Published online Aug 7, 2013. doi: 10.3748/wjg.v19.i29.4774
Revised: July 3, 2013
Accepted: July 17, 2013
Published online: August 7, 2013
AIM: To assess the value of ultrasonography (US) in evaluation of proximal gastric accommodation disorder in patients with functional dyspepsia (FD).
METHODS: Between April 2011 and March 2012, 45 patients with FD and 27 healthy volunteers were enrolled in this study. Two-dimensional ultrasound (2DUS) and 3-dimensional ultrasound (3DUS) were performed sequentially to measure proximal gastric area (PGA), maximal proximal gastric diameter (MPGD), and proximal gastric volume (PGV). These values were measured separately in the two groups every other 5 min for a duration of 25 min after the beginning of ingestion of a test meal. Air pocket grading was done separately for images of 2DUS and blocks of 3DUS obtained at five scanning time points.
RESULTS: Both PGA and PGV of patients were significantly smaller than healthy controls (P = 0.000 and 0.002, respectively). Comparing the two parameters between the groups at each time point, the differences were also statistically significant (P = 0.000-0.013), except at 10 min for the PGV (P = 0.077). However, no overall difference was found between the groups in the MPGD measurements (P = 0.114), though it was statistically significant at a 20-minute examination point (P = 0.026). A total of 360 sets or blocks of images were obtained for both 2DUS and 3DUS. For the images analyzed by 2DUS, none were excluded because of gastric gas, and 50 (13.9%) and 310 (86.1%) sets were determined as air pockets grades 1 and 2, respectively. For the images analyzed by 3DUS, 23 (6.4%) blocks were excluded from the measurement due to presence of a large fundus air pocket (grade 3); fifty (13.9%) and 287 (79.7%) blocks were also graded as 1 and 2, respectively.
CONCLUSION: Measurement of both PGA and PGV by 2DUS and 3DUS could be useful for assessment of the proximal gastric accommodation.
Core tip: We adopted 2-dimensional and 3-dimensional ultrasonography to measure area and volume of the proximal stomach in patients with functional dyspepsia; a condition whereby patients can experience impaired gastric accommodation. Area and volume could be used to assess accommodation impairment, because both area and volume of the patients were smaller than the controls (P < 0.05). Therefore, the ultrasound measurement of gastric area and volume could help predict the functional dyspepsia.
- Citation: Fan XP, Wang L, Zhu Q, Ma T, Xia CX, Zhou YJ. Sonographic evaluation of proximal gastric accommodation in patients with functional dyspepsia. World J Gastroenterol 2013; 19(29): 4774-4780
- URL: https://www.wjgnet.com/1007-9327/full/v19/i29/4774.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i29.4774
Functional dyspepsia (FD) is the presence of symptoms thought to originate from the gastro-duodenal region, in the absence of organic, systemic, or metabolic disease that is likely to explain the symptoms[1]. The prevalence of FD is 24.4% in Australia and 23.5% in China, based on the Rome II criteria[2]. The pathogenesis of FD is still unknown, but several studies have indicated that the proximal stomach, which includes the fundus and the proximal one-third of the body, is the site of an accommodation disorder that is likely to substantially contribute to the pathogenesis of FD[3-5]. Cannon et al[6] first described gastric accommodation in 1911. It is thought to be a vagally mediated reflex that occurs postprandially and results in reduction of tone, providing a reservoir for the meal[7]. In patients with impaired gastric accommodation, the proximal stomach cannot relax and change its volume to the content following meal ingestion, and the subsequent increase of intragastric pressure contributes to postprandial discomfort[8]. The impairment of proximal gastric accommodation has been found in 40% of patients with FD[3]. Hence, it is likely that FD can be diagnosed through the recognition of impaired gastric accommodation.
There are two methods to measure proximal gastric accommodation. One method is the intragastric barostat technique, in which a polyethylene bag is directly placed into the proximal stomach via oral intubation. The intragastric barostat bag technique is regarded as the gold standard because it allows simultaneous acquisition of volume, pressure, and tone, and makes the user correlate these variables to sensory parameters[9]. The disadvantages of the method are its interventional and time-consuming nature, leading to discomfort of patients[10], and a likely interference with gastric physiology due to pressure caused by the bag[11]. The second method is imaging, such as magnetic resonance (MR) imaging, single photon emission computed tomography (SPECT), and ultrasonography (US). MR imaging and SPECT can estimate volumetric change of the stomach directly and accurately[12], but the equipment is not widely available and is costly, and the natural state of the stomach impacted by gravity is also neglected owing to the flat position of the examination. In addition, there is a problem of SPECT-associated radiation exposure[13].
US is widely available, inexpensive, non-radioactive, and can be performed repeatedly, even at the bedside, making it a much more attractive option for the measurement of proximal gastric accommodation. Moreover, because gravity plays a role in the propulsion of gastric contents, accommodation should be measured in a sitting or standing position that can be easily accomplished during the US examination[12,14,15]. However, published studies reviewing the feasibility of this method are limited, presumably due to the complex procedure of scanning the stomach with US. Therefore, the aim of the current study was to investigate the usefulness of US, including 2-dimensional US imaging (2DUS) and 3-dimensional US imaging (3DUS), in the measurement of proximal gastric accommodation disorders in patients with FD compared to healthy controls.
Between April 2011 and March 2012, 46 consecutive patients with FD underwent US scanning. One patient was excluded from the study because of nephroptosis, which obscured the left kidney as a landmark in obtaining a sagittal plane of the proximal stomach. Thus, 45 enrolled patients consisted of 17 men and 28 women, with an age range of 19-64 years (mean: 33.70 ± 9.86 years) and a body mass index (BMI) of 16.33-25.95 kg/m2 (mean: 20.67 ± 2.34 kg/m2). None of the patients had a history of other abdominal diseases, abnormal hepatic function tests, organic changes on gastroendoscopy, and positive findings on routine abdominal US scanning.
The Rome III classification system was the basis for the diagnostic criteria for inclusion of patients with FD[1]. According to these criteria, the patient must have one or more of the following symptoms: bothersome postprandial fullness, early satiation, epigastric pain, or epigastric burning. Further, the patient could have no evidence of structural gastrointestinal diseases on upper endoscopy likely to explain the symptoms, and the symptoms must have occurred 6 mo prior to diagnosis and be active for the last 3 mo. Of the 45 patients, 33 (73.3%), 20 (44.4%), 19 (42.2%), and 14 (31.1%) presented with postprandial fullness, epigastric pain, early satiation, and epigastric burning, respectively.
Twenty-seven healthy volunteers were examined by US. This sample included 14 men and 13 women with an age range of 19-75 years (mean: 38.07 ± 14.55 years) and a BMI of 18.02-24.21 kg/m2 (mean: 21.10 ± 1.74 kg/m2). Healthy controls had no symptoms and physical signs of gastrointestinal diseases in the past six months, history of other abdominal diseases, abnormal hepatic function tests, and positive findings on routine abdominal US examination.
There were no statistically significant differences between the patients with FD and control groups with respect to age and BMI. Informed consent was obtained from all of the subjects.
A 500 mL esculent liquid was used as the test meal, and was prepared by mixing 200 mL of nutrient emulsion (Enteral Nutritional Emulsion; Sino-Swed Pharmaceutical Corp, Beijing, China) with 300 mL of warm water. The emulsion contained 15 g of protein, 11.6 g of fat, and 34 g of carbohydrate (300 kcal). To decrease the presence of small bubbles in the nutridrink, the meal was allowed to sit stationary on a table for approximately 10 min before consumption.
A Voluson 730 expert system with a RAB 2-5 type probe with 3DUS imaging function was employed (GE Medical Systems, Milwaukee, WI, United States).
To avoid increscent gas within the stomach, the examination was performed before 10:00 am after an overnight fasting of > 8 h. Administration of medication affecting gastrointestinal motility was discontinued for at least 48 h prior to US. Smoking was not allowed on the day of examination. All the patients were examined within 7 d following gastric endoscopy.
The subjects were scanned in a half-sitting position, leaning back at an angle of approximately 80° on an examining couch. The antrum was observed 2-3 min before nutridrink ingestion to avoid antral contractions and emptying into duodenum, in which the elevation of proximal stomach tone is induced by an enterogastric reflex occurring in phase III of the migrating motor complex[16]. Thereafter, in the other phases without the contraction, a 500 mL meal was ingested with a straw within 4 min. The proximal stomach of each subject was scanned every other 5 min during 25 min after beginning ingestion.
To assess image quality, a grading system based on the amount of air pockets in the proximal stomach was established as follows: grade 1 (absence of visible air within the stomach); grade 2 (some air within the stomach, but the following measurements still being able to be proceed); and grade 3 (a great amount of gastric air so that the image would be excluded from the measurement). Grading was done separately using 2DUS and 3DUS using five examinations for each subject.
Subjects were instructed not to move and to hold their breath at the end of expiration to permit diaphragmatic rising and restoration of the gastric configuration.
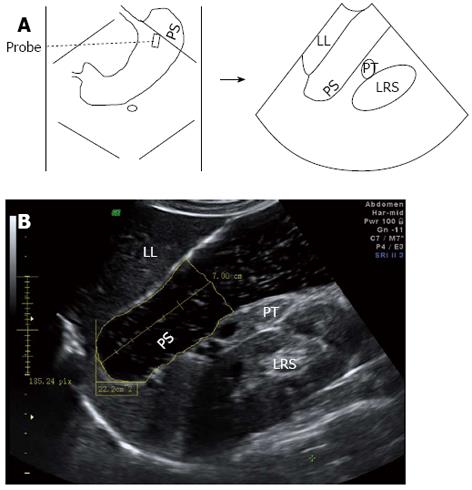
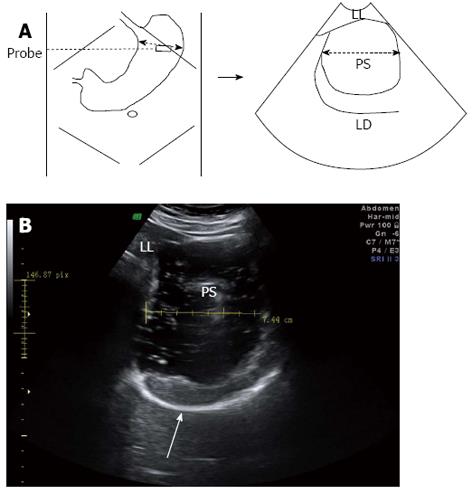
For 2DUS imaging, a scanning probe was placed longitudinally under the left subcostal margin and tilted cranially in the long axial direction to show the top of the gastric fundus. In this way, a sagittal section of the proximal stomach was visualized, in which the left renal sinus, the left lobe of the liver, and the tail of the pancreas served as anatomic landmarks (Figure 1). Then, the probe was rotated 90° and tilted cranially in the short axial direction to obtain a maximal transverse section of the proximal stomach, in which the left diaphragm and the left liver were landmarks (Figure 2). Image post-processing was done using image-processing software (4D View, version 5.0; GE Medical Systems). On the sagittal section, the proximal gastric area (PGA) was outlined by tracing along the luminal echogenic surface corresponding to the interface between the liquid and mucosa of the gastric wall, from the top margin of the fundus to 7 cm level inferiorly (Figure 1). On the transverse section, a maximal proximal gastric diameter (MPGD) was measured between the inner echogenic surfaces of the lesser and greater curvatures (Figure 2).
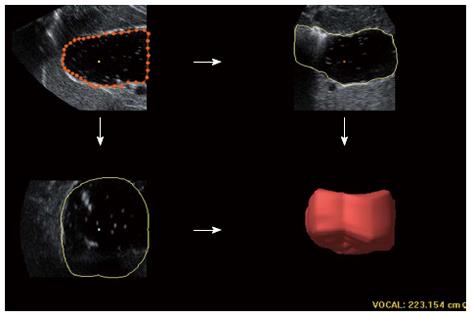
For 3DUS analysis, volumetric image data was acquired immediately following 2DUS using similar placement of the probe to that of the above sagittal section. A sweeping angle of 85° was set. The proximal stomach was scanned via automated sweeping between the curvatures over 5-10 s. The block cut at a distance of 7 cm inferior to the top of the fundus was saved for further processing on the workstation. Using a virtual organ computer-aided analysis (VOCAL) technique of the 4D View, six sections of one block were separately outlined manually along the echoic interface, with each rotating 30° from the previous section. The proximal gastric volume (PGV) was automatically calculated from these six highlighted areas and displayed as a reconstructive volume (Figure 3).
One physician (Wang L), blinded to the subject (FD patient or healthy control), completed the measurement. The mean value was calculated after two measurements.
The measurement values are presented as the mean ± SD. With repeated measures analysis of variance (ANOVA), the values of PGA, MPGD, and PGV were compared between two groups, as a total and at each scanning time within 25 min. Statistical significance was accepted as P < 0.05.
Three-hundred-and-sixty sets of 2DUS images (one sagittal and one transverse section), and the same number of 3DUS blocks, were graded. Of these, 225 were obtained from patients and 135 from controls in five time examinations. The 2DUS imaging revealed 50 (13.9%) and 310 (86.1%) sets of the image were determined as grades 1 and 2, respectively, and none were excluded due to grade 3. In 3DUS, 50 (13.9%) and 287 (79.7%) blocks were graded as 1 and 2, and the other 23 (6.4%) were grade 3 and in turn excluded from the measurement. Of these excluded blocks, 13 (56.5%) appeared at 10 min, and the remaining four (17.4%), three (13.0%), one (4.3%) and two (8.7%) occurred at 5, 15, 20 and 25 min after the meal, respectively.
The PGA and PGV of patients were significantly smaller than those of healthy controls (P = 0.000 and 0.002, respectively). When the two parameters were compared at each time point separately, the differences were also statistically significant between the two groups (P = 0.000-0.013), except at 10 min of the PGV.
The patients with FD revealed shorter MPGD than healthy controls postprandially; however, it was not statistically significant in the two groups, and the difference was significant (P = 0.026) only at 20 min when comparing each time point (Table 1).
| Time in min | Patients (n = 45) | Controls (n = 27) | t | P |
| PGA in cm2 | ||||
| 5 | 22.78 ± 6.59 | 30.68 ± 6.97 | 4.819 | 0.000 |
| 10 | 23.13 ± 6.39 | 29.52 ± 7.46 | 3.853 | 0.000 |
| 15 | 22.32 ± 5.93 | 27.51 ± 7.13 | 3.332 | 0.001 |
| 20 | 21.34 ± 6.34 | 28.25 ± 7.76 | 4.110 | 0.000 |
| 25 | 21.51 ± 6.02 | 26.35 ± 7.23 | 3.062 | 0.003 |
| F = 17.499 P = 0.000 | ||||
| MPGD in cm | ||||
| 5 | 6.77 ± 1.34 | 7.08 ± 1.10 | 1.035 | 0.304 |
| 10 | 6.92 ± 1.31 | 7.27 ± 1.00 | 1.185 | 0.240 |
| 15 | 6.66 ± 1.43 | 7.11 ± 1.03 | 1.432 | 0.157 |
| 20 | 6.33 ± 1.29 | 7.00 ± 1.06 | 2.275 | 0.026 |
| 25 | 6.31 ± 1.50 | 6.66 ± 1.24 | 1.032 | 0.305 |
| F = 2.562 P = 0.114 | ||||
| PGV in cm3 | ||||
| 5 | 145.75 ± 60.40 | 185.08 ± 60.81 | 2.645 | 0.010 |
| 10 | 152.91 ± 52.10 | 177.13 ± 59.10 | 1.797 | 0.077 |
| 15 | 142.46 ± 49.50 | 184.16 ± 52.28 | 3.358 | 0.001 |
| 20 | 132.45 ± 46.70 | 169.12 ± 48.64 | 3.147 | 0.002 |
| 25 | 126.15 ± 50.23 | 157.46 ± 49.97 | 2.544 | 0.013 |
| F = 10.319 P = 0.002 | ||||
Based on the theory that the impairment of proximal gastric accommodation is likely to lead to the pathogenesis of FD, both 2DUS and 3DUS imaging were utilized to measure the size of the proximal stomach. The data indicated that both PGA and PGV could help assess the proximal gastric accommodation.
US can provide an indirect evaluation of stomach relaxation and intragastric pressure by measuring the size of the stomach[17]. The 2DUS method of assessing gastric accommodation was developed first by Gilja et al[18]. According to their study, 2DUS could offer a geometric estimation of proximal gastric size by measuring PGA and MPGD. They also found that the patients with FD exhibited a smaller PGA and MPGD than controls (P = 0.018 and 0.046, respectively)[19]. Having adopted a similar method, our study showed that the PGA was significantly different between FD patients and healthy controls, but the MPGD was not different overall, being significant only at 20 min (P = 0.026). The reason why PGA was superior to MPGD could be related to an irregular shape of the proximal stomach. When estimating the size of an organ that had an irregular contour, using an area was likely more accurate than a diameter. The other reason might be that the left renal sinus, which served as a key landmark in a sagittal section used for PGA measurement, had a relatively narrow distance in a transverse direction and a constant relationship to the proximal stomach. Therefore, the measurement of PGA might tend to be of less operator variability in various examination time points. Besides, when MPGD was transversely measured, the right cursor was placed on or near the gastric lesser curvature with less expansive function[20], and hence the difference of the transverse dimension between the patient and the control would be decreased. This phenomenon also occurred in other previous studies[21,22]. Thus, the application value of MPGD was limited in assessment of gastric accommodation.
The feasibility of assessing the proximal gastric accommodation with PGA on 2DUS images has been confirmed by several studies[21-24]. The measuring of PGA was simple, with only one section being outlined in this process. It was less likely to be affected by gastric air, which was verified since no subject was excluded for this factor. However, it was difficult to find the landmarks in subjects with obese body types, nephroptosis, or renal ectopy. The volumetric estimation that was based on values of 2DUS, i.e., V = PGA × MPGD, and adopted by other studies before the advent of 3DUS could to some extent bring an error because of the irregular-shaped stomach[18].
In general, 3DUS has advantages over 2DUS, which can measure a volume directly and needs no landmarks. Using this technique, Gilja et al[25] obtained a good correlation (r = 0.997, P < 0.05) between the estimated volume of porcine stomach filled with water in vitro and the actual quantity of water injected. Another study demonstrated that 3DUS had a moderate correlation (r = 0.55, P = 0.002) with the barostat in measurement of proximal gastric volumes[26]. However, there were some drawbacks of the freehand 3DUS technique used in these studies[23,24]. An additional tracking device that consisted of a transmitter generating a pulse magnetic field and a position sensor attached to the probe was required. Therefore, the environment where the patient was examined requires magnetic shielding to avoid image distortion. The distortion also appeared while the data of both image and position could not be transformed simultaneously to a post-processing workstation. The time-consuming process of coordinating images with their spatial locations also restricted it. The previous method of 3DUS scanning was still limited by the complicated manipulation in which the operator had to scan several times to obtain an image with high quality, because the image was easily distorted in free-hand moving a probe on body surface at an appropriate speed. In the current study, a new type of 3DUS probe (RAB 2-5, Voluson 730) was used, in which a 1D transducer array moving mechanically through a designed trajectory was mounted together with integrated positioning system and sensor. Hence, the data acquisition was automatic rather than manual, could be done during a single breath-hold, and could be displayed on the monitor immediately after scanning. Using this type of transducer, PGV could be obtained in most (337/360, 93.6%; air pockets grading 1 and 2) of the 3DUS blocks at five examination time points. Consequently, the patients with FD showed smaller PGV than healthy controls (P = 0.002). Adopting the same transducer, Manini et al[27] measured the whole gastric volume accurately, with the results comparable to that from SPECT, establishing a reference standard for measuring gastric size.
An air grading system was designed to assess the image quality. Intragastric gas is a critical interference factor in US stomach examination, which may induce multiple reflection artifacts and limit the stomach outline. The accumulation of gas in the fundus was a gradually incremental process. Small bubbles could be seen in the entire stomach immediately after drinking a test meal, in addition to the usual existence of fundus air pockets, but these bubbles did not decline the image quality. Over time, they burst and mixed into the fundus air pockets, which to some degree obstructed the visualization of the gastric wall, especially the posterior wall. Within minutes, the gas reduced because of burping and the image quality was improved again. In our results, none of images were excluded from the 2DUS analysis but 23 (6.4%) 3DUS blocks could not be utilized due to the grade 3 air pockets. We hypothesize that the higher resolution of the single section 2DUS images[28], combined with fact that 3DUS was more subject to the intragastric gas explains this phenomenon. We also found that most of the grade 3 (13/23, 56.5%) appeared at 10 min after the beginning of ingestion. The decrease of sample size after excluding the 13 3DUS blocks might cause no significant difference in PGV measurement between two groups this time. These results suggested that 3DUS testing at the 10 min time point or with the appearance of fundus air pockets should be avoided.
There were some limitations in the current study. The limited sample size did not allow us to divide patients into two subgroups of postprandial distress syndrome and epigastric pain syndrome according to the Rome III criteria[1]. After fasting overnight, ingesting a 500 mL test meal in a short time would make subjects uncomfortable, and consequently the smaller amount of the meal should be tested. The observation duration of 25 min might not be enough to investigate into the gastric accommodation, which lasts in the entire process of postprandial digestion[29]. Because no reference method as the barostat procedure or SPECT was adopted in our study, the comparative study of 2DUS and 3DUS imaging could not be carried out to find out which one was more accurate.
In conclusion, we show that the impaired gastric accommodation to a test meal was present in patients with FD. Two parameters of PGA and PGV on 2DUS and 3DUS images could be used for assessing the proximal gastric accommodation in which 2DUS was simpler in manipulation and less likely to be degraded by gastric gas, and 3DUS had the merit of measuring volume directly, providing less gastric gas.
There is a high global incidence of functional dyspepsia (FD), with about half of the reported gastroenterological outpatients being in China. Several studies have indicated that impaired gastric accommodation is a major pathogenesis of the disease. Hence, assessment of proximal gastric accommodation disorder could help diagnose FD.
Imaging can provide an indirect evaluation of gastric relaxation in accommodation reflex by measuring the size (i.e., area and volume) of the proximal stomach. Ultrasonography is a preferable imaging method, which is accurate, easy to manipulate, non-invasive, and cost effective.
The proximal gastric accommodation was evaluated by measuring the proximal gastric area (PGA) and proximal gastric volume (PGV) in patients with FD using 2-dimensional and 3-dimensional ultrasound. PGA and PGV of the patients were significantly smaller than the controls. Therefore, the change of PGA or PGV could be pertinent to the impairment of gastric accommodation and utilized in predicting FD.
US is likely to be a convenient and accurate imaging modality to assess the gastric accommodation. It has the potential value of diagnosing FD and evaluating the effect of therapy.
The proximal stomach is a compartment consisting of the fundus and the upper one-third of gastric body, which has the function of gastric accommodation. The boundary between the proximal and distal stomach has not been definitely determined anatomically and physiologically. Gastric accommodation is a vagally mediated reflex that occurs postprandially, results in reduction of gastric tone, and provides a reservoir for the meal.
In this study, the authors found that both PGA and PGV on 2-dimensional ultrasound and 3-dimensional ultrasound images could be used in assessment of the proximal gastric accommodation. Very interesting study, it could give the readers some new information about the use of US in evaluation of proximal gastric accommodation disorder in patients with functional dyspepsia. The manuscript is well written.
P- Reviewers Flier SN, Malfertheiner P, Monnikes H S- Editor Wen LL L- Editor A E- Editor Zhang DN
| 1. | Tack J, Talley NJ, Camilleri M, Holtmann G, Hu P, Malagelada JR, Stanghellini V. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466-1479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1243] [Cited by in F6Publishing: 1154] [Article Influence: 64.1] [Reference Citation Analysis (0)] |
| 2. | Mahadeva S, Goh KL. Epidemiology of functional dyspepsia: a global perspective. World J Gastroenterol. 2006;12:2661-2666. [PubMed] [Cited in This Article: ] |
| 3. | Tack J, Piessevaux H, Coulie B, Caenepeel P, Janssens J. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology. 1998;115:1346-1352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 771] [Cited by in F6Publishing: 734] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 4. | Miwa H, Watari J, Fukui H, Oshima T, Tomita T, Sakurai J, Kondo T, Matsumoto T. Current understanding of pathogenesis of functional dyspepsia. J Gastroenterol Hepatol. 2011;26 Suppl 3:53-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Salet GA, Samsom M, Roelofs JM, van Berge Henegouwen GP, Smout AJ, Akkermans LM. Responses to gastric distension in functional dyspepsia. Gut. 1998;42:823-829. [PubMed] [Cited in This Article: ] |
| 6. | Cannon W, Lieb C. The receptive relaxation of the stomach. Am J Physiol. 1911;29:267-273. [Cited in This Article: ] |
| 7. | Choung RS, Talley NJ. Novel mechanisms in functional dyspepsia. World J Gastroenterol. 2006;12:673-677. [PubMed] [Cited in This Article: ] |
| 8. | Distrutti E, Azpiroz F, Soldevilla A, Malagelada JR. Gastric wall tension determines perception of gastric distention. Gastroenterology. 1999;116:1035-1042. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 172] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | van der Schaar PJ, Lamers CB, Masclee AA. The role of the barostat in human research and clinical practice. Scand J Gastroenterol Suppl. 1999;230:52-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Whitehead WE, Delvaux M. Standardization of barostat procedures for testing smooth muscle tone and sensory thresholds in the gastrointestinal tract. The Working Team of Glaxo-Wellcome Research, UK. Dig Dis Sci. 1997;42:223-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 252] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 11. | de Zwart IM, Haans JJ, Verbeek P, Eilers PH, de Roos A, Masclee AA. Gastric accommodation and motility are influenced by the barostat device: Assessment with magnetic resonance imaging. Am J Physiol Gastrointest Liver Physiol. 2007;292:G208-G214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Schwizer W, Steingötter A, Fox M, Zur T, Thumshirn M, Bösiger P, Fried M. Non-invasive measurement of gastric accommodation in humans. Gut. 2002;51 Suppl 1:i59-i62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | De Schepper HU, Cremonini F, Chitkara D, Camilleri M. Assessment of gastric accommodation: overview and evaluation of current methods. Neurogastroenterol Motil. 2004;16:275-285. [PubMed] [Cited in This Article: ] |
| 14. | Gilja OH, Lunding J, Hausken T, Gregersen H. Gastric accommodation assessed by ultrasonography. World J Gastroenterol. 2006;12:2825-2829. [PubMed] [Cited in This Article: ] |
| 15. | Gilja OH, Hatlebakk JG, Odegaard S, Berstad A, Viola I, Giertsen C, Hausken T, Gregersen H. Advanced imaging and visualization in gastrointestinal disorders. World J Gastroenterol. 2007;13:1408-1421. [PubMed] [Cited in This Article: ] |
| 16. | Savoye G, Bouin M, Hervé S, Denis P, Ducrotté P. Gastric tone variations during gastric infusion of fiber-supplemented formulas. J Gastroenterol Hepatol. 2005;20:409-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Moloo SK, Kutuza SB. Effect of Samorin administered to a bovine host on the survival and reproductive performance of female Glossina morsitans centralis. Ann Trop Med Parasitol. 1987;81:743-744. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 18. | Gilja OH, Hausken T, Odegaard S, Berstad A. Monitoring postprandial size of the proximal stomach by ultrasonography. J Ultrasound Med. 1995;14:81-89. [PubMed] [Cited in This Article: ] |
| 19. | Gilja OH, Hausken T, Wilhelmsen I, Berstad A. Impaired accommodation of proximal stomach to a meal in functional dyspepsia. Dig Dis Sci. 1996;41:689-696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 197] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Schulze-Delrieu K, Shirazi SS. Pressure and length adaptations in isolated cat stomach. Am J Physiol. 1987;252:G92-G99. [PubMed] [Cited in This Article: ] |
| 21. | Undeland KA, Hausken T, Gilja OH, Aanderud S, Berstad A. Gastric meal accommodation studied by ultrasound in diabetes. Relation to vagal tone. Scand J Gastroenterol. 1998;33:236-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 49] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Tefera S, Gilja OH, Hatlebakk JG, Berstad A. Gastric accommodation studied by ultrasonography in patients with reflux esophagitis. Dig Dis Sci. 2001;46:618-625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Olafsdottir E, Gilja OH, Aslaksen A, Berstad A, Fluge G. Impaired accommodation of the proximal stomach in children with recurrent abdominal pain. J Pediatr Gastroenterol Nutr. 2000;30:157-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Izbéki F, Kiss I, Wittmann T, Várkonyi TT, Légrády P, Lonovics J. Impaired accommodation of proximal stomach in patients with alcoholic liver cirrhosis. Scand J Gastroenterol. 2002;37:1403-1410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Gilja OH, Detmer PR, Jong JM, Leotta DF, Li XN, Beach KW, Martin R, Strandness DE. Intragastric distribution and gastric emptying assessed by three-dimensional ultrasonography. Gastroenterology. 1997;113:38-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 95] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Mundt MW, Samsom M. Fundal dysaccommodation in functional dyspepsia: head-to-head comparison between the barostat and three-dimensional ultrasonographic technique. Gut. 2006;55:1725-1730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Manini ML, Burton DD, Meixner DD, Eckert DJ, Callstrom M, Schmit G, El-Youssef M, Camilleri M. Feasibility and application of 3-dimensional ultrasound for measurement of gastric volumes in healthy adults and adolescents. J Pediatr Gastroenterol Nutr. 2009;48:287-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Brattain LJ, Howe RD. Real-time 4D ultrasound mosaicing and visualization. Med Image Comput Comput Assist Interv. 2011;14:105-112. [PubMed] [Cited in This Article: ] |
| 29. | Barrett KE, Malley J, Naglieri C. Gastrointestinal physiology. New York: McGraw-Hill 2006; . [Cited in This Article: ] |