M. Accuracy of endoscopic ultrasound elastography used for differential diagnosis of focal pancreatic masses: a multicenter study. Endoscopy. 2011;43:596-603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 73. | Săftoiu A, Dietrich CF, Vilmann P. Contrast-enhanced harmonic endoscopic ultrasound. Endoscopy. 2012;44:612-617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 74. | Hocke M, Ignee A, Topalidis T, Stallmach A, Dietrich CF. Contrast-enhanced endosonographic Doppler spectrum analysis is helpful in discrimination between focal chronic pancreatitis and pancreatic cancer. Pancreas. 2007;35:286-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 75. | Dietrich CF, Ignee A, Braden B, Barreiros AP, Ott M, Hocke M. Improved differentiation of pancreatic tumors using contrast-enhanced endoscopic ultrasound. Clin Gastroenterol Hepatol. 2008;6:590-597.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 76. | Park CH, Chung MJ, Oh TG, Park JY, Bang S, Park SW, Kim H, Hwang HK, Lee WJ, Song SY. Differential diagnosis between gallbladder adenomas and cholesterol polyps on contrast-enhanced harmonic endoscopic ultrasonography. Surg Endosc. 2013;27:1414-1421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 77. | Romagnuolo J, Hoffman B, Vela S, Hawes R, Vignesh S. Accuracy of contrast-enhanced harmonic EUS with a second-generation perflutren lipid microsphere contrast agent (with video). Gastrointest Endosc. 2011;73:52-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 78. | Xia Y, Kitano M, Kudo M, Imai H, Kamata K, Sakamoto H, Komaki T. Characterization of intra-abdominal lesions of undetermined origin by contrast-enhanced harmonic EUS (with videos). Gastrointest Endosc. 2010;72:637-642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 79. | Janssen J, Dietrich CF, Will U, Greiner L. Endosonographic elastography in the diagnosis of mediastinal lymph nodes. Endoscopy. 2007;39:952-957. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 80. | Larsen MH, Fristrup CW, Mortensen MB. Intra- and interobserver agreement of endoscopic sonoelastography in the evaluation of lymph nodes. Ultraschall Med. 2011;32 Suppl 2:E45-E50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 81. | Paterson S, Duthie F, Stanley AJ. Endoscopic ultrasound-guided elastography in the nodal staging of oesophageal cancer. World J Gastroenterol. 2012;18:889-895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 29] [Cited by in F6Publishing: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 82. | Knabe M, Günter E, Ell C, Pech O. Can EUS elastography improve lymph node staging in esophageal cancer. Surg Endosc. 2013;27:1196-1202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 83. | Xu W, Shi J, Zeng X, Li X, Xie WF, Guo J, Lin Y. EUS elastography for the differentiation of benign and malignant lymph nodes: a meta-analysis. Gastrointest Endosc. 2011;74:1001-1009; quiz 1115.e1-4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 84. | Omoto K, Matsunaga H, Take N, Hozumi Y, Takehara M, Omoto Y, Shiozawa M, Mizunuma H, Harashima H, Taniguchi N. Sentinel node detection method using contrast-enhanced ultrasonography with sonazoid in breast cancer: preliminary clinical study. Ultrasound Med Biol. 2009;35:1249-1256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 85. | De Giorgi V, Gori A, Grazzini M, Rossari S, Marino G, D’Elia G, Crocetti E, Roselli G, Innocenti P, Dini M. Contrast-enhanced ultrasound: a filter role in AJCC stage I/II melanoma patients. Oncology. 2010;79:370-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 86. | Sever A, Jones S, Cox K, Weeks J, Mills P, Jones P. Preoperative localization of sentinel lymph nodes using intradermal microbubbles and contrast-enhanced ultrasonography in patients with breast cancer. Br J Surg. 2009;96:1295-1299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 87. | Sever AR, Mills P, Weeks J, Jones SE, Fish D, Jones PA, Mali W. Preoperative needle biopsy of sentinel lymph nodes using intradermal microbubbles and contrast-enhanced ultrasound in patients with breast cancer. AJR Am J Roentgenol. 2012;199:465-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 88. | Sever AR, Mills P, Jones SE, Mali W, Jones PA. Sentinel node identification using microbubbles and contrast-enhanced ultrasonography. Clin Radiol. 2012;67:687-694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 89. | Sever AR, Mills P, Jones SE, Cox K, Weeks J, Fish D, Jones PA. Preoperative sentinel node identification with ultrasound using microbubbles in patients with breast cancer. AJR Am J Roentgenol. 2011;196:251-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 90. | Cui XW, Ignee A, Nielsen MB, Schreiber-Dietrich D, De Molo C, Pirri C, Jedrzejczyk M, Dietrich CF. Contrast enhanced ultrasound of sentinel lymph nodes. J Ultrason. 2013;13:73-81. [Cited in This Article: ] |
| 91. | Bialek EJ, Jakubowski W, Szczepanik AB, Maryniak RK, Bilski R, Prochorec-Sobieszek M, Serafin-Krol M. 3D ultrasound examination of the superficial lymph nodes--does it provide additional information. Ultraschall Med. 2006;27:467-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 92. | Dietrich CF. 3D real time contrast enhanced ultrasonography,a new technique. Rofo. 2002;174:160-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 93. | Hocke M, Dietrich CF. New technology--combined use of 3D contrast enhanced endoscopic ultrasound techniques. Ultraschall Med. 2011;32:317-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |